Abstract
Aims
Following injury, fibroblasts transform into myofibroblasts and produce extracellular matrix (ECM). Excess production of ECM associated with cardiac fibrosis severely inhibits cardiac function. Sphingosine-1-phosphate (S1P), a bioactive lysophospholipid, regulates the function of numerous cell types. In this study, we determined the role of S1P in promoting pro-fibrotic actions of cardiac fibroblasts (CFs).
Methods and results
S1P-mediated effects on myofibroblast transformation, collagen production, and cross-talk with transforming growth factor-β (TGF-β) using mouse CF were examined. S1P increased α-smooth muscle actin (a myofibroblast marker) and collagen expression in a S1P2 receptor- and Rho kinase-dependent manner. TGF-β increased sphingosine kinase 1 (SphK1; the enzyme responsible for S1P production) expression and activity. TGF-β-stimulated collagen production was inhibited by SphK1 or S1P2 siRNA, a SphK inhibitor, and an anti-S1P monoclonal antibody.
Conclusion
These findings suggest that TGF-β-stimulated collagen production in CF involves ‘inside-out’ S1P signalling whereby S1P produced intracellularly by SphK1 can be released and act in an autocrine/paracrine fashion to activate S1P2 and increase collagen production.
Keywords: α-Smooth muscle actin, Cardiac fibroblast, Collagen, Fibrosis, S1P, Sphingosine kinase, TGF-β
1. Introduction
Fibroblasts play a critical role in wound healing and tissue remodelling. Following injury, fibroblasts transform into myofibroblasts and produce extracellular matrix (ECM). Excess production of ECM can severely inhibit organ function such as in Dupuytren’s disease, scleroderma, hypertrophic scars, and cardiac fibrosis.1 Cardiac fibroblasts (CFs) are a predominant cell type in the heart, constituting between one-third and two-third of the cell population,2,3 and are widely recognized as the critical cell in cardiac repair following injury.4 Following myocardial injury, CFs undergo transformation to a myofibroblast phenotype and produce excess amounts of ECM, consisting predominantly of collagen types I and III,5 which contribute to deleterious cardiac fibrosis.3
Sphingosine-1-phosphate (S1P), a lysophospholipid produced by active sphingosine kinase (SphK), has been shown to regulate the function of numerous cell types,6 including certain types of fibroblasts.7 S1P can activate a set of five G-protein-coupled receptors, leading to regulation of numerous downstream signalling components. Activation of S1P-mediated signalling pathways is linked to regulation of cell proliferation, migration, differentiation, and survival.6,8 Importantly, S1P promotes differentiation of human lung fibroblasts to a pro-fibrotic, myofibroblast phenotype9 and stimulates collagen synthesis in foreskin fibroblasts.10
Transforming growth factor-β (TGF-β) has been shown to have a pivotal role in stimulation of excessive ECM production by fibroblasts.11 Recent literature reports have demonstrated a connection between TGF-β and S1P signalling pathways. SphK activity was necessary for TGF-β-stimulated ERK phosphorylation and migration in oesophageal cancer cells.12 In both fibroblasts and myofibroblasts, TGF-β stimulated SphK1 expression.13,14 Importantly, siRNA against SphK1 inhibited TGF-β-stimulated up-regulation of tissue inhibitor of metaloprotease 1 (TIMP-1), a molecule that decreases ECM degradation.14 Although these data implicate S1P in the regulation of connective tissue remodelling, the role of S1P and its possible integration with TGF-β signalling in regulating ECM production in the heart has yet to be defined.
We hypothesized that S1P stimulates pro-fibrogenic function of CF and that TGF-β-stimulated increases in SphK1 expression and/or activity could lead to increases in ECM production in an S1P-dependent manner. In this report, we demonstrate for the first time, that both exogenously and endogenously produced S1P stimulates myofibroblast transformation and collagen expression through the activation of S1P2. We investigated cross-talk between TGF-β and S1P signalling pathways and found that TGF-β increased SphK1 expression in CF. Importantly, TGF-β-stimulated increases in collagen production were both SphK1- and S1P2-dependent and could be inhibited with an anti-S1P monoclonal antibody (mAb) or a SphK inhibitor.
2. Methods
2.1. Materials
S1P was obtained from Avanti Polar Lipids (Alabaster, AL, USA) and prepared according to manufacturer’s recommendation. SphK inhibitor (F-12509a) was purchased from Calbiochem (Gibbstown, NJ, USA). Liberase Blendzyme 4 was obtained from Roche Applied Science (Indianapolis, IN, USA). Horseradish peroxidase-conjugated goat anti-rabbit and rabbit anti-mouse secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit polyclonal SphK1 antibody was from Abgent (San Diego, CA, USA). Collagenase type 2 was purchased from Worthington Biochemical Corporation (Lakewood, NJ, USA). Radiolabelled proline was obtained from Perkin Elmer (Wellesley, MA, USA). All other reagents were purchased from Sigma (St Louis, MO, USA).
