Abstract
Background
Enhanced production of endothelin-1 (ET-1), vasoconstrictive 21 amino acids produced by endothelial cells during ischemia and after reperfusion of the liver, is known to cause sinusoidal constriction and microcirculatory disturbances, which lead to severe tissue damage. Using a 2-hour hepatic vascular exclusion model in dogs, we tested our hypothesis that neutralization of ET-1 by monoclonal anti-ET-1 and anti-ET-2 antibody (AwETN40) abates vascular dysfunction and ameliorates ischemia/reperfusion injury of the liver.
Study Design
After skeletonization, the liver was made totally ischemic by cross-clamping the portal vein, the hepatic artery, and the vena cava (above and below the liver). Veno-venous bypass was used to decompress splanchnic and inferior systemic congestion. AwETN40, 5 mg/kg, was administered intravenously 10 minutes before ischemia (treatment group, n = 5). Nontreated animals were used as controls (control group, n = 10). Animal survival, hepatic tissue blood flow, liver function tests, total bile acid, high-energy phosphate, ET-1 levels, and liver histopathology were studied.
Results
Treatment with AwETN40 improved 2-week animal survival from 30% to 100%. Hepatic tissue blood flow after reperfusion was significantly higher in the treatment group. The treatment significantly attenuated liver enzyme release, total bile acid, and changes in adenine nucleotides. Immunoreactive ET-1 levels in the hepatic venous blood of the control group showed a significant increase and remained high for up to 24 hours after reperfusion. Histopathologic alterations were significantly lessened in the treatment group.
Conclusions
These results indicate that ET-1 is involved in ischemia/reperfusion injury of the liver, which can be ameliorated by the monoclonal anti-ET-1 and anti-ET-2 antibody AwETN40.
Endothelins (ETs), a group of vasoconstrictive 21 amino acids identified by Yanagisawa and colleagues (1), are involved in the pathogenesis of various disorders of the liver. They play regulatory roles in the hepatic microcirculation and exert glycogenolytic actions in the liver (2). In particular, ET-1, synthesized by sinusoidal cells and catabolized by hepatocytes (3), induces Ca2+ release and stimulation of glycogen phosphorylase in hepatocytes (4), constriction of Ito cells (5), and stimulation of platelet-activating factor in Kupffer cells (6). More important, production of ET-1 is enhanced during ischemia and after reperfusion in the liver and causes sinusoidal constriction, leading to severe microcirculatory disturbances and tissue damage (7).
In this study, we examined the role of ET-1 in ischemia-induced liver damage in dogs by blocking its action with a novel anti-ET-1 and anti-ET-2 monoclonal antibody, AwETN40.
Methods
Animals
Adult female beagle dogs weighing 8–12 kg were used. This study was conducted with the approval of the Institutional Animal Care and Use Committee, and all animals were cared for according to National Institutes of Health guidelines. After overnight fasting, the animals were anesthetized with thiopental sodium (25 mg/kg) for induction, and anesthesia was maintained with isoflurane, nitrous oxide, and oxygen by positive pressure mechanical ventilation. The right carotid artery and right jugular vein were cannulated for monitoring arterial pressure and central venous pressure and for obtaining blood samples. Electrocardiograph results and esophageal temperatures were monitored during the operation. Electrolyte solution (Plasmalyte, Travenol Laboratories, Deer-field, IL) was infused (20 mL·kg−1·h−1) through the operation to maintain adequate hydration and hemodynamic indices. Blood gases and electrolytes were measured frequently and corrected if necessary.
Operative procedures
After entering the abdomen through a midline incision, we skeletonized the liver by dividing all of the suspensory hepatic ligaments. A venous catheter, Intracath 16GA (Becton Dickinson Vascular Access, Sandy, UT), was inserted into the suprahepatic inferior vena cava and positioned in the left hepatic vein for collecting hepatic venous blood samples. The catheter was tunneled beneath the skin and exteriorized between the scapulae to allow serial blood sampling after the operation. Total hepatic vascular exclusion was initiated by cross-clamping the portal vein, hepatic artery, and infra- and supra-hepatic inferior vena cava. Pump-driven venovenous bypass (Biomedics, Minetonka, MN) was used to decompress the splanchnic venous bed and infrahepatic inferior vena cava (8). The bypass circuit connected the left femoral vein and the splenic vein to the left jugular vein. The animals were given heparin (50 U/kg) 5 minutes before the initiation of ischemia. After 2 hours of ischemia, the liver was reperfused, the bypass system was removed, and a splenectomy was performed. Cefamandole nafate (1 g) was administered intraoperatively and continued for 3 postoperative days. The animals were allowed to eat and drink the morning after the operation and were followed for 2 weeks.
Experimental groups
AwETN40, a monoclonal anti-ET-1 and anti-ET-2 antibody, was supplied by Takeda Chemical Industries Ltd. (Osaka, Japan). AwETN40 dissolved in saline was administered intravenously, at a dose of 5 mg/kg, 10 minutes before the initiation of ischemia (treatment group, n = 5). Animals receiving saline alone were used as controls (control group, n = 10).
