Abstract
FKBP52 is a tetratricopeptide repeat (TPR) protein with peptidyl-prolyl isomerase activity and is found in steroid receptor complexes, including glucocorticoid receptor (GR). It is generally accepted that FKBP52 has a stimulatory effect on GR transcriptional activity. However, the mechanism by which FKBP52 controls GR is not yet clear, with reports showing effects on GR hormone-binding affinity and/or hormone-induced nuclear translocation. To address this issue, we have generated mice with targeted ablation of the FKBP52 gene. To date, no overt defects of GR-regulated physiology have been found in these animals, demonstrating that FKBP52 is not an essential regulator of global GR activity. To better assess the impact of FKBP52 on GR, mouse embryonic fibroblasts (MEFs) were generated from wild-type (WT) and FKBP52-deficient (KO) animals. Analysis of GR activity at reporter genes showed an approximate 70% reduction of activity in 52KO MEF cells, with no effect of FKBP52 loss on thyroid receptor. Interestingly, GR activity at endogenous genes was not globally affected in 52KO cells, with reduced activity at GILZ and FKBP51, but not at SGK and p21. Thus, FKBP52 appears to be a gene-specific modulator of GR. To investigate the mechanism of this action, analyses of GR heterocomplex composition, hormone-binding affinity, and ability to undergo hormone-induced nuclear translocation and DNA-binding were performed. Interestingly, no effect of FKBP52 loss was found for any of these GR properties, suggesting that the main function of FKBP52 is a heretofore-unknown ability to control GR activity at target genes. Lastly, loss of FKBP52 did not affect the ability of GR to undergo hormone-induced autologous down-regulation, showing that FKBP52 does not contribute to all branches of GR signaling. The implications of these results to the potential actions of FKBP52 on GR activity in vivo are discussed.
1. Introduction
The FK506-binding Protein 52 (FKBP52) was discovered as a component of progesterone receptor complexes isolated from rabbit uterus [1]. Originally, the protein was called p59 but has since gone by several names, including p56 [2], HBI [3], HSP56 [4], and most recently, FKBP52, based on its ability to bind the immunosuppressive ligand FK506 [5-7]. Because of the latter property, FKBP52 is often referred to as an immunophilin, and like most members of this family, it has peptidy-prolyl cis-trans isomerase activity (PPIase) that is inhibited by the binding of FK506 [5]. However, there is little evidence that FKBP52 acts to effect immunosuppression in lymphocytes like the true immunophilin FKBP12. Instead, the best known function of FKBP52 is to control activity of steroid receptors (SR) [see Toft & Pratt for review [8]]. The latter activity derives principally from the ability of FKBP52 to interact with hormone-free receptor complexes utilizing conserved protein-protein interaction motifs known as tetratricopeptide repeats (TPR) [5, 9-12]. Hence, FKBP52 is more accurately referred to as TPR protein in the context of SR signaling.
As a hormone-activated transcription factor, glucocorticoid receptor (GR) is most often studied with respect to its transcriptional activation function [13]. Yet, the impact of FKBP52 on GR is generally thought to be a co-chaperone function controlling the early stages of receptor signaling. FKBP52 enters into receptor complexes by directly binding HSP90 [14], which in turn binds receptors at the conserved Signal Transduction Domain located within the larger ligand-binding region [15, 16]. Other members of the TPR family exist which similarly enter into mature SR complexes. The best known of these are FKBP51, cyclophilin-40 (Cyp40) and protein phosphatase 5 (PP5). Because HSP90 generates only one TPR acceptor site per receptor complex [14, 17], a variety of receptor complexes are possible, even within the same cell, based on TPR protein content. This heterogeneity of structure is an active area of investigation since it implies differential regulation of SR activity.
Most investigations of the GR/FKBP52 relationship have pointed to a role for FKBP52 in the regulation of GR hormone-binding function. In early work, FK506 treatment of cells was found to cause a potentiation of steroid-induced GR transcriptional activity [18]. Although this effect has been ascribed to inhibition of steroid export by p-glycoprotein membrane pumps [19], subsequent studies showed that the cellular FK506 potentiation effect is partly due to disruption of the FKBP51 and FKBP52 interaction with receptor, leading to recruitment of PP5 and increased affinity of GR for hormone [20]. Biochemical approaches have supported this conclusion, as treatment of cell-free lysates with FK506 increases the hormone-binding affinities of both GR [21] and PR [22]. More recent molecular approaches have yielded similar results. When GR and FKBP52 are expressed in yeast a potentiation of GR transcriptional activity is seen that correlates with increased affinity for hormone [23]. In mammalian cells, over-expression of FKBP52 causes a similar potentiation of both transactivity and hormone-binding function [20].
A role for FKBP52 in subcellular trafficking of receptors has also been proposed. In the intact cell FKBP52 is found diffusely distributed in the nucleus, but discretely localized to microtubule filaments in the cytoplasm [24-26]. Early purification attempts showed FKPB52 interaction with the microtubule-based motor protein dynein [25, 26] and that this interaction requires the PPIase domain of the protein [27, 28]. It is now known that the FKBP52/dynein interaction is indirect, requiring the intermediary protein dynamitin [29]. Indeed, the most recent data show that hormone-free GR can be recovered in a complex that contains most members of the dynein motor complex, yielding the following constitution: GR, HSP90, FKBP52, dynamitin, dynein and α-tubulin [30]. While these data point to FKBP52 as a central linker in the motor complex required for retrograde movement, there is even now evidence for an active role of FKBP52 in microtubule de-polymerization [31].
Although the above suggests an essential role for FKBP52 in GR nuclear translocation, functional evidence for this is more limited. Pratt and co-workers showed that expression of an FKBP52 PPIase domain fragment in cells blocked hormone-induced nuclear translocation of a green fluorescent protein (GFP)-GR construct [32]. Consistent with this, our laboratory has uncovered a switching mechanism in which hormone causes displacement of FKBP51 by FKBP52 in GR complexes [33]. As the newly-formed GR/HSP90/FKBP52 complex was found to accumulate in the nucleus, and as hormone-free GR complexes in most cells contain, not FKBP52, but FKBP51 (unpublished observation), which does not interact with dynein [34], this observation provides the reasonable next step by which the FKBP52/dynein interaction leads to GR translocation. Finally, Rein and co-workers have shown that over-expression of FKBP51 attenuates hormone-induced GR translocation [34]. Curiously, over-expression of FKBP52 did not increase GR translocation, although it did block the inhibitory effect of FKBP51.
Using targeted ablation, we have recently generated mice deficient in FKBP52. To our surprise, these animals have not yet shown dramatic defects of GR-regulated physiology. Instead, FKBP52-deficient females were found to be sterile due to a defect of progesterone receptor action in the uterus, leading to a failure of implantation [35]; while FKBP52-deficient males are infertile due to development of hypospadias arising from attenuation of androgen receptor activity [36]. Similar results in male and female mice have been reported by the Smith and Dey laboratories [37] [38]. The lack of GR phenotype in FKBP52-deficient animals suggests that other members of the TPR family may serve a compensatory role for the actions of FKBP52. This speculation is supported by the following. First, we now know that hormone-free GR complexes in most cells contain, not FKBP52, but FKBP51 and PP5 [39], and that increased amounts of PP5 serve to increase GR hormone-binding function [20]. Second, in addition to FKBP52, both Cyp40 and PP5 are known to interact with the dynein motor complex [29]. Thus, it is possible that these TPRs serve overlapping roles with FKBP52 to maintain GR activity by ensuring adequate hormone-binding and nuclear translocation functions.
With these concepts in mind, we have generated mouse embryonic fibroblast (MEF) cells from wild-type (WT) and FKBP52-knockout (KO) mice. The MEF cells were used to assess the impact of FKBP52 loss on the major stages of GR signaling. In keeping with the compensation model, we report that FKBP52 loss had no affect on the early stages of GR signaling, including composition of the hormone-free complex, hormone-binding function and nuclear translocation. In spite of this, an unexpected decrease in GR transcriptional enhancement activity was seen in the FKBP52 KO cells. However, the reduction was only partial and gene specific. We conclude that FKBP52 is a modulatory, rather than essential, factor controlling GR function at select genes. Such results are consistent with the lack of overt and deleterious phenotypes in the FKBP52 KO animals. More importantly, our results have uncovered a new FKBP52 function as a down-stream regulator of GR transcriptional activity. Although the exact mechanism by which FKBP52 controls GR gene activity is not yet clear, its eventual elucidation should have an important impact on endocrine physiology and disease.
