Abstract
Biologic sex and sex steroids are important factors in clinical and experimental stroke and traumatic brain injury (TBI). Laboratory data strongly show that progesterone treatment after TBI reduces edema, improves outcomes and restores blood brain barrier function. Clinical studies to date agree with these data, and there are ongoing human trials for progesterone treatment after TBI. Estrogen has accumulated an impressive reputation as a neuroprotectant when evaluated at physiologically relevant doses in laboratory studies of stroke, but translation to patients remains to be shown. The role of androgens in male stroke or TBI is understudied and important to pursue given the epidemiology of stroke and trauma in men. To date, male sex steroids remain largely evaluated at the bench rather than the bedside. This review evaluates key evidence and highlights the importance of the platform on which brain injury occurs, i.e. genetic sex and hormonal modulators.
Keywords: stroke, brain ischemia, estradiol, neurosteroids, progesterone, allopreganolone, androgens
1. Sex differences in brain injury
It is now clear that biological sex alters the incidence of, and outcome from, ischemic and traumatic brain injury. For example, male sex is an acknowledged risk factor for stroke, and in most epidemiological series, stroke occurs more frequently in men vs. women. This sexually dimorphic disease pattern remains apparent until ages well beyond the menopausal years 1, 2. Nevertheless, stroke risk increases with age in both sexes, and there is broad evidence that outcome from an ischemic event is worse in aged women than in their male counterparts. Knowledge of mechanisms of ischemic cell death and neuroprotection is important for both sexes, but current evidence suggests that these mechanisms are not identical in males and females.
a. Sex differences in ischemic outcomes: animal models
Animal models of brain injury, typically rodents, have been used to evaluate side-by-side outcomes from ischemia or trauma. In most reports, females fare better than do their age-matched male counterparts. Early evidence in female vs. male spontaneously hypertensive, genetically stroke prone rats uncovered the male phenotype of “ischemia-sensitivity”3. This landmark study of 2000 animals showed that life expectancy is longer in the female, and the development of cerebral hemorrhage is delayed until an advanced age 3. Subsequently, a number of studies have shown that outcome from experimental brain injury is clearly sex-linked. Female rats and mice of various inbred and outbred strains experience smaller tissue damage for an equivalent insult from focal or global cerebral ischemia 4-7 and improved functional outcome 8. Similarly, male animals sustain greater injury than do age-matched females after traumatic brain injury 9. We have explored complicated rodent models with genetic risk factors associated with human stroke, e.g. insulin-dependent genetic diabetes 10. non-insulin dependent diabetes 11 and hypertension 12. In each genetic strain and despite deleterious complications from diabetes or hypertension, females are less sensitive to cerebral ischemia than are males.
b. Sex-specific cultures: hormone independent cell death or survival
In vitro data more directly support the concept that cell death mechanisms can be sex-specific. This specificity is typically modeled in primary cell cultures grown without background steroids. Under such conditions, some molecular pathways of cell death or survival diverge based on the genetic sex of the tissue. For example, cultured female dopaminergic neurons tolerate exposure to toxic dopamine concentrations and survive twofold relative to male cells 13. Similarly, female neurons from the cortical plate or ventricular zone have greater longevity in culture than do male cells, and differentially express higher levels of phosphorylated kinases such as Akt 14. Sensitivity to glutamate, peroxynitrate (ONOO) and staurosporine in neuronal culture is sex specific, with male neurons being more susceptible to glutamate and ONOO than females. In contrast, response to oxidants such as H2O2 is gender neutral 15. These observations are not limited to neurons. Cell death resulting from oxygen-glucose deprivation is less in female vs male cultured astrocytes or hippocampal slices 16, 17.
In summary, these findings suggest that the response to cerebral injury in vivo and in vitro is partially a function of the sex of the cell. However, this in no way discounts the importance of gonadal steroids or brain-derived neurosteroids as modulators of oxidant, toxic and ischemic challenges to the brain. The role of progesterone, estradiol and testosterone in shaping neuro-injury is reviewed in subsequent sections.
2. Neuroprotective effects of Progesterone
Progesterone is synthesized from cholesterol by the gonads, adrenal gland or placenta. In addition, progesterone can be generated within the brain as a neurosteroid, i.e. steroid hormones that accumulate in the central nervous system (CNS) either by de novo synthesis from cholesterol or in situ metabolism of precursors from the blood 18. In contrast, the term neuro-active steroid refers to any steroid having a direct effect in the CNS, independent of source of production. Within brain, progesterone is metabolized to the highly neuro-active metabolites 5αDH-progesterone and 3α,5αTH-progesterone (allopregnanolone; ALLO). Progesterone's neuroprotective actions are mediated, in part, by these neuro-active metabolites.
Exogenous progesterone protects the CNS in a variety of experimental animal models of neurodegeneration, including spinal cord injury 19-24, penetrating or diffuse brain injury, traumatic brain injury (TBI) 25, transient global and focal cerebral ischemia {Stein, 2008 12702 /id;Schumacher, 2007 12749 /id}. Many of these experimental studies emphasize progesterone's attractive attributes from a clinical perspective, i.e. its relatively long therapeutic window that extends up to 2 hours after middle cerebral artery occlusion 27 and to 24 hrs after experimental TBI 28. The ability of the active metabolite ALLO to mimic the beneficial effects of progesterone in many models points to metabolism in the CNS as a key factor in the steroid's neuroprotection. In contrast, recent clinical data showed no benefit or even harmful effects on incidence of cerebrovascular disease in postmenopausal women using estrogen/progestin formulations 29-31. One explanation for this apparent discrepancy is that most synthetic progestins cannot be converted to neuro-active metabolites such as ALLO. In fact, the most commonly used progestin in hormone replacement therapy, medroxyprogesterone acetate (MPA), appears to antagonize the beneficial effects of estrogen against glutamate toxicity in hippocampal neuronal cultures 32, 33. In contrast, progesterone protects hippocampal neurons from glutamate toxicity and provides an additive benefit to estrogen-treated neurons 32, 34. Similarly, MPA decreases estrogen protection against cerebral ischemia in subcortical brain regions in rat 35, while progesterone does not 36. Therefore, our emerging knowledge of steroid synthesis and metabolism within the CNS may inform our clinical use of these complex substances.
a.Neurosteroids
Evidence began to emerge in the early 1980's that the brain is a steroidogenic organ, when Baulieu and co-workers found that the steroids dehydroepiandrosterone (DHEA) and pregnenolone, and their sulfated esters, were present in greater concentration in the brain than in circulation 37, 38. Importantly, brain concentrations of all these steroids remained very high after adrenalectomy and gondadectomy. This gave rise to the now well accepted concept that steroids are produced in the brain, termed neurosteroids 18, 22. The metabolic enzymes required to generate and metabolize steroids are present throughout the rodent and human central nervous system, although not expressed uniformly in all brain regions (For review see 39-41). The non-uniform distribution of the metabolic enzymes points to the possibility of regional differences in generation and metabolism of neurosteroids, although this has not yet been thoroughly investigated.
Synthesis of neurosteroids begins with the conversion of cholesterol to pregnenolone within the mitochondria by the enzyme cytochrome P450scc (cholesterol side-chain cleavage enzyme) (Fig. 1). P450scc is the rate limiting enzyme in neurosteroid biosynthesis. Interestingly, P450scc, and its human counterpart Cyp11a1, is more highly expressed in female brain as compared to male 42, 43, indicating the possibility of sexually dimorphic synthesis of neurosteroids. Pregnenolone is the precursor of all neurosteroids, being converted directly into DHEA or progesterone (Fig. 1). Further metabolism of DHEA leads to the production of androgens (testosterone and DHT) as well as 17β-estradiol and derivatives. On the other hand, progesterone is predominantly converted to its highly active metabolites 5α-DHP and 3α,5α-THP (ALLO). While the focus of this review is on the beneficial effects of estradiol and progesterone, it is important to note that DHEA and its sulfated ester DHEAS are neuroprotective in a variety of experimental models. DHEA and DHEAS protect rat hippocampal neurons against N-methyl-D-aspartate (NMDA)-induced excitotoxicity 44, 45, β-amyloid peptide toxicity 46 and oxygen-glucose deprivation 47. Importantly, DHEAS prevents ischemia-induced hippocampal neuron cell loss and impairment of hippocampal long-term potentiation following forebrain ischemia 48. In addition, the PROG precursor PREG and PREGS also exhibit beneficial effects in experimental models of neurodegeneration 49, 50.
