Abstract
Objectives:
We hypothesized that TNFα would be higher in obese versus lean preeclamptic subjects.
Methods:
Total plasma TNFα was measured in a nested case-control study of 123 nulliparous lean and obese control and preeclamptic subjects.
Results:
Adjusted mean TNFα concentrations were 0.97±0.11 (pg/ml ± SEM) in lean controls, 1.01±0.10 in obese controls, 1.43±0.11 in lean preeclamptics and 1.16±0.11 in obese preeclamptics. Pregnancy outcome was the single predictor of TNFα concentration in the general linear regression model (p=0.04).
Conclusion:
TNFα concentration was higher in preeclamptic compared to control subjects. Obesity was not associated with higher TNFα concentrations in preeclamptic or control subjects.
Keywords: Preeclampsia, tumor necrosis factor alpha, TNFα, obesity, BMI
Introduction
Preeclampsia, a leading cause of maternal and fetal morbidity and mortality,1 is a multisystemic disorder diagnosed by the new onset of hypertension and proteinuria after 20 weeks gestation.2 The pathophysiology of preeclampsia is incompletely understood, but inflammatory processes have been proposed to mediate maternal endothelial activation and dysfunction. The resulting vasospasm, activation of the coagulation cascade and increased microvascular permeability in this syndrome lead to end organ ischemia.3-5
The proinflammatory cytokine, tumor necrosis factor alpha (TNFα), produces endothelial dysfunction.3 Through innate and acquired immune processes, TNFα modulates growth, differentiation and metabolism of many cell types, affects lipid metabolism, coagulation, insulin resistance and inflammation.6, 7 Most studies of TNFα in preeclampsia report higher circulating concentrations in affected pregnancies compared with uncomplicated control pregnancies,3, 7 however TNFα was not greater in placental tissues from preeclamptic pregnancies compared to placentas from uncomplicated pregnancies.8, 9 Taken together, these findings may indicate that as in the metabolic syndrome,10-12 TNFα is produced in excess by adipocytes in preeclamptic women. Mounting evidence associates metabolic syndrome and preeclampsia.13, 14
Obesity is a significant risk factor for preeclampsia.4 Inflammation is a potential pathway through which obesity increases the risk of preeclampsia.15, 16 For example, C-reactive protein as a measure of inflammation accounted for over forty percent of the increased risk of preeclampsia by body mass index (BMI).15 TNFα was higher in nonpregnant obese subjects compared to normal weight individuals.17-19 Some studies of TNFα in preeclampsia have adjusted for BMI,20, 21 but little is known about the relationship between obesity and TNFα in preeclampsia. Taken together, previous studies suggest that elevated TNFα might explain the higher incidence of preeclampsia in obese women. We hypothesized that TNFα would be higher in 1) lean preeclamptic compared to lean control subjects, 2) obese preeclamptic compared to obese control subjects and 3) obese preeclamptic compared to lean preeclamptic subjects.
Materials and Methods
Study subjects and samples
This was a nested case-control study from an ongoing investigation of preeclampsia at the University of Pittsburgh, Magee-Womens Hospital (MWH) and Magee-Womens Research Institute. The study was approved by the institutional review board and informed consent was obtained from all subjects. Women 14–44 years of age with a singleton pregnancy and planning to deliver at MWH were recruited before 16 weeks gestation. Prepregnancy weight and height, blood pressures and urinary protein measurements throughout gestation, use of hypertensive medications, antepartum and delivery events, and neonatal outcomes were abstracted from medical records. Women with multiple fetuses, chronic hypertension, renal disease, diabetes, other preexisting medical conditions, or history of illicit drug use were excluded. All women were without clinical evidence of infection.