Determinations
Two-week animal survival, liver function tests, total bile acid (TBA), hepatic tissue blood flow (HTBF), tissue biochemistry, histopathology, and ET levels were used to evaluate the efficacy of the treatment in this study. Peripheral venous blood samples were collected serially for determination of liver enzymes, including aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase, by a Technicon RA500 autoanalyzer (Bayer, Tarrytown, NY). Serum TBA was measured by the enzymatic fluorometric method using the Sterongnost-alpha Flu Kit (Nyegaad, Oslo, Norway) (9). Serial measurements of HTBF were performed intraoperatively using a laser Doppler flowmeter (Advance Laser Flowmeter, ALF21; Advance Company Ltd., Tokyo, Japan). The probe was placed on three lobes of the liver until a stable flow value was obtained. To avoid a misreading in HTBF measurements related to respiratory motion, the ventilation was always stopped briefly. The mean of these three values was used as the representative value and was expressed as a percentage of the value of the preischemia level.
Wedge liver biopsies collected before ischemia, at the end of ischemia, and 15 minutes and 60 minutes after reperfusion were diced into small pieces and immediately transferred into liquid nitrogen for the determination of adenine nucleotides. Concentrations of adenine nucleotides were measured using a Waters high-performance liquid chromatography system (Waters Chromatography Division/Millipore Corp., Milford, MA; Model 484 absorbance module and Model 717 WISP system) at 254 nm (Waters 484, Tunable Absorbance Detector) (10). Energy charge was calculated using the equation [ATP + ½ ADP]/[ATP + ADP + AMP], in which ATP = adenosine triphosphate, ADP = adenosine diphosphate, and AMP = adenosine monophosphate. The other tissues were fixed in buffered formalin and stained with hematoxylin and eosin. Histopathology was examined by a single pathologist without knowledge of the groups or the timing of tissue sampling. Histologic assessment was performed semiquantitatively based on the degree of sinusoidal congestion, sinusoidal derangement, ischemic hepatocyte injury, and hepatocyte necrosis as follows: none = 0, mild = 1, moderate = 2, and severe = 3. The number of neutrophils infiltrated in the tissue was counted after Leder’s staining (11) and expressed as that per 1,000 hepatocyte nuclei.
Plasma concentration of immunoreactive ET-1 (irET-1) in the hepatic venous blood was measured by radioimmunoassay (12). The 4-mL blood samples, collected in a Vacutainar 6452 tube (Becton Dickinson Vacutainer Systems, Rutherford, NJ) with 7.5 mg of ethylenediamine tetraacetic acid, were centrifuged at 2,800 × g and 4°C for 10 minutes and were stored at − 80°C until the assay. Samples and standards (ET-1; Peninsula Laboratories, Belmont, CA) were reconstituted in assay buffer and incubated for 24 hours with rabbit anti-ET-1 serum (RIK-6901; Peninsula Laboratories) at 4°C. The addition of iodine 125 ET-1 (Peninsula Laboratories) was followed by a second 24-hour incubation, and bound and free radioligand were separated using the second antibody method. Bound radioactivity data were evaluated after logit/log transformation, and the irET-1 data were presented after correction for recovery. The rabbit antiserum exhibited a cross-reactivity of 17% with human big ET-1 and of 7% with ET-3, but no cross-reactivity with unrelated peptides (ie, atrial natriuretic peptide, brain natriuretic peptide, vasopressin, and angiotensins I, II, and III). The standard curve was very stable, with a midpoint (50% inhibitory concentration) of 14 pg/tube.
Statistical analysis
Values were expressed as mean ± standard error of the mean (SEM). Animal survival was compared using χ2 test. Intra-group analysis was performed using Wilcoxon signed-rank test; intergroup analysis was performed using Mann-Whitney U test. A p value < 0.05 was considered statistically significant.
Results
Survival
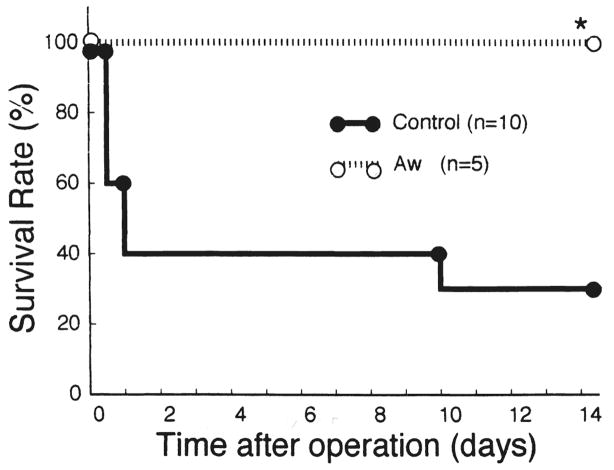
All of the animals treated with AwETN40 survived for 14 days after the operation, but 7 of 10 control dogs died of liver failure. The survival rate in the treatment group was significantly higher than that in the control group (p < 0.05) (Fig. 1).