2.2. Animals
Five-to-six-week-old, male C57BL/6 mice were purchased from Harlan (Indianapolis, IN, USA). Mice were housed in an air-conditioned room with a 12 h light–dark cycle and given standard chow with free access to water. Ethical approval for these studies was granted by the San Diego State University Office of Laboratory Animal Care. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
2.3. Preparation and culture of adult mouse cardiac fibroblast
CFs were prepared as follows: excised heart ventricles were minced and digested in 0.03 mg mL−1 Liberase Blendzyme 4 and 0.014% Trypsin at 37°C for 10 min, washed, and fresh digestion solution was added for an additional 75 min. Isolated cells were pelleted (1000 rpm for 5 min), washed three times, re-suspended in growth media [Dulbecco’s modified Eagle’s medium (DMEM)/10% fetal calf serum (FCS)/200 U mL−1 penicillin/200 µg mL−1 streptomycin] and incubated at 37°C with 90% air/10% CO2. Cells were allowed to adhere to T150 cm2 flasks for 2 h after which attached cells were supplemented with fresh media. All cells were used at passage 2. Cells were pre-treated for 30 min with Y-27632 (10 µM) to inhibit Rho kinase.
2.4. siRNA transfections
For collagen synthesis or immunoblot analysis, 3 × 104 cells were plated on 12-well plates, and for Rho assays 1 × 105 cells were plated on 6-well plates in growth media and allowed to adhere overnight. Lipid complexes of siRNA and Lipofectamine RNAiMax (Invitrogen, Carlsbad, CA, USA) were prepared, yielding 1 µM siRNA according to manufacturer’s directions, and added in DMEM (0% FCS) without antibiotics. The following siRNA constructs were used: s74219 for SphK1; s80820 for SphK2; 61102 for S1P1; 164753 for S1P2; 66573 for S1P3 (Ambion, Austin, TX, USA). After 4 h of transfection, 2% bovine serum albumin (BSA)/DMEM without antibiotics was added to yield a final concentration of 1% BSA. Cells were incubated for an additional 2 days before assays.
2.5. Polymerase chain reaction analysis
RNA was isolated from CF using Qiagen RNeasy kit (Valencia, CA, USA) according to the manufacturer’s protocol. cDNA was made using Reverse Transcriptase III from Invitrogen. For quantitative polymerase chain reaction (QPCR), Taqman-labelled primers for Collagen Iα1 (Invitrogen, MLUX3302453), S1P1, S1P2, S1P3, SphK1, SphK2, and GAPDH (Mm00514644_m1, Mm01177794_m1, Mm00515669_m1, Mm00474763_m1, Mm00448841_g1, Mm00445021_m1, and 4352339E, respectively) and real-time polymerase chain reaction master mix from Applied Biosystems (Foster City, CA, USA) were used for amplification of cDNA using iCycler from Bio-Rad (Hercules, CA, USA). Standard curves for each gene were created using the above-listed primers on mouse reference total RNA standard from Stratagene (La Jolla, CA, USA). Total cDNA representing each gene was back calculated using standard curve and normalized to GAPDH signal for that particular cDNA sample.
2.6. Immunoblot analysis
Immunoblot analysis was performed as previously described.15
2.7. Immunohistochemisty
Immunohistochemistry was performed essentially as previously described.15 Primary antibodies were used at the following dilutions: α-smooth muscle actin (α-SMA) antibody (Sigma) at 1:500; Collage Type I (Rockland, Gillbertsville, PA, USA) at 1:20; SphK1 (Abcam, Cambridge, MA, USA) at 1:20. FITC-labelled phalloidin (Invitrogen) was added to secondary antibodies at 1:50 to label actin filaments. Topro (Invitrogen) was used at 1:500 to label nuclei.
2.8. Collagen synthesis
Collagenase sensitive [3H]-proline incorporation assay was performed as previously described.15
2.9. Rho activation
CFs were plated overnight on 6-well plates at 1 × 105 cells per well followed by 48 h of serum starvation in DMEM (0% FCS). Cells were then treated with media alone (control) or with 1 µM S1P for indicated times. Rho activation was assessed using G-LISA Rho Activation Assay Biochem Kit from Cytoskeleton (Denver, CO, USA) using the manufacturer’s recommended protocol.
2.10. Sphingosine kinase assay
SphK assays were performed using a modified version of Olivera et al.16 A total of 1.7 × 105 cells were plated on 6-well plates in growth media and allowed to adhere overnight followed by 48 h of serum starvation in DMEM, treated with media alone (control) or appropriate treatments in duplicate wells, and lysates were made, assayed, and extracted. The organic phase was separated and dried down followed by resuspension in 400 µL of delipidized human serum. S1P levels were quantified using S1P ELISA.6
2.11. Statistical analysis
One-way analysis of variance (ANOVA) and graphical representations were performed using PRISM 4.0 from GraphPad (San Diego, CA, USA). Statistical significance was set at P < 0.05.
3. Results
3.1. Sphingosine-1-phosphate-stimulated myofibroblast transformation is Rho- and S1P2-dependent
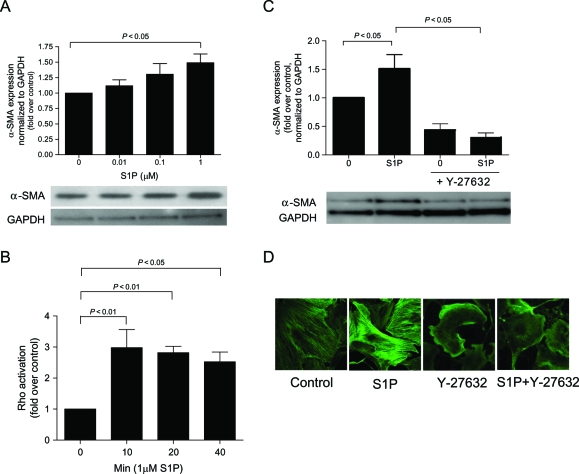
To determine the effects of S1P on transformation of CFs into collagen-producing myofibroblasts, we evaluated α-SMA expression (a myofibroblast marker) in CF. As Figure 1A demonstrates S1P stimulated α-SMA expression in a dose-dependent manner.
