Abstract
Myocardial phospholipids serve as primary reservoirs of arachidonic acid (AA), which is liberated through the rate-determining hydrolytic action of cardiac phospholipases A2 (PLA2s). A predominant PLA2 in myocardium is calcium-independent phospholipase A2β (iPLA2β), which, through its calmodulin (CaM) and ATP-binding domains, is regulated by alterations in local cellular Ca2+ concentrations and cardiac bioenergetic status, respectively. Importantly, iPLA2β has been demonstrated to be activated by ischaemia through elevation of the concentration of myocardial fatty acyl-CoA, which abrogates Ca2+/CaM-mediated inhibition of iPLA2β. AA released by PLA2-catalysed hydrolysis of phospholipids serves as a precursor for eicosanoids generated by pathways dependent on cyclooxygenases (COX), lipoxygenases (LOX), and cytochromes P450 (CYP). Eicosanoids initiate and propagate diverse signalling cascades, primarily through their interaction with cellular receptors and ion channels. However, during pathologic states such as ischaemia or congestive heart failure, eicosanoids contribute to multiple maladaptive changes including inflammation, alterations of cellular growth programmes, and activation of multiple transcriptional events leading to the deleterious sequelae of these pathologic states. This review summarizes the central roles of myocardial PLA2s in eicosanoid signalling in the heart, the major COX, LOX, and CYP pathways of eicosanoid generation in the myocardium, and the effects of important eicosanoids on receptor-, ion channel-, and transcription-mediated processes that facilitate cardiac hypertrophy, mediate ischaemic preconditioning, and precipitate arrhythmogenesis in response to pathologic stimuli.
Keywords: Myocardium, Arachidonic acid, Eicosanoid, Phospholipase A2, Ion channel, Cyclooxygenase, Lipoxygenase, Cytochrome P450
1. Introduction
Arachidonic acid (AA) and its eicosanoid metabolites occupy central roles in the regulation of myocardial physiology, bioenergetics, contractile function, and signalling pathways. The majority of AA in heart is esterified to the sn-2 position of myocardial phospholipids, in particular choline and ethanolamine plasmalogens. Activation of intracellular phospholipases that catalyse the release of AA from its endogenous phospholipid storage depots is the rate-determining step in the generation of eicosanoids in myocardium (Figure 1). The released non-esterified AA serves as substrate for oxidation by multiple cyclooxygenases (COXs), lipoxygenases (LOXs), and cytochrome P450 (CYP) enzymes in the heart thereby producing a complex spectrum of lipid second messengers. Specific eicosanoids have been demonstrated to regulate a diverse array of critical cellular processes, such as gene transcription, ion channel kinetics, and haemodynamic function, that promote salutary adaptive changes in myocardium during physiologic perturbations. However, chronic or persistent induction of these pathways, although initially adaptive in the preservation of normal myocardial function (e.g. ischaemic preconditioning), often eventually become maladaptive leading to multiple deleterious sequelae including dysfunctional excitation–contraction coupling due to alterations in ion channel kinetics, bioenergetic inefficiency, apoptosis, and accelerated necrosis, which collectively promote the development of congestive heart failure and tachyarrhythmias leading to sudden death.
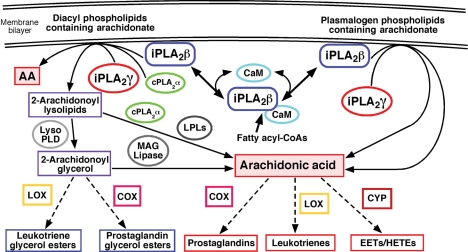
Figure 1.
Myocardial phospholipase A2 (PLA2) mediated generation of arachidonic acid (AA). Arachidonate-containing phospholipids are hydrolysed by myocardial PLA2s resulting in the release of free AA. Disruption of the calcium-activated calmodulin (CaM)-calcium-independent PLA2β (iPLA2β) inhibitory complex by increases in fatty acyl-CoA levels (e.g. in diabetic cardiomyopathy or ischaemia) leads to the activation of iPLA2β. Lipolysis of diacyl phospholipids by either iPLA2β or cytosolic PLA2α (cPLA2α) results in the direct liberation of AA while iPLA2γ predominantly catalyses the production of 2-arachidonoyl lysolipids. Subsequent action by lysophospholipase D (Lyso PLD) or lysophospholipases (LPLs) generates 2-arachidonoyl glycerol or AA from 2-arachidonoyl phospholipids, respectively. 2-Arachidonoyl-glycerol can be further metabolized by lipoxygenase (LOX), cyclooxygenase (COX), or monoacylglycerol (MAG) lipase enzymes. The vinyl ether linkage at the sn-1 position of plasmalogen phospholipids restricts iPLA2γ (and other PLA2s) to directly release AA which is further metabolized to the indicated eicosanoids. CYP, cytochrome P450 monooxygenases; EETs, epoxyeicosatrienoic acids; HETEs, hydroxyeicosatrienoic acids.
The metabolism of AA in the heart is primarily determined by three cell types (i.e. myocardial, endothelial, and vascular smooth muscle), which rely upon complex intercellular communication through paracrine signalling to coordinate blood flow, contractile state, and haemodynamic function. In addition, inflammatory cells such as neutrophils or macrophages can acutely infiltrate myocardium following ischaemic damage or chronically participate in inflammatory changes and the development of fibrosis during cardiomyopathic processes precipitating congestive heart failure. These and other cell types (e.g. platelets and fibroblasts) can contribute to the production of eicosanoids and other lipid second messengers inducing wound repair programmes that although designed to repair damaged tissue often have deleterious effects on atherosclerotic plaque formation and stability during chronic and prolonged stimulation. This review will first focus on the identification and characterization of the myocardial phospholipases A2 (PLA2s) that serve as the major mediators of AA release in heart and thus function as the rate-determining step in eicosanoid production. Secondly, the major metabolic nodes for myocardial eicosanoid signalling pathways as catalysed by COXs, LOXs, and CYPs will be discussed with respect to ischaemia/reperfusion injury, cardiac hypertrophy, haemodynamic function, and inflammation.
2. Regulation of calcium-independent phospholipase A2-mediated release of arachidonic acid for eicosanoid production in myocardium
2.1. Calcium-independent phospholipase A2β
Mammalian myocardial membranes (sarcolemma and sarcoplasmic reticulum) are electrophysiologically active membranes that are highly enriched in choline and ethanolamine plasmalogens (as well as smaller amounts of diacyl glycerophospholipids) containing AA esterified at the sn-2 position.1,2 In addition to serving as an important source of AA during cardiac myocyte activation, myocardial plasmalogens have profound effects on membrane dynamics, modulate the properties of membrane proteins (e.g. ion channels and ion pumps), and promote membrane fusion through the unique stereoelectronic structure imposed by the vinyl ether linkage at the sn-1 aliphatic chain.2–7 Phospholipases A2 (PLA2s) catalyse the hydrolysis of the sn-2 ester linkage of glycerophospholipids to generate free fatty acid (e.g. AA) and lysolipid products (Figure 2). Substantial experimental evidence supports the importance of myocardial PLA2 activation as the primary mediator of AA release from cellular phospholipids in response to a diverse array of physiologic and pathophysiologic stimuli.8–10 In the mid-1980s, a novel type of calcium-independent phospholipase A2 (iPLA2, now designated iPLA2β) present in canine and human myocardium was identified that did not require Ca2+ for membrane binding or catalysis, and selectively hydrolysed plasmenylcholine and plasmenylethanolamine substrates.11,12 Subsequent experiments demonstrated that myocardial iPLA2 activity was activated within minutes of cardiac ischaemia and that purified iPLA2β could produce alterations in both ion channel and mitochondrial function replicating those observed during ischaemia.8,9,13 Furthermore, mechanism-based inhibition of iPLA2β activity by (E)-6-(bromomethylene)-3-(1-naphthalenyl)-2-H-tetrahydropyran-2-one (BEL) prior to the induction of myocardial ischaemia was shown to have salutary effects on infarct size, mitochondrial degeneration, and arrhythmogenesis.10,14 To further substantiate the importance of iPLA2β activation during ischaemia and clarify its role in the pathogenesis of ischaemic damage, transgenic (TG) mice were generated that overexpressed iPLA2β in a cardiac myocyte-restricted manner.10 Although normally perfused TG iPLA2β myocardium possessed a lipid profile similar to that of its non-TG counterpart, the induction of myocardial ischaemia resulted in the robust activation of iPLA2β activity as demonstrated by a massive release of fatty acids into the venous effluent and the accumulation of both fatty acid and lysophosphatidylcholine (LPC) in ischaemic tissue.10 The ischaemia-mediated activation of iPLA2β was accompanied by a dramatic induction of ventricular arrhythmias including multiple episodes of tachycardia within minutes of ischaemia. Both premature ventricular contraction and ventricular tachycardia were completely suppressed by inhibition of iPLA2β activity with BEL. In sharp contrast, non-TG murine myocardium, which has virtually undetectable levels of iPLA2β activity, did not exhibit arrhythmias and neither released fatty acids into the venous effluent nor accumulated LPC when subjected to ischaemia. These results demonstrated that iPLA2β was activated during ischaemia resulting in the release of fatty acids and the generation of lysolipids leading to dual signalling pathways with multiple downstream effectors emanating from a single reaction. Moreover, activation of myocardial phospholipases during myocardial ischaemia modulates membrane dynamics leading to alterations in the interactions of membrane-associated protein complexes that modulate myocardial metabolism and bioenergetics.