2. Materials and Methods
2.1 Materials
[3H]acetate was from ICN Radiochemicals (Cleveland, OH). [3H]Dexamethasone (Dex) was purchased from Dupont-New England Nuclear (Boston, MA). ATP, acetyl coenzyme A (CoA) synthetase, acetyl CoA, Tris, HEPES, EDTA, sodium molybdate, Hank's balanced salts, protease inhibitor cocktail, hydrogen peroxide, dexamethasone, NaCl, sodium acetate, chloramphenicol, phosphate buffered saline, dextran, luminol, p-courmaric, technical grade mouse IgG2a, protein A-Sepharose, DMEM powered medium, and goat anti-mouse IgG horseradish peroxidase-conjugate were from Sigma Chemical Co. (St. Louis, MO). Lipofectamine 2000, OPTI-MEM, glycerol, and goat serum were from Invitrogen Corp (Carlsbad, CA). Iron-supplemented bovine calf serum was from Hyclone Laboratories, Inc (Logan, UT). Immobilon-P membrane was from Millipore Corp (Bedford, MA). BCA protein assay kit was from Pierce Chemical Co. (Rockford, IL). FiGR monoclonal antibody was a gift from Jack Bodwell (Dartmouth Medical School, Hanover, NH). PP5 antibody was a gift from Michael Chinkers (University of South Alabama, College of Medicine, Mobile, AL). Cyp40 (PA3-022) and FKBP52 (UPJ52) antibodies were from Affinity Bioreagents (Golden, CO). Antibodies to FKBP51 (sc-11518), HSP90 (sc-13119), bovine anti-goat IgG horseradish peroxidase-conjugate (sc-2350) were from Santa Cruz Biotechnologies (Santa Cruz, CA). Luciferase Assay kit was from Promega (Madison, WI). Fluorescein-conjugated goat anti-mouse IgG was obtained from Calbiochem (San Diego, CA).
2.2 Cell Lines and Culture
Mouse embryonic fibroblast (MEF) cells were obtained from wild-type (WT) and FKBP52-deficient (KO) embryos at day 13.5 of gestation [35]. Embryonic cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with 15% fetal bovine serum until fibroblasts attached and proliferated. Primary fibroblasts were immortalized via transfection with SV40 large T antigen. Mouse L929 fibroblast cells were obtained from American Type Culture Collection (ATCC), Inc. Established cell lines were maintained in DMEM with 10% bovine calf serum in an atmosphere of 5% CO2 at 37°C. All cell treatments were done at or near confluence. For measurement of steroid responses, cells were grown in DMEM containing 10% charcoal-stripped serum 24 h prior to treatment.
2.3 Gel Electrophoresis and Western Blotting
Whole cell extracts (WCE) were prepared by freezing cell pellet for 30 minutes to overnight at −80°C. Pellet was then resuspended in 3 volumes of WCE buffer (20 mM HEPES, 0.42 M NaCl, 0.2 M EDTA, 25% glycerol, pH 7.4) plus protease inhibitor cocktail and incubated on ice for ten min followed by 100,000 × g centrifugation at 4°C. Protein content was determined by BCA method of Pierce. Samples were resolved on denaturing SDS gels and transferred to Immobilon-P membranes. Immunoblotting was achieved by probing of membranes with appropriate primary and peroxidase-conjugated secondary antibodies, followed by enhanced chemiluminescence.
2.4 Immunoadsorption of GR Complexes
Cells were harvested in HEMG (10 mM HEPES, 3 mM EDTA, 20 mM sodium molybdate, 10% glycerol, pH 7.4) plus protease inhibitor cocktail and set on ice for ten min followed by Dounce homogenization. Supernatants (cytosol) were collected proceeding a 30 min 4°C centrifugation at 20,800 × g, then pre-cleared with protein A-Sepharose nutating for 1 h at 4°C. Samples were spun down, split into equal aliquots of cytosol, and immunoadsorbed overnight with FiGR antibody against GR or non-immune mouse IgG at 4°C under constant rotation. Pellets were washed 5-7 times with TEG (10 mM Tris, 3 mM EDTA, 10% glycerol, 50 mM NaCl, 20 mM sodium molybdate, pH 7.4) and complexes were eluted with 4XSDS sample buffer.
2.5 Cellular Fractionation
MEF cells were treated cells with 1 μM Dex or vehicle control at 37°C for 1 hour, followed by Dounce homogenization in HEMG buffer plus protease inhibitors and a 20,800 × g centrifugation for 30 min at 4°C. Supernatant (cytosolic fraction) was saved, while nuclear pellet was extracted with HEMG containing 0.5 M NaCl and protease inhibitors for 1 hour on ice, followed by centrifugation at 20,800 × g for 30 minutes at 4°C. Prior to immunoadsorption, the cytosolic fractions were normalized to 0.5 M NaCl. Cytosolic and nuclear fractions were immunoadsorbed using protein A-sepharose and FiGR antibody by rotating at 4°C for two hours. Pellets were washed three times with TEG (10 mM Tris, 3 mM EDTA, 10% glycerol, 50 mM NaCl, 20 mM sodium molybdate, pH 7.4) and GR was eluted using 4XSDS sample buffer.
2.6 Indirect Immunofluorescence
MEF cells were grown in DMEM containing 10% charcoal-stripped serum on coverslips to 50% confluence. Cells were treated with either 1 μM Dex or vehicle at designated times over the course of one h at 37°C. Coverslips were washed 2X in HBSS then fixed and permeabilized with methanol for 15 min at 25°C. Permeabilized cells were blocked in 10% goat serum in PBS for 20 min at 25°C. Cells were incubated for 1 h at 25°C with FiGR antibody at 1:100 dilution, washed 3X in blocking buffer, then incubated with fluorescein-conjugated secondary antibody at 1:20 dilution for 1 h. Cells were visualized with 100X objective lens on a Nikon Eclipse E800 microscope and photographed with a Sensys digital camera.
2.7 Saturation Binding Assay
MEF cell cytosolic lysates were prepared, as described above. Replicate aliquots of equal protein were incubated at 4°C with increasing concentrations of [3H]Dex in the absence or presence of 50 μM radioinert Dex for 2 h. Free radiolabeled ligand was extracted with 1% dextran-coated charcoal in 10 mM HEPES buffer. Radioactivity was measured by a scintillation counter and specific binding values were calculated then converted to fmol GR per mg protein. Calculations of dissociation constants (Kd) and maximal binding (Bmax) were determined through non-linear regression analysis using a one-site binding equation (Graph Pad Prism software; GraphPad, San Diego, CA).
2.8 Transfection and Reporter Assay
MEF cells were grown to 90% confluence in 6-well plates then transiently transfected with expression vectors for GR or TR and RXR using Lipofectamine 2000, according to manufacturer's protocol. MMTV-CAT, pGRE2EIB-Luc, or TRE-Luc reporters were co-transfected, as appropriate, along with a CMV-galactosidase reporter to normalize for transfection efficiency. Twenty-four h post-transfection cells were treated with vehicle or hormone at the indicated concentrations for an additional 24 h until harvest. Cell lysates were prepared by repeated freezing and thawing in HEG (10 mM HEPES, 3 mM EDTA, 10% glycerol, pH 7.4) plus protease inhibitor cocktail, followed by a 20 min 20,800 × g centrifugation at 4°C. CAT enzyme activity was measured by the method of Nordeen et al [40] using [3H]acetyl-CoA as substrate, while luciferase activity was measured using Promega's luciferase assay system.
