Abstract
The nuclear hormone receptor DAF-12 from Caenorhabditis elegans is activated by dafachronic acids, which derive from sterols upon oxidation by DAF-9, a cytochrome P450. DAF-12 activation is a critical checkpoint in C. elegans for acquisition of reproductive competence and for entry into adulthood rather than dauer diapause. Previous studies implicated the (25S)-Δ7-dafachronic acid isomer as the most potent compound, but the (25S)-Δ4-isomer was also identified as an activator of DAF-12. To explore the tolerance of DAF-12 for structural variations in the ligand and to enable further studies requiring large amounts of ligands for DAF-12 and homologs in other nematodes, we synthesized (25R)- and (25S)-isomers of five dafachronic acids differing in A/B-ring configurations. Both the (25S)- and (25R)-Δ7-dafachronic acids are potent transcriptional activators in a Gal4-transactivation assay using HEK-293 cells, with EC50 values of 23 and 33 nm, respectively, as are (25S)- and (25R)-Δ4-dafachronic acids, with EC50 values of 23 and 66 nm, respectively. The (25S)- and (25R)-Δ5-isomers were much less potent, with EC50 values approaching 1000 nm, and saturated 5α- and 5β-dafachronic acids showed mostly intermediate potencies. Rescue assays using daf- 9-null mutants confirmed the results from transactivation experiments, but this in vivo assay accentuated the greater potencies of the (25S)-epimers, particularly for the (25S)-Δ7-isomer. We conclude that DAF-12 accommodates a large range of structural variation in ligand geometry, but (25S)-Δ7-dafachronic acid is the most potent and probably biologically relevant isomer. Potency derives more from the A/B-ring configuration than from the stereochemistry at C-25.
The Δ4- Δ7-dafachronic acids are the most potent and biologically relevant isomers, due principally to A/B-ring structure. Comprehensive synthetic methods to multiple isomers are described.
The life cycle of Caenorhabditis elegans allows for arrested development in a protected dormant or dauer diapause during periods of stress or starvation. When favorable conditions return, a pathway is activated to complete the second larval molt and to restore reproductive competence. The genes responsible for the entry and exit from the dauer diapause, termed the daf genes (for dauer formation), have been the topic of intense study, and the functions of many proteins encoded by these genes have been identified (1). In particular, two of the terminal genes in this pathway, daf-9 and daf-12, encode a cytochrome P450 enzyme and a nuclear hormone receptor, respectively (2,3,4).
Given that C. elegans is auxotrophic for cholesterol (2), we reasoned that the environmental nutrient necessary for the cascade of events culminating in exit from the dauer diapause was a sterol, which was oxygenated by DAF-9 to form the ligand for DAF-12. Using this model, which intriguingly parallels the oxygenation of cholesterol to androgens and estrogens during reproductive maturation in mammals, we previously identified two compounds extracted from worms, which we have called the Δ7- and Δ4-dafachronic acids (7- and 4-cholesten-3-one-26-oic acids, I and II, Fig. 1). Our prior studies suggested that (25S)-Δ7-dafachronic acid was the most potent and important endogenous ligand with nanomolar affinity; however, the dafachronic acids were previously unknown compounds (3). We synthesized authentic Δ4-dafachronic acid and confirmed its potency, but we did not have access to synthetic Δ7-dafachronic acid, and we did not test other sterol acids with additional configurations of the A/B ring system.
Figure 1.
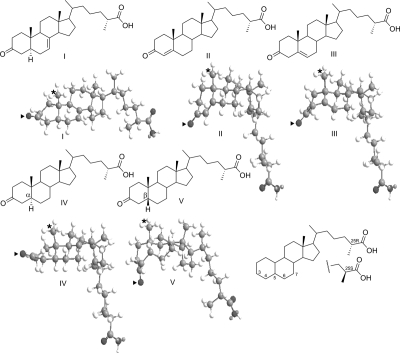
Structures of dafachronic acids synthesized in this study. The 25R isomers of each compound are shown in I-V, with important carbon atoms numbered and stereochemical features defined at C-5 and C-25. Beneath the structures are ball-and-stick models, which illustrate the differences in A/B-ring geometries among these compounds. The 19-methyl groups are highlighted by asterisks, and the 3-ketone oxygen atoms are indicated by arrowheads. Models were rendered by ChemBio 3D Ultra 11.0 after energy minimization with MM2.
Consequently, we have developed a versatile synthetic scheme suitable for large-scale preparation of multiple dafachronic acids from readily available and inexpensive diosgenin (VI). After synthesizing compounds I–V, we explored how the position of a double bond in the A/B ring system and the stereochemistry at C-5 and C-25 influence the potency of dafachronic acid ligands for DAF-12 using transactivation and dauer rescue assays.
Results
Synthesis of dafachronic acids
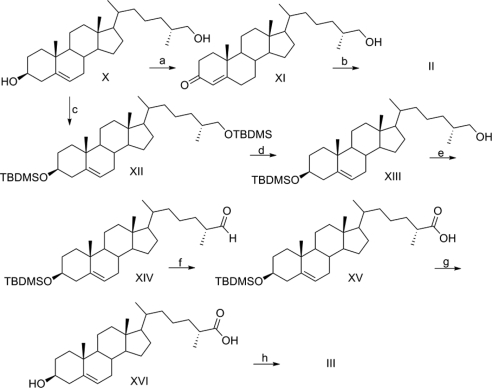
The strategy employed to access a repertoire of dafachronic acids centered on the common intermediate X, which derives from diosgenin (VI) in four steps (Scheme 1 in Fig. 2). Conversion of X to (25R)-Δ5- and Δ4-dafachronic acids (III and II) require only a few additional steps (Scheme 2 in Fig. 3). The main hurdle in preparation of (25R)-III and II is to oxidize the 3-hydroxyl without isomerization of the double bond using 2-iodoxybenzoic acid to access III or with clean isomerization to the conjugated enone in II using Oppenauer oxidation. The terminal steps in conversion of intermediate 3-keto-26-alcohols to the corresponding 3-keto-26-acids differs for the two compounds to prevent isomerization of the double bond en route to (25R)-III.
Figure 2.
Scheme 1: a, Zn(Hg), HCI, ethanol; b, tert-butyl-dimethylsilyl chloride (TBDMSCI), DBU, THF; c, Dess-Martin periodinane, CH2Cl2; d, NH2NH2 · HCI, NH2NH2 · H2O diethylene glycol.
Figure 3.
Scheme 2: a, Al(Oi-Pr)3, 1-methyl-4-piperidone, toluene; b, Jones reagent, 0 C; c, tert-butyl-dimethylsilyl chloride (TBDMSCl), DBU, THF, 24 h; d, CSA, MeOH, 0 C; e, Dess-Martin periodinane, CH2Cl2; f, NaClO2, NaH2PO4, 2-methyl-2-butene; g, TBAF, THF; h, iodoxybenzoic acid (lBX), dimethylsulfoxide/THF (1:1).