Figure 1.
Sphingosine-1-phosphate (S1P)-stimulated myofibroblast transformation is Rho-dependent. α-SMA expression was measured using immunoblot or immunohistochemical analysis in cardiac fibroblasts that were (A) serum starved for 48 h and then stimulated for 48 h with serum-free media alone (control) or 0.01, 0.1, or 1 µM sphingosine-1-phosphate and (C and D) 1 µM sphingosine-1-phosphate with or without Y-27632 (Rho kinase inhibitor). (B) Rho activation was measured using Rho ELISA on cells that were serum starved for 48 h then stimulated with or without 1 µM sphingosine-1-phosphate for 10, 20 or 40 min. Data are represented as fold change over control. Values represent mean ± SEM of at least four independent experiments. Statistical analyses were performed using one-way analysis of variance with Bonferroni post hoc analysis.
Activation of the low molecular weight G-protein, Rho, is required for myofibroblast transformation,17 and S1P has been shown in CF (Figure 1B) and non-CFs to activate Rho.18 To examine the role of Rho in S1P-induced myofibroblast transformation, cells were pre-treated for 30 min with the Rho kinase inhibitor, Y-27632, followed by S1P. Addition of the Rho kinase inhibitor decreased S1P-induced α-SMA expression (Figure 1C and D).
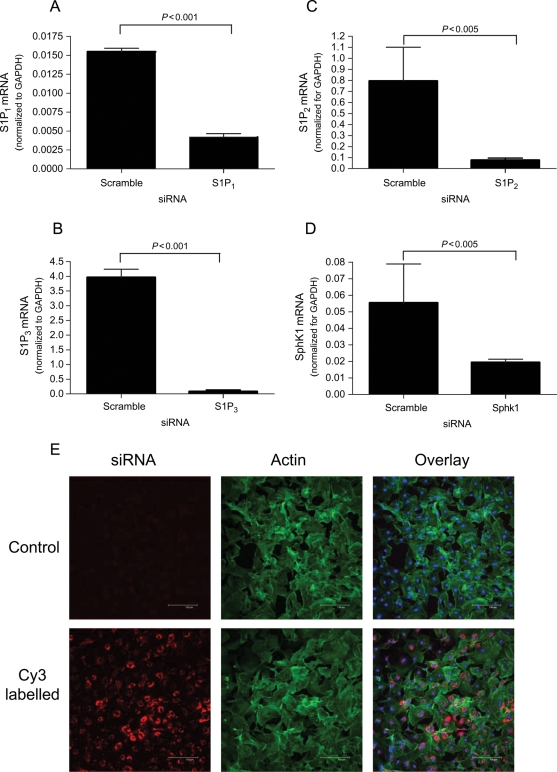
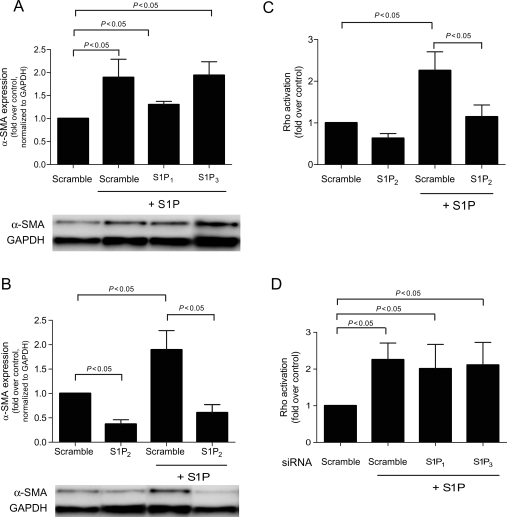
To determine which receptor is responsible for S1P-stimulated α-SMA expression and Rho activation, siRNAs were directed against the predominant receptors for S1P: S1P1, S1P2, or S1P3.19 siRNA transfection efficiency was nearly 100% as judged by transfection with fluorescently tagged scramble siRNA (Figure 2). As seen in Figure 2, when evaluated with gene-specific QPCR, the RNA levels for S1P1, S1P2, S1P3, and SphK1 were significantly knocked down to 26, 13, 2, and 33% of scrambled siRNA control, respectively. No reduction in α-SMA expression was observed in response to knockdown of S1P1 or S1P3 (Figure 3A). In contrast, silencing of the S1P2 gene by siRNA completely abrogated S1P-stimulated α-SMA expression (Figure 3B), suggesting that this isoform of the receptor is responsible for α-SMA protein expression and the differentiation of fibroblasts to the myofibroblast phenotype. Consistent with the requirement with the role of Rho in S1P-stimulated α-SMA expression, Figure 3C shows that S1P-stimulated Rho activation was also inhibited S1P2 knockdown. Once again, S1P1 and S1P3 siRNAs had no effect on Rho activation (Figure 3D). In addition, no reduction in cell viability was observed in response to these siRNA constructs.