Fig 1.
Animal survival after the operation. *p < 0.05 versus control. Aw, AwETN40 (treatment group).
Mean arterial pressure and hepatic tissue blood flow
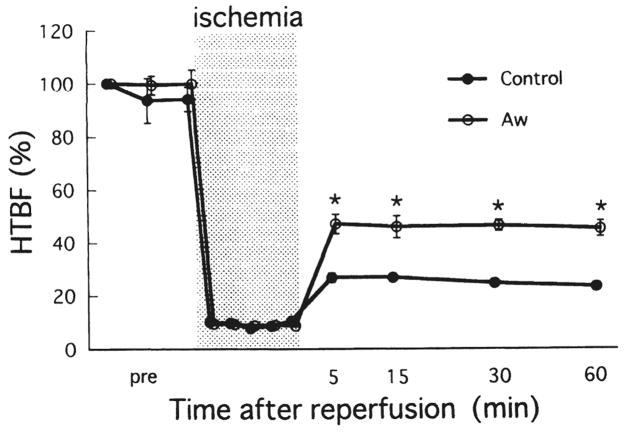
The mean arterial pressure of both groups fell moderately soon after the initiation of ischemia and fell considerably immediately after reperfusion. Our operational procedure produced complete liver ischemia, but there was false-positive tissue blood flow by Doppler flow measurement during ischemia, ranging from 8–11% of the pre-ischemia levels (Fig. 2). The restoration of tissue blood flow after reperfusion was significantly better in the treated animals than in the controls.
Fig 2.
Changes in hepatic tissue blood flow (HTBF) during ischemia and after reperfusion. Values are expressed as a percentage of the preischemic HTBF level. Bars express SEM. *p < 0.01 versus control. Aw, AwETN40.
Liver enzyme levels
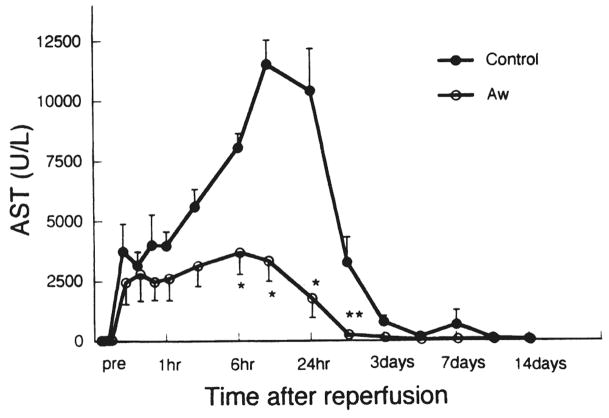
After reperfusion, levels of aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase were significantly lower in the AwETN40-treated animals than in control animals (Fig. 3; Table 1).
Fig 3.
Changes in aspartate aminotransferase (AST) after reperfusion. Bars express SEM; only one-sided SEM is presented. *p < 0.05 versus control: **p < 0.01 versus control. Aw, AwETN40.
Table 1.
Alanine Aminotransferase, Lactate Dehydrogenase, and Total Bile Acid After Reperfusion
| Measurement | Group | Preischemia | 30 min | 60 min | 3h | 6h | 12 h | 24 h |
|---|---|---|---|---|---|---|---|---|
| ALT (U/L) | Control | 32.2 ± 2.2 | 4,846 ± 1.678 | 4,961 ± 798 | 6,575 ± 1,007 | 9,332 ± 1,128 | 13,563 ± 1,379 | 12,680 ± 1,451 |
| Aw | 32.0 ± 4.5 | 2,222 ± 699 | 2,373 ± 897 | 2,842 ± 807* | 3,344 ± 914† | 3,505 ± 925† | 3,439 ± l,612† | |
| LDH (U/L) | Control | 80.6 ± 15.7 | 3,209 ± 1,076 | 3,385 ± 474 | 3,128 ± 479 | 2,906 ± 559 | 3,045 ± 634 | 1,440 ± 216 |
| Aw | 53.0 ± 15.7 | 2,378 ± 624 | 2,004 ± 732 | 1,533 ± 423 | 1,148 ± 161* | 561 ± 125* | 261 ± 25.5† | |
| TBA (μmol/L) | Control | 0.3 ± 0.3 | 36.3 ± 7.9 | 48.5 ± 11.7 | 100 ± 11.0 | 133.8 ± 11.8 | 124.3 ± 13.1 | 175.4 ± 39.0 |
| Aw | 2.4 ± 1.0 | 67.2 ± 5.0* | 59.4 ± 14.9 | 97.4 ± 31.1 | 102.4 ± 24.6 | 44.6 ± 14.9† | 30.4 ± 12.3* |
p < 0.05 versus control value.
p < 0.01 versus control value.
