Abstract
We describe intracranial local field potentials (LFP) recorded in the anterior cingulate cortex (ACC) of macaque monkeys performing a saccade countermanding task. The most prominent feature at ∼70% of sites was greater negative polarity after errors than after rewarded correct trials. This negative polarity was also evoked in unrewarded correct trials. The LFP evoked by the visual target was much less polarized, and the weak presaccadic modulation was insufficient to control the initiation of saccades. When saccades were cancelled, LFP modulation decreased slightly with the magnitude of response conflict that corresponds to the coactivation of gaze-shifting and -holding neurons estimated from the probability of canceling. However, response time adjustments on subsequent trials were not correlated with LFP polarity on individual trials. The results provide clear evidence that error- and feedback-related, but not conflict-related, signals are carried by the LFP in the macaque ACC. Finding performance monitoring field potentials in the ACC of macaque monkeys establishes a bridge between event-related potential and functional brain-imaging studies in humans and neurophysiology studies in non-human primates.
INTRODUCTION
Human errors in reaction time tasks are associated with the error-related negativity (referred to as ERN or Ne) and a later positive deflection (Pe) (e.g., Falkenstein et al. 1991; Gehring et al. 1993). The ERN has a frontocentral distribution over the scalp and peaks ∼100 ms after the incorrect response in choice-reaction time tasks or the uninhibited response on no-go trials (Scheffers et al. 1996). A dipole for the ERN can be located in the anterior cingulate cortex (ACC) (e.g., Dehaene et al. 1994; Miltner et al. 1997; van Veen and Carter 2002). At least three hypotheses have been proposed to explain this signal and the function it performs.
First, the error-monitoring hypothesis proposes that the ERN/Ne reflects a comparison between the representations of the overt error response and the correct response (Falkenstein et al. 1991; Gehring et al. 1993), a function comparable to other midline negativities signaling mismatch (Näätänen et al. 1978) and the N400 (Kutas and Hillyard 1984). However, the presence of frontocentral negativities during correct trials, albeit of smaller amplitude (e.g., Falkenstein et al. 2000; Vidal et al. 2000) is difficult for the error-monitoring hypothesis to account for (Coles et al. 2001).
Second, the reinforcement-feedback hypothesis proposes that this frontocentral negativity is elicited by feedback indicating error, loss, or punishment (Gehring and Willoughby 2002; Miltner et al. 1997). In particular, Holroyd and Coles (2002) hypothesize that the mesencephalic dopamine system conveys a reinforcement learning signal to the frontal cortex when participants commit errors. According to this model, the ERN is generated because the inhibitory influence of the dopaminergic innervation in the ACC is modulated, fine-tuning the ACC to enable more appropriate choices in the subsequent trial.
Third, the conflict-monitoring hypothesis proposes that control is recruited based on the coactivation of mutually incompatible response processes (Botvinick et al. 2001, 2004). This hypothesis was formulated originally based on fMRI evidence for a conflict-monitoring function of the ACC (Botvinick et al. 1999; Carter et al. 1998, 1999). Subsequent work suggested that response conflict was also reflected in the frontocentral N2 event-related potential component (Yeung et al. 2004), which is similar to the ERN and can be localized to an ACC-generator comparable to that of the ERN (Kopp et al. 1996).
Numerous experiments have sought to test the error-monitoring, reinforcement-feedback, and conflict-monitoring hypotheses (reviewed by Botvinick et al. 2004; Ridderinkhof et al. 2004; van Veen and Carter 2006). This extensive literature can be summarized with the statement that each hypothesis remains plausible, and none can be excluded entirely. One reason for this lack of conceptual resolution is the low spatial or temporal resolution of event related potentials (ERP) and functional magnetic resonance imaging (fMRI) measures. The opportunity to carry out invasive studies in non-human primates can contribute to resolving among these alternative hypotheses. In fact, in monkeys performing a saccade stop signal task, single-unit activity signaling errors, reinforcement, and response conflict has been observed in the supplementary eye field (SEF) (Stuphorn et al. 2000). Similarly, single-unit activity signaling errors and reinforcement, but not response conflict, has been observed in the dorsal bank of the ACC (Ito et al. 2003). These results are consistent with other results showing SEF and ACC unit modulation correlated with monitoring performance in macaque monkeys performing other tasks (Amiez et al. 2006; Isomura et al. 2003; Koyama et al. 2000, 2001; Nakamura et al. 2005; Niki and Watanabe 1979; Procyk and Joseph 2001; Procyk et al. 2000; Shidara and Richmond 2002; Shidara et al. 2005). However, scalp potentials are the summation of intracranial local field potentials and not unit discharges (reviewed by Nunez and Srinivasan 2005). Therefore drawing conclusions based on converging evidence from single unit studies in non-human primates and ERP or fMRI studies in humans entails several uncertain inferences.
The goal of this study was to lay the first planks in a bridge between monkey single-unit data and human ERP and fMRI data by determining whether local field potentials (LFPs) signaling error, reinforcement, or conflict are observed in the ACC of macaque monkeys performing a saccade stop signal (or countermanding) task. This task requires subjects to produce speeded responses that countermand, or cancel, a partially prepared movement to a target when a stop signal is presented at various stages of preparation (Hanes and Schall 1995; Logan 1994; Logan and Cowan 1984). A saccade version of the stop signal task has been used to examine the role of the frontal eye field and superior colliculus in controlling the initiation of saccades (Hanes et al. 1998; Paré and Hanes 2003; J. W. Brown, D. P. Hanes, J. D. Schall, and V. Stuphorn, unpublished results) and the role of the SEF and the ACC in monitoring performance (Ito et al. 2003; Stuphorn et al. 2000) but not controlling saccade initiation (Stuphorn et al. 2007).
The present study reports the characteristics of LFPs that were recorded simultaneously with single units in the ACC of monkeys performing the saccade stop signal task. We determined whether intracerebral negativities (like the ERN/Ne) and positivities (like the Pe) occur in the ACC when monkeys made countermanding errors. We also investigated whether the pre-movement LFPs were modulated in a manner sufficient to control saccade initiation. Finally, we determined whether LFPs in the ACC were modulated in a manner consistent with signaling response conflict. The results provide clear evidence that LFP in the ACC do not contribute to saccade initiation and that error- and feedback-related, but not conflict-related, LFP modulation occur in the ACC of macaque monkeys.
METHODS
Data were collected from two male bonnet monkeys (Macaca radiata: 8−10 kg) that were cared for in accordance with U. S. Department of Agriculture and Public Health Service Policy on the humane care and use of laboratory animals. Each animal was tested for ∼4 h/day, 5 day/wk. During testing, water or fruit juice was given as positive reinforcement. Access to water in the home cage was controlled and monitored. Fluids were supplemented as needed. Detailed descriptions of all surgical procedures, electrophysiological techniques behavioral training, and tasks have appeared previously (Hanes and Schall 1995; Hanes et al. 1998).
The experiments were under computer control to present stimuli, record eye movements, and deliver liquid reinforcement. Stimuli were presented on a video monitor (48 × 48°) using computer-controlled raster graphics (Peritek VCH-Q, 512 × 512 resolution or TEMPO Videosync 1,280 × 1,040 resolution). The fixation spot subtended 0.37 of visual angle, and the target stimuli subtended from 0.3 to 3° of visual angle, depending on their eccentricity and had a luminance of 10 or 30 cd/m2 on a 1-cd/m2 background. Eye position was monitored via a scleral search coil or a video-based infrared eye tracker (ASL, Bedford, MA) while monkeys were head-restrained and seated in an enclosed chair within a magnetic field. Saccades were detected using a computer algorithm that searched for significantly elevated velocity (30°/s). Saccade initiation and termination were defined as the beginning and end of the monotonic change in eye position during the high-velocity gaze shift.
The countermanding task provided the data for this study. All trials began when the monkey shifted gaze to fixate a centrally located stimulus for a variable interval (500−800 ms; Fig. 1). Following this fixation interval, the central stimulus was removed and simultaneously a peripheral target was presented at one of two locations in opposite hemifields cuing the monkey to make a single saccade to the target. Targets were located along the horizontal axis and 10° from the fixation target in the vast majority of sessions. For trials with no stop signal, monkeys were reinforced for making a saccade within 700 ms. In each behavioral session, the delay between fixation of the target and delivery of reinforcement was constant at 400 ms. On 20−50% of the trials, after a delay, referred to as the stop signal delay (SSD), the central fixation target reappeared, instructing the monkey to inhibit saccade initiation. Two outcomes were possible on these stop signal trials. Maintaining fixation on the stop signal for 700 ms after the target appeared was reinforced as correct; these trials were referred to as cancelled trials. On stop signal trials, a saccade to the target was considered incorrect, and thus resulted in a 1,500-ms timeout with no reinforcecment. These trials were referred to as noncancelled trials. In each behavioral session, three to six SSDs of constant value ranging from 25 to 450 ms were used. The values were adjusted across sessions and monkeys to adjust for overall changes in response time so that, on average, monkeys failed to inhibit approximately half the stop signal trials.
FIG. 1.
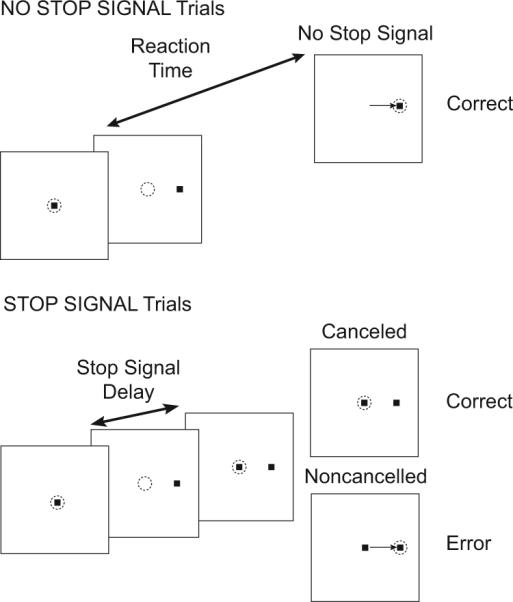
Trial displays for saccade countermanding task. Dotted circle indicates focus of gaze at each interval; arrow, the saccade. All trials began with presentation of a central fixation spot. After fixation of this spot for a variable interval, it disappeared simultaneous with presentation of a target on the left or right. In no-stop signal trials, a single saccade to the peripheral target was reinforced as the correct response. In stop signal trials, the fixation spot reappeared after a variable stop signal delay. Maintained fixation was reinforced as the correct response; these are referred to as cancelled (or signal-inhibit) trials. If a saccade was produced in spite of the stop signal, no reinforcement was given; these errors are referred to as noncancelled (or signal-respond) trials.