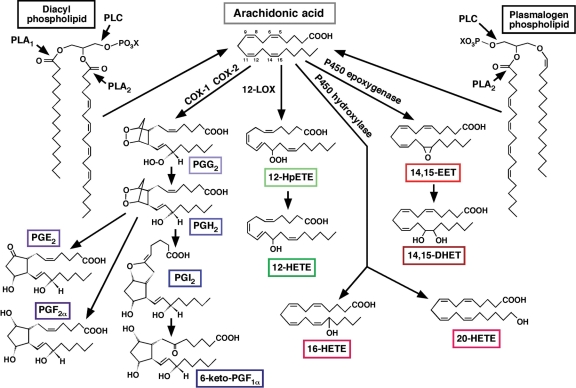
Figure 2.
Myocardial eicosanoid signalling pathways. Phospholipase A2 (PLA2) catalysed release of arachidonic acid (AA) from cellular phospholipids [X = polar head group (e.g. choline, ethanolamine, serine, etc.)] results in the production of prostaglandin (PG) G2 (PGG2) and PGH2 by either COX-1 or COX-2. PGH2 is further metabolized to PGE2, PGF2α, and PGI2, by their corresponding synthases. PGI2 is further metabolized to form 6-keto-PGF1α. Oxidation of AA to 12-hydroperoxyeicosatrienoic acid (12-HpETE) by 12-lipoxygenase (12-LOX), the most abundant LOX in myocardium, and subsequent conversion to 12-hydroxyeicosatrienoic acid (12-HETE) is shown as an example. Representative epoxidation and hydroxylation reactions as catalysed by cytochrome P450 epoxigenase and hydroxylase enzymes to form 14,15-epoxyeicosatrienoic acid (14,15-EET), 16-HETE, and 20-HETE are shown as indicated. Formation of 14,15-dihydroxyeicosatrienoic acid (14,15-DHET) from 14,15-EET is catalysed by epoxide hydrolase.
During the course of these experiments, a protein factor from myocardium was purified, which inhibited iPLA2β activity in the presence, but not in the absence, of Ca2+. This protein factor was identified as calmodulin (CaM).15 Importantly, iPLA2β activity could be incrementally regulated within the physiological range of calcium ion concentrations (50 nM–1 mM Ca2+) in the presence of CaM (indicating a direct physical interaction between the two proteins) and CaM-mediated inhibition was rapidly reversed by either EGTA or CaM antagonists (e.g. W-7) in vitro. The physiologic importance of this interaction was identified by the demonstration that iPLA2β activity was tonically inhibited in intact cells and could be fully activated by cell-permeable CaM antagonists resulting in fatty acid release.16 Thus, iPLA2β was tightly associated with CaM in vivo and therefore constitutively inactive until released from CaM-mediated inhibition.
In addition to its phospholipase activity, iPLA2β was recently found to hydrolyse fatty acyl-CoAs.17 This was of particular relevance since acyl-CoA accumulates dramatically within minutes of myocardial ischaemia. Accordingly, it was hypothesized that the binding of acyl-CoA to iPLA2β could potentially reverse the tonic inhibition of PLA2 activity by CaM and was potentially responsible for the rapid activation of iPLA2β in ischaemic myocardium leading to membrane dysfunction. To test this hypothesis, real-time kinetic analyses in conjunction with mass spectrometry were used to demonstrate that low micromolar concentrations of acyl-CoA could reverse the CaM-mediated inhibition of phospholipase activity.17 These results collectively identified acyl-CoA as both a substrate for and regulator of iPLA2β (Figure 1).
Although in vitro assays of purified iPLA2β and other iPLA2s have not revealed greater than two- to three-fold selectivity for arachidonate-containing phospholipids, ventricular myocytes have been demonstrated to robustly release AA within minutes of hypoxia or agonist stimulation. Work by McHowat et al.18,19 has demonstrated that both hypoxia and thrombin-induced AA release from rabbit ventricular myocytes are sensitive to the iPLA2 selective inhibitor BEL. Furthermore, measurement of membrane-associated and cytosolic PLA2 activities in vitro revealed selective hydrolysis of plasmalogen- and arachidonate-containing phospholipid substrates that were predominantly calcium-independent and BEL sensitive.18–21
2.2. Calcium-independent phospholipase A2γ
In addition to iPLA2β, a second major phospholipase A2 in myocardium, iPLA2γ, has been identified which contains both N-terminal mitochondrial localization and C-terminal (-SKL) peroxisomal localization sequences.22,23 Multiple mRNA splice and proteolytically processed variants of iPLA2γ have been identified, indicating the regulatory complexity of iPLA2γ.23 Experiments with the recombinant purified 63 kDa peroxisomal isoform of iPLA2γ in combination with arachidonate-containing phospholipids (e.g. 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine, PAPC) have revealed a novel role for this enzyme in eicosanoid signalling (Figure 1). Mass spectral analyses demonstrated that 2-arachidonoyl lysophosphatidylcholine (2-AA-LPC) was the major hydrolytic product generated from PAPC substrate following incubation with iPLA2γ.24 Importantly, significant amounts of 2-arachidonoyl-LPC were identified in human myocardium.24 The sequential hydrolysis of diacyl arachidonate-containing phospholipids by iPLA2γ followed by the rapid cPLA2α- or lysophospholipase (LPL)-mediated hydrolysis of 2-AA-LPC would be predicted to generate robust quantities of AA for eicosanoid production in myocardium.25 Alternatively, 2-arachidonoyl-LPC may serve as a substrate for enzymes with lysophospholipase C activity, such as nucleotide pyrophosphatase/phosphodiesterase 6,26 thereby generating the endocannabinoid 2-arachidonoyl-glycerol. Notably, 2-arachidonoyl-glycerol is a substrate for COX-2 (but not COX-1),27 12-LOX,28 and 15-LOXs.29 In addition, iPLA2γ was demonstrated to directly release AA from arachidonate-containing plamenylcholine.24 These results identified a novel mechanism for eicosanoid generation which place 2-arachidonoyl LPC as a key metabolic node in the cardiac myocyte signalling system that generates multiple discrete lipid second messengers.
Recently, to determine the function of iPLA2γ in myocardium, both TG mice overexpressing the full-length isoform of iPLA2γ in a cardiac myocyte-selective manner30 and knockout (KO) mice null for the iPLA2γ gene31 have been generated and initially characterized. Analysis of the TG iPLA2γ mice revealed a central role of iPLA2γ in myocardial lipid metabolism. Specifically, myocardial phospholipid mass was substantially depleted (≈35% in comparison to control mice) and brief caloric restriction resulted in dramatic triglyceride accumulation (50% of total lipid mass) in mice selectively overexpressing iPLA2γ in cardiac myocytes. Immunohistochemical analysis localized iPLA2γ to both the peroxisomal and mitochondrial compartments. The presence of elevated levels of iPLA2γ in mitochondria led to marked morphological alterations (e.g. loosely packed and disorganized cristae) and defects in mitochondrial bioenergetic efficiency. Importantly, TG iPLA2γ myocardium contained significantly elevated levels of 2-arachidonoyl-LPC and 2-docosahexaenoyl-LPC, demonstrating the ability of iPLA2γ to generate these signalling molecules in vivo. In contrast, myocardial mitochondria from iPLA2γ KO mice possessed only 35% of the phospholipase A1 activity of wild-type mitochondria utilizing PAPC as substrate further substantiating a predominant role of iPLA2γ in the generation of 2-AA-LPC and its downstream bioactive metabolites in myocardium.31
2.3. Cytosolic phospholipase A2α
Although present at lower levels relative to the iPLA2s, cPLA2α may play an important role in myocardial eicosanoid signalling. Cytosolic PLA2α displays approximately five-fold selectivity for AA at the sn-2 position of phosphatidylcholine relative to oleic acid.32 By virtue of its C2 calcium-binding domain, cPLA2α is activated by increases in intracellular Ca2+ which results in translocation of the enzyme to cellular membranes.33,34 In addition, phosphorylation of cPLA2α by various protein kinases, such as MAPKs and CaMKII, is believed to be important for modulating cellular AA release, although identification of the precise phosphorylation sites leading to its activation is still an area of active investigation.35–37 At the present time, the roles of Ser505 and Ser727 have been most extensively studied and implicated in cPLA2α activation.38
In myocardium, genetic knockout of cPLA2α results in significant myocardial hypertrophy which is increased following the stress of pressure overload in comparison to wild-type control mice.39 In this study, cPLA2α was implicated as a negative regulator of striated muscle growth due to enhanced insulin growth factor (IGF)-1-mediated phosphorylation of Akt, GSK-3β, and p38-MAPK in cPLA2α KO hearts (Figure 3). The basis for the cPLA2α-mediated regulation of this process was proposed to occur through PDK-1/PKCζ (also a negative regulatory pathway of striated muscle hypertrophic growth) signalling. Specifically, the absence of cPLA2α was shown to interfere with PDK-1 recruitment and activation of PKCζ which normally down-regulates IGF-1-mediated hypertrophic growth. AA supplementation restored IGF-1-mediated PKCζ phosphorylation, possibly through enhancement of PDK-1/PKCζ interactions.