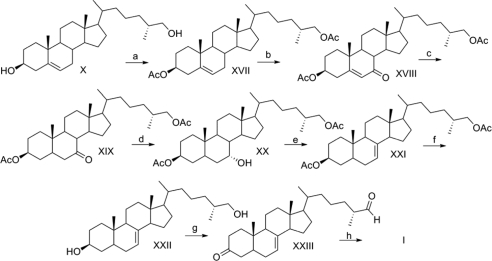
Several routes were attempted to introduce the 5α-reduced-Δ7-system found in (25R)-I. Hydroboration and oxidation of X gave the 5α-reduced-6-ketosterol, which could be functionalized to the Δ7-enone via bromination and elimination but not using phenylselenyl chloride/hydrogen peroxide. Deoxygenation of this enone, however, could not be achieved. Allylic bromination of X with 1,3-dibromo-5, 5-dimethylhydantoin, elimination to the 5,7-diene, and selective 5α-hydrogenation of the Δ5-olefin afforded the 5α-reduced Δ7-system found in (25R)-I, but the bromination gave low yields and could not be scaled to more than 15 mg V. In contrast, allylic oxidation, hydrogenation, reduction, and elimination (4) installed the 5α- reduced Δ7-system in good yield (Scheme 3 in Fig. 4).
Figure 4.
Scheme 3: a, Ac2O, pyridine, dimethylaminopyridine (DMAP); b, 3,5 dimethylpyrazole, CrO3, CH2Cl2, −15 C; c, Pd/C, H2, EtOAc; d, L-selectride, THF, −78 C; e, Burgess reagent, benezene, reflux; f, KOH, MeOH; g, PCC, NaOAc; h, NaCIO2, NaH2PO4, 2-methyl-2-butene.
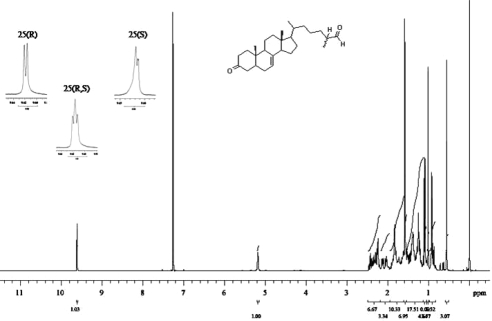
These routes to dafachronic acids retain the (25R)-stereochemistry found in diosgenin, necessitating a procedure for inverting the stereochemistry at C-25. Routes via the Δ25-olefins using chiral boranes to selectively hydroborate the terminal olefin were envisioned; however, elimination of the 26-alcohols proceeded in low yield with side products under several conditions. The 26-alcohols were then oxidized to the 26-aldehydes and isomerized using 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) to 1:1 mixtures of the (25R)- and (25S)-aldehydes by nuclear magnetic resonance (NMR) (Fig. 5), which were converted to the 26-alcohols or acids by further reduction or oxidation, respectively (Scheme 4 in Fig. 6). These mixtures of diastereomers could not be separated using silica gel chromatography or reverse-phase HPLC, and attempts to form esters with chiral alcohols or acids to improve separations gave poor yields. Instead, the diastereomeric 26-alcohols were kinetically resolved by selective acetylation of the (25S)-isomers with vinyl acetate catalyzed by Pseudomonas cepacia lipase in chloroform. The (25S)-acetates were easily separated from the unreacted (25R)-alcohols and carried forward to the (25S)-dafachronic acids (see supplemental data and schemes, especially Schemes 4a and 4b, published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org).
Figure 5.
Epimerization and resolution of dafachronic acid intermediates. The 1H-NMR spectrum of aldehydes XXIII before and after epimerization and resolution. Inset shows expansion of aldehyde (CHO) proton resonance, which is a clean doublet as predicted for a single 25(R)-isomer and becomes overlapping doublets (pseudotriplet) after isomerization. The 25(S)-isomer, obtained after enzymatic kinetic resolution of epimeric alcohols and oxidation, also shows a doublet.
Figure 6.
Scheme 4: a, DBU, THF, 24 h; b, NaBH4, methanol, 0 C; c, P. cepacia lipase, vinyl acetate, CHCl3; d, KOH, MeOH, reflux; e, PCC, NaOAc; f, NaClO2, NaH2PO4, 2-methyl-2-butene.
Finally, generation of the (25R)- and (25S)-5α-reduced dafachronic acids IV was achieved by catalytic hydrogenation of any Δ4-intermediates or II with Pd/CaCO3. The corresponding 5β-reduced dafachronic acids V were prepared from II using ammonium formate reduction catalyzed by Pd on carbon black (Scheme 5 in Fig. 7).
Figure 7.
Scheme 5: a, PD/CaCO3, isopropanol, H2; b, Jones reagent, 0 C; c, Pd/C, ammonium formate, MeOH, reflux; d, Pd/CaCO3, acetonitrile:isopropanol (2:1), H2; e, same as b; f, same as c.
Gal4-transactivation assays
In our previous study, we identified two dafachronic acids characterized by having 3-keto-Δ4- or Δ7-configurations, but authentic standards of neither Δ7-dafachronic acids nor structurally similar compounds were available. In addition, 5-cholesten-3β-ol-26-oic acid also activated DAF-12 and rescued the dauer phenotype (3,5), suggesting that DAF-12 could be activated by compounds with other A/B ring configurations. We first employed the convenient Gal4-transactivation assay to test the activation of DAF-12 by the panel of dafachronic acids I-V in HEK-293 cells to characterize the structural features required for DAF-12 transactivation.
All dafachronic acids transactivated the nuclear receptor DAF-12 but with a wide range of potencies (Table 1). As proposed previously, Δ7-dafachronic acids I are the most potent activators of DAF-12 in this assay, with EC50 values of 23 and 33 nm for the (25S)- and (25R)-isomers, respectively. The (25S)-Δ4-dafachronic acid II is equipotent to Δ7-acids I, with an EC50 of 23 nm, but the (25R)-isomer of II is significantly less potent, with an EC50 of 66 nm. In contrast, the Δ5-dafachronic acids III are significantly less potent than acids I and II, with EC50 values approaching 1000 nm. These data quantitatively confirm our proposal that the most potent endogenous activators of DAF-12 are the Δ7- and Δ4-dafachronic acids I and II, with the (25S)-isomers significantly more potent than the (25R)-isomers only for II. Although DAF-12 is activated by compounds III, metabolism of these 3-hydroxy-Δ5-sterols to more potent ligands in vivo might account for much of their biological activity.
Table 1.
