Abstract
Heart failure (HF) afflicts about 5 million people and causes 300 000 deaths a year in the United States alone. An integral part of the pathogenesis of HF is cardiac remodelling, and the signalling events that regulate it are a subject of intense research. Cardiac remodelling is the sum of responses of the heart to causes of HF, such as ischaemia, myocardial infarction, volume and pressure overload, infection, inflammation, and mechanical injury. These responses, including cardiomyocyte hypertrophy, myocardial fibrosis, and inflammation, involve numerous cellular and structural changes and ultimately result in a progressive decline in cardiac performance. Pharmacological and genetic manipulation of cultured heart cells and animal models of HF and the analysis of cardiac samples from patients with HF are all used to identify the molecular and cellular mechanisms leading to the disease. Protein kinase C (PKC) isozymes, a family of serine–threonine protein kinase enzymes, were found to regulate a number of cardiac responses, including those associated with HF. In this review, we describe the PKC isozymes that play critical roles in specific aspects of cardiac remodelling and dysfunction in HF.
Keywords: Protein kinase C, Heart failure, Cardiac remodeling, Hypertrophy, Fibrosis and inflammation
1. Protein kinase C: an introduction
Protein kinase C (PKC) is a group of closely related serine–threonine protein kinases, further classified as (1) the classical PKCs (α, βI, βII, and γ), the diacylglycerol (DAG)-, and calcium-dependent enzymes, (2) the novel PKCs (δ, ϵ, θ, and η), which require DAG, but not calcium, for activity, and (3) the atypical PKCs (ζ, λ), which are not stimulated by DAG or calcium, but are stimulated by other lipid-derived second messengers.
1.1. Protein kinase C in the normal and diseased myocardium
PKC isozymes are expressed in all tissues. mRNA expression of α, δ, ϵ, η, and ζPKCs is found in rat cultured cardiomyocytes.1,2 Abundant expression of both βI and βIIPKC in human and rat cardiomyocytes has also been reported,3–6 whereas the mouse myocardium expresses low levels of these βPKCs.7 Further species-specific differences in the expression of η, θ, and ϵPKC were also reported.8 Therefore, the interpretation of animal studies must be done with caution as species to species variation in PKC isozyme expression is substantial. Western blot analyses of human cardiac tissue using polyclonal antibodies (PKC-α, -βI, -βII -ε, -δ,-γ, and -η) or monoclonal antibodies (PKC-λ, -μ, and -θ) demonstrated the presence of these isozymes in human heart tissue.4 This study also demonstrated differences in the distribution of PKC isozymes between the atria and ventricles. The calcium-dependent isozymes, α, βI, and βIIPKC, reside predominantly in the ventricle, whereas δ and ζPKC are mainly expressed in the atria and ε and λPKC are evenly distributed in both atria and ventricles.
PKC isozymes are involved in a variety of chronic cardiac diseases9 as well as in acute cardiac injuries and preconditioning.10 We and others have demonstrated that select PKC isozymes contribute to heart failure (HF).9,11–16 Isozyme-selective tools that were generated in the past few years, including pharmacological peptide regulators (Figure 1) and use of genetic manipulation and RNAi, demonstrated that the same isozyme may mediate different functions in acute vs. chronic heart diseases. For example, εPKC activation prior to MI is protective,17 whereas in hypertension-induced HF εPKC activation is detrimental.15 Here we review the role of PKC isozymes in cardiac remodelling and HF.
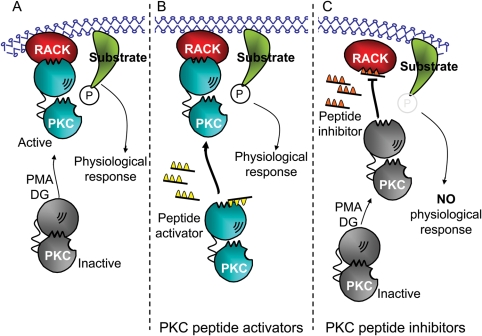
Figure 1.
Protein kinase C peptide modulators. (A) Inactive protein kinase C (gray) undergoes a conformational change exposing both the RACK-binding site and the active site when diacylglycerol (DG) or PMA are elevated. Active protein kinase C (blue) binds to its RACK (red), anchoring the activated isozyme near its substrate (green). Phosphorylation (P) of that substrate leads to the physiological responses of that isozyme. (B) Alternatively, a peptide that mimics the RACK-binding site, pseudo-RACK (ΨRACK, yellow) can also cause these conformational changes. ΨRACK binds to protein kinase C with a lower affinity than the intact RACK and thus does not always occupy the RACK-binding site on the enzyme. During the time that the peptide is not bound, the activated enzyme may bind to its RACK (red), resulting in anchoring of the activated isozyme near its substrate (green) followed by substrate phosphorylation (P) and physiological responses. This process is isozyme-specific. (C) A peptide corresponding to the RACK-biding site on protein kinase C (orange) inhibits translocation and function of its corresponding isozyme. The translocation inhibitor peptide binds to the RACK and blocks binding of the activated isozyme to that RACK. Therefore, the physiological responses mediated by that isozyme are blocked.
2. Heart failure: an introduction
HF is a clinical syndrome characterized by impaired ability of the left ventricle to fill or eject blood.18 Currently, the life-time risk to develop HF after the age of 40 is ∼20% for men or women.19 The aetiology of HF is diverse and includes ischaemia, hypertension, idiopathic cardiomyopathy, bacterial endocarditis, congenital cardiovascular defects, and valvular diseases. However, the most common aetiologies of HF are coronary artery disease and myocardial infarction (MI);20 each year, about one million people suffer from acute MI and within 6 years, HF will disable about 300 000 of these patients.19
2.1. Cardiac remodelling
Cardiac remodeling is an early and progressive response of the heart to insults, such as ischaemia, volume and pressure overload, infection, inflammation, mechanical injury, and stimulation by cytokines and enzymes. Depending on the extent of cardiomyocyte loss by these insults, fibroblast proliferation and collagen secretion, namely fibrosis, is triggered to maintain the shape and structure of the myocardium. Injuries to coronary vasculature, stimulation by stress factors, and fibrosis also result in changes in structure and function of blood vessels. Infiltration of inflammatory cells into the jeopardized myocardium leads to continuous release of cytokines, chemokines, enzymes, and growth factors, which further contribute to the remodelling process. Therefore, a better understanding of the cellular and molecular basis of cardiac remodelling events, including adaptive and maladaptive hypertrophy, perivascular and interstitial fibrosis, and inflammation, will further clarify the pathogenesis of HF.
3. Cardiac hypertrophy: adaptive and maladaptive responses
Adaptive cardiac hypertrophy is characterized by an increase in heart mass and wall thickening due to an increase in cardiac myocyte size and protein synthesis, and is associated with improved cardiac function.21 If cardiac overload continues, a transition from adaptive to maladaptive hypertrophy takes place. This is associated with left ventricle dilation, a decrease in contractile elements, and reduced cardiac output.21 The use of culture studies, animal models, and human samples with HF provided insight into the role of PKC in this pathology.
3.1. Protein kinase C isozymes in cardiomyocyte hypertrophy in cell culture models
Neonatal cardiomyocytes are commonly used to study hypertrophic signalling. Whether this model represents developmental hypertrophy only or whether it also provides an appropriate model of pathological hypertrophy is debated. However, because cardiac remodelling activates many of the cardiac embryonic developmental programs, this culture model may provide an insight into HF. A variety of stimulants, such as phorbol myristate acetate (PMA), angiotensin-II (AngII), phenylephrine (PE), and endothelin-1 (ET1), are used to induce hypertrophy in culture (Table 1). PMA, which activates both conventional and novel PKC isozymes, or transfection of either wild-type (WT) or dominant-negative (DN) αPKC mutant demonstrated that αPKC is both necessary and sufficient to induce certain features of cardiomyocyte hypertrophy including increases in protein synthesis, the protein-to-DNA ratio, and cell surface area.22 Further, αPKC antisense treatment reduced PE-induced increases in α-actin mRNA and atrial natriuretic peptide (ANP) secretion, but not PE-induced β-myosin heavy chain, ANP, or B-type natriuretic peptide (BNP) gene expression,23 therefore causing a loss of only some to the pathological hypertrophic markers. In other studies, overexpression of αPKC increased cell surface area, [3H]-leucine incorporation, and mRNA levels of ANP,24 together indicating that αPKC activation induced cardiomyocyte hypertrophy in cultured cardiomyocytes. We found that βI and βIIPKC are required for PMA-induced cardiomyocyte hypertrophy.25 Later, we substantiated that RBCK-1 (RBCC protein that interacts with βIPKC) is essential for PE-induced cardiomyocyte hypertrophy.26 Overexpression of RBCK1 increased the cardiac myocyte cell surface by 50% in the absence of PE treatment and the βI- and βIIPKC-specific peptide inhibitors prevented that effect.26 A role for εPKC has also been suggested; treatment with εPKC antisense reduced myotrophin-induced stimulation of protein synthesis in neonatal myocytes.27 This study also found that δ and ζPKC are not involved in this process. Therefore, at least four PKC isozymes, α, βI, βII, and ε induce hypertrophy in neonatal cardiac myocytes.
