The β- and γ-secretases responsible for the formation of the amyloid β-peptide (Aβ) have been top targets for the development of Alzheimer therapeutics even before the identification of these proteases. Indeed, secretase inhibitors have been sought ever since the discovery of AD-causing missense mutations in APP [1-3] and the realization that these mutations alter Aβ production [4-6]. Cell-based screens netted a number of compounds that blocked Aβ formation, and virtually all of these initial leads appeared to work at the level of γ-secretase [7-11]. The identification of β- and γ-secretases ultimately revealed why this was the case. β-Secretase is a membrane-tethered pepsin-like aspartyl protease [12-15] that cleaves APP on the lumenal/extracellular side at the juxtamembrane region. The enzyme has a long and shallow active site [16], and molecules that inhibit β-secretase are typically not very cell permeable. In contrast, γ-secretase cleaves within the transmembrane region of APP and contains a membrane-embedded active site [17]. Thus, compounds that inhibit γ-secretase are typically quite hydrophobic and readily traverse the cell membrane. For these reasons, γ-secretase inhibitors have advanced further along the drug development pipeline than β-secretase inhibitors, which are only just emerging from the preclinical stage.
γ-Secretase is nevertheless a difficult drug target in some other respects. In stark contrast to β-secretase, γ-secretase is a large protein complex composed of four different membrane proteins [18-20], and knowledge of structural details that would allow rational drug design is not available. Moreover, γ-secretase processes a number of different type I integral membrane proteins, so many that this protease has been dubbed “the proteasome of the membrane” [21]. Many of these processing events are part of critical pathways in cell biology that may have toxic consequences if blocked. Indeed, this is already well known to be the case with one particular γ-secretase substrate, the Notch receptor. Thus, modulation rather than inhibition of γ-secretase is preferred, to alter Aβ production in a therapeutically relevant way without interfering with essential cellular processes. This minireview provides an overview of the various types of γ-secretase inhibitors and modulators and what is known about how they interact with the protease complex, their mechanisms of action, and their potential as disease-modifying therapies for AD.
Transition-State Analogues
Among the first types of specific γ-secretase inhibitors were peptide analogues containing classical transition-state mimicking moieties for aspartyl proteases. In aspartyl protease catalysis, two aspartates activate water for direct attack on the amide bond fated for cleavage, forming an unstable gem-diol intermediate (in which the carbonyl carbon becomes transiently attached to two hydroxyl groups upon attack by water). Transition-state analogue inhibitors of aspartyl proteases are chemically stable and contain one or two hydroxyl groups attached to a carbon, and this moiety replaces an amide bond in the peptide analogue. The net result is a compound that interacts well with the two aspartates in the active site but is not susceptible to cleavage by the protease.
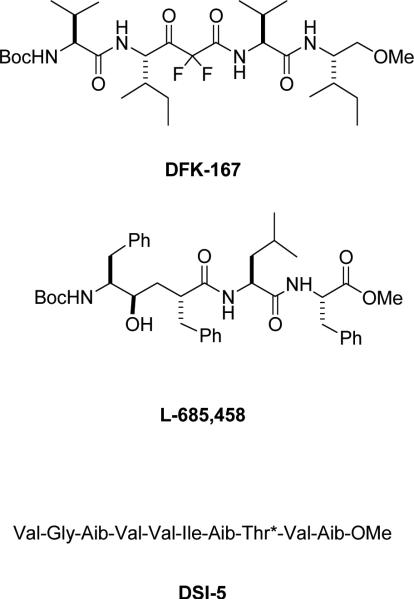
The first reported inhibitors of γ-secretase were peptide aldehydes originally developed for the proteasome [7]. These aldehydes are readily hydrated to form a stable gem-diol structure similar to that formed during aspartyl protease catalysis. Subsequently, difluoro ketone peptidomimetic inhibitors (e.g., DFK-167; Fig. 1) were developed that were specifically designed from the sequence of the APP transmembrane domain containing the γ-secretase site [8], and these difluoro ketones likewise become hydrated to form a chemically stable gem-diol. Unlike the peptide aldehydes, the difluoro ketone peptidomimetics contained residues corresponding to the P' side as well as on the P side (that is, on the C-terminal side relative to the cleavage site as well as on the N-terminal side). However, both types of compounds (aldehydes and ketones) can also react with serine or cysteine proteases, in which the nucleophilic serine or cysteine residue in the active site attacks the electrophilic carbonyl of the aldehyde or ketone functionality. Swapping the ketone with an alcohol moiety, a group unable to react with serine or cysteine proteases, retained inhibitory activity toward γ-secretase, evidence that γ-secretase might be an aspartyl protease [22]. Confirmatory results were soon reported with other alcohol-containing aspartyl protease peptidomimetics [9] (e.g., L-685,458; Fig.1.
Figure 1.
Chemical structures of difluoro ketone transition-state analogue γ-secretase inhibitor DFK-167, hydroxyl-containing transition-state analogue L-685,458, and aminoisobutyric acid (Aib)-containing helical peptide DSI-1.
