Abstract
Sensitivity amplification has long been regarded as a virtually universal property of signal transduction cascades, yet a comprehensive parameter analysis remains a challenge even for relatively simple networks. We use a fast and accurate method to compute properties of multilevel cascades of activation-inactivation cycles and show that the monocyclic cascades amplify sensitivity only under specific conditions. In particular, it is found that efficient sensitivity amplification in a cascade, relative to the sensitivities of individual cycles, requires asymmetry in saturation of converter enzymes, with inhibitors much more saturated than activators.
Keywords: cascades of activation-inactivation cycles, sensitivity amplification, converter enzymes, mathematical model, parameter analysis
Introduction
Activation-inactivation cycles, such as phosphorylation and dephosphorylation of proteins, have long been recognized to play an important role in signal transduction and metabolic regulation [1, 2]. In a seminal study [3], Goldbeter and Koshland have shown that in addition to allosteric changes in protein conformation and cooperative interactions, threshold phenomena in cell signaling, such as switch-like behavior and bistability [4, 5], can emerge as a result of competition within an enzymatic activation-inhibition cycle. This mechanism involves covalent modifications of proteins and does not necessarily require cooperativity or allosteric changes. Goldbeter and Koshland also argued that if a signal-response curve of an individual cycle is only moderately steep, a cascade of such cycles, in which a protein activated in one cycle acts as a converter enzyme for the subsequent cycle, can exhibit an amplified sensitivity.
Ferrell and co-workers [6, 7] employed these ideas to explain the enhanced sensitivity of the mitogen-activated protein kinase (MAPK) cascade in Xenopus oocyte extracts. Kholodenko and colleagues [8] applied methods of metabolic control analysis to quantifying sensitivity in signal transduction cascades and showed that multilevel structure of signaling cascades provides conditions for the multiplicative amplification of sensitivity. In their original calculations [9], Kholodenko et al. used an approximation that ignores sequestration of the activated proteins in the cascade. Later they argued [10] that sequestration in cells reduces sensitivity amplification and that the Goldbeter - Koshland mechanism is unlikely to account for the situation in vivo. However, Huang and Ferrell [6] successfully reproduced ultrasensitivity in the MAPK cascade, with possible sequestration taken into account, but systematic parameter analysis has not been published.
In this study, we investigate conditions for sensitivity amplification in a cascade of activation-inactivation cycles [3] by means of in-depth parameter analyses based on various definitions of sensitivity. Our results show, for the first time, that asymmetry in saturation of converter enzymes - saturated inhibition vs. unsaturated activation – is required for the cascade to work as an efficient sensitivity amplifier.
Quantifying sensitivity
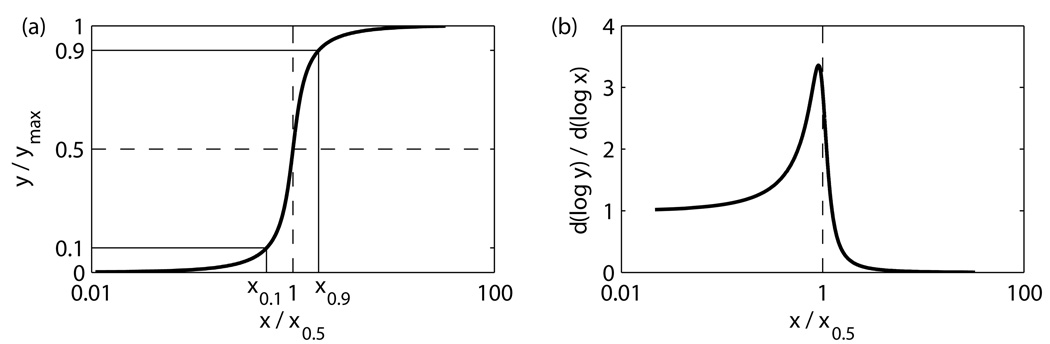
A commonly used approach to evaluate sensitivity of a signaling pathway is to approximate a signal-response curve (Fig 1(a)) by a Hill function, y (x) = (1 + x−n)−1, and to identify sensitivity by the “Hill coefficient” n, which characterizes the steepness of the curve. With this method, however, if data are fitted to the Hill equation by different approaches, the results may vary. Goldbeter and Koshland [3] define sensitivity of the input-output relation as a parameter n in the Hill equation that has the same ratio x0.9 / x0.1 as the experimental curve. Here, x0.9 and x0.1 are the signal values corresponding to the 90% and 10% of maximal response (Fig 1(a)). Sensitivity defined in this way will be termed the “Hill sensitivity” and denoted as SH: SH = log 81 / log(x0 9 / x0.1). This is the definition of sensitivity used in [3, 6].
Figure 1.
A typical input-output curve of a signaling pathway (a) and its response coefficients (b). The input values yielding 10% and 90% outputs, x0.1 and x0.9, are used to define Hill sensitivities, SH; the logarithmic sensitivity, SL, is defined as a maximal response coefficient in (b).
An alternative approach [9, 10] is based on local sensitivities, d log y / d log x, known as response coefficients in a metabolic control analysis [11, 12]. The response coefficient yields local steepness at a given input value. To characterize the full input-output curve, we introduce here a “logarithmic sensitivity”, SL, defined as maximum of d log y / d log x over the entire range of inputs, SL = max {d log y / d log x} (Fig 1(b)); note that for the Hill equation, SL = n. The relationship between SH and SL for activation-inactivation cycles (discussed in detail in the next section) is essentially monotone and close to linear when the cycles are sufficiently sensitive, see Fig 4 below.