Table 1.
The role of individual protein kinase C isozymes in cardiac remodelling and heart failure
| Model | Cardiac phenotype | PKC isozyme | Stimulus/treatment | Main response | Reference |
|---|---|---|---|---|---|
| Streptozotocin-induced diabetic rats | Hypertrophy | cPKCs | Increased cardiac ßIIPKC activity | 86 | |
| Transgenic mice | Hypertrophy | εPKC | Cardiac-specific expression of εPKC inhibitor, εV1 | Lethal dilated cardiomyopathy | 36 |
| Transgenic mice | Hypertrophy | εPKC | Cardiac-specific expression of εPKC activator, ψεRACK | Concentric cardiac hypertrophy | 36 |
| Transgenic mice | Hypertrophy | εPKC | Over-expression constitutively active εPKC | Concentric cardiac hypertrophy | 87 |
| Transgenic mice | Hypertrophy | cPKCs and nPKCs | Active calcineurin over-expression | Increased cardiac α and βPKC translocation | 88 |
| Pressure-overload aortic banding rats | Hypertrophy | cPKCs and nPKCs | Increased cardiac βIPKC, βIIPKC, εPKC, and θPKC translocation | 89 | |
| Transgenic mice | Hypertrophy | βIIPKC and εPKC | Over-expression constitutively active εPKC | Pathological cardiac hypertrophy | 90 |
| Adult rat ventricle myocyte | Hypertrophy | εPKC | Pharmacological: εV1-2 (specific εPKC isozyme inhibitor) | Attenuated isoproterenol-induced apoptosis | 91 |
| Dahl salt-sensitive hypertensive rats | Hypertrophy | cPKCs and nPKCs | Increased cardiac εPKC levels in compensatory stage and βIIPKC levels during cardiac dysfunction | 14 | |
| Pressure-overload aortic banding rats | Hypertrophy | αPKC and δPKC | Increased cardiac levels of αPKC and δPKC | 92 | |
| Adult guinea pig heart (ex vivo) | Hypertrophy | cPKCs and nPKCs | Perfusion with angiotensin II | Increased cardiac αPKC, βIIPKC, and γPKC | 93 |
| Dahl Salt hypertensive rats | Hypertrophy/heart failure | cPKCs, nPKCs, and aPKCs | Increased cardiac levels of cPKC, nPKC, and aPKC | 94 | |
| Pressure-overload heart failure rats | Hypertrophy/heart failure | αPKC and εPKC | Sustained treatment with ACE inhibitor | ACE inhibitor attenuates increased αPKC and εPKC translocation | 95 |
| βIIPKC transgenic mice | Hypertrophy/heart Failure | βIIPKC | Cardiac-specific βIIPKC over-expression | Pathological cardiac hypertrophy | 32 |
| αPKC transgenic mice | Hypertrophy/heart failure | αPKC | Wild-type and dominant negative αPKC expression | Increased contractility and cardioprotection | 29,96 |
| Human end-stage dilated or ischaemic cardiomyopathy | Heart failure | cPKCs | Increased cardiac βPKC activity | 3 | |
| Human end-stage Dilated cardiomyopathy | Heart failure | cPKCs and nPKCs | Increased cardiac βIIPKC levels | 4 | |
| Dahl Salt hypertensive rats | Heart failure | cPKCs and nPKCs | Increased cardiac βIPKC, βIIPKC levels, and translocation | 14 | |
| MLP transgenic mice | Heart failure | Ro-31-8220 (cPKC and εPKC inhibitors) | PKC inhibition reverted cardiac hypertrophy | 34 | |
| Dahl Salt hypertensive rats | Fibrosis/heart failure | εPKC | Sustained treatment with εV1-2 (specific εPKC inhibitor) | Decreased cardiac fibrosis | 15 |
| βIIPKC transgenic mice | Hypertrophy/fibrosis | βIIPKC | Cardiac-specific over-expression of βIIPKC | Pathological cardiac hypertrophy and fibrosis | 11 |
| Pressure-overload aortic banding mice | Fibrosis | δPKC and εPKC | εPKC knock-out mouse | Increased fibrosis | 54 |
| Neonatal rat cardiac fibroblast | Fibroblast proliferation | δPKC and ζPKC | TGFβ1 and isozyme-specific inhibitors | Stimulated cardiac fibroblast proliferation | 39 |
| Neonatal rat cardiac fibroblast | Fibroblast proliferation | cPKC, nPKC, and aPKC | Endothelin-1 | Increased cardiac fibroblast proliferation | 49 |
| Adult rat cardiac fibroblast | Fibroblast proliferation | δPKC | Angiotensin II | Increased δPKC and fibroblast proliferation | 97 |
| Adult rat cardiac fibroblast | Collagen synthesis | cPKCs and nPKC | Mechanical load and non-specific PKC inhibitor (RO-31-8220) | Non-specific PKC inhibition and decreased collagen synthesis | 98 |
| Myocardial infarction-induced heart failure rats | Fibroblast proliferation | αPKC, βIPKC, and βIIPKC | Non-specific PKC inhibitor (LY333531) | Decreased fibrosis and TGFβ1 expression | 12 |
PKC, protein kinase C.
3.2. Protein kinase C in cardiac hypertrophy of animal models
αPKC expression and activity were unaltered in early HF but were up-regulated in two distinct rat models of end-stage HF.28 Depletion of myocardial αPKC by gene knock-out increased myocardial contractility, whereas transgenic overexpression of αPKC led to marked ventricular dysfunction and alterations in Ca2+ homeostasis.29 Phosphorylation studies suggest that αPKC depresses myofilament contractility through phosphorylation of cTnI and/or cTnT.30 Skinned left-ventricular myocytes isolated from rats subjected to chronic (8–9 months) pressure overload or MI-induced HF in rats supported these conclusions; myofilament function is severely depressed in these experimental HF models.28
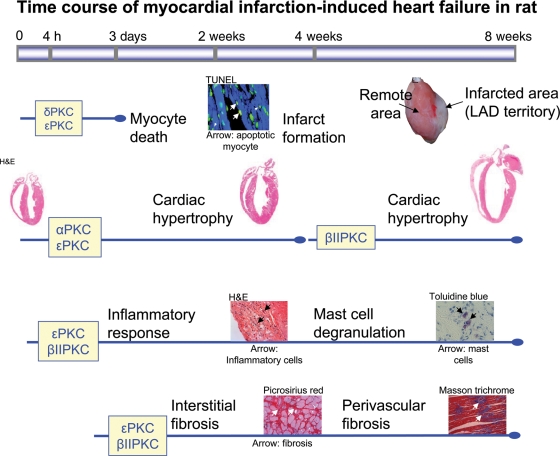
In an earlier study, βPKC levels were elevated in hypertension-induced HF rats.14 Treatment with angiotensin-receptor blocker improved cardiac function and decreased βPKC activation and levels.14 In a recent study, we found that a selective inhibition of βIIPKC improved cardiac function and calcium handling in rats with post-MI HF, and improved function and prolonged the life span of rats with hypertension-induced HF.31 In addition to reducing mortality, selective and sustained inhibition of βIIPKC by βIIV5-3 (a selective inhibitor of βIIPKC25) in rats with post-MI HF improved cardiac function compared with that prior to treatment initiation.31 The beneficial effect was associated with enhanced calcium handling and normalization of the levels and phosphorylation of SERCA2, NCX, and troponin I.31 Further, the reduction in cardiomyocyte width, HW/BW, and increased fractional shortening following βIIV5-3 treatment in rats with end-stage pathological hypertrophy were observed, thereby indicating that βIIPKC activation is a critical mediator of cardiac hypertrophy in rats (Figure 2).
