Abstract
Aims
The mechanisms involved in hypoxic pulmonary vasoconstriction (HPV) are not yet fully defined. The aim of the study was to determine the role of protein kinase C ζ (PKCζ) and neutral sphingomyelinase (nSMase) in HPV.
Methods and results
Ceramide content was measured by immunocytochemistry and voltage-gated potassium channel (KV) currents were recorded by the patch clamp technique in isolated rat pulmonary artery smooth muscle cells (PASMC). Contractile responses were analysed in rat pulmonary arteries mounted in a wire myograph. Pulmonary pressure was recorded in anesthetized open-chest rats. Protein and mRNA expression were measured by western blot and RT–PCR, respectively. We found that hypoxia increased ceramide content in PASMC which was abrogated by inhibition of nSMase, but not acid sphingomyelinase (aSMase). The hypoxia-induced vasoconstrictor response in isolated pulmonary arteries and the inhibition of KV currents were strongly reduced by inhibition of PKCζ or nSMase but not aSMase. The nSMase inhibitor GW4869 prevented HPV in vivo. The vasoconstrictor response to hypoxia was mimicked by exogenous addition of bacterial Smase and ceramide. nSMase2 mRNA expression was ∼10-fold higher in pulmonary compared with mesenteric arteries. In mesenteric arteries, hypoxia failed to increase ceramide but exogenous SMase induced a contractile response.
Conclusion
nSMase-derived ceramide production and the activation of PKCζ are early and necessary events in the signalling cascade of acute HPV.
Keywords: Hypoxic pulmonary vasoconstriction, Neutral sphingomyelinase, Protein kinase C ζ, Pulmonary arteries
1. Introduction
Hypoxic pulmonary vasoconstriction (HPV) is an adaptive physiological mechanism that optimizes blood oxygen saturation by increasing pulmonary vascular resistance in poorly aerated lung regions, thereby diverting pulmonary blood flow to the better ventilated ones.1–7 HPV reflects an intrinsic property of the small pulmonary artery (PA) smooth muscle cells (PASMC) in response to alveolar hypoxia. Generalized alveolar hypoxia associated with altitude, atelectasis, chronic obstructive pulmonary disease, or sleep apnea induces HPV which, if sustained, may lead to pulmonary hypertension and right ventricular failure. On the contrary, failure of HPV, as occurs in adult respiratory distress syndrome, pneumonia, sepsis, or liver cirrhosis is often a critical determinant of ventilation–perfusion mismatch and, hence, hypoxaemia.8
Despite intensive research, the molecular basis of HPV remains one of the most enduring mysteries of cell physiology and several, sometimes contradictory, hypotheses have emerged to explain it.1–7,9–12 Nevertheless, there is consensus about the involvement of a putative redox-based O2 sensor, i.e. mitochondrial electron transport chain2,4,5 and/or the membrane NADPH oxidase,5,7 regulating the activity of effector proteins and there is a large body of evidence indicating a role of the reactive oxygen species (ROS) as signalling intermediates. Voltage-gated K+ (KV) channels are known effector proteins which are inhibited by hypoxia, leading to cell membrane depolarization and opening of voltage-dependent L-type Ca2+ channels.1,7,9 Additional effectors include twin pore domain K+ channels whose inhibition also depolarizes the membrane, store-operated Ca2+ channels (SOCs) leading to increased capacitative Ca2+ entry and Rho kinase which is involved in Ca2+-sensitization.10–12
We have reported that protein kinase C ζ (PKCζ) is involved in the KV channel inhibition and the pulmonary vasoconstriction induced by the thromboxane A2 mimetic U46619.13 The role of this kinase, as well as its adaptor protein p62, was recently confirmed using PKCζ−/− and p62−/− mice.14 PKCζ is directly activated by ceramide,15 a sphingolipid-derived second messenger molecule. Ceramide is synthesized from membrane sphingomyelin by sphingomyelin phosphodiesterases (SMPD: SMPD1, SMPD2, SMPD3, and SMPD4), also known as acid or neutral sphingomyelinases (aSMase, nSMase1, nSMase2, and nSMase3, respectively) which are activated by multiple membrane receptors and non-receptor stimuli.16 SMases-derived ceramide production is an attractive candidate mechanism in HPV because aSMase and nSMase are activated by ROS16 and SMase and ceramide have been shown to inhibit KV channels.17,18
Herein, we show for the first time that the activation of nSMase and PKCζ are necessary events in the signalling of HPV.
2. Methods
The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996) and approved by our institutional review board. A detailed description of the experimental methods is available in previous studies.13,14,19,20 Third generation PA (250–450 µm) and mesenteric arteries of similar diameter were isolated from male Wistar rats and smooth muscle cells were enzymatically isolated.
2.1. Solutions and hypoxia
Hepes-buffered solution contained (in mmol/L): NaCl 130, KCl 5, MgCl2 1.2, CaCl2 1.5, glucose 10, HEPES 10, pH 7.3, and Krebs solution (in mmol/L): NaCl 118, KCl 4,75, NaHCO3 25, MgSO4 1,2, CaCl2 2,0, KH2PO4 1,2, and glucose 11 bubbled with 21% O2, 5%CO2. For the in vitro experiments, hypoxia was induced by bubbling the Hepes solution with 100% N2 or the Krebs solution with 95%N2–5% CO2 to achieve an oxygen concentration of 3–4% (24 ± 1 Torr) in the chamber. For the in vivo experiments, hypoxia was induced by switching the inspiratory input from room air (∼21% O2, normoxia) to a 10% O2–90% N2 gas.