Figure 4.
Comparison of the two definitions of cycle sensitivity for varying asymmetry in Michaelis - Menten constants for activation and inhibition.
An activation-inactivation cycle: sensitivity and efficacy
We start with a single activation-inactivation cycle (Fig 2), a building block of a signal transduction cascade [3]. This relatively simple network has been widely studied (see, e.g. [10]), but we present here a first detailed parameter analysis of its sensitivity and efficacy.
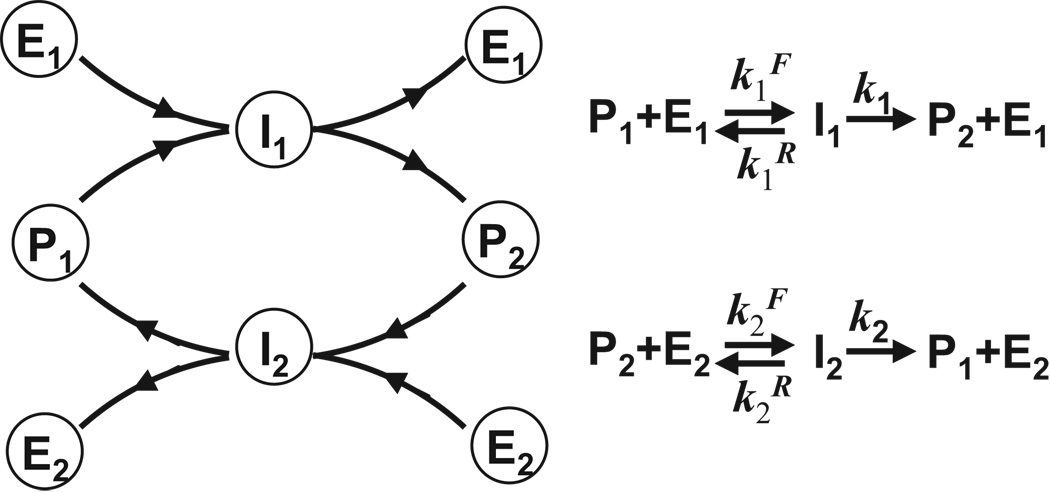
Figure 2.
A single cycle diagram and description of reactions.
The output, a steady-state concentration [P2] of an active form of the protein P, is a function of the input, a total concentration of the converter enzyme, E1,tot. The steady state of the network is determined by three dimensionless parameters (see Methods section for derivation): normalized equilibrium constants for binding of the protein to the inhibiting and activating converter enzymes: and the normalized concentration of the inactivating enzyme, c = (1 + k2 / k1)E2,tot / Ptot. Parameters a and b are also known as the normalized Michaelis – Menten constants.
The normalized input and output are: x = k1E1,tot /(k2E2,tot) and y = [P2] / Ptot, respectively, where E1,tot = [E1] + [I1], E2,tot = [E2] + [I2], and Ptot = [P1] + [P2]+ [I1] + [I2]. The maximum dimensionless output, ymax, also characterizes efficacy of a signal transduction pathway; this quantity will be monitored along with sensitivities.
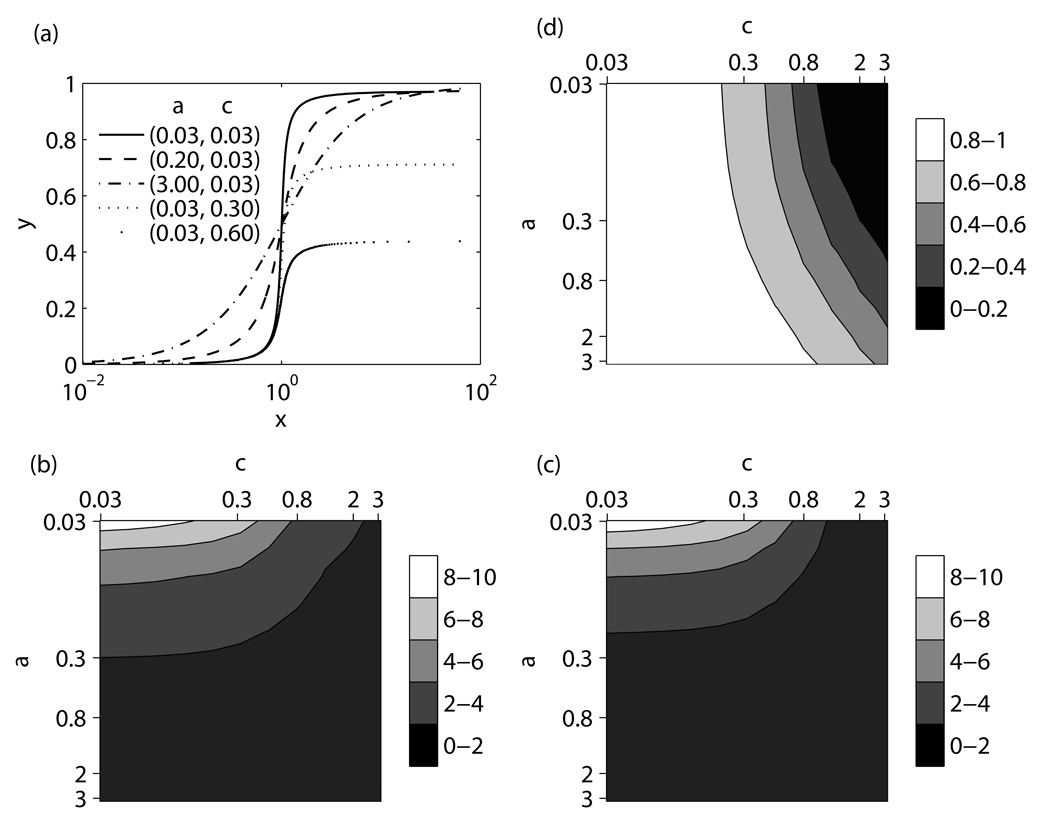
Fig 3 summarizes results for a symmetric activation-inactivation cycle, a = b. The input-output relationships for selected parameter sets are illustrated in Fig 3(a), and the sensitivities and the efficacy are plotted in Figs 3(b)–(d) as functions of network parameters. The sensitivity levels in Figs 3(b) and (c) change most rapidly when parameter a is varied, whereas the efficacy levels in Fig 3(d) are most responsive to variations in c. This can also be deduced from Fig 3(a): the signal-response curves obtained by varying a at fixed c have different slopes but similar efficacies, whereas the curves for varying c at the fixed value of a have different maximum outputs but similar slopes. We therefore conclude that the cycle sensitivities are controlled mainly by the dissociation constants, whereas the efficacy of the cycle depends largely on the normalized concentration of the inactivating converter enzyme, E2,tot / Ptot.
