Abstract
Plasma epinephrine and heart rate are elevated in metabolic syndrome, suggesting enhanced catecholamine secretion from the adrenal medulla. Canonical transient receptor potential (TRPC) channels are implicated in mediating hormone-induced Ca2+ influx and catecholamine secretion in adrenomedullary chromaffin cells. We studied the pattern of TRPC expression in the pig adrenal medulla and investigated whether adrenal TRPC expression is altered in prediabetic metabolic syndrome Ossabaw miniature pigs. We used a combination of molecular biological, biochemical, and fluorescence imaging techniques. We determined the sequence of pig TRPC1 and TRPC3-7 channels. We found that the pig adrenal medulla expressed predominantly TRPC1, TRPC5, and TRPC6 transcripts. The expression level of these TRPCs was significantly elevated in the adrenal medulla from pigs with metabolic syndrome. Interestingly, aldosterone, which is endogenously secreted in the adjacent adrenal cortex, increased TRPC1, TRPC5, and TRPC6 expression in adrenal chromaffin cells isolated from metabolic syndrome but not control pigs. Spironolactone, a blocker of mineralocorticoid receptors, inhibited the aldosterone effect. Dexamethasone also increased TRPC5 expression in metabolic syndrome chromaffin cells. The amplitude of hormone-induced divalent cation influx correlated with the level of TRPC expression in adrenal chromaffin cells. Orai1/Stim1 protein expression was not significantly altered in the metabolic syndrome adrenal medulla when compared with the control. We propose that in metabolic syndrome, abnormally elevated adrenal TRPC expression may underlie increased plasma epinephrine and heart rate. The excess of plasma catecholamines and increased heart rate are risk factors for cardiovascular disease. Thus, TRPCs are potential therapeutic targets in the fight against cardiovascular disease.
TRPC channels’ expression is increased in the adrenal medulla of metabolic syndrome pigs. The involvement of elevated plasma aldosterone, a metabolic syndrome signature, is proposed.
The modern diet with an excess of trans-fatty acids and cholesterol leads to increased incidences of obesity. Severely obese patients often present with high blood pressure, insulin resistance, and glucose intolerance as well as elevated triglycerides and low-density lipoprotein cholesterol. Such a combination of clinical conditions has been termed metabolic syndrome or prediabetes. In the United States, the number of diagnosed metabolic syndrome cases has increased rapidly over the last decade (1,2). The metabolic syndrome is a risk factor for cardiovascular disease (1,3,4).
Elevated plasma epinephrine and resting heart rate are observed in metabolic syndrome patients (5,6,7,8). Similar findings are reported in animal models of metabolic syndrome (9,10). Plasma epinephrine originates predominantly from the adrenal medulla; therefore, elevated plasma epinephrine suggests enhanced catecholamine exocytosis from adrenal chromaffin cells. It is well established that plasma catecholamines are involved in controlling vascular tone, cardiac output, and heart rate. Excessive release of epinephrine from the adrenal medulla is known to increase the risk of myocardial infarction, cerebrovascular accident, arrhythmias, stroke, and sudden death. Indeed, β-adrenoreceptor antagonists have been used for hypertension, angina, and myocardial infarction for a century and are one of the most effective current therapies (11,12,13).
The release of catecholamines from the adrenal medulla is a Ca2+-dependent process (14,15). Voltage-gated Ca2+ channels are the major Ca2+ influx pathway in adrenal chromaffin cells. Acetylcholine released from the preganglionic sympathetic nerve endings activates the nicotinic acetylcholine receptor channel in the plasma membrane of chromaffin cells. This depolarizes chromaffin cells and subsequently activates voltage-gated Na+ and, in turn, voltage-gated Ca2+ channels. It is well documented, however, that circulating hormones, such as histamine and angiotensin II, can also stimulate catecholamine exocytosis from adrenal chromaffin cells by activating voltage-independent circulating hormone-operated cation channels (16,17,18). Circulating hormones stimulate Gq/11-protein-coupled receptors that are present on adrenal chromaffin cells. This leads to the dissociation of heterotrimeric Gq/11-proteins. The Gαq/11- and Gβγ-subunits activate phospholipase Cβ (PLC), which cleaves a membrane lipid, phosphatidylinositol-4,5-bisphosphate, to produce inositol 1,4,5-trisphosphate (IP3) and diacylglycerol. IP3 stimulates Ca2+ release from the intracellular Ca2+ stores, whereas diacylglycerol activates protein kinase C. PLC activation may also result in Ca2+ influx into chromaffin cells, although the mechanism is not fully understood. Ca2+ influx is crucial for initiating the fusion of catecholamine containing dense-core vesicles with the plasma membrane of chromaffin cells and the release of catecholamines into the bloodstream.
Canonical transient receptor potential (TRPC) proteins form receptor- and/or store-operated, PLC-dependent Ca2+-permeable channels in various cellular systems. The TRPC protein subfamily contains seven members (TRPC1–TRPC7), with the TRPC2 gene being a pseudogene in humans. It was shown that the transient overexpression of some TRPCs in adrenal chromaffin cells results in markedly increased hormone-induced exocytosis (19). PC12 (pheochromocytoma 12) cells, a tumor cell line derived from adrenal chromaffin cells, appear to predominantly express endogenous TRPC6 channels (20,21,22,23,24,25). In addition, one report demonstrated that all seven TRPC transcripts can be found in another subclone of PC12 cells (26). TRPC6 can mediate hormone-induced Ca2+ influx in PC12 cells (21,22,26).
Little is known about the expression pattern of endogenous TRPCs in the adrenal medulla in health and disease. Specifically, it is not clear whether the expression level of TRPCs is altered during metabolic syndrome, which is associated with increased plasma epinephrine and heart rate. In this study, we determined the pattern of TRPC expression in the pig adrenal medulla and demonstrated that TRPC expression is markedly elevated in the adrenal medulla in the Ossabaw miniature swine model of metabolic syndrome. We also defined a possible mechanism that can contribute to regulating the expression of TRPC channels in adrenal chromaffin cells of metabolic syndrome pigs. Some of the preliminary results were reported previously (27).
Results
The molecular cloning of pig TRPCs
Because the sequences of pig TRPCs were not known, we first set out to determine them. We designed primer sets to highly homologous regions among known mammalian TRPCs from different species and established whether these primer sets could be employed to amplify TRPC cDNA from the total pig brain RNA as a template (Fig. 1A; the primer sequences are available on request). The sequencing of the amplified pig TRPC cDNAs confirmed their high homology to other mammalian TRPCs. To extend the ends of the cDNAs, if needed, we used the rapid amplification of cDNA ends (RACE) approach. The TRPC cDNA and protein sequence data were deposited to GenBank with the accession numbers indicated in Table 1. Aligning the DNA sequences of TRPCs from human, rat, mouse, and pig using the Lasergene 7 software showed that pig TRPC cDNAs resemble their human homologs more than the others (supplemental Fig. 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals. org; human and pig TRPC cDNAs share from 88.8–95.5% identity).
Figure 1.