ALT, alanine aminotransferase; Aw, AwETN40; LDH, lactate dehydrogenase; TBA, total bile acid.
Total bile acid
Table 1 details the changes in the TEA levels in the control and treatment groups. TEA levels in the treatment group reached a peak value at 6 hours after reperfusion and declined thereafter; levels in the control group continued to increase until 24 hours after reperfusion.
Endothelin levels
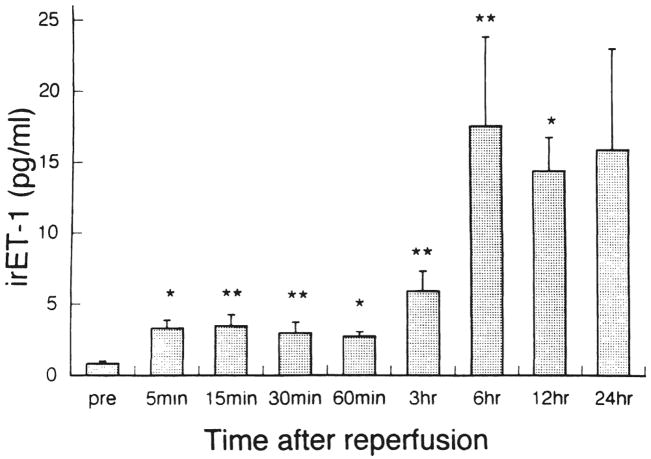
Changes in irET-1 levels in the hepatic venous blood in the control group are shown in Figure 4. Reperfusion of the liver after 2 hours of ischemia induced a significant rise in irET levels, by 4 times from 5 minutes to 60 minutes, 7 times at 3 hours, and > 18 times thereafter. Measurement of irET-1 in the treatment group failed because of the competitive nature of AwETN40 against the radioimmunoassay method used in this study.
Fig 4.
Concentration of immunoreactive endothelin-1 (irET-l) in the hepatic venous blood alter reperfusion. Bars express SEM. *p < 0.05 versus preischemic value; **p < 0.01 versus preischemic value.
Adenine nucleotides
Treatment with AwETN40 slowed the degradation of adenine nucleotides during ischemia and enhanced energy resynthesis after reperfusion (Table 2). Adenosine triphosphate levels and total adenine nucleotide levels in the treatment group at 15 minutes and 60 minutes after reperfusion were significantly higher than those in the control group. Energy charge showed no significant difference between the groups.
Table 2.
Tissue Concentrations of Adenine Nucleotides During Ischemia and After Reperfusion
| Time | Group | ATP (nmol/g tissue) | ADP (nmol/g tissue) | AMP (nmol/g tissue) | TAN (nmol/g tissue) | EC (nmol/g tissue) |
|---|---|---|---|---|---|---|
| Preischemia | Control | 2,666.2 ± 283.5 | 894.0 ± 111.2 | 213.6 ± 26.4 | 3,773.7 ± 286.7 | 0.8229 ± 0.017 |
| 2 h after ischemia | Control | 236.9 ± 40.4 | 335.0 ± 29.1 | 1,147.1 ±95.9 | 1,718.9 ± 132.2 | 0.2337 ± 0.024 |
| Aw | 357.0 ± 61.5 | 422.6 ± 94.2 | 1,537.7 ± 98.2* | 2,317.3 ± 165.1* | 0.2401 ± 0.031 | |
| 15 min after reperfusion | Control | 846.8 ± 152.2 | 409.9 ± 65.6 | 160.0 ± 15.9 | 1,416.8 ± 209.3 | 0.7229 ± 0.036 |
| Aw | 1,905.3 ± 233.9† | 623.7 ± 82.3* | 252.7 ± 21.1* | 2,781.6 ± 275.2† | 0.7943 ± 0.015 | |
| 60 min after reperfusion | Control | 1,227.2 ± 123.7 | 329.4 ± 33.4 | 112.2± 13.1 | 1,668.8 ± 143.2 | 0.8324 ± 0.014 |
| Aw | 1,994.2 ± 171.8* | 517.9 ± 73.0* | 268.4 ± 48.5 | 2,780.6 ± 137.1* | 0.8093 ± 0.030 |
p < 0.05 versus control value.
p < 0.01 versus control value.
ATP, adenosine triphosphate; ADP, adenosine diphosphate; AMP, adenosine monophosphate; TAN, total adenine nucleotides; EC, energy charge; Aw, AwETN40.
Histology
Although no significant histologic differences were found at the end of 2-hour ischemia and at 15 minutes after reperfusion, the treatment group showed significantly less structural damage and neutrophil infiltration in the liver than the control group. In particular, hepatocyte necrosis was not seen in any of the treatment-group animals (Table 3).
Table 3.