2.2. Ceramide content
Freshly isolated cells adhered to gelatine-coated coverslips were perfused with Hepes solution for 15 min with or without inhibitors and subsequently with hypoxic solution during 0, 1, 3, or 5 min and rapidly fixed with 4% paraformaldehyde. Cells were stained using an anti-ceramide antibody (15B4) and then with donkey anti-rabbit FITC conjugated antibodies. Immunofluorescence was quantified using ImageJ (ver. 1.32j, NIH).
2.3. Contractile responses
PA rings were mounted in a wire myograph in Krebs solution and stretched to give an equivalent transmural pressure of 30 mmHg.21 Each vessel was exposed to three hypoxic challenges of 10 min duration each, leaving a 45 min incubation period in normoxia between hypoxic challenges. The third hypoxic response was examined after 45 min incubation with vehicle (control) or different inhibitors and the contractile responses were expressed as a percentage of the second exposure to hypoxia.
2.4. Pulmonary arterial pressure
Pressure was recorded with a pressure transducer in anesthetized (100 mg kg−1 ketamine plus 5 mg kg−1 diazepam) open chest rats via a catheter advanced through the right ventricle and placed into the main PA.
2.5. Electrophysiological studies
Membrane currents were recorded using the whole-cell configuration of the patch clamp technique. Cells were superfused with an external Ca2+-free Hepes solution and a Ca2+-free pipette (internal) solution containing (mmol/L): KCl 110, MgCl2 1.2, Na2ATP 5, HEPES 10, EGTA 10, pH adjusted to 7.3 with KOH. Currents were evoked following the application of 200 ms depolarizing pulses from −60 mV to test potentials from −60 mV to +60 mV in 10 mV increments.
2.6. Protein expression
Protein expression was quantified by western blotting using anti-Kv1.5 (Alomone, Israel), anti-PKCζ (Santa Cruz Biotechnology), anti-α-actin (Sigma) antibodies, or an affinity purified rabbit anti-nSMase2 antibody (prepared by Genscript). The synthetic oligopeptide used for immunization was CRRRHPDEAFDHEVS (identical to the 335–348 amino acids of rat nSMase2 plus an N-terminal cystein and similar to that used by Tani and Hannun22).
2.7. Real-time RT–PCR
Total RNA was isolated and purified from arterial homogenates using RNeasy Mini kit (Qiagen). Real-time PCR was performed using a Taqman system (Roche Diagnostics, Mannheim, Germany) in the Unidad de Genomica (Universidad Complutense de Madrid). Specific primers were designed for rat aSMase (right 5′- TTTCCCGAGCCCTGTAGA-3′ and left 5′-ATCTGACCCACGCCAATG-3′), nSMase1 (right 5′-CCCCGTCCACTCTTTCAGTA-3′ and left 5′- GTGCGGGGATCTCAACAT-3), nSMase2 (right 5′- TGAAAACATTATTGAGCCTTGC-3′ and left 5′-CTTTGCCACAGCCAATGTC-3′), and β-actin (right 5′-TCAGGCAGCTCATAGCTCTTC-3′ and left 5′- GCCCTAGACTTCGAGCAAGA-3′).
2.8. Statistical analysis
Data are expressed as means ± SEM; n indicates the number of animals, arteries, or cells tested. For multiple comparisons (e.g. the effects of various inhibitors against a control), statistical analysis was performed using a one-way ANOVA followed by a Bonferroni post hoc test, otherwise (e.g. control vs. single treatment) using a two-tailed Student's t-test for paired or unpaired observations. Differences were considered statistically significant when P < 0.05.
3. Results
3.1. Effects of hypoxia on ceramide production in isolated pulmonary artery smooth muscle cells
Exposure to hypoxia induced an increase in ceramide content in freshly isolated PASMC (Figure 1A) which was significant after 1 min of perfusion with the hypoxic solution and remained elevated for at least 5 min. We analysed the role of SMases using GW4869,23 a specific inhibitor of nSMase, and desipramine, specific for aSMase. GW4869, but not desipramine (Figure 1B), inhibited hypoxia-induced ceramide production, suggesting that hypoxia-induced ceramide production is derived from nSMase.
Figure 1.