Figure 2.
siRNA transfection efficiency and mRNA knockdown. (A–D) Quantitative polymerase chain reaction was used to evaluate mRNA of targeted genes after 48 h transfection with specific siRNAs or scramble siRNA. (E) Cardiac fibroblasts were transfected with Cy3 tagged scrambled siRNA or lipofectamine only (control) and imaged. Phalloidin (green) and Topro (blue) were used to label actin and nuclei, respectively. Data are represented as fold change over negative control siRNA only. Values represent mean ± SEM of at least three independent experiments. Statistical analyses were performed using Student’s t-test.
Figure 3.
Sphingosine-1-phosphate (S1P)-stimulated myofibroblast transformation and Rho activation are S1P2-dependent. α-SMA expression was measured using immunoblot analysis in cardiac fibroblasts that were transfected with (A) S1P2 siRNA or (B) S1P1 or S1P3 siRNA for 48 h then treated for 48 h with serum-free media alone or 1 µM sphingosine-1-phosphate. Rho activation was measured using Rho ELISA on cells that were transfected with (C) S1P2 siRNA or (B) S1P1 or S1P3 siRNA for 48 h then stimulated with or without 1 µM sphingosine-1-phosphate. Data are represented as fold change over control. Values represent mean ± SEM of at least four independent experiments. Statistical analyses were performed using one-way analysis of variance with Bonferroni post hoc analysis.
3.2. Sphingosine-1-phosphate-stimulated collagen production by cardiac fibroblast is Rho- and S1P2-dependent
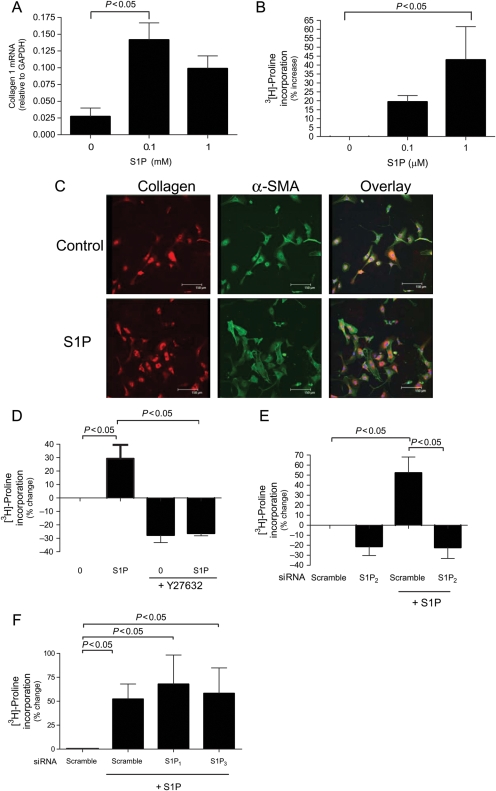
Since myofibroblasts are known to be a collagen-producing phenotype, we assessed the ability of S1P to stimulate collagen production. For these studies, assays for both quantitative collagen Iα1 mRNA expression and collagenase sensitive [3H]-proline incorporation into collagen were employed. Figure 4A demonstrates that S1P induced a dose-dependent increase in collagen RNA expression as measured by QPCR with a maximal increase observed at 0.1 µM S1P. There were no changes in collagen III (data not shown). Consistently, S1P induced a dose-dependent increase in collagen protein production by CF (as measured by proline incorporation), demonstrating a 43% increase in response to 1 µM S1P (Figure 4B). Immunofluorescence was used to validate these results and those from Figure 1A, as seen in Figure 4C, whereby 1 µM S1P increased both α-SMA and collagen production. Basal levels of both proteins were seen in untreated cells. As seen with α-SMA protein expression (Figure 1C), the Rho kinase inhibitor, Y-27632, abrogated S1P-induced collagen protein expression (Figure 4D).
Figure 4.
Sphingosine-1-phosphate (S1P) increases collagen production in a Rho-dependent manner in cardiac fibroblast (CF). (A) Cardiac fibroblasts were serum starved for 48 h and then stimulated for 48 h with serum-free media alone (control) or 0.1, or 1 µM sphingosine-1-phosphate and collagen mRNA was measured by quantitative polymerase chain reaction using gene-specific primers. (C) Cardiac fibroblasts were serum starved for 48 h and then stimulated for 48 h with serum-free media alone (control) or 1 µM sphingosine-1-phosphate and collagen (red) and α-SMA (green) were measured using immunofluorescence and co-stained with Topro for nuclei (blue). Collagen expression was measured using collagenase sensitive [3H]-proline incorporation assay in cells that were (B) serum starved for 48 h and treated with serum-free media alone (control) or 0.1, or 1 µM sphingosine-1-phosphate (D) serum starved for 48 h followed by treatment with serum-free media alone or 1 µM sphingosine-1-phosphate with or without Y-27632 (Rho kinase inhibitor) and (E and F) transfected with S1P1, S1P2, or S1P3 siRNA for 48 h then treatment with or without 1 µM sphingosine-1-phosphate. Data are represented as percentage change from control. Values represent mean ± SEM of at least three independent experiments. Statistical analyses were performed using one-way analysis of variance with Bonferroni post hoc analysis.
In order to determine the receptor-selective effects of S1P on collagen production by CF, we investigated the effects of siRNA-mediated knockdown of S1P1, S1P2, or S1P3 on S1P-stimulated [3H]-proline incorporation. Figure 4E shows that knockdown of S1P2 abrogated S1P-stimulated collagen expression. Silencing of either S1P1 or S1P3 had no effect on S1P-mediated [3H]-proline incorporation (Figure 4F).