Histologic Findings
| Finding | Group | Preischemia | 120 min of ischemia | 15 min after reperfusion | 60 min after reperfusion |
|---|---|---|---|---|---|
| Histologic abnormality | |||||
| Sinusoidal congestion | Control | 0 | 1.0 ± 0.15 | 1.4 ± 0.31 | 2.0 ± 0.21 |
| Aw | 0.2 ± 0.2 | 1.0 ± 0 | 1.6 ± 0.24 | 1.6 ± 0.24 | |
| Sinusoidal derangement | Control | 0 | 1.5 ± 0.17 | 1.3 ± 0.15 | 2.1 ± 0.1 |
| Aw | 0 | 1.2 ± 0.2 | 1.6 ± 0.24 | 1.8 ± 0.2 | |
| Ischemic hepatocyte injury | Control | 0 | 0 | 1.1±0.18 | 1.9 ± 0.18 |
| Aw | 0 | 0 | 0.6 ± 0.24 | 1.0 ± 0.32* | |
| Hepatocyte necrosis | Control | 0 | 0 | 0.1 ± 0.1 | 1.3 ± 0.15 |
| Aw | 0 | 0 | 0 | 0† | |
| Total score | Control | 0 | 2.5 ± 0.22 | 3.8 ± 0.42 | 7.3 ± 0.33 |
| Aw | 0.2 ± 0.2 | 2.2 ± 0.2 | 3.8 0.73 | 4.4 ± 0.6† | |
| Number of PMNs | Control | 16.7 ± 2.2 | 15.7 ± 2.2 | 39.4 ± 5.5 | 57.2 ± 6.4 |
| Aw | 17.3 ± 3.4 | 18.2 ± 4.2 | 22.5 ± 5.2 | 22.3 ± 3.2* | |
p < 0.05 versus control value.
p < 0.01 versus control value.
Aw, AwETN40; PMN, polymorphonuclear neutrophil.
Discussion
In this study, we demonstrated that the neutralization of ET-1 by AwETN40 lessened microcirculatory disturbance, attenuated ischemia/reperfusion injury, and improved animal survival. In addition, pretreatment with AwETN40 slowed enzyme leakage, inhibited adenine nucleotide degradation in ischemic tissues, enhanced ATP resynthesis, and improved histopathologic findings.
Endothelins are a group of related 21-amino acid peptides (ET-1, ET-2, and ET-3), which bind to three different types of ET receptors (ETA, ETB, and ETC) (13). In addition, two subtypes of ETB receptors have been identified, ETB1 and ETB2 (14). ETA has a high affinity for ET-1 and ET-2~ but a low affinity for ET-3. ETB has an equally potent affinity for ET-1, -2, and -3 (15). The ETA receptor generally has been accepted as the predominant receptor subtype associated with vasoconstriction. The ETB1 receptor is located in the endothelial cell and mediates vasodilation through the release of nitric oxide and prostacyclin. The ETB2 receptor is located on the vascular smooth muscle cells and mediates vasoconstriction directly (16). In the liver, the number of these ET receptors per cell is 30–60-fold higher on Ito cells than on sinusoidal endothelial cells or hepatocytes (17). The abundance of ET receptors on Ito cells suggests that these cells are a major target for ET in the liver. Ito cells extend their numerous long cytoplasmic processes to surround the sinusoidal wall and are involved in reducing the hepatic sinusoidal microcirculation by contraction (18). Among various ETs, it has been shown that ET-1 is a major ET produced by ischemia and reperfusion, and that both ETA and ETB receptors are located on the cell membrane of Ito cells (15).
Endothelin can be divided into two structural portions: (1) the N-terminal 15 amino acids, having a rigid structure with two disulfide bonds; and (2) the C-terminal heptapeptide, consisting of relatively hydrophobic amino acids. AwETN40 is a monoclonal antibody for ET-1 and ET-2 that recognizes only the N terminal and not the C terminal. Although AwETN40 was first developed for the sandwich enzyme immunoassay for ET-1 (19), it has also proved effective in preventing myocardial infarction (20), acute renal failure (21), liver ischemia (22, 23), and endotoxin-induced liver injury (24) by its neutralizing activity against ET-1 and ET-2.
The dosage of AwETN40 used in this experiment was based on previous reports. Watanabe and associates (20) showed that the effect of exogenous ET-1 administration (0.75 mg/kg) on blood pressure was neutralized by pretreating animals with 1.5–4.5 mg/kg of AwETN40. They also found that the effect of 22.5 mg/kg AwETN40 lasted for > 24 hours. Using a 1-hour partial (70%) liver ischemia model in dogs, Kawamura and colleagues (23) reported that pretreatment with 2 mg/kg of AwETN40 was effective in attenuating ischemia/reperfusion injury. Thus, we considered that the dosage of AwETN40 in this study, 5 mg/kg, would be sufficient for neutralization of ET-1 in ischemia/reperfusion injury in the 2-hour total hepatic vascular exclusion model in dogs.