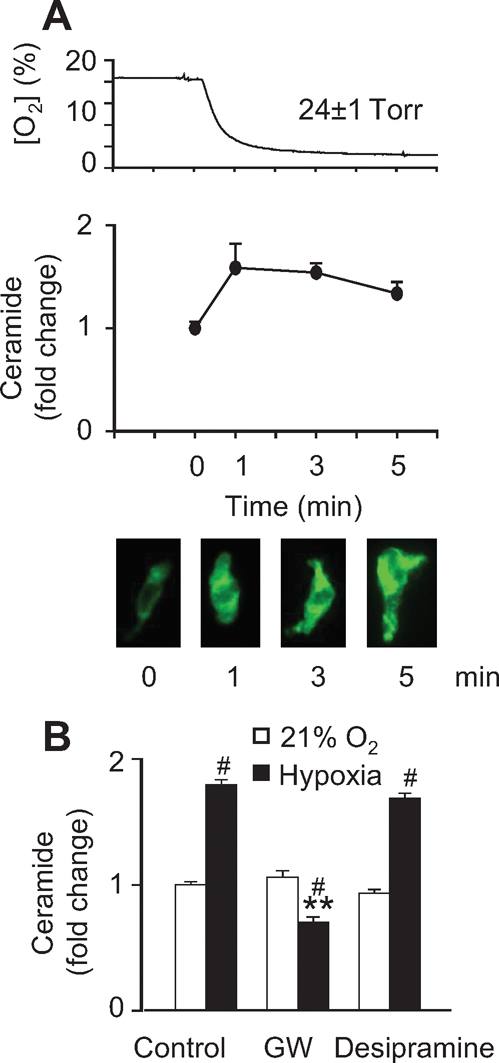
Hypoxia induces ceramide production by the activation of nSMase. (A) Top panel: time course of the decay in O2 as measured by a Clark electrode in the chamber. Medium panel: cellular ceramide content determined by immunostaining of PASMC with a monoclonal ceramide-specific antibody 15B4. Bottom panel: representative pictures of immunostained cells after 0, 1, 3, and 5 min of hypoxia. (B) Effects of 10 µmol/L GW4869 (GW) and 10 µmol/L desipramine, inhibitors of nSMase and aSMase, respectively, on ceramide production stimulated by 3 min hypoxia. Results are means ± SEM (averaged FITC-fluorescence intensity relative to cell surface measured in three to four coverslips with at least 10 cells each), # indicates P < 0.05 normoxia vs. hypoxia (unpaired t-test), **indicates P < 0.01 vs. control (ANOVA followed by a Bonferroni's test).
3.2. Role of protein kinase C ζ and nSMase in hypoxia-induced contraction in isolated pulmonary artery
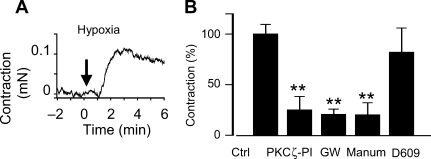
Hypoxia induced a contractile response in isolated small pulmonary arteries. In the absence of other vasoconstrictor agent, this response was small in magnitude but rapid, sustained, and reproducible (Figure 2A) and could also be observed in endothelium-denuded arteries (not shown). The magnitude of this response (0.08 ± 0.01 mN, n = 10) is approximately 20% of the maximal response induced by the thromboxane A2 mimetic U46619. This hypoxic contraction was strongly inhibited by the PKCζ pseudosubstrate inhibitory peptide (PKCζ-PI) (Figure 2B) suggesting a role for this kinase in HPV. GW4869 and the chemically unrelated nSMase inhibitor manumycin A also inhibited the hypoxic-induced contraction (Figure 1B). The effects of desipramine as aSMase inhibitor were not analysed because it is a known Ca2+ channel antagonist and inhibits agonist- and depolarization-induced contractions in vascular smooth muscle.24 However, D609,25 another inhibitor of aSMase, had no effect on hypoxic contraction (Figure 2B).
Figure 2.
Inhibition of protein kinase C ζ (PKCζ) and nSMase but not aSMase prevents hypoxia-induced vasoconstriction in pulmonary artery (PA) in vitro. (A) Time course of the contractile response in PA mounted in a wire myograph exposed to hypoxia at time 0 (representative tracing). (B) Effects of PKCζ-pseudosubstrate inhibitory peptide (10 µmol/L), the nSMase inhibitors GW4869 (10 µmol/L, GW), and manumycin A (5 µmol/L, Manum) or the aSMase inhibitor D609 (100 µmol/L) on the hypoxia-induced PA contraction. Results are means ± SEM. **indicates P < 0.01 vs. control (ANOVA followed by a Bonferroni's test).
3.3. Role of nSMase in hypoxic pulmonary vasoconstriction in vivo
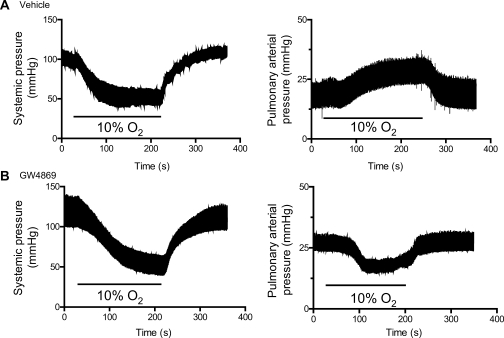
To analyse the role of nSMase in HPV in vivo, we recorded PA pressure via a catheter located in the PA through the right ventricle in open-chest ventilated rats. As expected, hypoxia (10% O2) led to a reversible decrease in systemic pressure and an increase in PA pressure, i.e. HPV (Figure 3A). Interestingly, in rats treated with GW4869, hypoxia not only failed to increase PA pressure but it even reduced it (Figure 3B). On average, hypoxia increased by 7.3 ± 1.3 mmHg (n = 9) and decreased by 5.3 ± 1.1 mmHg (n = 11) mean PA pressure in vehicle and GW4869-treated rats, respectively (P < 0.05 vehicle vs. GW4869, unpaired Student's t-test). The hypoxic-induced pulmonary decrease in PA pressure in GW4869-treated rats was reversible and reproducible (not shown). In other set of experiments in closed chest rats, GW4869 did not modify the hypoxia-induced decrease in systemic pressure (n = 5, Figure 3B).