Transactivation of Gal4-DAF-12-LBD by dafachronic acid isomers in HEK-293 cells
| Isomer | EC50 (nm) | Replicates | P value [25(S) vs. 25(R)] |
|---|---|---|---|
| Δ7- (I) | 0.2 | ||
| (25S) | 23 ± 11 | 3 | |
| (25R) | 33 ± 6 | 3 | |
| Δ4- (II) | 0.00001 | ||
| (25S) | 23 ± 6 | 6 | |
| (25R) | 66 ± 12 | 6 | |
| Δ5- (III) | 0.0002 | ||
| (25S) | 940 ± 75 | 5 | |
| (25R) | 524 ± 124 | 5 | |
| 5α- (IV) | 0.005 | ||
| (25S) | 474 ± 119 | 4 | |
| (25R) | 192 ± 50 | 4 | |
| 5β- (V) | 0.001 | ||
| (25S) | 329 ± 183 | 5 | |
| (25R) | 1847 ± 647 | 5 |
Data represent means ± sd.
To determine whether the superior potency of the Δ7-dafachronic acids compared with the Δ4-isomers derives from the 5α-reduced trans-ring fusion or the location of the double bond in the A/B-ring system, we prepared the saturated 5α- and 5β-reduced dafachronic acids IV and V and tested these compounds as DAF-12 activators. Both the 5α- and 5β-reduced acids showed potencies mostly intermediate to the Δ4- and Δ5-isomers in the Gal4-transactivation assay, with EC50 values of 200-1800 nm (Table 1). The 5β-dafachronic acids were the only compounds for which the (25S)-isomer was convincingly more potent than the (25R)-isomer in this assay. These data demonstrate that both the 5α-reduced trans-ring fusion and the Δ7-olefin both contribute to the potency of dafachronic acids, more than the stereochemistry at C-25.
Dauer rescue assays
Because Gal4-transactivation assays employ mammalian cells using a convenient, but artificial Gal4-DBD/DAF-12- ligand-binding domain (LBD) fusion construct, it is possible that results from these chimeric receptors may not precisely reflect the potencies of ligands with endogenous DAF-12 in C. elegans. Therefore, we performed dauer rescue assays to assess the potencies of these ligands in vivo. In this experiment, we supplemented the daf-9-null, constitutive dauer mutants with dafachronic acids and scored the percentage of worms rescued from dauer diapause. Similar to the results obtained from the Gal4-transactivation assay, the Δ7- and Δ4-dafachronic acids I and II showed significantly higher potency than the Δ5-acids III (Table 2). In this assay, however, the (25S)-isomers were substantially more potent than the (25R)-isomers, and the Δ7-dafachronic acids were convincingly more potent than the Δ4-isomers. The 5α- and 5β-reduced dafachronic acids were likewise intermediate in potency between the Δ5-isomers and the Δ7-isomers, with the (25R)-5β-reduced compound being the least potent. These data, obtained with a more physiological assay, confirm that the 5α-reduced trans-ring fusion, the Δ7-configuration in the A/B ring, and the (25S)-stereochemistry all contribute to the potency of DAF-12 ligands.
Table 2.
Rescue of daf-9 (dh6) dauer phenotype by dafachronic acid isomers
| Treatment | Dose (nm) | Rescue (%) | n |
|---|---|---|---|
| Vehicle | 0 | 287 | |
| Δ7-Dafachronic acid (I) | |||
| (25S) | 10 | 16 | 43 |
| 50 | 98 | 158 | |
| 100 | 100 | 210 | |
| 500 | 100 | 57 | |
| 1000 | 100 | 72 | |
| (25R) | 10 | 0 | 95 |
| 50 | 0 | 87 | |
| 100 | 1 | 138 | |
| 500 | 99 | 204 | |
| 1000 | 100 | 199 | |
| Δ4-Dafachronic acid (II) | |||
| (25S) | 10 | 0 | 38 |
| 50 | 0 | 46 | |
| 100 | 17 | 42 | |
| 500 | 100 | 182 | |
| 1000 | 100 | 141 | |
| (25R) | 500 | 16 | 110 |
| 1000 | 50 | 84 | |
| 10000 | 100 | 113 | |
| Δ5-Dafachronic acid (III) | |||
| (25S) | 1000 | 0 | 146 |
| 2500 | 95 | 88 | |
| 5000 | 94 | 96 | |
| 10000 | 100 | 129 | |
| (25R) | 1000 | 0 | 137 |
| 2500 | 97 | 145 | |
| 5000 | 93 | 98 | |
| 10000 | 100 | 111 | |
| 5α-Dafachronic acid (IV) | |||
| (25S) | 10 | 0 | 48 |
| 50 | 0 | 77 | |
| 100 | 0 | 89 | |
| 500 | 19 | 78 | |
| 1000 | 97 | 202 | |
| 5000 | 98 | 174 | |
| 10000 | 98 | 104 | |
| (25R) | 10 | 0 | 81 |
| 50 | 0 | 79 | |
| 100 | 1 | 113 | |
| 500 | 29 | 121 | |
| 1000 | 99 | 181 | |
| 5000 | 96 | 130 | |
| 10000 | 97 | 149 | |
| 5β-Dafachronic acid (V) | |||
| (25S) | 10 | 0 | 92 |
| 50 | 0 | 58 | |
| 100 | 0 | 82 | |
| 500 | 18 | 66 | |
| 1000 | 97 | 172 | |
| 5000 | 99 | 139 | |
| 10000 | 97 | 138 | |
| (25R) | 1000 | 0 | 76 |
| 5000 | 6 | 87 | |
| 10000 | 79 | 66 |
Discussion
Previously, we showed that in the presence of DAF-9, the 3- keto-Δ7-sterol lathosterone was more potent than either lathosterol or 4-cholesten-3-one in DAF-12 transactivation assays and in rescue assays with daf-9-null, constitutive dauer mutants. Compounds with mass spectra consistent with monounsaturated dafachronic acids were isolated from worms, and microsomes containing DAF-9 metabolized 4-cholesten-3-one primarily to the (25S)-26-alcohol and then to the 26-acid (3). These data suggested but did not prove that (25S)-Δ7-dafachronic acid was the major endogenous ligand for the C. elegans DAF-12 nuclear hormone receptor. The structure-activity studies presented here support this conclusion, at least considering the series of structurally similar compounds analyzed. We obtained equivalent results with another sample of (25S)-Δ7-dafachronic acid (kindly provided by Dr. Elias J. Corey) (4), which was prepared in a different laboratory by a different route.
In addition to the Δ7-isomers, we also show that DAF-12 accommodates a variety of compounds similar to dafachronic acid ligands, an observation that parallels the relaxed substrate specificity of the cytochrome P450 DAF-9 (3) (Cummins, C. L., D. L. Motola, and D. J. Mangelsdorf, unpublished data). This flexibility in ligand processing and binding, in turn, might enable the animal to use a variety of sterols as raw materials to signal the return of favorable conditions and to initiate exit from the dauer diapause.