Here we report data from 130 sites in the ACC of two monkeys. Data were recorded serially along acute single penetrations. An individual site consisted of all the behavioral and neurophysiological data recorded from a single location in the cortex. Some of the behavioral and neurophysiological data from these monkeys have appeared in other publications (Hanes et al. 1998; Ito et al. 2003; Stuphorn et al. 2000, 2007; Brown, D. P. Hanes, J. D. Schall, and V. Stuphorn, unpublished data).
Data acquisition
LFPs were recorded using single tungsten microelectrodes (impedance: 2−5 MΩ at 1 kHz), nonreferenced single ended. The electrode signals were amplified with a high-input impedance head stage (>1GΩ, ∼2 pF of parallel input capacitance) and filtered by a Multichannel Acquisition Processor (Plexon, Dallas, TX). The LFP data were filtered between 0.7 and 170 Hz with two cascaded one-pole low-cut Butterworth filters and a four-pole high-cut Butterworth filter and was sampled at 1 kHz. The reference used for both spikes and LFP was the same ground wire on the head-stage.
Data analysis
All recording sites were assessed for the occurrence of excessive noise. Recordings with recurring artifacts during time intervals of interest were excluded from analysis. The mean voltage in the 300 ms preceding target presentation for each valid trial was defined as the baseline and subtracted from the voltage for each trial. SSDs were varied according to the monkeys’ performance so that at the shortest SSD, monkeys generally inhibited the movement in >75% of the stop signal trials and at the longest delay, monkeys inhibited the movement in <25% of the stop signal trials. No selection was made on the basis of whether or not the LFP displayed task-related activity.
To identify intervals of significant LFP modulation across different trial types, single trial LFPs were time synchronized to stimulus presentation or saccade initiation and then time averaged for each trial type. The event-related LFPs were then filtered using a 50th-order low-pass finite impulse response digital filter with a cutoff of 20 Hz. A difference wave was produced by subtracting the time-synchronized activity in one condition from the other (e.g., noncancelled – latency-matched no stop). For all comparisons between trial types, the onset of a significant difference was defined as the instant the difference wave exceeded ±2 SD for ≥50 ms and achieved a difference of ±3 SD during that interval. This criterion was used to compare the LFP on trials with no stop signal to the LFP on cancelled and noncancelled trials.
The rationale and approach for the race model analysis of the countermanding data have been described in detail previously (Hanes and Schall 1995; Hanes et al. 1998; Logan and Cowan 1984). Briefly, the data obtained in the countermanding task are the inhibition function and the distribution of reaction times in no-stop signal trials. Inhibition functions plot the probability of noncancelled trials as a function of SSD and were fit with a cumulative Weibull function. The stop signal reaction time (SSRT), the length of time that was required to cancel the saccade, was estimated using two methods (reviewed by Band et al. 2003; Logan 1994). The first assumes that SSRT is a random variable, whereas the second method assumes that SSRT is constant (reviewed by Band et al. 2003). We obtained an overall estimate of SSRT estimates derived from both methods. An analysis of these data based on the race model was done to estimate the SSRT from the behavioral data collected while recording from each site in the ACC. Hanes et al. (1998) established the central benefit of the countermanding paradigm as capable of determining whether neural activity generates signals sufficient to control the production of movements. For some neural activity to play a direct role in controlling the initiation of an eye movement, it must be different during trials in which a saccade is initiated as compared with trials in which the saccade is inhibited. Moreover, this difference in activity must occur by the time the movement was cancelled.
To determine if LFPs recorded from the ACC were modulated in a manner sufficient to control the production of saccades, we compared the LFP on cancelled trials to the LFP on no-stop signal trials with saccade latencies greater than the SSD plus the SSRT. According to the race model, these are the no-stop signal trials in which the go process was slow enough that the stop process would have finished before the go process if the stop signal had occurred. The onset of significant differential activity was measured for each SSD collected at each site in the ACC. If significant modulation was measured, the time of that modulation was compared with the SSRT estimated from the behavioral data collected during each recording. To determine if LFP modulation was proportional to response conflict, the average polarity difference between cancelled and latency-matched no-stop signal trials was measured following the analysis of Stuphorn et al. (2000). To determine if the LFP signaled error or feedback, we measured polarization following saccade initiation and reward delivery. For each site, the LFP synchronized on saccade initiation on noncancelled trials was compared with the LFP synchronized on saccade initiation on no-stop signal trials. Response-synchronized LFPs were produced for saccades to each target separately and collapsed across targets.
RESULTS
Event-related LFP in ACC
In macaque monkeys performing the saccade stop signal task, the LFP recorded from the dorsal bank of the ACC exhibited weak stimulus-related and presaccadic negative polarization and pronounced postsaccadic modulation (Fig. 2). Note that in this and all subsequent figures plotting voltage on the ordinate, negative is up. Stimulus-evoked modulation of the intracranial LFP was common but of low magnitude. Stimulus-evoked LFP modulation was equally common for targets presented contralateral (77/130) or ipsilateral (70/130) to the recording site. The mean latency of the LFP modulation evoked by contralateral targets was 188 ± 101 (SD) ms and that for ipsilateral targets was 201 ± 77 ms. The onset latency was not different for ipsiversive versus contraversive saccades (P = 0.30 χ2 = 1.06, Kruskal-Wallis rank sum test).
FIG. 2.
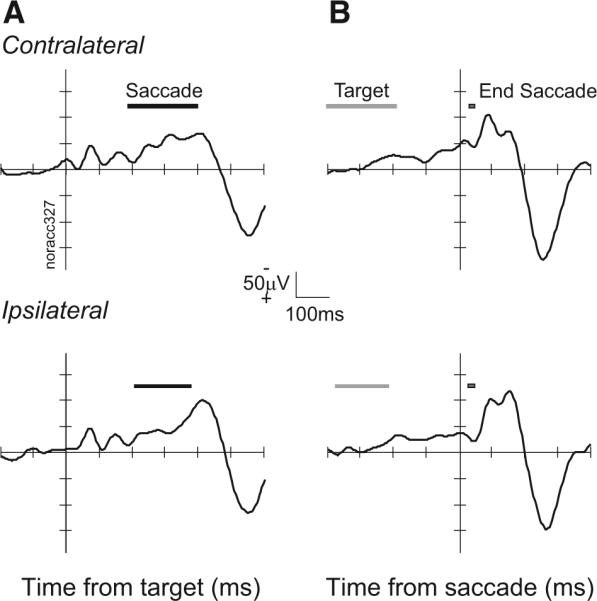
Event-related local field potentials (LFP) in anterior cingulate cortex (ACC) from representative site. A: LFP from no-stop signal trials synchronized on target presentation for contralateral (top, 149 trials) and ipsilateral (bottom, 143 trials) target. ■, range of saccade latencies. B: LFP synchronized on initiation of saccade to contralateral (top) and ipsilateral (bottom) target. ■, range of target onset times.
The LFP at a minority of sites in the ACC tended to become more negative immediately preceding saccade initiation, corresponding to a readiness potential (Evdokimidis et al. 1992; Everling et al. 1996). This was observed at 19% (25/130 sites) for contraversive and 9% (12/130sites) for ipsiversive saccades. The mean onset of this modulation relative to saccade initiation was −21 ± 14 ms for contraversive and −29 ± 16 ms for ipsiversive saccades.
Postsaccadic modulation of the LFP in the ACC was almost always observed and stronger than the presaccadic modulation. Overall we identified LFP modulations in the interval following the saccade in 91% (117/130) of the sites. LFP modulation was equally common following contraversive (106/130 sites) and ipsiversive (114/130 sites) saccades. This modulation began 47 ± 42 ms after contraversive and 69 ± 45 ms after ipsiversive saccades. The latency was significantly earlier after contraversive saccades (P < 0.01; χ2 = 30.73, Kruskal-Wallis rank sum test).
Effects of stop signal on stimulus-evoked LFP
The logic of the stop signal task and the measurement of SSRT using the race model suggest particular comparisons between stop signal and no-stop signal trials. First, cancelled stop signal trials can be compared with those no-stop signal trials with latencies long enough that the saccade would have been cancelled if a stop signal had occurred. Specifically, the LFP from cancelled stop signal trials can be compared with the LFP from no-stop signal trials with saccade latencies greater than SSD + SSRT. Second, noncancelled stop signal trials can be compared with those no-stop signal trials with latencies short enough that the saccade would not have been cancelled if a stop signal had occurred. Specifically, the LFP from noncancelled stop signal trials can be compared with the LFP from no-stop signal trials with saccade latencies less than SSD + SSRT. We refer to the subset of no-stop signal trials compared with either cancelled or noncancelled stop signal trials as latency-matched.
Figure 3 illustrates these comparisons for stimulus-aligned LFPs from a representative site in the ACC. Consider first the comparison between cancelled trials and latency-matched no-stop signal trials (Fig. 3A). When examined in this manner, movement- and fixation-related but not visual neurons in the FEF and the SC exhibit a pronounced modulation in cancelled trials occurring before the SSRT (Hanes et al. 1998; Paré and Hanes 2003). This modulation occurs in a manner and at a time sufficient to be interpreted as controlling whether the saccade is initiated. We observed a significant difference between the LFP recorded on cancelled trials and that recorded on latency-matched no-stop signal trials in only 38% (206/537) of the SSDs sampled across 130 sites in the ACC. In approximately half of these SSDs (21%, 104/537), the LFP polarity on cancelled trials was more negative than on no-stop trials, and in the other half (20%, 102/537), the LFP on cancelled trials was more positive than on no-stop trials. However, this polarity difference occurred on average 220 ± 98 ms after the SSRT. A significant polarization difference between cancelled trials and no-stop trials before the SSRT occurred for only 2 of the 537 SSDs sampled. This result clearly demonstrates that presaccadic LFPs in the ACC do not modulate in a manner sufficient to control the initiation of saccades.
FIG. 3.