Plasma samples available from admission for delivery were selected for preeclamptic cases and nonpreeclamptic controls and for lean and obese prepregnancy BMI. Preeclampsia was diagnosed by the presence of gestational hypertension, proteinuria, and hyperuricemia beginning after the 20th week of pregnancy with resolution of blood pressure and proteinuria postpartum. Gestational hypertension was defined as an absolute blood pressure ≥ 140 mmHg systolic and/or ≥ 90 mmHg diastolic after 20 weeks of gestation. Proteinuria was defined as ≥ 300 mg per 24-hour urine collection, ≥ 2+ protein on voided urine sample, ≥ 1+ protein on catheterized urine specimen, or a protein-creatinine ratio of ≥ 0.3. 2 Hyperuricemia was defined as plasma uric acid concentration ≥ 1 SD above reference values at the gestational age the sample was obtained (e.g. term, > 5.5 mg/dL). Prepregnancy BMI was based on measured height and maternal self report of prepregnancy weight at the initial visit. Prepregnancy BMI was categorized as lean (19-24.9 kg/m2) or obese (≥ 30 kg/m2); overweight BMI (25.0–29.9 kg/m2) was excluded. Power analysis for a two-way analysis of variance with 80 percent power to detect a moderate effect size (0.75) at 0.05 alpha would require 40 subjects in each group and to detect a large effect size (1.00) would require 24 subjects per group (SAS Institute Inc.; Cary, NC). Four groups were studied: samples from 31 lean control women, 31 obese control women, 31 lean preeclamptic women, and 30 obese preeclamptic women met inclusion criteria for diagnosis and weight status. Gestational age was determined by the best obstetrical estimate at delivery.
Quantitation of plasma TNFα
Blood samples were collected in EDTA at the time of admission for delivery. Plasma was stored at −80C degrees until assayed. Investigators conducting the assay were blinded to cases, controls and BMI categories. TNFα concentrations were determined in duplicate by enzyme-linked immunoabsorbent assay using a commercially available reagent kit (Quantikine HS High Sensitivity Human TNFα, R&D Systems, Minneapolis, MN). The coefficient of variation averaged 6.6% intra-assay and 13.4% inter-assay. Sensitivity was 0.12 pg/ml.
Statistical analyses
Descriptive analysis was initially performed to characterize the study population. Summary statistics, including means and standard deviations for all continuous variables were calculated. Frequency distributions were determined for categorical variables. Demographic and clinical characteristics of case and control groups were compared using univariate analysis of variance (ANOVA) and chi square tests. The General Linear Model (GLM) was used to evaluate the association between TNFα concentration and weight status (lean vs. obese) and diagnostic status (control vs. preeclampsia) controlling for race, smoking status, labor at sampling, entry age and gestational age. The possible interaction between covariates was also examined and Bonferroni adjustment was used for multiple comparisons. Verification of model assumptions and fit was carried out via examination of residual plots. Means plus/minus standard error of the mean (± SEM) adjusted for other covariates are reported for weight status and/or preeclampsia status. All analyses were performed as two-tailed tests using SAS Version 8 (SAS Institute, Inc., Cary, NC, USA). The level of significance was set a priori at p < 0.05.
Results
By design in this nested case-control study, preeclamptic and control groups differed significantly in meeting diagnostic criteria, e.g. uric acid concentrations and systolic and diastolic blood pressures at delivery (p < 0.05; Table 1). Systolic and diastolic blood pressures were greater before 20 weeks gestation in the preeclamptic compared to control groups and in the obese compared to the lean groups. Obese preeclamptic and control groups differed from lean preeclamptic and control groups in weight status as designed by BMI criteria. Thirty Black women and three Asian women were grouped as “Non-white” and there was no difference among the four study groups by racial composition (p = 0.28). The four study groups differed in smoking status and labor at sampling.
Table 1.