2.9 Electrophoretic Mobility Shift Assay
Nuclear extracts from vehicle- or Dex-treated WT52 and KO52 cells were prepared by suspension of cells in pellet lysis buffer (10 mM Tris, pH 7.4, 3 mM MgCl2, 10 mM NaCl, and 0.5% Nonidet-P40), followed by centrifugation at 3000×g for 15 min. The nuclear pellets were treated with Buffer A (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT) and centrifuged at 3000×g for 15 min. The nuclear pellets were further treated by stirring for 60 min at 4°C in Buffer B (20 mM HEPES, pH 7.9, 1.5 mM MgCl2, 0.2 mM EDTA, 0.42 M NaCl, 25% glycerol, 0.5 mM DTT, 0.5 mM PMSF). Nuclear extracts were obtained by centrifugation for 60 min at 25,000×g, followed by measurement of protein content. Electrophoretic mobility shift assays were performed as originally described by Sen and Baltimore [41], using a synthetic oligonucleotide corresponding to the GRE at position −191 to −159 of the MMTṼLTR promoter [42]. Sequence of the oligonucleotide is as follows: 5′-GTT, TAT, GGT, TAC, AAA, CTG, TTC, TTA, AAA, CAA, GGA-3′. The GRE was end-labeled with [γ-32P] using T4 polynucleotide kinase. EMSA assays were performed by mixing 10 μg of nuclear extract with 0.1 ng (40,000 cpm) of 32P-labeled GRE oligonucleotide and 1 μg of poly(dI-dC) in 1× gel shift buffer (10 mM Tris–HCl, pH 7.5, 50 mM NaCl, 1 mM MgCl2, 0.5 mM EDTA, 0.5 mM dithiothreitol, 4% glycerol) in a final volume of 20 μl. The reactions were carried out at room temperature for 30 min, and protein–DNA complexes were analyzed on a 4% polyacrylamide gel in 0.5× TBE.
2.10 Quantitative Real-Time PCR
Total RNA was isolated from MEF cells using 5 Prime PerfectPure RNA Cell Kit (Fisher Scientific Company, LLC) according to the manufacturer's instructions. Total RNA concentration and purity was determined by measuring absorbance at 260/280 nm and confirmed on an RNA denaturing formaldehyde gel. cDNA was synthesized using iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA). PCR amplification of the cDNA was performed using qPCR Core kit for SYBR Green I (Bio-Rad). Primers were designed using Primer Express 3.0 software (Applied Biosystems). The following primers were used in this study: GILZ (TGACTGCAACGCCAAAGC and CTGATACATTTCGGTGTTCATGGTT), SGK (GAGAAGGATGGGCCTGAACGAT and CGGACCCAGGTTGATTTGTTGA), p21 (GGCAGACCAGCCTGACAGAT and TTCAGGGTTTTCTCTTGCAGAAG), and FKBP51 (GCTGGCAAACAACACGAGAG and GAGGAGGGCCGAGTTCATT). For normalization, in separate reactions, primers were used to amplify 18S mRNA (TTCGAACGTCTGCCCTATCAA and ATGGTAGGCACGGCGACTA). The thermocycling protocol consisted of 10 min at 95 °C, 40 cycles of 1 sec at 95 °C, 1 min at 60 °C, and 15 sec at 72 °C and finished with a melting curve ranging from 60–95 °C to allow discrimination of specific products. All data were normalized to 18S.
3. Results
3.1 Analysis of GR and immunophilin content in wild-type and FKBP52-deficient MEF cells
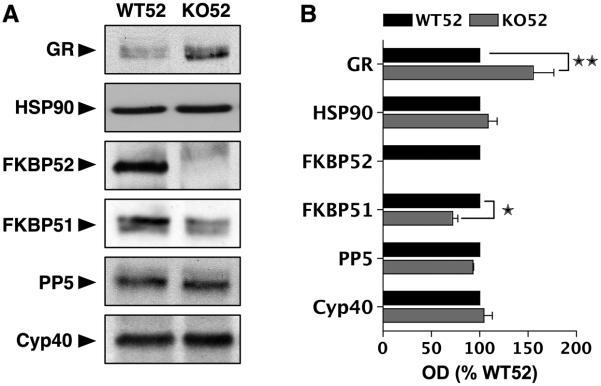
As a first step to assess the impact of FKBP52 loss, we analyzed the expression profile of GR, HSP90 and TPRs in wild-type (WT52) and FKBP52 KO (KO52) MEF cells. Fig. 1 shows quantitative Western-blot analysis of the proteins present in whole cell extracts derived from untreated cells. Both WT52 and KO52 cells were found to express endogenous GR, abrogating the need, under most circumstances, to transfect the receptor. Interestingly, the KO52 cells had more GR than WT52 cells (1.75 fold increase). At present we do not know if there is a functional relationship between FKBP52 and equilibrium levels of GR protein. Expression levels of HSP90, PP5 and Cyp40 in the KO52 cells were normal. However, FKBP51 expression was significantly decreased in the KO52 cells. Because FKBP51 expression is regulated by several steroid receptors, including androgen receptor [43, 44], progesterone receptor [45] and GR [46], we suspected that reduced FKBP51 was an indirect effect of compromised GR activity due to FKBP52 loss (see below). All MEF cells so far tested do not express androgen or progesterone receptors.
Fig. 1.
Expression profile of GR and TPR proteins in wild-type and FKB52-deficient MEF Cells. Panel A: Western blot of GR, HSP90, FKBP52, FKBP51, PP5 and Cyp40 in whole-cell extracts from wild-type (WT52) and FKBP52-deficient (KO52) MEF cells. Panel B: Quantitation of results in Panel A was achieved by densitometric scanning of films. Values for each protein expressed as percentage of WT52 control and represent means +/− S.E.M. of three independent experiments. P > 0.05 vs WT; P > 0.01 vs WT.
3.2 Interaction of hormone-free GR with FKBP51 and PP5 is unaffected by loss of FKBP52
As a next step, we analyzed the composition of hormone-free GR complexes in WT52 and KO52 MEF cells. Recently, we have undertaken a systematic approach to determination of TPR protein composition in steroid receptor complexes by testing for the presence of all four TPRs under standard conditions. This approach has become necessary due to conflicting reports in the literature concerning SR/TPR interaction. For example, many reports exist showing an apparently strong interaction between hormone-free GR and FKBP52. Yet, our efforts have yielded a weak signal for FKBP52 compared to FKBP51. Moreover, the FKBP52 signal has been shown to increase in response to hormone-binding at both the GR [20, 33] and mineralocorticoid receptor [47]. Thus, it has become apparent to us that simple pair-wise comparisons by co-IP approaches of SR/TPR interactions are not very meaningful when trying to assess the relative contribution to SR activity of one TPR protein over another. With our systematic approach, we have shown that hormone-free GR complexes from L929 and COS cells contain FKBP51 and PP5 as their principal TPR components, with only minimal participation by FKBP52 and none by Cyp40 [39]. In contrast, progesterone receptor expressed in the same cells was found to preferentially interact with FKBP52.
With the above concepts in mind, we assayed for the TPR preference profile of GR in WT and 52KO MEF cells (Fig. 2). As previously seen in COS and L929 cells [39], the hormone-free GR of WT MEF cells had a distinct preference for FKBP51 and PP5, but little or no preference for FKBP52. Not surprisingly, perhaps, lack of FKBP52 did not affect GR composition in the KO52 cells, as the GR interaction with FKBP51 and PP5 was unaltered. It should also be noted that levels of FKBP51 bound to GR were not reduced in the KO52 cells, even though these cells express lower amounts of the protein (Fig. 1). Lastly, no alteration to the GR/HSP90 interaction was seen in the KO52 cells. Thus, the hormone-free GR of MEF cells exhibits a distinct preference for FKBP51 and PP5 – a property that is not altered by loss of FKBP52. In response to hormone, we and others have shown that FKBP52 is recruited to the GR complex. We therefore tested whether another TPR fills this role in KO52 cells. Other than lack of FKBP52 recruitment, no changes in TPR profile were seen in response to hormone binding (data not shown). Thus, FKBP51, Cyp40 and PP5 appear unable to compensate for FKBP52 in this function.
Fig. 2.
Loss of FKBP52 does not alter composition of GR heterocomplex. Cytosolic lysates from untreated WT52 and KO52 MEF cells were immunoadsorbed with FiGR antibody against GR (I) or non-immune mouse IgG (NI). Samples were analyzed by Western blotting for the presence of GR, HSP90, FKBP52, FKBP51, PP5 and Cyp40. Results are representative of three independent experiments. Quantitative data are not shown, as no statistically significant differences were found.