Figure 2.
Protein kinase C isozymes are closely involved with different remodelling events in myocardial infarction induced-heart failure. Heart failure progression is noticeably characterized by cardiac remodelling, whereas specific protein kinase C isozyme plays a crucial role in this time-related event. Cardiomyocyte death, inflammation, cardiac hypertrophy, and fibrosis are directly regulated by specific protein kinase C isozymes such as α, βII, δ, and ε protein kinase C as depicted in the figure. The TUNEL staining image is from Murriel et al. (2004)33 and the hypertrophy image from www.ipmc.cnrs.fr.
Although the basal level of βPKC in the hearts of adult mice is low,13 a number of reports suggest that βPKC is an important isozyme in cardiac diseases in mice, as well.11,32 Targeted over-expression of βIIPKC in mice resulted in cardiac hypertrophy with myocardial dysfunction similar to that of HF.11 Over-expression of activated βIIPKC in neonatal mice is fatal and, in the case of adult mice, it induces hypertrophy and myocardial dysfunction.32 Pharmacological inhibition of α and βPKC by Ro-32-0432 improved myocardial contractility and left-ventricular developed pressure in mouse hearts.34 In contrast to these observations, another study using βPKC knockout mice demonstrated no role for βPKC in HF development.35 Hence the role of βPKC in cardiac hypertrophy in mice using genetic manipulation is controversial.
Transgenic mice that express ε or δPKC activator or inhibitor peptides only postnatally and only in cardiac myocytes (using the αMHC promoter)36 revealed potential redundant roles for these enzymes in cardiac hypertrophy. Mice expressing the εPKC-selective inhibitor, εV1, developed dilated eccentric cardiomyopathy and HF, an effect associated with a 10% increase in myocyte size when compared with the non-transgenic mice. Transgenic mice expressing the εPKC-selective activator, ψεRACK, exhibited normal cardiac function, increased cardiac muscle mass (concentric hypertrophy) (Figure 2), and had no increase in fibrosis. However, upon examination of cardiac myocyte cell size, it was found that myocytes were 10% smaller (P < 0.01). Since the number and size of other cardiac cells remained unchanged, this indicated that the number of cardiomyocytes has increased. As a result of εPKC activation, hyperplasia, a phenomenon restricted mainly to perinatal cardiac development, may have occurred.36 These data suggest that εPKC signalling may be part of a compensatory signalling pathway that is pro-proliferative at least during early post-natal development. This conclusion was further supported by work examining a cross between Gαq over-expressing mice, which develop a dilated cardiomyopathy phenotype, and transgenic mice with mild activation of εPKC. The double-transgenic progeny exhibited a reduction in cardiac hypertrophy and an improvement in cardiac function compared with the Gαq over-expressing mice.37 Therefore, εPKC appears to be a positive modulator of compensatory cardiac hypertrophy in this mouse model. Because in this model activation of εPKC and Gαq were induced from day 1 after birth, the phenotype may reflect restoration of εPKC activity in the Gαq-phenotype.37
In hypertensive rats, εPKC levels increase during the compensatory stage of cardiac hypertrophy,14 but εPKC was detrimental in this response. Further activation of εPKC (by a sustained treatment with the PKC-selective activator, ψεRACK) increased cardiac fibrosis and HF, whereas εPKC inhibition (by sustained treatment with the εPKC-selective inhibitor, εV1-2) prolonged survival, reduced hypertrophy, excessive fibrosis, vascular remodelling, and inflammation and corrected cardiac dysfunction.15,16
Thus studies in mice and rats are not in agreement, suggesting either species differences and/or differences due to the tools that were used (pharmacological vs. genetic manipulation of the animals) as well as the timing of regulation of PKC (i.e. before and/or during the disease course.). A summary of a number of studies using pharmacological and genetic approaches to determine the role of PKC isozymes in hypertrophy and in HF is provided in Table 1.
3.3. Protein kinase C in human hypertrophy and heart failure
Although animal studies were inconclusive using genetic manipulations and pharmacologcial PKC modulators (Table 1), studies characterizing the level and activity of PKC isozymes in human HF3,4 provide insight into which isozymes should be focused on as therapeutic targets. For instance, αPKC was found to be critical in cardiomyocyte hypertrophy by knock-out and over-expression studies in mice.22,28,29 Though activation of αPKC is critical in mouse model of HFs,29,34 activated αPKC levels were found to be low in samples of patients with end-stage HF when compared with normal subjects.4 Further this study demonstrated that αPKC is activated in the myocardium of patients with aortic stenosis, a condition in which heart functions are not jeopardized. In contrast, both Bowling et al.3 and Simonis et al. also4 found a significant 70 and 150% increase in activation of βPKC, respectively and imunohistochemical staining and mRNA labelling indicated that βPKC is elevated in cardiomyocytes of human HF samples.3,4 LY333531, an inhibitor reported initially to be specific for βPKC,38 reduced total PKC activity in membrane fractions of failing hearts by 209 pmol min−1mg−1 suggesting that βPKC constitutes for the majority PKC activity in the failing hearts. Together, these studies indicate that changes in βPKC correlate better with the human HF, suggesting that focusing on this PKC isozyme in considering therapeutic intervention is advisable.
4. Cardiac fibrosis
Fibrosis refers to accumulation of fibroblasts due to increased proliferation, migration, and adhesion of fibroblasts to the site of injury and/or leading to the accumulation of extracellular matrix proteins, such as collagen, by augmented release from fibroblasts or reduced degradation of collagen. Replacement fibrosis, interstitial fibrosis, and perivascular fibrosis are different types of myocardial fibrotic processes, which may occur sequentially or simultaneously. However, an excess of any of these processes interferes with myocardial metabolism, particularly the supply of oxygen and removal of cellular metabolic waste, leading to myocardial malfunctioning, and thus posing detrimental effects to failing hearts. Excess fibrosis can also decrease cardiac elasticity and thus affect cardiac contraction. A variety of pathological stressors, such as ischaemia and hypertension, can trigger cardiac fibrosis. The occurrence of cardiac fibrosis requires a series of coordinated molecular and cellular events that alter the properties of the extracellular matrix (ECM) and cardiac fibroblasts. PKC has been shown to regulate the specific events leading to the deposition of collagen.
4.1. Protein kinase C isozymes in cultured cardiac fibroblasts
α, βI, βII, δ, ε, and ζPKC have been found in both neonatal and adult cardiac fibroblasts and the non-selective PKC activator, PMA, inhibits basal and TGFβ-induced thymidine incorporation in these rat fibroblasts.39 Using isozyme-selective inhibitors, we found that δPKC and ζPKC have opposing roles in TGFβ-induced fibroblast proliferation, whereas other PKC isozymes have no role in this process.39 We showed that selective inhibition of ζPKC blocked TGF-β1-induced cardiac fibroblast proliferation. In contrast, the δPKC-selective peptide inhibitor, δV1-1, had an opposite effect to that of the ζPKC inhibitor; it increased TGF-β1-induced proliferation. Therefore, δ and ζPKC act downstream of TGF-β1, yielding opposing roles in fibroblast proliferation.
PKC regulates the levels and activity of matrix metalloproteinases (MMP), a family of zinc-containing proteases, that degrade ECM and facilitate the motility of cardiac fibroblasts.40–44 For example, α and βIPKC increase the activity of MMP-9, but not MMP-2, primarily through the JNK-dependent pathway.44 Other PKCs, such as θ and ζPKC, increase both MMP-2 and MMP-9 via ERK and NFκB pathways in adult rat cardiac fibroblasts.44 Ang II binds to angiotensin-type 1 receptor and activates PKC45 which ultimately leads to fibroblast proliferation.46–48 The pro-proliferative effects of other profibrotic stimuli, such as ET-1, were also attenuated by inhibition of PKC with either chelerythrine or staurosporine in neonatal cardiac fibroblasts.49
A critical role for εPKC in regulating fibroblast adhesion and migration has also been reported; the effect of Ang II treatment, which induces adhesion and migration in cardiac fibroblasts, was blocked by PKC inhibition, and was abolished in cardiac myofibroblasts obtained from εPKC knockout mice.48 Additional mechanistic studies demonstrated that εPKC forms a tight complex with β1-integrin to regulate the interaction between the cell and ECM.50–52 These findings corroborate a role for εPKC in mediating cardiac fibroblast adhesion and migration.