Figure 3.
The nSMase inhibitor GW4869 reverses hypoxic pulmonary vasoconstriction in vivo. Recordings of systemic (left panel, in closed chest rats via a catheter in the carotid artery) and pulmonary (right panel, in open chest rats via a catheter advanced through the right ventricle into the main pulmonary artery) arterial pressure in anesthetized ventilated rats treated with DMSO (vehicle) (A) or 1 mg Kg−1 of GW4869 (B) administered intraperitoneally. Rats were exposed to alveolar hypoxia (10% O2) as indicated.
3.4. Effects of exogenous SMase
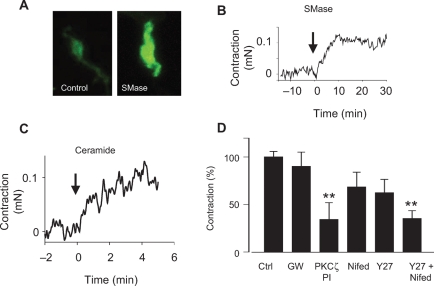
As expected, exogenous addition of SMase from Bacillus cereus, a homologue of mammalian nSMase, produced a marked increase in ceramide content in isolated PASMC (Figure 4A). Moreover, SMase mimicked the effects of hypoxia, i.e. it produced a sustained contractile response (Figure 4B) of a similar magnitude (0.10 ± 0.04 mN, n = 5) to the response induced by hypoxia. However, the time course of this contraction was slower than that induced by hypoxia. This response was also present in endothelium-denuded arteries (not shown). Exogenous addition of C6-ceramide also induced a contractile response (0.05 ± 0.01 mN, n = 32, Figure 4C). This response was inhibited by the PKCζ-PI but not by the nSMase inhibitor GW4869. The inhibitory effect of the Rho kinase inhibitor Y27632 and the L-type calcium channel blocker nifedipine on ceramide-induced contraction did not reach statistical significance, but the effects of both drugs combined were highly significant (Figure 4D).
Figure 4.
Exogenous addition of bacterial SMase reproduces the effects of hypoxia on ceramide content and induces a contractile response in isolated pulmonary artery (PA). (A) Effects of SMase (100 mU mL−1 from Bacillus cereus) on cellular ceramide content determined by immunostaining of pulmonary artery smooth muscle cells with a monoclonal ceramide-specific antibody 15B4. Cells were exposed to SMase for 15 min. (B and C) Time course of the contractile response in PA mounted in a wire myograph exposed to SMase (B) and C6-ceramide (C) at time 0 (representative tracings). (C) Effects of the nSMase inhibitor GW4869 (10 µmol/L, GW), protein kinase C ζ-pseudosubstrate inhibitory peptide (10 µmol/L), the L-type Ca2+ channel inhibitor nifedipine (1 µmol/L, Nifed), the Rho kinase inhibitor Y27632 (10 µmol/L), or the combination of Nifed +Y27632 on the contraction induced by C6-ceramide (10 µmol/L) in PA. Results are means ± SEM of 6–10 experiments (except controls where n = 32). * and ** indicates P < 0.05 and 0. 0.01, respectively, vs. control (ANOVA followed by a Bonferroni's test).
3.5. Hypoxic KV channel inhibition
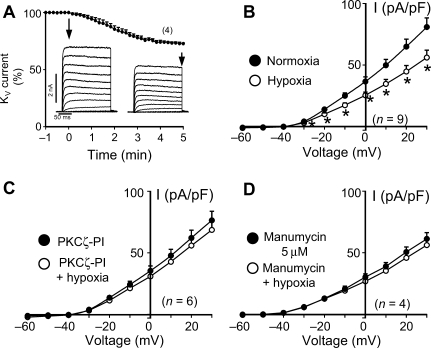
As expected,1,2 we found that hypoxia inhibited KV currents (Figure 5) with a similar time course to the hypoxia-induced ceramide production and contraction. These effects, as shown above for hypoxia-induced vasoconstriction, were prevented by PKCζ-PI (included in patch pipette solution) and manumycin A.
Figure 5.
Hypoxia-induced inhibition of KV currents in pulmonary artery smooth muscle cells (PASMC) is prevented by inhibition of protein kinase C ζ (PKCζ) and nSMase. (A) Time course of hypoxia-induced KV current inhibition as measured in the whole-cell configuration of the patch-clamp technique in PASMC (average percent change at +30 mV depolarizing pulses and representative current traces for 200 ms depolarization pulses from −60 to +60 mV in 10 mV increments from a holding potential of −60 mV before and after 5 min of hypoxia), (B–D) Current–voltage relationship calculated from experiments as in (A) performed in the absence (B) or in the presence of PKCζ-pseudosubstrate inhibitory peptide (0.1 µmol/L in the internal solution) (C) or manumycin (5 µmol/L) (D). Results are means ± SEM. *indicates P < 0.05 vs. control (paired t-test).