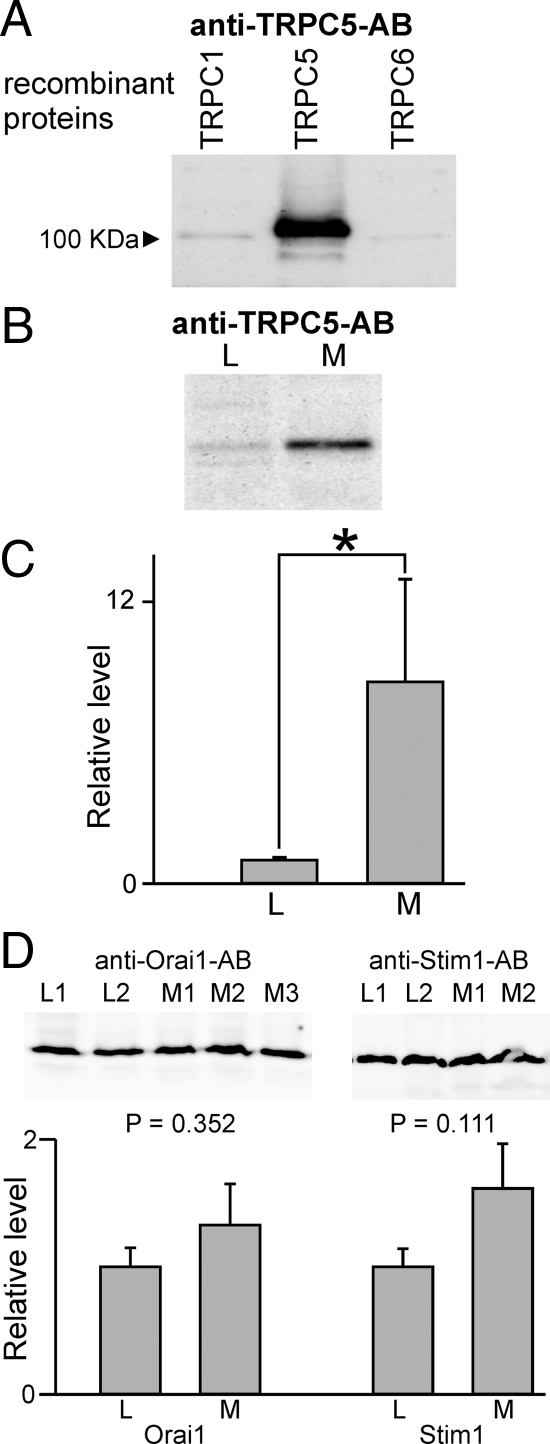
The pattern of TRPC expression in the pig adrenal medulla tissue. A, RT-PCR analysis of TRPC expression in the brain from lean/control Ossabaw pigs. An ethidium bromide-stained agarose gel is shown. B, RT-PCR analysis of TRPC expression in the snap-frozen adrenal medullae from lean/control (L) and metabolic syndrome (M) pigs. C, TRPC channel expression is elevated in the adrenal medulla from obese pigs exhibiting the metabolic syndrome. A comparison of relative (normalized to β-actin) TRPC expression levels in lean vs. obese/metabolic syndrome pig adrenal medullae is shown. *, Significant difference between the tested groups (P < 0.05; n = 5). D, RT-qPCR analysis of TRPC5 expression in the adrenal medullae from control and metabolic syndrome pigs. A comparison of relative TRPC5 expression in the adrenal medullae from control (n = 6) and metabolic syndrome (n = 6) pigs is shown. *, Means are significantly different (P < 0.05).
Table 1.
Pig TRPC GenBank accession numbers
| Pig TRPC channels | GenBank accession nos. |
|---|---|
| TRPC1 | FJ205710 |
| TRPC3 | FJ205711 |
| TRPC4 | FJ205712 |
| TRPC5 | EF101315 |
| TRPC6 | FJ205713 |
| TRPC7 | FJ205714 |
TRPC1, TRPC5, and TRPC6 are expressed in the pig adrenal medulla, and their expression level is elevated in metabolic syndrome
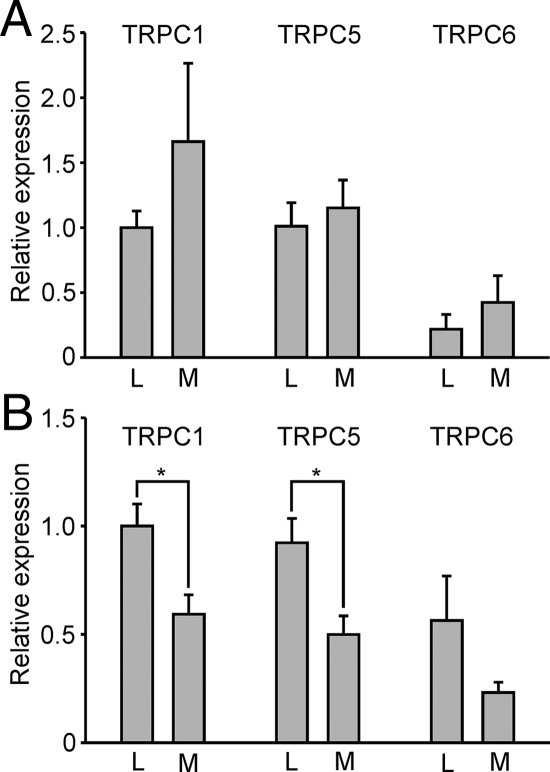
After we determined the sequence of pig TRPCs, we studied whether TRPCs are expressed in the adrenal medulla of control lean miniature Ossabaw pigs. We isolated the total RNA from the adrenal medulla as described in Materials and Methods and performed the semiquantitative RT-PCR analysis to determine the expression pattern of TRPCs. We found that TRPC1, TRPC5, and TRPC6 channels are predominantly expressed in the pig adrenal medulla (Fig. 1B). Although TRPC1 and TRPC5 transcripts were detected in almost equal amounts, the relative abundance of TRPC6 transcripts was much lower. It is not yet clear whether it represents a lower level of the TRPC6 mRNA in the total RNA or lower efficiency of the TRPC6 primer set we used, because the TRPC6 primer set also yielded a weaker band when the total brain RNA was used as a template.
Next, we investigated whether the expression level of TRPCs is altered in the adrenal medulla of pigs exhibiting metabolic syndrome. Figure 1, B and C, demonstrates that the metabolic syndrome adrenal medulla also predominantly expressed TRPC1, TRPC5, and TRPC6. In one case, we were able to amplify TRPC4 from the total RNA of metabolic syndrome adrenal medullae, but in other experiments no TRPC4 transcripts were found. Semiquantitative RT-PCR revealed that the relative level of TRPC1, TRPC5, and TRPC6 expression was 2- to 3-fold higher in the adrenal medulla from pigs manifesting the metabolic syndrome. For TRPC5, these results were further confirmed by real-time quantitative PCR (RT-qPCR) analysis (Fig. 1D). It is important to note that all these findings were from adrenal medullae that were rapidly frozen within only several minutes after the harvest from the pig.
The amount of the TRPC protein, but not Orai1/Stim1 proteins, is elevated during the metabolic syndrome in the adrenal medulla
To examine TRPC protein expression in control and the metabolic syndrome adrenal medulla, we used a commercial anti-TRPC5 antibody (Alomone Labs, Jerusalem, Israel). We were unable to evaluate TRPC1 and TRPC6 protein levels, because the commercial anti-TRPC1 and anti-TRPC6 antibodies did not recognize pig proteins. We used sections of the adrenal medulla that were snap frozen after harvesting from the pig. Figure 2A shows that the anti-TRPC5 antibody specifically detects the human TRPC5 recombinant protein but not TRPC1 or TRPC6. We found that the amount of TRPC5 protein was elevated about 3- to 4-fold in the metabolic syndrome adrenal medulla (Fig. 2, B and C). The adrenal medulla also expresses the store-operated Orai1 channel and the store-depletion sensor Stim1 protein. We found that the amount of Orai1/Stim1 proteins was not significantly increased in the metabolic syndrome adrenal medulla (Fig. 2D).
Figure 2.
TRPC protein expression levels are higher in the pig adrenal medulla frozen within several minutes of harvest from metabolic syndrome pigs. A, An anti-TRPC5 antibody (Alomone Labs) specifically stains the recombinant human TRPC5, but not TRPC1 or TRPC6, proteins isolated from HEK cells transfected with human TRPC1, TRPC5, and TRPC6. B, Representative Western blot of protein extracts from the adrenal medullae isolated from lean (L) and metabolic syndrome (M) pigs. C, Quantitation of TRPC protein amounts in lean/control vs. metabolic syndrome pigs (normalized to β-actin). *, Significant difference between the tested groups (P < 0.05; n = 4). D, Quantification of Orai1 and Stim1 protein amounts in lean (L, n = 8) vs. metabolic syndrome (M, n = 7) pig adrenal medullae (normalized to β-actin). The insets in the upper panel show representative Western blots with anti-Orai1 and anti-Stim1 antibodies (both from Alomone Labs). L1 and L2, Lean pigs; M1–M3, metabolic syndrome obese pigs.