4.2. Protein kinase C in cardiac fibrosis, in vivo
A role for PKC in cardiac fibrosis has also been suggested by in vivo studies using animal models of HF. Inhibition of classical PKCs, namely, α and βPKC, by ruboxistaurin attenuated pathological fibrosis and improved cardiac function following MI in rats, suggesting a role for classical PKCs in fibrogenesis in the heart.12 In a recent study, selective βIIPKC inhibition with βIIV5-3 attenuated collagen deposition in the remote region of the myocardium of post-MI HF rats53 (Figure 2). Hearts from εPKC knock-out mice demonstrated elevated interstitial fibrosis when subjected to pressure overload by transverse aortic constriction.54 In contrast, sustained inhibition of εPKC by its isozyme-selective peptide inhibitor, εV1-2, suppressed cardiac fibrosis and ameliorated cardiac function, in part by inhibiting MMP-2 activity, in a rat model of hypertension-induced HF.15 Moreover, ψεRACK, an activator of εPKC, augmented the fibrotic process and accelerated mortality in these hypertensive animals. Further, in a rat or mouse cardiac transplantation model, we found that inhibition of εPKC by εV1-2 also blocked parenchymal fibrosis and the increase in TGF-β1 in the grafted heart, corroborating a role for εPKC in cardiac fibrosis in vivo55,56 (Figure 2). The contradicting findings using εPKC knock-out mice and pharmacological regulation of εPKC in rats suggest that regulation by PKC isozymes may differ according to the aetiology of fibrosis, the species, and/or the extent of activation of compensatory mechanisms (i.e. εPKC null mice have 60% increase in δPKC activity, which may have compensated for εPKC,45 whereas there is no change in the levels or activity of any PKC isozyme, other than εPKC, when using the εPKC-selective inhibitor15). (These and other studies are also listed in Table 1.). Understanding the exquisite control of cardiac fibrosis by PKC could potentially translate to novel effective treatments for cardiac dysfunction and HF.
5. Cardiac inflammation
Irrespective of aetiology, myocardial inflammation is an integral part of HF (i.e. inflammation is found in the myocardium following ischaemia, cardiac infection, autoimmune response, pressure and volume overload, etc.). Though inflammation is often secondary to the trigger for specific cardiac disease, inflammatory cells are a constant source for cytokines, enzymes, and growth factors, which regulate remodelling events such as hypertrophy, fibrosis, and vascularization.
5.1. Protein kinase C in cardiac inflammation
Numerous in vitro studies point out the role of PKC isozymes in pro-inflammatory mediator production (transcription and translation) and release.57–62 One aspect of cardiac inflammation that is better described is the induction of cell damage by pro-inflammatory cytokines in cardiac diseases or cardiac cells. For example, TNF-α induced apoptosis in coronary vascular endothelial cells seems to be a PKC-mediated event.63 Sustained inhibition of εPKC by εV1-2 decreased the infiltration of inflammatory cells into the myocardium in the hearts of hypertensive rats with HF.16 In an MI-induced HF rat model, βIIV5-3, the specific βIIPKC inhibitor, also attenuated infiltration of inflammatory cells53 (Figure 2). Both of these PKC inhibitors attenuated degranulation of mast cells (MCs), the important inflammatory cells that are involved in HF progression (Figure 2). Likewise, TNF-α production in macrophages is blocked by PKC inhibition with bisindolylmaleimide, a pan PKC inhibitor, or by Go-6976, a classical PKC inhibitor.64 Further studies on the role of PKC isozymes in the function of important inflammatory cells such as macrophages, T-cells, MCs, and neutrophils in HF progression are needed, because these cells are an integral part of cardiac remodelling and HF.65,66
6. Downstream targets of protein kinase C in cardiac remodelling
As discussed earlier, PKC isozymes regulate fibrosis, inflammation, and cardiac muscle dysfunction and a number of downstream mediators of PKC effects have been identified. Stimulation of primary cultures of adult feline cardiomyocytes with ET-1, PE, PMA, or insulin resulted in phosphorylation and activation of several pro-survival kinases, including mTOR (AKA mammalian Target of Rapamycin; at S2248) and S6K1 (at and T389 S421/T424) via PKC activation. Expression of DN-εPKC abolished ET-1-stimulated mTOR and S6K1 phosphorylation, but not insulin-stimulated S6K1 phosphorylation. ET-1- and insulin-stimulated mTOR and S6K1 phosphorylation in cardiomyocytes was inhibited by expression of DN-δPKC or pre-treatment with rottlerin, a δPKC inhibitor. However, treatment with Gö6976, a specific classical PKC (cPKC) inhibitor did not affect mTOR/S6K1 activation. This study demonstrates that ε and δPKC activate mTOR and S6 kinase and subsequently lead to cardiomyocyte hypertrophy in cultured feline cardiomyocytes.67 Another important kinase that has been implicated in hypertrophic signalling is glycogen synthase kinase (GSK). Up-regulation of GSK-3α suppresses cardiac growth and pressure-overload-induced cardiac hypertrophy in mice. Knock-down of GSK-3α increased the phosphorylation of ERK (extracellular signal-regulated kinase), an effect that was inhibited by pharmacological inhibitors of ε and δPKC and MEK (mitogen-activated protein kinase kinase), suggesting that GSK-3α inhibits ERK through PKC-MEK-dependent mechanisms and further regulates cardiac hypertrophy.68 In another study, involvement of PKC in TGF-β1-induced cardiac hypertrophic responses by activating TAK1 (another member of the mitogen-activated kinase kinase kinase family) and ultimately activating transcription factor (ATF). The PKC inhibitors, GO6976 and GF109203X, blocked TGF-β1-induced TAK1 kinase activity and subsequent downstream signalling pathways including ATF-2 phosphorylation, leading to suppression of ATF-2 transcriptional activity.69 The transcription factor GATA-4 plays a key role in ANF promoter activation in response to pro-hypertrophic Ang II through PKC activation and ultimately resulting in enhanced DNA binding activity.70 Inhibition of PKC prevents nuclear export of histone deacetylase 5 (HDAC5, a protein regulating myogenesis) in response to hypertrophic agonists. Moreover, a mutation in HDAC5 is refractory to PKC activation. Protein kinase D (PKD), another downstream effector of PKC, directly phosphorylates HDAC5 and stimulates its nuclear export.71 The stretch-induced increase in cardiac hypertrophy is blocked by inhibition of the small G protein, Rho, or by overexpression of dominant negative α and δPKC, suggesting that α and δPKC are both required for stretch-induced hypertrophy, through Rho GTPase-mediated signalling pathways. Also, phosphorylation of MEK1/ERK1/2 and the MEK kinase, MKK4, and jun kinase, JNK, was inhibited by over-expression of dominant negative α and δPKC.72
Myocyte dysfunction in αPKC transgenic mice was caused by alterations in Ca2+ homeostasis.29 As discussed, in mice, αPKC depresses myofilament contractility through site-specific phosphorylation of cTnT at the threonine-206 residue in cardiomyocytes.30 On the other hand, a decreased myofilament responsiveness to Ca2+ was seen in the myocardium of βIIPKC overexpressing transgenic mice as well as a significant increase in the degree of phosphorylation of troponin I. The depressed cardiomyocyte function improved after the sequential superfusion of LY333531, a βPKC inhibitor. This study shows that βIIPKC-mediated phosphorylation of troponin I in vivo may decrease myofilament Ca2+ responsiveness, and thus causes cardiomyocyte dysfunction.73
Other protein targets of PKC are those involved in ECM regulation. εPKC regulates β1-integrin complex formation with ECM and further participates in the fibrotic events.50–52 α, βI, ε, θ, and ζPKC isozymes activate different MMPs to degrade ECM, thereby facilitating the motility of cardiac fibroblasts44 and inflammatory cells. In inflammatory cells, PKC activation enhances the transcription of cytokine production through phosphorylation of the inhibitor of transcription factor NFkB, IkB.74 A summary of these and other downstream targets of PKC activation leading to HF is given in Figure 3.
Figure 3.
Schematic protein kinase C isozyme signalling pathways and downstream targets in the heart. The activation of different protein kinase C isozymes contributes to the establishment of heart failure through phosphorylation of isozyme-selective substrates in the failing heart.