Figure 1. Overview of Neurosteroid Synthesis.
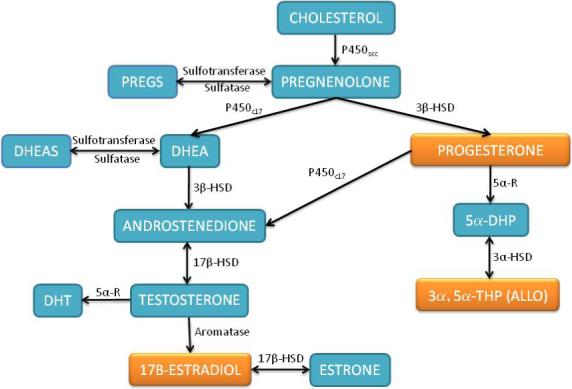
Cholesterol is converted into pregnenolone within the mitochondria and then further converted into DHEA via P450c17 or progesterone by 3β-HSD. DHEA is converted into testosterone via the intermediate androstenedione. Progesterone can also be converted to testosterone by P450c17. Testosterone is converted to its more potent analog DHT by 5α-R or converted into 17β-estradiol by the enzyme Aromatase. Progesterone is converted to its highly active metabolites 5α-DHP and 3α,5α-THP (ALLO) by consecutive reductions by 5α-R and then 3α-HSD. Both pregnenolone and DHEA can be interconverted to sulfated esthers by sulfotransferase and sulfatase enzymes. P450scc, mitochondrial cholesterol side-chain cleavage enzyme; P450c17, microsomal 17 hyrdoxylase; 3β-HSD, 3β-hydroxysteroid dehydrogenase; 5α-R, 5α-reductase; 5α-HSD, 5α-hyrdoxysteroid dehydrogenase; 17β-HSD, 17β-hyrdoxysteroid dehydrogenase; Aromatase, P450-aromatase; DHT, dihydrotestosterone; 3α,5α-THP, Allopregnanolone.
b. Progesterone and Stroke
Acute administration of progesterone either immediately before or after transient focal cerebral ischemia decreases infarct volume and improves behavioral outcome in male mice 51, 52 and rats 53-57 and reduces hippocampal neuron loss following global cerebral ischemia (four vessel occlusion; 4-VO) in male rats 58. The therapeutic window for acute progesterone in male rats has been shown to be 2 hours after focal cerebral ischemia 27 and 24 hr after global ischemia58, making it a promising molecule for pharmacological intervention following stroke. Notably, progesterone is an effective neuroprotectant in the more complex cat brain 59. While most of the studies to date have focused on male animals, progesterone is effective in reproductively senescent aged and young ovariectomized female rats 53, 54. However, others have observed either no benefit 36 or worse outcome 60 following chronic progesterone treatment, suggesting that dose and duration of steroid administration is important to outcome.
c. Progesterone and Traumatic Brain Injury
Progesterone has been well-studied in models of TBI, for example controlled contusion of the medial frontal cortex (For review see 25). Combined pre-and post-injury treatment decreases cerebral edema and neuronal loss at 72 hours in both young and aged male and female animals61, 62. Similar results are observed with doses administered up to 48 hours post-injury, although the steroid was most effective when administered within 2 hr of trauma28. Similarly, physiological levels of progesterone provide protection in ovariectomized female rodents63. The main effect observed in these studies is that of restricting post-injury edema. As with ischemic brain injury, progesterone's benefits are dose dependent 64 and may be lost at very high doses 65. Importantly, progesterone treatment improves functional recovery following experimental TBI 62, 66-68.
These experimental observations have been extended to clinical trials involving patients with TBI 69. Patients of both sexes (n=100) with moderate to severe brain damage from blunt head trauma were enrolled in a prospective, randomized, placebo controlled study. Progesterone was continuously administered by intravenous infusion for 3 days post-injury, and mortality was reduced in patients receiving progesterone as compared to placebo-treatment. Functional recovery was also significantly better in moderately injured patients who received progesterone. Similar positive results have been recently reported with severe traumatic brain injury 70. While these results must be confirmed in a larger, fully powered trial, they are the first evidence that progesterone may be effective for the treatment of clinical TBI.
3. Mechanisms of Progesterone Neuroprotection
Studies of experimental TBI again provide the best clues as to progesterone's mechanisms of protection. For example, PROG decreases vasogenic and cytotoxic cerebral edema following TBI 28 and focal stroke 51, 52, 71. Recent evidence suggests that the steroid regulates expression of aquaporin-4, a water permeable channel that likely plays a significant role in edema formation following brain injury 72. Additional evidence implicates progesterone's transcriptional actions, by increasing expression of anti-apoptotic genes such as Bcl-2, and decreasing pro-apoptotic genes such as bax, bad and caspase-3 activity 73, 74. Inhibition of sigma1 receptors has also been implicated 75, as has anti-oxidant actions as revealed by a reduction in lipid peroxidation after TBI 76. Progesterone is thought to have anti-inflammatory actions by reducing microglia activation, toll-like receptor expression 68 and pro-inflammatory cytokines 77 78 67, 79, 80.
In addition to neuroprotection, progesterone may play a role in post-injury remyelination and repair 26. The steroid increases the rate of myelin formation in cultured Schwann cells 81 and improves regeneration of lesioned mouse sciatic nerve 82. Within the central nervous system, progesterone stimulated oligodendrocyte proliferation and subsequent myelination 20, 83-85, possibly by progesterone receptor dependent mechanisms 83. Interestingly, the latter is the only effect to date directly attributed to classical nuclear progesterone receptor signaling.
4. Allopreganolone
The ability of the nervous system to metabolize progesterone complicates our understanding of its mechanism of protection. To date few studies have demonstrated that progesterone, rather than a metabolite, is the active molecule responsible for protection in brain ischemia and injury. In fact, Sayeed et al. recently demonstrated that ALLO is more effective than progesterone in transient ischemic injury, suggesting that it is the metabolite that is of greatest potency 55. Further, we have observed that progesterone protection of cerebellar Purkinje cells treated with oxygen and glucose deprivation requires metabolism to ALLO 86.
ALLO is synthesized in the brain by sequential reductions by 5α-R and 3α-HSD. 5α-R reduces progesterone to 5αDH-PROG in a uni-directional reaction that is rate-limiting in the production of ALLO. 3α-HSD converts 5αDH-PROG into 3α,5αTH-PROG (ALLO), and is also capable of oxidizing ALLO into 5αDH-PROG. ALLO is neuroprotective in several animal models of neurodegeneration and injury, including Alzheimers 87, Niemann-Pick C disease 88, kainate excitotoxicity 89, focal cerebral ischemia 55, and TBI 90. ALLO improves post-injury neurogenesis 91, decrease apoptosis via activation of PKB/Akt kinase 92, and like progesterone, increases anti-apoptotic gene transcription 93, 94 and decreases inflammation 79. Because ALLO is among the most abundant and potent endogenous positive modulators of GABAA receptors, most physiological effects of ALLO are attributed to this mechanism (For review see 95). Interaction with GABAA receptors may also hold the key to ALLO's neuroprotection, as we have recently demonstrated in cultured neurons treated with in vitro “ischemic” insults 86.
5. Summary
The brain is a steroidogenic organ, possessing the metabolic enzymes necessary to produce most steroid hormones. Progesterone, among the most abundant neurosteroids in the brain, is neuroprotective in a variety of experimental animal settings. This protection may be mediated by altering specific gene expression via binding to the classical progesterone receptor (PR). Alternatively, neuro-active progesterone metabolites interact directly with neuronal membrane receptors and intracellular signaling cascades. In fact, the active metabolite ALLO mimics PROG neuroprotection in most experimental settings. Few studies have attempted to determine the relation between the parent hormone progesterone and it conversion to metabolites in animal studies of brain injury, but our recent data suggests that progesterone neuroprotection is completely mediated by metabolism to active metabolites, likely ALLO.