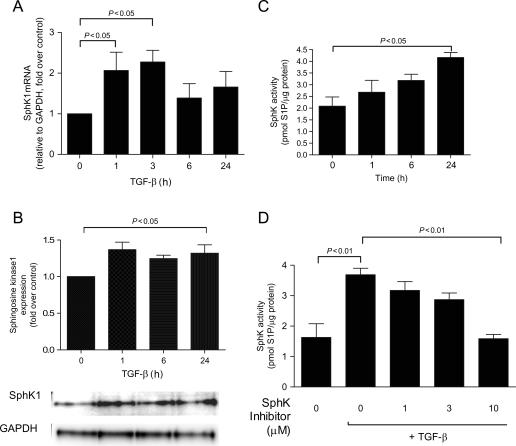
3.3. Transforming growth factor-β increases sphingosine kinase expression and activity
The well-known pro-fibrotic mediator, TGF-β, has been shown to increase SphK1 expression in other cell types.14 To investigate whether TGF-β regulates SphK in CF, SphK RNA, protein, and activity levels were evaluated. Figure 5A shows there was greater than two-fold increase in SphK1 mRNA expression after 1 and 3 h of TGF-β (10 ng mL−1) treatment, whereas no change in SphK2 mRNA expression was observed (data not shown). Co-ordinately, TGF-β significantly increased SphK1 protein expression (Figure 5B). In addition to regulating both SphK1 message and protein expression, TGF-β stimulated SphK enzymatic activity in a time-dependent manner (Figure 5C). The TGF-β-increased SphK activity was abolished by the addition of the SphK inhibitor (Figure 5D). The SphK inhibitor had no effect on cellular viability (data not shown).
Figure 5.
Transforming growth factor-β (TGF-β) stimulates sphingosine kinase 1 (SphK1) mRNA, expression, and activity. Cardiac fibroblasts were serum starved for 48 h, treated with transforming growth factor-β (10 ng/mL) for the indicated times and (A) mRNA was harvested and evaluated for sphingosine kinase 1 expression, (B) protein was harvested and evaluated for sphingosine kinase 1 expression, (C) cell lysates were subjected to sphingosine kinase activity assay, and (D) cells were pre-treated for 30 min with various sphingosine kinase inhibitor concentrations followed by 24 h of transforming growth factor-β treatment. Data are represented as fold change over control or picomoles of sphingosine-1-phosphate/microgram protein. Values represent mean ± SEM of at least four independent experiments. Statistical analyses were performed using one-way analysis of variance with Bonferroni post hoc analysis.
3.4. Transforming growth factor-β-induced collagen expression is dependent on sphingosine kinase 1
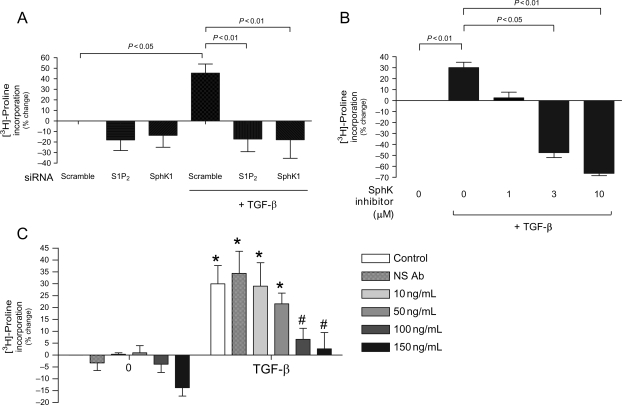
Considering the ability of TGF-β to regulate SphK1 expression and activity in CF, we next evaluated whether TGF-β-stimulated collagen production involved the activation of SphK1. For these studies, SphK1 action was blocked using siRNA against the SphK1 or the use of a SphK inhibitor. In addition, we employed an anti-S1P mAb6 to determine whether the mechanism for TGF-β-stimulated collagen production in CF involved increased S1P production and subsequent autocrine activation of S1P receptors. As shown in Figure 6A, knockdown of either SphK1 or S1P2 using siRNA completely suppressed TGF-β-stimulated collagen expression as measured by the proline incorporation assay. Consistently, TGF-β-stimulated proline incorporation into collagen was also blocked by the use of the SphK inhibitor (Figure 6B).
Figure 6.
Transforming growth factor-β (TGF-β-stimulated collagen expression is sphingosine kinase 1 (SphK1)- and S1P2-dependent. Collagen expression was measured using collagenase sensitive [3H]-proline incorporation assay in cells that were (A) transfected for 48 h with scramble control, S1P2, or sphingosine kinase 1 siRNA and then treated with serum-free media alone (control) or 10 ng/mL transforming growth factor-β (B) serum starved for 48 h followed by treatment with serum-free media alone or 10 ng/mL transforming growth factor-β with varying concentrations of sphingosine kinase inhibitor and (C) serum starved for 48 h followed by treatment with serum-free media alone or 10 ng/mL transforming growth factor-β with or without varying concentrations of anti-sphingosine-1-phosphate monoclonal antibody (S1P Ab) or non-specific (NS) Ab. Data are represented as percentage change from control. Values represent mean ± SEM of at least four independent experiments. Asterisk indicates significance from comparable treatment without transforming growth factor-β. Hash (#) indicates significant reduction from transforming growth factor-β without anti-sphingosine-1-phosphate monoclonal antibody. Statistical analyses were performed using one-way analysis of variance with Bonferroni post hoc analysis.