These findings, along with the discovery that presenilins are essential for γ-secretase activity [23-25], prompted the identification of two conserved transmembrane aspartates in presenilin that are essential for activity and the initial suggestion that presenilin is a novel membrane-embedded aspartyl protease [26]. Indeed, the conversion of the alcohol-containing transition-state analogue inhibitors of γ-secretase to affinity labeling reagents led to covalent and specific binding to the two presenilin subunits (the N-terminal and C-terminal fragments, NTFs and CTFs, that form upon endoproteolysis of the presenilin holoprotein), strong evidence that the active site of γ-secretase resides at the subunit interface [27, 28]. Indeed, each subunit contains one of the two conserved aspartates, suggesting that a catalytic pair of aspartates is formed at the interface between the two presenilin subunits.
The transition-state analogue inhibitors also proved useful for isolating the γ-secretase complex. Although presenilin appeared to be the catalytic component, it was clear that there were other unidentified components. First, presenilin did not possess proteolytic activity on its own. Second, the protein assembled into high molecular weight complexes [29, 30]. Third, the endoproteolysis of presenilin into a stable heterodimeric form is tightly regulated by limiting cellular factors [31-33]. Thus, attempts were made to purify γ-secretase in the hope of identifying the missing members of what is now called the γ-secretase complex. Immobilization of a transition-state analogue inhibitor provided a means of affinity isolating the enzyme [34], and this purification step was one means by which the membrane proteins nicastrin, Aph-1 and Pen-2 were confirmed as bona fide γ-secretase complex members.
Docking Site Inhibitors
The isolation of γ-secretase using the immobilized transition-state analogue also helped understand how the protease interacts with substrate, because an endogenous APP substrate copurified with the enzyme [34]. Because the active site should be occupied by the immobilized transition-state analogue, the copurification of the substrate suggested the existence of a substrate binding site that is distinct from the active site. This other substrate binding site was dubbed the docking site, because this was envisioned to be where substrate initially interacts before entry into an internal, water-containing active site that is sequestered from the hydrophobic environment of the lipid bilayer.
Substrate-based peptides were designed to interact with this docking site. Peptides based on the transmembrane domain of APP and constrained in a helical conformation (by incorporation of the helix-inducing aminoisobutyric acid, or Aib; e.g., DSI-1, Fig. 1) can potently inhibit γ-secretase, apparently by interacting with this docking site [35]. Conversion of these helical peptide inhibitors into affinity labeling reagents led to the localization of the substrate docking site to the presenilin NTF/CTF interface [36]. As mentioned above, transition-state analogue inhibitors also bind directly to the NTF/CTF interface, but these bind at a site distinct from that of helical peptide inhibitors. These findings suggest a pathway for γ-secretase substrate from docking site to active site: upon binding to the outer surface of presenilin at the NTF/CTF interface, the substrate can pass, either in whole or in part, between these two presenilin subunits to access the internal active site. Interestingly, extension of a ten-residue helical peptide inhibitor by just three additional residues resulted in a potent inhibitor [37] apparently capable of binding both docking site and active site [36], suggesting that these two substrate binding sites are relatively close.
In Vivo Active Agents and Clinical Candidates
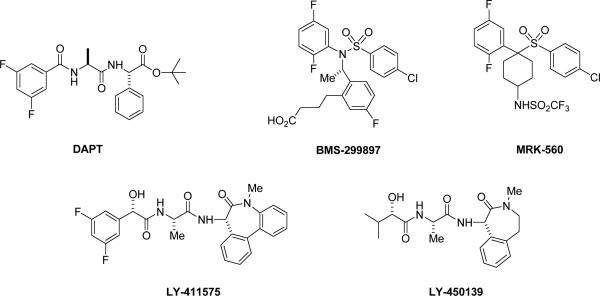
In addition to the transition-state analogues and helical substrate analogues, a number of other potent inhibitors of γ-secretase have been identified. Among the earliest was a dipeptide analogue called DAPT (Fig. 2), and this compound was the first reported in vivo active agent, inhibiting Aβ production in plasma and brain in an APP transgenic mouse model [11, 38]. C-terminal modification of DAPT with benzodiazepine-like moieties led to the development of the highly potent inhibitor compound E [10] and related analogues with better in vivo properties, including LY-411,575 [39] and the clinical candidate LY-450,139 [40, 41] (Fig. 2). In addition, certain sulfonamides displayed potent inhibition (BMS-299897, MRK-560; Fig. 2) [42, 43] and had been considered as clinical candidates. All of these compounds (DAPT, benzodiazepines, sulfonamides) are apparently noncompetitive inhibitors of γ-secretase [44] and interact at a site distinct from docking and active site [36, 45]. Nevertheless, the conversion of some of these inhibitors into affinity labeling reagents revealed that presenilin is thedirect target [10, 46, 47]. To date, no inhibitors have been reported to interact directly with any of the other members of the γ-secretase complex. In principle though, it should be possible to target nicastrin, as the ectodomain of this type I integral membrane protein apparently plays a direct role in substrate recognition, binding to the lumenal/extracellular N-terminus of substrates [48].
Figure 2.
Chemical structures of in vivo active γ-secretase inhibitors.
Reports of the effects of benzodiazepine-type compound LY-411-575 in mice show their ability to acutely and chronically lower Aβ in plasma and in brain. However, elevated doses result in severe gastrointestinal toxicity and interfere with the maturation of B- and T-lympocytes in mice, effects that are due to inhibition of processing of another γ-secretase substrate, the Notch receptor [49, 50]. These mechanism-based toxicities are the most serious stumbling block to the development of γ-secretase inhibitors as AD therapeutics. Clinical studies on only one γ-secretase inhibitor, LY-4150,139, have been reported in the literature [40, 41]. These reports describe reduction of Aβ in plasma, but little or no effect in the CSF, suggesting that the doses might be too low. Increasing the dose must be done cautiously, however, with special attention toward gastrointestinal effects, such as severe cramps and diarrhea, and immunosuppressive effects.