3.3 Normal hormone-binding affinity of GR in FKBP52 KO cells
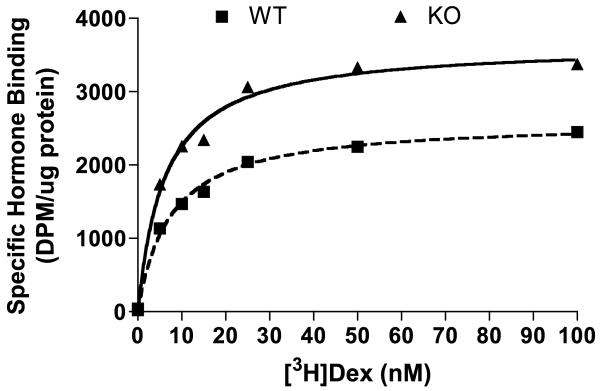
As mentioned above, there is considerable evidence that FKBP52 has a stimulatory effect on GR hormone-binding function, while the closely-related FKBP51 exerts the opposite effect [48, 49]. If true, loss of FKBP52 should manifest in reduced ability of GR to bind hormone. We, therefore, subjected cytosolic GR derived from WT52 and KO52 cells to a saturation hormone-binding assay using increasing amounts of [3H]Dex (Fig. 3). This assay was chosen, as measurement of dissociation constants (Kd) is not dependent upon receptor protein levels. Dissociation constants were not significantly different between WT52 and KO52 GRs (Kd = 7.375 and 6.125, respectively). Thus, intrinsic hormone-binding affinity is not altered by loss of FKBP52. Although the GR of KO52 cells was found to have a higher βmax value than GR in WT52 cells (βmax = 3644 and 2598, respectively), the difference most likely reflects the higher levels of GR protein found in the KO52 cells (Fig. 1). These results were somewhat surprising, given prior reports [20, 23]. As elaborated in the Discussion, we propose that lack of an effect in the KO52 cells is probably indicative of a strong contribution to basal hormone-binding function by FKBP51 and PP5, as well as the intrinsic differences between over-expression versus knock-out approaches to the study of target activity.
Fig. 3.
Normal GR hormone-binding function in FKBP52-Deficient MEF cells. Cytosolic lysates from WT52 and KO52 MEF cells were subjected to a saturation hormone binding assay. Aliquots of MEF cell lysates were incubated for 2 h at 4°C with increasing concentrations of [3H]Dex in the absence or presence of excess unlabeled ligand. Values for specific hormone binding were analyzed by nonlinear regression based on one-site competition. Wild-type values: Kd = 7.375, βmax = 2598. KO values: Kd = 6.125, βmax = 3644. Results are representative of three independent saturation binding experiments.
3.4 GR in FKBP52 KO cells retains normal rate of hormone-induced translocation and localization to intra-nuclear foci
We have described a model for hormone-induced TPR exchange in which FKBP51 is replaced by FKBP52 upon the binding of Dex to GR [20, 33]. Because the recruitment of FKBP52 to GR correlated with co-recruitment of dynein, and because the GR/FKBP52 complex could be seen to accumulate in the nucleus, we speculated that hormone-induced TPR exchange was the necessary first step of GR nuclear translocation. As a test of this model, we have measured hormone-induced GR translocation in the KO52 cells (Fig. 4). In the first approach, we used indirect immunofluorescence to follow GR over a time-course of Dex treatment. A time-course was employed because of the documented ability of GR to undergo nuclear translocation by the slower process of diffusion, even in the complete absence of cytoskeleton [50]. The results show that GR nuclear translocation is unimpeded in the absence of FKBP52 (Fig. 4A). To confirm these results, we also assayed for GR translocation using a fractionation assay. Here again GR movement to the nuclear pellet fraction was unaffected in the KO52 cells (Fig. 4C), demonstrating that the nuclear GR of KO52 cells seen in Fig. 4A is tightly bound within the nucleus, presumably because of localization to chromatin. To test the latter, we examined localization of GR to intra-nuclear foci, proposed to be sites of active GR transcription [51]. Fig. 4B shows that GR of KO52 cells localizes to foci to the same extent as GR in WT52 cells. As a final test, we measured GR binding to DNA using the gel shift assay (Fig. 4D). Here, too, no effect of FKBP52 loss was seen. Thus, we must conclude that deficiency of FKBP52 does not inhibit GR movement to the nucleus, nor its intrinsic DNA-binding function.
Fig. 4.
Hormone-induced nuclear translocation and DNA-binding properties of GR are unaffected in FKBP52-deficient cells. Panel A: Immunofluorescence analysis of steroid-dependent GR movement from cytoplasm to nucleus. WT52 and KO52 MEF cells were incubated with 1 μM Dex for the indicated time intervals, followed by indirect immunofluorescence using FiGR monoclonal antibody against GR. Result is representative of two independent time-course experiments. Panel B: Enlargement of 15 min images seen in Panel A demonstrating localization of GR to nuclear foci in both WT52 and KO52 cells. Panel C: Analysis of GR nuclear translocation by fractionation assay. WT52 and KO52 MEF cells were treated or not with 1 μM Dex for 30 min, followed by fractionation to yield cytosolic (C) and nuclear (N) extracts. GR in each fraction was detected by Western-blotting following immunoadsorption of GR with FiGR antibody. Result is representative of two independent experiments. Panel D: Gel shift analysis of GR DNA-binding function. WT52 (WT) and KO52 (KO) cells were treated with vehicle or 1.0 μM Dex for 3 h, followed by preparation of nuclear extracts and EMSA assay for GR DNA-binding. Result is representative of three independent experiments.
3.5 FKBP52 loss reduces GR but not TR activity at reporter genes
We next tested the contribution of FKBP52 to GR transcription enhancement activity by transfecting WT52 and KO52 cells with pMMTV-CAT – a widely used GR reporter driven by the complex mouse mammary tumor virus promoter. Surprisingly, a strong reduction of GR activity was seen in the KO52 cells at this reporter (Fig. 5A). To eliminate the possibility that FKBP52 may be regulating other factors that contribute to activity at MMTV, WT52 and KO52 cells were transfected with the pGRE2EIB-Luc reporter – a minimal promoter composed of two synthetic glucocorticoid response elements driving expression of luciferase. The latter experiment was done in the presence of increasing concentrations of Dex and the results were normalized to amount of GR (Fig. 5B). The results show decreased GR activity at all concentrations of hormone (0.01, 0.1 and 1.0 μM) in the KO52 cells (Fig. 5B). At 0.1 and 1.0 μM Dex, GR activity was reduced by 83.3 and 70.1%, respectively. We therefore conclude that FKBP52 is a positive regulator of GR transcriptional activity at both physiologic and pharmacologic concentrations of hormone.
Fig. 5.
Attenuation of GR reporter gene activity in FKBP52-deficient MEF cells. Panel A: WT52 and KO52 MEF cells were transiently-transfected with pMMTV-CAT reporter, followed by treatment with vehicle or 1 μM Dex for 24 h. Values represent the means +/− S.E.M. of six independent experiments. Panel B: WT52 and KO52 MEF cells were transiently-transfected with the minimal pGRE2E1B-Luc reporter, followed by treatment with the indicated concentrations of Dex for 24 h. Values were normalized to amount of GR protein and represent the means +/− S.E.M. of four independent experiments. Panel C: WT52 and KO52 MEF cells were co-transfected with the TR, RXR and TRE-Luc vectors, followed by treatment with vehicle or 100 nM T3 for 24 h. Values represent the means +/− S.E.M. of four independent experiments.
To assess the specificity of FKBP52 actions, we first measured transcription activity of the p65 subunit of NF-κB in WT52 and KO52 cells using an NF-κB driven luciferase reporter. No effect of FKBP52 loss was seen (data not shown). Activity of thyroid receptor (TR) in the KO52 cells was also measured. As a member of the nuclear receptor family, TR, like GR, acts as a hormone-activated transcription factor [13]. However, hormone-free TR is known to bind DNA immediately after de novo synthesis [13] and does not enter into complexes with HSP90 [52, 53]. Thus, loss of FKBP52 should not have an effect on TR activity. This was tested by transfecting WT52 and KO52 cells with TR and its heterodimeric binding partner RXR, followed by treatment with thyroid hormone (T3) and assay at a TR-responsive reporter (Fig. 5C). The results show no decrease of TR activity in response to thyroid hormone.