7. Summary and conclusion: should protein kinase C be a target for heart failure treatment?
Preventing maladaptive cardiac remodelling is a goal of therapy for HF.75 In this review, we provide evidence that select PKC isozymes play different roles in many aspects of cardiac remodelling in HF (see Table 1 and Figures 2 and 3). Because modulators of PKC isozymes are already in clinical trials for a variety of indications,76–84 it may now be possible to consider using such PKC isozyme regulators to treat human HF. Although PKC isozymes are present in many tissues, recent clinical trials suggest that systemic delivery of inhibitors and activators of PKC isozymes is well tolerated (see studies with the βPKC small molecule inhibitor,77,78 with the peptide inhibitor of δPKC.76 Further, new developments in drug delivery suggest that organ-selective delivery may also be possible in the future, for example by delivering slow drug releasing particles to the organ of interest.85 Therefore, clinical trials with PKC inhibitors to address HF should be considered using either systemic or cardiac-specific drug delivery. Further in vivo studies using animal models, including larger animals like dogs, sheep, and pigs, will help guide the identification of the right PKC isozymes that should serve as therapeutic targets for the treatment of pathological cardiac remodelling and HF in humans.
Conflict of interest: D.M.-R. is a founder, stock option holder, paid consultant, chair of the scientific advisory board, and member of the board of directors of KAI Pharmaceuticals, a company whose goal is to bring peptide regulators of PKC to the clinic. However, none of the research proposed here and none of the detail of the work done in my laboratory at Stanford is disclosed to the company before it is disclosed publicly elsewhere. D.M.-R. has provided reports on her relationship with KAI to Stanford University (last report was provided in April 2008). S.S.P., L.S., and J.C.B.F. have no conflict of interest to disclose.
Funding
This work was supported by The National Institutes of Health grant HL76675 to D.M.-R.
References
- 1.Kohout TA, Rogers TB. Use of a PCR-based method to characterize protein kinase C isoform expression in cardiac cells. Am J Physiol. 1993;264:C1350–C1359. doi: 10.1152/ajpcell.1993.264.5.C1350. [DOI] [PubMed] [Google Scholar]
- 2.Erdbrugger W, Keffel J, Knocks M, Otto T, Philipp T, Michel MC. Protein kinase C isoenzymes in rat and human cardiovascular tissues. Br J Pharmacol. 1997;120:177–186. doi: 10.1038/sj.bjp.0700877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowling N, Walsh RA, Song G, Estridge T, Sandusky GE, Fouts RL, et al. Increased protein kinase C activity and expression of Ca2+-sensitive isoforms in the failing human heart. Circulation. 1999;99:384–391. doi: 10.1161/01.cir.99.3.384. [DOI] [PubMed] [Google Scholar]
- 4.Simonis G, Briem SK, Schoen SP, Bock M, Marquetant R, Strasser RH. Protein kinase C in the human heart: differential regulation of the isoforms in aortic stenosis or dilated cardiomyopathy. Mol Cell Biochem. 2007;305:103–111. doi: 10.1007/s11010-007-9533-3. [DOI] [PubMed] [Google Scholar]
- 5.Shin HG, Barnett JV, Chang P, Reddy S, Drinkwater DC, Pierson RN, et al. Molecular heterogeneity of protein kinase C expression in human ventricle. Cardiovasc Res. 2000;48:285–299. doi: 10.1016/s0008-6363(00)00185-1. [DOI] [PubMed] [Google Scholar]
- 6.Disatnik MH, Buraggi G, Mochly-Rosen D. Localization of protein kinase C isozymes in cardiac myocytes. Exp Cell Res. 1994;210:287–297. doi: 10.1006/excr.1994.1041. [DOI] [PubMed] [Google Scholar]
- 7.Schreiber KL, Paquet L, Allen BG, Rindt H. Protein kinase C isoform expression and activity in the mouse heart. Am J Physiol Heart Circ Physiol. 2001;281:H2062–H2071. doi: 10.1152/ajpheart.2001.281.5.H2062. [DOI] [PubMed] [Google Scholar]
- 8.Rouet-Benzineb P, Mohammadi K, Perennec J, Poyard M, Bouanani Nel H, Crozatier B. Protein kinase C isoform expression in normal and failing rabbit hearts. Circ Res. 1996;79:153–161. doi: 10.1161/01.res.79.2.153. [DOI] [PubMed] [Google Scholar]
- 9.Churchill E, Budas G, Vallentin A, Koyanagi T, Mochly-Rosen D. PKC isozymes in chronic cardiac disease: possible therapeutic targets? Annu Rev Pharmacol Toxicol. 2008;48:569–599. doi: 10.1146/annurev.pharmtox.48.121806.154902. [DOI] [PubMed] [Google Scholar]
- 10.Budas GR, Churchill EN, Mochly-Rosen D. Cardioprotective mechanisms of PKC isozyme-selective activators and inhibitors in the treatment of ischemia-reperfusion injury. Pharmacol Res. 2007;55:523–536. doi: 10.1016/j.phrs.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Wakasaki H, Koya D, Schoen FJ, Jirousek MR, Ways DK, Hoit BD, et al. Targeted overexpression of protein kinase C beta2 isoform in myocardium causes cardiomyopathy. Proc Natl Acad Sci USA. 1997;94:9320–9325. doi: 10.1073/pnas.94.17.9320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyle AJ, Kelly DJ, Zhang Y, Cox AJ, Gow RM, Way K, et al. Inhibition of protein kinase C reduces left ventricular fibrosis and dysfunction following myocardial infarction. J Mol Cell Cardiol. 2005;39:213–221. doi: 10.1016/j.yjmcc.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Sabri A, Steinberg SF. Protein kinase C isoform-selective signals that lead to cardiac hypertrophy and the progression of heart failure. Mol Cell Biochem. 2003;251:97–101. [PubMed] [Google Scholar]
- 14.Inagaki K, Iwanaga Y, Sarai N, Onozawa Y, Takenaka H, Mochly-Rosen D, et al. Tissue angiotensin II during progression or ventricular hypertrophy to heart failure in hypertensive rats; differential effects on PKC epsilon and PKC beta. J Mol Cell Cardiol. 2002;34:1377–1385. doi: 10.1006/jmcc.2002.2089. [DOI] [PubMed] [Google Scholar]
- 15.Inagaki K, Koyanagi T, Berry NC, Sun L, Mochly-Rosen D. Pharmacological inhibition of epsilon-protein kinase C attenuates cardiac fibrosis and dysfunction in hypertension-induced heart failure. Hypertension. 2008;51:1565–1569. doi: 10.1161/HYPERTENSIONAHA.107.109637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palaniyandi SS, Inagaki K, Mochly-Rosen D. Mast cells and varepsilonPKC: A role in cardiac remodeling in hypertension-induced heart failure. J Mol Cell Cardiol. 2008;45:779–786. doi: 10.1016/j.yjmcc.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inagaki K, Begley R, Ikeno F, Mochly-Rosen D. Cardioprotection by epsilon-protein kinase C activation from ischemia: continuous delivery and antiarrhythmic effect of an epsilon-protein kinase C-activating peptide. Circulation. 2005;111:44–50. doi: 10.1161/01.CIR.0000151614.22282.F1. [DOI] [PubMed] [Google Scholar]
- 18.Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS, et al. ACC/AHA Guidelines for the Evaluation and Management of Chronic Heart Failure in the Adult: Executive Summary. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure): Developed in Collaboration With the International Society for Heart and Lung Transplantation; Endorsed by the Heart Failure Society of America. Circulation. 2001;104:2996–3007. doi: 10.1161/hc4901.102568. [DOI] [PubMed] [Google Scholar]
- 19.AHA heart disease and stroke statistics; 2003 update [Google Scholar]
- 20.Gheorghiade M, Bonow RO. Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation. 1998;97:282–289. doi: 10.1161/01.cir.97.3.282. [DOI] [PubMed] [Google Scholar]