Figure 3.
Parameter analysis of a symmetric activation-inactivation cycle, a=b: input-output curves for selected parameter sets (a), sensitivities SH (b) and SL (c), and efficacy ymax (d) as functions of cycle parameters.
This conclusion holds for asymmetric cycles as well. Unlike the symmetric case, SH and SL can differ significantly for a large asymmetry coefficient, α=b/a (Fig 4). Nevertheless, their values, when sufficiently large, are, for a given α, in direct proportion to one another. Their dependencies on parameter values are therefore qualitatively similar. Note that in the range of relatively low values, of interest for building ulltrasensitive cascades, the Hill sensitivity of the cycle can be substantially greater than its logarithmic sensitivity. This observation will be referred to as “the low-range anomaly”.
Bi-cyclic cascade: conditions for sensitivity amplification
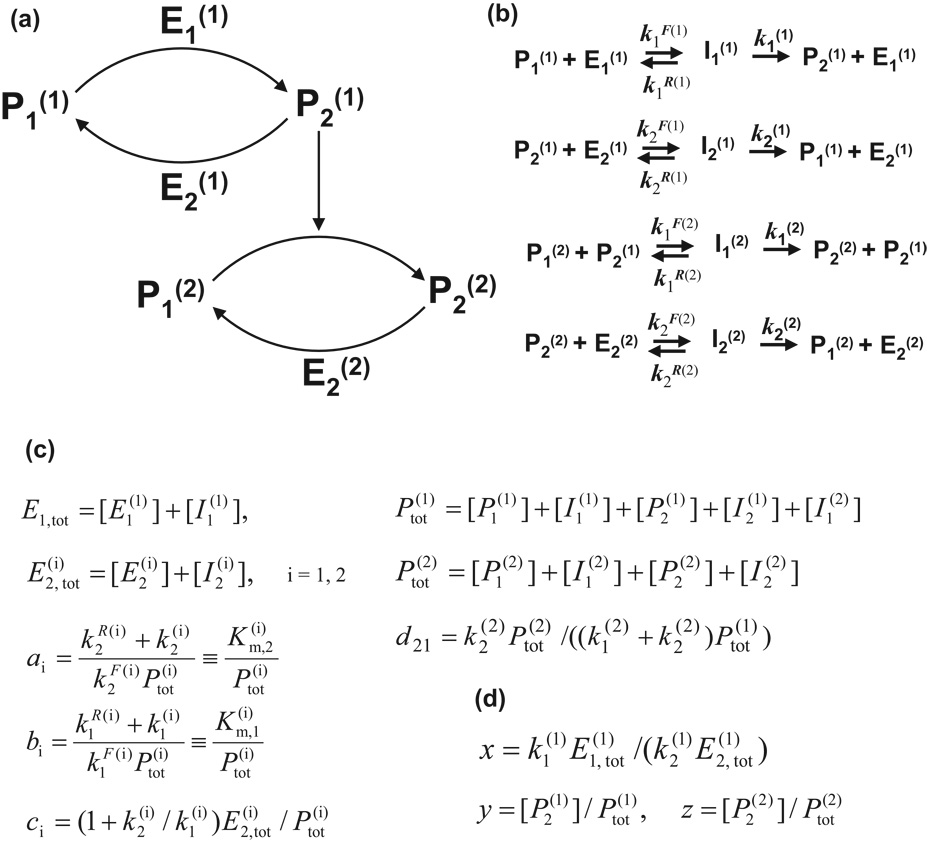
In a cascade of two cycles, the protein activated in the first cycle acts as an activating enzyme for the second cycle (Fig 5). The network, which is now described by two parameter subsets, (a1,b1,c1), (a2,b2,c2), also includes an additional parameter d21 which is associated with the ratio of total protein concentrations in the two cycles, (see notations in Fig 5).