6. Estradiol: The best studied sex steroid in neuroprotection
The estrogens, particularly E2, have been widely studied in experimental ischemic and hemorrhagic brain injury. Almost without exception, these studies report that E2 reduces tissue damage, improves functional recovery and may stimulate repair processes. Interest in the estrogen steroid family arose, in part, from clinical use of contraceptives and of perimenopausal hormone replacement therapy in women with potential cerebrovascular disease. In addition, two key observations fueled ongoing interest in estrogens as neuroprotectants. First, outcome from experimental stroke in female animals is influenced by the stage of the estrous cycle. Proestrus is associated with smaller tissue damage (quantified as infarct size) after focal cerebral ischemia in rat, and this association is generally attributed to the high levels of E2 produced during this stage. In contrast, metestrus (low E2 production) is associated with greater brain damage 96. Second, female animals sustain less tissue damage than do males after similar ischemic or traumatic insults 5, 6, 53, 97. The benefit of female gender is lost in reproductively senescent animals or after ovariectomy but can be restored by estrogen supplementation 5, 53. These data emphasize that estrogens are highly important to outcome from ischemic brain injury in the female.
a. Basis for estrogen as a neuroprotective therapy
Subsequently, a large number of laboratories focused on the estrogens as a form of endogenous neuroprotection or as a potential therapy for stroke 98. Estrogen therapy of a variety of types and formulations has been shown to be beneficial in the male ischemic brain 99-101. While most of these animal studies emphasized tissue damage quantified as infarct size or cell loss early after the insult, chronic E2 supplementation also improves functional outcome 8, 102.
Despite the mass of evidence that E2 is neuroprotective, it should be noted that some investigators have found no effect or even detrimental effects of E2 in experimental stroke. Such findings may be related to the dose of E2 used since neuroprotection may be lost or detrimental effects may occur at higher doses 12 103. E2 may also be less beneficial with increased severity of injury, e.g., prolonged or permanent vessel occlusion as opposed to transient occlusion 104, 105. Age may also be a confounding factor, as steroid receptor expression is altered with reproductive senescence 106. A recent study showed that E2 replacement reduced infarct size, systemic and brain inflammation only if it was initiated immediately after ovariectomy, but not after a prolonged period of hypoestrogenicity 107. This observation, if translated to the human, may have clinical implications for postmenopausal women. Overall, experimental data supports a beneficial effect of E2, however dissenting findings suggest that E2 mediated neuroprotection may depend on the specifics of the experimental and clinical situation.
One final consideration is that acute steroid injection has been protective in some studies, while in others only chronic therapy produces beneficial results 108, 109. The latter issue influenced subsequent investigations into E2's protective mechanisms, i.e. if such mechanisms are mediated by the steroid's cognate estrogen receptors (ER) of either subtype and if transcriptional processes are required for benefit. A profound understanding of these mechanisms is required if we are to develop drugs that mirror estrogen's neuroprotection without the undesirable hormonal effects.
7. Mechanisms of Estrogenic Neuroprotection
It is now well-recognized that E2 elicits both genomic and non-genomic protective actions following an ischemic insult (Fig.2). These actions include stabilizing the blood-brain barrier and subsequently reducing brain edema 110 111, increasing blood flow during and after the ischemic insult 112, 113, engaging anti-inflammatory molecules 114, 115, and increasing expression of cell-survival mediators such as bcl2 and the novel cocaine and amphetamine regulated transcript (CART) 116, 117. In addition, E2 can act as a concentration-dependent antioxidant and anti-lipid peroxidation agent 118-120. The steroid also interacts with N-methyl-D-aspartate (NMDA) receptors, producing receptor activation at low doses (Connell BJ et al., 2007), but inhibiting receptors and subsequently blunting excitotoxicity at higher E2 doses 121. Lastly, E2 not only acts as an acute protectant after ischemia but can also improve regeneration and plasticity of new neurons 107. This may contribute to improved post-ischemic memory after ischemia that is seen in estrogen-supplemented animals 102.
A plethora of literature documents E2 mechanisms that are transcriptional, involving genes that do or do not carry estrogen response elements (EREs), as well as non-transcriptional rapid signaling action through interaction with membrane-bound G-proteins, modification of protein phosphorylation and of intracellular second messenger levels such as cAMP or calcium. The relative importance of these mechanisms in explaining E2's ability to improve ischemic and traumatic neuronal injury is unclear. For example, whether classical nuclear ERs are essential for neuroprotection has remained controversial for some time, and there is disagreement amongst studies that use pharmacological ER agonists vs. genetically ER deficient mice. It is known that both ER-α and ER-β are widely expressed in all cell types throughout the brain, i.e., neurons, glia, and endothelial cells, and are present in ischemia-sensitive areas such as neocortex and hippocampus 122-124. Not surprisingly, concentrations of ER-α and ER-β are higher in adult females as compared to males 122. We showed in a very early study that generalized pharmacological blockade of ERs with ICI182,780 exacerbated ischemic brain injury in female mice, suggesting that nuclear receptors play a role 125. However, ERα gene deletion in female mice was paradoxically associated with decreased infarct size, which may be related to the enhanced brain tissue perfusion observed in ERα knockout mice 126. In a model of permanent cerebral vessel occlusion, E2 failed to protect cortical tissue in ERα, but not ERβ, knockout mice, suggesting that E2 may signal through ERα to reduce stroke damage 127. However, a selective ERβ, but not ERα, agonist reduced hippocampal ischemic damage in ovariectomized mice 12, suggesting that different receptor subtypes may mediate E2 signaling in a model-specific and possible brain-region specific manner. Clearly, further work is required to translate our extensive understanding of E2 signaling under physiological conditions to those of brain injury.
8. Androgens and Brain Injury in the Male
Despite the relative paucity of data that address “male sensitivity” to brain injury, emerging evidence suggests that androgens strongly impact outcome and mechanisms of cell death. In men, low testosterone levels have been associated with poor outcome after acute ischemic events 128. Androgen levels are inversely associated with stroke severity, infarct size, and 6-month mortality; and total and free testosterone levels tend to normalize within 6 months following stroke. These data do not necessarily suggest a direct causal relationship because brain injury provokes an acute stress reaction that causes a reduction in plasma testosterone. Animal studies are starting to address this issue. In male rats, androgen replacement in castrates increases histological damage from stroke 100, 129, 130, while stressors that reduce pre-ischemic testosterone level also reduce stroke damage 131. In contrast, testosterone replacement in male castrates after stroke accelerates functional recovery 67. Beneficial effects of androgens following peripheral nerve damage or brain trauma have also been reported in animals 132-134. These apparently conflicting results may be reconciled by the hypothesis that androgens are deleterious during acute injury but beneficial during the recovery phase. Potential mechanisms by which androgens could enhance post-stroke recovery include normalization of reperfusion, promotion of axonal regeneration, synaptogenesis, and neurogenesis 135.
9. Conclusion
Biologic sex and sex steroids are important factors in clinical and experimental brain injury. Our understanding of these factors is relatively recent, in part, because many pre-clinical and mechanistic studies have been carried out only in male animals and mixed sex cell cultures. Estrogen has accumulated an impressive reputation as a neuroprotectant in physiologically relevant doses in laboratory studies, but translation to patients remains to be shown. Laboratory data strongly show that progesterone treatment after TBI reduces edema, improves outcomes and restores blood brain barrier function. Clinical studies to date agree with these data, and there are ongoing human trials for progesterone treatment after TBI. The role of androgens in male stroke or TBI is understudied and important to pursue given the epidemiology of stroke and trauma in men. To date, male sex steroids remain largely evaluated at the bench rather than the bedside. Further studies of genetic sex and of the hormonal platform on which brain injury occurs are needed and should provide new insights for science and health care.