- 21.Hunter JJ, Chien KR. Signaling pathways for cardiac hypertrophy and failure. N Engl J Med. 1999;341:1276–1283. doi: 10.1056/NEJM199910213411706. [DOI] [PubMed] [Google Scholar]
- 22.Vijayan K, Szotek EL, Martin JL, Samarel AM. Protein kinase C-alpha-induced hypertrophy of neonatal rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2004;287:H2777–H2789. doi: 10.1152/ajpheart.00171.2004. [DOI] [PubMed] [Google Scholar]
- 23.Kerkela R, Ilves M, Pikkarainen S, Tokola H, Ronkainen J, Vuolteenaho O, et al. Identification of PKCalpha isoform-specific effects in cardiac myocytes using antisense phosphorothioate oligonucleotides. Mol Pharmacol. 2002;62:1482–1491. doi: 10.1124/mol.62.6.1482. [DOI] [PubMed] [Google Scholar]
- 24.Braz JC, Bueno OF, De Windt LJ, Molkentin JD. PKC alpha regulates the hypertrophic growth of cardiomyocytes through extracellular signal-regulated kinase1/2 (ERK1/2) J Cell Biol. 2002;156:905–919. doi: 10.1083/jcb.200108062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stebbins EG, Mochly-Rosen D. Binding specificity for RACK1 resides in the V5 region of beta II protein kinase C. J Biol Chem. 2001;276:29644–29650. doi: 10.1074/jbc.M101044200. [DOI] [PubMed] [Google Scholar]
- 26.Vallentin A, Mochly-Rosen D. RBCK1, a protein kinase CbetaI (PKCbetaI)-interacting protein, regulates PKCbeta-dependent function. J Biol Chem. 2007;282:1650–1657. doi: 10.1074/jbc.M601710200. [DOI] [PubMed] [Google Scholar]
- 27.Sil P, Kandaswamy V, Sen S. Increased protein kinase C activity in myotrophin-induced myocyte growth. Circ Res. 1998;82:1173–1188. doi: 10.1161/01.res.82.11.1173. [DOI] [PubMed] [Google Scholar]
- 28.Belin RJ, Sumandea MP, Allen EJ, Schoenfelt K, Wang H, Solaro RJ, et al. Augmented protein kinase C-alpha-induced myofilament protein phosphorylation contributes to myofilament dysfunction in experimental congestive heart failure. Circ Res. 2007;101:195–204. doi: 10.1161/CIRCRESAHA.107.148288. [DOI] [PubMed] [Google Scholar]
- 29.Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, et al. PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat Med. 2004;10:248–254. doi: 10.1038/nm1000. [DOI] [PubMed] [Google Scholar]
- 30.Sumandea MP, Pyle WG, Kobayashi T, de Tombe PP, Solaro RJ. Identification of a functionally critical protein kinase C phosphorylation residue of cardiac troponin T. J Biol Chem. 2003;278:35135–35144. doi: 10.1074/jbc.M306325200. [DOI] [PubMed] [Google Scholar]
- 31.Ferreira JC, TK, Inagaki K, Fajardo G, Churchill EN, Budas G, et al. Pharmacological inhibition of βIIPKC is cardioprotective in late-stage hypertrophy and end-stage heart failure. (Unpublished observation) [Google Scholar]
- 32.Bowman JC, Steinberg SF, Jiang T, Geenen DL, Fishman GI, Buttrick PM. Expression of protein kinase C beta in the heart causes hypertrophy in adult mice and sudden death in neonates. The Journal of clinical investigation. 1997;100:2189–2195. doi: 10.1172/JCI119755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murriel CL, Churchill E, Inagaki K, Szweda LI, Mochly-Rosen D. Protein kinase Cdelta activation induces apoptosis in response to cardiac ischemia and reperfusion damage: a mechanism involving BAD and the mitochondria. J Biol Chem. 2004;279:47985–47991. doi: 10.1074/jbc.M405071200. [DOI] [PubMed] [Google Scholar]
- 34.Hambleton M, Hahn H, Pleger ST, Kuhn MC, Klevitsky R, Carr AN, et al. Pharmacological- and gene therapy-based inhibition of protein kinase Calpha/beta enhances cardiac contractility and attenuates heart failure. Circulation. 2006;114:574–582. doi: 10.1161/CIRCULATIONAHA.105.592550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roman BB, Geenen DL, Leitges M, Buttrick PM. PKC-beta is not necessary for cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2001;280:H2264–H2270. doi: 10.1152/ajpheart.2001.280.5.H2264. [DOI] [PubMed] [Google Scholar]
- 36.Mochly-Rosen D, Wu G, Hahn H, Osinska H, Liron T, Lorenz JN, et al. Cardiotrophic effects of protein kinase C epsilon: analysis by in vivo modulation of PKCepsilon translocation. Circ Res. 2000;86:1173–1179. doi: 10.1161/01.res.86.11.1173. [DOI] [PubMed] [Google Scholar]
- 37.Wu G, Toyokawa T, Hahn H, Dorn GW., 2nd Epsilon protein kinase C in pathological myocardial hypertrophy. Analysis by combined transgenic expression of translocation modifiers and Galphaq. J Biol Chem. 2000;275:29927–29930. doi: 10.1074/jbc.C000380200. [DOI] [PubMed] [Google Scholar]
- 38.Ishii H, Jirousek MR, Koya D, Takagi C, Xia P, Clermont A, et al. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC beta inhibitor. Science. 1996;272:728–731. doi: 10.1126/science.272.5262.728. [DOI] [PubMed] [Google Scholar]
- 39.Braun MU, Mochly-Rosen D. Opposing effects of delta- and zeta-protein kinase C isozymes on cardiac fibroblast proliferation: use of isozyme-selective inhibitors. Journal of molecular and cellular cardiology. 2003;35:895–903. doi: 10.1016/s0022-2828(03)00142-1. [DOI] [PubMed] [Google Scholar]
- 40.Lee HS, Park SY, Lee HW, Choi HS. Secretions of MMP-9 by soluble glucocorticoid-induced tumor necrosis factor receptor (sGITR) mediated by protein kinase C (PKC)delta and phospholipase D (PLD) in murine macrophage. J Cell Biochem. 2004;92:481–490. doi: 10.1002/jcb.20099. [DOI] [PubMed] [Google Scholar]
- 41.Park MJ, Park IC, Lee HC, Woo SH, Lee JY, Hong YJ, et al. Protein kinase C-alpha activation by phorbol ester induces secretion of gelatinase B/MMP-9 through ERK 1/2 pathway in capillary endothelial cells. Int J Oncol. 2003;22:137–143. [PubMed] [Google Scholar]
- 42.Reuben PM, Cheung HS. Regulation of matrix metalloproteinase (MMP) gene expression by protein kinases. Front Biosci. 2006;11:1199–1215. doi: 10.2741/1873. [DOI] [PubMed] [Google Scholar]
- 43.Xie B, Laouar A, Huberman E. Fibronectin-mediated cell adhesion is required for induction of 92-kDa type IV collagenase/gelatinase (MMP-9) gene expression during macrophage differentiation. The signaling role of protein kinase C-beta. J Biol Chem. 1998;273:11576–11582. doi: 10.1074/jbc.273.19.11576. [DOI] [PubMed] [Google Scholar]
- 44.Xie Z, Singh M, Singh K. Differential regulation of matrix metalloproteinase-2 and -9 expression and activity in adult rat cardiac fibroblasts in response to interleukin-1beta. J Biol Chem. 2004;279:39513–39519. doi: 10.1074/jbc.M405844200. [DOI] [PubMed] [Google Scholar]
- 45.Gray MO, Long CS, Kalinyak JE, Li HT, Karliner JS. Angiotensin II stimulates cardiac myocyte hypertrophy via paracrine release of TGF-beta 1 and endothelin-1 from fibroblasts. Cardiovasc Res. 1998;40:352–363. doi: 10.1016/s0008-6363(98)00121-7. [DOI] [PubMed] [Google Scholar]
- 46.Booz GW, Dostal DE, Singer HA, Baker KM. Involvement of protein kianse C and Ca2+ in angiotensin II-induced mitogenesis of cardiac fibroblasts. Am J Physiol. 1994;267:C1308–C1318. doi: 10.1152/ajpcell.1994.267.5.C1308. [DOI] [PubMed] [Google Scholar]
- 47.Hou M, Pantev E, Moller S, Erlinge D, Edvinsson L. Angiotensin II type 1 receptors stimulate protein synthesis in human cardiac fibroblasts via a Ca2+-sensitive PKC-dependent tyrosine kinase pathway. Acta Physiol Scand. 2000;168:301–309. doi: 10.1046/j.1365-201x.2000.00684.x. [DOI] [PubMed] [Google Scholar]