Figure 5.
Bi-cyclic cascade: minimal diagram (a), detailed description of reactions (b), parameters (c), and dimensionless input and outputs (d). Notation in (d) is used below in Fig 6(a) and in Methods section: x denotes the input to the cascade, z stands for the cascade output, and y is the output of the first cycle (and, at the same time, the input to the second cycle). Efficacy of the cascade is defined as zmax.
If consumption of the active form of the protein by the second cycle can be ignored [3, 9], each cycle will operate in a “single-cycle” mode. One would then expect the logarithmic sensitivity to increase in a multiplicative manner, based on the calculus chain rule of differentiation [8, 9]. However, even under these, most favorable, conditions, sensitivity amplification is not guaranteed unless the cycles are “synchronized” in the way illustrated in Fig 6(a). Indeed, even if individual cycles are sufficiently sensitive, there will be no sensitivity amplification in the cascade, if the “steep” segment of the input-output curve of the second cycle falls out of the appropriate range dictated by the analogous segment of the first cycle. The condition for such synchronization is particularly simple for the cascade of identical cycles: d21 c = y* / x*, where (x*,y*) is the point on a single cycle signal-response curve corresponding to the maximum logarithmic derivative (see Methods section for derivation). For the cascade to produce any noticeable sensitivity amplification, each cycle should be at least moderately sensitive and in this case, the ratio y*/ x* is practically independent of c (see the results of the previous section); its dependence on dissociation constants is presented in Table 1.
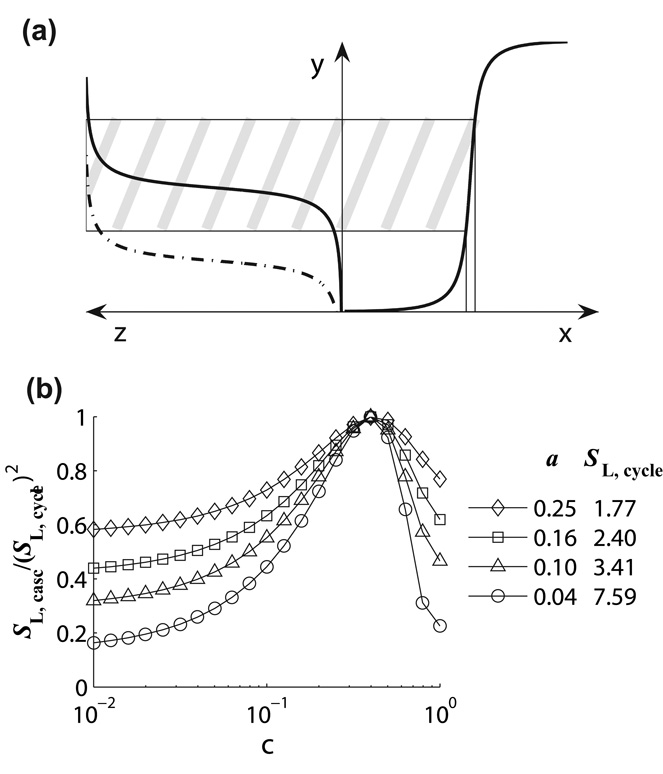
Figure 6.
Optimizing sensitivity of the cascade: cycles must be “synchronized” so that the steep segment of the input-output curve z(y) of the second cycle maps onto the “most sensitive” range of inputs to the first cycle (a); amplification of logarithmic sensitivity of the cascade of two identical cycles with b = a (sequestration is ignored, d21 = 1) (b).
Table 1.
Synchronization conditions for cascades of identical cycles (sequestration is ignored)
| y*/x*(=d21c) | |||
|---|---|---|---|
| a | b = a | b = 10a | b = 100a |
| 0.0125 | 0.397511 | 0.190621 | 0.052482 |
| 0.025 | 0.398474 | 0.179266 | 0.040399 |
| 0.05 | 0.400541 | 0.161732 | 0.029368 |
| 0.1 | 0.405134 | 0.138972 | 0.020747 |
| 0.2 | 0.415784 | 0.115589 | 0.014859 |
| 0.4 | 0.440852 | 0.097892 | 0.011409 |
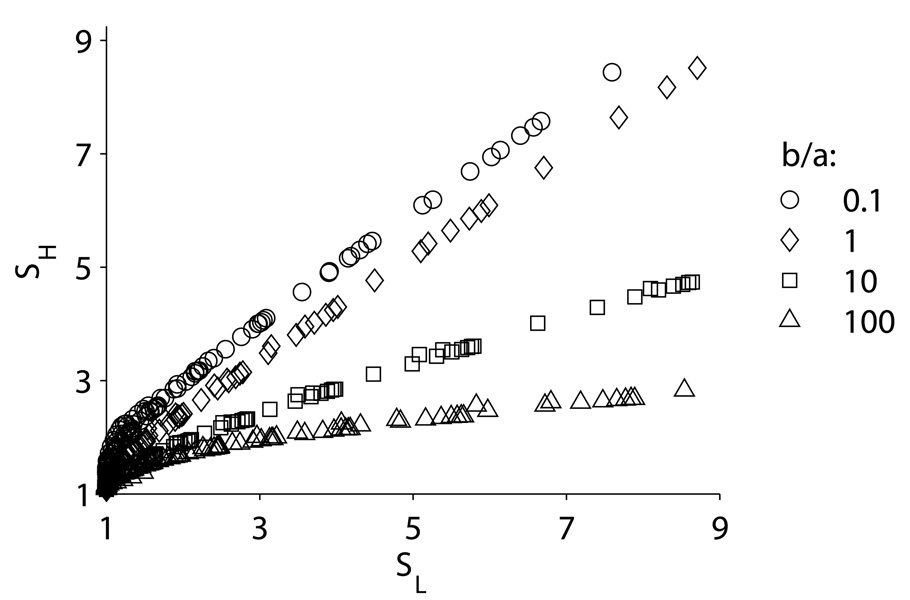
According to experimental data for the MAPK cascade [13], the protein concentrations in different levels of the cascade are of the same order of magnitude in many cell types [10]. This implies that d21 ~1, and the optimal sensitivity amplification in a cascade of symmetric cycles is achieved at c ≈ 0.4 (Fig 6(b) and Table 1). The efficacy of a symmetric cycle with c = 0.4 is only 60–70%. Higher efficacy, along with the optimal sensitivity amplification, can be achieved in a cascade of asymmetric cycles, because the ratio y*/ x* decreases as the asymmetry coefficient α (=b/a) grows (Table 1).