Toward γ-Secretase Modulators
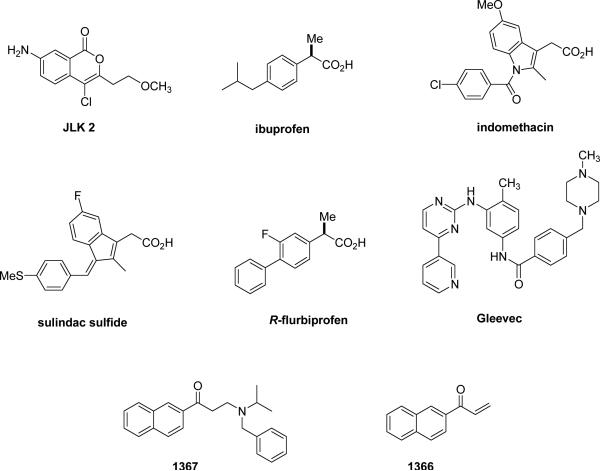
Although γ-secretase has in many ways been an attractive target for Alzheimer therapeutics, interference with Notch processing and signaling may lead to toxicities that preclude clinical use of inhibitors of this protease. However, compounds that can modulate the enzyme to alter or block Aβ production with little or no effect on Notch would bypass this potential roadblock to therapeutics. Among the first reported modulators were isocoumarin JLK compounds (e.g., JLK2, Fig. 3), which blocked Aβ production at the γ-secretase level but did not affect Notch processing [51]. However, the direct target of these compounds remains unclear, as they do not affect γ-secretase activity in biochemical assays [52]. Nevertheless, other studies suggest that the protease complex contains allosteric binding sites that can alter substrate selectivity and the sites of substrate proteolysis. Certain non-steroidal anti-inflammatory drugs (NSAIDs; e.g., ibuprofen, indomethacin, and sulindac sulfide; Fig. 3) can reduce the production of the highly aggregation-prone Aβ42 peptide and increase a 38-residue form of Aβ, a pharmacological property independent of inhibition of cyclooxygenase [53]. The alteration of the proteolytic cleavage site is observed with isolated or purified γ-secretase [54, 55], suggesting that the compounds may interact directly with the protease complex to exert these effects. Enzyme kinetic studies and displacement experiments show that the selective NSAIDs can be noncompetitive with respective to APP substrate and to a transition-state analogue inhibitor, suggesting interaction with a site distinct from the active site [56]. The site of cleavage within the Notch transmembrane domain is similarly affected, but this subtle change does not inhibit the release of the intracellular domain and thus does not affect Notch signaling [57]. For this reason, these agents may be safer as Alzheimer therapeutics than inhibitors that block the active site or the docking site. Indeed, one compound, R-flurbiprofen (Fig. 3), has advanced to Phase III clinical trials.
Figure 3.
Chemical structures of γ-secretase modulators.
Another type of modulator are compounds that resemble kinase inhibitors and interact with a nucleotide binding site on the γ-secretase complex. The discovery that ATP can increase Aβ production in membrane preparations prompted the testing of a variety of compounds that interact with ATP binding sites on other proteins [58]. In this focused screen, the Abl kinase inhibitor Gleevec (Fig. 3) emerged as a selective inhibitor of Aβ production in cells without affecting the proteolysis of Notch. In light of these findings, ATP and other nucleotides were tested for effects on purified γ-secretase preparations and found to selectively increase the proteolytic processing of a purified recombinant APP-based substrate without affecting the proteolysis of a Notch counterpart [59]. Furthermore, certain compounds known to interact with ATP binding sites were found to selectively inhibit APP processing vis-à-vis Notch in purified protease preparations (e.g., naphthylketones 1367 and 1366; Fig. 3). The γ-secretase complex could be pulled down with beads containing immobilized ATP, and the presenilin-1 CTF was specifically photolabeled by 8-azido-ATP. This labeling was not blocked by a transition-state analogue inhibitor or by the recombinant APP- and Notch-based substrates; however, the APP-selective inhibitors could prevent photolabeling by 8-azido-ATP. Taken together, these results suggest that the γ-secretase complex contains a nucleotide binding site, to which the presenilin-1 CTF is at least a contributor, and that this site may allow allosteric regulation of γ-secretase processing of APP with respect to Notch. Whether such regulation is physiologically important is unclear, but the pharmacological relevance is profound and may lead to new therapeutic candidates for Alzheimer's disease. This hope is tempered by the fact that γ-secretase cleaves numerous other type I membrane protein stubs that result from ectodomain shedding. Agents selective for APP versus Notch may reveal new long-term toxicities due to blocking proteolysis of these other substrates, toxicities masked by the severe Notch-related effects with nonselective inhibitors.