TRPC1, TRPC5, and TRPC6 transcripts are also predominant in isolated pig adrenal chromaffin cells
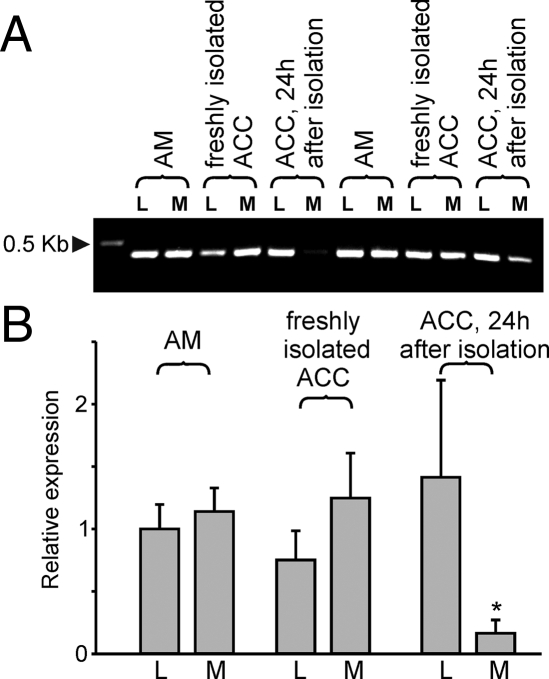
We next determined the pattern of TRPC expression in enzymatically dissociated pig adrenal chromaffin cells freshly isolated or maintained for 24 h in a serum-free medium (see supplemental Fig. 2) by using the semiquantitative RT-PCR analysis. We found that chromaffin cells freshly isolated from lean and obese/metabolic syndrome pigs also expressed only TRPC1, TRPC5, and TRPC6 transcripts, similar to tissue samples. To our surprise, however, the relative expression level of TRPCs was not significantly different in cells freshly isolated from metabolic syndrome and control pigs (Fig. 3A). Moreover, when chromaffin cells were maintained in serum-free medium for an additional 24 h, we observed a marked decrease of TRPC relative expression in cells isolated from metabolic syndrome pigs but not in cells obtained from control pigs (Fig. 3B).
Figure 3.
Relative TRPC expression levels in enzymatically isolated adrenal chromaffin cells. Semiquantitative RT-PCR was used to assess TRPC mRNA levels in freshly isolated chromaffin cells (A, about 3–4 h after the adrenal gland dissection, n = 5) or cells incubated for 24 h in serum-free media (B, n = 5–9). L, Cells isolated from lean control pig adrenal medulla; M, cells isolated from metabolic syndrome adrenal medulla. β-Actin normalized data are shown.
A continuous transcription of TRPCs is required to maintain high levels of TRPC expression in isolated adrenal chromaffin cells
Next, we tested whether de novo synthesis of RNA is required to maintain the observed levels of TRPC expression in chromaffin cells. To determine this, we treated the cells with an inhibitor of transcription, actinomycin D. Figure 4A shows that a 24-h actinomycin D pretreatment markedly decreased the amount of TRPC transcripts in the total RNA isolated from isolated control and metabolic syndrome chromaffin cells as compared with untreated cells. This indicates that TRPC transcripts are quickly degraded and that a continuous transcription of TRPC genes is required to maintain a constant amount of the TRPC mRNA.
Figure 4.
Adrenocorticoids affect the expression of TRPCs in isolated chromaffin cells from the adrenal medulla of metabolic syndrome pigs. A, TRPC RNAs are quickly degraded in isolated chromaffin cells. RT-PCR analysis of TRPC expression in isolated chromaffin cells treated with actinomycin D (AMD) for 24 h vs. untreated cells. B and C, Relative expression of TRPCs in dissociated adrenal chromaffin cells. RT-PCR analysis was performed using total RNA isolated from chromaffin cells treated with indicated chemicals for 24 h in a serum-free medium. A, Aldosterone; C, control; DM, dexamethasone; SL, spironolactone. β-Actin normalized data are plotted. Representative images of ethidium bromide-containing agarose gels are shown in B. *, Significant difference between tested groups (P < 0.05; n = 4–9).
Aldosterone increases the expression level of TRPCs in chromaffin cells isolated from the adrenal medulla of metabolic syndrome pigs but not in chromaffin cells from lean pigs
The marked decrease of TRPC expression specifically in chromaffin cells freshly isolated from metabolic syndrome pigs puzzled us. We reasoned that the chromaffin cell enzymatic isolation procedure takes about 3–4 h and includes multiple washes with an SMEM-based (MEM for suspension cultures; Invitrogen, Carlsbad, CA) buffer. Therefore, we hypothesized that there might be a diffusible molecular factor that maintains the relatively high expression of TRPCs in metabolic syndrome chromaffin cells while the cells are in the adrenal gland, but the factor is present in much lower levels or even absent in isolated cells.
In the adrenal gland, the adrenal medulla is surrounded by the adrenal cortex. The outer zone of the adrenal cortex, zona glomerulosa, produces aldosterone. Because the blood flow in the adrenal gland is centripetal, the adrenal chromaffin cells are exposed to very high concentrations of aldosterone. Aldosterone is a known regulator of cation channel gene expression (28,29). Therefore, we hypothesized that aldosterone may regulate the expression of TRPCs in adrenal chromaffin cells.
To test this hypothesis, we first incubated isolated metabolic syndrome chromaffin cells in the presence or absence of aldosterone and determined the expression level of TRPCs using semiquantitative RT-PCR. The acute treatment (1 h) of metabolic syndrome chromaffin cells with aldosterone did not alter the expression level of TRPCs (data not shown). However, 24 h preincubation with aldosterone significantly increased the expression level of TRPC1, TRPC5, and TRPC6 in metabolic syndrome chromaffin cells maintained in a serum-free medium (Fig. 4B). Aldosterone is an agonist of the mineralocorticoid receptors. To determine whether the mineralocorticoid receptors mediate the aldosterone effects, we employed spironolactone, a mineralocorticoid receptor inhibitor. In the absence of aldosterone, spironolactone itself did not alter TRPC expression in chromaffin cells (Fig. 4B). On the other hand, it completely antagonized the effect of aldosterone on TRPC expression in isolated chromaffin cells from metabolic syndrome pigs (Fig. 4B). In a separate set of experiments, we tested whether dexamethasone, a nonspecific agonist of glucocorticoid and mineralocorticoid receptors, affects the level of TRPC expression in metabolic syndrome chromaffin cells. Dexamethasone also significantly increased the expression level of TRPC5. However, it did not greatly affect the expression levels of TRPC1 and TRPC6 (Fig. 4C), suggesting that this nonspecific agonist was less effective.
Next, we investigated whether aldosterone regulates the expression of TRPC channels in control chromaffin cells isolated from lean pigs. To test that, we treated isolated control chromaffin cells with or without aldosterone for 24 h in the serum-free medium. Surprisingly, we found that the level of TRPC expression was not altered by aldosterone in control cells (Fig. 5). Spironolactone also did not affect the background TRPC expression level in control chromaffin cells, ruling out that mineralocorticoid receptors were activated by an unknown factor secreted from the cells.
Figure 5.
RT-PCR analysis of TRPC expression of adrenal chromaffin cells isolated from lean/control pigs. Cells were treated with aldosterone (A) or spironolactone (SL) for 24 h in the serum-free medium. β-Actin normalized data are plotted (n = 4).
Control chromaffin cells express higher levels of the mineralocorticoid receptors than metabolic syndrome chromaffin cells
The lack of aldosterone effects may also be due to an altered expression of the mineralocorticoid receptors in control chromaffin cells. Figure 6 shows that the pig adrenal medulla and freshly isolated adrenal chromaffin cells expressed similar levels of the mineralocorticoid receptor. After 24 h incubation in serum-free medium, the mineralocorticoid receptor expression increased slightly, although not significantly, in cells isolated from control pigs, whereas in cells isolated from metabolic syndrome pigs, expression was markedly attenuated (P < 0.05).
Figure 6.
RT-PCR analysis of mineralocorticoid receptor expression in the adrenal medulla and isolated chromaffin cells. A, Mineralocorticoid receptor transcripts were analyzed in total RNA isolated from the adrenal medulla (AM) and chromaffin cells (freshly isolated or incubated for 24 h in the serum-free medium). Two independent experiments are shown. The agarose gel was stained with ethidium bromide. ACC, Adrenal chromaffin cells; L, lean control; M, metabolic syndrome. B, Quantification of the data shown in A. Mineralocorticoid receptor expression levels were normalized to a β-actin control. *, Significant difference between the tested groups (P < 0.05; n = 5).