The real problem with cascades of symmetric cycles is that they do not amplify sensitivity! Indeed, if sequestration of the active form of a protein by a subsequent cycle is factored in, these cascades are actually less sensitive than individual cycles (Fig 7(a)). This result agrees with the findings of Bluthgen et al [10] and can be explained qualitatively as follows. A monocyclic signal transduction cascade can amplify sensitivity only if the sensitivities of individual cycles are noticeably greater than unity. Based on the properties of a single cycle (see the previous section), this implies significant binding affinities and, when the cycles are built in a cascade, leads to substantial sequestration of the active form by a subsequent cycle. This, in turn, effectively desensitizes the upstream cycle. The fact that it now contains only a fraction of the total amount of the protein is equivalent to larger normalized dissociation constants and therefore to a lower sensitivity. The effect is so strong that the cascade sensitivity becomes lower than that of individual cycles.
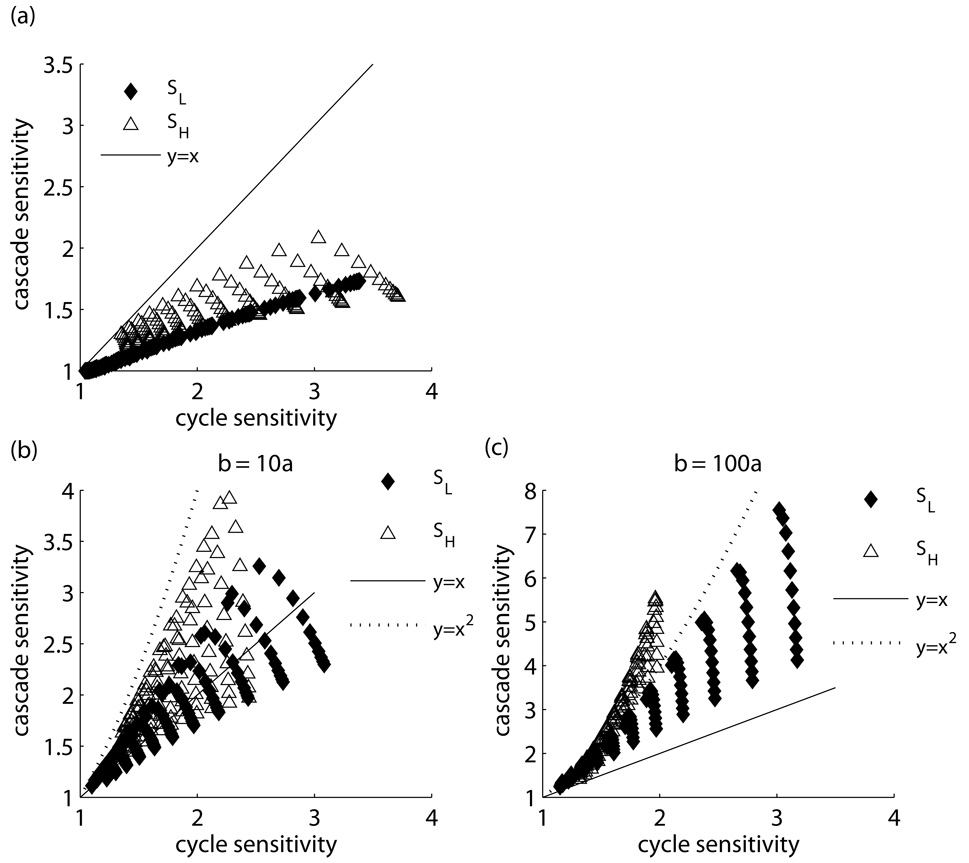
Figure 7.
Sensitivity amplification in a cascade of two identical cycles with the account of sequestration. In the case of symmetric cycles, the cascade sensitivity is lower than that of an individual cycle (a); however, for asymmetric cycles, with activators less saturated than inhibitors, the cascade acquires an amplified sensitivity that with increasing asymmetry approaches (and even exceeds, for the Hill sensitivities) the multiplicative limit (b). The data are shown for logarithms of a and c (base 10) that equidistantly sample the following ranges: in panel (a), [−1, −0.1] for log a and [−2, −0.5] for log c; in panel (b), b=10a, same as in (a) except for log a which was in the range [−1.25, −0.5] for SL; in panel (b), b=100a, [−1.5, −0.5] for log a and [−2, −1.2] for log c.