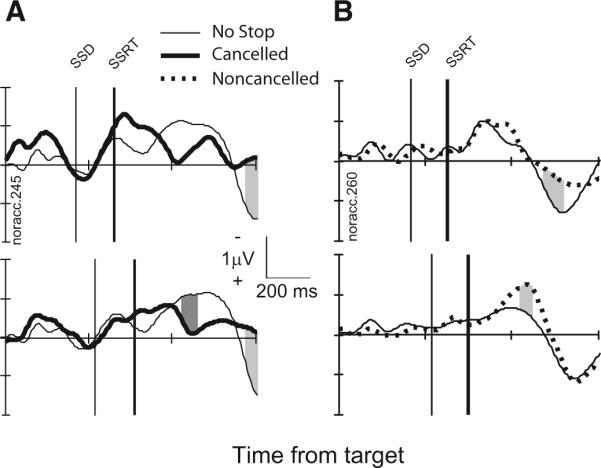
LFP in stop signal trials from a representative site. A: comparison of LFP in cancelled stop signal trials (thick) and latency-matched no-stop signal trials (thin) with stop signal delays of 169 ms (top, 198 no-stop trials trials; 26 cancelled trials) and 217 ms (bottom, 137 no-stop trials; 58 cancelled trials). Intervals in stop signal trials in which polarity is significantly more negative are highlighted by dark gray. Intervals in stop signal trials in which polarity is significantly more positive are highlighted by light gray. B: comparison of LFP in noncancelled stop signal trials (thick, dotted) and latency-matched no-stop signal trials (thin) with stop signal delays of 217 ms (top, 67 no-stop trials; 19 cancelled trials) and 269 ms (bottom, 165 no-stop trials; 49 cancelled trials).
Consider next the comparison between noncancelled trials and latency-matched no-stop signal trials (Fig. 3B). A critical assumption of the race model is that the go and stop processes are independent (Logan and Cowan 1984). Hanes et al. (1998) tested the assumption of independence (whether the presence of the stop process affected the timing of the go process) by comparing the target aligned neural activity on noncancelled trials to latency-matched no-stop signal trials. When examined in this manner, neurons in the FEF and the SC exhibit identical activation in noncancelled and no-stop signal trials (Hanes et al. 1998; Paré and Hanes 2003). We compared the LFP polarization on noncancelled trials to that on no-stop signal trials with saccade latencies less than SSD + SSRT. These are the no-stop signal trials in which the go process was fast enough that the go process would have finished before the stop process if the stop signal had been presented. On 37% (222/589) of the SSDs across 130 sites in the ACC, we observed a significant LFP modulation for noncancelled trials versus latency-matched no-stop signal trials with half (110/589) showing greater negativity and half (112/589) showing greater positivity in noncancelled trials. The overall latency of this modulation was 246 ± 139 ms after the SSRT. Thus presentation of the foveal visual stop signal does not influence ACC LFP polarization on noncancelled trials before the SSRT.
Tests of ACC LFP conflict signal
Botvinick et al. (2001) postulated that conflict between incompatible response processes signals the need for control by the executive system. This hypothesis can be evaluated using behavioral performance and physiological data from the saccade stop signal task in two ways.
The first test involves relating LFP signals in the ACC to the amount of response conflict in different trials. Performance in countermanding tasks can be accounted for by a race between go and stop processes (Logan and Cowan 1984); in the saccade stop signal task this race is accomplished through the interaction between gaze-shifting and -holding circuits in the FEF and SC (Hanes et al. 1998; Paré and Hanes 2003). In fact, an interactive race model with mutual inhibition between a go unit and a stop unit fits performance data as well as the independent race if and only if the timing of modulation of the go and stop units correspond to the actual modulation times of movement and fixation neurons (Boucher et al. 2007). In this framework, the coactivation of movement (go) and fixation (stop) units engenders response conflict. Now, cancelled trials include a period during which movement (go) and fixation (stop) neurons are coactive; this period of coactivation does not occur in noncancelled error trials because the fixation neurons (and the stop unit in the model) do not turn on before the movement neurons (and the go unit in the model) reach the threshold of activation to trigger the movement. Furthermore, the magnitude of coactivation of movement (go) and fixation (stop) units in cancelled trials increases as the probability of a noncancelled saccade increases; this occurs because the activation of the movement (go) units grow progressively closer to the threshold. Thus a given amount of activation of fixation (stop) units sufficient to inhibit the growing activation of movement (go) units multiplied by the magnitude of activation of movement (go) units will result in higher response conflict. A population of neurons in the SEF of monkeys performing the saccade stop signal task was modulated after SSRT to a degree that was proportional to the probability of a noncancelled saccade and so may signal response conflict (Stuphorn et al. 2000). Thus the first test of the conflict-monitoring theory is to determine whether the LFP exhibits polarity differences in cancelled as compared with latency-matched no-stop signal trials that vary systematically with the probability of a noncancelled saccade.
Figure 4 plots the stimulus-evoked LFPs for cancelled stop signal trials and for latency-matched no-stop signal trials at a single site in the dorsal bank of the ACC for the three of four SSDs with sufficient trials (>10) to provide a reliable value. The average difference in LFP polarity between the trial types was measured in the interval starting 50 ms before to 150 ms after SSRT. This interval was chosen because it corresponds to the interval in which single-unit modulation related to response conflict was observed in the SEF (Stuphorn et al. 2000). For this site, the LFP polarity difference between cancelled and latency-matched no-stop signal trials became more positive with SSD and increasing probability of producing an errant noncancelled saccade (Fig. 4C). To determine whether the variation in LFP polarity difference was related to SSD or to performance, we analyzed the regression of the LFP polarity difference between trial types as a function of SSD and of the probability of producing a noncancelled saccade. The polarity difference in the LFP between cancelled and no-stop signal trials did not vary with SSD (slope = 0.0005, t = 0.61, P = 0.29; Fig. 4D, top), but it did vary significantly with the probability of producing a noncancelled saccade in a stop signal trial (slope = −0.36, t = 4.17, P < 0.01; Fig. 4D, bottom). However, the polarity difference between cancelled and latency-matched no-stop signal trials decreased with the probability of failing to cancel the saccade. This is opposite the pattern of modulation of SEF neurons signaling conflict (Stuphorn et al. 2000) and also incompatible with the variation of response conflict in this task, which increases on canceled trials as a function of the decreasing probability of canceling.
FIG. 4.
First test for conflict-related activity. A: inhibition function plots characteristic increasing probability of a noncancelled saccade as a function of stop signal delay. B: LFPs from representative site synchronized on stimulus onset for cancelled trials (thick solid line) at stop signal delays of 168, 216, and 268 ms (labeled in A) were compared with latency-matched no-stop signal trials (thin solid line). Average polarity difference between LFPs in cancelled and latency-matched no-stop signal trials in the interval from 50 ms before to 100 ms after stop signal reaction time (SSRT, highlighted by gray box) was measured. The vertical thin and thick black lines represent the stop signal delay (SSD) and SSRT, respectively. (B1: 215 no-stop trials, 51 cancelled trials; B2: 153 no-stop trials, 47 cancelled trials; B3: 55 no-stop trials, 15 cancelled trials). C: average polarity difference between cancelled and latency-matched no-stop signal trials plotted as a function of P (noncancelled|stop signal). The decreasing trend is significant. D: Z-scored average voltage difference across 314 stop signal delays plotted as function of SSD (top) and P (noncancelled|stop signal) (bottom). The polarity difference became significantly less negative with increasing P (noncancelled|stop signal).
The second test involves determining whether LFP signals in the ACC relate to adjustments of performance; specifically, the magnitude of the response time adjustment on a given trial should increase with the magnitude of conflict on the previous trial (e.g., Kerns et al. 2004). Consistent with this, saccade latency increases significantly following cancelled stop signal trials, which are the type of trial in which conflict between the go and stop units occurs (e.g., Emeric et al. 2007a). We tested this prediction by measuring the trial-by-trial correlation between the LFP signal in the interval around SSRT in trial N and the response time adjustment in trial n + 1 (Fig. 5). For each trial, the maximum negative-going deflection in the interval from 50 ms before to 150 ms after the SSRT was plotted against the adjustment in reaction time on the subsequent no-stop trial. Although a significant correlation was observed at some sites, across all the sites examined, response time adjustments were not correlated with the magnitude of the LFP negativity on cancelled trials. Thus according to another criterion, LFPs in the ACC do not appear to signal response conflict.
FIG. 5.
Second test for conflict related activity. A: LFP aligned on the estimate of SSRT for the subset of 45 cancelled stop signal trials that were followed by no-stop signal trials from a single session. Red circles mark peak negative polarity in the interval from 50 ms before to 150 ms after SSRT. B: peak negative polarity plotted as a function of the response time adjustment on the subsequent no-stop trial. No trend was evident. C: distribution of correlations between peak negativity in cancelled trials and response time adjustment in next trial. No relationship was found across all the sites examined.
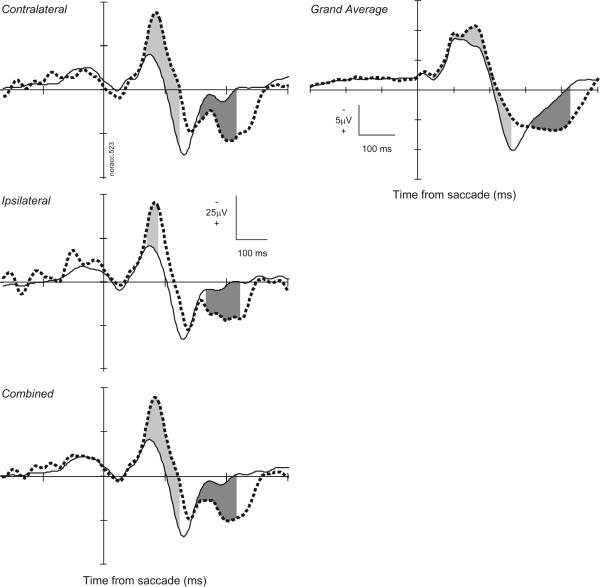
Tests of ACC LFP error signal
Modulation of the intracranial LFP following saccade production was common for both no-stop signal trials and noncancelled trials. Figure 6 plots comparisons of the response-synchronized LFPs from the ACC on noncancelled trials and all no-stop signal trials. The intracranial error-related potential was defined as the onset of the first significant negative-going potential following the saccade. Overall, an intracranial error-related potential was identified in 69% (89/130) of the sites when the LFP was combined across targets. This prevalence is evident by the clear polarization observed in the grand average LFP. In this grand average, a statistically significant negativity began 40 ms after the saccade; however, the largest quantitative negativity began ∼125 ms after the saccade. Measured across individual sites, this potential began 148 ± 77 ms after saccade initiation. LFP modulation was equally common following contraversive (78/130 sites) or ipsiversive (74/130 sites) saccades. Measured site by site, the latency of this modulation following contraversive saccades was 181 ± 89 ms and that following ipsiversive saccades was 178 ± 57 ms; these distributions were not significantly different (P = 0.31; χ2 = 1.04, Kruskal-Wallis rank sum test). The earlier onset for the grand average and combined data as compared with the site-by-site values is a simple result of improving signal-to-noise through averaging.