3.6 FKBP52 control of GR is gene specific
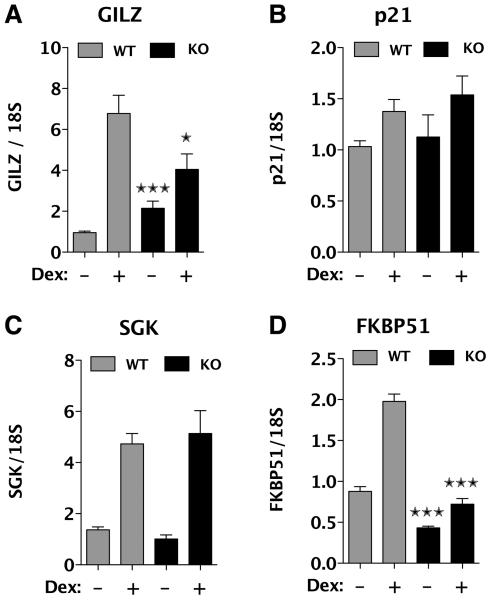
To assess the effect of FKBP52 loss on GR control of endogenous genes, we performed quantitative real-time PCR analysis of the following genes: glucocorticoid-inducible leucine zipper (GILZ), p21, serum- and glucocorticoid-regulated kinase (SGK) and FKBP51. All four of these genes are known to be under GR control. FKBP51, in particular, was chosen because we had seen reduced expression of FKBP51 protein in untreated KO cells (Fig. 1), indicating that FKBP52 loss was affecting basal activity of GR at this gene. The results presented an unexpected but interesting pattern (Fig. 6). In the KO cells, GILZ showed increased basal expression but a decrease in Dex-induced expression. For p21 and SGK, no changes in either basal or hormone-induced activities were seen. For FKBP51, both basal and Dex-induced activities were reduced. Calculation of fold increases (dex / control) also showed a significant decrease of Dex-induced expression for this gene in KO cells compared to WT (1.68 fold +/− 0.183 SEM vs 2.29 +/− 0.171 SEM, P<0.05). In the case of FKBP51, we have confirmed the qRT-PCR results by Western-blot analysis of protein levels. Here, too, FKBP51 protein levels were reduced in KO cells under both basal and Dex-induced conditions (data not shown). An interesting question that arises from the FKBP51 results is whether reduced expression of this TPR somehow contributes to the reduced GR activity seen at GILZ (Fig. 6) or at the reporter genes (Fig. 5). Although we cannot eliminate this possibility, it does not seem likely for the following reasons. First, all reported evidence suggests that FKBP51, unlike FKBP52, is inhibitory of GR activity [48, 49]. Second, in spite of reduced basal expression of FKBP51, the amount of FKBP51 in the hormone-free GR complex was unchanged in the KO52 cells (Fig. 2). Thus, at least with respect to this parameter and its subsequent ramifications, it is unlikely that FKBP51 is sufficiently reduced to have a large effect on GR one way or the other.
Fig. 6.
FKBP52 control of GR transcriptional activity is gene specific. Dex-induced GR-mediated expression of GILZ, p21, SGK and FKBP51 in WT and KO52 MEF cells was measured by quantitative real-time PCR, as described under Materials & Methods. Values were normalized to 18S mRNA following treatment with or without 100 nM Dex for 2 h, and represent the means +/− S.E.M. of 5-6 independent experiments. P<0.001 vs WT, ĔP<0.05 vs WT.
Taken as a whole, these results suggest a novel and important role for FKBP52 in the gene-specific regulation of GR transcription enhancement activity. This observation has important implications for glucocorticoid control of cellular and physiologic processes, as FKBP52 may now serve as a means to selectively target only a subset of GR responses.
3.7 FKBP52 loss does not affect GR autologous down-regulation
The above results provide strong evidence that FKBP52 is required for optimal activity of GR as a transcription enhancement factor. But GR is also known to exert other functions within the nucleus, such as transrepression and autogous down-regulation [54]. Although there is evidence that GR down-regulation in response to steroids occurs through multiple mechanisms, including direct GR inhibition of transcription, decreased stability of GR mRNA and decreased stability of GR protein [54], all of these mechanism are eventually manifested as reduced GR protein following hormone treatment of intact cells. For this reason, we measured GR protein by Western-blotting in WT52 and KO52 cells subjected to 24 h treatment with Dex (Fig. 7). But because KO52 cells express more steady-state GR than WT52 cells (Fig. 1), we could not be sure that down-regulation would be independent of starting GR levels. We therefore measured GR down-regulation in L929 fibroblast cells, which express levels of steady-state GR comparable to KO52 fibroblasts (Fig. 7A). The results show a strong reduction of total GR protein in WT52, KO52 and L929 cells in response to Dex (Fig. 7A). Expression of the data as percentage of starting GR levels shows that Dex treatment yields approximately 50% reduction of GR in all cases (Fig. 7B). The fact that FKBP52 loss had no affect on down-regulation while impairing GR transactivation suggests that FKBP52 may be a critical factor by which to dissociate the transactivation and down-regulation properties of GR.
Fig. 7.

Analysis of hormone-induced GR autologous down-regulation in FKBP52-deficient MEF cells. Panel A: Western blot of GR from whole cell extracts made from WT52, KO52 and L929 fibroblasts treated with vehicle or 1 μM Dex for 24 h. Panel B: Quantitation of results in Panel A was achieved by densitometric scanning of films. Values are percent loss of GR protein compared to vehicle-treated control and represent means +/− S.E.M. of three independent experiments.
4. Discussion
In this work, we have analyzed the contribution of FKBP52 to the major stages of GR signaling. We report that loss of FKBP52 had no effect, contrary to expectations, on the composition of hormone-free GR heterocomplexes, hormone-binding function and ability of GR to move to sites of chromatin action within the nucleus. Instead, we found that FKBP52 plays an unexpected role as a gene-specific modulator of GR transcriptional activity. This new role of FKBP52 appears to be specific for steroid receptors, as no effect of FKBP52 loss was observed on thyroid receptor action, a member of the nuclear receptor family not chaperoned by the HSP90/TPR machinery. We also show that autologous down-regulation of GR was not altered in the FKBP52-deficient cells. The latter result is particularly intriguing, as it suggests that FKBP52 may serve as a modulatory factor specific for only the transactivation branch of GR signaling.
The major new observation of this work is an apparent ability of FKBP52 to regulate GR transactivation function without affecting hormone-binding or translocation. Although we do not yet know the mechanism responsible for this new role, some interesting inferences can be made. For example, we found that hormone caused GR localization to nuclear foci in the KO52 cells. As there is good evidence that these foci represent sites of active GR transcription at chromatin [51], this means that FKBP52 is either playing a direct role at chromatin to regulate transactivation, or that it has a downstream effect on this activity. Because FKBP52 is principally known to interact with GR prior to DNA-binding, either as part of inactive complexes or as an intermediate complex in the process of hormone-induced translocation, a downstream effect seems the most plausible. We speculate that the PPIase function of FKBP52 could achieve this, perhaps by altering conformation of GR's AF-1 or AF-2 domains. Both the Smith and Rein laboratories have shown that potentiation of GR activity requires the PPIase domain [23, 34]. Yet, the latest data suggest that mutations which abrogate the PPIase function of FKBP52 do not block the potentiation of GR [55]. Thus, the PPIase domain must be contributing in another fashion, perhaps as the site of FKBP52 interaction with dynein. Thus, future studies to prove a role for the FKBP52 PPIase domain in our novel transactivation function will require the development of precise PPIase domain mutations that differentiate between dynein binding and enzymatic activity.
Evidence also exists that FKBP52 could play a direct role on GR activity at chromatin. First, it is clear that a good portion of cellular FKBP52 is found in the nucleus [24, 26]. Second, a role for HSP90 in nuclear regulation of GR has been reported. Kang et al showed that nuclear-targeting of HSP90 caused inhibition of GR transcriptional activity and binding to DNA [56]. Using the chromatin immunoprecipitation assay, Freeman & Yamamoto showed hormone-dependent localization of HSP90 to GR-regulated promoters [57]. Although both of these reports showed that HSP90 was inhibitory of GR activity within the nucleus, it would not be unreasonable to imagine a sub-population of HSP90 interacting with FKBP52 that is stimulatory. Last but not least, there are several reports demonstrating FKBP52 to be the adeno-associated virus D-sequence-binding protein, ssD-BP, which binds single-stranded DNA to prevent viral replication [58, 59]. If FKBP52 can bind single-stranded DNA, why not double-stranded DNA, perhaps at GR-regulated promoters.