Acknowledgements
Support for this research was provided by NIH grants NS049210, NS 058792, and the Medical Research Foundation of Oregon. The authors gratefully acknowledge Executive Specialist Ashley Branch for expert manuscript preparation.
References
- 1.Giroud M, Milan C, Beuriat P, et al. Incidence and survival rates during a two-year period of intracerebral and subarachnoid haemorrhages, cortical infarcts, lacunes and transient ischaemic attacks. the stroke registry of dijon: 1985−1989. Int J Epidemiol. 1991;20(4):892–899. doi: 10.1093/ije/20.4.892. [DOI] [PubMed] [Google Scholar]
- 2.Sacco RL, Boden-Albala B, Gan R, et al. Stroke incidence among white, black, and hispanic residents of an urban community: The northern manhattan stroke study. Am J Epidemiol. 1998;147(3):259–268. doi: 10.1093/oxfordjournals.aje.a009445. [DOI] [PubMed] [Google Scholar]
- 3.Yamagata K, Tagami M, Ikeda K, Yamori Y, Nara Y. Altered gene expressions during hypoxia and reoxygenation in cortical neurons isolated from stroke-prone spontaneously hypertensive rats. Neurosci Lett. 2000;284(3):131–134. doi: 10.1016/s0304-3940(00)00936-8. [DOI] [PubMed] [Google Scholar]
- 4.Hall ED, Pazara KE, Braughler JM. Effects of tirilazad mesylate on postischemic brain lipid peroxidation and recovery of extracellular calcium in gerbils. Stroke. 1991;22:361–366. doi: 10.1161/01.str.22.3.361. [DOI] [PubMed] [Google Scholar]
- 5.Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–166. doi: 10.1161/01.str.29.1.159. [DOI] [PubMed] [Google Scholar]
- 6.Carswell HV, Anderson NH, Clark JS, et al. Genetic and gender influences on sensitivity to focal cerebral ischemia in the stroke-prone spontaneously hypertensive rat. Hypertension. 1999;33(0194−911 2):681–685. doi: 10.1161/01.hyp.33.2.681. [DOI] [PubMed] [Google Scholar]
- 7.Alkayed NJ, Murphy SJ, Traystman RJ, Hurn PD, Miller VM. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke. 2000;31(0039−2499 1):161–168. doi: 10.1161/01.str.31.1.161. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Blizzard KK, Zeng Z, DeVries AC, Hurn PD, McCullough LD. Chronic behavioral testing after focal ischemia in the mouse: Functional recovery and the effects of gender. Exp Neurol. 2004;187(1):94–104. doi: 10.1016/j.expneurol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Bramlett HM, Dietrich WD. Neuropathological protection after traumatic brain injury in intact female rats versus males or ovariectomized females. J Neurotrauma. 2001;18(0897−7151 9):891–900. doi: 10.1089/089771501750451811. [DOI] [PubMed] [Google Scholar]
- 10.Toung TK, Hurn PD, Traystman RJ, Sieber FE. Estrogen decreases infarct size after temporary focal ischemia in a genetic model of type 1 diabetes mellitus. Stroke. 2000;31(1524−4628 11):2701–2706. doi: 10.1161/01.str.31.11.2701. [DOI] [PubMed] [Google Scholar]
- 11.Vannucci SJ, Willing LB, Goto S, et al. Experimental stroke in the female diabetic, db/db, mouse. J Cereb Blood Flow Metab. 2001;21(0271−678 1):52–60. doi: 10.1097/00004647-200101000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Carswell HV, Macrae IM, Gallagher L, Harrop E, Horsburgh KJ. Neuroprotection by a selective oestrogen receptor {beta} agonist in a mouse model of global ischaemia. Am J Physiol Heart Circ Physiol. 2004;(0363−6135) doi: 10.1152/ajpheart.00227.2004. [DOI] [PubMed] [Google Scholar]
- 13.Lieb K, Andrae J, Reisert I, Pilgrim C. Neurotoxicity of dopamine and protective effects of the NMDA receptor antagonist AP-5 differ between male and female dopaminergic neurons. Exp Neurol. 1995;134(0014−4886 2):222–229. doi: 10.1006/exnr.1995.1052. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Li PP, Feng X, Barker JL, Smith SV, Rubinow DR. Sex-related differences in neuronal cell survival and signaling in rats. Neurosci Lett. 2003;337(0304−3940 2):65–68. doi: 10.1016/s0304-3940(02)01179-5. [DOI] [PubMed] [Google Scholar]
- 15.Du L, Bayir H, Lai Y, et al. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J Biol Chem. 2004;(1083−351) doi: 10.1074/jbc.M405461200. [DOI] [PubMed] [Google Scholar]
- 16.Liu M, Hurn PD, Roselli CE, Alkayed NJ. Role of P450 aromatase in sex-specific astrocytic cell death. J Cereb Blood Flow Metab. 2007;27(1):135–141. doi: 10.1038/sj.jcbfm.9600331. 10.1038/sj.jcbfm.9600331. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Pin S, Zeng Z, Wang MM, Andreasson KA, McCullough LD. Sex differences in cell death. Ann Neurol. 2005;58(0364−5134 2):317–321. doi: 10.1002/ana.20538. [DOI] [PubMed] [Google Scholar]
- 18.Baulieu EE, Robel P, Schumacher M. Neurosteroids: Beginning of the story. Int Rev Neurobiol. 2001;46(0074−7742):1–32. doi: 10.1016/s0074-7742(01)46057-0. [DOI] [PubMed] [Google Scholar]
- 19.Schumacher M, Baulieu EE. Neurosteroids: Synthesis and functions in the central and peripheral nervous systems. Ciba Found Symp. 1995;191(0300−5208):90–106. doi: 10.1002/9780470514757.ch6. [DOI] [PubMed] [Google Scholar]
- 20.Jung-Testas I, Schumacher M, Robel P, Baulieu EE. The neurosteroid progesterone increases the expression of myelin proteins (MBP and CNPase) in rat oligodendrocytes in primary culture. Cell Mol Neurobiol. 1996;16(0272−4340 3):439–443. doi: 10.1007/BF02088110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schumacher M, Robel P, Baulieu EE. Development and regeneration of the nervous system: A role for neurosteroids. Dev Neurosci. 1996;18(1−2):6–21. doi: 10.1159/000111391. [DOI] [PubMed] [Google Scholar]
- 22.Baulieu EE. Neurosteroids: Of the nervous system, by the nervous system, for the nervous system. Recent Prog Horm Res. 1997;52(0079−9963):1–32. [PubMed] [Google Scholar]
- 23.De Nicola AF, Gonzalez SL, Labombarda F, et al. Progesterone treatment of spinal cord injury: Effects on receptors, neurotrophins, and myelination. J Mol Neurosci. 2006;28(0895−8696 1):3–15. doi: 10.1385/jmn:28:1:3. [DOI] [PubMed] [Google Scholar]
- 24.Labombarda F, Gonzalez S, Gonzalez Deniselle MC, et al. Progesterone increases the expression of myelin basic protein and the number of cells showing NG2 immunostaining in the lesioned spinal cord. J Neurotrauma. 2006;23(0897−7151 2):181–192. doi: 10.1089/neu.2006.23.181. [DOI] [PubMed] [Google Scholar]
- 25.Stein DG. Progesterone exerts neuroprotective effects after brain injury. Brain Res Rev. 2008;57(0165−0173 2):386–397. doi: 10.1016/j.brainresrev.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schumacher M, Guennoun R, Stein DG, De Nicola AF. Progesterone: Therapeutic opportunities for neuroprotection and myelin repair. Pharmacol Ther. 2007;116(0163−7258 1):77–106. doi: 10.1016/j.pharmthera.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Jiang N, Chopp M, Stein D, Feit H. Progesterone is neuroprotective after transient middle cerebral artery occlusion in male rats. Brain Res. 1996;735(0006−8993 1):101–107. doi: 10.1016/0006-8993(96)00605-1. [DOI] [PubMed] [Google Scholar]