- 48.Stawowy P, Margeta C, Blaschke F, Lindschau C, Spencer-Hansch C, Leitges M, et al. Protein kinase C epsilon mediates angiotensin II-induced activation of beta1-integrins in cardiac fibroblasts. Cardiovasc Res. 2005;67:50–59. doi: 10.1016/j.cardiores.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 49.Piacentini L, Gray M, Honbo NY, Chentoufi J, Bergman M, Karliner JS. Endothelin-1 stimulates cardiac fibroblast proliferation through activation of protein kinase C. J Mol Cell Cardiol. 2000;32:565–576. doi: 10.1006/jmcc.2000.1109. [DOI] [PubMed] [Google Scholar]
- 50.Besson A, Wilson TL, Yong VW. The anchoring protein RACK1 links protein kinase Cepsilon to integrin beta chains. Requirements for adhesion and motility. J Biol Chem. 2002;277:22073–22084. doi: 10.1074/jbc.M111644200. [DOI] [PubMed] [Google Scholar]
- 51.Heidkamp MC, Bayer AL, Scully BT, Eble DM, Samarel AM. Activation of focal adhesion kinase by protein kinase C epsilon in neonatal rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2003;285:H1684–H1696. doi: 10.1152/ajpheart.00016.2003. [DOI] [PubMed] [Google Scholar]
- 52.Ivaska J, Whelan RD, Watson R, Parker PJ. PKC epsilon controls the traffic of beta1 integrins in motile cells. EMBO J. 2002;21:3608–3619. doi: 10.1093/emboj/cdf371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palaniyandi SS, Ferreira JCB, Brum P, Mochly-Rosen D. βIIPKC inhibition reduces post-myocardial infarction-induced end-stage heart failure in rats. 2008 (Unpublished observation) [Google Scholar]
- 54.Klein G, Schaefer A, Hilfiker-Kleiner D, Oppermann D, Shukla P, Quint A, et al. Increased collagen deposition and diastolic dysfunction but preserved myocardial hypertrophy after pressure overload in mice lacking PKCepsilon. Circ Res. 2005;96:748–755. doi: 10.1161/01.RES.0000161999.86198.1e. [DOI] [PubMed] [Google Scholar]
- 55.Tanaka M, Gunawan F, Terry RD, Inagaki K, Caffarelli AD, Hoyt G, et al. Inhibition of heart transplant injury and graft coronary artery disease after prolonged organ ischemia by selective protein kinase C regulators. J Thorac Cardiovasc Surg. 2005;129:1160–1167. doi: 10.1016/j.jtcvs.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 56.Koyanagi T, Noguchi K, Ootani A, Inagaki K, Robbins RC, Mochly-Rosen D. Pharmacological inhibition of epsilon PKC suppresses chronic inflammation in murine cardiac transplantation model. J Mol Cell Cardiol. 2007;43:517–522. doi: 10.1016/j.yjmcc.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 57.Shapira L, Sylvia VL, Halabi A, Soskolne WA, Van Dyke TE, Dean DD, et al. Bacterial lipopolysaccharide induces early and late activation of protein kinase C in inflammatory macrophages by selective activation of PKC-epsilon. Biochem Biophys Res Commun. 1997;240:629–634. doi: 10.1006/bbrc.1997.7717. [DOI] [PubMed] [Google Scholar]
- 58.Paul A, Doherty K, Plevin R. Differential regulation by protein kinase C isoforms of nitric oxide synthase induction in RAW 264.7 macrophages and rat aortic smooth muscle cells. Br J Pharmacol. 1997;120:940–946. doi: 10.1038/sj.bjp.0700976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.West MA, LeMieur T, Clair L, Bellingham J, Rodriguez JL. Protein kinase C regulates macrophage tumor necrosis factor secretion: direct protein kinase C activation restores tumor necrosis factor production in endotoxin tolerance. Surgery. 1997;122:204–211. doi: 10.1016/s0039-6060(97)90010-6. discussion 211–202. [DOI] [PubMed] [Google Scholar]
- 60.Devaraj S, Venugopal SK, Singh U, Jialal I. Hyperglycemia induces monocytic release of interleukin-6 via induction of protein kinase c-{alpha} and -{beta} Diabetes. 2005;54:85–91. doi: 10.2337/diabetes.54.1.85. [DOI] [PubMed] [Google Scholar]
- 61.Nechushtan H, Leitges M, Cohen C, Kay G, Razin E. Inhibition of degranulation and interleukin-6 production in mast cells derived from mice deficient in protein kinase Cbeta. Blood. 2000;95:1752–1757. [PubMed] [Google Scholar]
- 62.Nikodemova M, Watters JJ, Jackson SJ, Yang SK, Duncan ID. Minocycline down-regulates MHC II expression in microglia and macrophages through inhibition of IRF-1 and protein kinase C (PKC)alpha/betaII. J Biol Chem. 2007;282:15208–15216. doi: 10.1074/jbc.M611907200. [DOI] [PubMed] [Google Scholar]
- 63.Li D, Yang B, Mehta JL. Tumor necrosis factor-alpha enhances hypoxia-reoxygenation-mediated apoptosis in cultured human coronary artery endothelial cells: critical role of protein kinase C. Cardiovasc Res. 1999;42:805–813. doi: 10.1016/s0008-6363(98)00342-3. [DOI] [PubMed] [Google Scholar]
- 64.Meldrum DR, Meng X, Sheridan BC, McIntyre RC, Jr, Harken AH, Banerjee A. Tissue-specific protein kinase C isoforms differentially mediate macrophage TNFalpha and IL-1beta production. Shock. 1998;9:256–260. doi: 10.1097/00024382-199804000-00004. [DOI] [PubMed] [Google Scholar]
- 65.Petrovic D. Cytopathological basis of heart failure–cardiomyocyte apoptosis, interstitial fibrosis and inflammatory cell response. Folia Biol (Praha) 2004;5:58–62. [PubMed] [Google Scholar]
- 66.Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res. 2002;91:988–998. doi: 10.1161/01.res.0000043825.01705.1b. [DOI] [PubMed] [Google Scholar]
- 67.Moschella PC, Rao VU, McDermott PJ, Kuppuswamy D. Regulation of mTOR and S6K1 activation by the nPKC isoforms, PKCepsilon and PKCdelta, in adult cardiac muscle cells. J Mol Cell Cardiol. 2007;43:754–766. doi: 10.1016/j.yjmcc.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhai P, Gao S, Holle E, Yu X, Yatani A, Wagner T, et al. Glycogen synthase kinase-3alpha reduces cardiac growth and pressure overload-induced cardiac hypertrophy by inhibition of extracellular signal-regulated kinases. J Biol Chem. 2007;282:33181–33191. doi: 10.1074/jbc.M705133200. [DOI] [PubMed] [Google Scholar]
- 69.Lim JY, Park SJ, Hwang HY, Park EJ, Nam JH, Kim J, et al. TGF-beta1 induces cardiac hypertrophic responses via PKC-dependent ATF-2 activation. J Mol Cell Cardiol. 2005;39:627–636. doi: 10.1016/j.yjmcc.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 70.Wang J, Paradis P, Aries A, Komati H, Lefebvre C, Wang H, et al. Convergence of protein kinase C and JAK-STAT signaling on transcription factor GATA-4. Mol Cell Biol. 2005;25:9829–9844. doi: 10.1128/MCB.25.22.9829-9844.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vega RB, Harrison BC, Meadows E, Roberts CR, Papst PJ, Olson EN, et al. Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol Cell Biol. 2004;24:8374–8385. doi: 10.1128/MCB.24.19.8374-8385.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pan J, Singh US, Takahashi T, Oka Y, Palm-Leis A, Herbelin BS, et al. PKC mediates cyclic stretch-induced cardiac hypertrophy through Rho family GTPases and mitogen-activated protein kinases in cardiomyocytes. J Cell Physiol. 2005;202:536–553. doi: 10.1002/jcp.20151. [DOI] [PubMed] [Google Scholar]
- 73.Takeishi Y, Chu G, Kirkpatrick DM, Li Z, Wakasaki H, Kranias EG, et al. In vivo phosphorylation of cardiac troponin I by protein kinase Cbeta2 decreases cardiomyocyte calcium responsiveness and contractility in transgenic mouse hearts. J Clin Invest. 1998;102:72–78. doi: 10.1172/JCI2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reiner G, Oliver M, Skamene E, Radzioch D. Induction of tumor necrosis factor alpha gene expression by lipoprotein lipase requires protein kinase C activation. J Lipid Res. 1994;35:1413–1421. [PubMed] [Google Scholar]