In this study, the concentration of irET-1 in hepatic venous blood increased immediately after reperfusion and remained markedly elevated for up to 24 hours above 15.9 ± 7.1 pg/mL. Nakamura and associates (22) reported that irET-1 increased to 12 pg/mL at 3 hours after reperfusion using a 60-minute total liver ischemia model in rats. Kawamura and colleagues (23) reported that irET-1 increased to 6 pg/mL at 3 hours after reperfusion using the 1-hour partial liver ischemia model in dogs. The different concentrations of irET-1 between these studies may depend on the difference in species, the duration of ischemic time, the timing of the measurement, and the amount of ischemic liver damage. Levels of ET-1 in the circulation after ischemia and reperfusion are influenced by the balance between production and its clearance by the injured liver. It has been demonstrated that hypoxia induces ET-1 gene expression and secretion in endothelial cells (25), which is regulated by the degree of ischemia and the duration of reperfusion. In addition, interleukin-1, tumor necrosis factor, and transforming growth factor-β, all of which are up-regulated by ischemia and reperfusion, stimulate ET-1 production and release from sinusoidal endothelial cells (26). Clearance of ET is markedly reduced in injured livers as compared with normal livers (23).
Exogenous ET-1 has been shown to cause a pronounced elevation of intrahepatic vascular resistance, resulting in a profound decrease in hepatic blood flow and a refractory increase in portal pressure (2). In this study, although there was no difference in mean arterial pressure between the groups, preischemic administration of AwETN40 markedly improved HTBF after reperfusion. However, HTBF did not reach the preischemic level during the 1-hour observation period, suggesting that the effect of this agent may not be sufficient or that there are other factors involved. First, AwETN40 barely moves from the bloodstream into the interstitial space because of its large molecular weight. It may bind easily to ET in the circulation, but it is more difficult to trap ET in the interstitial space, even if a sufficient dose of the agent is given intravenously. Second, ET-1 exerts its action by an autocrine or paracrine mechanism. Ito cells have a very low level of ET-1 mRNA in the normal liver, but it increases significantly once the liver receives ischemic insults (27). In addition, when ET-1 is produced by endothelial cells, 80% of the ET-1 is released into the interstitial space rather than into the circulation (28). Finally, insufficient restoration of HTBF in this study may relate to other impaired endothelial cell functions, such as reduced synthesis of nitric oxide and prostaglandins (29).
The determination of serum TBA is a sensitive and specific index for hepatobiliary disorders that depends on the synthesis and excretory functions of hepatocytes and the hepatic blood flow (30). As shown in the present study, TBA increased immediately after reperfusion and remained high in the control group for up to 24 hours; TBA levels in the treatment group began to decrease after 6 hours, indicating better hepatocyte function and greater hepatic blood flow.
Adenosine triphosphate is the most basic factor in maintaining functional and structural integrity of hepatocytes (31). Reduction of ATP levels leads directly to the disturbance of cellular integrity and exacerbation of cellular dysfunction. Endothelin-1 lowers ATP levels by increased consumption (2) or inhibits ATP synthesis by suppressing the oxygen supply through vasoconstriction (32). Pretreatment with AwETN40 abated these adverse effects of ET-1 in this study.
The number of polymorphonuclear neutrophils (PMNs) infiltrated in postischemic liver tissue was significantly reduced by AwETN40 treatment. Polymorphonuclear neutrophils play a crucial role in the progression of ischemia/reperfusion injury (33). During interactions of PMNs and ET-1, ET-1 has been shown to stimulate migration and aggregation of PMNs (34) and to enhance superoxide anion production by the chemotactic peptide N-formyl-methionyl-leucyl-phenylalanine (35). On the other hand, PMNs increase the induction of ET-1 mRNA expression in endothelial cells (36) and convert big ET to ET-1 (37). Furthermore, ET-1 stimulates the expression of adhesive molecules, CD11/CD18 integrin, on the neutrophil surface (38). The expression of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 on cerebromicrovascular endothelial cell lines is also up-regulated by ET-1 (39). These observations suggest that ET-1 exerts influence on PMNs by facilitating their adhesion to endothelial cells and infiltration into the tissue. Consequently, the neutralization of ET-1 by AwETN40 appears, in part, to suppress PMNs’ adhesion to endothelial cells, lessen PMNs’ activation, and attenuate liver injury.
We postulated the following scenario on the basis of our findings. Two hours of ischemia causes extensive damage to sinusoidal endothelial cells in the liver. The damage triggers the production and release of endogenous ET-1 that induces the constriction of Ito cells, leading to sinusoidal constriction and microcirculatory disturbances. The reduction in blood flow augments postischemic tissue injury. In addition, ET-1 enhances the various adverse actions of PMNs. The neutralization of ET-1 by the monoclonal antibody AwETN40 is considered to contribute to the attenuation of liver damage by inhibiting sinusoidal constriction and PMN action, which are involved in the pathogenesis of ischemia/reperfusion injury of the liver.
Acknowledgments
Aided by Research Grants from the Veterans Administration and Project Grant No. DK-29961 from the National Institutes of Health, Bethesda. Maryland.