Structural Model for γ-Secretase
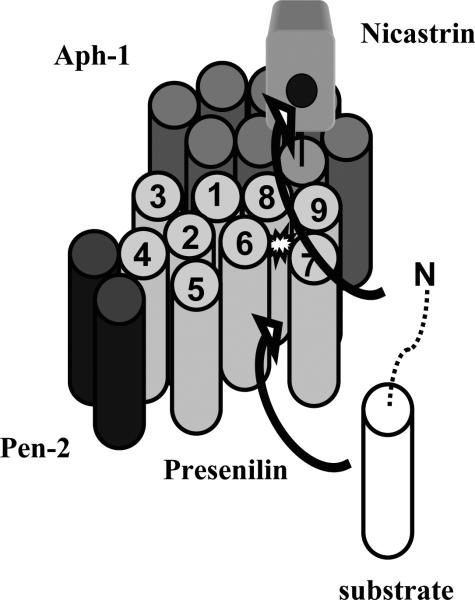
The design of more potent and selective modulators would be greatly facilitated by a detailed structure of the γ-secretase complex, ideally interacting with a modulator at a specific site. Elucidation of structure is quite difficult and represents the next frontier in understanding this biologically important and therapeutically relevant protease complex. A schematic structural model for the γ-secretase complex and how it interacts with substrate is offered in Figure 4. This model, while partially hypothetical, is consistent with the following observations. First, dissociation into partial complexes using the detergent DDM revealed that Aph-1 interacts with nicastrin, Pen-2 interacts with the PS1 NTF, and the PS1 CTF associated separately with NTF and with Aph-1/nicastrin [60]. Second, nicastrin and Aph-1 as a subcomplex form an initial subcomplex during intracellular assembly of the protease [61], and is consistent with the nicastrin/Aph-1 partial complex observed upon detergent dissocation. Third, the finding that Pen-2 interacts with TMD4 of presenilin [62, 63] confirmed the PS1 NTF/Pen-2 interaction seen upon detergent dissociation and identified a specific region of presenilin contributing to the interface. Fourth, evidence suggests that the C-terminus of presenilin interacts with the nicastrin TMD [64]. Fifth, cysteine cross-linking studies indicate that presenilin TMD1 and TMD8 are proximal [65]. Sixth, both the docking site and the active site lies at the interface between the presenilin NTF and CTF [26, 28, 66, 67]. Seventh, studies with helical peptide inhibitors suggest that the docking and active sites are relatively close, within the length of three amino acid residues [67]. Eighth, the extracellular domain of nicastrin also plays a role in substrate recognition, binding to the N-terminus of the substrate [48]. This model for γ-secretase structure and function will undoubtedly prove to be imperfect but should provide a useful framework to ask specific questions and design appropriate experiments going forward. The ultimate goal, a high resolution structure, remains quite challenging, but already electron microscopy has provided the first glimpses of the protease complex, albeit at 10-15 Å resolution. Despite the low resolution, certain features can be visualized, including an interior chamber, which is likely where the active site resides, and two ports into this chamber, which may allow access for catalytic water.
Figure 4.
Model for the γ-secretase complex and its interaction with substrate. The transmembrane region of the substrate initially docks at the presenilin NTF/CTF interface, while the N-terminus of the substrate interacts with the nicastrin ectodomain. The substrate, either in whole or in part, then accesses the internal active site, which contains water and two aspartates. The γ-secretase complex is drawn to take into account molecular and biochemical evidence as described in the text.
Perspective
Our knowledge of γ-secretase and its role in AD and in biology has increased dramatically in the past ten years. This enzyme is a complex of four different integral membrane proteins with a membrane-embedded active site. At present, the possibility of a detailed structure of this complex seems like a distance dream, and little is known about the shape and character of any of the drug binding sites. Also a hindrance to the development of clinically useful γ-secretase inhibitors is the critical importance of this protease in the Notch signaling pathway. It will likely be necessary to avoid interfering with Notch proteolysis by γ-secretase. Despite these hurdles, γ-secretase continues to be pursued as a top therapeutic target for AD and in many respects has advantages over the more classical aspartyl protease β-secretase. Indeed, γ-secretase inhibitors and modulators are well into human trials, while no β-secretase inhibitors have only just begun to pass through preclinical testing. Answers should soon be forthcoming about the safety and efficacy of the first-generation compounds, but whatever the result, it will be critically important to continue development of more potent and selective agents with better pharmaceutical properties. Despite the difficulties, working toward a better structural and mechanistic understanding of the protease complex should ultimately aid drug discovery, allowing structure-based design of γ-secretase modulators.