These findings suggest that SphK may regulate agonist-mediated increases in collagen production by CF via ‘inside-out’ autocrine S1P signalling. Previous studies have shown that SphK1 activation results in the production of a pool of S1P that can be released from cells during this inside-out signalling process act in an autocrine fashion on S1P receptors.20 To evaluate whether TGF-β-stimulated collagen production by CF results, in part, from this inside-out signalling process, we employed a highly specific anti-S1P mAb6 to neutralize extracellular S1P signalling that might have been released into the cell-conditioned medium. As the data in Figure 6C clearly demonstrate, addition of the anti-S1P mAb was capable of inhibiting TGF-β-stimulated collagen production in a dose-dependent manner. At 150 µg mL−1 of anti-S1P mAb, TGF-β-stimulated increases in proline incorporation were substantially abrogated. Addition of a non-specific isotype-matched antibody had no significant effect.
4. Discussion
S1P is a pleiotropic signalling molecule that can regulate multiple, sometimes divergent, downstream signalling pathways. S1P receptors differentially couple to three G proteins, Gi, Gq, and G12/13.21 Although S1P1 couples exclusively to Gi, S1P2 and S1P3 can couple to all three G proteins.22 G12/13 exclusively activates the small GTPase, Rho, which is necessary for fibroblast transformation and function of fully mature myofibroblasts.23 Constitutively, active Rho up-regulates α-SMA promoter activity, and Rho is necessary for stress fibre and focal adhesion formation.17 Rho kinase knockout mice exhibit dramatic decreases in cardiac fibrosis,24 and the Rho kinase inhibitor (Y-27632) decreases interstitial fibrosis in mice post-MI.25 Consistent with these findings, the data presented here demonstrate that S1P-stimulated myofibroblast transformation and collagen production are dependent upon activation of Rho kinase, likely via signalling through G12/13.
Figure 1 clearly demonstrates that S1P promotes myofibroblast transformation of CF (as indicated by increased α-SMA expression). Reports using fibroblasts from other tissues have demonstrated that the pro-fibrotic function of S1P is dependent on either S1P2 or S1P3.26,27 Specifically, S1P2 knockout mice have reduced hepatic wound healing as quantified by a reduction in the presence of α-SMA expressing hepatic myofibroblasts.26 Thus, in order to understand which S1P receptors were involved in the observed S1P- stimulated α-SMA expression in CF, siRNA knockdown techniques were employed for all three S1P receptors expressed by CF. Although knockdown of S1P1 and S1P3 had no effect on S1P-stimulated myofibroblast transformation or collagen production, knockdown of S1P2 abrogated S1P-mediated increases in α-SMA expression as well as collagen mRNA and protein expression, indicating that S1P2 is a key mediator of S1P-induced pro-fibrotic signalling in CF.
One of the more interesting findings outlined in this report is the observation that S1P appears to facilitate the action of the well-known pro-fibrotic factor, TGF-β. TGF-β is one of the most widely studied and recognized contributors to fibrosis and the heart28 and other tissues.29 TGF-β increases both production and secretion of collagens11 and promotes transformation of fibroblasts and epithelial cells into fibro-contractile myofibroblasts.30,31 Original work done by Trojanowska and colleagues established a connection between S1P and TGF-β in fibroblasts.32 Using YSR2, the yeast equivalent to SphK, fused to a luciferase reporter, they found that TGF-β stimulated kinase activity. In addition, TGF-β up-regulated SphK1 expression and activity leading to increased expression of TIMP-1, a protein that inhibits ECM degradation.14
In light of these findings, and the S1P-stimulated collagen production seen in Figure 4, we sought to investigate the potential for TGF-β/S1P cross-talk in CF. As seen in Figure 6, when the TGF-β-stimulated increase in SphK1 expression was inhibited with siRNA or a SphK inhibitor, TGF-β-stimulated collagen expression was abrogated. This suggests that endogenous S1P production is necessary for TGF-β-stimulated collagen expression. To further elucidate this idea, we used siRNA knockdown techniques targeted to the specific S1P receptor that we linked to S1P-stimulated collagen expression: S1P2. This also inhibited TGF-β-stimulated collagen expression. Further, the addition of the anti-S1P mAb to TGF-β-treated cells abrogated collagen expression. This demonstrates that neutralizing by molecular absorption any endogenously produced S1P from the cell culture media could prevent ‘inside-out’ S1P signalling. Further, the anti-S1P mAb was titrated to approximate the concentration of S1P released in response to TGF-β treatment (Figure 6C). Based on a molecular weight of 150 kDa, the anti-S1P mAb at 150 µg mL−1 is equivalent to 1 µM of full-length antibody. The anti-S1P mAb has at least two binding sites for S1P (one for each Fab’) and, therefore, at 150 µg mL−1 could bind 2 µM S1P. As seen in Figure 6C, 100 and 150 µg mL−1 of anti-S1P mAb effectively inhibit TGF-β-stimulated increases in proline incorporation. At 50 µg mL−1, over half of the effective inhibition is lost, and at 10 µg/mL there is no detectable inhibition. This suggests an approximate IC50 of 0.66 µM anti-S1P mAb for the inhibition of proline incorporation and indicates that these cells release a substantial amount of S1P into the cell-conditioned media approximating 1.32 µM. This substantial extracellular S1P production accounts for the observation that, in the absence of any agonist, siRNA to S1P2 is able to knockdown α-SMA (Figure 3B) and collagen expression (Figure 4E). We postulate that CFs constitutively produce and release S1P and that likely accounts for the transformation of CFs to the myoblast phenotype if cells are cultured for longer periods in minimal media. It is, therefore, surprising that we can observe additional stimulatory effects of endogenous S1P considering the robust constitutive nature of S1P release. An ‘inside-out’ mechanism has previously been identified for platelet-derived growth factor (PDGF) signalling whereby PDGF stimulated fibroblast migration7 and mast cell chemotaxis.33 This is the first time that TGF-β has been shown to utilize this type of signalling in association with S1P.