The question then arises whether sequestration can be avoided and yet cycles in the cascade retain moderate sensitivity. The answer is yes, if the cycles are asymmetric and involve saturated inactivation and much less saturated activation.
Sequestration by a downstream cycle can be ignored if activation is unsaturated, but it is less obvious that the cycle with unsaturated activation (large b) can still remain sensitive. In fact, it is easy to show that saturated inactivation alone is sufficient for the cycle to be moderately sensitive. When b is large, the logarithmic sensitivity of a cycle is
and becomes significant when a is small (see Methods section for derivation). Fig 7(b) shows that the “asymmetry condition” requiring unsaturated activation and saturated inactivation is sufficient for sensitivity amplification and that under this condition, the sensitivity of the cascade can be greater than that of an individual cycle, with the amplification approaching the multiplicative limit as α increases.
Sampling parameter spaces of bi-cyclic and tri-cyclic cascades
For insight into whether the asymmetry with α>s1 is also a necessary condition for an efficient sensitivity amplification, we performed uniform random sampling of the “free energy space” of a cascade (the log-uniform sampling). The resulting normalized parameters covered several orders of magnitude: log ai and log bi were randomly chosen between −2 and 2, whereas the values of log ci were picked from the interval [−3, 0]. Here the index “i” enumerates the cascade levels, so that i = 1, 2 for bi-cyclic cascades and i = 1, 2, 3 for tri-cyclic cascades. The latter are characterized by three parameter subsets, (ai,bi,ci), and two additional dimensionless parameters,
associated with the ratios of protein concentrations in the two adjacent levels. On the assumption that protein concentrations at different levels of a cascade are of the same order of magnitude (see discussion in the previous section), parameters d21 and d32 in the numerical experiments were set at 1, both for two- and three-level cascades.
Randomly generated cascades were evaluated with respect to their sensitivity and efficacy. The ultrasensitive cascades with sufficiently high efficacy and substantial sensitivity amplification were studied with respect to the activation - inactivation asymmetry of their cycles. The following cut-offs were used: 0.75 for efficacy and S0=4.0 for sensitivity of the cascade, both for SH or SL. The amplification of sensitivity was regarded as sufficiently high when the cascade was substantially more sensitive than its most sensitive cycle. Specifically, for the two-level cascades, the sensitivity of the cascade had to be at least times the maximum sensitivity of its individual cycles. For the three-level cascades, the amplification coefficient was set at . The number of samples routinely used in the numerical experiments was 106 and, on occasion, 107.
First, we engineered cascades of identical cycles. For this, only one set of parameters a, b, and c had to be randomly generated per sample. The cascades that satisfied the conditions for high efficacy, ultrasensitivity, and amplification, as specified above, appeared with a frequency of: 3.3% (bi-cyclic, SH), 2.4% (bi-cyclic, SL), 4.4% (tri-cyclic, SH), 3.1% (tri-cyclic, SL). In all these cases, the cycles were asymmetric with α>1. That the Hill definition of sensitivity is more permissive is due to the low-range anomaly of the Hill sensitivity described at the end of the Single activation-inactivation cycle: sensitivity and efficacy section.
We then proceeded with building cascades of different cycles. To generate a two-level cascade of this type, the six-dimensional parameter space was randomly sampled. The cascades met the cut-off criteria for 0.70% (SH) and 0.27% (SL) cases. All of these cascades had at least one cycle that involved unsaturated activation and saturated inhibition, with 95–97% of them having both such cycles. Similar statistics were observed for the tri-cyclic cascades generated by sampling the nine-dimensional parameter space. In this case frequencies, with which the generated cascades satisfied the criteria of sufficient sensitivity amplification and efficacy, were 0.30% for SH and 0.11% for SL. Of these cascades, all had at least one cycle with α>1, while 73% had all three and ~27% cases had two such cycles. For the Hill sensitivity, a very small fraction, ~0.03%, had only one asymmetric cycle with α>1 (see Table 2 for the parameters of one such cascade). Interestingly, sequestration in these instances is negligible because of strong asymmetry between bi+1 and ai (all ratios bi+1/ai were above 10, see Table 2), while some cycles with α<1 had substantial sensitivities due to the low-range anomaly of Hill sensitivities.
Table 2.
Parameters of a tri-cyclic cascade with substantial amplification of Hill sensitivity and one asymmetric cycle with b>a (α>1)
| Cascade sensitivity, SH | 13.47563 | ||
|---|---|---|---|
| Cycle #, i | 1 | 2 | 3 |
| ai | 0.09149 | 0.010735 | 15.97172 |
| bi | 0.049533 | 3.967634 | 5.487359 |
| ci | 0.001439 | 0.087075 | 0.423306 |
| Cycle sensitivities, | 4.868984 | 2.056108 | 1.042588 |
Overall, the analysis in this section indicates that asymmetry in saturation of converter enzymes is a likely necessary condition for the monocyclic cascade to substantially amplify sensitivity in an efficient manner.