References
- 1.Yanagisawa M, Kurihara H, Kimura S, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 2.Tran-Thi TA, Kawada N, Decker K. Regulation of endothelin-1 action on the perfused rat liver. FEBS Letts. 1993;318:353–357. doi: 10.1016/0014-5793(93)80544-5. [DOI] [PubMed] [Google Scholar]
- 3.Gandhi CR, Harvev SAK, Olson MS. Hepatic effects of endothelin. Mechanism of [125I] endothelin-1 by liver derived cells. Arch Biochem Biophys. 1993;305:38–46. doi: 10.1006/abbi.1993.1390. [DOI] [PubMed] [Google Scholar]
- 4.Serradeil-Le Gal C, Jouneaux C, Sanchez-Bueno A, et al. Endothelin action in rat liver. J Clin Invest. 1991;87:133–138. doi: 10.1172/JCI114962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakamoto M, Ueno T, Kin M, et al. Ito cell contraction in response to endothelin-1 and substance P. Hepatology. 1993;18:978–983. doi: 10.1002/hep.1840180432. [DOI] [PubMed] [Google Scholar]
- 6.Mustafa SB, Gandhi CR, Harvey SAK, Olson MS. Endothelin stimulates platelet activating factor synthesis by cultured rat Kupffer cells. Hepatology. 1995;21:545–553. [PubMed] [Google Scholar]
- 7.Zhang JX, Pegoli W, Clemens MG. Endothelin-1 induces direct constriction of hepatic sinusoids. Am J Physiol. 1994;266:G624–G632. doi: 10.1152/ajpgi.1994.266.4.G624. [DOI] [PubMed] [Google Scholar]
- 8.Todo S, Zhu Y, Zhang S, et al. Attenuation of ischemic liver injury by augmentation of endogenous adenosine. Transplantation. 1997;63:217–223. doi: 10.1097/00007890-199701270-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mashige F, Imai K, Osuga T. A simple and sensitive assay of total serum bile acids. Clin Chim Acta. 1976;70:79–86. doi: 10.1016/0009-8981(76)90007-3. [DOI] [PubMed] [Google Scholar]
- 10.Hamamoto I, Takaya S, Todo S, et al. Can adenine nucleotides predict primary nonfunction of the human liver homograft? Transplant Int. 1994;7:89–95. doi: 10.1007/bf00336468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevens A. Enzyme histochemistry: diagnostic applications. In: Bancroft JD, Stevens A, editors. Theory and Practice of Histologic Techniques. New York: Churchill Livingstone; 1990. pp. 401–411. [Google Scholar]
- 12.Xuan YT, Whorton AR, Shearer-Poor E, et al. Determination of immunoreactive endothelin in medium from cultured endothelial cells and human plasma. Biochem Biophys Res Commun. 1989;164:326–332. doi: 10.1016/0006-291x(89)91721-x. [DOI] [PubMed] [Google Scholar]
- 13.Karne S, Jayawickreme CK, Lerner MR. Cloning and characterization of an endothelin-3 specific receptor (ETC receptor) from xenopus laevis dermal melanophores. J Biol Chem. 1993;268:19126–19133. [PubMed] [Google Scholar]
- 14.Sokolovsky M, Amber I, Galron R. A novel subtype of endothelin receptors. J Biol Chem. 1992;267:20551–20554. [PubMed] [Google Scholar]
- 15.Rocky D. The cellular pathogenesis of portal hypertension: stellate cell contractility, endothelin, and nitric oxide. Hepatology. 1997;25:2–5. doi: 10.1053/jhep.1997.v25.ajhep0250002. [DOI] [PubMed] [Google Scholar]
- 16.Ohlstein EH, Nambi P, Ruffolo RR. Endothelin receptor subclassification. In: Ruffolo RR, editor. Endothelin Receptors From the Gene to the Human. New York: CRC Press; 1995. pp. 15–36. [Google Scholar]
- 17.Housset CN, Rockey DC, Friedman SL, Bissell DM. Hepatic lipocytes: a major target for endothelin-1. J Hepatol. 1995;22(Suppl 2):55–60. [PubMed] [Google Scholar]
- 18.Tanikawa K. Hepatic sinusoidal cells and sinusoidal circulation. J Gastroenterol Hepatol. 1995;10:S8–S11. doi: 10.1111/j.1440-1746.1995.tb01806.x. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki N, Matsumoto H, Kitada C, et al. A sensitive sandwich-enzyme immunoassay for human endothelin. J Immunol Methods. 1989;118:245–250. doi: 10.1016/0022-1759(89)90012-4. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe T, Suzuki N, Shimamoto N, et al. Contribution of endogenous endothelin to the extension of myocardial infarct size in rats. Circ Res. 1991;69:370–377. doi: 10.1161/01.res.69.2.370. [DOI] [PubMed] [Google Scholar]