- 28.Roof RL, Duvdevani R, Heyburn JW, Stein DG. Progesterone rapidly decreases brain edema: Treatment delayed up to 24 hours is still effective. Exp Neurol. 1996;138(0014−4886 2):246–251. doi: 10.1006/exnr.1996.0063. [DOI] [PubMed] [Google Scholar]
- 29.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the women's health initiative randomized controlled trial. JAMA. 2002;288(0098−7484 3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 30.Simon JA, Hsia J, Cauley JA, et al. Postmenopausal hormone therapy and risk of stroke: The heart and estrogen-progestin replacement study (HERS). Circulation. 2001;103(1524−4539 5):638–642. doi: 10.1161/01.cir.103.5.638. [DOI] [PubMed] [Google Scholar]
- 31.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. heart and Estrogen/progestin replacement study (HERS) research group. JAMA. 1998;280(0098−7484 7):605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 32.Nilsen J, Brinton RD. Divergent impact of progesterone and medroxyprogesterone acetate (provera) on nuclear mitogen-activated protein kinase signaling. Proc Natl Acad Sci U S A. 2003;100(0027−8424 18):10506–10511. doi: 10.1073/pnas.1334098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nilsen J, Morales A, Brinton RD. Medroxyprogesterone acetate exacerbates glutamate excitotoxicity. Gynecol Endocrinol. 2006;22(0951−3590 7):355–361. doi: 10.1080/09513590600863337. [DOI] [PubMed] [Google Scholar]
- 34.Nilsen J, Brinton RD. Impact of progestins on estradiol potentiation of the glutamate calcium response. Neuroreport. 2002;13(0959−4965 6):825–830. doi: 10.1097/00001756-200205070-00018. [DOI] [PubMed] [Google Scholar]
- 35.Littleton-Kearney MT, Klaus JA, Hurn PD. Effects of combined oral conjugated estrogens and medroxyprogesterone acetate on brain infarction size after experimental stroke in rat. J Cereb Blood Flow Metab. 2005;25(0271−678 4):421–426. doi: 10.1038/sj.jcbfm.9600052. [DOI] [PubMed] [Google Scholar]
- 36.Toung TJ, Chen TY, Littleton-Kearney MT, Hurn PD, Murphy SJ. Effects of combined estrogen and progesterone on brain infarction in reproductively senescent female rats. J Cereb Blood Flow Metab. 2004;24(0271−678 10):1160–1166. doi: 10.1097/01.WCB.0000135594.13576.D2. [DOI] [PubMed] [Google Scholar]
- 37.Corpechot C, Robel P, Axelson M, Sjovall J, Baulieu EE. Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proc Natl Acad Sci U S A. 1981;78(0027−8424 8):4704–4707. doi: 10.1073/pnas.78.8.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corpechot C, Synguelakis M, Talha S, et al. Pregnenolone and its sulfate ester in the rat brain. Brain Res. 1983;270(0006−8993 1):119–125. doi: 10.1016/0006-8993(83)90797-7. [DOI] [PubMed] [Google Scholar]
- 39.Mellon SH, Vaudry H. Biosynthesis of neurosteroids and regulation of their synthesis. Int Rev Neurobiol. 2001;46(0074−7742):33–78. doi: 10.1016/s0074-7742(01)46058-2. [DOI] [PubMed] [Google Scholar]
- 40.Baulieu EE, Robel P. Dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) as neuroactive neurosteroids. Proc Natl Acad Sci U S A. 1998;95(0027−8424 8):4089–4091. doi: 10.1073/pnas.95.8.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stoffel-Wagner B. Neurosteroid biosynthesis in the human brain and its clinical implications. Ann N Y Acad Sci. 2003;1007(0077−8923):64–78. doi: 10.1196/annals.1286.007. [DOI] [PubMed] [Google Scholar]
- 42.Watzka M, Bidlingmaier F, Schramm J, Klingmuller D, Stoffel-Wagner B. Sex- and age-specific differences in human brain CYP11A1 mRNA expression. J Neuroendocrinol. 1999;11(12):901–905. doi: 10.1046/j.1365-2826.1999.00407.x. [DOI] [PubMed] [Google Scholar]
- 43.Lavaque E, Mayen A, Azcoitia I, Tena-Sempere M, Garcia-Segura LM. Sex differences, developmental changes, response to injury and cAMP regulation of the mRNA levels of steroidogenic acute regulatory protein, cytochrome p450scc, and aromatase in the olivocerebellar system. J Neurobiol. 2006;66(0022−3034 3):308–318. doi: 10.1002/neu.20221. [DOI] [PubMed] [Google Scholar]
- 44.Kimonides VG, Khatibi NH, Svendsen CN, Sofroniew MV, Herbert J. Dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEAS) protect hippocampal neurons against excitatory amino acid-induced neurotoxicity. Proc Natl Acad Sci U S A. 1998;95(0027−8424 4):1852–1857. doi: 10.1073/pnas.95.4.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mao X, Barger SW. Neuroprotection by dehydroepiandrosterone-sulfate: Role of an NFkappaB-like factor. Neuroreport. 1998;9(0959−4965 4):759–763. doi: 10.1097/00001756-199803090-00036. [DOI] [PubMed] [Google Scholar]
- 46.Cardounel A, Regelson W, Kalimi M. Dehydroepiandrosterone protects hippocampal neurons against neurotoxin-induced cell death: Mechanism of action. Proc Soc Exp Biol Med. 1999;222(0037−9727 2):145–149. doi: 10.1046/j.1525-1373.1999.d01-124.x. [DOI] [PubMed] [Google Scholar]
- 47.Kaasik A, Kalda A, Jaako K, Zharkovsky A. Dehydroepiandrosterone sulphate prevents oxygen-glucose deprivation-induced injury in cerebellar granule cell culture. Neuroscience. 2001;102(2):427–432. doi: 10.1016/s0306-4522(00)00489-9. [DOI] [PubMed] [Google Scholar]
- 48.Li Z, Zhou R, Cui S, et al. Dehydroepiandrosterone sulfate prevents ischemia-induced impairment of long-term potentiation in rat hippocampal CA1 by up-regulating tyrosine phosphorylation of NMDA receptor. Neuropharmacology. 2006;51(0028−3908 5):958–966. doi: 10.1016/j.neuropharm.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 49.Weaver CE, Jr., Marek P, Park-Chung M, Tam SW, Farb DH. Neuroprotective activity of a new class of steroidal inhibitors of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A. 1997;94(0027−8424 19):10450–10454. doi: 10.1073/pnas.94.19.10450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gursoy E, Cardounel A, Kalimi M. Pregnenolone protects mouse hippocampal (HT-22) cells against glutamate and amyloid beta protein toxicity. Neurochem Res. 2001;26(0364−3190 1):15–21. doi: 10.1023/a:1007668213330. [DOI] [PubMed] [Google Scholar]
- 51.Gibson CL, Murphy SP. Progesterone enhances functional recovery after middle cerebral artery occlusion in male mice. J Cereb Blood Flow Metab. 2004;24(0271−678 7):805–813. doi: 10.1097/01.WCB.0000125365.83980.00. [DOI] [PubMed] [Google Scholar]
- 52.Gibson CL, Constantin D, Prior MJ, Bath PM, Murphy SP. Progesterone suppresses the inflammatory response and nitric oxide synthase-2 expression following cerebral ischemia. Exp Neurol. 2005;193(0014−4886 2):522–530. doi: 10.1016/j.expneurol.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 53.Alkayed NJ, Murphy SJ, Traystman RJ, Hurn PD, Miller VM. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke. 2000;31(0039−2499 1):161–168. doi: 10.1161/01.str.31.1.161. [DOI] [PubMed] [Google Scholar]
- 54.Murphy SJ, Littleton-Kearney MT, Hurn PD. Progesterone administration during reperfusion, but not preischemia alone, reduces injury in ovariectomized rats. J Cereb Blood Flow Metab. 2002;22(10):1181–1188. doi: 10.1097/01.WCB.0000037990.07114.07. [DOI] [PubMed] [Google Scholar]