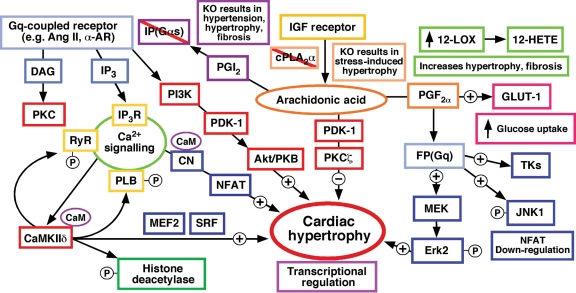
Figure 3.
Effects of eicosanoid mediated signalling on cardiac hypertrophy. G-protein (Gq)-coupled receptor and insulin growth factor (IGF) receptor pathways are central to development of cardiac hypertrophy. Activation of Gq-coupled receptors results in IP3 generation triggering intracellular Ca2+ release [increasing CaMKIIδ, calcineurin (CN), and NFAT (nuclear factor of activated T-cells) pathways] and DAG generation activating PKC. Stimulation of CaMKIIδ increases phosphorylation of the ryanodine receptor (RyR), phospholamban (PLB), and histone deacetylase (activating MEF2 and SRF transcription of hypertrophic genes).84 In addition, Gq-coupled receptors increase hypertrophic signalling through PI3K, PDK-1, and Akt/PKB. Knockout of the IP receptor (Gαs-coupled)57 or cPLA2α39 have been found to result cardiac hypertrophy. Stimulation of the FP receptor through PGF2α mediates the hypertrophic response through increased phosphorylation of Erk2 (through MEK) and JNK1 and activation of cytosolic tyrosine kinases (TKs).52,53 JNK1 may suppress hypertrophic signalling through phosphorylation of NFAT, thereby preventing its nuclear translocation and activation. PGF2α has also been demonstrated to up-regulate GLUT-1 expression in cardiac myocytes.55 Increased levels of 12-HETE through overexpression of 12-LOX leads to a hypertrophic and fibrotic phenotype.66
Experiments by Pavoine and co-workers have implicated a role for cPLA2α in the inhibition of cardiac β2-adrenergic receptor-mediated activation of adenylate cyclase and protein kinase A.40 Stimulation of cardiac myocytes with the β2-adrenergic receptor selective agonist zinterol results in the induction of [Ca2+]i transients and fractional shortening which were enhanced by pre-treatment with arachidonyl trifluoromethyl ketone (AACOCF3), indicating the role of a PLA2 in the down-regulation of these processes.40 Although Pavoine et al. emphasize that AACOCF3 is a cPLA2 inhibitor, it should be recognized that AACOCF3 inhibits both cPLA2 and iPLA2 enzymes with similar effectiveness.
2.4. Conclusions
Recent studies regarding genetic ablation and/or cardiac specific overexpression of established myocardial intracellular PLA2s have provided important insight into the function of these enzymes in myocardium. Various members of the iPLA2 and cPLA2 families likely have central roles both in the parallel processing and sequential integrated generation of free AA for eicosanoid production as well as the generation of multiple lysolipid signalling molecules. Alterations in membrane dynamics and resultant maladaptive changes in membrane function are also likely mediators of the sequelae of myocardial ischaemia and congestive heart failure. Future work will depend on multiple genetic approaches in conjunction with enantiospecific inhibition to mechanistically define the roles of each of these enzymes, their splice variants, and post-translational modifications in myocardial signal transduction pathways during ischaemia and congestive heart failure.
3. Myocardial cyclooxygenases and prostanoid signalling
An abundance of recent pharmacologic evidence has demonstrated that selective COX-2 inhibition (e.g. administration of celecoxib or rofecoxib) can be detrimental to myocardial function, whereas non-selective COX inhibition can be cardioprotective as in the case of aspirin.41,42 One possible explanation for this observed effect has been that specific COX-2 inhibition results in decreased prostacyclin (PGI2) synthesis (contributing to increased thrombogenesis, hypertension, and atherogenesis), while not reducing the production of the pro-thrombotic eicosanoid thromboxane A2 (TXA2).43 Human myocardium has been demonstrated to express both COX-1 and COX-2,44,45 each of which has been proposed to have distinct functions. COXs catalyse the incorporation of two molecular oxygens per AA molecule to form prostaglandin G2 which is then converted to the prostanoid series-2 precursor prostaglandin H2 by the peroxidase activity of the enzyme (Figure 2). COX-1 is believed to be constitutively expressed and likely serves to maintain normal cardiac homeostasis.45 In contrast, although COX-2 is induced in various pathophysiologic states as a mediator of inflammatory responses which can lead to cardiac fibrosis,46–48 increasing evidence indicates that it may also serve a cardioprotective role. In ischaemic preconditioning of rabbit myocardium, COX-2 mRNA is rapidly (<1 h) induced followed by increased COX-2 protein expression and PGE2 and 6-keto-PGF1α production49 at 24 h following ischaemia/reperfusion. Selective inhibition of COX-2 using NS-398 or celecoxib abolished the cardioprotective effects of ischaemic preconditioning, indicating that PGE2 and/or PGI2 are the likely mediators of this process.49 COX-2 appears to be up-regulated during ischaemic preconditioning through PKC-, Src-PTK, and NF-κB pathways and requires iNOS-generated NO in the heart.50,51
Multiple effects of downstream prostanoids generated by myocardial COXs have been documented. PGF2α is produced by both COX-1 and COX-2 and is involved in inflammation and hypertrophy. In addition, PGF2α is known to activate several signalling pathways in cardiac myocytes including the PGF2α receptor (Gq coupled), JNK1, and c-Jun pathways, although not all activated kinases (e.g. PKC, ERK, and p38) were required for the hypertrophic response52–54 (Figure 3). Administration of PGF2α to rat ventricular myocytes has been demonstrated to result in a dramatic increase in GLUT-1 expression and glucose uptake.55 However, glucose was not required for the hypertrophic response induced by PGF2α. Targeted knockout of the receptors for PGF2α and TXA2 (FP and TP, respectively), and not those for other prostanoids [DP, EP (isoforms 1–4), and IP], has shown that their respective ligands mediate the tachycardia that accompanies inflammatory changes in myocardium.56 Notably, lipopolysaccharide-stimulated tachycardia was diminished in FP and TP null mice and absent in FP, TP double knockout animals. PGI2 has also been documented to have substantial effects on myocardial physiology. Mice null for the receptor for PGI2 (IP) have been demonstrated to exhibit salt-sensitive hypertension, cardiac hypertrophy, and severe cardiac fibrosis.57 IP KO mice also exhibited exacerbated cardiac hypertrophy in response to pressure overload in comparison to wild-type controls.58
3.1. Conclusions
The complexity of eicosanoid signalling downstream of the COX enzymes, although necessary to balance and maintain normal physiologic function in myocardium, has complicated efforts to develop specific COX-2 inhibitors designed to reduce inflammation and platelet aggregation in patients with cardiovascular disease. Similarities between the detrimental effects of selective COX-2 inhibition and the phenotypes of mice null for different prostanoid receptors will continue to provide valuable insight into the relative influence of eicosanoid-mediated signalling pathways involved in vascular tone, hypertension, atherosclerosis, and thrombogenesis as well as ischaemic heart disease and congestive heart failure.