References
- 1.Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991;349:704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 2.Chartier-Harlin MC, Crawford F, Houlden H, Warren A, Hughes D, Fidani L, Goate A, Rossor M, Roques P, Hardy J, et al. Early-onset Alzheimer's disease caused by mutations at codon 717 of the beta-amyloid precursor protein gene. Nature. 1991;353:844–846. doi: 10.1038/353844a0. [DOI] [PubMed] [Google Scholar]
- 3.Murrell J, Farlow M, Ghetti B, Benson MD. A mutation in the amyloid precursor protein associated with hereditary Alzheimer's disease. Science. 1991;254:97–99. doi: 10.1126/science.1925564. [DOI] [PubMed] [Google Scholar]
- 4.Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, Vigo-Pelfrey C, Lieberburg I, Selkoe DJ. Mutation of the beta-amyloid precursor protein in familial Alzheimer's disease increases beta-protein production. Nature. 1992;360:672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki N, Cheung TT, Cai XD, Odaka A, Otvos L, Jr., Eckman C, Golde TE, Younkin SG. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science. 1994;264:1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- 6.Cai XD, Golde TE, Younkin SG. Release of excess amyloid beta protein from a mutant amyloid beta protein precursor. Science. 1993;259:514–516. doi: 10.1126/science.8424174. [DOI] [PubMed] [Google Scholar]
- 7.Higaki J, Quon D, Zhong Z, Cordell B. Inhibition of beta-amyloid formation identifies proteolytic precursors and subcellular site of catabolism. Neuron. 1995;14:651–659. doi: 10.1016/0896-6273(95)90322-4. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe MS, Citron M, Diehl TS, Xia W, Donkor IO, Selkoe DJ. A substrate-based difluoro ketone selectively inhibits Alzheimer's γ-secretase activity. J Med Chem. 1998;41:6–9. doi: 10.1021/jm970621b. [DOI] [PubMed] [Google Scholar]
- 9.Shearman MS, Beher D, Clarke EE, Lewis HD, Harrison T, Hunt P, Nadin A, Smith AL, Stevenson G, Castro JL. L-685,458, an Aspartyl Protease Transition State Mimic, Is a Potent Inhibitor of Amyloid beta-Protein Precursor gamma-Secretase Activity. Biochemistry. 2000;39:8698–8704. doi: 10.1021/bi0005456. [DOI] [PubMed] [Google Scholar]
- 10.Seiffert D, Bradley JD, Rominger CM, Rominger DH, Yang F, Meredith JE, Jr., Wang Q, Roach AH, Thompson LA, Spitz SM, et al. Presenilin-1 and -2 are molecular targets for gamma -secretase inhibitors. J Biol Chem. 2000;275:34086–34091. doi: 10.1074/jbc.M005430200. [DOI] [PubMed] [Google Scholar]
- 11.Dovey HF, John V, Anderson JP, Chen LZ, de Saint Andrieu P, Fang LY, Freedman SB, Folmer B, Goldbach E, Holsztynska EJ, et al. Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. J Neurochem. 2001;76:173–181. doi: 10.1046/j.1471-4159.2001.00012.x. [DOI] [PubMed] [Google Scholar]
- 12.Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, et al. beta-Secretase Cleavage of Alzheimer's Amyloid Precursor Protein by the Transmembrane Aspartic Protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 13.Sinha S, Anderson JP, Barbour R, Basi GS, Caccavello R, Davis D, Doan M, Dovey HF, Frigon N, Hong J, et al. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature. 1999;402:537–540. doi: 10.1038/990114. [DOI] [PubMed] [Google Scholar]
- 14.Yan R, Bienkowski MJ, Shuck ME, Miao H, Tory MC, Pauley AM, Brashier JR, Stratman NC, Mathews WR, Buhl AE, et al. Membrane-anchored aspartyl protease with Alzheimer's disease beta- secretase activity. Nature. 1999;402:533–537. doi: 10.1038/990107. [DOI] [PubMed] [Google Scholar]
- 15.Hussain I, Powell D, Howlett DR, Tew DG, Meek TD, Chapman C, Gloger IS, Murphy KE, Southan CD, Ryan DM, et al. Identification of a Novel Aspartic Protease (Asp 2) as beta-Secretase. Mol Cell Neurosci. 1999;14:419–427. doi: 10.1006/mcne.1999.0811. [DOI] [PubMed] [Google Scholar]
- 16.Hong L, Koelsch G, Lin X, Wu S, Terzyan S, Ghosh AK, Zhang XC, Tang J. Structure of the protease domain of memapsin 2 (beta-secretase) complexed with inhibitor. Science. 2000;290:150–153. doi: 10.1126/science.290.5489.150. [DOI] [PubMed] [Google Scholar]
- 17.Wolfe MS. The gamma-secretase complex: membrane-embedded proteolytic ensemble. Biochemistry. 2006;45:7931–7939. doi: 10.1021/bi060799c. [DOI] [PubMed] [Google Scholar]
- 18.Takasugi N, Tomita T, Hayashi I, Tsuruoka M, Niimura M, Takahashi Y, Thinakaran G, Iwatsubo T. The role of presenilin cofactors in the gamma-secretase complex. Nature. 2003;422:438–441. doi: 10.1038/nature01506. [DOI] [PubMed] [Google Scholar]