- 55.Sayeed I, Guo Q, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, is more effective than progesterone in reducing cortical infarct volume after transient middle cerebral artery occlusion. Ann Emerg Med. 2006;47(1097−6760 4):381–389. doi: 10.1016/j.annemergmed.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 56.Chen Z, Yuhanna IS, Galcheva-Gargova Z, Karas RH, Mendelsohn ME, Shaul PW. Estrogen receptor alpha mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J Clin Invest. 1999;103(0021−9738 3):401–406. doi: 10.1172/JCI5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumon Y, Kim SC, Tompkins P, Stevens A, Sakaki S, Loftus CM. Neuroprotective effect of postischemic administration of progesterone in spontaneously hypertensive rats with focal cerebral ischemia. J Neurosurg. 2000;92(0022−3085 5):848–852. doi: 10.3171/jns.2000.92.5.0848. [DOI] [PubMed] [Google Scholar]
- 58.Morali G, Letechipia-Vallejo G, Lopez-Loeza E, Montes P, Hernandez-Morales L, Cervantes M. Post-ischemic administration of progesterone in rats exerts neuroprotective effects on the hippocampus. Neurosci Lett. 2005;382(0304−3940 3):286–290. doi: 10.1016/j.neulet.2005.03.066. [DOI] [PubMed] [Google Scholar]
- 59.Cervantes M, Gonzalez-Vidal MD, Ruelas R, Escobar A, Morali G. Neuroprotective effects of progesterone on damage elicited by acute global cerebral ischemia in neurons of the caudate nucleus. Arch Med Res. 2002;33(0188−4409 1):6–14. doi: 10.1016/s0188-4409(01)00347-2. [DOI] [PubMed] [Google Scholar]
- 60.Murphy SJ, Traystman RJ, Hurn PD, Duckles SP. Progesterone exacerbates striatal stroke injury in progesterone-deficient female animals. Stroke. 2000;31(0039−2499 5):1173–1178. doi: 10.1161/01.str.31.5.1173. [DOI] [PubMed] [Google Scholar]
- 61.Roof RL, Duvdevani R, Stein DG. Gender influences outcome of brain injury: Progesterone plays a protective role. Brain Res. 1993;607(0006−8993 1−2):333–336. doi: 10.1016/0006-8993(93)91526-x. [DOI] [PubMed] [Google Scholar]
- 62.Roof RL, Duvdevani R, Braswell L, Stein DG. Progesterone facilitates cognitive recovery and reduces secondary neuronal loss caused by cortical contusion injury in male rats. Exp Neurol. 1994;129(0014−4886 1):64–69. doi: 10.1006/exnr.1994.1147. [DOI] [PubMed] [Google Scholar]
- 63.Robertson CL, Puskar A, Hoffman GE, Murphy AZ, Saraswati M, Fiskum G. Physiologic progesterone reduces mitochondrial dysfunction and hippocampal cell loss after traumatic brain injury in female rats. Exp Neurol. 2006;197(0014−4886 1):235–243. doi: 10.1016/j.expneurol.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 64.Wright DW, Bauer ME, Hoffman SW, Stein DG. Serum progesterone levels correlate with decreased cerebral edema after traumatic brain injury in male rats. J Neurotrauma. 2001;18(0897−7151 9):901–909. doi: 10.1089/089771501750451820. [DOI] [PubMed] [Google Scholar]
- 65.Goss CW, Hoffman SW, Stein DG. Behavioral effects and anatomic correlates after brain injury: A progesterone dose-response study. Pharmacol Biochem Behav. 2003;76(0091−3057 2):231–242. doi: 10.1016/j.pbb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 66.Shear DA, Galani R, Hoffman SW, Stein DG. Progesterone protects against necrotic damage and behavioral abnormalities caused by traumatic brain injury. Exp Neurol. 2002;178(0014−4886 1):59–67. doi: 10.1006/exnr.2002.8020. [DOI] [PubMed] [Google Scholar]
- 67.Pan DS, Liu WG, Yang XF, Cao F. Inhibitory effect of progesterone on inflammatory factors after experimental traumatic brain injury. Biomed Environ Sci. 2007;20(0895−3988 5):432–438. [PubMed] [Google Scholar]
- 68.Chen G, Shi J, Jin W, et al. Progesterone administration modulates TLRs/NF-kappaB signaling pathway in rat brain after cortical contusion. Ann Clin Lab Sci. 2008;38(0091−7370 1):65–74. [PubMed] [Google Scholar]
- 69.Wright DW, Kellermann AL, Hertzberg VS, et al. ProTECT: A randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emerg Med. 2006;(1097−6760) doi: 10.1016/j.annemergmed.2006.07.932. [DOI] [PubMed] [Google Scholar]
- 70.Xiao G, Wei J, Yan W, Wang W, Lu Z. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: A randomized controlled trial. Crit Care. 2008;12(1466−609 2):R61. doi: 10.1186/cc6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Betz AL, Coester HC. Effect of steroids on edema and sodium uptake of the brain during focal ischemia in rats. Stroke. 1990;21(0039−2499 8):1199–1204. doi: 10.1161/01.str.21.8.1199. [DOI] [PubMed] [Google Scholar]
- 72.Guo Q, Sayeed I, Baronne LM, Hoffman SW, Guennoun R, Stein DG. Progesterone administration modulates AQP4 expression and edema after traumatic brain injury in male rats. Exp Neurol. 2006;198(0014−4886 2):469–478. doi: 10.1016/j.expneurol.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 73.Yao XL, Liu J, Lee E, Ling GS, McCabe JT. Progesterone differentially regulates pro- and anti-apoptotic gene expression in cerebral cortex following traumatic brain injury in rats. J Neurotrauma. 2005;22(0897−7151 6):656–668. doi: 10.1089/neu.2005.22.656. [DOI] [PubMed] [Google Scholar]
- 74.Djebaili M, Guo Q, Pettus EH, Hoffman SW, Stein DG. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J Neurotrauma. 2005;22(0897−7151 1):106–118. doi: 10.1089/neu.2005.22.106. [DOI] [PubMed] [Google Scholar]
- 75.Cai W, Zhu Y, Furuya K, Li Z, Sokabe M, Chen L. Two different molecular mechanisms underlying progesterone neuroprotection against ischemic brain damage. Neuropharmacology. 2008;(0028−3908) doi: 10.1016/j.neuropharm.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 76.Roof RL, Hoffman SW, Stein DG. Progesterone protects against lipid peroxidation following traumatic brain injury in rats. Mol Chem Neuropathol. 1997;31(1044−7393 1):1–11. doi: 10.1007/BF02815156. [DOI] [PubMed] [Google Scholar]
- 77.Miller L, Hunt JS. Regulation of TNF-alpha production in activated mouse macrophages by progesterone. J Immunol. 1998;160(10):5098–5104. [PubMed] [Google Scholar]
- 78.Drew PD, Chavis JA. Female sex steroids: Effects upon microglial cell activation. J Neuroimmunol. 2000;111(1−2):77–85. doi: 10.1016/s0165-5728(00)00386-6. [DOI] [PubMed] [Google Scholar]
- 79.He J, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, enhances behavioral recovery and decreases neuronal loss after traumatic brain injury. Restor Neurol Neurosci. 2004;22(0922−6028 1):19–31. [PubMed] [Google Scholar]
- 80.Pettus EH, Wright DW, Stein DG, Hoffman SW. Progesterone treatment inhibits the inflammatory agents that accompany traumatic brain injury. Brain Res. 2005;1049(1):112–119. doi: 10.1016/j.brainres.2005.05.004. 10.1016/j.brainres.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 81.Chan JR, Phillips LJ, 2nd, Glaser M. Glucocorticoids and progestins signal the initiation and enhance the rate of myelin formation. Proc Natl Acad Sci U S A. 1998;95(18):10459–10464. doi: 10.1073/pnas.95.18.10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koenig HL, Schumacher M, Ferzaz B, et al. Progesterone synthesis and myelin formation by schwann cells. Science. 1995;268(0036−8075 5216):1500–1503. doi: 10.1126/science.7770777. [DOI] [PubMed] [Google Scholar]