Another issue that arises from this work is why no effects of FKBP52 loss on GR hormone-binding and translocation functions were found. As already stated, prior work from our laboratory and others have shown a stimulatory contribution of FKBP52 to both of these GR functions. Although a definitive answer to this question does not yet exist, we propose that the discrepancy may have arisen from a combination of two factors: 1) inherent limitations of knock-out versus over-expression studies, and 2) potential compensation for FKBP52 by other TPRs. On the technical side, most of the prior work in the area has utilized over-expression techniques to demonstrate the FKBP52 contribution to GR function. The ubiquitous over-expression approach has always had the inherent risk of inducing a super-physiologic (pharmacologic) effect not normally seen in the homeostatic cell. Although over-expression is clearly useful to the discovery of intrinsic activities, such effects could be achieved by completely overwhelming balancing forces within the cell. In contrast, the targeted ablation approach used in this work has a finite limit below which no further cellular alteration can occur. Thus, it may simply be that we saw no effect on GR binding function or translocation because we did not sufficiently alter the ratio of FKBP52 to other key factors.
Of course, it is likely that these “factors” are other members of the TPR protein family known to interact with steroid receptors. Thus, if FKBP51, PP5 or Cyp40 have overlapping functions with FKBP52, then targeted ablation would be less likely to yield measurable differences. We refer to this process as the Compensation Model. With respect to hormone-binding function, FKBP51 is generally viewed as an inhibitor of GR. Thus, it is possible that reduced expression of FKBP51 in the KO cells (Fig. 1) may be compensating for loss of stimulatory FKBP52, thereby maintaining homeostasis for ability to bind hormone. However, this mechanism does not seem likely because loss of FKBP52 did not alter the interaction of hormone-free GR with FKBP51 or PP5 (Fig. 2). Thus, FKBP51 levels in the KO cells are not sufficiently low to affect the GR/FKBP51 interaction. Instead, we propose that the failure of a dramatic drop in GR hormone-binding function in FKBP52 KO cells is simply because FKBP51 and PP5 are the major contributors to this GR function in most cells.
We have speculated that compensation may also be occurring with respect to hormone-induced translocation of GR, as both PP5 and Cyp40 can bind the motor protein dynein [28]. To test this, we assayed for hormone-induced changes in TPR content, but no increase in PP5 or Cyp40 were found in the GR complex of 52 KO cells (data not shown). When we published our TPR swap model [33], we suggested that recruitment of FKBP52 and dynein could be the mechanism by which GR nuclear translocation is achieved. Given our latest results, we must now conclude that the TPR swap model as a mechanism of translocation may not be correct. Instead, it is more likely that hormone-induced recruitment of FKBP52 to the GR, rather than being causative, simply correlates with nuclear translocation. If so, recruitment of FKBP52 to the GR, either before or after movement of GR to the nucleus, would be consistent with our new observation that FKBP52 contribute to the transactivation function of the receptor.
As already mentioned, we were surprised to see no obvious defects of GR-regulated physiology in FKBP52-deficient mice. If FKBP52 played a global and essential role in GR activity, the FKBP52 KO animals should have shown defects similar to those seen in the GRKO mouse – most notably, peri-natal lethality due to atelectasia [60]. Clearly, this is not the case, as most FKBP52 KO animals are overtly healthy, except for reduced fertility, and have about normal life-spans [35, 36]. How then can the observed defect of GR transcription activity seen here be reconciled against the apparent lack of physiological phenotypes? We propose three non-exclusive mechanisms. First and foremost, FKBP52 loss affected GR activity at only a subset of endogenous genes. Thus, if FKBP52 is not a major contributor to GR action at genes controlling neo-natal lung development, for example, then atelectasia would not occur. Second, at genes affected by FKBP52 loss, reduced expression was not absolute, making it more likely that FKBP52 mutant mice would be neutral with respect to viability. Lastly, it is important to remember that glucocorticoid hormones (cortisol in humans, corticosterone in rodents) are properly referred to as “stress” hormones, whose main function is to moderate primary physiological responses to stress, such as the inflammatory response [61, 62]. Thus, an initial stress event may be needed to uncover the true extent of defective GR signaling in FKBP52 KO animals. Studies are underway, using various forms to stress, to connect the results of this work to GR-controlled physiology in vivo.
Acknowledgements
We thank Drs. Michael Chinkers (University of South Alabama) and Jack Bodwell (Dartmouth University) for the gifts of PP5 and FiGR antibodies, respectively. We also acknowledge the generosity of Dr. Ronald Koenig (University of Michigan) for the gift of TR and RXR expression plasmids. This study was supported, in part, by a National Institute of Health grant (DK70127) to E.R.S. and W.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tai PK, Maeda Y, Nakao K, Wakim NG, Duhring JL, Faber LE. A 59-kilodalton protein associated with progestin, estrogen, androgen, and glucocorticoid receptors. Biochemistry. 1986;25:5269–5275. doi: 10.1021/bi00366a043. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez ER, Faber LE, Henzel WJ, Pratt WB. The 56-59-kilodalton protein identified in untransformed steroid receptor complexes is a unique protein that exists in cytosol in a complex with both the 70- and 90-kilodalton heat shock proteins. Biochemistry. 1990;29:5145–5152. doi: 10.1021/bi00473a021. [DOI] [PubMed] [Google Scholar]
- 3.Callebaut I, Renoir JM, Lebeau MC, Massol N, Burny A, Baulieu EE, Mornon JP. An immunophilin that binds M(r) 90,000 heat shock protein: main structural features of a mammalian p59 protein. Proc Natl Acad Sci U S A. 1992;89:6270–6274. doi: 10.1073/pnas.89.14.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanchez ER. Hsp56: a novel heat shock protein associated with untransformed steroid receptor complexes. J Biol Chem. 1990;265:22067–22070. [PubMed] [Google Scholar]
- 5.Peattie DA, Harding MW, Fleming MA, DeCenzo MT, Lippke JA, Livingston DJ, Benasutti M. Expression and characterization of human FKBP52, an immunophilin that associates with the 90-kDa heat shock protein and is a component of steroid receptor complexes. Proc Natl Acad Sci U S A. 1992;89:10974–10978. doi: 10.1073/pnas.89.22.10974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tai PK, Albers MW, Chang H, Faber LE, Schreiber SL. Association of a 59-kilodalton immunophilin with the glucocorticoid receptor complex. Science. 1992;256:1315–1318. doi: 10.1126/science.1376003. [DOI] [PubMed] [Google Scholar]
- 7.Yem AW, Tomasselli AG, Heinrikson RL, Zurcher-Neely H, Ruff VA, Johnson RA, Deibel MR., Jr. The Hsp56 component of steroid receptor complexes binds to immobilized FK506 and shows homology to FKBP-12 and FKBP-13. J Biol Chem. 1992;267:2868–2871. [PubMed] [Google Scholar]