- 21.Shibouta Y, Suzuki N, Shino A, et al. Pathophysiological role of endothelin in acute renal failure. Life Sci. 1990;46:1611–1618. doi: 10.1016/0024-3205(90)90392-5. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura S, Nishiyama R, Serizawa A, et al. Hepatic release of endothelin-1 after warm ischemia. Transplantation. 1995;59:679–684. doi: 10.1097/00007890-199503150-00006. [DOI] [PubMed] [Google Scholar]
- 23.Kawamura E, Yamanaka N, Okamoto E, et al. Response of plasma and tissue endothelin-1 to liver ischemia and its implication in ischemia-reperfusion injury. Hepatology. 1995;21:1138–1143. [PubMed] [Google Scholar]
- 24.Ohuchi T, Tada K, Akamatsu K. Endogenous ET-1 contributes to liver injury induced by galactosamine and endotoxin in isolated perfused rat liver. Am J Physiol. 1995;268:G997–G1003. doi: 10.1152/ajpgi.1995.268.6.G997. [DOI] [PubMed] [Google Scholar]
- 25.Rakugi H, Tabuchi Y, Nakamaru M, et al. Evidence for endothelin-1 release from resistance vessels of rats in response to hypoxia. Biochem Biophys Res Commun. 1990;169:973–977. doi: 10.1016/0006-291x(90)91989-6. [DOI] [PubMed] [Google Scholar]
- 26.Yoshizumi M, Kurihara H, Morita T, et al. Interleukin-1 increases the production of endothelin-1 by cultured endothelial cells. Biochem Biophys Res Commun. 1990;166:324–329. doi: 10.1016/0006-291x(90)91948-r. [DOI] [PubMed] [Google Scholar]
- 27.Housset CN, Rockey DC, Bissell DM. Endothelin receptors in rat liver: lipocytes as a contractile target for endothelin-1. Proc Nad Acad Sci USA. 1993;90:9266–9270. doi: 10.1073/pnas.90.20.9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagner OF, Christ G, Wojta J, et al. Polar secretion of endothelin-1 by cultured endothelial cells. J Biol Chem. 1992;267:16066–16068. [PubMed] [Google Scholar]
- 29.Pinsky DJ, Oz MC, Koga S, et al. Cardiac preservation is enhanced in a heterotopic rat transplant model by supplementing the nitric oxide pathway. J Clin Invest. 1994;93:2291–2297. doi: 10.1172/JCI117230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herrera FJ, Codoceo R, Cienfuegos J, et al. Bile acid profile as early indicator of allograft function during orthotopic liver transplantation. Eur Surg Res. 1990;22:19–26. doi: 10.1159/000129078. [DOI] [PubMed] [Google Scholar]
- 31.Kamiike W, Burdelski M, Steinhoff G, et al. Adenine nucleotide metabolism and its relation to organ viability in human liver transplantation. Transplantation. 1988;45:138–143. doi: 10.1097/00007890-198801000-00030. [DOI] [PubMed] [Google Scholar]
- 32.Okumura S, Takei Y, Kawano S, et al. Vasoactive effect of endothelin-1 on rat liver in vivo. Hepatology. 1994;19:155–161. doi: 10.1016/0270-9139(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 33.Jaeschke H, Farhood A, Smith CW. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB J. 1990;4:3355–3359. [PubMed] [Google Scholar]
- 34.McMillen MA, Huribal M, Sumpio B. Common pathway of endothelial-leukocyte interaction in shock, ischemia, and reperfusion. Am J Surg. 1993;166:557–562. doi: 10.1016/s0002-9610(05)81153-5. [DOI] [PubMed] [Google Scholar]
- 35.Ishida K, Takeshige K, Minakami S. Endothelin-1 enhances superoxide generation of human neutrophils stimulated by the chemotactic peptide N-formyl-methionyl-leucyl- phenylalanine. Biochem Biophys Res Commun. 1990;173:496–500. doi: 10.1016/s0006-291x(05)80061-0. [DOI] [PubMed] [Google Scholar]
- 36.Morita T, Kurihara H, Yoshizumi M, et al. Human polymorphonuclear leukocytes have dual effects on endothelin-1. Heart Vessels. 1993;8:l–6. [PubMed] [Google Scholar]
- 37.Uprichard ACG, Chi L, Lucchesi BR. Functional consequence of big endothelin conversion. Pharmacology. 1993;47:277–285. doi: 10.1159/000139108. [DOI] [PubMed] [Google Scholar]
- 38.Lopez Farre A, Riesco A, Espinosa G, et al. Effect of endothelin-1 on neutrophil adhesion to endothelial cells and perfused heart. Circulation. 1993;88:1661–1671. doi: 10.1161/01.cir.88.3.1166. [DOI] [PubMed] [Google Scholar]
- 39.MacCarron RM, Wang L, Stanimirovic DB, Spatz M. Endothelin induction of adhesion molecule expression on human brain microvascular endothelial cells. Neurosci Lett. 1993;156:31–34. doi: 10.1016/0304-3940(93)90432-k. [DOI] [PubMed] [Google Scholar]