Interestingly, knockdown of SphK1 and S1P2 slightly reduced basal proline incorporation in the absence of either exogenous S1P or TGF-β addition (Figure 6A). Likewise, molecular absorption of endogenously produced S1P using an anti-S1P mAb slightly reduced basal collagen production. These findings suggest a role for basal S1P release in collagen production by CF through ‘inside-out’ signalling. Changes in basal levels of Rho were not observed as a result of siRNA knockdown of S1P2 in the Rho activation assay, suggesting that low levels of Rho activation may be facilitated through S1P receptors other than S1P2.
The data presented in this study suggest that S1P is an important, previously unrecognized, mediator of cardiac fibrosis through its direct and indirect effects on CF and its coordination with TGF-β. Our data suggest that S1P plays a very important role in stimulation of cardiac fibrosis by TGF-β through an ‘inside-out’ signalling. These data support the hypothesis that TGF-β stimulates production of S1P, via increased SphK1 activity and expression, which is then released and, in an autocrine and paracrine fashion, acts on the S1P2 receptor to promote pro-fibrotic cellular transformation and function. Not only is this the first time that S1P has been identified as having a direct role in CF function, but this is also the first report demonstrating a link between S1P and TGF-β-associated fibrosis in the heart.
Conflict of interest: N.G.L., K.M.M., J.S.S., and R.A.S. are shareholders in Lpath Inc., whose proprietary anti-S1P mAb is utilized in this manuscript.
Funding
Grant from the National Institute of Health (HL075906-01). N.G.L. is a Fellow of the Rees-Stealy Research Foundation and the San Diego State University Heart Institute.
References
- 1.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol. 2007;293:H1883–H1891. doi: 10.1152/ajpheart.00514.2007. [DOI] [PubMed] [Google Scholar]
- 3.Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005;45:657–687. doi: 10.1146/annurev.pharmtox.45.120403.095802. [DOI] [PubMed] [Google Scholar]
- 4.Sun Y, Kiani MF, Postlethwaite AE, Weber KT. Infarct scar as living tissue. Basic Res Cardiol. 2002;97:343–347. doi: 10.1007/s00395-002-0365-8. [DOI] [PubMed] [Google Scholar]
- 5.Long CS, Brown RD. The cardiac fibroblast, another therapeutic target for mending the broken heart? J Mol Cell Cardiol. 2002;34:1273. doi: 10.1006/jmcc.2002.2090. [DOI] [PubMed] [Google Scholar]
- 6.Visentin B, Vekich JA, Sibbald BJ, Cavalli AL, Moreno KM, Matteo RG, et al. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell. 2006;9:225–238. doi: 10.1016/j.ccr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 7.Hobson JP, Rosenfeldt HM, Barak LS, Olivera A, Poulton S, Caron MG, et al. Role of the sphingosine-1-phosphate receptor EDG-1 in PDGF-induced cell motility. Science. 2001;291:1800–1803. doi: 10.1126/science.1057559. [DOI] [PubMed] [Google Scholar]
- 8.Karliner JS. Mechanisms of cardioprotection by lysophospholipids. J Cell Biochem. 2004;92:1095–1103. doi: 10.1002/jcb.20129. [DOI] [PubMed] [Google Scholar]
- 9.Urata Y, Nishimura Y, Hirase T, Yokoyama M. Sphingosine 1-phosphate induces alpha-smooth muscle actin expression in lung fibroblasts via Rho-kinase. Kobe J Med Sci. 2005;51:17–27. [PubMed] [Google Scholar]
- 10.Youm JK, Jo H, Hong JH, Shin DM, Kwon MJ, Jeong SK, et al. K6PC-5, a sphingosine kinase activator, induces anti-aging effects in intrinsically aged skin through intracellular Ca2+ signaling. J Dermatol Sci. 2008;51:89–102. doi: 10.1016/j.jdermsci.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Lijnen P, Petrov V. Induction of cardiac fibrosis by aldosterone. J Mol Cell Cardiol. 2000;32:865–879. doi: 10.1006/jmcc.2000.1129. [DOI] [PubMed] [Google Scholar]
- 12.Miller AV, Alvarez SE, Spiegel S, Lebman DA. Sphingosine kinases and sphingosine-1-phosphate are critical for transforming growth factor beta-induced extracellular signal-regulated kinase 1 and 2 activation and promotion of migration and invasion of esophageal cancer cells. Mol Cell Biol. 2008;28:4142–4151. doi: 10.1128/MCB.01465-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kono Y, Nishiuma T, Nishimura Y, Kotani Y, Okada T, Nakamura S, et al. Sphingosine kinase 1 regulates differentiation of human mouse lung fibroblasts mediated by TGF-beta1. Am J Respir Cell Mol Biol. 2007;37:395–404. doi: 10.1165/rcmb.2007-0065OC. [DOI] [PubMed] [Google Scholar]