Methods
Mathematically, a steady state of a reaction network is described by a system of algebraic equations that are generally nonlinear. A single activation-inactivation cycle depicted in Fig 2 is described by the following set of equations:
| (1) |
with three mass conservation relationships: E1,tot = [E1]+[I1], E2,tot = [E2]+[I2], and Ptot = [P1]+[P2]+[I1]+[I2]. Excluding [I1] and [I2] from (1) and introducing the normalized input x = k1E1,tot /(k2E2,tot) and output y = [P2] / Ptot, one obtains
| (2) |
Equations (2) include three dimensionless parameters: a = Km,2 / Ptot, b = Km,1 / Ptot, and c = (1+k2 / k1)E2,tot / Ptot, and further reduce to
| (3) |
A multi-level cascade of such cycles is described by a system of multiple polynomial equations of this type. For each parameter set, the system must be solved for a sufficiently large range of x. Sensitivity should be evaluated using the physically meaningful branch of roots y. Given the high polynomial power, the only practical option would be to seek a highly accurate numerical approximation for an appropriate root, which makes a large scale parameter analysis a daunting task.
The problem simplifies, however, upon the observation that Eq (3) is linear with respect to x and permits obtaining a closed explicit expression for the response-signal relation:
| (4) |
Sampling the output with sufficiently small steps in the range y>0, (y + a)(1 − y) − cy>0 and using Eq (4) for evaluating the corresponding input automatically yields a physically appropriate branch of solution, free of numerical errors. As a result, the problem becomes manageable and scales easily for the case of multilevel cascades. For the bi-cyclic cascade, the equations are:
where z stands for the output of the cascade whereas y is now the output of the first cycle (see notation in Fig 5(d)).
For the tri-cyclic cascade, the corresponding equations can be written as
where xi (i=1, 2, 3) denotes the output of the i-th level of the cascade. Further generalization, if desired, is straightforward. The algorithm always starts with a uniform sampling, in an appropriate range, of a final output, followed by sequential computation of intermediate outputs in a reverse order, and concludes with the evaluation of an input. Programming of the algorithm is straightforward and computations are fast, which makes extensive sampling of the multidimensional parameter space feasible.
The conditions for cycle synchronization can be derived analytically for the cascades of identical cycles, provided sequestration is negligible. Let x = F (y) be the output-input relationship for the upstream cycle. A similar relationship should hold for the subsequent cycle. Indeed, in the absence of sequestration, the cycles in the cascade are independent, and by assumption, have the same parameters. Because the input to the first cycle, E1,tot, is normalized by k2E2,tot/k1 and the input to the subsequent cycle, , is normalized by , the output-input relationship for the second cycle is y = d21cF(z) with . Let (x*, y*) be the point on the curve x = F(y) that corresponds to the maximum of the logarithmic derivative. Then the condition for perfect synchronization is y* = d21cF(y*) or d21c = y* / x*.
Finally, we derive the logarithmic sensitivity of a cycle in the case of unsaturated activation. Note that for large b, Eq (4) becomes quadratic with respect to y: x ≈ by /((y + a)(1 − y) − cy) and the physical root is where w = a + c + x̃−1 −1 and x̃ = x/b . Further calculations are straightforward: the response coefficient,
reaches maximum of (1+ (a + c − 1)2 /(4a))−1/2 at x̃max = (1 − a − c) /((a + c − 1)2 + 4a).
Conclusions and Outlook
Cascades of activation-inactivation cycles are a ubiquitous feature of intracellular signaling pathways. Why is this particular network design so important for signal transduction? Experimental studies of the MAPK cascade in Xenopus oocyte extracts [4, 6] indicate that the signal transduction cascades may serve as effective sensitivity amplifiers as first proposed by Goldbeter and Koshland in their pioneering theoretical study [3]. Quantitative arguments provided by Kholodenko and colleagues [8, 9] support the idea. But is the sensitivity amplification a universal property of a signaling cascade or is it realized only under specific conditions?
This report presents an in-depth parameter analysis of a relatively simple model of a monocyclic signal transduction cascade so as to determine the conditions for the cascade to be an efficient sensitivity amplifier. Protein sequestration, which desensitizes cycles in the cascade and is detrimental for sensitivity amplification [10], can be avoided if b>a where b and a are the normalized Michaelis–Menten constants for protein activation and inactivation. This means the saturation of converter enzymes must be asymmetric: activators must be less (in fact, much less) saturated than inhibitors.
Even in the absence of sequestration, a cascade can amplify sensitivity only if the cycles are appropriately synchronized. While this imposes an additional constraint on the normalized concentrations of inactivating converter enzymes, c, affecting in turn cycle efficacy, once conditions of asymmetry are satisfied, synchronization goes hand in hand with efficacy.
Log-uniform parameter sampling has been performed to show that the condition of “saturation asymmetry” is not only sufficient but necessary. In addition, these numerical experiments have shown that only ~0.1–1% of all randomly generated two-level cascades satisfy this condition. Given the low probabilities of favorable parameter sets, the system must be fine-tuned for efficient sensitivity amplification. One can argue that if this property is essential for survival, it may take only a few generations for the system to be fine-tuned by natural selection so as to elevate these probabilities to >90%. But it is also possible that the properties other than sensitivity amplification, such as multiple regulatory sites, make the cascade structure important for signal transduction. Quantitative information about concentrations and affinities of signaling enzymes is scarce [10], but according to the experimental data available for Xenopus oocyte extracts [6], the inactivating enzymes of the MAPK cascade do function in a partially saturated regime. In physiological conditions, apparent Km for the MAPK phosphatase activity is ≈300 nM and the total concentration of p42 MAPK is estimated to be 1.2 µM, yielding a ≈ 0.25. It is therefore desirable for the “saturation asymmetry” predicted by this study to be tested experimentally.