The potencies of the structurally similar ligands studied herein, however, vary over two orders of magnitude. In most but not all cases, the (25S)-isomers are more potent than the (25R)-isomers, but the magnitude of this difference varies with the A/B-ring configuration. For compounds I–IV, the ring fusion forces the molecule into the flat geometry of a twist-boat conformation or a trans-decalin. For compound V, the 5β-reduction forces the A-ring nearly perpendicular to the B-ring as found in bile acids, yet DAF-12 is still activated despite this large structural change. Nevertheless, III and V are the least potent compounds in this study, particularly the (25R)-isomers. Although DAF-12 tolerates significant structural variation in the A/B-ring geometry, the 5α-reduced stereochemistry and trans-decalin ring system, particularly the (25S)-isomer with the Δ7-olefin, optimizes potency.
The qualitative agreement between the Gal4-transactivation and dauer rescue assays is reassuring, and the quantitative differences do not detract from our conclusions. Reasons for these discrepancies might include differences in permeability and metabolism of these ligands in C. elegans compared with HEK-293 cells. In addition, only the DAF-12 LBD is used in the Gal-4 transactivation assay, precluding activation via the amino-terminal (AF-1) domain in these experiments. Finally, the endogenous coactivators and transcriptional machinery in C. elegans and HEK-293 cells are different, so we would not anticipate exactly the same results in both assay systems.
Homologs of DAF-12 appear to be present in several nematode species, including hookworms and roundworms that infect human beings, livestock, and crops. Access to a repertoire of dafachronic acids, therefore, might translate to important medicinal and agricultural uses, particularly in developing countries. Our versatile synthetic scheme allows for production of many different dafachronic acids from inexpensive starting materials using methods that are suitable for expansion to large-scale production; we have prepared (25R/S)-I on a gram scale. Our data also indicate that, at least for the most potent Δ4- and Δ7-isomers, the more accessible (25R)-epimers are sufficiently potent to be used experimentally and pharmacologically. Additional synthetic approaches to specific dafachronic acids are emerging (4,6,7,8), indicating interest in these compounds and suggesting that our methods could be optimized further. Consequently, this signaling pathway and the physiology elucidated in the study of a model organism may have important implications in agriculture and medicine.
Materials and Methods
General methods and reagents
The firefly luciferase substrate luciferin is from Molecular Probes (Eugene, OR); the β-galactosidase substrate O-nitrophenyl-β-d-galactopyranoside is from Diagnostic Chemicals Ltd. (Oxford, CT). Diosgenin was obtained from Steraloids (Newport, RI), and most chemicals and other reagents were obtained from Sigma-Aldrich (St. Louis, MO) and Pierce (Rockford, IL).
Gal4 transactivation assay
Gal4 transactivation of DAF-12 was performed as described (3). Briefly, HEK-293 cells were transfected in 96-well plates with plasmids as follows: 50 ng MH100x4-tk-luc reporter, 10 ng CMX-β-galactosidase to control for transfection efficiency, and 15 ng CMX-Gal4-DAF-12LBD, which expresses a fusion protein of the Gal4 DNA-binding domain with the LBD of the nuclear hormone receptor DAF-12 (referred to as Gal4-DAF-12-LBD). The ligands were added 6 h after calcium phosphate transfection, and luciferase and β-galactosidase activities were measured 16–24 h after the addition of dafachronic acids or vehicle control.
Dauer rescue assay
Dauer rescue assay was performed as described (3). Vehicle control or dafachronic acid stock solutions were diluted with 0.1 ml 5×OP50 bacteria from an overnight culture to achieve the indicated concentration of ligands, and the mixtures were loaded on agar plates prepared with 4 ml nematode growth medium. After the bacteria pads were formed, about 15 daf-9 (dh6, dhEx24) gravid adults were introduced to the plates and allowed to lay eggs for 3–6 h, after which the adults were removed. The plates were stored at 23 C for 48 h, and the green fluorescent protein-positive progeny were identified under fluorescence dissection microscope. Rescue of daf-9 (dh6) dauer phenotype was scored after 12–24 h and represented as the percentage of gravid adults from all progeny.
Synthesis of dafachronic acids from diosgenin
Scheme 1
(25R)-Cholest-5-ene-3β,16β,26-triol (VII).
To a refluxing solution of diosgenin (VI) (4.0 g, 9.6 mmol) and zinc amalgam (120 g, freshly prepared from 2 g HgCl2 and 120 g mossy zinc (Aldrich) in 300 ml water with 400 ml 95% ethanol) was added concentrated HCl (120 ml) over 4 h at room temperature (RT), and heating was then continued for 30 min. The reaction mixture was decanted from zinc, cooled to RT, diluted with water, and thoroughly extracted with CHCl3. The combined extracts were washed with water, dried over Na2SO4, and evaporated to white solid. The solid was subjected to medium-pressure liquid chromatography (MPLC) on silica gel with 3:2 ethyl acetate-hexanes. Evaporation of solvent gave VII (9) (2.94 g; 74%).
(25R)-3β,26-Bis(tert-butyldimethylsilyloxy)cholest-5-en-16β-ol (VIII).
To a solution of VII (2.92 g, 7.0 mmol) in 40 ml tetrahydrofuran (THF) was added tert-butyl dimethylsilylchloride (4.2 g, 27.9 mmol) under nitrogen. The mixture was stirred for 15 min at RT, and DBU (4.03 g, 26.5 mmol) was slowly added, and the resulting mixture was stirred at RT for 16 h. The reaction mixture was diluted with 100 ml water and extracted with ethyl acetate. The combined extracts were washed with water, dried over Na2SO4, and evaporated to dryness. The residue was subjected to MPLC on silica gel with 5% ethyl acetate in hexanes. Evaporation of solvent afforded VIII (10) (4.2 g, 95%).
(25R)-3β,26-Bis(tert-butyldimethylsilyoxy)cholest-5-en-16-one (IX).
A solution of VIII (3.31 g, 5.1 mmol) in methylene chloride (50 ml) was treated with Dess Martin periodinane (2.38 g, 5.6 mmol). The reaction was stirred at RT for 6 h, quenched with saturated Na2S2O3 solution, diluted with water, and extracted with ethyl acetate. The organic layers were dried over Na2SO4, filtered, and evaporated to dryness. The crude product was subjected to MPLC on silica gel with 4% ethyl acetate in hexanes; evaporation of solvent gave IX (11) (2.91 g, 88%).
(25R)-Cholest-5-ene-3β,26-diol (X).