FIG. 6.
Error-related LFP. Left: LFP from a representative site aligned on saccade initiation for error noncancelled stop signal trials (thick dashed) and correct no-stop signal trials (solid) for contraversive (top, 172 no-stop trials; 17 cancelled trials), ipsiversive (middle, 168 no-stop trials; 11 cancelled trials), and both (bottom, 340 no-stop trials; 28 cancelled trials) saccades. Right: grand average LFP from 130 sites in the dorsal bank of the ACC aligned on saccade initiation for error noncancelled and correct no-stop signal trials. Intervals in which the polarity of noncancelled error LFP was significantly more negative than that in no-stop signal trials indicated by light gray fill. Intervals in which polarity of noncancelled error LFP was significantly more positive than that in no-stop signal trials indicated by dark gray fill.
We also observed a later, positive-going potential following errors. This was defined as the onset of the first significant positive-going potential following the saccade. Overall an intracranial error-related positive potential was identified in 82% (106/130) of the sites when the LFP was combined across targets. The error-related positivity in the grand average began 316 ms and peaked 424 ms after the onset of the error saccade. Measured across sites, this potential began 319 ± 84 ms after saccade initiation. The positivity was equally common following contraversive (84/130 sites) and ipsiversive (89/130 sites) saccades. Its latency following contraversive saccades (329 ± 64 ms) was not significantly different from that following ipsiversive saccades (334 ± 69 ms; P = 0.41 χ2 = 0.67, Kruskal-Wallis rank sum test).
We compared the latency of these negative- and positive-going error-related potentials to the onset of error-related spike rate modulation in the SEF and the ACC (Fig. 7). Error-related unit modulation occurs earlier in the SEF than in the ACC (Ito et al. 2003). The negative-going potential in the ACC occurred later than the SEF error cell modulation (P < 0.01; χ2 = 16.66, Kruskal-Wallis rank sum test) and synchronously with the ACC error cell modulation (P = 0.55; χ2 = 0.36, Kruskal-Wallis rank sum test). The positive-going error-related potential in the ACC occurred later than the SEF error cell modulation (P < 0.01; χ2 = 87.38, Kruskal-Wallis rank sum test) and also later than the ACC error cell modulation (P < 0.01; χ2 = 55.91, Kruskal-Wallis rank sum test).
FIG. 7.
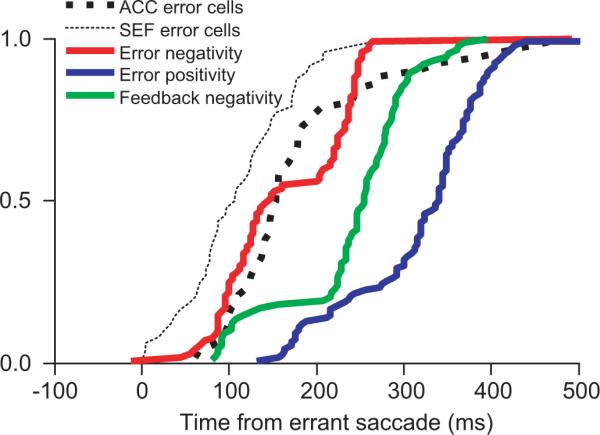
Cumulative distributions of onset of error-related negative polarity LFP (red), error-related positive polarity LFP (blue), and the feedback-related negative polarity LFP (green). These are compared with latency of error related units in supplementary eye field (SEF, thin black) and in ACC (thick black).
Several studies have examined the relationship between the ERN and posterior adjustments (e.g., Debener et al. 2005). We examined the trial-by-trial covariation of the error-related LFP and the response time adjustment on the n + 1 trial (Fig. 8). For each noncancelled trial, the maximum negative-going deflection in the 0- to 250-ms interval and the maximum positive-going deflection in the 200- to 500-ms interval after the errant saccade were plotted against the difference in reaction time on the subsequent no-stop trial. Although significant correlations were observed at some recording sites, response time adjustments were not correlated with the LFP peak negativity (t = 0.88, P = 0.38) or peak positivity (t = 0.32, P = 0.75) across all the sites examined after errors had been produced (Fig. 8B).
FIG. 8.

Error-related LFP and the response time adjustment. A: response-synchronized LFP for noncancelled stop signal trials that were followed by no stop signal trials (top). •, peak negative value in the 250-ms interval following the response on each of the 37 individual trials. ▲, peak positive value in 250- to 500-ms interval following the response on individual trials. Peak negative and positive polarization plotted against the response time adjustment on the subsequent no stop trial (bottom). B: correlation coefficients for peak negativity (top) and peak positivity (bottom) as a function of RT adjustment.
Tests of ACC LFP reinforcement-feedback signal
To determine whether LFPs in the ACC were modulated by feedback about reinforcement, we compared the LFPs synchronized on the time of reinforcement when it was delivered and when it was withheld in correct no-stop signal trials (Fig. 9). This could be done because the delay between the end of the saccade to the target and delivery of reinforcement was fixed at 400 ms and therefore entirely predictable. A significant negative-going potential was measured in 40% (46/116) of the sites with the LFP combined across contraversive and ipsiversive saccades; this modulation began 256 ± 204 ms after the time when reinforcement would have been delivered. This latency was significantly longer than the error-related modulation after saccades (P < 0.01; χ2 = 40.64, Kruskal-Wallis rank sum test). Thus LFPs in macaque ACC also signal reinforcement feedback.
FIG. 9.
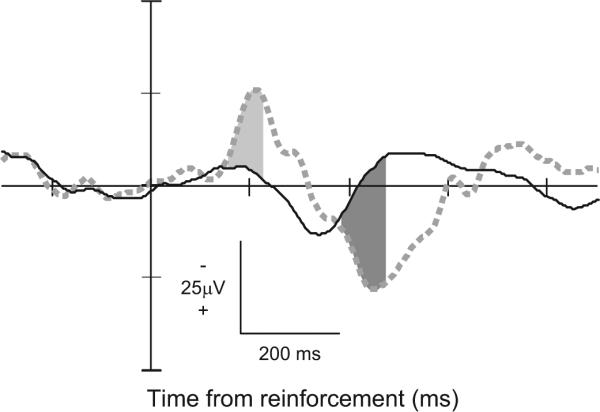
Feedback-related LFP from a representative site. LFP aligned on time of reinforcement following contra- and ipsiversive saccades in reinforced (solid) and unreinforced no-stop signal trials (461 rewarded no-stop trials; 35 unrewarded no-stop trials). The pattern of polarization resembles that observed following stop trial errors. Intervals on unreinforced trials in which the polarity was significantly more negative than that on reinforced trials is indicated by light gray fill; intervals of significantly more positive polarity indicated by dark gray fill. The significantly more negative polarity began 156 ms following time that reinforcement would have been delivered. The significantly more positive polarity began 388 ms after scheduled reinforcement.
Location of intracranial potentials
Nearly all of the intracranial error-related potentials were recorded from the dorsal bank of the anterior cingulate sulcus within area 24c as judged by depth relative to the overlying SEF and other landmarks. The sites with intracranial error-related potentials were distributed in a strip extending from 3 mm caudal to 4 mm rostral of the SEF (Fig. 10). This region is coextensive with an area of the ACC that is reciprocally connected with the SEF (Huerta and Kaas 1990), in which single units signal errors and the receipt of reinforcement (Ito et al. 2003).
FIG. 10.
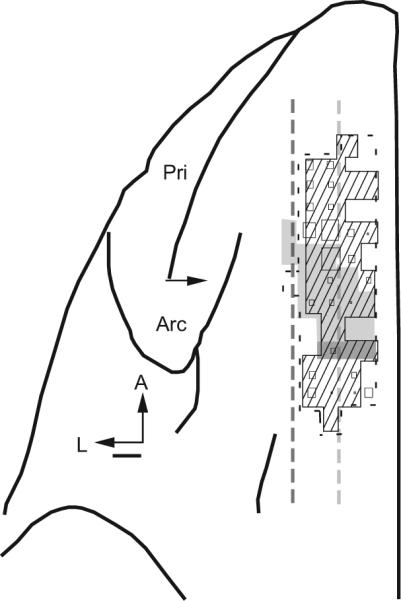
Location of sites with error LFP signals. Top view of the left frontal lobe of monkey N. Neural activity was sampled within the region bounded by the thin dashed line. The area in which error-related and reinforcement-related single-unit activity was encountered in ACC indicated by cross-hatching (from Ito et al. 2003). Number of error-related LFPs recorded indicated by the size of the squares. Single-unit and LFP signals were concentrated in the dorsal bank of the cingulate sulcus. Other landmarks include the extent of the SEF defined by low thresholds (<50 μA) for eliciting saccades with intracortical electrical stimulation (light gray fill), the rostral extent of the forelimb representation in the supplementary motor area (dark gray fill), the lateral extent of the gray matter in the medial wall (light gray dashed line), and the fundus of the cingulate sulcus (dark gray dashed line). These lines appear straight because the mediolateral extent of the cingulate sulcus varies little in the frontal lobe of macaques. The arcuate (Arc) and principal (Pri) sulci are labeled. Horizontal arrow marks 27 mm anterior to the interaural line. Scale bar, 1 mm.
DISCUSSION
We observed error-related and reinforcement-feedback potentials in the dorsal bank of the ACC in macaque monkeys performing a saccade stop signal task. However, the error-related potentials did not covary with response time adjustments. Moreover, vanishingly few sites exhibited LFP modulation sufficient to control the initiation of saccades. Finally, the LFPs recorded from the ACC yielded no evidence of a signal consistent with conflict monitoring.