The promoter regions of TRPC1, TRPC5, and TRPC6 channels possess half-site glucocorticoid response elements (GREs)
To regulate gene expression, both mineralocorticoid and glucocorticoid nuclear receptors bind to the same short promoter segment known as GRE. The classical GRE is a palindromic sequence of 15 nucleotides (AGAACAnnnTGTTCT) (30,31) that contains two half-sites. The dimerized aldosterone-mineralocorticoid receptor complex binds simultaneously to both half-sites of GRE. It is reported, however, that some GREs may also be activated by binding a monomeric ligand-nuclear receptor complex (32) to only one half-site of the GRE. We investigated whether TRPC1, TRPC5, and TRPC6 promoter regions contain any GRE-like motifs. To perform the promoter region analysis, we used the TESS software (33) (http://www.cbil. upenn.edu/cgi-bin/tess/tess). The software was designed to search for the transcription factor binding sites in promoter regions (33). To test the software, we first analyzed the promoter region of phenylethanolamine-N-methyl transferase (PNMT), an enzyme known to be activated by the glucocorticoid receptors via classical GRE-1 (AGAACAgagTGTCCT) (32). The TESS software correctly recognized several GRE-like sequences in the promoter of PNMT, including the classical PNMT-GRE-1 binding site (32). Next, we analyzed the promoter regions of TRPC1, TRPC5, and TRPC6. The TESS software identified no complete classical GRE sequences in the promoter region of the channels; however, several GRE half-sites were noted (TESS) (33,34): TRPC1 (CTCTG) and TRPC6 (TGTGC). A very prominent half-site GRE was found at two positions in the TRPC5 promoter region (AGAACA, at −358 to −352 and −856 to −850; GenBank accession no. NM_012471, human). No GRE-like half-sites were identified in the promoter region of the Orai1 channel (NM_032790, human), a store-operated channel expressed in the adrenal medulla.
Adrenal chromaffin cells express several G-protein-coupled receptors capable of activating TRPC channels
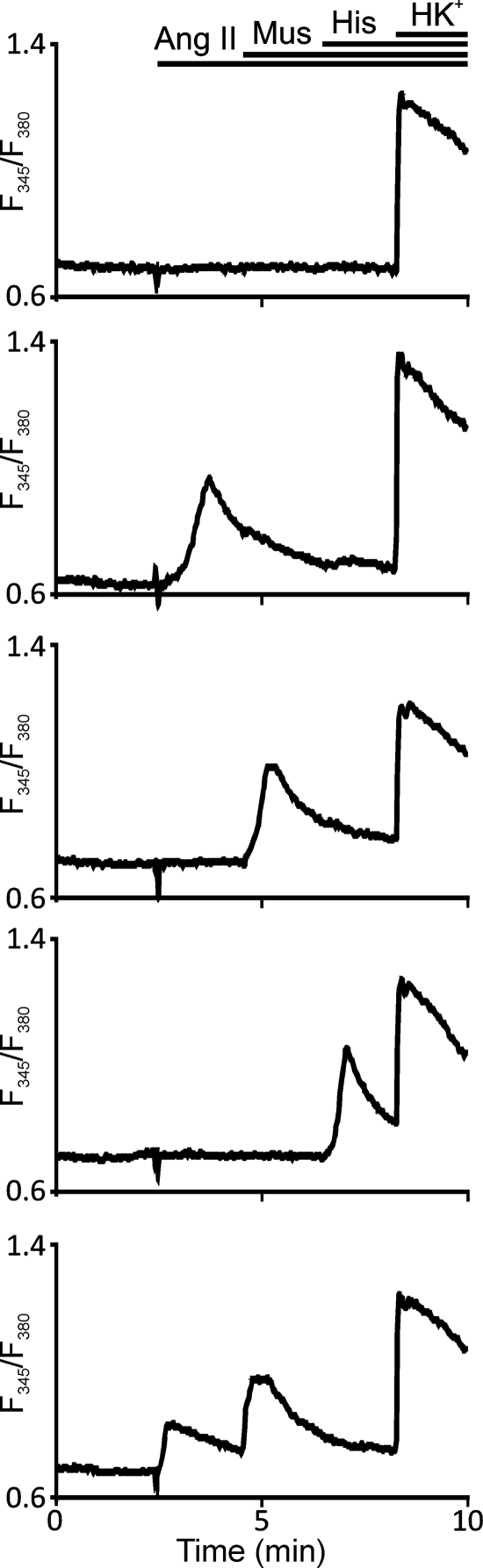
TRPC channels are activated by hormones of Gq,11-protein-coupled receptors in a PLC-dependent manner. To determine which functional Gq,11-protein-coupled receptors are expressed in pig adrenal chromaffin cells, we used the fluorescence imaging technique. During these experiments, we challenged fura-2-loaded adrenal chromaffin cells with angiotensin II (up to 0.5 μm), muscarine (10 μm), or histamine (40 μm). At the end of each experiment, an application of the high-potassium solution was used to identify chromaffin cells. Figure 7 shows the hormone- and high potassium-induced changes of intracellular [Ca2+] in five representative cells. Hormones stimulated slow intracellular [Ca2+] changes due to Ca2+ release from the IP3-sensitive stores and Ca2+ entry through, presumably, Ca2+-permeable receptor/store-operated channels, whereas the high-potassium solution induced a robust and rapid Ca2+ entry through voltage-gated Ca2+ channels. Control adrenal chromaffin cells (211 tested cells, four independent experiments) responded to the tested hormones as follows: 31.7 ± 11.9% of the cells were sensitive to angiotensin II, 48.7 ± 2.9% of the cells were sensitive to muscarine, and 37.1 ± 8.7% of the cells were sensitive to histamine. Of the tested control cells, 13.9 ± 2.9% were not sensitive to any of the above listed hormones. In the metabolic syndrome group (207 tested cells, four independent experiments), 19.2 ± 0.6% of the tested cells responded to angiotensin II, 58.5 ± 12.1% of the tested cells responded to muscarine, and 31.6 ± 7.9% of the tested cells responded to histamine. Of the metabolic syndrome chromaffin cells, 14.5 ± 3.2% did not respond to the above listed hormones. Up to 15% of the tested cells responded to more than one hormone in both the control and metabolic syndrome groups. The number of chromaffin cells sensitive to angiotensin II, muscarine, or histamine was not significantly different (P > 0.05) between the control and metabolic syndrome groups.
Figure 7.
Circulating hormones of Gq/11-protein-coupled receptors induce intracellular Ca2+ increases in isolated adrenal chromaffin cells. Isolated chromaffin cells were loaded with fura-2 to image intracellular calcium changes as described in Materials and Methods. Representative traces of the F345/F380 fluorescence ratio from five different cells are shown. Some cells responded to more than one hormone. Chromaffin cells from the adrenal medullae of lean/control and obese/metabolic syndrome pigs exhibited a comparable percentage of responding cells. Horizontal bars indicate times when agonists and the high-potassium solution were added.
Hormone-stimulated Mn2+ influx correlates with the relative expression level of TRPCs in isolated chromaffin cells
TRPC channels mediate divalent cation influx in numerous cell types. Therefore, we tested the hypothesis that TRPC channels contribute to hormone-induced divalent cation influx in adrenal chromaffin cells. Using the fluorescence imaging technique, we measured intracellular Ca2+ changes by calculating the ratio of fura-2 fluorescence (F345/F380) and Mn2+ influx by recording the decrease of fura-2 fluorescence excited at 360 nm (Mn2+ quench) in fura-2-loaded chromaffin cells that were incubated in serum-free medium for 24 h and then stimulated with histamine, a well-known adrenal secretagogue. Mn2+ influx under these conditions provides a measure of the activity of plasma membrane calcium influx channels.
Although the extracellularly applied 1 mm Mn2+ solution caused no intracellular Ca2+ changes or Mn2+ influx in unstimulated cells, histamine application induced slowly developing intracellular Ca2+ increases and slow Mn2+ influx in isolated control chromaffin cells (Fig. 8, A left, B left, and D). Subsequent application of the high-potassium solution resulted in an abrupt increase of intracellular Ca2+ due to massive Ca2+ influx through voltage-gated Ca2+ channels. In chromaffin cells isolated from metabolic syndrome pigs and incubated in serum-free medium for 24 h, histamine-induced intracellular Ca2+ changes and Mn2+ influx were smaller than in cells isolated from control pigs (Fig. 8, A middle, B middle, and D), whereas the rate of high-potassium application-activated intracellular Ca2+ rise was unaltered. This correlates well with the lower levels of TRPC transcripts in the cells isolated from metabolic syndrome pigs. In accordance with the similar expression levels of TRPC channels in freshly isolated cells from control and metabolic syndrome pigs, there was no difference in amplitude of the Mn2+ influx in these chromaffin cells (Fig. 8D, bars labeled F).
Figure 8.