4. Hydroxyeicosatrienoic acids mediated myocardial signalling
Lipoxygenases catalyse the stereospecific oxidation of the olefinic linkages of AA to produce hydroperoxyeicosatetraenoic acids (HpETEs), which are then reduced to form their hydroxyeicosatrienoic (HETE) derivatives (Figure 2). LOX activity has been previously detected in subcellular fractions of rat heart and cultured rat myocytes.59 The majority of the myocardial LOX activity measured in this study was determined to be 12-LOX (i.e. 12-HETE produced from AA) with trace amounts of 15-LOX (15-HETE production).
Myocardial ischaemia results in LOX activation in myocytes as well as recruitment of leucocytes to damaged areas, thereby leading to an increased production of a wide variety of oxidized biologically active eicosanoids. For example, 5-HETE and 12-HETE levels were found to dramatically increase in cultured canine myocytes following 45 min of hypoxia and 5 h re-oxygenation.60 Similarly, ischaemic rabbit myocardium produced greater amounts of leukotriene B4, 5-HETE, and 12-HETE than non-ischaemic controls, with the latter being the major product.61 Recently, 12-HETE has been demonstrated to increase mitochondrial Ca2+ concentration and mtNOS activity (NO production) in isolated rat heart mitochondria and in HL-1 cardiac myocytes which underwent apoptosis following treatment with 12-HETE.62 Elevated levels of NO under these conditions were found to decrease mitochondrial respiratory efficiency and transmembrane potential, thereby leading to cell death. In addition, 15(S)-HETE, 11(S)-HETE, and 5(S),15(S)-diHETE have been shown to increase the sensitivity of the isoproterenol-mediated β-adrenergic response in cardiomyocytes.63 Of particular interest in this study was the selective incorporation of 15-HETE into cellular phosphatidylinositols and the activation of PKC in 15-HETE-mediated enhancement of β-adrenergic signalling.
Lipoxygenase-mediated production of HETEs likely participates in maintaining normal myocardial function and its alteration in multiple disease states. Inhibition of LOXs inhibits insulin-stimulated GLUT-4-mediated glucose uptake by cultured ventricular myocytes through prevention of actin cytoskeletal rearrangement, but not through alterations in Akt phosphorylation or association of phosphoinositide 3-kinase with IRS-1,64,65 suggesting a potential role of HETEs in maintaining sensitivity to insulin. Separately, eicosanoid products of 12-LOX have been implicated in the development of cardiac fibrosis and hypertrophy.66,67 Overexpression of 12-LOX in cardiac fibroblasts resulted in increased incorporation of leucine, thymidine, and uridine, increased cell size, and a reduction in the rate of cell division in comparison to control cells. The presence of 12-LOX also resulted in a significant elevation of collagen and fibronectin levels, indicative of a fibrotic phenotype.
4.1. Conclusions
The roles of LOXs and their eicosanoid products in myocardial function remain relatively unexplored. Initial investigations have revealed evidence of the up-regulation of LOX enzymes and/or LOX products following myocardial ischaemia/infarction and may contribute to cardiac hypertrophy, myocyte apoptosis, and fibrosis. Alternatively, LOXs and their eicosanoid products may facilitate β-adrenergic signalling and glucose transport. Future studies using genetic models in conjunction with specific pharmacologic agents will help deconvolute the role of each specific HETE moiety in physiologic adaptation in addition to their contribution to compromised myocardial function in pathologic states.
5. Epoxyeicosatrienoic and hydroxyeicosatrienoic acids produced by myocardial cytochromes P450
Multiple members of the CYP superfamily (e.g. CYP1B, CYP2A, CYP2B, CYP2C, CYP2E, CYP2J, CYP4A, and CYP11) have been identified in human heart and their expression and resultant AA metabolites have been found to be generally increased during cardiac hypertrophy and heart failure.68,69 Myocardial CYPs which metabolize AA are haem protein monooxygenases which require O2, CYP reductase, and NADPH for catalysis. Notably, CYP inhibitors (chloroamphenicol, cimetidine, and sulfaphenazole) have been demonstrated to decrease myocardial ischaemia/reperfusion damage, presumably through attenuation of CYP-mediated reactive oxygen species (ROS) generation although other mechanisms are possible.70 Members of the CYP 2C and 2J subfamilies are believed to be the predominant P450 isoforms involved in eicosanoid signalling in the heart. Murine myocardium contains at least three CYP 2C members (CYP2C29, CYP2C40, and CYP2C50) that predominantly catalyse the production of epoxyeicosatrienoic acids [EETs, primarily 8(S),9(R)-EET and 14(R),15(S)-EET], HETEs (primarily 16-HETE with minor amounts of 20-HETE), as well as a variety of lower abundance EETs and HETEs, respectively.71,72 The epoxide linkage of EETs can be further metabolized by soluble epoxide hydrolases to dihydroxyeicosatrienoic acids which generally possess less potent biologic effects. Zeldin and co-workers73 identified a novel CYP epoxygenase, CYP2J2, abundantly expressed in human myocardium which was highly enantioselective for the production of 14(R),15(S)-EET from AA. Other EET enantiomeric pairs (e.g. 8,9-EET and 11,12-EET) were produced in nearly equal racemic distributions. Interestingly, the EET composition of human myocardium paralleled that of AA metabolites generated with purified recombinant CYP2J2, suggesting that this is a predominant pathway in eicosanoid generation.73 However, it should be noted that other eicosanoids, although present in smaller amounts, may be of equal or greater importance in signalling depending on their relative affinities for target proteins and subsequent mechanisms used for signal amplification.
Endogenous generation of EETs has been demonstrated to be cardioprotective by reduction of ischaemia–reperfusion damage and rescue of myocardial function. Selective TG overexpression of CYP2J2 in heart results in improved post-myocardial infarction (MI) recovery of left ventricular developed pressure, an effect that was reversed by administration of the CYP2J2 inhibitor N-methanesulfonyl-6-(2-propargyloxyphenyl)hexanamide (MS-PPOH).74 The CYP2J2-dependent recovery of heart function was also blocked by either the sarcolemmal KATP channel inhibitor glibenclamide or the mitochondrial KATP channel inhibitor 5-hydroxydecanoate. Mitochondrial KATP channel activity was found to be higher in TG CYP2J2 cardiomyocytes in comparison to their non-TG controls and low micromolar concentrations of P450 2J2 generated EETs increased mitochondrial redox potential as measured by flavoprotein fluorescence (indicative of KATP channel activity) in non-TG animals. Although the phosphorylation state of MAPK p42/p44 was similar between CYP2J2 TG and non-TG hearts under basal and ischaemic conditions, reperfusion induced increased levels of MAPK p42/p44 phosphorylation in TG vs. non-TG controls. Importantly, selective inhibition of MEK with PD98059 abolished the CYP2J2-dependent post-MI recovery of left ventricular developed pressure.74
In contrast to EETs, 20-HETE has been found to be detrimental to cardiac function. The CYP ω-hydroxylases CYP4A1, CYP4A2, and CYP4F have been demonstrated to be expressed in canine heart75 and produce 20-HETE, a potent vasoconstrictor that acts through inhibition of Ca2+-sensitive K+ channels.76 High concentrations of 20-HETE are released in the coronary venous effluent during reperfusion of canine hearts rendered ischaemic.75 Pre-treatment with the specific P450 ω-hydroxylase inhibitors, 17-octadecanoic acid (17-ODYA) and N-methylsulfonyl-12,12-dibromododec-11-enamide (DDMS), significantly inhibited 20-HETE production and reduced infarct size compared with the total area at risk. Consistent with this, infusion of exogenous 20-HETE prior to left anterior descending coronary artery occlusion resulted in a significant increase in infarct size. Further studies demonstrated that the putative 20-HETE antagonist 20-hydroxyeicosa-6(Z),15(Z)-dienoic acid also decreased infarct size similar to DDMS which was augmented by ischaemic preconditioning.77 Additional experiments utilizing ischaemic/reperfused rat hearts confirmed that 17-ODYA and DDMS dose-dependently reduced ischaemic damage, but that prior treatment with the P450 epoxygenase inhibitor MS-PPOH before induction of ischaemia or reperfusion was without effect.78
5.1. Conclusions
Cytochrome P450 monooxygenases represent important participants in myocardial eicosanoid signalling. Increased expression of the epoxygenase CYP2J2 in mouse myocardium decreased ischaemia–reperfusion damage through production of EETs which modulate KATP channel function and MAPK signalling. Conversely, production of HETEs (in particular 20-HETE) during ischaemia by CYP4 family members likely promotes ischaemic damage either through elevated vasoconstriction and/or potentially increased ROS generation, although this remains to be determined.