These results constitute an initial step toward bridging human electrophysiology and monkey neurophysiology. Several reports have described ERPs from human subjects performing stop signal tasks (Bekker et al. 2005; De Jong et al. 1990, 1995; Dimoska et al. 2006; Kok et al. 2004; Naito and Matsumura 1994, 1996; Pliszka et al. 2000; Ramautar et al. 2004, 2006a,b; Stahl and Gibbons 2006; van Boxtel et al. 2001). Although these have employed variations in task demand, stop stimulus modality, and effector, some general conclusions seem plausible. Larger N2 and P3 components are observed in stop signal as compared with no-stop signal trials. Latency and some magnitude differences in components are observed when comparing canceled and noncancelled stop signal trials. An enhanced N2 on noncancelled trials may be identified with the ERN. However, the N2 observed on canceled trials is difficult to identify conclusively with a measure of conflict. Also clear modulation of ERP components before SSRT when movements are canceled in stop signal trials as compared with produced in no-stop signal trials has not been consistently reported. Source localization identifies the N2 and P3 components on canceled and noncancelled trials with different parts of the brain with the medial frontal cortex among other loci contributing. Although the results presented herein complement these observations, taken as a whole careful analysis of this body of work highlights the need for further investigation coordinated across species, task conditions, and effectors.
Stimulus-related and postsaccadic modulation
LFPs in the ACC were much more polarized in the interval following saccade initiation than in the interval following stimulus onset. This is consistent with single-unit studies observing increased activity related to trial outcome following responses (Amiez et al. 2005, 2006; Isomura et al. 2003; Ito et al. 2003; Niki and Watanabe 1979; Procyk and Joseph 2001; Procyk et al. 2000; Shidara and Richmond 2002). However, visual responses have been observed in the ACC that are contingent on the probability of reward (e.g., Koyama et al. 2001; Shidara and Richmond 2002; Shima et al. 1991) as well as in the context of the saccade stop signal task (Pouget et al. 2005). The ACC receives few visual afferents, mainly from area PO, area 7a in the inferior parietal lobule, and inferotemporal area TG (Van Hoesen et al. 1993), the SEF (Huerta and Kaas 1990; Luppino et al. 1990) and a diffuse connection with FEF (Huerta et al. 1987; Stanton et al. 1993; Wang et al. 2004). This may account for the result that the LFP at fewer sites in the ACC were modulated in the interval following the stimulus as compared with the interval following the saccade.
Response control
Anatomical data have been interpreted as evidence for the ACC contributing to high level response control (e.g., Dum and Strick 1991; Morecraft and Van Hoesen 1992, 1993; Paus 2001). Apparent movement-related single-unit activity has been described in the ACC for self-paced and stimulus-triggered arm movements (Shima et al. 1991). Skeletal and ocular movements can be evoked by electrical microstimulation of the ACC (Hughes and Mazurowski 1962; Luppino et al. 1991; Mitz and Godschalk 1989; Showers 1959; Talairach et al. 1973). Thus ACC can be described as an ocular motor cortical area like FEF or SEF.
The countermanding paradigm provides a clear criterion for determining whether neural activity generates signals sufficient to control the production of movements. The key test is whether the activity of neurons if different between trials with a movement (no-stop signal or noncancelled trials) and trials with no movement (cancelled trials), and, critically, whether such a difference occurs before SSRT. If some neural modulation occurs after SSRT, then according to the race model that identifies SSRT with the time of inhibition of the movement the modulation is too late (Boucher et al. 2007; Logan and Cowan 1984).
Prior studies showed that movement and fixation but not visual neurons in the frontal eye fields and superior colliculi provide signals sufficient to control gaze (Hanes et al. 1998; Paré and Hanes 2003). Specifically, on both no-stop signal and noncancelled trials, the activity of movement neurons increases until the saccade is triggered, and the activity of fixation neurons decreases after the target is presented. In contrast, on cancelled trials, the activity of movement neurons approaches but does not achieve the level of activity at which the saccade is triggered, and the activity of fixation neurons, which had decreased after the target was presented, increases before the SSRT.
The present analysis of the ACC field potentials, revealed vanishingly few sites with LFP modulation when movements were canceled that was early enough to contribute to controlling the initiation of the saccades. At sites with a significant LFP modulation on cancelled trials, the latency was much longer than the SSRT (Fig. 3). This is consistent with the observation that saccades can be evoked by stimulation of few sites in the ACC (Luppino et al. 1991; Mitz and Godschalk 1989; Talairach et al. 1973).
This evidence against the ACC having a direct role in the control of gaze shifts is generally consistent with the results of lesion studies in both humans and monkeys. Human ACC lesion patients are not deficient in producing simple saccades to visual stimuli but are deficient in the ability to voluntarily inhibit reflexive saccades (Paus et al. 1991) and in the production of antisaccades, memory guided saccades, and sequences of visually guided saccades (Gaymard et al. 1998). Macaques with ACC lesions have deficits specific to the maintenance and selection of responses associated with different rewards but not in basic task performance (Kennerley et al. 2006; Rushworth et al. 2003).
Performance monitoring
A dipole for the ERN can be located in the ACC (e.g., Dehaene et al. 1994; Miltner et al. 1997; van Veen and Carter 2002). Both Falkenstein et al. (1991) and Gehring et al. (1993) initially proposed that the ERN/Ne reflects a comparison between the representations of the overt error response and the correct response. An ERN-like potential has also been identified in human intracerebral EEG recording (Brázdil et al. 2002, 2005) and error-related field potentials in the medial frontal cortex of monkeys (Gemba et al. 1986) but not in monkeys performing a task that requires executive control. The ERN was originally interpreted as an error-detection signal resulting from a mismatch between the response and the outcome of response selection (Falkenstein et al. 1990, 1991; Gehring et al. 1993). However, alternate accounts view the ERN as a brain signal reflecting detection of response conflict (Botvinick et al. 2001; Yeung et al. 2004) or representing the dopaminergic input to the ACC (Holroyd and Coles 2002). Such hypotheses ultimately require measurements of single units and field potentials that can be compared with the surface field potentials. Finding an intracranial homologue of the ERN is a necessary bridge.
Response conflict
The absence of field potentials in the ACC signaling conflict during the saccade stop signal task is incompatible with the general conflict-monitoring hypothesis of ACC function. The modulation of the N2 event-related potential during high-conflict trials have been emphasized as evidence for this conflict hypothesis (Botvinick et al. 2004; Yeung et al. 2004). According to this interpretation, the N2 and the ERN originate from the same neural process but are just observed at different times; response conflict on correct trials is supposed to precede the response and is manifested as the N2, whereas response conflict on error trials follows the response and is manifested as the ERN. Central tenets of the conflict hypothesis are that conflict is produced when mutually incompatible responses are active and response times increase following trials with high conflict.
We tested both of these predictions. First, the magnitude of ACC field potential modulation did not increase with the probability of noncancelled saccades (Fig. 4). In fact, the magnitude of the modulation decreased as the probability of noncancelled saccades approached 1.0. This result is contrary to other studies that have observed ERPs that increase with the level of response conflict (Gehring and Fencsik 2001; Yeung et al. 2004). Second, response time adjustment did not covary with the magnitude of ACC field potential modulation on the preceding cancelled trial (Fig. 5).
Examinations of single-unit activity in the medial frontal cortex of monkeys performing the saccade stop signal task have reported distinct populations of neurons that are modulated for errors, reinforcement, and response conflict (Ito et al. 2003; Stuphorn et al. 2000). Stuphorn et al. (2000) identified single units in the SEF modulated by response conflict on cancelled trials that were not modulated on noncancelled trials as well as separate SEF neurons modulated by noncancelled errors and reinforcement. Ito et al. (2003) identified single units in the ACC modulated by errors and reinforcement but not response conflict. Field potentials, both those recorded from the scalp and intracranially, are hypothesized to be produced by standing synaptic dipoles, a signal to which action potentials may not contribute. Therefore further work is required to examine field potentials in the medial frontal cortex for components that may contribute to conflict-related potentials recorded from the scalp.
The possibility exists that species, task, and effector differences may contribute to the differences observed for countermanding saccades in macaque monkeys versus human manual responses in the context of a flanker or stroop task. However, the ERN is evoked by saccade errors in the stop signal and antisaccade tasks (Endrass et al. 2005; Nieuwenhuis et al. 2001), and functional imaging has revealed that the ACC is active for cancelled and noncancelled saccades (Curtis et al. 2004). Therefore it is unlikely that the absence of ACC field potentials modulated by conflict is due to effector or task differences. Stahl and Gibbons (2007) have proposed an alternative account of conflict monitoring in the context of the manual version of the stop signal task. In their account, conflict is greater on noncancelled trials than on cancelled trials. Only one saccade can be produced at a time, but multiple simultaneous manual responses are common. Further investigation is required to determine if conflict produced for competing bimanual response representations differs from the conflict between competing gaze-shifting and -holding processes. This does not, however, rule out the possibility that conflict occurs in other parts of the medial frontal cortex. Conflict-related single-unit activity in the SEF and activation in the supplementary motor area have also been observed under conditions of response conflict (Garavan et al. 2003; Stuphorn et al. 2000).
Error monitoring
Converging evidence from imaging, ERPs, and intracranial field potentials have implicated the ACC as the generator of the ERN (reviewed by Bush et al. 2000). In this investigation, we consistently observed negative-going potentials followed by positive-going potentials after noncancelled errors throughout the dorsal bank of the ACC in monkeys performing a saccade stop signal task. This LFP modulation was not observed when comparing correct cancelled stop signal trials to correct no-stop signal trials. Therefore the LFP modulation was not evoked by the stop signal. The LFP modulation occurred after both contra- and ipsiversive errant noncancelled saccades. Therefore it is unlikely that this modulation is due to a sensory or movement-evoked potential. We therefore interpret this LFP modulation as signaling the occurrence of an error. Intracranial error-related potentials have been previously observed in the ACC of macaque monkeys (Gemba et al. 1986). In addition, intracranial error-related potentials have been observed in humans that covary in time with potentials recorded at the scalp (Brázdil et al. 2002, 2005). This evidence leads us to the conclusion that the error-related potentials observed in this study are intracranial analogs of the ERN/Ne and the Pe. Further work is required, though, to confirm that an ERN can be recorded extracranially in macaques.
Further evidence supporting this conclusion is found in the timing of the intracranial field potential relative to the human ERN. In humans performing manual stop signal tasks, an ERN is recorded that exhibits a peak negative deflection 80 ms after the error response (Kok et al. 2004; Ramautar et al. 2004, 2006a,b). Similarly, the ERN measured during an antisaccade task peaked ∼80 ms after error saccades (Nieuwenhuis et al. 2001). ERP components measured from the scalp are derived from LFPs distributed within some volume of tissue. We found that across individual sites, the ERN occurred as early as 12 ms before and as late as 300 ms after the errant saccade. Averaged across individual sessions, the intracranial error-related field potential began ∼150 ms after the errant saccade; however, in the grand average field potential, a significant negative-going polarization was measured beginning 40 ms and peaking 104 ms after the saccade. Given known conduction time differences between larger human and smaller macaque brains, these time values are very comparable.