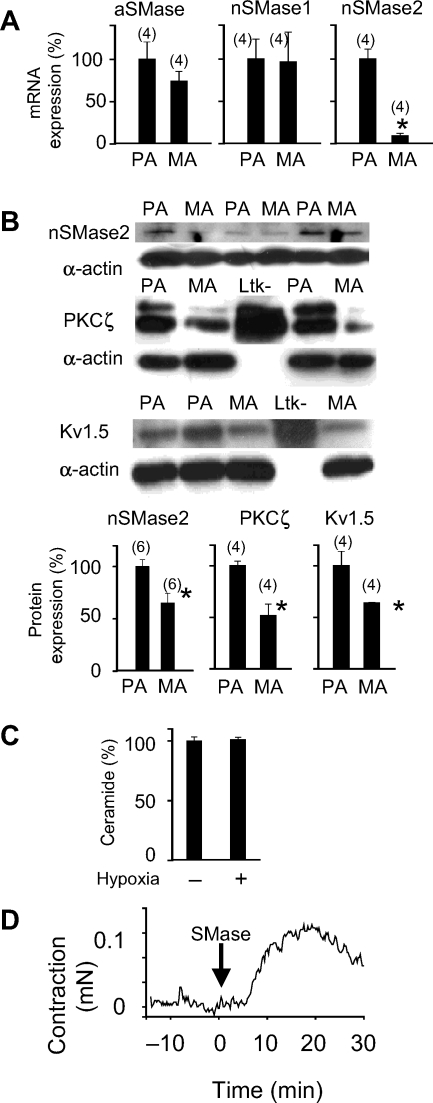
3.6. Pulmonary selectivity
The mRNAs of aSMase and nSMase1 were similarly expressed in PA and mesenteric arteries as measured by real-time RT–PCR (Figure 6A). However, the mRNA expression of nSMase2 was much higher in PA when compared with mesenteric arteries. The protein expression of nSMase2, PKCζ, and Kv1.5 was also higher in PA as measured by western blot (Figure 6B). In addition, hypoxia failed to generate ceramide (Figure 6C) in mesenteric arteries. However, exogenous SMase induced a contractile response in isolated mesenteric arteries which was similar to that induced in PA (Figure 6D).
Figure 6.
Pulmonary selectivity. (A) Expression of aSMase, nSMase1, and nSMase2 analysed by RT–PCR. Results are normalized to β-actin and expressed as a percent of mean values of PA. (B) Protein expression of nSMase2, protein kinase C ζ (PKCζ), and KV1.5 analysed by western blot in pulmonary artery (PA) and mesenteric arteries. Blots were re-probed with smooth muscle α-actin as a loading control. Homogenates of Ltk− cells expressing KV1.5 were also loaded in some gels as positive controls. Results are normalized to α-actin and expressed as a percent of mean values of PA. *indicates P < 0.05 vs. PA. (C) Ceramide production in mesenteric arteries, under normoxia (n = 14) and after 3 min hypoxia (n = 19), determined by immunostaining with a monoclonal ceramide-specific antibody (15B4). (D) Time course of the contractile response in mesenteric arteries mounted in a wire myograph exposed to SMase (100 mU mL−1 from Bacillus cereus) at time 0 (representative tracing). Results are means ± SEM, n is shown in parenthesis.
4. Discussion
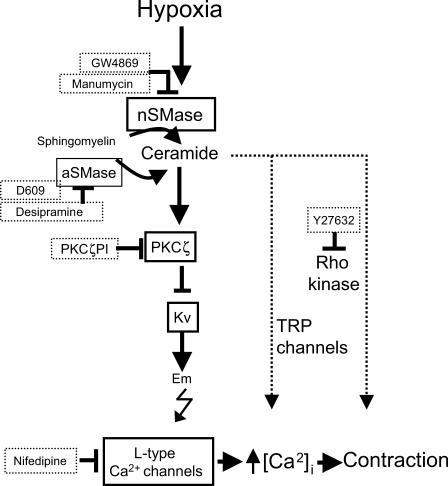
Herein we show that the activation of nSMase is required for acute HPV (Figure 7). The specific inhibitors of nSMase but not of aSMase strongly inhibited the constrictor response to hypoxia in small PA. The time course of ceramide generation induced by hypoxia in isolated PASMC and the mimicking effect of exogenous addition of SMase or ceramide are consistent with this view. These events were observed in non-genetically modified systems in vitro (rat PA or freshly isolated PASMC) and the role of nSMase was also confirmed in vivo by using the specific inhibitor GW4869. The hypoxia-induced increase in ceramide was not observed in mesenteric arteries. In addition, the expression of nSMase2 as well as other signalling proteins PKCζ and KV1.5 was higher in small PA vs. mesenteric arteries.
Figure 7.
Diagram illustrating the proposed signalling cascade involved in hypoxic pulmonary vasoconstriction. The drugs used in the present work as well as their targets are also shown.