A mixture of IX (2.71 g, 4.2 mmol), hydrazine hydrochloride (3.25 g), and 85% hydrazine hydrate (16.7 g) in diethylene glycol (50 ml) was heated at 135 C for 90 min. After the addition of KOH (8.2 g), the resulting mixture was heated at 220 C for 3.5 h with removal of water by distillation (9). After cooling to room temperature, the mixture was diluted with water and extracted with CHCl3. The extracts were washed with water, dried over Na2SO4, and evaporated to dryness. The crude product was subjected to MPLC on silica gel with 40% ethyl acetate in hexanes; evaporation of solvent gave X (1.21 g, 71%).
Scheme 2
(25R)-Cholest-4-ene-3-one-26-ol (XI).
A solution of X (0.84 g, 2.0 mmol) in 55 ml toluene and 2 ml 1-methyl-4-piperidone was refluxed under a Dean-Stark trap until 3 ml distillate had collected. After cooling to RT, aluminum isopropoxide (0.92 g, 2.3 mmol) was added. The mixture was refluxed for 8 h, cooled, diluted with 100 ml diethyl ether, washed twice with 50 ml each time with 1 m HCl and twice with 75 ml saturated NaCl each time, and dried over Na2SO4. The crude product was purified by MPLC on silica gel with ethyl acetate in hexanes. Evaporation of solvent afforded XI (12) (0.72 g, 86%).
(25R)-Cholest-4-ene-3-one-26-oic acid (Δ4-dafachronic acid, II).
Jones reagent (0.4 ml, 0.50 mmol) was added slowly and dropwise to a stirred solution of XI (40.7 mg, 0.10 mmol) in acetone (8 ml) at 0 C. After stirring for 1 h, the reaction was quenched by addition of excess 2-propanol, and the product was extracted with diethyl ether. The organic phase was washed with 10% NaHCO3, dried over Na2SO4, filtered and concentrated. The crude material was purified by MPLC on silica gel with 40% ethyl acetate in hexanes, and evaporation of solvent gave II (3) (38 mg, 90%).
(25R)-3β,26-Bis(tert-butyldimethylsilyloxy)cholest-5-ene (XII).
Similar to the procedure for VIII, X (0.652 g, 1.6 mmol) was diprotected with tert-butyl dimethylsilylchloride (0.94 g, 6.40 mmol) and DBU (0.90 g, 6.07 mmol) in 20 ml THF at RT for 16 h to afford XII (900 mg, 88%).
(25R)-Cholest-5-ene-3β-tert-butyldimethylsilyloxy-26-ol (XIII).
To the solution of XII (1.1 g, 1.7 mmol) in 10 ml each methanol and dichloromethane was added portionwise camphorsulphonic acid (0.236 g, 1.0 mmol). After 0.5 h, the reaction was quenched with saturated NaHCO3 and extracted twice with 5 ml ethyl acetate, dried over Na2SO4, concentrated, and purified by MPLC on silica column with 8% ethyl acetate in hexanes. Evaporation of the solvent afforded XIII (0.304 g, 82%).
(25R)-Cholest-5-ene-3β-tert-butyldimethylsilyloxy-26-al (XIV).
A stirred solution of XIII (0.114 g, 0.22 mmol) in 20 ml dichloromethane was treated with Dess Martin periodinane (0.25 g, 0.46 mmol) at RT for 4 h. The reaction was quenched with saturated Na2S2O3 solution, diluted with water, and extracted with ethyl acetate. The organic layers were dried over Na2SO4, and the crude product was subjected to MPLC on silica gel with ethyl acetate in hexanes. Evaporation of solvent afforded XIV (0.102 g, 90%).
(25R)-Cholest-5-ene-3β-tert-butyldimethylsilyloxy-26-oic acid (XV).
A solution of XIV (18 mg, 0.035 mmol) in 4 ml methanol was treated with 2-methyl 2-butene (40 μl, 0.35 mmol) and stirred for 5 min at RT. A freshly prepared solution of 1.25 m NaClO2 in 20% NaH2PO4 (6.4 μl, 0.070 mmol) was added, and the reaction was stirred at RT for 1 h. After completion of reaction, water (4 ml) was added, and the product was extracted twice into 20 ml ethyl acetate, which was dried over Na2SO4. Purification by MPLC silica gel with 12% ethyl acetate in hexanes and removal of solvent afforded XV (16 mg, 86%).
(25R)-Cholest-5-ene-3β-ol-26-oic acid (XVI).
A solution of XV (16 mg, 0.030 mmol) in 3 ml THF was treated with 1 ml HF (48% in H2O) at 0 C, slowly brought to RT, and stirred for 4 h. After completion, the reaction mixture was extracted with ether (2 × 20 ml), dried over Na2SO4, concentrated, and purified by MPLC on silica gel with 30% ethyl acetate in hexanes. Evaporation of solvent afforded XV (10 mg, 80%).
(25R)-Cholest-5-ene-3-one-26-oic acid (Δ5-dafachronic acid, III).
A solution of XVI (10 mg, 0.024 mmol) in 2 ml 1:1 dimethylsulfoxide/THF was stirred at RT with iodoxybenzoic acid (53 mg, 0.192 mmol) for 8 h. The reaction was quenched with saturated NH4Cl solution, diluted with water, extracted into ethyl acetate, dried over Na2SO4, and purified by MPLC on silica gel with 20% ethyl acetate in hexanes. Evaporation of solvent afforded III (8 mg, 80%).
Scheme 3
(25R)-3β,26-Bisacetoxy-cholest-5-ene (XVII).
To a solution of X (1.31 g, 3.25 mmol) in dry pyridine (15 ml) was added acetic anhydride (3 ml) and N,N-dimethylaminopyridine (4 mg, 0.027 mmol). The mixture was stirred at RT under nitrogen for 18 h. Water (5 ml) was added, and the product was extracted with ethyl acetate. The organic layer was washed with NaHCO3 and water and then dried over Na2SO4 and purified by MPLC on silica gel with 6% ethyl acetate in hexanes. Evaporation of solvent gave XVII (13,14) (1.21 g, 76%).
(25R)-3β,26-Bisacetoxy-cholest-5-ene-7-one (XVIII).
To a solution of XVII (0.405 g, 0.89 mmol) in dichloromethane (50 ml) was added 3,5-dimethyl pyrazole (1.46 g, 15.3 mmol) and chromium trioxide (1.52 g, 15.3 mmol) at −20 C. The reaction mixture was slowly brought to RT, stirred overnight, filtered through a small plug of silica gel, and extracted into ethyl acetate (200 ml), which was washed with water (3 × 50 ml) and dried over Na2SO4. The crude product (0.82 g) was subjected to MPLC on silica gel with ethyl acetate in hexanes, and evaporation of solvent gave XVIII (0.41 g, 90%).
(25R)-3β,26-Bisacetoxy-cholest-7-one (XIX).
To a solution of XVIII (0.40 g, 0.86 mmol) in ethyl acetate (50 ml) was added Pd/C (20 mg, 5% wt/wt). A hydrogen-filled balloon (1 atm) was connected over the solution, which was stirred overnight. The reaction mixture was filtered through a pad of silica gel, which was washed with ethyl acetate, and the combined solutions were concentrated and subjected to MPLC on silica gel with 20% ethyl acetate in hexanes. Evaporation of the solvent afforded XIX (0.38 g, 95%).