- 83.Ghoumari AM, Ibanez C, El-Etr M, et al. Progesterone and its metabolites increase myelin basic protein expression in organotypic slice cultures of rat cerebellum. J Neurochem. 2003;86(0022−3042 4):848–859. doi: 10.1046/j.1471-4159.2003.01881.x. [DOI] [PubMed] [Google Scholar]
- 84.Ghoumari AM, Baulieu EE, Schumacher M. Progesterone increases oligodendroglial cell proliferation in rat cerebellar slice cultures. Neuroscience. 2005;135(0306−4522 1):47–58. doi: 10.1016/j.neuroscience.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 85.Ibanez C, Shields SA, El-Etr M, Baulieu EE, Schumacher M, Franklin RJ. Systemic progesterone administration results in a partial reversal of the age-associated decline in CNS remyelination following toxin-induced demyelination in male rats. Neuropathol Appl Neurobiol. 2004;30(0305−1846 1):80–89. doi: 10.1046/j.0305-1846.2003.00515.x. [DOI] [PubMed] [Google Scholar]
- 86.Ardeshiri A, Kelley MH, Korner IP, Hurn PD, Herson PS. Mechanism of progesterone neuroprotection of rat cerebellar purkinje cells following oxygen-glucose deprivation. Eur J Neurosci. 2006;24(0953−816 9):2567–2574. doi: 10.1111/j.1460-9568.2006.05142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brinton RD, Wang JM. Therapeutic potential of neurogenesis for prevention and recovery from alzheimer's disease: Allopregnanolone as a proof of concept neurogenic agent. Curr Alzheimer Res. 2006;3(1567−2050 3):185–190. doi: 10.2174/156720506777632817. [DOI] [PubMed] [Google Scholar]
- 88.Mellon SH, Gong W, Schonemann MD. Endogenous and synthetic neurosteroids in treatment of niemann-pick type C disease. Brain Res Rev. 2008;57(0165−0173 2):410–420. doi: 10.1016/j.brainresrev.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ciriza I, Azcoitia I, Garcia-Segura LM. Reduced progesterone metabolites protect rat hippocampal neurones from kainic acid excitotoxicity in vivo. J Neuroendocrinol. 2004;16(0953−8194 1):58–63. doi: 10.1111/j.1365-2826.2004.01121.x. [DOI] [PubMed] [Google Scholar]
- 90.Djebaili M, Hoffman SW, Stein DG. Allopregnanolone and progesterone decrease cell death and cognitive deficits after a contusion of the rat pre-frontal cortex. Neuroscience. 2004;123(0306−4522 2):349–359. doi: 10.1016/j.neuroscience.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 91.Wang JM, Johnston PB, Ball BG, Brinton RD. The neurosteroid allopregnanolone promotes proliferation of rodent and human neural progenitor cells and regulates cell-cycle gene and protein expression. J Neurosci. 2005;25(1529−2401 19):4706–4718. doi: 10.1523/JNEUROSCI.4520-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xilouri M, Avlonitis N, Calogeropoulou T, Papazafiri P. Neuroprotective effects of steroid analogues on P19-N neurons. Neurochem Int. 2007;50(0197−0186 4):660–670. doi: 10.1016/j.neuint.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 93.Charalampopoulos I, Tsatsanis C, Dermitzaki E, et al. Dehydroepiandrosterone and allopregnanolone protect sympathoadrenal medulla cells against apoptosis via antiapoptotic bcl-2 proteins. Proc Natl Acad Sci U S A. 2004;101(21):8209–8214. doi: 10.1073/pnas.0306631101. 10.1073/pnas.0306631101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Charalampopoulos I, Alexaki VI, Tsatsanis C, et al. Neurosteroids as endogenous inhibitors of neuronal cell apoptosis in aging. Ann N Y Acad Sci. 2006;1088:139–152. doi: 10.1196/annals.1366.003. 10.1196/annals.1366.003. [DOI] [PubMed] [Google Scholar]
- 95.Belelli D, Lambert JJ. Neurosteroids: Endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6(1471−003 7):565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- 96.Carswell HV, Dominiczak AF, Macrae IM. Estrogen status affects sensitivity to focal cerebral ischemia in stroke-prone spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2000;278(0363−6135 1):H290–H294. doi: 10.1152/ajpheart.2000.278.1.H290. [DOI] [PubMed] [Google Scholar]
- 97.Hall ED, Pazara KE, Linseman KL. Sex differences in postischemic neuronal necrosis in gerbils. J Cereb Blood Flow Metab. 1991;11(2):292–298. doi: 10.1038/jcbfm.1991.61. [DOI] [PubMed] [Google Scholar]
- 98.Hurn PD, Macrae IM. Estrogen as a neuroprotectant in stroke. J Cereb Blood Flow Metab. 2000;20(4):631–652. doi: 10.1097/00004647-200004000-00001. 10.1097/00004647−200004000−00001. [DOI] [PubMed] [Google Scholar]
- 99.Toung TJ, Traystman RJ, Hurn PD. Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke. 1998;29(8):1666–1670. doi: 10.1161/01.str.29.8.1666. [DOI] [PubMed] [Google Scholar]
- 100.Hawk T, Zhang YQ, Rajakumar G, Day AL, Simpkins JW. Testosterone increases and estradiol decreases middle cerebral artery occlusion lesion size in male rats. Brain Res. 1998;796(1−2):296–298. doi: 10.1016/s0006-8993(98)00327-8. [DOI] [PubMed] [Google Scholar]
- 101.Jover T, Tanaka H, Calderone A, et al. Estrogen protects against global ischemia-induced neuronal death and prevents activation of apoptotic signaling cascades in the hippocampal CA1. J Neurosci. 2002;22(1529−2401 6):2115–2124. doi: 10.1523/JNEUROSCI.22-06-02115.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gulinello M, Lebesgue D, Jover-Mengual T, Zukin RS, Etgen AM. Acute and chronic estradiol treatments reduce memory deficits induced by transient global ischemia in female rats. Horm Behav. 2006;49(2):246–260. doi: 10.1016/j.yhbeh.2005.07.010. 10.1016/j.yhbeh.2005.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bingham D, Macrae IM, Carswell HV. Detrimental effects of 17beta-oestradiol after permanent middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2005;25(3):414–420. doi: 10.1038/sj.jcbfm.9600031. 10.1038/sj.jcbfm.9600031. [DOI] [PubMed] [Google Scholar]
- 104.Vergouwen MD, Anderson RE, Meyer FB. Gender differences and the effects of synthetic exogenous and non-synthetic estrogens in focal cerebral ischemia. Brain Res. 2000;878(0006−8993 1−2):88–97. doi: 10.1016/s0006-8993(00)02713-x. [DOI] [PubMed] [Google Scholar]
- 105.Gordon CP, Keller PA. Control of hepatitis C: A medicinal chemistry perspective. J Med Chem. 2005;48(0022−2623 1):1–20. doi: 10.1021/jm0400101. [DOI] [PubMed] [Google Scholar]
- 106.Sohrabji F, Bake S. Age-related changes in neuroprotection: Is estrogen pro-inflammatory for the reproductive senescent brain? Endocrine. 2006;29(2):191–197. doi: 10.1385/ENDO:29:2:191. 10.1385/ENDO:29:2:191. [DOI] [PubMed] [Google Scholar]
- 107.Suzuki S, Brown CM, Dela Cruz CD, Yang E, Bridwell DA, Wise PM. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proc Natl Acad Sci U S A. 2007;104(14):6013–6018. doi: 10.1073/pnas.0610394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rusa R, Alkayed NJ, Crain BJ, et al. 17beta-estradiol reduces stroke injury in estrogen-deficient female animals. Stroke. 1999;30(8):1665–1670. doi: 10.1161/01.str.30.8.1665. [DOI] [PubMed] [Google Scholar]