Because of double phosphorylation, the MAPK cascade involves a more complex network than considered here. While similarities in the network structure and mechanisms make it likely that the conditions for sensitivity amplification are qualitatively similar, it would be interesting to explore whether the method developed here is applicable to the networks with multiple phosphorylation.
In summary, the asymmetry between saturated inhibiting enzymes and unsaturated activators has been shown to be a sufficient condition for optimal sensitivity amplification. Random parameter sampling strongly suggests that this condition is also necessary.
Acknowledgments
We thank Felix Bronner for the careful reading of the manuscript and helpful comments. The work has been supported by National Institutes of Health grants 1U54 RR022232 and P41 RR13186.
Glossary
- Response coefficients
The response coefficient, or the local sensitivity, of a signaling cascade is the relative change in response divided by the corresponding relative change in signal. Mathematically, this is a logarithmic derivative, d(log y)/d(log x), where x and y are the signal and response values, respectively. The response coefficient can be different for different signal levels.
- Logarithmic sensitivity
The logarithmic sensitivity of a signaling cascade or a cycle is defined as the maximum response coefficient over the entire range of inputs.
- Michaelis-Menten constant
The Michaelis-Menten constant is a parameter of the Michaelis-Menten kinetics, a frequently used approximation for the rate of an enzymatic reaction. The Michaelis-Menten constant, having the units of concentration, can be interpreted as a concentration threshold beyond which the substrate increasingly saturates the enzyme and the reaction rate approaches its maximum value.
- Efficacy
The efficacy of a cascade or a cycle is defined as the maximum normalized output over the entire range of inputs. The normalized output is the fraction of the protein activated at the last level of the cascade.
Contributor Information
Eva Racz, Email: re551@hszk.bme.hu.
Boris M. Slepchenko, Email: boris@neuron.uchc.edu.
References
- 1.Stadtman ER, Chock PB. Superiority of interconvertible enzyme cascades in metabolic regulation: Analysis of monocyclic systems. Proc Natl Acad Sci USA. 1977;74:2761–2765. doi: 10.1073/pnas.74.7.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greengard P. Phosphorylated Proteins as Physiological Effectors. Science. 1978;199:146–152. doi: 10.1126/science.22932. [DOI] [PubMed] [Google Scholar]
- 3.Goldbeter A, D. E. Koshland J. An amplified sensitivity arising from covalent modification in biological systems. Proc Natl Acad Sci USA. 1981;78:6840–6844. doi: 10.1073/pnas.78.11.6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrell JE, Jr, Machleder EM. The Biochemical Basis of an All-or-None Cell Fate Switch in Xenopus Oocytes. Science. 1998;280:895–898. doi: 10.1126/science.280.5365.895. [DOI] [PubMed] [Google Scholar]
- 5.Ferrell JE, Jr, Xiong W. Bistability in cell signaling: How to make continuous processes discontinuous, and reversable processes irreversable. Chaos. 2001;11:227–236. doi: 10.1063/1.1349894. [DOI] [PubMed] [Google Scholar]
- 6.Huang C-YF, J. E. Ferrell J. Ultrasensitivity in the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1996;93:10078–10083. doi: 10.1073/pnas.93.19.10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrell JE., Jr How responses get more switch-like as you move down a protein kinase cascade. Trends Biochem. Sci. 1997;22:288–289. doi: 10.1016/s0968-0004(97)82217-7. [DOI] [PubMed] [Google Scholar]
- 8.Brown GC, Hoek JB, Kholodenko BN. Why do protein kinase cascades have more than one level? Trends Biochem. Sci. 1997;22:288. doi: 10.1016/s0968-0004(97)82216-5. [DOI] [PubMed] [Google Scholar]
- 9.Kholodenko BN, Hoek JB, Westerhoff HV, Brown GC. Quantification of information transfer via cellular signal transduction pathways. FEBS Lett. 1997;414:430–434. doi: 10.1016/s0014-5793(97)01018-1. [DOI] [PubMed] [Google Scholar]
- 10.Bluthgen N, Bruggeman FJ, Legewie S, Herzel H, Westerhoff HV, Kholodenko BN. Effects of sequestration on signal transduction cascades. FEBS J. 2006;273:895–906. doi: 10.1111/j.1742-4658.2006.05105.x. [DOI] [PubMed] [Google Scholar]
- 11.Fell DA. Metabolic control analysis: a survey of its theoretical and experimental development. Biochem. J. 1992;286:313–330. doi: 10.1042/bj2860313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Small JR, Fell DA. Covalent modification and metabolic control analysis: modification to the theorems and their applications to metabolic systems containing covalently modifiable enzymes. Eur. J. Biochem. 1990;191:405–411. doi: 10.1111/j.1432-1033.1990.tb19136.x. [DOI] [PubMed] [Google Scholar]
- 13.Yeung K, Seitz T, Li S, Janosch P, McFerran B, Kaiser C, Fee F, Katsanakis KD, Rose DW, Mischak H, Sedivy JM, Kolch W. Suppression of Raf-1 kinase activity and MAP kinase signaling by RKIP. Nature. 1999;401:173–177. doi: 10.1038/43686. [DOI] [PubMed] [Google Scholar]