Demographic and clinical characteristics
|
Variable |
Lean Control N=31 |
Obese Control N=31 |
Lean Preeclamptic N=31 |
Obese Preeclamptic N=30 |
p-value |
|---|---|---|---|---|---|
| Maternal age (years) |
22.2 ± 5.4a | 23.2 ± 6.3a | 24.7 ± 5.9a | 28.1 ± 6.7b | 0.0015 |
| Gestational age at delivery (weeks) |
39.9 ± 1.1a | 39.7 ± 2.4a | 34.8 ± 3.5b | 35.1 ± 4.2b | < 0.0001 |
| Race (N/%) | 0.28* | ||||
| Non-white | 6 (19) | 12 (39) | 9 (29) | 6 (20) | |
| White | 25 (81) | 19 (61) | 22 (71) | 24 (80) | |
| Smoking (N %) | 0.005* | ||||
| Yes | 18 (58.1) | 15 (48.4) | 6 (19.9) | 8 (29.0) | |
| No | 13 (41.9) | 16 (51.6) | 25 (80.1) | 22 (71.0) | |
| Labor at sampling (N %) |
< 0.001* | ||||
| Yes | 22 (71.0) | 10 (32.3) | 19 (61.3) | 4 (13.3) | |
| No | 9 (29.0) | 21 (67.7) | 12 (38.7) | 26 (86.7) | |
| Pre-pregnancy BMI (kg/m2) |
21.5 ± 1.6a | 36.5 ± 5.7b | 21.7 ± 1.4a | 35.9 ± 4.8b | < 0.0001 |
| Blood pressure < 20 weeks (mmHg) |
< 0.0001 | ||||
| 107.8 ± 7.0a | 117.7 ± 7.2b | 113.7 ± 9.4b | 124.0 ± 9.9c | ||
| 65.9 ± 4.2a | 71.6 ± 5.8b | 68.4 ± 6.8a | 77.3 ± 6.1c | ||
| Blood pressure in labor (mmHg) |
116.1 ± 9.0a | 123.5 ± 6.9a | 155.3 ± 11.6b | 161.6 ± 14.3b | < 0.0001 |
| 69.4 ± 7.2a | 75.7 ± 5.5b | 93.3 ± 9.0c | 90.9 ± 10.0c | ||
| Uric acid (mg/dl) | 4.3 ± 0.77a | 4.6 ± 0.74a | 6.2 ± 0.79b | 6.7 ± 1.4c | < 0.0001 |
| Birth weight (grams) |
3391.2 ± 314.3a | 3537.0 ± 691.7a | 2102.2 ± 824.3b | 2256.5 ± 919.7b | < 0.0001 |
| Birth weight centile (%) |
49.2 ± 22.9a | 64.7 ± 22.9b | 25.3 ± 21.6c | 33.3 ± 28.0c | < 0.0001 |
Data are mean ± SD; ANOVA
Chi square analysis
Variables within rows that do not share the same letter are statistically different, p < 0.05.
Distributions of TNFα concentrations are shown in Figure 1. Although the study groups differed in composition by smoking status and labor at sampling, in univariate analyses, TNFα concentration was not significantly different by smoking status (p = 0.11) or labor (p = 0.15) among study groups. The GLM was significant for association of plasma TNFα concentration with diagnostic status but not weight status, controlling for race, smoking status, labor at sampling, entry age and gestational age (overall p = 0.04). All the covariates including race, smoking status, labor at sampling, entry age and gestational age were not significantly related to plasma TNFα concentration (p > 0.20). There was marginal interaction between weight status and diagnostic status on TNFα concentration (p = 0.10). GLM showed no difference in TNFα concentrations between lean and obese subjects (adjusted mean ± SEM: 1.21 ± 0.11 vs. 1.11 ± 0.12; p = 0.27; Figure 2). The preeclamptic subjects had higher TNFα concentrations than control subjects (adjusted mean ± SEM: 1.32 ± 0.13 vs. 1.00 ± 0.12; p = 0.01; Figure 3). Adjusted mean TNFα concentrations were 0.97 ± 0.11 pg/ml in the lean controls, 1.01 ± 0.10 pg/ml in the obese controls, 1.43 ± 0.11 pg/ml in the lean preeclamptics, and 1.16 ± 0.11 pg/ml in the obese preeclamptics. Adjusted mean TNFα concentrations were higher in the lean preeclamptic compared to the lean control subjects (p = 0.03) and not higher in the obese preeclamptics compared to obese control subjects (p = 1.00). No difference in TNFα was found between obese control and lean control (p = 1.00) or between obese preeclamptic and lean preeclamptic subjects (p = 0.27).
Figure 1.