Another line of evidence concerns the morphology of the polarization. Similar to the grand average error-related LFP reported here, the ERN waveform for saccades appears double-peaked (Nieuwenhuis et al. 2001; Van't Ent and Apkarian 1999). However, the response-locked ERN may overlap with the stop signal-locked N2, therefore the negative-going potentials observed following noncancelled errors may reflect both stop signal and error-related processing (e.g., Dimoska et al. 2006; Ramautar et al. 2004, 2006a,b). Thus the topographic and temporal similarity between the human ERN and the intracranial error-related negative-going field potential in the macaque ACC suggests that the intracranial potential contributes to the dipole producing the surface potential.
The error-detection hypothesis originally included the premise that ERN magnitude relates to response time adjustments (Coles et al. 1995; Gehring et al. 1993). Several studies have examined this relationship with diverse results using ERPs (Debener et al. 2005; Gehring and Fencsik 2001; Gehring et al. 1993; Scheffers et al. 1996) and fMRI (Debener et al. 2005; Garavan et al. 2003). We found that the variations in response time adjustment did not covary with the magnitude of error-related field potential modulation (negative- or positive-going) on the preceding noncancelled trial similar to other recent studies of human subjects (Gehring and Fencsik 2001; Nieuwenhuis et al. 2001). However, the general interpretation of these results should acknowledge that response times do not increase systematically following noncancelled saccade errors (Cabel et al. 2000; Emeric et al. 2007a), and the overwhelming majority of these saccades are not followed by an immediate corrective saccade back to the initial fixation (Ito et al. 2003). Thus it is possible that medial frontal error signals are not used to control response times in subjects performing the saccade stop signal task and are instead a generic monitor of the occurrence of errors (e.g., Holroyd et al. 1998).
Reinforcement learning
The reinforcement learning hypothesis proposes that the frontocentral negativity is elicited by events signaling error, loss of reinforcement, or punishment (e.g., Gehring and Willoughby 2002; Miltner et al. 1997). Holroyd and Coles (2002) hypothesize that the mesencephalic dopamine system conveys a negative reinforcement learning signal to the frontal cortex when human participants commit errors in reaction time tasks. They also proposed that errors induce phasic changes in mesencephalic dopaminergic activity that is manifest through ACC activity producing the ERN. Consistent with this, single units in ACC that discharge after errors are also active when earned reinforcement is withheld (Ito et al. 2003; see also Niki and Watanabe 1979). Also in monkeys performing the saccade stop signal task, other neurons in ACC modulate in a manner directly paralleling dopamine neurons (Ito et al. 2003). In other words, single units in the ACC signal whether ongoing events are better or worse than expected.
Consistent with the single-unit data, we observed feedback-related modulation on correct no-stop signal trials when reinforcement was withheld. However, further examination is required to test whether these LFPs are modulated in a way consistent with the reinforcement learning hypothesis. In particular, if the reinforcement learning hypothesis were true, then the amplitude of the LFP in the ACC should be modulated by reinforcement predictability, being large for unexpected errors and absent or possibly of reversed polarity for unexpected rewards (e.g., Holroyd et al. 2003).
Source localization
The ERN has a frontocentral distribution over the scalp such that a dipole for the ERN can be located in the ACC (e.g., Dehaene et al. 1994; Miltner et al. 1997; van Veen and Carter 2002). However, being an inverse problem (Helmholtz 1853), an effectively infinite number of dipoles can account for a given scalp potential topography. Intracranial recordings can contribute useful data to constrain the range of plausible solutions. We found prominent, mostly biphasic, LFPs resembling human scalp ERN/Pe potentials in the monkey ACC after noncancelled errors on stop signal trials. Our results are consistent with previous reports of error-related field potentials in the medial frontal lobe of macaques (Gemba et al. 1986). In addition, intracranial ERPs resembling scalp Ne/Pe potentials have been observed in ACC as well as several other cortical locations after incorrect trials in humans (Brázdil et al. 2002, 2005).
These observations must be viewed with appropriate skepticism though. Due to superposition, potentials generated by local and remote sources and sinks add algebraically at any given point so interpreting field potentials entirely in terms of local generators is uncertain. Thus it is possible that the field potentials we observed in the dorsal bank of the ACC arose from dipoles in, for example, the ventral bank of the ACC or more dorsally in the SEF. Evidence against this concern, though, includes preliminary results we have obtained showing attenuated or absent error-related field potentials in the ventral bank of the ACC (Emeric et al. 2003) and significantly less common error-related negative polarization in the SEF (Emeric et al. 2007b). Nevertheless, to resolve this localization problem most definitely, it will be necessary to record current source density across the medial frontal cortex, spanning the layers of the dorsal and ventral banks of the ACC (e.g., Dias et al. 2006).
Cingulate cortex and gaze control
We now consider how signals in the portion of the dorsal bank of the ACC, in which we found these LFP signals, might influence the ocular motor system. In doing so, though, it is critical to recognize that anatomical tracer studies have not been performed that restrict tracer injections to this portion of area 24c. Granting this, signals in the ACC can influence the ocular motor system because the rostral cingulate cortex of monkeys is oligosynaptically connected to extraocular motoneurons (Moschovakis et al. 2004). The ACC is only weakly connected with the FEF (Barbas and Mesulam 1981; Huerta and Kaas 1990; Stanton et al. 1993; Van Hoesen et al. 1993; Vogt and Pandya 1987; Vogt et al. 1987) and does not project to the SC (Fries 1983).
Other routes for the ACC to influence saccade production are available. First, the region of the ACC in which we recorded performance monitoring LFP signals is reciprocally connected with the SEF (e.g., Huerta and Kaas 1990; Luppino et al. 2003). Previous work has shown that subthreshold microstimulation of the SEF improves performance of the stop signal task by monkeys by delaying saccade initiation (Stuphorn and Schall 2006). Second, the ACC might also influence performance through connections with prefrontal areas 9 and 46 (Barbas and Pandya 1989; Selemon and Goldman-Rakic 1988; Vogt and Pandya 1987), but the role of these areas in saccade countermanding has not been investigated so no more can be inferred at this time. Third, Aron and Poldrak (2006) have emphasized a critical role of the subthalamic nucleus in response inhibition during a manual stop signal task. The subthalamic nucleus is innervated by the FEF and SEF but not ACC (e.g., Frankle et al. 2006; Huerta and Kaas 1990; Huerta et al. 1986). Finally, the ACC can exert a more subtle influence through its projections to the locus coeruleus (reviewed by Aston-Jones and Cohen 2005). Clearly, much more work is needed to determine the relative contributions of each of these pathways in the executive control of gaze.
Conclusion
This study provides evidence of an analog of the ERN in the ACC field potentials of monkeys performing a stop signal task. Electrophysiological studies have led to the current view that electrical potentials recorded at the scalp are the result of summed cortical LFPs, which are generated by the synchronous synaptic activity of populations of neurons. Finding error-related field potentials concomitantly with unit activity in the ACC provides a bridge between the human ERN literature and the monkey neurophysiology literature. These findings provide an avenue for more closely examining the neural events that give rise to human ERPs.
ACKNOWLEDGMENTS
We thank E. Crowder, A. Garr, and J. Jewett for assistance with manuscript preparation and M. Feurtado for help with animal care.
GRANTS
This work was supported by National Institutes of Health Grants F32-EY-017765, T32-MH-065782, R01-MH-55806, P30-EY-08126, and P30-HD-015052 and by Robin and Richard Patton through the E. Bronson Ingram Chair in Neuroscience.