Aldosterone regulates histamine-induced Mn2+ influx in adrenal chromaffin cells isolated from metabolic syndrome pigs but not from lean pigs. Intracellular [Ca2+] changes were measured using the fluorescence imaging technique in fura-2-loaded cells. A, Intracellular [Ca2+] changes are shown in adrenal chromaffin cells from lean (left) and metabolic syndrome (MetS, middle and right) pigs. In A, right, chromaffin cells were incubated with aldosterone for 24–30 h before measurements. Mn2+, histamine (His), and the high-potassium solution were added at times indicated with horizontal bars. Representative experiments are shown. F345/F380 ratios are plotted vs. time. The solid lines are the means of F345/F380 ratio values recorded in 50–100 cells. The vertical lines are sem. B, The amplitude of Mn2+ influx was determined by measuring the ability of extracellular Mn2+ to quench the fura-2 fluorescence at 360 nm. Mn2+ and histamine (His) are added at times indicated by the horizontal bars. Normalized data are shown. The groups are the same as in A. C, La3+ (100 μm) inhibited high-potassium-activated Ca2+ increases in chromaffin cells but not the histamine-induced release of Ca2+ from the intracellular Ca2+ stores. D, The relative, normalized Mn2+ influx values computed 2 min after a histamine application. The means and error bars indicating sem are plotted. *, Significant difference between tested groups (P < 0.05). Aldo24, Groups of chromaffin cells treated with aldosterone for 24 h; C24, chromaffin cells incubated for 24 h in the serum-free medium; F, freshly isolated chromaffin cells.
Aldosterone modulates hormone-induced Mn2+ influx in isolated metabolic syndrome chromaffin cells
TRPC channels are implicated in mediating the hormone-induced divalent cation influx. Because our data indicated that aldosterone regulates the expression of TRPC1, TRPC5, and TRPC6 channels, we also conducted fluorescence imaging experiments to explore the role of aldosterone in modulating histamine-induced intracellular Ca2+ changes and Mn2+ influx in chromaffin cells. Histamine-induced intracellular Ca2+ increases and Mn2+ influx were significantly larger in 24-h aldosterone-treated metabolic syndrome chromaffin cells as compared with untreated cells (Fig. 8, A right, B right, and D). This is in good agreement with the observed aldosterone-dependent up-regulation of TRPC expression in metabolic syndrome chromaffin cells. In contrast, aldosterone pretreatment did not modulate histamine-induced Ca2+ changes and Mn2+ influx in control chromaffin cells. The averaged peak amplitude of high-potassium-stimulated Ca2+ influx through voltage-gated Ca2+ channels was not significantly different in control vs. metabolic syndrome experimental groups (1.107 ± 0.021 vs. 1.088 ± 0.024, respectively, P > 0.05). Also, aldosterone pretreatment did not significantly affect high-potassium-induced changes of intracellular Ca2+ (F345/F380 = 1.113 ± 0.026, P > 0.05) in isolated chromaffin cells from metabolic syndrome pigs.
La3+ inhibits histamine-induced Mn2+ influx
Because there are no specific inhibitors of TRPC channels, we tested whether an unspecific inhibitor of cation channels, lanthanum, blocks histamine-activated Ca2+ changes and Mn2+ influx mediated fura-2 quenching. We found that La3+ (100 μm) did not inhibit histamine-induced intracellular Ca2+ increases due to Ca2+ release from the intracellular Ca2+ stores. In contrast, La3+ significantly inhibited histamine-induced Mn2+ influx and high-potassium-induced Ca2+ rises (Fig. 8, C and D) in chromaffin cells. A small fraction of histamine-induced Mn2+ influx was La3+ resistant (100 μm, Fig. 8D). The La3+-resistant component was larger in metabolic syndrome adrenal chromaffin cells. Interestingly, TRPC5, which is up-regulated in metabolic syndrome, was shown to be resistant to 100 μm La3+ (35).
Discussion
These studies demonstrate that the adrenal medullae of obese pigs manifesting metabolic syndrome express much higher levels of TRPC1, TRPC5, and TRPC6 channels than their lean and healthy counterparts. Our data indicate that aldosterone increased the expression level of TRPC1, TRPC5, and TRPC6 channels in adrenal chromaffin cells isolated from metabolic syndrome pigs but not in those from lean controls. We also found that histamine-induced divalent cation influx correlated with the expression level of TRPCs in isolated pig adrenal chromaffin cells. Orai1/Stim1 proteins, mediating store-operated Ca2+ influx (36), were also expressed in the adrenal medulla. However, the amount of Orai1/Stim1 proteins was not significantly altered in the metabolic syndrome adrenal medulla.
The lack of exercise and an unhealthy diet with an excess of fat and cholesterol leads to obesity. Obesity may result in the metabolic syndrome, which is a risk factor of developing cardiovascular disease. The metabolic syndrome affects a large part of the world’s population, striking regardless of age or gender. Elevated heart rate during metabolic syndrome is known to be a contributor leading to higher mortality rates due to cardiovascular disease (37). Higher heart rate may be in part due to increased plasma epinephrine in the metabolic syndrome (9).
Chromaffin cells of the adrenal medulla are the major production sites of epinephrine in mammals. The secretion of epinephrine from the adrenal medulla depends on Ca2+ influx. Voltage-gated Ca2+ channels are the main pathway for extracellular Ca2+ entry into a chromaffin cell. However, circulating hormone-operated Ca2+ influx can also mediate exocytosis in the adrenal medulla. TRPCs are candidates for the circulating hormone-operated channels in chromaffin cells. The enhanced adrenal expression of TRPC channels during metabolic syndrome may underlie elevated epinephrine release from the adrenal medulla.
The Tepel group (38) recently published a convincing report indicating that high plasma glucose may specifically enhance TRPC6, but not TRPC1 or TRPC5, protein expression and promote TRPC6 surface localization in human platelets. Obese Ossabaw pigs are a prediabetic metabolic syndrome model (plasma glucose is about 100 mg/dl) (10). Therefore, it is unlikely that slightly elevated glucose concentrations in Ossabaw pigs significantly contribute to regulating the adrenal TRPC6 expression in our metabolic syndrome model.
In the current study, we found that aldosterone increases the expression level of TRPC1, TRPC5, and TRPC6 channels in chromaffin cells isolated from metabolic syndrome pigs. Aldosterone is well known to modulate gene expression. For instance, aldosterone markedly potentiates the expression of the α-subunit of epithelial sodium channels (ENaC) in the collecting ducts of the kidney that is crucial for salt retention (29). Interestingly, a recent elegant study by Bae et al. (39) indicates that aldosterone is also involved in up-regulating TRPC6 expression in rat aortic smooth muscle cells. The renin-angiotensin-aldosterone system activity is markedly increased in metabolic syndrome (40,41), and patients exhibiting the metabolic syndrome have elevated plasma aldosterone. Ossabaw pigs manifesting metabolic syndrome also exhibit more than 5-fold elevated levels of plasma aldosterone (42). Aldosterone is secreted in the zona glomerulosa, a peripheral zone of the adrenal gland. Because the direction of blood flow is from the adrenal cortex to the central adrenal medulla in the adrenal gland, adrenal chromaffin cells may be exposed to very high concentrations of aldosterone, especially during the metabolic syndrome.
Aldosterone regulates gene expression by acting via the mineralocorticoid receptor. Our data indicate that adrenal medullae from both control and metabolic syndrome pigs expressed similar levels of the mineralocorticoid receptor. Mineralocorticoid and glucocorticoid receptors bind to the same response element in the promoter, the GRE. Although no classical 15-nucleotide GRE sequence was present in the putative promoter region of TRPC1, TRPC5, and TRPC6 channels, we identified several GRE-like half-site sequences. The strongest matches were in the TRPC5 gene that contains two classical half-site GRE motifs (AGAACA). A nuclear receptor binding to a single GRE half-site motif was shown to regulate gene expression (32,34). Some of these GRE half-sites were classified as delayed secondary elements that may be less effective than the classical GRE in regulating gene expression. Most likely, nuclear receptors may require some extent of phosphorylation to interact with delayed secondary elements before inducing transcription activation (34). Notably, the promoter analysis revealed no GRE-like sites in the promoter region of Orai1. It is in good agreement with our observation that the protein is not up-regulated in the metabolic syndrome adrenal medulla.
Surprisingly, we found that aldosterone significantly increases the expression level of TRPC1, TRPC5, and TRPC6 only in adrenal chromaffin cells isolated from metabolic syndrome pigs but not in chromaffin cells from control pigs. This is not due to differences in the mineralocorticoid receptor expression because control cells actually express more mineralocorticoid receptors than the cells isolated from metabolic syndrome pigs. A reduced expression level of the mineralocorticoid receptors in isolated metabolic syndrome chromaffin cells may explain why aldosterone only rescued the control cell level of TRPC expression in isolated metabolic syndrome cells but did not restore the original elevated TRPC expression levels observed in the adrenal medulla tissue from metabolic syndrome pigs.