HPV in vivo is a rapid and sustained response. Because HPV responses are more readily and consistently observed when the small PAs are pre-constricted, the addition of a pre-constrictor agent has been a common practice in many studies analysing HPV in vitro. In pre-constricted rat PA, hypoxia produces a tri-phasic response, with an initial vasoconstriction followed by vasodilation and a late slow-developing contraction. However, under the right conditions of stretch, a sustained constrictor response to hypoxia has been reported with no added constrictor agent.21 In order to avoid a possible influence of a pre-constriction agent on the intracellular signalling mediating HPV, in the present study hypoxic contractions were carried out in the absence of an agonist.
Several broad inhibitors of PKC isoforms have demonstrated to be effective in inhibiting HPV in isolated perfused lungs.26 PKCζ is activated by hypoxia in alveolar epithelial cells27 and is a known modulator of KV currents in PA.13,14 Our data showing that PKCζ-PI prevented the vasoconstriction induced by hypoxia strongly suggest that PKCζ is involved in the signalling pathway of HPV.
PKCζ is directly activated by ceramide.15 Herein, we show that hypoxia induced a rapid increase in ceramide content. The role of nSMase as a source of ceramide was confirmed using the specific inhibitor GW4869,23 whereas inhibition of aSMase with desipramine was without effect. GW4869 and the chemically unrelated nSMase inhibitor manumycin (but not the aSMase inhibitor D609) inhibited the vasoconstrictor response induced by hypoxia in isolated PA. Moreover, the vasoconstrictor response to hypoxia was mimicked by exogenous addition of bacterial SMase or ceramide. The effects of ceramide were prevented by PKCζ-PI. However, GW4869 did not modify ceramide-induced contractions suggesting that the effects of GW4869 are related to the inhibition of nSMase and not to a non-specific effect on smooth muscle contraction. Interestingly, GW4869 completely abolished the hypoxia-induced increase in PA pressure in vivo. Moreover, after nSMase inhibition, hypoxia induced a reversible and reproducible decrease in pulmonary arterial pressure, i.e. a characteristic feature of systemic vascular beds. Taken together, these results indicate that nSMase plays a fundamental role in HPV.
KV channels are known targets of hypoxia in PA.1,2 Hypoxia inhibited KV currents in PASMC and this effect was prevented by PKCζ-PI and manumycin A, implying that hypoxic regulation of KV channels is mediated via PKCζ and nSMase. However, our data do not exclude that other effector proteins such as Rho kinase, twin pore domain K+, or voltage-independent Ca2+ channels, possibly regulated via nSMAse-derived ceramide, might also be involved in HPV.11,12,28 Accordingly, examples of Rho kinase or SOC activation by ceramide have been reported in other cell types.29,30 Herein we also show that the contractile responses induced by ceramide were inhibited by the Rho kinase inhibitor Y27632 and the Ca2+ channel blocker nifedipine.
Because vasoconstriction induced by hypoxia is a unique property of the small pulmonary vasculature,1–7 the potential mechanisms involved in HPV should be also exclusive to small PA. Thus, we analysed the expression of signalling proteins and the functional differences between PA and mesenteric arteries as prototype systemic arteries. The failure of isolated smooth muscle cells from mesenteric arteries to generate ceramide in response to hypoxia indicates that hypoxic activation of nSMAse is specific of PA. This specificity may be related in part to a higher expression of nSMase2 in small PA. However, it seems also possible that differences in the upstream mechanisms activating nSMase (e.g. the oxygen sensor or the redox response to hypoxia) may account for the pulmonary specificity. The expression of other signalling proteins involved, i.e. PKCζ and KV1.5 (a specific KV channel protein suggested to be involved in HPV31), was also higher in PA compared with mesenteric arteries. However, exogenous addition of SMase also induced a contractile response in mesenteric arteries suggesting that pulmonary selectivity is related to differences in the activation of nSMase rather than in the response to SMase.
Generalized alveolar hypoxia associated with altitude, atelectasis, chronic obstructive pulmonary disease, or sleep apnea leads to HPV and, hence, pulmonary hypertension.8 The present study identifies nSMase and PKCζ as two novel mediators of acute HPV. It is noteworthy that nSMase does not appear to play a role in the control of systemic vascular tone as opposed to its downstream effectors KV or L-type Ca2+ channels, suggesting that their inhibitors may selectively inhibit HPV without inducing systemic hypotension, a common side effect of vasodilators used in pulmonary hypertension. Interestingly, aSMase and PKCζ have been also proposed as therapeutic targets in acute lung injury25 and asthma,32 respectively.
Funding
This work was supported by the Spanish Ministerio de Ciencia e Innovación [research grants SAF2005-03770 to F.P.-V., SAF2008-03948 to F.P.-V., AGL2004-06685 to F.P.-V., BFU2007-61848 to C.G., and predoctoral grants: J.M.-S. and G.F. (FPU grants) and C.M. (FPI grant)], by Instituto de Salud Carlos III (CibeRes to F.P.-V. and C.G.) and by Fundación Mutua Madrileña to A.C. L.M. is funded by a Marie Curie grant. Funding to Pay the Open Access Charges for this article was provided by Ciberes.
Acknowledgements
The authors are grateful to Enrique Moreno and Ana Isabel Merino for excellent technical assistance.