- 8.Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18(3):306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- 9.Sikorski RS, Boguski MS, Goebl M, Hieter P. A repeating amino acid motif in CDC23 defines a family of proteins and a new relationship among genes required for mitosis and RNA synthesis. Cell. 1990;60:307–317. doi: 10.1016/0092-8674(90)90745-z. [DOI] [PubMed] [Google Scholar]
- 10.Goebl M, Yanagida M. The TPR snap helix: a novel protein repeat motif from mitosis to transcription. Trends Biochem Sci. 1991;16(5):173–177. doi: 10.1016/0968-0004(91)90070-c. [DOI] [PubMed] [Google Scholar]
- 11.Radanyi C, Chambraud B, Baulieu EE. The ability of the immunophilin FKBP59-HBI to interact with the 90-kDa heat shock protein is encoded by its tetratricopeptide repeat domain. Proc Natl Acad Sci U S A. 1994;91:11197–11201. doi: 10.1073/pnas.91.23.11197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Owens-Grillo JK, Czar MJ, Hutchison KA, Hoffmann K, Perdew GH, Pratt WB. A model of protein targeting mediated by immunophilins and other proteins that bind to hsp90 via tetratricopeptide repeat domains. J Biol Chem. 1996;271:13468–13475. doi: 10.1074/jbc.271.23.13468. [DOI] [PubMed] [Google Scholar]
- 13.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Owens-Grillo JK, Hoffmann K, Hutchison KA, Yem AW, Deibel MRJ, Handschumacher RE, Pratt WB. The cyclosporin A-binding immunophilin CyP-40 and the FK506-binding immunophilin hsp56 bind to a common site on hsp90 and exist in independent cytosolic heterocomplexes with the untransformed glucocorticoid receptor. J Biol Chem. 1995;270:20479–20484. doi: 10.1074/jbc.270.35.20479. [DOI] [PubMed] [Google Scholar]
- 15.Pratt WB, Jolly DJ, Pratt DV, Hollenberg SM, Giguere V, Cadepond FM, Schweizer-Groyer G, Catelli MG, Evans RM, Baulieu EE. A region in the steroid binding domain determines formation of the non-DNA-binding, 9 S glucocorticoid receptor complex. J Biol Chem. 1988;263:267–273. [PubMed] [Google Scholar]
- 16.Housley PR, Sanchez ER, Danielsen M, Ringold GM, Pratt WB. Evidence that the conserved region in the steroid binding domain of the glucocorticoid receptor is required for both optimal binding of hsp90 and protection from proteolytic cleavage. A two-site model for hsp90 binding to the steroid binding domain. J Biol Chem. 1990;265:12778–12781. [PubMed] [Google Scholar]
- 17.Renoir JM, Mercier-Bodard C, Hoffmann K, Le BS, Ning YM, Sanchez ER, Handschumacher RE, Baulieu EE. Cyclosporin A potentiates the dexamethasone-induced mouse mammary tumor virus-chloramphenicol acetyltransferase activity in LMCAT cells: a possible role for different heat shock protein-binding immunophilins in glucocorticosteroid receptor-mediated gene expression. Proc Natl Acad Sci U S A. 1995;92:4977–4981. doi: 10.1073/pnas.92.11.4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ning YM, Sanchez ER. Potentiation of glucocorticoid receptor-mediated gene expression by the immunophilin ligands FK506 and rapamycin. J Biol Chem. 1993;268:6073–6076. [PubMed] [Google Scholar]
- 19.Kralli A, Yamamoto KR. An FK506-sensitive transporter selectively decreases intracellular levels and potency of steroid hormones. J Biol Chem. 1996;271:17152–17156. doi: 10.1074/jbc.271.29.17152. [DOI] [PubMed] [Google Scholar]
- 20.Davies TH, Ning YM, Sanchez ER. Differential Control of Glucocorticoid Receptor Hormone-Binding Function by Tetratricopeptide Repeat (TPR) Proteins and the Immunosuppressive Ligand FK506. Biochemistry. 2005;44:2030–2038. doi: 10.1021/bi048503v. [DOI] [PubMed] [Google Scholar]
- 21.Ning YM, Sanchez ER. Stabilization in vitro of the untransformed glucocorticoid receptor complex of S49 lymphocytes by the immunophilin ligand FK506. J Steroid Biochem Mol Biol. 1995;52:187–194. doi: 10.1016/0960-0760(94)00162-f. [DOI] [PubMed] [Google Scholar]
- 22.Renoir JM, Le Bihan S, Mercier-Bodard C, Gold A, Arjomandi M, Radanyi C, Baulieu EE. Effects of immunosuppressants FK506 and rapamycin on the heterooligomeric form of the progesterone receptor. J Steroid Biochem Mol Biol. 1994;48:101–110. doi: 10.1016/0960-0760(94)90256-9. [DOI] [PubMed] [Google Scholar]
- 23.Riggs DL, Roberts PJ, Chirillo SC, Cheung-Flynn J, Prapapanich V, Ratajczak T, Gaber R, Picard D, Smith DF. The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. EMBO J. 2003;22:1158–1167. doi: 10.1093/emboj/cdg108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruff VA, Yem AW, Munns PL, Adams LD, Reardon IM, Deibel MR, Jr., Leach KL. Tissue distribution and cellular localization of hsp56, an FK506-binding protein. Characterization using a highly specific polyclonal antibody. J Biol Chem. 1992;267:21285–21288. [PubMed] [Google Scholar]
- 25.Czar MJ, Owens-Grillo JK, Yem AW, Leach KL, Deibel MR, Jr., Welsh MJ, Pratt WB. The hsp56 immunophilin component of untransformed steroid receptor complexes is localized both to microtubules in the cytoplasm and to the same nonrandom regions within the nucleus as the steroid receptor. Mol Endocrinol. 1994;8:1731–1741. doi: 10.1210/mend.8.12.7708060. [DOI] [PubMed] [Google Scholar]
- 26.Perrot-Applanat M, Cibert C, Geraud G, Renoir JM, Baulieu EE. The 59 kDa FK506-binding protein, a 90 kDa heat shock protein binding immunophilin (FKBP59-HBI), is associated with the nucleus, the cytoskeleton and mitotic apparatus. J Cell Sci. 1995;108:2037–2051. doi: 10.1242/jcs.108.5.2037. [DOI] [PubMed] [Google Scholar]
- 27.Silverstein AM, Galigniana MD, Kanelakis KC, Radanyi C, Renoir JM, Pratt WB. Different regions of the immunophilin FKBP52 determine its association with the glucocorticoid receptor, hsp90, and cytoplasmic dynein. J Biol Chem. 1999;274:36980–36986. doi: 10.1074/jbc.274.52.36980. [DOI] [PubMed] [Google Scholar]
- 28.Galigniana MD, Harrell JM, Murphy PJ, Chinkers M, Radanyi C, Renoir JM, Zhang M, Pratt WB. Binding of hsp90-associated immunophilins to cytoplasmic dynein: direct binding and in vivo evidence that the peptidylprolyl isomerase domain is a dynein interaction domain. Biochemistry. 2002;41:13602–13610. doi: 10.1021/bi020399z. [DOI] [PubMed] [Google Scholar]
- 29.Galigniana MD, Morishima Y, Gallay PA, Pratt WB. Cyclophilin-A is bound through its peptidylprolyl isomerase domain to the cytoplasmic dynein motor protein complex. J Biol Chem. 2004;279:55754–55759. doi: 10.1074/jbc.M406259200. [DOI] [PubMed] [Google Scholar]
- 30.Harrell JM, Murphy PJ, Morishima Y, Chen H, Mansfield JF, Galigniana MD, Pratt WB. Evidence for glucocorticoid receptor transport on microtubules by dynein. J Biol Chem. 2004;279:54647–54654. doi: 10.1074/jbc.M406863200. [DOI] [PubMed] [Google Scholar]
- 31.Chambraud B, Belabes H, Fontaine-Lenoir V, Fellous A, Baulieu EE. The immunophilin FKBP52 specifically binds to tubulin and prevents microtubule formation. FASEB J. 2007 doi: 10.1096/fj.06-7667com. [DOI] [PubMed] [Google Scholar]
- 32.Galigniana MD, Radanyi C, Renoir JM, Housley PR, Pratt WB. Evidence that the peptidylprolyl isomerase domain of the hsp90-binding immunophilin FKBP52 is involved in both dynein interaction and glucocorticoid receptor movement to the nucleus. J Biol Chem. 2001;276:14884–14889. doi: 10.1074/jbc.M010809200. [DOI] [PubMed] [Google Scholar]
- 33.Davies TH, Ning YM, Sanchez ER. A new first step in activation of steroid receptors: hormone-induced switching of FKBP51 and FKBP52 immunophilins. J Biol Chem. 2002;277:4597–4600. doi: 10.1074/jbc.C100531200. [DOI] [PubMed] [Google Scholar]
- 34.Wochnik GM, Ruegg J, Abel GA, Schmidt U, Holsboer F, Rein T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J Biol Chem. 2005;280:4609–4616. doi: 10.1074/jbc.M407498200. [DOI] [PubMed] [Google Scholar]