(25R)-3β,26-Bisacetoxy-cholest-7α-ol (XX).
To a stirred solution of XIX (0.33 g, 0.67 mmol) in THF (15 ml) was added L-Selectride (Aldrich; 1 m, 0.82 ml, 0.82 mmol) at −78 C. The resulting solution was stirred at −78 C for 3 h and quenched with 3.5 ml saturated NaHCO3 and 10 ml 35% aqueous H2O2. The solution was stirred at RT for 1 h, and the aqueous phase was extracted with ethyl acetate (3 × 15 ml). The combined organic phases were dried over Na2SO4 and evaporated to dryness. The residue was purified by MPLC on silica gel with 20% ethyl acetate in hexanes; evaporation of the solvent gave XX (0.26 g, 78%), a single diastereomer by NMR.
(25R)-3β,26-Bisacetoxy-cholest-7-ene (XXI).
To a stirred solution of XX (0.18 g, 0.38 mmol) in benzene (5 ml) was added Burgess reagent (0.18 g, 0.76 mmol) at RT. The solution was refluxed for 3 h, quenched with water, extracted with ethyl acetate (20 × 3 ml), dried over Na2SO4, and subjected to MPLC on silica gel with 10% ethyl acetate in hexanes. Evaporation of the solvent gave XXI (0.15 g, 86%).
(25R)-Cholest-7-ene-3β,26-diol (XXII).
To a stirred solution of XXI (0.15 g, 0.33 mmol) in 5 ml methanol was added KOH (100 mg), and the solution was refluxed for 4 h. The reaction mixture was neutralized with 1 N HCl, and methanol was removed under reduced pressure. The residue was and extracted with ethyl acetate, which was dried with Na2SO4 and purified by MPLC with ethyl acetate in hexanes. Evaporation of the solvent afforded XXII (0.12 g, 90%).
(25R)-Cholest-7-ene-3-one-26-al (XXIII).
A stirred solution of XXII (0.15 g, 0.37 mmol) in 20 ml dichloromethane was treated with Dess Martin periodinane (0.33 g, 0.78 mmol) at RT for 4 h. The reaction was quenched with saturated Na2S2O3 solution, diluted with water, and extracted with ethyl acetate. The organic layers were dried over Na2SO4, and the crude product was subjected to MPLC on silica gel with ethyl acetate in hexanes. Evaporation of solvent afforded XXIII (0.12 g, 81%).
(25R)-Cholest-7-ene-3-one-26-oic acid (Δ7-dafachronic acid, I).
A solution of XXIII (51 mg, 0.126 mmol) in 4 ml methanol was treated with 2-methyl 2-butene (90 μl, 1.27 mmol) and stirred for 5 min at RT. A freshly prepared solution of 1.25 m NaClO2 in 20% NaH2PO4 (24 μl, 0.026 mmol) was added, and the reaction was stirred at RT for 1 h. After completion of reaction, water (10 ml) was added, and the product was extracted twice into 30 ml ethyl acetate, which was dried over Na2SO4. Purification by MPLC silica gel with 24% ethyl acetate in hexanes afforded I (42 mg, 80%).
Scheme 4: Δ7 series
(25R,S)-Cholest-7-ene-3-one-26-al (R/S-XXIII).
A solution of XXIII (0.14 g, 0.35 mmol) in 5 ml THF with 1 ml DBU was stirred at RT for 48 h. Water was added, and the products were extracted with ethyl acetate, which was dried over Na2SO4. The crude product was purified by MPLC on silica gel with ethyl acetate in hexanes, and evaporation of solvent afforded R/S-XXIII (0.13 g, 93%).
(25R,S)-Cholest-7-ene-3,26-diol (R/S-XXII).
To a stirred solution of R/S-XXIII (0.13 g, 0.33 mmol) in 6 ml methanol at 0 C was added NaBH4 (0.026 g, 0.67 mmol), and the reaction was brought slowly to RT. After 1 h, water was added, and the product was extracted with ethyl acetate. The organic layer was dried over Na2SO4 and purified by MPLC on silica gel with ethyl acetate in hexanes; evaporation of solvent afforded R/S-XXII (0.12 g, 92%). The stereochemistry of the 3- hydroxyl, which was lost in subsequent steps, was not determined.
(25S)-Cholest-7-ene-26-acetoxy-3-ol (XXIV).
A solution of R/S-XXII (0.12 g, 0.30 mmol) in 8 ml CHCl3 was stirred at RT for 5 min and treated with Pseudomonas cepacia lipase (10 mg) and then 5 min later with vinyl acetate (0.5 ml) (15). The reaction mixture was stirred at RT until thin-layer chromatography indicated that approximately 50% of starting material was converted to product (48 h). The reaction mixture was filtered and purified by MPLC on silica gel with ethyl acetate in hexanes; evaporation of solvent afforded XXIV (0.055 g, 42% based on starting material, 84% of theoretical).
(25S)-Cholest-7-ene-3,26-diol (S-XXII).
A methanolic solution of XXIV (0.052 g, 0.117 mmol) was refluxed with KOH under conditions described for XXII, yielding S-XXII (0.045 g, 92%).
(25S)-Cholest-7-ene-3-one-26-al (S-XXIII).
The S-XXII (0.045 g 0.11 mmol) in dichloromethane was oxidized to S-XXIII with Dess Martin periodinane according to the procedure for XXIII (0.035 g, 92%).
(25S)-Cholest-7-ene-3-one-26-oic acid (Δ7-dafachronic acid, S-I).
A solution of S-XXIII (32 mg, 0.08 mmol) in 5 ml methanol was treated with 2-methyl 2-butene (56 μl, 0.8 mmol) and stirred for 5 min at RT. A freshly prepared solution of 1.25 m NaClO2 in 20% NaH2PO4 (14.4 μl, 0.16 mmol) was added, and the reaction was stirred at RT for 1 h. Water (10 ml) was added, and the product was extracted twice into 30 ml ethyl acetate, which was dried over Na2SO4. Purification by MPLC silica gel with 24% ethyl acetate in hexanes afforded S-I (4) (0.030 mg, 90%).
Scheme 4: Δ4 series
(25R)-Cholest-4-ene-3-one-26-al (XXV).
Compound XI (0.10 g, 0.024 mmol) was oxidized with Dess Martin periodinane as described for other alcohols above (0.082 g, 82%).
(25R,S)-Cholest-4-ene-3-one-26-al (R/S-XXV).
The aldehyde XXV (0.082 g 0.20mmol) was epimerized with DBU as described for XXIII to give R/S-XXV (0.064 g, 78%).