The reason for the ineffectiveness of aldosterone in isolated control chromaffin cells remains to be determined. Interestingly, aldosterone also did not significantly modulate the expression levels of TRPC channels in PC12 cells, a tumor cell line derived from adrenal chromaffin cells (supplemental Fig. 3). A recent report highlighted the importance of chromatin remodeling for nuclear receptor activation (43). The authors attribute the tissue-selective nuclear receptor activity to the differences in the local chromatin structure (the chromatin landscape) (43). It is possible that some mechanisms involved in chromatin remodeling are altered in metabolic syndrome chromaffin cells. Another possible explanation may be the difference in the availability of nuclear receptor coactivators such as TIF2/GRIP1 (transcriptional intermediary factor 2/glucocorticoid receptor interacting protein 1) and steroid receptor coactivator-1 (SRC-1) (44,45). The coactivators bind to hormone-nuclear receptor complexes and then enhance transcriptional activation. SRC can recruit the coactivator-associated arginine methyltransferase that modulates chromatin remodeling and GRE availability via histone methylation (44). It is not known whether any of the SRC family proteins are involved in mediating aldosterone effects in metabolic syndrome chromaffin cells.
It is not yet clear whether aldosterone is the only regulator of TRPC expression in the adrenal medulla. Additional molecular factors that further up-regulate TRPC channels during metabolic syndrome may exist. In vivo, metabolic syndrome milieu includes increased adipokines, low-density lipoprotein/high-density lipoprotein, triglycerides, insulin, etc. (46,47), which may tonically regulate the expression of TRPC. The extremely labile nature of TRPC expression in metabolic syndrome is quite striking, because TRPC mRNA markedly decreased in isolated metabolic syndrome chromaffin cells after only several hours of isolation from the pig (Fig. 3). On the other hand, the modulation by aldosterone treatment in vitro suggests that some other factors in isolated metabolic syndrome chromaffin cells have maintained them more responsive to aldosterone. Certainly, additional experiments will be needed to further explore other possible candidates.
There are no specific inhibitors for TRPCs that can aid in the pharmacological assessment of the contribution of different TRPC channels to histamine-induced divalent influx in pig adrenal chromaffin cells. Because chromaffin cells are difficult to transfect, the small interfering RNA approach is complicated. In addition, the pig genome is not fully sequenced yet, which would further complicate the design of TRPC-specific small interfering RNA. We found that La3+, an unspecific inhibitor of cation channels, significantly inhibited histamine-induced divalent influx in pig adrenal chromaffin cells in our fluorescence imaging experiments. However, La3+ also inhibits voltage-gated Ca2+ channels, which are highly expressed in pig adrenal chromaffin cells. Because TRPCs form nonselective cation channels, the channels’ activation would depolarize chromaffin cells and might activate voltage-gated Ca2+ channels. Thus, TRPC activation may indirectly stimulate voltage-gated Ca2+ channels in chromaffin cells during histamine stimulation.
Thus, we demonstrated that TRPC1, TRPC5, and TRPC6 are up-regulated in the adrenal medullae of obese pigs exhibiting the metabolic syndrome. Aldosterone emerged as a possible modulator of TRPC1, TRPC5, and TRPC6 expression in chromaffin cells isolated from metabolic syndrome pigs. Given that isolated chromaffin cells may lose some of their in vivo characteristics during dissociation, future in vivo experiments would be needed to establish whether similar mechanisms function in vivo in intact adrenal medulla during metabolic syndrome. Also, additional experiments will be required to verify that the expression level of the histamine receptors and/or PLC is not altered in the metabolic syndrome adrenal medulla. We propose that an abnormal up-regulation of adrenal TRPCs may underlie the elevated plasma epinephrine and heart rate observed during the metabolic syndrome (3,10). Therefore, TRPC proteins may be a possible target for therapeutic interventions in metabolic syndrome patients with elevated heart rate and plasma epinephrine.
Materials and Methods
Ossabaw miniature swine origin and phenotype
Ossabaw miniature pigs adapted to the unique feast and famine ecology of Ossabaw Island, GA, by acquiring the ability to accumulate fat when food is abundant to survive during times of food scarcity. Sustained fat accumulation in captivity, however, results in severe cardiovascular disease (46). Ossabaw pigs develop all major characteristics of the metabolic syndrome if they are fed with an excess-kilocalorie high-fat/high-cholesterol atherogenic diet for a long period of time (6–9 months) (3,4,10,46).
Ossabaw miniature swine maintenance and tissue collection
Control Ossabaw pigs were fed standard chow containing 22% kcal from protein, 70% kcal from carbohydrates, and 8% kcal from fat (Purina TestDiet, Richmond, IN). The obese Ossabaw pig group was fed standard chow supplemented with 2.0% cholesterol, 46% kcal from fat, 20% kcal from fructose for 25–37 wk. Control group pigs ate approximately 700 g/d, whereas obese group pigs ate approximately 1000 g/d. Obese Ossabaw pigs weighed 81 ± 5 kg and displayed all features of metabolic syndrome as documented previously (10), whereas the lean control pigs weighed 60 ± 3 kg. All procedures for tissue collection were approved by the Indiana University Animal Care and Use Committee. The adrenal glands were dissected and cleaned from fatty tissue. The adjusted weight of the adrenal glands was not significantly different in obese and control pig groups (22.71 ± 1.04 and 23.98 ± 0.88 mg/kg, correspondingly, P > 0.05). The adrenal medulla was isolated from the adrenal cortex. A part of the medulla was snap frozen in liquid N2 and stored at −80 C for future biochemical experiments. The other part of the medulla was used to isolate chromaffin cells. All steps were performed in the standard PBS solution containing no Ca2+ or Mg2+ (Invitrogen).
Molecular biology
Total RNA was isolated from freshly collected and snap-frozen tissues of lean or metabolic syndrome Ossabaw pigs by employing the Trizol method (Invitrogen). A deoxyribonuclease treatment step was used to eliminate any possible traces of the genomic DNA in the total RNA. The pig TRPC channels were cloned using a combination of homologous cloning and the RNA ligase mediated rapid amplification of cDNA ends method (RLM-RACE, Ambion, Austin, TX) from the pig brain total RNA. A one-step RT-PCR kit (Invitrogen) was used to amplify the cDNAs; 25 ng of the total RNA per reaction was used. During the PCR amplification, we used 30–33 cycles to ensure that the PCR remains in its linear amplification stage. The relative quantification of TRPC expression after RT-PCR analysis was done by normalizing the fluorescence intensities of the TRPC cDNA stained with ethidium bromide and resolved on an agarose gel to that of β-actin amplified from the same total RNA. To present the data, all values were normalized to the mean value of the control group. To validate this semiquantitative approach, we performed an RT-PCR analysis of TRPC5 expression in a total RNA after varying the template amount from 1–100 ng. We found that throughout this range, a change in the mRNA input produced a linear change in the PCR signal. We also performed a RT-qPCR analysis. We used the Bio-Rad iScript cDNA synthesis kit to reverse-transcribe cDNA using total RNA from 12 pigs (six control and six metabolic syndrome pigs) as templates. The Applied Biosystems (Foster City, CA) 7500 Real Time PCR System was used to perform RT-qPCR. The data were quantified using the standard ΔΔCt method. The endogenous control (18S rRNA) was amplified with TaqMan Universal PCR Master Mix (Applied Biosystems), whereas TRPC5 was amplified using SYBR Green Master mix (Applied Biosystems). The dissociation curves were analyzed for all SYBR Green reagent products yielding a single maximum with the mean melting temperature of 83.0 ± 0.1 C for TRPC5 control cell products and of 83.1 ± 0.1 C for TRPC5 metabolic syndrome cell products (P > 0.05). The no-reverse-transcriptase control was negative. The RT-qPCR required a large amount of cDNA to achieve a reasonable signal-to-noise ratio (1 μg of the initial total RNA). Because our usual yield of the total RNA from isolated cells was always low, it was not possible to use the RT-qPCR analysis for quantifying TRPCs in isolated cell samples. In contrast, semiquantitative RT-PCR worked well even if we used only 1 ng total RNA as a template (data not shown). The PCR products were subcloned into the pSC-A cloning vector using the TOPO-UA technology (Stratagene, Cedar Creek, CA) and sequenced.