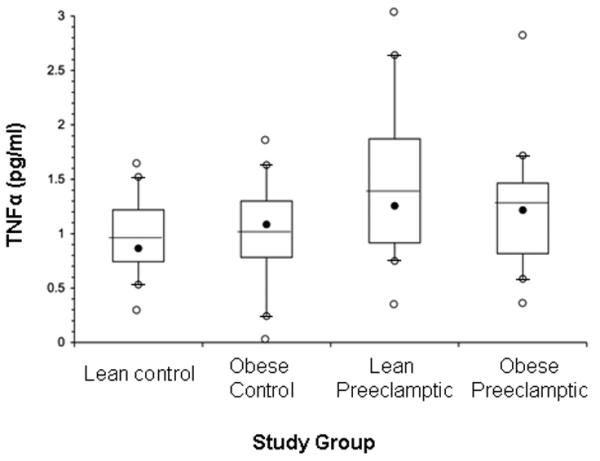
Box plot chart of TNFα distributions by study group. Box plots of TNFα concentrations show top, bottom, and line through the interior of the box corresponding to the 75th percentile, 25th percentile, and mean, respectively; and the black dot represents the median. The whiskers (t bars) on bottom denote the 10th percentile, and on the top, denote the 90th percentile. Open dots above and below plots represent minimum and maximum data points.
Figure 2.
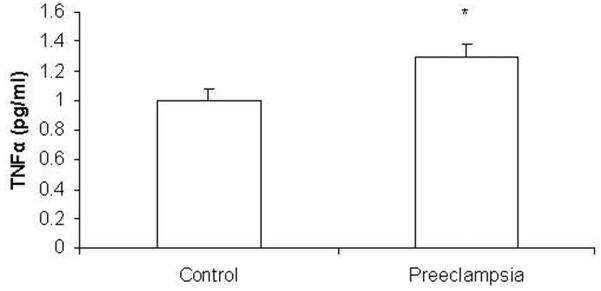
Adjusted means of TNFα concentration by diagnostic status. * TNFα concentrations significantly differed by diagnosis between the two groups of 62 uncomplicated control and 61 preeclamptic subjects (GLM; p = 0.02).
Comment
In this study we observed that TNFα was higher in women with preeclampsia compared to women with uncomplicated pregnancies. The lean preeclamptic group had higher TNFα concentrations compared to the lean control subjects. Our hypothesis that TNFα would be elevated by obesity in preeclampsia was not supported. Diagnosis of preeclampsia was the single predictor of elevated TNFα in the four study groups. Obesity was not associated with higher TNFα in preeclamptic subjects.
Higher circulating TNFα concentrations in this sample of preeclamptic subjects are consistent with concentrations found by others.3, 9, 20, 22 In previous studies, BMI was adjusted for statistically, matched, or not considered in studies of TNFα during pregnancy. Our study analyzed TNFα in preeclampsia and uncomplicated pregnancies specifically by lean and obese BMI categories.
We expected elevated TNFα in obese preeclamptics based on adipocyte TNFα secretion and population studies of inflammation markers in obesity.10-12, 17 Reproductive age, nonpregnant obese women were found to have higher TNFα concentrations compared to nonpregnant lean subjects of similar age.18, 19, 24 In these studies, TNFα concentrations correlated directly with waist to hip ratio (WHR) and glucose tolerance test insulin levels, but not with BMI, and was higher in obese women with a WHR greater than 0.90.24 This was not the case in our study of pregnant women. Neither control nor preeclamptic obese subjects had elevated TNFα compared to the appropriate lean subset. It is possible that the simple assessment of obesity, that is, by prepregnancy BMI without attention to visceral as opposed to subcutaneous fat may not have been sufficiently sensitive.17
The nonsignificant difference found in plasma TNFα by BMI during pregnancy and preeclampsia in our study may be a result of the generalized inflammatory response in pregnancy, characterized by innate immune system (Type 1) activation and adaptive immune system (Type 2) suppression.25 Type 1 cytokines, such as TNFα, IFN-γ, and IL-2 that are augmented in preeclampsia may overcome any additional effect of obesity. Relative proportions rather than the absolute levels of cytokines may lead to the greater effect of preeclampsia than obesity on TNFα concentrations.25-27 Alternatively, our findings may corroborate others' suggestion that adipose cytokines act locally without elevating plasma concentrations.10, 12
The assay used in this experiment measured total TNFα (Quantikine HS), including free and receptor-bound protein. It may be that soluble TNF receptors are more reliable markers for TNFα activity.28 Theoretically TNFα level may be lowered, but its bioactive effect paradoxically increases when the cytokine is sequestered by its receptor and stabilized at the cell membrane. Nonetheless, when measured on the same samples there is a strong correlation between immunoreactive TNFalha and circulating receptor concentration (29)
Our findings are limited by the cross sectional design. The nested case-control study showed association or lack of association among weight status as BMI, diagnostic status and TNFα, but could not demonstrate cause and effect. A longitudinal study with serial sampling of lean and obese women at risk of developing preeclampsia could provide more conclusive data on causality.