- 75.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling–concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35:569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 76.Bates E, Bode C, Costa M, Gibson CM, Granger C, Green C, et al. Intracoronary KAI-9803 as an adjunct to primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. Circulation. 2008;117:886–896. doi: 10.1161/CIRCULATIONAHA.107.759167. [DOI] [PubMed] [Google Scholar]
- 77.The PKC-DRS Study Group. The effect of ruboxistaurin on visual loss in patients with moderately severe to very severe nonproliferative diabetic retinopathy: initial results of the Protein Kinase C beta Inhibitor Diabetic Retinopathy Study (PKC-DRS) multicenter randomized clinical trial. Diabetes. 2005;54:2188–2197. doi: 10.2337/diabetes.54.7.2188. [DOI] [PubMed] [Google Scholar]
- 78.The PKC-DRS Study Group. Effect of ruboxistaurin in patients with diabetic macular edema: thirty-month results of the randomized PKC-DMES clinical trial. Arch Ophthalmol. 2007;125:318–324. doi: 10.1001/archopht.125.3.318. [DOI] [PubMed] [Google Scholar]
- 79.Advani R, Peethambaram P, Lum BL, Fisher GA, Hartmann L, Long HJ, et al. A Phase II trial of aprinocarsen, an antisense oligonucleotide inhibitor of protein kinase C alpha, administered as a 21-day infusion to patients with advanced ovarian carcinoma. Cancer. 2004;100:321–326. doi: 10.1002/cncr.11909. [DOI] [PubMed] [Google Scholar]
- 80.Ajani JA, Jiang Y, Faust J, Chang BB, Ho L, Yao JC, et al. A multi-center phase II study of sequential paclitaxel and bryostatin-1 (NSC 339555) in patients with untreated, advanced gastric or gastroesophageal junction adenocarcinoma. Invest New Drugs. 2006;24:353–357. doi: 10.1007/s10637-006-6452-1. [DOI] [PubMed] [Google Scholar]
- 81.Casellini CM, Barlow PM, Rice AL, Casey M, Simmons K, Pittenger G, et al. A 6-month, randomized, double-masked, placebo-controlled study evaluating the effects of the protein kinase C-beta inhibitor ruboxistaurin on skin microvascular blood flow and other measures of diabetic peripheral neuropathy. Diabetes Care. 2007;30:896–902. doi: 10.2337/dc06-1699. [DOI] [PubMed] [Google Scholar]
- 82.Grossman SA, Alavi JB, Supko JG, Carson KA, Priet R, Dorr FA, et al. Efficacy and toxicity of the antisense oligonucleotide aprinocarsen directed against protein kinase C-alpha delivered as a 21-day continuous intravenous infusion in patients with recurrent high-grade astrocytomas. Neuro Oncol. 2005;7:32–40. doi: 10.1215/S1152851703000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Herbst RS, Oh Y, Wagle A, Lahn M. Enzastaurin, a protein kinase Cbeta- selective inhibitor, and its potential application as an anticancer agent in lung cancer. Clin Cancer Res. 2007;13:s4641–s4646. doi: 10.1158/1078-0432.CCR-07-0538. [DOI] [PubMed] [Google Scholar]
- 84.Ritch P, Rudin CM, Bitran JD, Edelman MJ, Makalinao A, Irwin D, et al. Phase II study of PKC-alpha antisense oligonucleotide aprinocarsen in combination with gemcitabine and carboplatin in patients with advanced non-small cell lung cancer. Lung Cancer. 2006;52:173–180. doi: 10.1016/j.lungcan.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 85.Mayer CR, Bekeredjian R. Ultrasonic gene and drug delivery to the cardiovascular system. Adv Drug Deliv Rev. 2008;60:1177–1192. doi: 10.1016/j.addr.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 86.Inoguchi T, Battan R, Handler E, Sportsman JR, Heath W, King GL. Preferential elevation of protein kinase C isoform beta II and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantation. Proc Natl Acad Sci USA. 1992;89:11059–11063. doi: 10.1073/pnas.89.22.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Takeishi Y, Ping P, Bolli R, Kirkpatrick DL, Hoit BD, Walsh RA. Transgenic overexpression of constitutively active protein kinase C epsilon causes concentric cardiac hypertrophy. Circ Res. 2000;86:1218–1223. doi: 10.1161/01.res.86.12.1218. [DOI] [PubMed] [Google Scholar]
- 88.De Windt LJ, Lim HW, Haq S, Force T, Molkentin JD. Calcineurin promotes protein kinase C and c-Jun NH2-terminal kinase activation in the heart. Cross-talk between cardiac hypertrophic signaling pathways. J Biol Chem. 2000;275:13571–13579. doi: 10.1074/jbc.275.18.13571. [DOI] [PubMed] [Google Scholar]
- 89.Gu X, Bishop SP. Increased protein kinase C and isozyme redistribution in pressure-overload cardiac hypertrophy in the rat. Circ Res. 1994;75:926–931. doi: 10.1161/01.res.75.5.926. [DOI] [PubMed] [Google Scholar]
- 90.Pass JM, Gao J, Jones WK, Wead WB, Wu X, Zhang J, et al. Enhanced PKC beta II translocation and PKC beta II-RACK1 interactions in PKC epsilon-induced heart failure: a role for RACK1. Am J Physiol. 2001;281:H2500–H2510. doi: 10.1152/ajpheart.2001.281.6.H2500. [DOI] [PubMed] [Google Scholar]
- 91.Shizukuda Y, Buttrick PM. Protein kinase C(epsilon) modulates apoptosis induced by beta -adrenergic stimulation in adult rat ventricular myocytes via extracellular signal-regulated kinase (ERK) activity. J Mol Cell Cardiol. 2001;33:1791–1803. doi: 10.1006/jmcc.2001.1442. [DOI] [PubMed] [Google Scholar]
- 92.Braun MU, LaRosee P, Schon S, Borst MM, Strasser RH. Differential regulation of cardiac protein kinase C isozyme expression after aortic banding in rat. Cardiovasc Res. 2002;56:52–63. doi: 10.1016/s0008-6363(02)00511-4. [DOI] [PubMed] [Google Scholar]
- 93.Takeishi Y, Jalili T, Ball NA, Walsh RA. Responses of cardiac protein kinase C isoforms to distinct pathological stimuli are differentially regulated. Circ Res. 1999;85:264–271. doi: 10.1161/01.res.85.3.264. [DOI] [PubMed] [Google Scholar]
- 94.Koide Y, Tamura K, Suzuki A, Kitamura K, Yokoyama K, Hashimoto T, et al. Differential induction of protein kinase C isoforms at the cardiac hypertrophy stage and congestive heart failure stage in Dahl salt-sensitive rats. Hypertens Res. 2003;26:421–426. doi: 10.1291/hypres.26.421. [DOI] [PubMed] [Google Scholar]
- 95.Takeishi Y, Bhagwat A, Ball NA, Kirkpatrick DL, Periasamy M, Walsh RA. Effect of angiotensin-converting enzyme inhibition on protein kinase C and SR proteins in heart failure. Am J Physiol. 1999;276:H53–H62. doi: 10.1152/ajpheart.1999.276.1.H53. [DOI] [PubMed] [Google Scholar]
- 96.Hambleton M, York A, Sargent MA, Kaiser RA, Lorenz JN, Robbins J, et al. Inducible and myocyte-specific inhibition of PKCalpha enhances cardiac contractility and protects against infarction-induced heart failure. Am J Physiol. 2007;293:H3768–H3771. doi: 10.1152/ajpheart.00486.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Olson ER, Shamhart PE, Naugle JE, Meszaros JG. Angiotensin II-induced extracellular signal-regulated kinase 1/2 activation is mediated by protein kinase Cdelta and intracellular calcium in adult rat cardiac fibroblasts. Hypertension. 2008;51:704–711. doi: 10.1161/HYPERTENSIONAHA.107.098459. [DOI] [PubMed] [Google Scholar]
- 98.Husse B, Briest W, Homagk L, Isenberg G, Gekle M. Cyclical mechanical stretch modulates expression of collagen I and collagen III by PKC and tyrosine kinase in cardiac fibroblasts. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1898–R1907. doi: 10.1152/ajpregu.00804.2006. [DOI] [PubMed] [Google Scholar]