(25R,S)-Cholest-4-ene-3,26-diol (XXVI).
Aldehydes R/S-XXV (0.064 g 0.164 mmol) were reduced with NaBH4 in methanol similar to the preparation of R/S-XXII (0.052 g, 80%). The stereochemistry of the 3-hydroxyl, which was lost in subsequent steps, was not determined.
(25S)-Cholest-4-ene-26-acetoxy-3-ol (XXVII).
Stereospecific acetylation of XXVI (0.064 g, 0.158 mmol) with lipase was performed as with R/S-XXII to afford XXVII (0.052 g, 80%).
(25S)-Cholest-4-ene-3,26-diol (S-XXVI).
Saponification of XXVII (0.060 g, 0.135 mmol) with methanolic KOH was performed as with S-XXII to afford S-XXVI (0.48 g, 87%).
(25S)-Cholest-4-ene-3-one-26-oic acid (Δ4-dafachronic acid, S-II).
Jones reagent (0.44 ml, 0.55 mmol) was added slowly and dropwise to a stirred solution of S-XXVI (40.7 mg, 0.10 mmol) in acetone (8 ml) at 0 C. After stirring for 1 h, the reaction was quenched by addition of excess 2-propanol, and the product was extracted with diethyl ether. The organic phase was washed with 10% NaHCO3, dried over Na2SO4, filtered, and concentrated. The crude material was purified by MPLC on silica gel with 40% ethyl acetate in hexanes, and evaporation of solvent gave II (8) (42 mg, 90%).
Scheme 4: Δ5 series
(25RS)-Cholest-5-ene-3β-tert-butyldimethylsilyloxy-26-al (R/S-XIV).
The aldehyde XIV (0.102 g, 0.19 mmol) was epimerized with DBU as described for XXIII to give R/S-XIV (0.096 g, 94%).
(25RS)-Cholest-5-ene-3β-tert-butyldimethylsilyloxy-26-ol (R/S-XIII).
To a stirred solution of R/S-XIV (0.094 g, 0.18 mmol) in 3 ml methanol at 0 C was added NaBH4 (0.008 g, 0.22 mmol), and the reaction was brought slowly to RT. After 1 h, water was added, and the product was extracted with ethyl acetate. The organic layer dried over Na2SO4 and purified by MPLC on silica gel with ethyl acetate in hexanes. Evaporation of solvent afforded R/S-XIII (84 mg, 82% based on starting material recovery). The stereochemistry of the 3-hydroxyl, which was lost in subsequent steps, was not determined.
(25S)-Cholesta-3β-tert-butyldimethylsilyloxy-26-acetoxy-5-ene (XXVIII).
Enzymatic acetylation of R/S-XIII (82 mg, 0.16 mmol) using the procedure for R/S-XXII afforded XXVIII (35 mg, 40% based on starting material, 80% of theoretical).
(25S)-Cholesta-5-ene-3β-tert-butyldimethylsilyloxy-26-ol (S-XIII).
Saponification of S-XIII (0.018 g, 0.032 mmol) with methanolic KOH according to the procedure for XXIV afforded S-XIII (0.015 g, 90%).
(25S)-Cholest-5-ene-3β-tert-butyldimethylsilyloxy-26-oic acid (S-XV).
Two-step oxidation of S-XIII (12 mg, 0.023 mmol) as described for conversion of XIII to XV afforded S-XV (9 mg, 73%).
(25S)-Cholest-5-ene-3β-ol-26-oic acid (S-XVI).
A solution of S-XVI (8 mg, 0.015 mmol) in 4 ml THF was stirred with tetrabutyl ammonium fluoride (1 m, 0.1 ml) at RT for 8 h. The reaction was quenched with saturated NH4Cl solution, diluted with water, extracted into ethyl acetate, dried over Na2SO4, and purified by MPLC on silica gel with ethyl acetate in hexanes to afford S-XVI (6 mg, 95%).
(25S)-Cholest-5-ene-3-keto-26-acid (S-III).
A solution of S-XVI (4 mg, 0.009 mmol) in 4 ml 1:1 dimethylsulfoxide/THF was stirred at RT with iodoxybenzoic acid (201 mg, 0.072 mmol) for 8 h. The reaction was quenched with saturated NH4Cl solution, diluted with water, extracted into ethyl acetate, dried over Na2SO4, and purified by MPLC on silica gel with ethyl acetate in hexanes to afford S-III (3.6 mg, 90%).
Scheme 5
(25R)-5α-Cholest-26-ol-3-one (XXIX).
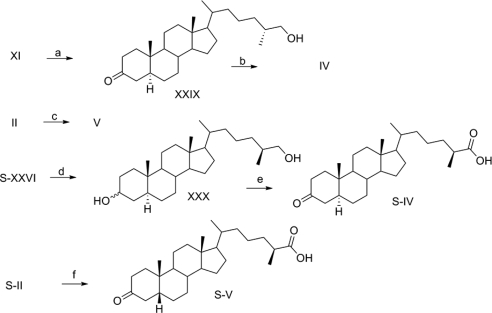
To a solution of XI (16 mg, 0.040 mmol) in 3 ml isopropanol was added Pd/CaCO3 (4 mg, 25% wt/wt). A hydrogen-filled balloon (1 atm) was connected over the solution, which was stirred overnight. The reaction mixture was filtered through a pad of silica gel, which was washed with ethyl acetate, and the combined solutions were concentrated and subjected to MPLC on silica gel with 16% ethyl acetate in hexanes. Evaporation of solvent afforded XXIX (14 mg, 87%).
(25R)-5α-Cholest-3-one-26-oic acid (IV).
Jones reagent (0.12 ml, 0.15 mmol) was added slowly and dropwise to a stirred solution of XXIX (12 mg, 0.029 mmol) in 4 ml acetone at 0 C. After stirring for 1 h, the reaction was quenched by addition of excess 2-propanol, and the product was extracted with diethyl ether. The organic phase was washed with 10% NaHCO3, dried over Na2SO4, filtered, and concentrated. The crude material was purified by MPLC on silica gel with 36% ethyl acetate in hexanes. Evaporation of solvent afforded IV (42 mg, 90%).
(25R)-5β-Cholest-3-one-26-oic acid (V).
A solution of II (5 mg, 0.012 mmol) in 5 ml methanol was treated with Pd/C (2 mg, 40% wt/wt) and ammonium formate (12 mg, 0.24 mmol), and the solution was refluxed for 6 h (16). The reaction mixture was filtered through a pad of silica gel, which was washed with ethyl acetate, and the combined solutions were concentrated and subjected to MPLC on silica gel with 20% ethyl acetate in hexanes. Evaporation of solvent afforded V (4 mg, 80%).
(25S)-5α-Cholest-3,26-diol (S-XXX).