Chromaffin cell isolation
Porcine adrenal medullae were digested using 2 mg/ml collagenase in the presence of 0.1 mg/ml of the soybean trypsin inhibitor (both from Roche Diagnostics, Indianapolis, IN). The digestion yielded isolated adrenal chromaffin cells about 3 h after harvest from the pig. The cells were plated on 25-mm round glass coverslips precoated with poly-l-lysine (Sigma, St. Louis, MO) and used either freshly after 1 h fura-2AM loading or maintained for 24 h in a MEM (Invitrogen) supplemented with 0.1% BSA and 50 U/ml penicillin/streptomycin in a moisturized 5% CO2 incubator at 37 C with no serum added to avoid exposure of isolated chromaffin cells to serum growth factors.
Immunoblotting
The protein extracts were subjected to 7.5% SDS-PAGE separating gel (4% stacking gel). The resolved proteins were transferred to the nitrocellulose membranes in a Bio-Rad wet transfer apparatus. The membrane (blocked with 5% casein) were incubated with TRPC5 antibodies (1:1000; Alomone Labs) and then with an antirabbit horseradish peroxidase-conjugated antibody (Pierce, Rockford, IL; 1:20,000). Bound horseradish peroxidase conjugates were detected using a SuperSignal West Femto Kit (Pierce) according to the manufacturer’s instructions.
Fluorescence imaging
A monochromator-equipped imaging system (TILL-Photonics, Martinsreid, Germany) was used to monitor fluorescence changes of cells loaded with fura-2 (Molecular Probes, Inc., Eugene, OR). Cells were loaded with fura-2/AM (2–5 μm) for 45–60 min in PBS containing Ca2+, Mg2+, and 0.1% BSA at room temperature. After loading, the cells were washed three times with the same PBS solution without fura-2/AM and then incubated for an additional 30 min at room temperature. Fura-2 fluorescence was excited at 345 and 380 nm for determining intracellular Ca2+ changes and at the Ca2+-insensitive isosbestic wavelength of 360 nm for Mn2+ quench measures of divalent cation influx in chromaffin cells. The Mn2+ quench technique evaluates Mn2+ influx with greater sensitivity than net measures of bulk cytosolic Ca2+ (F345/F380 ratio) because: 1) fura-2 has a 40-fold greater affinity for Mn2+ than Ca2+, 2) Mn2+ is not transported by other Ca2+ transporters, and 3) because there are no Mn2+ stores, no intracellular release is measured. Depending on loading, exposure times were set from 10–100 msec. Emitted light was collected with a 510-nm long-pass filter. Only cells exhibiting Ca2+ influx upon high-potassium-solution-induced depolarization were taken for analysis. Data were analyzed using the TILLvisION software. Background fluorescence was subtracted. Standard extracellular solution for fluorescence measurements contained (mm): 150 NaCl, 1 MgCl2, 1.2 CaCl2, 5.5 glucose, 10 HEPES (pH 7.2). This solution was supplemented with 1 mm MnCl2 at times indicated in Fig. 8. The high-potassium solution contained (mm): 70 NaCl, 80 KCl, 1 MgCl2, 1.2 CaCl2, 5.5 glucose, 10 HEPES (pH 7.2).
Chemicals
All chemicals were purchased from Sigma. Drugs were applied in the following concentrations (μm): 40 histamine, 0.5 angiotensin II, 10 muscarine, 1 aldosterone, 10 spironolactone, 5 dexamethasone, 0.4 actinomycin D.
Statistics
The Student’s t test or the ANOVA was used to determine whether the means of the tested groups were significantly different (assessed with the SigmaPlot 11 software suite). The results were considered significantly different if the P value was <0.05. All data are expressed as mean ± sem.
Supplementary Material
Acknowledgments
We thank Dr. B. Paul Herring for his critical reading and valuable comments on the manuscript, Ms. Alice Nakatsuka for technical assistance, Mr. Zachary P. Neeb, Mrs. Xin Long, and Drs. Mouhamad A. Alloosh, Jason M. Edwards, and Ian N. Bratz from the Sturek lab for tissue collection. We thank Dr. Saikat Chakraborty for the Western blot analysis of Stim1 expression.
Footnotes
This work was supported by National Institutes of Health grants to M.S. (RR013223 and HL062552), a National Institutes of Health grant to A.G.O. (HL083381), and the Purdue-Indiana University Comparative Medicine Program.
Disclosure Summary: The authors have nothing to disclose.
First Published Online February 12, 2009
Abbreviations: GRE, Glucocorticoid response element; GRIP1, glucocorticoid receptror interacting protein 1; IP3, inositol 1,4,5-trisphosphate; PLC, phospholipase C; PNMT, phenylethanolamine-N-methyl transferase; RT-qPCR, real-time quantitative PCR; SMEM, MEM for suspension cultures; SRC-1, steroid receptor coactivator-1; TIF2, transcriptional intermediary factor 2; TRPC, canonical transient receptor potential channels; SMEM, MEM for suspension cultures; TIF2, transcriptional intermediary factor 2; GRIP1, glucocorticoid receptor interacting protein 1.
References
- Flier JS 2004 Obesity wars: molecular progress confronts an expanding epidemic. Cell 116:337–350 [DOI] [PubMed] [Google Scholar]
- Rendell M, Gurwitz D 2006 Metabolic syndrome: a wake-up call. Drug Dev Res 67:535–538 [Google Scholar]
- Dyson MC, Alloosh M, Vuchetich JP, Mokelke EA, Sturek M 2006 Components of metabolic syndrome and coronary artery disease in female Ossabaw swine fed excess atherogenic diet. Comp Med 56:35–45 [PubMed] [Google Scholar]
- Knudson JD, Dincer UD, Bratz IN, Sturek M, Dick GM, Tune JD 2007 Mechanisms of coronary dysfunction in obesity and insulin resistance. Microcirculation 14:317–338 [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Quarti-Trevano F, Scopelliti F, Dell'Oro R, Bolla G, Mancia G 2007 Excessive sympathetic activation in heart failure with obesity and metabolic syndrome: characteristics and mechanisms. Hypertension 49:535–541 [DOI] [PubMed] [Google Scholar]
- Gilardini L, Parati G, Sartorio A, Mazzilli G, Pontiggia B, Invitti C 2008 Sympathoadrenergic and metabolic factors are involved in ambulatory blood pressure rise in childhood obesity. J Hum Hypertens 22:75–82 [DOI] [PubMed] [Google Scholar]
- Inoue T, Iseki K, Iseki C, Ohya Y, Kinjo K, Takishita S 2008 Association between heart rate and multiple risk factor syndrome: cross-sectional analysis of a screened cohort in Okinawa, Japan. Circ J 72:454–457 [DOI] [PubMed] [Google Scholar]
- Inoue T, Iseki K, Iseki C, Kinjo K, Ohya Y, Takishita S 2007 Higher heart rate predicts the risk of developing hypertension in a normotensive screened cohort. Circ J 71:1755–1760 [DOI] [PubMed] [Google Scholar]
- Dincer UD, Araiza AG, Knudson JD, Molina PE, Tune JD 2006 Sensitization of coronary α-adrenoceptor vasoconstriction in the prediabetic metabolic syndrome. Microcirculation 13:587–595 [DOI] [PubMed] [Google Scholar]
- Bratz IN, Dick GM, Tune JD, Edwards JM, Neeb ZP, Dincer UD, Sturek M 2008 Impaired capsaicin-induced relaxation of coronary arteries in a porcine model of the metabolic syndrome. Am J Physiol: Heart Circ Physiol 294: H2489–H2496 [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo Jr JL, Jones DW, Materson BJ, Oparil S, Wright Jr JT, Roccella EJ 2003 The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 289:2560–2572 [DOI] [PubMed] [Google Scholar]
- Broadley KJ, Penson PE 2004 The roles of α- and β-adrenoceptor stimulation in myocardial ischaemia. Auton Autacoid Pharmacol 24:87–93 [DOI] [PubMed] [Google Scholar]
- Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS; COURAGE Trial Research Group 2007 Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 356:1503–1516 [DOI] [PubMed] [Google Scholar]