6. Arachidonic acid and eicosanoid-mediated regulation of myocardial ion channel function
Disruption of normal ion channel function represents a primary mechanism for induction of the lethal ventricular arrhythmias which occur following myocardial ischaemia. Definition of the influence of AA on this process is complicated by differential pro- and anti-arrhythmogenic effects of AA and other polyunsaturated fatty acids as well as their oxidized metabolites on ion channel function. In addition, it has been difficult to differentiate the direct effects of AA and its oxidized metabolites on ion channel function from those mediated through receptor-activated pathways. Specific AA-mediated effects have been demonstrated in the case of the human delayed rectifier K+ channel, Kv 1.1, which undergoes significant increases in both macroscopic voltage-dependent activation and inactivation kinetics following administration of micromolar concentrations of AA13 (Figure 4). In addition, the effects of free AA on Kv 1.1 function could be recapitulated by the intracellular introduction of iPLA2β and were dependent upon the presence of AA-containing membrane phospholipids. Importantly, these effects were observed in Sf9 cells which are not known to oxidize the olefinic linkages of AA (as determined by autoradiographic thin layer chromatography), thereby ruling out the potential influence of downstream eicosanoids.
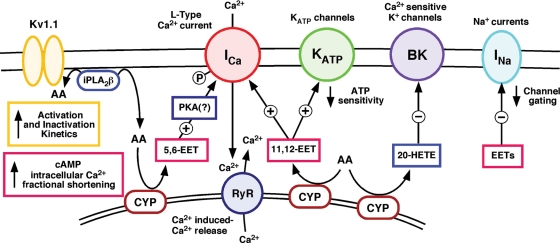
Figure 4.
Influence of arachidonic acid, EETs, and HETEs on myocardial ion channels/currents. Arachidonic acid generated by iPLA2β has been demonstrated to increase activation and deactivation kinetics of Kv1.1 ion channels.13 Cytochrome P450 epoxygenases (CYPs) catalyse the production of 5,6-EET and 11,12-EET regioisomers which elevate cAMP and intracellular Ca2+ levels through Ca2+-mediated activation of the ryanodine receptor, resulting in increased fractional shortening.79,80 L-type Ca2+ current may be enhanced by direct EET-ion channel interactions or through protein kinase A mediated Ca2+ channel phosphorylation.85 11,12-EET has been demonstrated to decrease KATP channel sensitivity to ATP,86 while several EETs have been shown to attenuate the gating of Na+ channels.83 The potent vasoconstrictive and pro-ischaemic effects of 20-HETE are proposed to occur through its inhibition of Ca2+-sensitive K+ (BK) channels.76
Epoxyeicosatrienoic acids have been demonstrated to have varying effects on different cardiac ion channels (Figure 4). Addition of low nanomolar concentrations of exogenous 5,6- and 11,12-EET significantly increased shortening of isolated ventricular myocytes and intracellular Ca2+ concentrations, while 8,9- and 14,15-EETs were without effect.79 Treatment of rat ventricular myocytes with the imidazole-based P450 inhibitors clotrimazole, econazole, or miconazole significantly blocked L-type Ca2+ current (ICa), intracellular Ca2+ signalling, and cell shortening.80 Clotrimazole suppressed ICa was reversed by administration of the β-adrenergic agonist isoprenaline or by incubation with 8-bromo-cAMP. Levels of cAMP in cells treated with clotrimazole were found to be significantly reduced while incubation of myocytes with 11,12-EET markedly increased the concentration of intracellular cAMP. From these and other data, it was concluded that P450-generated arachidonate metabolites modulated cAMP levels, Ca2+ channel phosphorylation (presumably protein kinase A mediated), Ca2+ signalling, and cellular contraction. In a separate study, cardiac L-type Ca2+ channels reconstituted into planar lipid bilayers were found to be inhibited by nanomolar concentrations of EETs which decreased channel open probability, accelerated channel inactivation, and decreased the unitary current amplitude of open channels.81 Interestingly, the same effects on L-type Ca2+ channel function were mediated by 11,12-EET when it was esterified at the sn-2 position of surrounding phosphatidylcholine molecules, suggesting a direct EET/Ca2+ channel interaction within the lipid phase of the bilayer. Utilizing an inside-out patch clamp technique, Lu et al.82 demonstrated that low micromolar concentrations of 11,12-EET markedly increased the activity (open channel probability) of ATP-sensitive K+ channels by reducing sensitivity of the channels to ATP in a dose- and voltage-dependent manner. Intact myocyte Na+ currents were found to be inhibited by low micromolar concentrations of 8,9-EET as well as 5,6-, 11,12-, and 14,15-EETs in a channel-use and voltage-dependent manner.83 Single channel recordings indicated that 8,9-EET markedly reduced the duration and probability of Na+ channel opening.
6.1. Conclusions
Arachidonic acid and its EET derivatives have been demonstrated to significantly alter myocardial ion channel function. Although the precise mechanisms involved in eiconsanoid regulation of myocardial ion channels have not been demonstrated in molecular detail, they likely involve both direct (e.g. binding and conformational alterations) and indirect (e.g. elevation of cAMP levels followed by PKA-mediated signalling) processes.
7. Concluding remarks
Elucidation of the cardiovascular effects of AA and its eicosanoid derivatives has led to a new appreciation of the importance of enzymes mediating their generation and their influence on cardiac physiology in health and disease. Multiple enzymes are involved in the release of AA (i.e. PLA2s) and its stereospecific oxidation (i.e. COXs, LOXs, and CYPs) to generate this diverse array of bioactive signalling molecules. Moreover, our understanding of the role played by eicosanoids in altering ion channel function, transcriptional programming, and ventricular remodelling, and in orchestrating the pleiotropic responses to cellular perturbations has begun to evolve. Prominent examples include the roles of eicosanoids in activating protein kinase cascades and ion channels and their resultant effects on cardiac hypertrophy, myocardial preconditioning, infarction, and arrhythmogenesis. Although considerable progress has been made, much work remains to be done to identify the complex interwoven pathways that integrate eicsosanoid signalling pathways with finely tuned myocardial responses to alterations in metabolic state, haemodynamic burden, or nutritional history of the cardiac myocyte. Continuing advances in pharmacologic and genetic approaches will allow greater insight into these mechanisms, thereby providing a foundation for the development of targeted pharmaceutical approaches for limiting ischaemic damage, attenuating the deleterious sequelae of cardiac hypertrophy, and preventing arrhythmogenesis.
Funding
This work was supported by the National Institutes of Health grants 2P01HL057278-11 and R01HL041250-14A1.
Acknowledgements
We thank David J. Mancuso and Harold F. Sims for their critical reading of this review.
Conflict of interest: none declared.