A solution of S-XXVI (12 mg, 0.029 mmol) in 2 ml acetonitrile and 1 ml isopropanol with Pd/CaCO3 (2 mg, 16% wt/wt) was stirred overnight under 1 atm of hydrogen, which was maintained by a hydrogen-filled balloon. The reaction mixture was filtered through a pad of silica gel, which was washed with ethyl acetate, and the combined solutions were concentrated and subjected to MPLC on silica gel with 20% ethyl acetate in hexanes. Evaporation of solvent afforded S-XXX (10 mg, 82%).
(25S)-5α-Cholest-3-one-26-oic acid (S-IV).
Jones reagent (0.028 ml, 0.035 mmol) was added slowly and dropwise to a stirred solution of S-XXX (3 mg, 0.007 mmol) in 2 ml acetone at 0 C. After stirring for 1 h, the reaction was quenched by addition of excess 2-propanol, and the product was extracted with diethyl ether. The organic phase was washed with 10% NaHCO3, dried over Na2SO4, filtered, and concentrated. The crude material was purified by MPLC on silica gel with 32% ethyl acetate in hexanes. Evaporation of solvent afforded S-IV (8) (2 mg, 65%).
(25S)-5β-Cholest-3-one-26-oic acid (S-V).
To a solution of S-II (5 mg, 0.012 mmol) in 5 ml methanol was treated with Pd/C (2 mg, 40% wt/wt) and ammonium formate (15 mg, 0.29 mmol), and the solution was refluxed for 6 h (16). The reaction mixture was filtered through a pad of silica gel, which was washed with ethyl acetate, and the combined solutions were concentrated and subjected to MPLC on silica gel with 20% ethyl acetate in hexanes. Evaporation of solvent afforded S-V (4 mg, 80%).
NMR and optical rotation data, as well as schemes, are found in the supplemental data file.
Supplementary Material
Acknowledgments
We thank Drs. Leon Avery and Scott Cameron for allowing us to use their GFP dissection scope and Dr. Stefan Andersson for helpful discussions.
Footnotes
This work was supported by the Howard Hughes Medical Institute (D.J.M.) and Grants I-1493 (to R.J.A.) and I-1275 (to D.J.M.) from the Robert A. Welch Foundation.
Disclosure Summary: The authors have nothing to disclose.
First Published Online February 5, 2009
Abbreviations: DBU, 1,8-Diazabicyclo[5.4.0]undec-7-ene; LBD, ligand-binding domain; MPLC, medium-pressure liquid chromatography; NMR, nuclear magnetic resonance; RT, room temperature; THF, tetrahydrofuran.
References
- Riddle DL, Albert PS 1997 Genetic and environmental regulation of dauer larva development. In: Riddle DL, Blumenthal TJ, eds. C. elegans II. New York: Cold Spring Harbor Press [PubMed] [Google Scholar]
- Crowder CM, Westover EJ, Kumar AS, Ostlund Jr RE, Covey DF 2001 Enantiospecificity of cholesterol function in vivo. J Biol Chem 276:44369–44372 [DOI] [PubMed] [Google Scholar]
- Motola DL, Cummins CL, Rottiers V, Sharma KK, Li T, Li Y, Suino-Powell K, Xu HE, Auchus RJ, Antebi A, Mangelsdorf DJ 2006 Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell 124:1209–1223 [DOI] [PubMed] [Google Scholar]
- Giroux S, Corey EJ 2007 Stereocontrolled synthesis of dafachronic acid A, the ligand for the DAF-12 nuclear receptor of Caenorhabditis elegans. J Am Chem Soc 129:9866–9867 [DOI] [PubMed] [Google Scholar]
- Broué F, Liere P, Kenyon C, Baulieu EE 2007 A steroid hormone that extends the lifespan of Caenorhabditis elegans. Aging Cell 6:87–94 [DOI] [PubMed] [Google Scholar]
- Giroux S, Bethke A, Fielenbach N, Antebi A, Corey EJ 2008 Syntheses and biological evaluation of B-ring-modified analogues of dafachronic acid A. Org Lett 10:3643–3645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroux S, Corey EJ 2008 An efficient, stereocontrolled synthesis of the 25-(R)-diastereomer of dafachronic acid A from β-ergosterol. Org Lett 10:801–802 [DOI] [PubMed] [Google Scholar]
- Martin R, Däbritz F, Entchev EV, Kurzchalia TV, Knölker HJ 2008 Stereoselective synthesis of the hormonally active (25S)-Δ7-dafachronic acid, (25S)-Δ4-dafachronic acid, (25S)-dafachronic acid, and (25S)-cholestenoic acid. Org Biomol Chem 6:4293–4295 [DOI] [PubMed] [Google Scholar]
- Kim HS, Wilson WK, Needleman DH, Pinkerton FD, Wilson DK, Quiocho FA, Schroepfer Jr GJ 1989 Inhibitors of sterol synthesis. Chemical synthesis, structure, and biological activities of (25R)-3β,26-dihydroxy-5α-cholest-8(14)-en-15-one, a metabolite of 3β-hydroxy-5α-cholest-8(14)-en-15-one. J Lipid Res 30:247–261 [PubMed] [Google Scholar]
- Williams JR, Chai D, Bloxton II JD, Gong H, Solvibile WR 2003 Synthesis of the aglycone of 26-O-deacetyl pavoninin-5. Tetrahedron 59:3183–3188 [Google Scholar]
- Williams JR, Chai D, Gong H, Zhao W, Wright D 2002 Studies toward the synthesis of the shark repellent pavoninin-5. Lipids 39:1193–1195 [DOI] [PubMed] [Google Scholar]
- Raggio ML, Watt DS 1976 A synthesis of progesterone from dehydroepiandrosterone. J Org Chem 41:1873–1875 [DOI] [PubMed] [Google Scholar]
- Guo LW, Wilson WK, Pang J, Shackleton CH 2003 Chemical synthesis of 7- and 8-dehydro derivatives of pregnane-3,17α,20-triols, potential steroid metabolites in Smith-Lemli-Opitz syndrome. Steroids 68:31–42 [DOI] [PubMed] [Google Scholar]
- Siddiqui AU, Wilson WK, Swaminathan S, Schroepfer Jr GJ 1992 Efficient preparation of steroidal 5,7-dienes of high purity. Chem Phys Lipids 63:115–129 [DOI] [PubMed] [Google Scholar]
- Ferraboschi P, Rezaelahi S, Verza E, Santaniello E 1998 Lipase-catalyzed resolution of stereogenic centers in steroid side chains by transesterification in organic solvents: the case of a 26-hydroxycholesterol. Tetrahedron Asymmetry 9:2193–2196 [Google Scholar]
- Paryzek Z, Koenig H, Tabaczka B 2003 Ammonium formate/palladium on carbon: a versatile system for catalytic hydrogen transfer reductions of carbon-carbon double bonds. Synthesis 13:2023–2026 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.