REFERENCES
- Amiez C, Joseph JP, Procyk E. Anterior cingulate error-related activity is modulated by predicted reward. Eur J Neurosci. 2005;21:3447–3452. doi: 10.1111/j.1460-9568.2005.04170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiez C, Joseph JP, Procyk E. Reward encoding in the monkey anterior cingulate cortex. Cereb Cortex. 2006;16:1040–1055. doi: 10.1093/cercor/bhj046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Band GP, van der Molen MW, Logan GD. Horse-race model simulations of the stop-signal procedure. Acta Psychol. 2003;112:105–142. doi: 10.1016/s0001-6918(02)00079-3. [DOI] [PubMed] [Google Scholar]
- Barbas H, Mesulam MM. Organization of afferent input to subdivisions of area 8 in the rhesus monkey. J Comp Neurol. 1981;200:407–431. doi: 10.1002/cne.902000309. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1989;286:353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- Bekker EM, Kenemans JL, Hoeksma MR, Talsma D, Verbaten MN. The pure electrophysiology of stopping. Int J Psychophysiol. 2005;55:191–198. doi: 10.1016/j.ijpsycho.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Boucher L, Logan GD, Palmeri TJ, Schall JD. Inhibitory control in mind and brain: an interactive race model of countermanding saccades. Psychol Rev. 2007;114:376–397. doi: 10.1037/0033-295X.114.2.376. [DOI] [PubMed] [Google Scholar]
- Brázdil M, Roman R, Daniel P, Rektor I. Intracerebral error-related negativity in a simple go/nogo task. J Psychophysiol. 2005;19:244–255. [Google Scholar]
- Brázdil M, Roman R, Falkenstein M, Daniel P, Jurak P, Rektor I. Error processing–evidence from intracerebral ERP recordings. Exp Brain Res. 2002;146:460–466. doi: 10.1007/s00221-002-1201-y. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cabel DW, Armstrong IT, Reingold E, Munoz DP. Control of saccade initiation in a countermanding task using visual and auditory stop signals. Exp Brain Res. 2000;133:431–441. doi: 10.1007/s002210000440. [DOI] [PubMed] [Google Scholar]
- Carter CS, Botvinick MM, Cohen JD. The contribution of the anterior cingulate cortex to executive processes in cognition. Rev Neurosci. 1999;10:49–57. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Coles MG, Scheffers MK, Fournier L. Where did you go wrong? Errors, partial errors, and the nature of human information processing. Acta Psychol. 1995;90:129–144. doi: 10.1016/0001-6918(95)00020-u. [DOI] [PubMed] [Google Scholar]
- Coles MG, Scheffers MK, Holroyd CB. Why is there an ERN/Ne on correct trials? Response representations, stimulus-related components, and the theory of error-processing. Biol Psychol. 2001;56:173–189. doi: 10.1016/s0301-0511(01)00076-x. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Cole MW, Rao VY, D'Esposito M. Canceling planned action: an fMRI study of countermanding saccades. Cereb Cortex. 2004;15:1281–1289. doi: 10.1093/cercor/bhi011. [DOI] [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J Neurosci. 2005;25:11730–11737. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psych Sci. 1994;5:303–305. [Google Scholar]
- De Jong R, Coles MG, Logan GD. Strategies and mechanisms in nonselective and selective inhibitory motor control. J Exp Psychol Hum Percept Perform. 1995;21:498–511. doi: 10.1037//0096-1523.21.3.498. [DOI] [PubMed] [Google Scholar]
- De Jong R, Coles MG, Logan GD, Gratton G. In search of the point of no return: the control of response processes. J Exp Psychol Hum Percept Perform. 1990;16:164–182. doi: 10.1037/0096-1523.16.1.164. [DOI] [PubMed] [Google Scholar]
- Dias EC, McGinnis T, Smiley JF, Foxe JJ, Schroeder CE, Javitt DC. Changing plans: neural correlates of executive control in monkey and human frontal cortex. Exp Brain Res. 2006;174:279–291. doi: 10.1007/s00221-006-0444-4. [DOI] [PubMed] [Google Scholar]
- Dimoska A, Johnstone SJ, Barry RJ. The auditory-evoked N2 and P3 components in the stop-signal task: indices of inhibition, response-conflict or error-detection? Brain Cogn. 2006;62:98–112. doi: 10.1016/j.bandc.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Dum R, Strick P. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991;11:667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeric EE, Brown JW, Boucher L, Carpenter RH, Hanes DP, Harris R, Logan GD, Mashru RN, Pare M, Pouget P, Stuphorn V, Taylor TL, Schall JD. Influence of history on saccade countermanding performance in humans and macaque monkeys. Vision Res. 2007a;47:35–49. doi: 10.1016/j.visres.2006.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeric EE, Leslie M, Pouget P, Schall JD. Local field potentials in supplementary eye field of macaque monkeys during a saccade stop signal task: Performance monitoring. Soc Neurosci Abstr. 2007b;398.9 [Google Scholar]
- Emeric EE, Stuphorn V, Schall JD. Error-related local field potentials in medial frontal lobe of macaques during saccade countermanding. Soc Neurosci Abstr. 2003;79.20 [Google Scholar]
- Endrass T, Franke C, Kathmann N. Error awareness in a saccade counter-manding task. J Psychophysiol. 2005;19:275–280. [Google Scholar]
- Evdokimidis I, Mergner T, Lucking CH. Dependence of presaccadic cortical potentials on the type of saccadic eye movement. Electroencephalogr Clin Neurophysiol. 1992;83:179–191. doi: 10.1016/0013-4694(92)90143-6. [DOI] [PubMed] [Google Scholar]
- Everling S, Krappmann P, Spantekow A, Flohr H. Cortical potentials during the gap prior to express saccades and fast regular saccades. Exp Brain Res. 1996;111:139–143. doi: 10.1007/BF00229563. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of errors in choice reaction tasks on the ERP under focused and divided attention. In: Brunia CHM, Gaillard AWK, Kok A, editors. Psychophysiological Brain Research. Tilburg Univesity Press; Tilburg, The Netherlands: 1990. pp. 192–195. [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalogr Clin Neurophysiol. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: a tutorial. Biol Psychol. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Frankle WG, Laruelle M, Haber SN. Prefrontal cortical projections to the midbrain in primates: evidence for a sparse connection. Neuropsychopharmacology. 2006;31:1627–1636. doi: 10.1038/sj.npp.1300990. [DOI] [PubMed] [Google Scholar]
- Fries W. Cortical projections to the superior colliculus in the macaque monkey: a retrograde study using horseradish peroxidase. J Comp Neurol. 1984;230:55–76. doi: 10.1002/cne.902300106. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Kaufman J, Stein EA. A midline dissociation between error-processing and response-conflict monitoring. Neuroimage. 2003;20:1132–1139. doi: 10.1016/S1053-8119(03)00334-3. [DOI] [PubMed] [Google Scholar]
- Gaymard B, Rivaud S, Cassarini JF, Dubard T, Rancurel G, Agid Y, Pierrot-Deseilligny C. Effects of anterior cingulate cortex lesions on ocular saccades in humans. Exp Brain Res. 1998;120:173–183. doi: 10.1007/s002210050391. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Fencsik DE. Functions of the medial frontal cortex in the processing of conflict and errors. J Neurosci. 2001;21:9430–9437. doi: 10.1523/JNEUROSCI.21-23-09430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Gross B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychol Sci. 1993;4:385–390. [Google Scholar]
- Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295:2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Gemba H, Sasaki K, Brooks VB. ‘Error’ potentials in limbic cortex (anterior cingulate area 24) of monkeys during motor learning. Neurosci Lett. 1986;70:223–227. doi: 10.1016/0304-3940(86)90467-2. [DOI] [PubMed] [Google Scholar]
- Goldman PS, Nauta WJ. Columnar distribution of cortico-cortical fibers in the frontal association, limbic, and motor cortex of the developing rhesus monkey. Brain Res. 1977;122:393–413. doi: 10.1016/0006-8993(77)90453-x. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Patterson WF, 2nd, Schall JD. Role of frontal eye fields in countermanding saccades: visual, movement, and fixation activity. J Neurophysiol. 1998;79:817–834. doi: 10.1152/jn.1998.79.2.817. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Schall JD. Countermanding saccades in macaque. Vis Neurosci. 1995;12:929–937. doi: 10.1017/s0952523800009482. [DOI] [PubMed] [Google Scholar]
- Helmholtz HV. Ueber einige Gesetze der Vertheilung elektrischer Ströme in körperlichen Leitern mit Anwendung auf die thierisch-elektrischen Versuche. Ann Physik Chemie. 1853;89:211–233. 354–377. [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Dien J, Coles MG. Error-related scalp potentials elicited by hand and foot movements: evidence for an output-independent error-processing system in humans. Neurosci Lett. 1998;242:65–68. doi: 10.1016/s0304-3940(98)00035-4. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Nieuwenhuis S, Yeung N, Cohen JD. Errors in reward prediction are reflected in the event-related brain potential. Neuroreport. 2003;14:2481–2484. doi: 10.1097/00001756-200312190-00037. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Kaas JH. Supplementary eye field as defined by intracortical microstimulation: connections in macaques. J Comp Neurol. 1990;293:299–330. doi: 10.1002/cne.902930211. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Krubitzer LA, Kaas JH. Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkeys. I. Subcortical connections. J Comp Neurol. 1986;253:415–439. doi: 10.1002/cne.902530402. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Krubitzer LA, Kaas JH. Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkeys. II. Cortical connections. J Comp Neurol. 1987;265:332–361. doi: 10.1002/cne.902650304. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Mazurowski JA. Studies on the supracallosal mesial cortex of unanesthetized, conscious mammals. II. Monkey. A. Movements elicited by electrical stimulation. Electroencephalogr Clin Neurophysiol. 1962;14:477–485. doi: 10.1016/0013-4694(62)90053-6. [DOI] [PubMed] [Google Scholar]
- Isomura Y, Ito Y, Akazawa T, Nambu A, Takada M. Neural coding of “attention for action” and “response selection” in primate anterior cingulate cortex. J Neurosci. 2003;23:8002–8012. doi: 10.1523/JNEUROSCI.23-22-08002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Stuphorn V, Brown JW, Schall JD. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 2003;302:120–122. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- Kennerley SW, Walton ME, Behrens TE, Buckley MJ, Rushworth MF. Optimal decision making and the anterior cingulate cortex. Nat Neurosci. 2006;9:940–947. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, III, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kok A, Ramautar JR, De Ruiter MB, Band GP, Ridderinkhof KR. ERP components associated with successful and unsuccessful stopping in a stop-signal task. Psychophysiology. 2004;41:9–20. doi: 10.1046/j.1469-8986.2003.00127.x. [DOI] [PubMed] [Google Scholar]
- Kopp B, Rist F, Mattler UN. 200 in the flanker task as a neurobehavioral tool for investigating executive control. Psychophysiology. 1996;33:282–294. doi: 10.1111/j.1469-8986.1996.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Koyama T, Kato K, Mikami A. During pain-avoidance neurons activated in the macaque anterior cingulate and caudate. Neurosci Lett. 2000;283:17–20. doi: 10.1016/s0304-3940(00)00894-6. [DOI] [PubMed] [Google Scholar]
- Koyama T, Kato K, Tanaka YZ, Mikami A. Anterior cingulate activity during pain-avoidance and reward tasks in monkeys. Neurosci Res. 2001;39:421–430. doi: 10.1016/s0168-0102(01)00197-3. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Brain potentials during reading reflect word expectancy and semantic association. Nature. 1984;307:161–163. doi: 10.1038/307161a0. [DOI] [PubMed] [Google Scholar]