- 19.Kimberly WT, LaVoie MJ, Ostaszewski BL, Ye W, Wolfe MS, Selkoe DJ. γ-Secretase is a membrane protein complex comprised of presenilin, nicastrin, aph-1, and pen-2. Proc Natl Acad Sci U S A. 2003;100:6382–6387. doi: 10.1073/pnas.1037392100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C. Reconstitution of gamma-secretase activity. Nat Cell Biol. 2003;5:486–488. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- 21.Kopan R, Ilagan MX. Gamma-secretase: proteasome of the membrane? Nat Rev Mol Cell Biol. 2004;5:499–504. doi: 10.1038/nrm1406. [DOI] [PubMed] [Google Scholar]
- 22.Wolfe MS, Xia W, Moore CL, Leatherwood DD, Ostaszewski B, Donkor IO, Selkoe DJ. Peptidomimetic probes and molecular modeling suggest Alzheimer's γ-secretases are intramembrane-cleaving aspartyl proteases. Biochemistry. 1999;38:4720–4727. doi: 10.1021/bi982562p. [DOI] [PubMed] [Google Scholar]
- 23.De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- 24.Herreman A, Serneels L, Annaert W, Collen D, Schoonjans L, De Strooper B. Total inactivation of gamma-secretase activity in presenilin-deficient embryonic stem cells. Nat Cell Biol. 2000;2:461–462. doi: 10.1038/35017105. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Nadeau P, Song W, Donoviel D, Yuan M, Bernstein A, Yankner BA. Presenilins are required for gamma-secretase cleavage of beta-APP and transmembrane cleavage of Notch-1. Nat Cell Biol. 2000;2:463–465. doi: 10.1038/35017108. [DOI] [PubMed] [Google Scholar]
- 26.Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature. 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- 27.Li YM, Xu M, Lai MT, Huang Q, Castro JL, DiMuzio-Mower J, Harrison T, Lellis C, Nadin A, Neduvelil JG, et al. Photoactivated gamma-secretase inhibitors directed to the active site covalently label presenilin 1. Nature. 2000;405:689–694. doi: 10.1038/35015085. [DOI] [PubMed] [Google Scholar]
- 28.Esler WP, Kimberly WT, Ostaszewski BL, Diehl TS, Moore CL, Tsai J-Y, Rahmati T, Xia W, Selkoe DJ, Wolfe MS. Transition-state analogue inhibitors of γ-secretase bind directly to presenilin-1. Nature Cell Biology. 2000;2:428–434. doi: 10.1038/35017062. [DOI] [PubMed] [Google Scholar]
- 29.Capell A, Grunberg J, Pesold B, Diehlmann A, Citron M, Nixon R, Beyreuther K, Selkoe DJ, Haass C. The proteolytic fragments of the Alzheimer's disease-associated presenilin-1 form heterodimers and occur as a 100-150-kDa molecular mass complex. J Biol Chem. 1998;273:3205–3211. doi: 10.1074/jbc.273.6.3205. [DOI] [PubMed] [Google Scholar]
- 30.Yu G, Chen F, Levesque G, Nishimura M, Zhang DM, Levesque L, Rogaeva E, Xu D, Liang Y, Duthie M, et al. The presenilin 1 protein is a component of a high molecular weight intracellular complex that contains beta-catenin. J Biol Chem. 1998;273:16470–16475. doi: 10.1074/jbc.273.26.16470. [DOI] [PubMed] [Google Scholar]
- 31.Thinakaran G, Borchelt DR, Lee MK, Slunt HH, Spitzer L, Kim G, Ratovitsky T, Davenport F, Nordstedt C, Seeger M, et al. Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron. 1996;17:181–190. doi: 10.1016/s0896-6273(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 32.Ratovitski T, Slunt HH, Thinakaran G, Price DL, Sisodia SS, Borchelt DR. Endoproteolytic processing and stabilization of wild-type and mutant presenilin. J Biol Chem. 1997;272:24536–24541. doi: 10.1074/jbc.272.39.24536. [DOI] [PubMed] [Google Scholar]
- 33.Thinakaran G, Harris CL, Ratovitski T, Davenport F, Slunt HH, Price DL, Borchelt DR, Sisodia SS. Evidence that levels of presenilins (PS1 and PS2) are coordinately regulated by competition for limiting cellular factors. J Biol Chem. 1997;272:28415–28422. doi: 10.1074/jbc.272.45.28415. [DOI] [PubMed] [Google Scholar]
- 34.Esler WP, Kimberly WT, Ostaszewski BL, Ye W, Diehl TS, Selkoe DJ, Wolfe MS. Activity-dependent isolation of the presenilin/γ-secretase complex reveals nicastrin and a γ substrate. Proc Natl Acad Sci U.S.A. 2002;99:2720–2725. doi: 10.1073/pnas.052436599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Das C, Berezovska O, Diehl TS, Genet C, Buldyrev I, Tsai JY, Hyman BT, Wolfe MS. Designed helical peptides inhibit an intramembrane protease. J Am Chem Soc. 2003;125:11794–11795. doi: 10.1021/ja037131v. [DOI] [PubMed] [Google Scholar]
- 36.Kornilova AY, Bihel F, Das C, Wolfe MS. The initial substrate-binding site of gamma-secretase is located on presenilin near the active site. Proc Natl Acad Sci U S A. 2005;102:3230–3235. doi: 10.1073/pnas.0407640102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bihel F, Das C, Bowman MJ, Wolfe MS. Discovery of a subnanomolar helical D-tridecapeptide inhibitor of γ-secretase. J Med Chem. 2004;47:3931–3933. doi: 10.1021/jm049788c. [DOI] [PubMed] [Google Scholar]