- 14.Yamanaka M, Shegogue D, Pei H, Bu S, Bielawska A, Bielawski J, et al. Sphingosine kinase 1 (SPHK1) is induced by transforming growth factor-beta mediates TIMP-1 up-regulation. J Biol Chem. 2004;279:53994–54001. doi: 10.1074/jbc.M410144200. [DOI] [PubMed] [Google Scholar]
- 15.Swaney JS, Roth DM, Olson ER, Naugle JE, Meszaros JG, Insel PA. Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase. Proc Natl Acad Sci USA. 2005;102:437–442. doi: 10.1073/pnas.0408704102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olivera A, Barlow KD, Spiegel S. Assaying sphingosine kinase activity. Methods Enzymol. 2000;311:215–223. doi: 10.1016/s0076-6879(00)11084-5. [DOI] [PubMed] [Google Scholar]
- 17.Anderson S, DiCesare L, Tan I, Leung T, SundarRaj N. Rho-mediated assembly of stress fibers is differentially regulated in corneal fibroblasts and myofibroblasts. Exp Cell Res. 2004;298:574–583. doi: 10.1016/j.yexcr.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Sugimoto N, Takuwa N, Okamoto H, Sakurada S, Takuwa Y. Inhibitory and stimulatory regulation of Rac and cell motility by the G12/13-Rho and Gi pathways integrated downstream of a single G protein-coupled sphingosine-1-phosphate receptor isoform. Mol Cell Biol. 2003;23:1534–1545. doi: 10.1128/MCB.23.5.1534-1545.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landeen LK, Aroonsakool N, Haga JH, Hu BS, Giles WR. Sphingosine-1-phosphate receptor expression in cardiac fibroblasts is modulated by in vitro culture conditions. Am J Physiol Heart Circ Physiol. 2007;292:H2698–H2711. doi: 10.1152/ajpheart.01065.2006. [DOI] [PubMed] [Google Scholar]
- 20.Takabe K, Paugh SW, Milstien S, Spiegel S. ‘Inside-out’ signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60:181–195. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92:913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- 22.Kluk MJ, Hla T. Signaling of sphingosine-1-phosphate via the S1P/EDG-family of G-protein-coupled receptors. Biochim Biophys Acta. 2002;1582:72–80. doi: 10.1016/s1388-1981(02)00139-7. [DOI] [PubMed] [Google Scholar]
- 23.Sah VP, Seasholtz TM, Sagi SA, Brown JH. The role of Rho in G protein-coupled receptor signal transduction. Annu Rev Pharmacol Toxicol. 2000;40:459–489. doi: 10.1146/annurev.pharmtox.40.1.459. [DOI] [PubMed] [Google Scholar]
- 24.Rikitake Y, Oyama N, Wang CY, Noma K, Satoh M, Kim HH, et al. Decreased perivascular fibrosis but not cardiac hypertrophy in ROCK1+/− haploinsufficient mice. Circulation. 2005;112:2959–2965. doi: 10.1161/CIRCULATIONAHA.105.584623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hattori T, Shimokawa H, Higashi M, Hiroki J, Mukai Y, Tsutsui H, et al. Long-term inhibition of Rho-kinase suppresses left ventricular remodeling after myocardial infarction in mice. Circulation. 2004;109:2234–2239. doi: 10.1161/01.CIR.0000127939.16111.58. [DOI] [PubMed] [Google Scholar]
- 26.Serriere-Lanneau V, Teixeira-Clerc F, Li L, Schippers M, de Wries W, Julien B, et al. The sphingosine 1-phosphate receptor S1P2 triggers hepatic wound healing. FASEB J. 2007;21:2005–2013. doi: 10.1096/fj.06-6889com. [DOI] [PubMed] [Google Scholar]
- 27.Keller CD, Rivera Gil P, Tolle M, van der Giet M, Chun J, Radeke HH, et al. Immunomodulator FTY720 induces myofibroblast differentiation via the lysophospholipid receptor S1P3 and Smad3 signaling. Am J Pathol. 2007;170:281–292. doi: 10.2353/ajpath.2007.060485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin L, Zhou Z, Zheng L, Alber S, Watkins S, Ray P, et al. Cross talk between Id1 and its interactive protein Dril1 mediate fibroblast responses to transforming growth factor-beta in pulmonary fibrosis. Am J Pathol. 2008;173:337–346. doi: 10.2353/ajpath.2008.070915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elberg G, Chen L, Elberg D, Chan MD, Logan CJ, Turman MA. MKL1 mediates TGF-{beta}1-induced {alpha}-smooth muscle actin expression in human renal epithelial cells 10.1152/ajprenal.00142.2007. Am J Physiol Renal Physiol. 2008;294:F1116–F1128. doi: 10.1152/ajprenal.00142.2007. [DOI] [PubMed] [Google Scholar]
- 31.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato M, Markiewicz M, Yamanaka M, Bielawska A, Mao C, Obeid LM, et al. Modulation of transforming growth factor-beta (TGF-beta) signaling by endogenous sphingolipid mediators. J Biol Chem. 2003;278:9276–9282. doi: 10.1074/jbc.M211529200. [DOI] [PubMed] [Google Scholar]
- 33.Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, Rivera J, et al. Transactivation of sphingosine-1-phosphate receptors by FcepsilonRI triggering is required for normal mast cell degranulation and chemotaxis. J Exp Med. 2004;199:959–970. doi: 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]