References
- 1.Gross RW. High plasmalogen and arachidonic acid content of canine myocardial sarcolemma: a fast atom bombardment mass spectroscopic and gas chromatography-mass spectroscopic characterization. Biochemistry. 1984;23:158–165. doi: 10.1021/bi00296a026. [DOI] [PubMed] [Google Scholar]
- 2.Gross RW. Identification of plasmalogen as the major phospholipid constituent of cardiac sarcoplasmic reticulum. Biochemistry. 1985;24:1662–1668. doi: 10.1021/bi00328a014. [DOI] [PubMed] [Google Scholar]
- 3.Bick RJ, Youker KA, Pownall HJ, Van Winkle WB, Entman ML. Unsaturated aminophospholipids are preferentially retained by the fast skeletal muscle Ca2+ATPase during detergent solubilization. Evidence for a specific association between aminophospholipids and the calcium pump protein. Arch Biochem Biophys. 1991;286:346–352. doi: 10.1016/0003-9861(91)90050-s. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Gross RW. Phospholipid subclass-specific alterations in the kinetics of ion transport across biologic membranes. Biochemistry. 1994;33:13769–13774. doi: 10.1021/bi00250a030. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Gross RW. Potassium flux through gramicidin ion channels is augmented in vesicles comprised of plasmenylcholine: correlations between gramicidin conformation and function in chemically distinct host bilayer matrices. Biochemistry. 1995;34:7356–7364. doi: 10.1021/bi00022a008. [DOI] [PubMed] [Google Scholar]
- 6.Ford DA, Hale CC. Plasmalogen and anionic phospholipid dependence of the cardiac sarcolemmal sodium-calcium exchanger. FEBS Lett. 1996;394:99–102. doi: 10.1016/0014-5793(96)00930-1. [DOI] [PubMed] [Google Scholar]
- 7.Duhm J, Engelmann B, Schonthier UM, Streich S. Accelerated maximal velocity of the red blood cell Na+/K+ pump in hyperlipidemia is related to increase in 1-palmitoyl,2-arachidonoyl-plasmalogen phosphatidylethanolamine. Biochim Biophys Acta. 1993;1149:185–188. doi: 10.1016/0005-2736(93)90040-7. [DOI] [PubMed] [Google Scholar]
- 8.Hazen SL, Ford DA, Gross RW. Activation of a membrane-associated phospholipase A2 during rabbit myocardial ischemia which is highly selective for plasmalogen substrate. J Biol Chem. 1991;266:5629–5633. [PubMed] [Google Scholar]
- 9.Ford DA, Hazen SL, Saffitz JE, Gross RW. The rapid and reversible activation of a calcium-independent plasmalogen-selective phospholipase A2 during myocardial ischemia. J Clin Invest. 1991;88:331–335. doi: 10.1172/JCI115296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mancuso DJ, Abendschein DR, Jenkins CM, Han X, Saffitz JE, Schuessler RB, et al. Cardiac ischemia activates calcium-independent phospholipase A2β, precipitating ventricular tachyarrhythmias in transgenic mice: rescue of the lethal electrophysiologic phenotype by mechanism-based inhibition. J Biol Chem. 2003;278:22231–22236. doi: 10.1074/jbc.C300033200. [DOI] [PubMed] [Google Scholar]
- 11.Wolf RA, Gross RW. Identification of neutral active phospholipase C which hydrolyzes choline glycerophospholipids and plasmalogen selective phospholipase A2 in canine myocardium. J Biol Chem. 1985;260:7295–7303. [PubMed] [Google Scholar]
- 12.Hazen SL, Gross RW. Human myocardial cytosolic Ca2+-independent phospholipase A2 is modulated by ATP. Concordant ATP-induced alterations in enzyme kinetics and mechanism-based inhibition. Biochem J. 1991;280:581–587. doi: 10.1042/bj2800581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gubitosi-Klug RA, Yu SP, Choi DW, Gross RW. Concomitant acceleration of the activation and inactivation kinetics of the human delayed rectifier K+ channel (Kv1.1) by Ca2+-independent phospholipase A2. J Biol Chem. 1995;270:2885–2888. doi: 10.1074/jbc.270.7.2885. [DOI] [PubMed] [Google Scholar]
- 14.Sargent CA, Wilde MW, Dzwonczyk S, Tuttle JG, Murray HN, Atwal K, et al. Glyburide-reversible cardioprotective effects of calcium-independent phospholipase A2 inhibition in ischemic rat hearts. Cardiovasc Res. 1996;31:270–277. [PubMed] [Google Scholar]
- 15.Wolf MJ, Gross RW. The calcium-dependent association and functional coupling of calmodulin with myocardial phospholipase A2. Implications for cardiac cycle-dependent alterations in phospholipolysis. J Biol Chem. 1996;271:20989–20992. doi: 10.1074/jbc.271.35.20989. [DOI] [PubMed] [Google Scholar]
- 16.Wolf MJ, Wang J, Turk J, Gross RW. Depletion of intracellular calcium stores activates smooth muscle cell calcium-independent phospholipase A2. A novel mechanism underlying arachidonic acid mobilization. J Biol Chem. 1997;272:1522–1526. doi: 10.1074/jbc.272.3.1522. [DOI] [PubMed] [Google Scholar]
- 17.Jenkins CM, Yan W, Mancuso DJ, Gross RW. Highly selective hydrolysis of fatty acyl-CoAs by calcium-independent phospholipase A2β. Enzyme autoacylation and acyl-CoA-mediated reversal of calmodulin inhibition of phospholipase A2 activity. J Biol Chem. 2006;281:15615–15624. doi: 10.1074/jbc.M511623200. [DOI] [PubMed] [Google Scholar]
- 18.McHowat J, Creer MH. Thrombin activates a membrane-associated calcium-independent PLA2 in ventricular myocytes. Am J Physiol. 1998;274:C447–C454. doi: 10.1152/ajpcell.1998.274.2.C447. [DOI] [PubMed] [Google Scholar]
- 19.McHowat J, Liu S, Creer MH. Selective hydrolysis of plasmalogen phospholipids by Ca2+-independent PLA2 in hypoxic ventricular myocytes. Am J Physiol. 1998;274:C1727–C1737. doi: 10.1152/ajpcell.1998.274.6.C1727. [DOI] [PubMed] [Google Scholar]
- 20.McHowat J, Creer MH. Calcium-independent phospholipase A2 in isolated rabbit ventricular myocytes. Lipids. 1998;33:1203–1212. doi: 10.1007/s11745-998-0324-5. [DOI] [PubMed] [Google Scholar]
- 21.McHowat J, Creer MH. Selective plasmalogen substrate utilization by thrombin-stimulated Ca2+-independent PLA2 in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2000;278:H1933–H1940. doi: 10.1152/ajpheart.2000.278.6.H1933. [DOI] [PubMed] [Google Scholar]
- 22.Mancuso DJ, Jenkins CM, Gross RW. The genomic organization, complete mRNA sequence, cloning, and expression of a novel human intracellular membrane-associated calcium-independent phospholipase A2. J Biol Chem. 2000;275:9937–9945. doi: 10.1074/jbc.275.14.9937. [DOI] [PubMed] [Google Scholar]
- 23.Mancuso DJ, Jenkins CM, Sims HF, Cohen JM, Yang J, Gross RW. Complex transcriptional and translational regulation of iPLA2γ resulting in multiple gene products containing dual competing sites for mitochondrial or peroxisomal localization. Eur J Biochem. 2004;271:4709–4724. doi: 10.1111/j.1432-1033.2004.04435.x. [DOI] [PubMed] [Google Scholar]
- 24.Yan W, Jenkins CM, Han X, Mancuso DJ, Sims HF, Yang K, et al. The highly selective production of 2-arachidonoyl lysophosphatidylcholine catalyzed by purified calcium-independent phospholipase A2γ: identification of a novel enzymatic mediator for the generation of a key branch point intermediate in eicosanoid signaling. J Biol Chem. 2005;280:26669–26679. doi: 10.1074/jbc.M502358200. [DOI] [PubMed] [Google Scholar]
- 25.Pete MJ, Exton JH. Purification of a lysophospholipase from bovine brain that selectively deacylates arachidonoyl-substituted lysophosphatidylcholine. J Biol Chem. 1996;271:18114–18121. doi: 10.1074/jbc.271.30.18114. [DOI] [PubMed] [Google Scholar]
- 26.Sakagami H, Aoki J, Natori Y, Nishikawa K, Kakehi Y, Natori Y, et al. Biochemical and molecular characterization of a novel choline-specific glycerophosphodiester phosphodiesterase belonging to the nucleotide pyrophosphatase/phosphodiesterase family. J Biol Chem. 2005;280:23084–23093. doi: 10.1074/jbc.M413438200. [DOI] [PubMed] [Google Scholar]
- 27.Kozak KR, Rowlinson SW, Marnett LJ. Oxygenation of the endocannabinoid, 2-arachidonylglycerol, to glyceryl prostaglandins by cyclooxygenase-2. J Biol Chem. 2000;275:33744–33749. doi: 10.1074/jbc.M007088200. [DOI] [PubMed] [Google Scholar]
- 28.Moody JS, Kozak KR, Ji C, Marnett LJ. Selective oxygenation of the endocannabinoid 2-arachidonylglycerol by leukocyte-type 12-lipoxygenase. Biochemistry. 2001;40:861–866. doi: 10.1021/bi002303b. [DOI] [PubMed] [Google Scholar]