Conclusion
In summary, TNFα was not higher by obesity in preeclamptic or uncomplicated pregnancies. Based on these findings, it is unlikely that the mechanism of obesity's association with preeclampsia is related to a simple effect of fat mass increasing TNFα concentrations. Investigations of proinflammatory cytokines such as TNFα in preeclampsia should account for interactions of fat distribution and normal pregnancy immune adaptations with inflammation.
Acknowledgments
Funded in part by the National Institutes of Health, NIH-2PO1-HD30367 (Preeclampsia Program Project) and NIH-5MO1-RR00056 (Magee-Womens CRC).
References
- 1.WHO . Beyond the numbers: Reviewing maternal deaths and complications to make pregnancy safer. Geneva: 2004. [DOI] [PubMed] [Google Scholar]
- 2.NHBPEP Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183(1):S1–S22. [PubMed] [Google Scholar]
- 3.Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol. 1997;37(3):240–9. doi: 10.1111/j.1600-0897.1997.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 4.Roberts J. Endothelial dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998;16(1):5–15. doi: 10.1055/s-2007-1016248. [DOI] [PubMed] [Google Scholar]
- 5.Sacks G, Sargent I, Redman C. An innate view of human pregnancy. Immunol Today. 1999;20(3):114–8. doi: 10.1016/s0167-5699(98)01393-0. [DOI] [PubMed] [Google Scholar]
- 6.Moldawer LL. In: The Tumor Necrosis Factor Superfamily and its Receptors. 5th ed. Paul WE, editor. Lippincott Williams & Wilkins; Philadelphia: 2003. [Google Scholar]
- 7.Schipper EJ, Bolte AC, Schalkwijk CG, Van Geijn HP, Dekker GA. TNF-receptor levels in preeclampsia--results of a longitudinal study in high-risk women. J Matern Fetal Neonatal Med. 2005;18(5):283–7. doi: 10.1080/14767050500246466. [DOI] [PubMed] [Google Scholar]
- 8.Benyo DF, Smarason A, Redman CW, Sims C, Conrad KP. Expression of inflammatory cytokines in placentas from women with preeclampsia. J Clin Endocrinol Metab. 2001;86(6):2505–12. doi: 10.1210/jcem.86.6.7585. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi M, Ueda Y, Yamaguchi T, Sohma R, Shibazaki M, Ohkura T, et al. Tumor necrosis factor-alpha in the placenta is not elevated in pre-eclamptic patients despite its elevation in peripheral blood. Am J Reprod Immunol. 2005;53(3):113–9. doi: 10.1111/j.1600-0897.2005.00253.x. [DOI] [PubMed] [Google Scholar]
- 10.Hauner H. The new concept of adipose tissue function. Physiol Behav. 2004;83(4):653–8. doi: 10.1016/j.physbeh.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi K. Adipokines: therapeutic targets for metabolic syndrome. Curr Drug Targets. 2005;6(4):525–9. doi: 10.2174/1389450054021972. [DOI] [PubMed] [Google Scholar]
- 12.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92(3):347–55. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 13.Evans RW, Powers RW, Ness RB, Cropcho LJ, Daftary AR, Harger GF, et al. Maternal and fetal amino acid concentrations and fetal outcomes during pre-eclampsia. Reproduction. 2003;125(6):785–90. doi: 10.1530/rep.0.1250785. [DOI] [PubMed] [Google Scholar]
- 14.Kaaja RJ, Poyhonen-Alho MK. Insulin resistance and sympathetic overactivity in women. J Hypertens. 2006;24(1):131–41. doi: 10.1097/01.hjh.0000194121.19851.e5. [DOI] [PubMed] [Google Scholar]
- 15.Bodnar LM, Ness RB, Harger GF, Roberts JM. Inflammation and triglycerides partially mediate the effect of prepregnancy body mass index on the risk of preeclampsia. Am J Epidemiol. 2005;162(12):1198–206. doi: 10.1093/aje/kwi334. [DOI] [PubMed] [Google Scholar]