- 38.Lanz TA, Himes CS, Pallante G, Adams L, Yamazaki S, Amore B, Merchant KM. The gamma-secretase inhibitor N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester reduces A beta levels in vivo in plasma and cerebrospinal fluid in young (plaque-free) and aged (plaque-bearing) Tg2576 mice. J Pharmacol Exp Ther. 2003;305:864–871. doi: 10.1124/jpet.102.048280. [DOI] [PubMed] [Google Scholar]
- 39.Best JD, Jay MT, Otu F, Ma J, Nadin A, Ellis S, Lewis HD, Pattison C, Reilly M, Harrison T, et al. Quantitative measurement of changes in amyloid-beta(40) in the rat brain and cerebrospinal fluid following treatment with the gamma-secretase inhibitor LY-411575 [N2-[(2S)-2-(3,5-difluorophenyl)-2-hydroxyethanoyl]-N1-[(7S)-5-methyl-6-ox o-6,7-dihydro-5H-dibenzo[b,d]azepin-7-yl]-L-alaninamide] J Pharmacol Exp Ther. 2005;313:902–908. doi: 10.1124/jpet.104.081174. [DOI] [PubMed] [Google Scholar]
- 40.Siemers E, Skinner M, Dean RA, Gonzales C, Satterwhite J, Farlow M, Ness D, May PC. Safety, tolerability, and changes in amyloid beta concentrations after administration of a gamma-secretase inhibitor in volunteers. Clin Neuropharmacol. 2005;28:126–132. doi: 10.1097/01.wnf.0000167360.27670.29. [DOI] [PubMed] [Google Scholar]
- 41.Siemers ER, Quinn JF, Kaye J, Farlow MR, Porsteinsson A, Tariot P, Zoulnouni P, Galvin JE, Holtzman DM, Knopman DS, et al. Effects of a gamma-secretase inhibitor in a randomized study of patients with Alzheimer disease. Neurology. 2006;66:602–604. doi: 10.1212/01.WNL.0000198762.41312.E1. [DOI] [PubMed] [Google Scholar]
- 42.Anderson JJ, Holtz G, Baskin PP, Turner M, Rowe B, Wang B, Kounnas MZ, Lamb BT, Barten D, Felsenstein K, et al. Reductions in beta-amyloid concentrations in vivo by the gamma-secretase inhibitors BMS-289948 and BMS-299897. Biochem Pharmacol. 2005;69:689–698. doi: 10.1016/j.bcp.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 43.Barten DM, Guss VL, Corsa JA, Loo AT, Hansel SB, Zheng M, Munoz B, Srinivasan K, Wang B, Robertson BJ, et al. Dynamics of {beta}-Amyloid Reductions in Brain, Cerebrospinal Fluid and Plasma of {beta}-Amyloid Precursor Protein Transgenic Mice Treated with a {gamma}-Secretase Inhibitor. J Pharmacol Exp Ther. 2004;27:27. doi: 10.1124/jpet.104.075408. [DOI] [PubMed] [Google Scholar]
- 44.Tian G, Sobotka-Briner CD, Zysk J, Liu X, Birr C, Sylvester MA, Edwards PD, Scott CD, Greenberg BD. Linear non-competitive inhibition of solubilized human gamma-secretase by pepstatin A methylester, L685458, sulfonamides, and benzodiazepines. J Biol Chem. 2002;277:31499–31505. doi: 10.1074/jbc.M112328200. Epub 32002 Jun 31418. [DOI] [PubMed] [Google Scholar]
- 45.Tian G, Ghanekar SV, Aharony D, Shenvi AB, Jacobs RT, Liu X, Greenberg BD. The mechanism of gamma-secretase: multiple inhibitor binding sites for transition state analogs and small molecule inhibitors. J Biol Chem. 2003;278:28968–28975. doi: 10.1074/jbc.M300905200. [DOI] [PubMed] [Google Scholar]
- 46.Morohashi Y, Kan T, Tominari Y, Fuwa H, Okamura Y, Watanabe N, Sato C, Natsugari H, Fukuyama T, Iwatsubo T, et al. C-terminal fragment of presenilin is the molecular target of a dipeptidic gamma-secretase-specific inhibitor DAPT (N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester) J Biol Chem. 2006;281:14670–14676. doi: 10.1074/jbc.M513012200. [DOI] [PubMed] [Google Scholar]
- 47.Fuwa H, Takahashi Y, Konno Y, Watanabe N, Miyashita H, Sasaki M, Natsugari H, Kan T, Fukuyama T, Tomita T, et al. Divergent synthesis of multifunctional molecular probes to elucidate the enzyme specificity of dipeptidic gamma-secretase inhibitors. ACS Chem Biol. 2007;2:408–418. doi: 10.1021/cb700073y. [DOI] [PubMed] [Google Scholar]
- 48.Shah S, Lee SF, Tabuchi K, Hao YH, Yu C, LaPlant Q, Ball H, Dann CE, 3rd, Sudhof T, Yu G. Nicastrin functions as a gamma-secretase-substrate receptor. Cell. 2005;122:435–447. doi: 10.1016/j.cell.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 49.Searfoss GH, Jordan WH, Calligaro DO, Galbreath EJ, Schirtzinger LM, Berridge BR, Gao H, Higgins MA, May PC, Ryan TP. Adipsin: a biomarker of gastrointestinal toxicity mediated by a functional gamma secretase inhibitor. J Biol Chem. 2003;29:29. doi: 10.1074/jbc.M307757200. [DOI] [PubMed] [Google Scholar]
- 50.Wong GT, Manfra D, Poulet FM, Zhang Q, Josien H, Bara T, Engstrom L, Pinzon-Ortiz M, Fine JS, Lee HJ, et al. Chronic treatment with the gamma-secretase inhibitor LY-411,575 inhibits beta-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J Biol Chem. 2004;279:12876–12882. doi: 10.1074/jbc.M311652200. [DOI] [PubMed] [Google Scholar]
- 51.Petit A, Bihel F, Alves da Costa C, Pourquie O, Checler F, Kraus JL. New protease inhibitors prevent gamma-secretase-mediated production of Abeta40/42 without affecting Notch cleavage. Nat Cell Biol. 2001;3:507–511. doi: 10.1038/35074581. [DOI] [PubMed] [Google Scholar]