- 109.Dubal DB, Kashon ML, Pettigrew LC, et al. Estradiol protects against ischemic injury. J Cereb Blood Flow Metab. 1998;18(0271−678 11):1253–1258. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- 110.Liu R, Wen Y, Perez E, et al. 17beta-estradiol attenuates blood-brain barrier disruption induced by cerebral ischemia-reperfusion injury in female rats. Brain Res. 2005;1060(1−2):55–61. doi: 10.1016/j.brainres.2005.08.048. 10.1016/j.brainres.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 111.O'Donnell ME, Lam TI, Tran LQ, Foroutan S, Anderson SE. Estradiol reduces activity of the blood-brain barrier na-K-cl cotransporter and decreases edema formation in permanent middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2006;26(10):1234–1249. doi: 10.1038/sj.jcbfm.9600278. 10.1038/sj.jcbfm.9600278. [DOI] [PubMed] [Google Scholar]
- 112.Pelligrino DA, Santizo R, Baughman VL, Wang Q. Cerebral vasodilating capacity during forebrain ischemia: Effects of chronic estrogen depletion and repletion and the role of neuronal nitric oxide synthase. Neuroreport. 1998;9(0959−4965 14):3285–3291. doi: 10.1097/00001756-199810050-00026. [DOI] [PubMed] [Google Scholar]
- 113.Hurn PD, Littleton-Kearney MT, Kirsch JR, Dharmarajan AM, Traystman RJ. Postischemic cerebral blood flow recovery in the female: Effect of 17 beta-estradiol. J Cereb Blood Flow Metab. 1995;15(4):666–672. doi: 10.1038/jcbfm.1995.82. [DOI] [PubMed] [Google Scholar]
- 114.Mori M, Tsukahara F, Yoshioka T, Irie K, Ohta H. Suppression by 17beta-estradiol of monocyte adhesion to vascular endothelial cells is mediated by estrogen receptors. Life Sci. 2004;75(0024−3205 5):599–609. doi: 10.1016/j.lfs.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 115.Wen Y, Perez EJ, Green PS, Sarkar SN, Simpkins JW. nNOS is involved in estrogen mediated neuroprotection in neuroblastoma cells. Neuroreport. 2004;15(0959−4965 9):1515–1518. doi: 10.1097/01.wnr.0000131674.92694.96. [DOI] [PubMed] [Google Scholar]
- 116.Alkayed NJ, Goto S, Sugo N, et al. Estrogen and bcl-2: Gene induction and effect of transgene in experimental stroke. J Neurosci. 2001;21(19):7543–7550. doi: 10.1523/JNEUROSCI.21-19-07543.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu Y, Zhang W, Klaus J, et al. Role of cocaine- and amphetamine-regulated transcript in estradiol-mediated neuroprotection. Proc Natl Acad Sci U S A. 2006;103(39):14489–14494. doi: 10.1073/pnas.0602932103. 10.1073/pnas.0602932103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Keller JN, Germeyer A, Begley JG, Mattson MP. 17Beta-estradiol attenuates oxidative impairment of synaptic Na+/K+-ATPase activity, glucose transport, and glutamate transport induced by amyloid beta-peptide and iron. J Neurosci Res. 1997;50(0360−4012 4):522–530. doi: 10.1002/(SICI)1097-4547(19971115)50:4<522::AID-JNR3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 119.Vedder H, Anthes N, Stumm G, Wurz C, Behl C, Krieg JC. Estrogen hormones reduce lipid peroxidation in cells and tissues of the central nervous system. J Neurochem. 1999;72(0022−3042 6):2531–2538. doi: 10.1046/j.1471-4159.1999.0722531.x. [DOI] [PubMed] [Google Scholar]
- 120.Behl C, Manthey D. Neuroprotective activities of estrogen: An update. J Neurocytol. 2000;29(0300−4864 5−6):351–358. doi: 10.1023/a:1007109222673. [DOI] [PubMed] [Google Scholar]
- 121.Weaver CE, Jr., Marek P, Park-Chung M, Tam SW, Farb DH. Neuroprotective activity of a new class of steroidal inhibitors of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A. 1997;94(0027−8424 19):10450–10454. doi: 10.1073/pnas.94.19.10450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shughrue PJ, Bushnell CD, Dorsa DM. Estrogen receptor messenger ribonucleic acid in female rat brain during the estrous cycle: A comparison with ovariectomized females and intact males. Endocrinology. 1992;131(1):381–388. doi: 10.1210/endo.131.1.1612018. [DOI] [PubMed] [Google Scholar]
- 123.Mor G, Nilsen J, Horvath T, et al. Estrogen and microglia: A regulatory system that affects the brain. J Neurobiol. 1999;40(0022−3034 4):484–496. doi: 10.1002/(sici)1097-4695(19990915)40:4<484::aid-neu6>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 124.Azcoitia I, Sierra A, Garcia-Segura LM. Localization of estrogen receptor beta-immunoreactivity in astrocytes of the adult rat brain. Glia. 1999;26(0894−1491 3):260–267. [PubMed] [Google Scholar]
- 125.Sawada M, Alkayed NJ, Goto S, et al. Estrogen receptor antagonist ICI182,780 exacerbates ischemic injury in female mouse. J Cereb Blood Flow Metab. 2000;20(1):112–118. doi: 10.1097/00004647-200001000-00015. 10.1097/00004647−200001000−00015. [DOI] [PubMed] [Google Scholar]
- 126.Sampei K, Goto S, Alkayed NJ, et al. Stroke in estrogen receptor-alpha-deficient mice. Stroke. 2000;31(3):738–43. doi: 10.1161/01.str.31.3.738. discussion 744. [DOI] [PubMed] [Google Scholar]
- 127.Dubal DB, Zhu H, Yu J, et al. Estrogen receptor alpha, not beta, is a critical link in estradiol- mediated protection against brain injury. Proc Natl Acad Sci U S A. 2001;98(0027−8424 4):1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jeppesen LL, Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS, Winther K. Decreased serum testosterone in men with acute ischemic stroke. Arterioscler Thromb Vasc Biol. 1996;16(1079−5642 6):749–754. doi: 10.1161/01.atv.16.6.749. [DOI] [PubMed] [Google Scholar]
- 129.Yang SH, Perez E, Cutright J, et al. Testosterone increases neurotoxicity of glutamate in vitro and ischemia- reperfusion injury in an animal model. J Appl Physiol. 2002;92(8750−7587 1):195–201. doi: 10.1152/jappl.2002.92.1.195. [DOI] [PubMed] [Google Scholar]
- 130.Cheng J, Alkayed NJ, Hurn PD. Deleterious effects of dihydrotestosterone on cerebral ischemic injury. J Cereb Blood Flow Metab. 2007;27(9):1553–1562. doi: 10.1038/sj.jcbfm.9600457. 10.1038/sj.jcbfm.9600457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang SH, Liu R, Wu SS, Simpkins JW. The use of estrogens and related compounds in the treatment of damage from cerebral ischemia. Ann N Y Acad Sci. 2003;1007(0077−8923):101–107. doi: 10.1196/annals.1286.010. [DOI] [PubMed] [Google Scholar]
- 132.Jones KJ. Gonadal steroids as promoting factors in axonal regeneration. Brain Res Bull. 1993;30(3−4):491–498. doi: 10.1016/0361-9230(93)90283-h. [DOI] [PubMed] [Google Scholar]
- 133.Kujawa KA, Jacob JM, Jones KJ. Testosterone regulation of the regenerative properties of injured rat sciatic motor neurons. J Neurosci Res. 1993;35(3):268–273. doi: 10.1002/jnr.490350306. 10.1002/jnr.490350306. [DOI] [PubMed] [Google Scholar]
- 134.Tanzer L, Jones KJ. Gonadal steroid regulation of hamster facial nerve regeneration: Effects of dihydrotestosterone and estradiol. Exp Neurol. 1997;146(1):258–264. doi: 10.1006/exnr.1997.6529. 10.1006/exnr.1997.6529. [DOI] [PubMed] [Google Scholar]
- 135.Chen R, Cohen LG, Hallett M. Nervous system reorganization following injury. Neuroscience. 2002;111(4):761–773. doi: 10.1016/s0306-4522(02)00025-8. [DOI] [PubMed] [Google Scholar]