- 16.Wolf M, Kettyle E, Sandler L, Ecker JL, Roberts J, Thadhani R. Obesity and preeclampsia: the potential role of inflammation. Obstet Gynecol. 2001;98(5):t–1. doi: 10.1016/s0029-7844(01)01551-4. [DOI] [PubMed] [Google Scholar]
- 17.Panagiotakos DB, Pitsavos C, Yannakoulia M, Chrysohoou C, Stefanadis C. The implication of obesity and central fat on markers of chronic inflammation: The ATTICA study. Atherosclerosis. 2005;183(2):308–15. doi: 10.1016/j.atherosclerosis.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez F, Minium J, Rote NS, Kirwan JP. Hyperglycemia alters tumor necrosis factor-alpha release from mononuclear cells in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90(9):5336–42. doi: 10.1210/jc.2005-0694. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez F, Minium J, Rote NS, Kirwan JP. Altered tumor necrosis factor alpha release from mononuclear cells of obese reproductive-age women during hyperglycemia. Metabolism. 2006;55(2):271–6. doi: 10.1016/j.metabol.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 20.Bartha JL, Romero-Carmona R, Escobar-Llompart M, Comino-Delgado R. The relationships between leptin and inflammatory cytokines in women with pre-eclampsia. BJOG. 2001;108(12):1272–6. doi: 10.1111/j.1471-0528.2001.00284.x. [DOI] [PubMed] [Google Scholar]
- 21.Freeman DJ, McManus F, Brown EA, Cherry L, Norrie J, Ramsay JE, et al. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension. 2004;44(5):708–14. doi: 10.1161/01.HYP.0000143849.67254.ca. [DOI] [PubMed] [Google Scholar]
- 22.Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol. 1998;40(2):102–11. doi: 10.1111/j.1600-0897.1998.tb00398.x. [DOI] [PubMed] [Google Scholar]
- 23.Hamai Y, Fujii T, Yamashita T, Nishina H, Kozuma S, Mikami Y, et al. Evidence for an elevation in serum interleukin-2 and tumor necrosis factor-alpha levels before the clinical manifestations of preeclampsia. Am J Reprod Immunol. 1997;38(2):89–93. doi: 10.1111/j.1600-0897.1997.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 24.Tsigos C, Kyrou I, Chala E, Tsapogas P, Stavridis JC, Raptis SA, et al. Circulating tumor necrosis factor alpha concentrations are higher in abdominal versus peripheral obesity. Metabolism. 1999;48(10):1332–5. doi: 10.1016/s0026-0495(99)90277-9. [DOI] [PubMed] [Google Scholar]
- 25.Luppi P. How immune mechanisms are affected by pregnancy. Vaccine. 2003;21(24):3352–7. doi: 10.1016/s0264-410x(03)00331-1. [DOI] [PubMed] [Google Scholar]
- 26.Dong M, He J, Wang Z, Xie X, Wang H. Placental imbalance of Th1- and Th2-type cytokines in preeclampsia. Acta Obstet Gynecol Scand. 2005;84(8):788–93. doi: 10.1111/j.0001-6349.2005.00714.x. [DOI] [PubMed] [Google Scholar]
- 27.Hunt JS, Chen HL, Miller L. Tumor necrosis factors: pivotal components of pregnancy? Biol Reprod. 1996;54(3):554–62. doi: 10.1095/biolreprod54.3.554. [DOI] [PubMed] [Google Scholar]
- 28.Dekker GA, Sibai BM. Etiology and pathogenesis of preeclampsia: current concepts. Am J Obstet Gynecol. 1998 Nov;179(5):1359–75. doi: 10.1016/s0002-9378(98)70160-7. [DOI] [PubMed] [Google Scholar]
- 29.Visser W, Beckmann I, Knook MA, Wallenburg HC. Soluble tumor necrosis factor receptor II and soluble cell adhesion molecule 1 as markers of tumor necrosis factor-alpha release in preeclampsia. Acta Obstet Gynecol Scand. 2002 Aug;81(8):713–9. [PubMed] [Google Scholar]