- Cheek TR, Barry VA 1993 Stimulus-secretion coupling in excitable cells: a central role for calcium. J Exp Biol 184:183–196 [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, Morgan A, Robinson I, Pender N, Cheek TR 1993 Exocytosis in adrenal chromaffin cells. J Anat 183:309–314 [PMC free article] [PubMed] [Google Scholar]
- Livett BG, Marley PD 1993 Noncholinergic control of adrenal catecholamine secretion. J Anat 183:277–289 [PMC free article] [PubMed] [Google Scholar]
- Sala F, Nistri A, Criado M 2008 Nicotinic acetylcholine receptors of adrenal chromaffin cells. Acta Physiol 192:203–212 [DOI] [PubMed] [Google Scholar]
- Olivos L, Artalejo AR 2008 Muscarinic excitation-secretion coupling in chromaffin cells. Acta Physiol 192:213–220 [DOI] [PubMed] [Google Scholar]
- Obukhov AG, Nowycky MC 2002 TRPC4 can be activated by G-protein-coupled receptors and provides sufficient Ca2+ to trigger exocytosis in neuroendocrine cells. J Biol Chem 277:16172–16178 [DOI] [PubMed] [Google Scholar]
- Mwanjewe J, Grover AK 2004 Role of transient receptor potential canonical 6 (TRPC6) in non-transferrin-bound iron uptake in neuronal phenotype PC12 cells. Biochem J 378:975–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Saffen D 2005 Activation of M1 muscarinic acetylcholine receptors stimulates the formation of a multiprotein complex centered on TRPC6 channels. J Biol Chem 280:32035–32047 [DOI] [PubMed] [Google Scholar]
- Zhang L, Guo F, Kim JY, Saffen D 2006 Muscarinic acetylcholine receptors activate TRPC6 channels in PC12D cells via Ca2+ store-independent mechanisms. J Biochem 139:459–470 [DOI] [PubMed] [Google Scholar]
- Caraveo G, van Rossum DB, Patterson RL, Snyder SH, Desiderio S 2006 Action of TFII-I outside the nucleus as an inhibitor of agonist-induced calcium entry. Science 314:122–125 [DOI] [PubMed] [Google Scholar]
- Leuner K, Kazanski V, Müller M, Essin K, Henke B, Gollasch M, Harteneck C, Müller WE 2007 Hyperforin: a key constituent of St. John's wort specifically activates TRPC6 channels. FASEB J 21:4101–4111 [DOI] [PubMed] [Google Scholar]
- Suzuki F, Morishima S, Tanaka T, Muramatsu I 2007 Snapin, a new regulator of receptor signaling, augments α1A-adrenoceptor-operated calcium influx through TRPC6. J Biol Chem 282:29563–29573 [DOI] [PubMed] [Google Scholar]
- Tesfai Y, Brereton HM, Barritt GJ 2001 A diacylglycerol-activated Ca2+ channel in PC12 cells (an adrenal chromaffin cell line) correlates with expression of the TRP-6 (transient receptor potential) protein. Biochem J 358:717–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Neeb ZP, Sturek MS, Obukhov AG 2008 Expression level of canonical transient receptor potential (TRPC) channels is increased in the adrenal medulla of Ossabaw miniature pigs manifesting the metabolic syndrome. FASEB J 22:1201.14 (Abstract) [Google Scholar]
- Beato M 1989 Gene regulation by steroid hormones. Cell 56:335–344 [DOI] [PubMed] [Google Scholar]
- Mick VE, Itani OA, Loftus RW, Husted RF, Schmidt TJ, Thomas CP 2001 The α-subunit of the epithelial sodium channel is an aldosterone-induced transcript in mammalian collecting ducts, and this transcriptional response is mediated via distinct cis-elements in the 5′-flanking region of the gene. Mol Endocrinol 15:575–588 [DOI] [PubMed] [Google Scholar]
- Lu NZ, Wardell SE, Burnstein KL, Defranco D, Fuller PJ, Giguere V, Hochberg RB, McKay L, Renoir JM, Weigel NL, Wilson EM, McDonnell DP, Cidlowski JA 2006 International Union of Pharmacology. LXV. The pharmacology and classification of the nuclear receptor superfamily: glucocorticoid, mineralocorticoid, progesterone, and androgen receptors. Pharmacol Rev 58:782–797 [DOI] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA 2008 Guide to receptors and channels (GRAC). 3rd ed. Br J Pharmacol 153(Suppl 2):S1–S209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams M, Meijer OC, Wang J, Bhargava A, Pearce D 2003 Homodimerization of the glucocorticoid receptor is not essential for response element binding: activation of the phenylethanolamine N-methyltransferase gene by dimerization-defective mutants. Mol Endocrinol 17:2583–2592 [DOI] [PubMed] [Google Scholar]
- Schug J 2008 Using TESS to predict transcription factor binding sites in DNA sequence. Curr Protoc Bioinformatics Chapter 2:Unit 2.6.1–2.6.15 [DOI] [PubMed] [Google Scholar]
- Chan GC, Hess P, Meenakshi T, Carlstedt-Duke J, Gustafsson JA, Payvar F 1991 Delayed secondary glucocorticoid response elements. Unusual nucleotide motifs specify glucocorticoid receptor binding to transcribed regions of α2u-globulin DNA. J Biol Chem 266:22634–22644 [PubMed] [Google Scholar]
- Schaefer M, Plant TD, Obukhov AG, Hofmann T, Gudermann T, Schultz G 2000 Receptor-mediated regulation of the nonselective cation channels TRPC4 and TRPC5. J Biol Chem 275:17517–17526 [DOI] [PubMed] [Google Scholar]
- Lewis RS 2007 The molecular choreography of a store-operated calcium channel. Nature 446: 284–287 [DOI] [PubMed] [Google Scholar]
- Dyer AR, Persky V, Stamler J, Paul O, Shekelle RB, Berkson DM, Lepper M, Schoenberger JA, Lindberg HA 1980 Heart rate as a prognostic factor for coronary heart disease and mortality: findings in three Chicago epidemiologic studies. Am J Epidemiol 112:736–749 [DOI] [PubMed] [Google Scholar]
- Liu D, Maier A, Scholze A, Rauch U, Boltzen U, Zhao Z, Zhu Z, Tepel M 2008 High glucose enhances transient receptor potential channel canonical type 6-dependent calcium influx in human platelets via phosphatidylinositol 3-kinase-dependent pathway. Arterioscler Thromb Vasc Biol 28:746–751 [DOI] [PubMed] [Google Scholar]
- Bae YM, Kim A, Lee YJ, Lim W, Noh YH, Kim EJ, Kim J, Kim TK, Park SW, Kim B, Cho SI, Kim DK, Ho WK 2007 Enhancement of receptor-operated cation current and TRPC6 expression in arterial smooth muscle cells of deoxycorticosterone acetate-salt hypertensive rats. J Hypertens 25:809–817 [DOI] [PubMed] [Google Scholar]
- Sarzani R, Salvi F, Dessì-Fulgheri P, Rappelli A 2008 Renin-angiotensin system, natriuretic peptides, obesity, metabolic syndrome, and hypertension: an integrated view in humans. J Hypertens 26:831–843 [DOI] [PubMed] [Google Scholar]
- Krug AW, Ehrhart-Bornstein M 2008 Aldosterone and metabolic syndrome: is increased aldosterone in metabolic syndrome patients an additional risk factor? Hypertension 51:1252–1258 [DOI] [PubMed] [Google Scholar]
- Alloosh M, Pratt JH, Sturek M, Basile D 2008 Elevated renin and enhanced adrenal steroidogenesis in the Ossabaw swine model of the metabolic syndrome. FASEB J 22:736.7 (Abstract) [Google Scholar]
- John S, Sabo PJ, Johnson TA, Sung MH, Biddie SC, Lightman SL, Voss TC, Davis SR, Meltzer PS, Stamatoyannopoulos JA, Hager GL 2008 Interaction of the glucocorticoid receptor with the chromatin landscape. Mol Cell 29:611–624 [DOI] [PubMed] [Google Scholar]
- Leo C, Chen JD 2000 The SRC family of nuclear receptor coactivators. Gene 245:1–11 [DOI] [PubMed] [Google Scholar]
- Wang Q, Anzick S, Richter WF, Meltzer P, Simons Jr SS 2004 Modulation of transcriptional sensitivity of mineralocorticoid and estrogen receptors. J Steroid Biochem Mol Biol 91:197–210 [DOI] [PubMed] [Google Scholar]
- Sturek M, Alloosh M, Wenzel J, Byrd JP, Edwards JM, Lloyd PG, Tune JD, March KL, Miller MA, Mokelke EA and Brisbin Jr IL 2007 Ossabaw Island miniature swine: cardiometabolic syndrome assessment. In: Swindle MM, ed. Swine in the laboratory: surgery, anesthesia, imaging, and experimental techniques. Boca Raton, FL: CRC Press; 397–402 [Google Scholar]
- Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S 2005 Adipokines: molecular links between obesity and atherosclerosis. Am J Physiol Heart Circ Physiol 288:H2031–H2041 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.