- 29.Kozak KR, Gupta RA, Moody JS, Ji C, Boeglin WE, DuBois RN, et al. 15-Lipoxygenase metabolism of 2-arachidonylglycerol. Generation of a peroxisome proliferator-activated receptor alpha agonist. J Biol Chem. 2002;277:23278–23286. doi: 10.1074/jbc.M201084200. [DOI] [PubMed] [Google Scholar]
- 30.Mancuso DJ, Han X, Jenkins CM, Lehman JJ, Sambandam N, Sims HF, et al. Dramatic accumulation of triglycerides and precipitation of cardiac hemodynamic dysfunction during brief caloric restriction in transgenic myocardium expressing human calcium-independent phospholipase A2γ. J Biol Chem. 2007;282:9216–9227. doi: 10.1074/jbc.M607307200. [DOI] [PubMed] [Google Scholar]
- 31.Mancuso DJ, Sims HF, Han X, Jenkins CM, Guan SP, Yang K, et al. Genetic ablation of calcium-independent phospholipase A2γ leads to alterations in mitochondrial lipid metabolism and function resulting in a deficient mitochondrial bioenergetic phenotype. J Biol Chem. 2007;282:34611–34622. doi: 10.1074/jbc.M707795200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clark JD, Lin LL, Kriz RW, Ramesha CS, Sultzman LA, Lin AY, et al. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca2+-dependent translocation domain with homology to PKC and GAP. Cell. 1991;65:1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- 33.Channon JY, Leslie CC. A calcium-dependent mechanism for associating a soluble arachidonoyl-hydrolyzing phospholipase A2 with membrane in the macrophage cell line RAW 264.7. J Biol Chem. 1990;265:5409–5413. [PubMed] [Google Scholar]
- 34.Nalefski EA, Sultzman LA, Martin DM, Kriz RW, Towler PS, Knopf JL, et al. Delineation of two functionally distinct domains of cytosolic phospholipase A2, a regulatory Ca2+-dependent lipid-binding domain and a Ca2+-independent catalytic domain. J Biol Chem. 1994;269:18239–18249. [PubMed] [Google Scholar]
- 35.Gijon MA, Spencer DM, Siddiqi AR, Bonventre JV, Leslie CC. Cytosolic phospholipase A2 is required for macrophage arachidonic acid release by agonists that Do and Do not mobilize calcium. Novel role of mitogen-activated protein kinase pathways in cytosolic phospholipase A2 regulation. J Biol Chem. 2000;275:20146–20156. doi: 10.1074/jbc.M908941199. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu M, Nakamura H, Hirabayashi T, Suganami A, Tamura Y, Murayama T. Ser515 phosphorylation-independent regulation of cytosolic phospholipase A2α (cPLA2α) by calmodulin-dependent protein kinase: possible interaction with catalytic domain A of cPLA2α. Cell Signal. 2008;20:815–824. doi: 10.1016/j.cellsig.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 37.Pavicevic Z, Leslie CC, Malik KU. cPLA2 phosphorylation at serine-515 and serine-505 is required for arachidonic acid release in vascular smooth muscle cells. J Lipid Res. 2008;49:724–737. doi: 10.1194/jlr.M700419-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Ghosh M, Tucker DE, Burchett SA, Leslie CC. Properties of the Group IV phospholipase A2 family. Prog Lipid Res. 2006;45:487–510. doi: 10.1016/j.plipres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Haq S, Kilter H, Michael A, Tao J, O'Leary E, Sun XM, et al. Deletion of cytosolic phospholipase A2 promotes striated muscle growth. Nat Med. 2003;9:944–951. doi: 10.1038/nm891. [DOI] [PubMed] [Google Scholar]
- 40.Ait-Mamar B, Cailleret M, Rucker-Martin C, Bouabdallah A, Candiani G, Adamy C, et al. The cytosolic phospholipase A2 pathway, a safeguard of β2-adrenergic cardiac effects in rat. J Biol Chem. 2005;280:18881–18890. doi: 10.1074/jbc.M410305200. [DOI] [PubMed] [Google Scholar]
- 41.Grosser T, Fries S, FitzGerald GA. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J Clin Invest. 2006;116:4–15. doi: 10.1172/JCI27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Funk CD, FitzGerald GA. COX-2 inhibitors and cardiovascular risk. J Cardiovasc Pharmacol. 2007;50:470–479. doi: 10.1097/FJC.0b013e318157f72d. [DOI] [PubMed] [Google Scholar]
- 43.McAdam BF, Catella-Lawson F, Mardini IA, Kapoor S, Lawson JA, FitzGerald GA. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc Natl Acad Sci USA. 1999;96:272–277. doi: 10.1073/pnas.96.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Testa M, Rocca B, Spath L, Ranelletti FO, Petrucci G, Ciabattoni G, et al. Expression and activity of cyclooxygenase isoforms in skeletal muscles and myocardium of humans and rodents. J Appl Physiol. 2007;103:1412–1418. doi: 10.1152/japplphysiol.00288.2007. [DOI] [PubMed] [Google Scholar]
- 45.Zidar N, Dolenc-Strazar Z, Jeruc J, Jerse M, Balazic J, Gartner U, et al. Expression of cyclooxygenase-1 and cyclooxygenase-2 in the normal human heart and in myocardial infarction. Cardiovasc Pathol. 2007;16:300–304. doi: 10.1016/j.carpath.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Wong SC, Fukuchi M, Melnyk P, Rodger I, Giaid A. Induction of cyclooxygenase-2 and activation of nuclear factor-κB in myocardium of patients with congestive heart failure. Circulation. 1998;98:100–103. doi: 10.1161/01.cir.98.2.100. [DOI] [PubMed] [Google Scholar]
- 47.Saito T, Rodger IW, Hu F, Robinson R, Huynh T, Giaid A. Inhibition of COX pathway in experimental myocardial infarction. J Mol Cell Cardiol. 2004;37:71–77. doi: 10.1016/j.yjmcc.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Z, Vezza R, Plappert T, McNamara P, Lawson JA, Austin S, et al. COX-2-dependent cardiac failure in Gh/tTG transgenic mice. Circ Res. 2003;92:1153–1161. doi: 10.1161/01.RES.0000071749.22027.45. [DOI] [PubMed] [Google Scholar]
- 49.Shinmura K, Tang XL, Wang Y, Xuan YT, Liu SQ, Takano H, et al. Cyclooxygenase-2 mediates the cardioprotective effects of the late phase of ischemic preconditioning in conscious rabbits. Proc Natl Acad Sci USA. 2000;97:10197–10202. doi: 10.1073/pnas.97.18.10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shinmura K, Xuan YT, Tang XL, Kodani E, Han H, Zhu Y, et al. Inducible nitric oxide synthase modulates cyclooxygenase-2 activity in the heart of conscious rabbits during the late phase of ischemic preconditioning. Circ Res. 2002;90:602–608. doi: 10.1161/01.res.0000012202.52809.40. [DOI] [PubMed] [Google Scholar]
- 51.Xuan YT, Guo Y, Zhu Y, Han H, Langenbach R, Dawn B, et al. Mechanism of cyclooxygenase-2 upregulation in late preconditioning. J Mol Cell Cardiol. 2003;35:525–537. doi: 10.1016/s0022-2828(03)00076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kunapuli P, Lawson JA, Rokach JA, Meinkoth JL, FitzGerald GA. Prostaglandin F2α (PGF2α) and the isoprostane, 8, 12-iso-isoprostane F2α-III, induce cardiomyocyte hypertrophy. Differential activation of downstream signaling pathways. J Biol Chem. 1998;273:22442–22452. doi: 10.1074/jbc.273.35.22442. [DOI] [PubMed] [Google Scholar]
- 53.Adams JW, Sah VP, Henderson SA, Brown JH. Tyrosine kinase and c-Jun NH2-terminal kinase mediate hypertrophic responses to prostaglandin F2α in cultured neonatal rat ventricular myocytes. Circ Res. 1998;83:167–178. doi: 10.1161/01.res.83.2.167. [DOI] [PubMed] [Google Scholar]
- 54.Adams JW, Migita DS, Yu MK, Young R, Hellickson MS, Castro-Vargas FE, et al. Prostaglandin F2α stimulates hypertrophic growth of cultured neonatal rat ventricular myocytes. J Biol Chem. 1996;271:1179–1186. doi: 10.1074/jbc.271.2.1179. [DOI] [PubMed] [Google Scholar]
- 55.Morissette MR, Howes AL, Zhang T, Heller Brown J. Upregulation of GLUT1 expression is necessary for hypertrophy and survival of neonatal rat cardiomyocytes. J Mol Cell Cardiol. 2003;35:1217–1227. doi: 10.1016/s0022-2828(03)00212-8. [DOI] [PubMed] [Google Scholar]
- 56.Takayama K, Yuhki K, Ono K, Fujino T, Hara A, Yamada T, et al. Thromboxane A2 and prostaglandin F2α mediate inflammatory tachycardia. Nat Med. 2005;11:562–566. doi: 10.1038/nm1231. [DOI] [PubMed] [Google Scholar]