- 35.Yang Z, Wolf IM, Chen H, Periyasamy S, Chen Z, Yong W, Shi S, Zhao W, Xu J, Srivastava A, Sanchez ER, Shou W. FK506-binding protein 52 is essential to uterine reproductive physiology controlled by the progesterone receptor a isoform. Mol Endocrinol. 2006;20:2682–2694. doi: 10.1210/me.2006-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yong W, Yang Z, Periyasamy S, Chen H, Yucel S, Li W, Lin LY, Wolf IM, Cohn MJ, Baskin LS, Sanchez ER, Shou W. Essential role for Co-chaperone Fkbp52 but not Fkbp51 in androgen receptor-mediated signaling and physiology. J Biol Chem. 2007;282:5026–5036. doi: 10.1074/jbc.M609360200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheung-Flynn J, Prapapanich V, Cox MB, Riggs DL, Suarez-Quian C, Smith DF. Physiological role for the cochaperone FKBP52 in androgen receptor signaling. Mol Endocrinol. 2005;19:1654–1666. doi: 10.1210/me.2005-0071. [DOI] [PubMed] [Google Scholar]
- 38.Tranguch S, Cheung-Flynn J, Daikoku T, Prapapanich V, Cox MB, Xie H, Wang H, Das SK, Smith DF, Dey SK. Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc Natl Acad Sci U S A. 2005;102:14326–14331. doi: 10.1073/pnas.0505775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banerjee A, Periyasamy S, Wolf IM, Hinds TDJ, Yong W, Shou W, Sanchez ER. Control of glucocorticoid and progesterone receptor subcellular localization by the ligand-binding domain is mediated by distinct interactions with tetratricopeptide repeat proteins. Biochemistry. 2008;47:10471–10480. doi: 10.1021/bi8011862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nordeen SK, Green PPIII, Fowlkes DM. A rapid, sensitive, and inexpensive assay for chloramphenicol acetyltransferase. DNA. 1987;6:173–178. doi: 10.1089/dna.1987.6.173. [DOI] [PubMed] [Google Scholar]
- 41.Sen R, Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986;47:921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- 42.Payvar F, DeFranco D, Firestone GL, Edgar B, Wrange O, Okret S, Gustafsson JA, Yamamoto KR. Sequence-specific binding of glucocorticoid receptor to MTV DNA at sites within and upstream of the transcribed region. Cell. 1983;35:381–392. doi: 10.1016/0092-8674(83)90171-x. [DOI] [PubMed] [Google Scholar]
- 43.Amler LC, Agus DB, LeDuc C, Sapinoso ML, Fox WD, Kern S, Lee D, Wang V, Leysens M, Higgins B, Martin J, Gerald W, Dracopoli N, Cordon-Cardo C, Scher HI, Hampton GM. Dysregulated expression of androgen-responsive and nonresponsive genes in the androgen-independent prostate cancer xenograft model CWR22-R1. Cancer Res. 2000;60:6134–6141. [PubMed] [Google Scholar]
- 44.Febbo PG, Lowenberg M, Thorner AR, Brown M, Loda M, Golub TR. Androgen mediated regulation and functional implications of fkbp51 expression in prostate cancer. J Urol. 2005;173:1772–1777. doi: 10.1097/01.ju.0000155845.44729.ba. [DOI] [PubMed] [Google Scholar]
- 45.Hubler TR, Denny WB, Valentine DL, Cheung-Flynn J, Smith DF, Scammell JG. The FK506-Binding Immunophilin FKBP51 Is Transcriptionally Regulated by Progestin and Attenuates Progestin Responsiveness. Endocrinology. 2003;144:2380–2387. doi: 10.1210/en.2003-0092. [DOI] [PubMed] [Google Scholar]
- 46.Hubler TR, Scammell JG. Intronic hormone response elements mediate regulation of FKBP5 by progestins and glucocorticoids. Cell Stress Chaperones. 2004;9:243–252. doi: 10.1379/CSC-32R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gallo LI, Ghini AA, Pilipuk GP, Galigniana MD. Differential Recruitment of Tetratricorpeptide Repeat Domain Immunophilins to the Mineralocorticoid Receptor Influences both Heat-Shock Protein 90-Dependent Retrotransport and Hormone-Dependent Transcriptional Activity. Biochemistry. 2007;46:14044–14057. doi: 10.1021/bi701372c. [DOI] [PubMed] [Google Scholar]
- 48.Reynolds PD, Ruan Y, Smith DF, Scammell JG. Glucocorticoid resistance in the squirrel monkey is associated with overexpression of the immunophilin FKBP51. J Clin Endocrinol Metab. 1999;84:663–669. doi: 10.1210/jcem.84.2.5429. [DOI] [PubMed] [Google Scholar]
- 49.Denny WB, Valentine DL, Reynolds PD, Smith DF, Scammell JG. Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology. 2000;141:4107–4113. doi: 10.1210/endo.141.11.7785. [DOI] [PubMed] [Google Scholar]
- 50.Galigniana MD, Scruggs JL, Herrington J, Welsh MJ, Carter-Su C, Housley PR, Pratt WB. Heat shock protein 90-dependent (geldanamycin-inhibited) movement of the glucocorticoid receptor through the cytoplasm to the nucleus requires intact cytoskeleton. Mol Endocrinol. 1998;12:1903–1913. doi: 10.1210/mend.12.12.0204. [DOI] [PubMed] [Google Scholar]
- 51.Htun H, Barsony J, Renyi I, Gould DL, Hager GL. Visualization of glucocortcoid receptor translocation and intranuclear organization in living cells with a green fluorescent protein chimera. Proc Natl Acad Sci U S A. 1996;93:4845–4850. doi: 10.1073/pnas.93.10.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dalman FC, Koenig RJ, Perdew GH, Massa E, Pratt WB. In contrast to the glucocorticoid receptor, the thyroid hormone receptor is translated in the DNA binding state and is not associated with hsp90. J Biol Chem. 1990;265:3615–3618. [PubMed] [Google Scholar]
- 53.Dalman FC, Sturzenbecker LJ, Levin AA, Lucas DA, Perdew GH, Petkovitch M, Chambon P, Grippo JF, Pratt WB. Retinoic acid receptor belongs to a subclass of nuclear receptors that do not form “docking” complexes with hsp90. Biochemistry. 1991;30:5605–5608. doi: 10.1021/bi00236a038. [DOI] [PubMed] [Google Scholar]
- 54.Schaaf MJ, Cidlowski JA. Molecular mechanisms of glucocorticoid action and resistance. J Steroid Biochem Mol Biol. 2002;83(15):37–48. doi: 10.1016/s0960-0760(02)00263-7. [DOI] [PubMed] [Google Scholar]
- 55.Riggs DL, Cox MB, Tardif HL, Hessling M, Buchner J, Smith DF. Noncatalytic role of the FKBP52 peptidyl-prolyl isomerase domain in the regulation of steroid hormone signaling. Mol Cell Biol. 2007;27:8658–8669. doi: 10.1128/MCB.00985-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kang KI, Meng X, Devin-Leclerc J, Bouhouche I, Chadli A, Cadepond F, Baulieu E-E, Catelli M-G. The molecular chaperone Hsp90 can negatively regulate the activity of a glucocorticosteroid-dependent promoter. Proc Natl Acad Sci U S A. 1999;96:1439–1444. doi: 10.1073/pnas.96.4.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Freeman BC, Yamamoto KR. Disassembly of transcriptional regulatory complexes by molecular chaperones. Science. 2002;296:2232–2235. doi: 10.1126/science.1073051. [DOI] [PubMed] [Google Scholar]
- 58.Qing K, Hansen J, Weigel-Kelley KA, Tan M, Zhou S, Srivastava A. Adeno-associated virus type 2-mediated gene transfer: role of cellular FKBP52 protein in transgene expression. J Virol. 2001;75:8968–8976. doi: 10.1128/JVI.75.19.8968-8976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qing K, Li W, Zhong L, Tan M, Hansen J, Weigel-Kelley KA, Chen L, Yoder MC, Srivastava A. Adeno-associated virus type 2-mediated gene transfer: role of cellular T-cell protein tyrosine phosphatase in transgene expression in established cell lines in vitro and transgenic mice in vivo. J Virol. 2003;77:2741–2746. doi: 10.1128/JVI.77.4.2741-2746.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cole TJ, Blendy JA, Monaghan AP, Krieglstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schutz G. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 1995;9:1608–1621. doi: 10.1101/gad.9.13.1608. [DOI] [PubMed] [Google Scholar]
- 61.Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984;5(1):25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- 62.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]