- Logan GD. On the abilty to inhibit thought and action: a user's guide to the stop signal paradigm. In: Dagenbach D, Carr TH, editors. Inhibitory Processes in Attention, Memory, and Language. Academic; San Diego: 1994. pp. 189–239. [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: a theory of an act of control. Psychol Rev. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Camarda RM, Gallese V, Rizzolatti G. Multiple representations of body movements in mesial area 6 and the adjacent cingulate cortex: an intracortical microstimulation study in the macaque monkey. J Comp Neurol. 1991;311:463–482. doi: 10.1002/cne.903110403. [DOI] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Rizzolatti G. Cortico-cortical connections of two electrophysiologically identified arm representations in the mesial agranular frontal cortex. Exp Brain Res. 1990;82:214–218. doi: 10.1007/BF00230855. [DOI] [PubMed] [Google Scholar]
- Luppino G, Rozzi S, Calzavara R, Matelli M. Prefrontal and agranular cingulate projections to the dorsal premotor areas F2 and F7 in the macaque monkey. Eur J Neurosci. 2003;17:559–578. doi: 10.1046/j.1460-9568.2003.02476.x. [DOI] [PubMed] [Google Scholar]
- Miltner WH, Baum CH, Coles MG. Event-related brain potentials following incorrect feedback in a time-estimation task: Evidence for a generic neural system for error detection. J Cogn Neurosci. 1997;9:788–798. doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- Mitz AR, Godschalk M. Eye-movement representation in the frontal lobe of rhesus monkeys. Neurosci Lett. 1989;106:157–162. doi: 10.1016/0304-3940(89)90219-x. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Van Hoesen GW. Cingulate input to the primary and supplementary motor cortices in the rhesus monkey: evidence for somatotopy in areas 24c and 23c. J Comp Neurol. 1992;322:471–489. doi: 10.1002/cne.903220403. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Van Hoesen GW. Frontal granular cortex input to the cingulate (M3), supplementary (M2) and primary (M1) motor cortices in the rhesus monkey. J Comp Neurol. 1993;337:669–689. doi: 10.1002/cne.903370411. [DOI] [PubMed] [Google Scholar]
- Moschovakis AK, Gregoriou GG, Ugolini G, Doldan M, Graf W, Guldin W, Hadjidimitrakis K, Savaki HE. Oculomotor areas of the primate frontal lobes: a transneuronal transfer of rabies virus and [14C]-2-deoxyglucose functional imaging study. J Neurosci. 2004;24:5726–5740. doi: 10.1523/JNEUROSCI.1223-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näätänen R, Gaillard AW, Mantysalo S. Early selective-attention effect on evoked potential reinterpreted. Acta Psychol. 1978;42:313–329. doi: 10.1016/0001-6918(78)90006-9. [DOI] [PubMed] [Google Scholar]
- Naito E, Matsumura M. Movement-related potentials associated with motor inhibition as determined by use of a stop signal paradigm in humans. Brain Res Cogn Brain Res. 1994;2:139–146. doi: 10.1016/0926-6410(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Naito E, Matsumura M. Movement-related potentials associated with motor inhibition under different preparatory states during performance of two visual stop signal paradigms in humans. Neuropsychologia. 1996;34:565–573. doi: 10.1016/0028-3932(95)00140-9. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Roesch MR, Olson CR. Neuronal activity in macaque SEF and ACC during performance of tasks involving conflict. J Neurophysiol. 2005;93:884–908. doi: 10.1152/jn.00305.2004. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GP, Kok A. Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task. Psychophysiology. 2001;38:752–760. [PubMed] [Google Scholar]
- Niki H, Watanabe M. Prefrontal and cingulate unit activity during timing behavior in the monkey. Brain Res. 1979;171:213–224. doi: 10.1016/0006-8993(79)90328-7. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. Electric Fields in the Brain: The Neurophysics of EEG. Oxford; New York: 2005. [Google Scholar]
- Pandya DN, Kuypers HG. Cortico-cortical connections in the rhesus monkey. Brain Res. 1969;13:13–36. doi: 10.1016/0006-8993(69)90141-3. [DOI] [PubMed] [Google Scholar]
- Paré M, Hanes DP. Controlled movement processing: superior colliculus activity associated with countermanded saccades. J Neurosci. 2003;23:6480–6489. doi: 10.1523/JNEUROSCI.23-16-06480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Paus T, Kalina M, Patockova L, Angerova Y, Cerny R, Mecir P, Bauer J, Krabec P. Medial vs lateral frontal lobe lesions and differential impairment of central-gaze fixation maintenance in man. Brain. 1991;114:2051–2067. doi: 10.1093/brain/114.5.2051. [DOI] [PubMed] [Google Scholar]
- Pliszka SR, Liotti M, Woldorff MG. Inhibitory control in children with attention-deficit/hyperactivity disorder: event-related potentials identify the processing component and timing of an impaired right-frontal response-inhibition mechanism. Biol Psychiatry. 2000;48:238–246. doi: 10.1016/s0006-3223(00)00890-8. [DOI] [PubMed] [Google Scholar]
- Pouget P, Emeric EE, Stuphorn V, Reis K, Schall JD. Chronometry of visual responses in frontal eye field, supplementary eye field, and anterior cingulate cortex. J Neurophysiol. 2005;94:2086–2092. doi: 10.1152/jn.01097.2004. [DOI] [PubMed] [Google Scholar]
- Procyk E, Ford Dominey P, Amiez C, Joseph JP. The effects of sequence structure and reward schedule on serial reaction time learning in the monkey. Brain Res Cogn Brain Res. 2000;9:239–248. doi: 10.1016/s0926-6410(00)00002-1. [DOI] [PubMed] [Google Scholar]
- Procyk E, Joseph JP. Characterization of serial order encoding in the monkey anterior cingulate sulcus. Eur J Neurosci. 2001;14:1041–1046. doi: 10.1046/j.0953-816x.2001.01738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramautar JR, Kok A, Ridderinkhof KR. Effects of stop-signal modality on the N2/P3 complex elicited in the stop-signal paradigm. Biol Psychol. 2006a;72:96–109. doi: 10.1016/j.biopsycho.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Ramautar JR, Kok A, Ridderinkhof KR. Effects of stop-signal probability in the stop-signal paradigm: the N2/P3 complex further validated. Brain Cogn. 2004;56:234–252. doi: 10.1016/j.bandc.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Ramautar JR, Slagter HA, Kok A, Ridderinkhof KR. Probability effects in the stop-signal paradigm: the insula and the significance of failed inhibition. Brain Res. 2006b;1105:143–154. doi: 10.1016/j.brainres.2006.02.091. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Hadland KA, Gaffan D, Passingham RE. The effect of cingulate cortex lesions on task switching and working memory. J Cogn Neurosci. 2003;15:338–353. doi: 10.1162/089892903321593072. [DOI] [PubMed] [Google Scholar]
- Scheffers MK, Coles MG, Bernstein P, Gehring WJ, Donchin E. Event-related brain potentials and error-related processing: an analysis of incorrect responses to go and no-go stimuli. Psychophysiology. 1996;33:42–53. doi: 10.1111/j.1469-8986.1996.tb02107.x. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J Neurosci. 1988;8:4049–4068. doi: 10.1523/JNEUROSCI.08-11-04049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shidara M, Mizuhiki T, Richmond BJ. Neuronal firing in anterior cingulate neurons changes modes across trials in single states of multitrial reward schedules. Exp Brain Res. 2005;163:242–245. doi: 10.1007/s00221-005-2232-y. [DOI] [PubMed] [Google Scholar]
- Shidara M, Richmond BJ. Anterior cingulate: single neuronal signals related to degree of reward expectancy. Science. 2002;296:1709–1711. doi: 10.1126/science.1069504. [DOI] [PubMed] [Google Scholar]
- Shima K, Aya K, Mushiake H, Inase M, Aizawa H, Tanji J. Two movement-related foci in the primate cingulate cortex observed in signal-triggered and self-paced forelimb movements. J Neurophysiol. 1991;65:188–202. doi: 10.1152/jn.1991.65.2.188. [DOI] [PubMed] [Google Scholar]
- Showers MJ. The cingulate gyrus: additional motor area and cortical autonomic regulator. J Comp Neurol. 1959;112:231–301. doi: 10.1002/cne.901120118. [DOI] [PubMed] [Google Scholar]
- Stahl J, Gibbons H. Dynamics of response-conflict monitoring and individual differences in response control and behavioral control: an electrophysiological investigation using a stop-signal task. Clin Neurophysiol. 2007;118:581–596. doi: 10.1016/j.clinph.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Stanton GB, Bruce CJ, Goldberg ME. Topography of projections to the frontal lobe from the macaque frontal eye fields. J Comp Neurol. 1993;330:286–301. doi: 10.1002/cne.903300209. [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Schall JD. Executive control of countermanding saccades by the supplementary eye field. Nat Neurosci. 2006;9:925–931. doi: 10.1038/nn1714. [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Taylor TL, Schall JD. Performance monitoring by the supplementary eye field. Nature. 2000;408:857–860. doi: 10.1038/35048576. [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Brown ET, Schall JD. Supplementary eye field does not initiate visually-guided saccades. Soc Neurosci Abst. 2003;187.15 [Google Scholar]
- Takada M, Tokuno H, Hamada I, Inase M, Ito Y, Imanishi M, Hasegawa N, Akazawa T, Hatanaka N, Nambu A. Organization of inputs from cingulate motor areas to basal ganglia in macaque monkey. Eur J Neurosci. 2001;14:1633–1650. doi: 10.1046/j.0953-816x.2001.01789.x. [DOI] [PubMed] [Google Scholar]
- Talairach J, Bancaud J, Geier S, Bordas-Ferrer M, Bonis A, Szikla G, Rusu M. The cingulate gyrus and human behavior. Electroencephalogr Clin Neurophysiol. 1973;34:45–52. doi: 10.1016/0013-4694(73)90149-1. [DOI] [PubMed] [Google Scholar]
- van Boxtel GJ, van der Molen MW, Jennings JR, Brunia CH. A psycho-physiological analysis of inhibitory motor control in the stop-signal paradigm. Biol Psychol. 2001;58:229–262. doi: 10.1016/s0301-0511(01)00117-x. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Morecraft RJ, Vogt BA. Connections of the monkey cingulate cortex. In: Vogt BA, Gabriel M, editors. Neurobiology of Cingulate Cortex and Limbic Thalamus: A Comprehensive Handbook. Birkhauser; Boston: 1993. pp. 249–284. [Google Scholar]
- Van't Ent D, Apkarian P. Motoric response inhibition in finger movement and saccadic eye movement: a comparative study. Clin Neurophysiol. 1999;110:1058–1072. doi: 10.1016/s1388-2457(98)00036-4. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav. 2002;77:477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. Error detection, correction, and prevention in the brain: a brief review of data and theories. Clin EEG Neurosci. 2006;37:330–335. doi: 10.1177/155005940603700411. [DOI] [PubMed] [Google Scholar]
- Vidal F, Hasbroucq T, Grapperon J, Bonnet M. Is the ‘error negativity’ specific to errors? Biol Psychol. 2000;51:109–128. doi: 10.1016/s0301-0511(99)00032-0. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey. II. Cortical afferents. J Comp Neurol. 1987;262:271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN, Rosene DL. Cingulate cortex of the rhesus monkey. I. Cytoarchitecture and thalamic afferents. J Comp Neurol. 1987;262:256–270. doi: 10.1002/cne.902620207. [DOI] [PubMed] [Google Scholar]
- Wang Y, Matsuzaka Y, Shima K, Tanji J. Cingulate cortical cells projecting to monkey frontal eye field and primary motor cortex. Neuroreport. 2004;15:1559–1563. doi: 10.1097/01.wnr.0000133300.62031.9b. [DOI] [PubMed] [Google Scholar]
- Yeung N, Cohen JD, Botvinick MM. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol Rev. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]