- 57.Francois H, Athirakul K, Howell D, Dash R, Mao L, Kim HS, et al. Prostacyclin protects against elevated blood pressure and cardiac fibrosis. Cell Metab. 2005;2:201–207. doi: 10.1016/j.cmet.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 58.Hara A, Yuhki K, Fujino T, Yamada T, Takayama K, Kuriyama S, et al. Augmented cardiac hypertrophy in response to pressure overload in mice lacking the prostaglandin I2 receptor. Circulation. 2005;112:84–92. doi: 10.1161/CIRCULATIONAHA.104.527077. [DOI] [PubMed] [Google Scholar]
- 59.Breitbart E, Sofer Y, Shainberg A, Grossman S. Lipoxygenase activity in heart cells. FEBS Lett. 1996;395:148–152. doi: 10.1016/0014-5793(96)01017-4. [DOI] [PubMed] [Google Scholar]
- 60.Kuzuya T, Hoshida S, Kim Y, Oe H, Hori M, Kamada T, et al. Free radical generation coupled with arachidonate lipoxygenase reaction relates to reoxygenation induced myocardial cell injury. Cardiovasc Res. 1993;27:1056–1060. doi: 10.1093/cvr/27.6.1056. [DOI] [PubMed] [Google Scholar]
- 61.Hughes H, Gentry DL, McGuire GM, Taylor AA. Gas chromatographic-mass spectrometric analysis of lipoxygenase products in post-ischemic rabbit myocardium. Prostaglandins Leukot Essent Fatty Acids. 1991;42:225–231. doi: 10.1016/0952-3278(91)90087-l. [DOI] [PubMed] [Google Scholar]
- 62.Nazarewicz RR, Zenebe WJ, Parihar A, Parihar MS, Vaccaro M, Rink C, et al. 12(S)-hydroperoxyeicosatetraenoic acid (12-HETE) increases mitochondrial nitric oxide by increasing intramitochondrial calcium. Arch Biochem Biophys. 2007;468:114–120. doi: 10.1016/j.abb.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wallukat G, Morwinski R, Kuhn H. Modulation of the β-adrenergic response of cardiomyocytes by specific lipoxygenase products involves their incorporation into phosphatidylinositol and activation of protein kinase C. J Biol Chem. 1994;269:29055–29060. [PubMed] [Google Scholar]
- 64.Dransfeld O, Rakatzi I, Sasson S, Eckel J. Eicosanoids and the regulation of cardiac glucose transport. Ann N Y Acad Sci. 2002;967:208–216. doi: 10.1111/j.1749-6632.2002.tb04277.x. [DOI] [PubMed] [Google Scholar]
- 65.Dransfeld O, Rakatzi I, Sasson S, Gruzman A, Schmitt M, Haussinger D, et al. Eicosanoids participate in the regulation of cardiac glucose transport by contribution to a rearrangement of actin cytoskeletal elements. Biochem J. 2001;359:47–54. doi: 10.1042/0264-6021:3590047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wen Y, Gu J, Liu Y, Wang PH, Sun Y, Nadler JL. Overexpression of 12-lipoxygenase causes cardiac fibroblast cell growth. Circ Res. 2001;88:70–76. doi: 10.1161/01.res.88.1.70. [DOI] [PubMed] [Google Scholar]
- 67.Wen Y, Gu J, Peng X, Zhang G, Nadler J. Overexpression of 12-lipoxygenase and cardiac fibroblast hypertrophy. Trends Cardiovasc Med. 2003;13:129–136. doi: 10.1016/s1050-1738(03)00027-6. [DOI] [PubMed] [Google Scholar]
- 68.Zordoky BN, El-Kadi AO. Modulation of cardiac and hepatic cytochrome P450 enzymes during heart failure. Curr Drug Metab. 2008;9:122–128. doi: 10.2174/138920008783571792. [DOI] [PubMed] [Google Scholar]
- 69.Delozier TC, Kissling GE, Coulter SJ, Dai D, Foley JF, Bradbury JA, et al. Detection of human CYP2C8, CYP2C9, and CYP2J2 in cardiovascular tissues. Drug Metab Dispos. 2007;35:682–688. doi: 10.1124/dmd.106.012823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Granville DJ, Tashakkor B, Takeuchi C, Gustafsson AB, Huang C, Sayen MR, et al. Reduction of ischemia and reperfusion-induced myocardial damage by cytochrome P450 inhibitors. Proc Natl Acad Sci USA. 2004;101:1321–1326. doi: 10.1073/pnas.0308185100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsao CC, Coulter SJ, Chien A, Luo G, Clayton NP, Maronpot R, et al. Identification and localization of five CYP2Cs in murine extrahepatic tissues and their metabolism of arachidonic acid to regio- and stereoselective products. J Pharmacol Exp Ther. 2001;299:39–47. [PubMed] [Google Scholar]
- 72.Wang H, Zhao Y, Bradbury JA, Graves JP, Foley J, Blaisdell JA, et al. Cloning, expression, and characterization of three new mouse cytochrome p450 enzymes and partial characterization of their fatty acid oxidation activities. Mol Pharmacol. 2004;65:1148–1158. doi: 10.1124/mol.65.5.1148. [DOI] [PubMed] [Google Scholar]
- 73.Wu S, Moomaw CR, Tomer KB, Falck JR, Zeldin DC. Molecular cloning and expression of CYP2J2, a human cytochrome P450 arachidonic acid epoxygenase highly expressed in heart. J Biol Chem. 1996;271:3460–3468. doi: 10.1074/jbc.271.7.3460. [DOI] [PubMed] [Google Scholar]
- 74.Seubert J, Yang B, Bradbury JA, Graves J, Degraff LM, Gabel S, et al. Enhanced postischemic functional recovery in CYP2J2 transgenic hearts involves mitochondrial ATP-sensitive K+ channels and p42/p44 MAPK pathway. Circ Res. 2004;95:506–514. doi: 10.1161/01.RES.0000139436.89654.c8. [DOI] [PubMed] [Google Scholar]
- 75.Nithipatikom K, Gross ER, Endsley MP, Moore JM, Isbell MA, Falck JR, et al. Inhibition of cytochrome P450ω-hydroxylase: a novel endogenous cardioprotective pathway. Circ Res. 2004;95:e65–e71. doi: 10.1161/01.RES.0000146277.62128.6f. [DOI] [PubMed] [Google Scholar]
- 76.Seubert JM, Zeldin DC, Nithipatikom K, Gross GJ. Role of epoxyeicosatrienoic acids in protecting the myocardium following ischemia/reperfusion injury. Prostaglandins Other Lipid Mediat. 2007;82:50–59. doi: 10.1016/j.prostaglandins.2006.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nithipatikom K, Endsley MP, Moore JM, Isbell MA, Falck JR, Campbell WB, et al. Effects of selective inhibition of cytochrome P-450 ω-hydroxylases and ischemic preconditioning in myocardial protection. Am J Physiol Heart Circ Physiol. 2006;290:H500–H505. doi: 10.1152/ajpheart.00918.2005. [DOI] [PubMed] [Google Scholar]
- 78.Gross ER, Nithipatikom K, Hsu AK, Peart JN, Falck JR, Campbell WB, et al. Cytochrome P450 ω-hydroxylase inhibition reduces infarct size during reperfusion via the sarcolemmal KATP channel. J Mol Cell Cardiol. 2004;37:1245–1249. doi: 10.1016/j.yjmcc.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 79.Moffat MP, Ward CA, Bend JR, Mock T, Farhangkhoee P, Karmazyn M. Effects of epoxyeicosatrienoic acids on isolated hearts and ventricular myocytes. Am J Physiol. 1993;264:H1154–H1160. doi: 10.1152/ajpheart.1993.264.4.H1154. [DOI] [PubMed] [Google Scholar]
- 80.Xiao YF, Huang L, Morgan JP. Cytochrome P450: a novel system modulating Ca2+ channels and contraction in mammalian heart cells. J Physiol. 1998;508:777–792. doi: 10.1111/j.1469-7793.1998.777bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen J, Capdevila JH, Zeldin DC, Rosenberg RL. Inhibition of cardiac L-type calcium channels by epoxyeicosatrienoic acids. Mol Pharmacol. 1999;55:288–295. doi: 10.1124/mol.55.2.288. [DOI] [PubMed] [Google Scholar]
- 82.Lu T, Hoshi T, Weintraub NL, Spector AA, Lee HC. Activation of ATP-sensitive K+ channels by epoxyeicosatrienoic acids in rat cardiac ventricular myocytes. J Physiol. 2001;537:811–827. doi: 10.1111/j.1469-7793.2001.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee HC, Lu T, Weintraub NL, VanRollins M, Spector AA, Shibata EF. Effects of epoxyeicosatrienoic acids on the cardiac sodium channels in isolated rat ventricular myocytes. J Physiol. 1999;519:153–168. doi: 10.1111/j.1469-7793.1999.0153o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang T, Kohlhaas M, Backs J, Mishra S, Phillips W, Dybkova N, et al. CaMKIIδ isoforms differentially affect calcium handling but similarly regulate HDAC/MEF2 transcriptional responses. J Biol Chem. 2007;282:35078–35087. doi: 10.1074/jbc.M707083200. [DOI] [PubMed] [Google Scholar]
- 85.Xiao YF, Ke Q, Seubert JM, Bradbury JA, Graves J, Degraff LM, et al. Enhancement of cardiac L-type Ca2+ currents in transgenic mice with cardiac-specific overexpression of CYP2J2. Mol Pharmacol. 2004;66:1607–1616. doi: 10.1124/mol.104.004150. [DOI] [PubMed] [Google Scholar]
- 86.Lu T, Ye D, Wang X, Seubert JM, Graves JP, Bradbury JA, et al. Cardiac and vascular KATP channels in rats are activated by endogenous epoxyeicosatrienoic acids through different mechanisms. J Physiol. 2006;575:627–644. doi: 10.1113/jphysiol.2006.113985. [DOI] [PMC free article] [PubMed] [Google Scholar]