Conflict of interest: The authors declare no competing financial interests.
References
- 1.Yuan X-J, Goldman W, Tod ML, Rubin LJ, Blaustein MP. Hypoxia reduces potassium currents in cultured rat pulmonary but not mesenteric arterial myocytes. Am J Physiol. 1993;264:L116–L123. doi: 10.1152/ajplung.1993.264.2.L116. [DOI] [PubMed] [Google Scholar]
- 2.Archer SL, Huang J, Henry T, Peterson D, Weir EK. A redox-based O2 sensor in rat pulmonary vasculature. Circ Res. 1993;73:1100–1112. doi: 10.1161/01.res.73.6.1100. [DOI] [PubMed] [Google Scholar]
- 3.Liu JQ, Sham JS, Shimoda LA, Kuppusamy P, Sylvester JT. Hypoxic constriction and reactive oxygen species in porcine distal pulmonary arteries. Am J Physiol Lung Cell Mol Physiol. 2003;285:L322–L333. doi: 10.1152/ajplung.00337.2002. [DOI] [PubMed] [Google Scholar]
- 4.Waypa GB, Chandel NS, Schumacker PT. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res. 2001;88:1259–1266. doi: 10.1161/hh1201.091960. [DOI] [PubMed] [Google Scholar]
- 5.Weissmann N, Zeller S, Schafer RU, Turowski C, Ay M, Quanz K, et al. Impact of mitochondria and NADPH oxidases on acute and sustained hypoxic pulmonary vasoconstriction. Am J Respir Cell Mol Biol. 2006;34:505–513. doi: 10.1165/rcmb.2005-0337OC. [DOI] [PubMed] [Google Scholar]
- 6.Killilea DW, Hester R, Balczon R, Babal P, Gillespie MN. Free radical production in hypoxic pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L408–L412. doi: 10.1152/ajplung.2000.279.2.L408. [DOI] [PubMed] [Google Scholar]
- 7.Marshall C, Mamary AJ, Verhoeven AJ, Marshall BE. Pulmonary artery NADPH-oxidase is activated in hypoxic pulmonary vasoconstriction. Am J Respir Cell Mol Biol. 1996;15:633–644. doi: 10.1165/ajrcmb.15.5.8918370. [DOI] [PubMed] [Google Scholar]
- 8.Marshall BE, Hanson CW, Frasch F, Marshall C. Role of hypoxic pulmonary vasoconstriction in pulmonary gas exchange and blood flow distribution. 2. Pathophysiology. Intensive Care Med. 1994;20:379–389. doi: 10.1007/BF01720916. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Barneo J, Lopez-Lopez JR, Urena J, Gonzalez C. Chemotransduction in the carotid body: K+ current modulated by PO2 in type I chemoreceptor cells. Science. 1988;241:580–582. doi: 10.1126/science.2456613. [DOI] [PubMed] [Google Scholar]
- 10.Gurney AM, Joshi S. The role of twin pore domain and other K+ channels in hypoxic pulmonary vasoconstriction. Novartis Found Symp. 2006;272:218–228. [PubMed] [Google Scholar]
- 11.Robertson TP, Hague D, Aaronson PI, Ward JP. Voltage-independent calcium entry in hypoxic pulmonary vasoconstriction of intrapulmonary arteries of the rat. J Physiol. 2000;525:669–680. doi: 10.1111/j.1469-7793.2000.t01-1-00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robertson TP, Dipp M, Ward JP, Aaronson PI, Evans AM. Inhibition of sustained hypoxic vasoconstriction by Y-27632 in isolated intrapulmonary arteries and perfused lung of the rat. Br J Pharmacol. 2000;131:5–9. doi: 10.1038/sj.bjp.0703537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cogolludo A, Moreno L, Bosca L, Tamargo J, Perez-Vizcaino F. Thromboxane A2-induced inhibition of voltage-gated K+ channels and pulmonary vasoconstriction. Role of protein kinase Cζ. Circ Res. 2003;93:656–663. doi: 10.1161/01.RES.0000095245.97945.FE. [DOI] [PubMed] [Google Scholar]
- 14.Moreno L, Frazziano G, Cogolludo A, Cobeno L, Tamargo J, Perez-Vizcaino F. Role of PKCζ and its adaptor protein p62 in KV channel modulation in pulmonary arteries. Mol Pharmacol. 2007;72:1301–1309. doi: 10.1124/mol.107.037002. [DOI] [PubMed] [Google Scholar]
- 15.Bourbon NA, Yun J, Kester M. Ceramide directly activates protein kinase C zeta to regulate a stress-activated protein kinase signaling complex. J Biol Chem. 2000;275:35617–35623. doi: 10.1074/jbc.M007346200. [DOI] [PubMed] [Google Scholar]
- 16.Gulbins E, Li PL. Physiological and pathophysiological aspects of ceramide. Am J Physiol Regul Integr Comp Physiol. 2006;290:R11–R26. doi: 10.1152/ajpregu.00416.2005. [DOI] [PubMed] [Google Scholar]