- 52.Esler WP, Das C, Campbell WA, Kimberly WT, Kornilova AY, Diehl TS, Ye W, Ostaszewski BL, Xia W, Selkoe DJ, et al. Amyloid-lowering isocoumarins are not direct inhibitors of γ-secretase. Nature Cell Biology. 2002;4:E110–111. doi: 10.1038/ncb0502-e110b. [DOI] [PubMed] [Google Scholar]
- 53.Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, Findlay KA, Smith TE, Murphy MP, Bulter T, et al. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414:212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- 54.Weggen S, Eriksen JL, Sagi SA, Pietrzik CU, Ozols V, Fauq A, Golde TE, Koo EH. Evidence that nonsteroidal anti-inflammatory drugs decrease amyloid beta 42 production by direct modulation of gamma-secretase activity. J Biol Chem. 2003;278:31831–31837. doi: 10.1074/jbc.M303592200. [DOI] [PubMed] [Google Scholar]
- 55.Fraering PC, Ye W, Strub JM, Dolios G, LaVoie MJ, Ostaszewski BL, Van Dorsselaer A, Wang R, Selkoe DJ, Wolfe MS. Purification and Characterization of the Human gamma-Secretase Complex. Biochemistry. 2004;43:9774–9789. doi: 10.1021/bi0494976. [DOI] [PubMed] [Google Scholar]
- 56.Beher D, Clarke EE, Wrigley JD, Martin AC, Nadin A, Churcher I, Shearman MS. Selected non-steroidal anti-inflammatory drugs and their derivatives target gamma-secretase at a novel site. Evidence for an allosteric mechanism. J Biol Chem. 2004;279:43419–43426. doi: 10.1074/jbc.M404937200. [DOI] [PubMed] [Google Scholar]
- 57.Okochi M, Fukumori A, Jiang J, Itoh N, Kimura R, Steiner H, Haass C, Tagami S, Takeda M. Secretion of the Notch-1 Abeta-like peptide during Notch signaling. J Biol Chem. 2006;281:7890–7898. doi: 10.1074/jbc.M513250200. [DOI] [PubMed] [Google Scholar]
- 58.Netzer WJ, Dou F, Cai D, Veach D, Jean S, Li Y, Bornmann WG, Clarkson B, Xu H, Greengard P. Gleevec inhibits beta-amyloid production but not Notch cleavage. Proc Natl Acad Sci U S A. 2003;100:12444–12449. doi: 10.1073/pnas.1534745100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fraering PC, Ye W, Lavoie MJ, Ostaszewski BL, Selkoe DJ, Wolfe MS. gamma -Secretase substrate selectivity can be modulated directly via interaction with a nucleotide binding site. J Biol Chem. 2005;280:41987–41996. doi: 10.1074/jbc.M501368200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fraering PC, LaVoie MJ, Ye W, Ostaszewski BL, Kimberly WT, Selkoe DJ, Wolfe MS. Detergent-dependent dissociation of active gamma-secretase reveals an interaction between Pen-2 and PS1-NTF and offers a model for subunit organization within the complex. Biochemistry. 2004;43:323–333. doi: 10.1021/bi035748j. [DOI] [PubMed] [Google Scholar]
- 61.Gu Y, Chen F, Sanjo N, Kawarai T, Hasegawa H, Duthie M, Li W, Ruan X, Luthra A, Mount HT, et al. APH-1 interacts with mature and immature forms of presenilins and nicastrin and may play a role in maturation of presenilin-nicastrin complexes. J Biol Chem. 2002;278:7374–7380. doi: 10.1074/jbc.M209499200. [DOI] [PubMed] [Google Scholar]
- 62.Watanabe N, Tomita T, Sato C, Kitamura T, Morohashi Y, Iwatsubo T. Pen-2 is incorporated into the gamma-secretase complex through binding to transmembrane domain 4 of presenilin 1. J Biol Chem. 2005;280:41967–41975. doi: 10.1074/jbc.M509066200. [DOI] [PubMed] [Google Scholar]
- 63.Kim SH, Sisodia SS. Evidence that the “NF” motif in transmembrane domain 4 of presenilin 1 is critical for binding with PEN-2. J Biol Chem. 2005;280:41953–41966. doi: 10.1074/jbc.M509070200. [DOI] [PubMed] [Google Scholar]
- 64.Kaether C, Capell A, Edbauer D, Winkler E, Novak B, Steiner H, Haass C. The presenilin C-terminus is required for ER-retention, nicastrin-binding and gamma-secretase activity. Embo J. 2004;23:4738–4748. doi: 10.1038/sj.emboj.7600478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kornilova AY, Kim J, Laudon H, Wolfe MS. Deducing the transmembrane domain organization of presenilin-1 in gamma-secretase by cysteine disulfide cross-linking. Biochemistry. 2006;45:7598–7604. doi: 10.1021/bi060107k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li YM, Lai MT, Xu M, Huang Q, DiMuzio-Mower J, Sardana MK, Shi XP, Yin KC, Shafer JA, Gardell SJ. Presenilin 1 is linked with gamma -secretase activity in the detergent solubilized state. Proc Natl Acad Sci U S A. 2000;97:6138–6143. doi: 10.1073/pnas.110126897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kornilova AY, Bihel F, Das C, Wolfe MS. The initial substrate binding site of γ-secretase is located on presenilin near the active site. Proc Natl Acad Sci U S A. 2005;102:3230–3235. doi: 10.1073/pnas.0407640102. [DOI] [PMC free article] [PubMed] [Google Scholar]