- 17.Gulbins E, Szabo I, Baltzer K, Lang F. Ceramide-induced inhibition of T lymphocyte voltage-gated potassium channel is mediated by tyrosine kinases. Proc Natl Acad Sci USA. 1997;94:7661–7666. doi: 10.1073/pnas.94.14.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Y, Ramu Y, Lu Z. Removal of phospho-head groups of membrane lipids immobilizes voltage sensors of K+ channels. Nature. 2008;451:826–829. doi: 10.1038/nature06618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cogolludo A, Moreno L, Lodi F, Frazziano G, Cobeno L, Tamargo J, et al. Serotonin inhibits voltage-gated K+ currents in pulmonary artery smooth muscle cells: role of 5-HT2A receptors, caveolin-1, and KV1.5 channel internalization. Circ Res. 2006;98:931–938. doi: 10.1161/01.RES.0000216858.04599.e1. [DOI] [PubMed] [Google Scholar]
- 20.Cogolludo A, Frazziano G, Cobeno L, Moreno L, Lodi F, Villamor E, et al. Role of reactive oxygen species in Kv channel inhibition and vasoconstriction induced by TP receptor activation in rat pulmonary arteries. Ann N Y Acad Sci. 2006;1091:41–51. doi: 10.1196/annals.1378.053. [DOI] [PubMed] [Google Scholar]
- 21.Ozaki M, Marshall C, Amaki Y, Marshall BE. Role of wall tension in hypoxic responses of isolated rat pulmonary arteries. Am J Physiol. 1998;275:L1069–L1077. doi: 10.1152/ajplung.1998.275.6.L1069. [DOI] [PubMed] [Google Scholar]
- 22.Tani M, Hannun YA. Neutral sphingomyelinase 2 is palmitoylated on multiple cysteine residues. Role of palmitoylation in subcellular localization. J Biol Chem. 2007;282:10047–10056. doi: 10.1074/jbc.M611249200. [DOI] [PubMed] [Google Scholar]
- 23.Luberto C, Hassler DF, Signorelli P, Okamoto Y, Sawai H, Boros E, et al. Inhibition of tumor necrosis factor-induced cell death in MCF7 by a novel inhibitor of neutral sphingomyelinase. J Biol Chem. 2002;277:41128–41139. doi: 10.1074/jbc.M206747200. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez del Pozo B, Perez-Vizcaino F, Fernandez C, Zaragoza F, Tamargo J. Effects of several class I antiarrhythmic drugs on isolated rat aortic vascular smooth muscle. Gen Pharmacol. 1997;29:539–543. doi: 10.1016/s0306-3623(96)00517-4. [DOI] [PubMed] [Google Scholar]
- 25.Goggel R, Winoto-Morbach S, Vielhaber G, Imai Y, Lindner K, Brade L, et al. PAF-mediated pulmonary edema: a new role for acid sphingomyelinase and ceramide. Nat Med. 2004;10:155–160. doi: 10.1038/nm977. [DOI] [PubMed] [Google Scholar]
- 26.Barman SA. Effect of protein kinase C inhibition on hypoxic pulmonary vasoconstriction. Am J Physiol Lung Cell Mol Physiol. 2001;280:L888–L895. doi: 10.1152/ajplung.2001.280.5.L888. [DOI] [PubMed] [Google Scholar]
- 27.Dada LA, Chandel NS, Ridge KM, Pedemonte C, Bertorello AM, Sznajder JI. Hypoxia-induced endocytosis of Na,K-ATPase in alveolar epithelial cells is mediated by mitochondrial reactive oxygen species and PKC-zeta. J Clin Invest. 2003;111:1057–1064. doi: 10.1172/JCI16826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weissmann N, Dietrich A, Fuchs B, Kalwa H, Ay M, Dumitrascu R, et al. Classical transient receptor potential channel 6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and alveolar gas exchange. Proc Natl Acad Sci USA. 2006;103:19093–19098. doi: 10.1073/pnas.0606728103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang J, Xie M, Shah VR, Schneider MD, Entman ML, Wei L, et al. Activation of Rho-associated coiled-coil protein kinase 1 (ROCK-1) by caspase-3 cleavage plays an essential role in cardiac myocyte apoptosis. Proc Natl Acad Sci USA. 2006;103:14495–14500. doi: 10.1073/pnas.0601911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colina C, Flores A, Rojas H, Acosta A, Castillo C, Garrido Mdel R, et al. Ceramide increase cytoplasmic Ca2+ concentration in Jurkat T cells by liberation of calcium from intracellular stores and activation of a store-operated calcium channel. Arch Biochem Biophys. 2005;436:333–345. doi: 10.1016/j.abb.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 31.Platoshyn O, Brevnova EE, Burg ED, Yu Y, Remillard CV, Yuan JX. Acute hypoxia selectively inhibits KCNA5 channels in pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol. 2006;290:C907–C916. doi: 10.1152/ajpcell.00028.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin P, Villares R, Rodriguez-Mascarenhas S, Zaballos A, Leitges M, Kovac J, et al. Control of T helper 2 cell function and allergic airway inflammation by PKCzeta. Proc Natl Acad Sci USA. 2005;102:9866–9871. doi: 10.1073/pnas.0501202102. [DOI] [PMC free article] [PubMed] [Google Scholar]