Abstract
Nuclear receptor (NR) transactivation involves multiple coactivators, and the molecular basis for how these are functionally integrated needs to be determined to fully understand the NR action. Activating signal cointegrator-2 (ASC-2), a transcriptional coactivator of many NRs and transcription factors, forms a steady-state complex, ASCOM (for ASC-2 complex), which contains histone H3-lysine-4 (H3K4) methyltransferase MLL3 or its paralog MLL4. Here, we show that ASCOM requires a functional cross talk with the ATPase-dependent chromatin remodeling complex Swi/Snf for efficient NR transactivation. Our results reveal that ASCOM and Swi/Snf are tightly colocalized in the nucleus and that ASCOM and Swi/Snf promote each other’s binding to NR target genes. We further show that the C-terminal SET domain of MLL3 and MLL4 directly interacts with INI1, an integral subunit of Swi/Snf. Our mutational analysis demonstrates that this interaction underlies the mutual facilitation of ASCOM and Swi/Snf recruitment to NR target genes. Importantly, this study uncovers a specific protein-protein interaction as a novel venue to couple two distinct enzymatic coactivator complexes during NR transactivation.
Specific protein-protein interactions functionally connect two enzymatic entities in nuclear receptor transactivation, histone methyltransferase ASCOM and the ATPase-dependent chromatin remodeler Swi/Snf.
Nuclear receptors (NRs) control transcription of their target genes in a ligand-dependent manner by binding DNA sequences named hormone response elements (1). Notably, NR transactivation involves a numerous number of coactivators (2). However, how these coactivators are functionally integrated remains poorly understood.
Functional analysis of NRs has shown that their ligand-binding domain (LBD) exhibits ligand-dependent transactivation function (AF2). The highly conserved AF-2 core region (1), located at the extreme C terminus of the LBDs, mediates NR transactivation by interacting with coactivators in a ligand- induced manner (2). These AF2-dependent coactivators have LxxLL signature motifs named NR boxes, which directly recognize the ligand-induced structural changes around the AF2 core region (helix 12) (2).
ASC-2 (also named AIB3, TRBP, RAP250, NRC, PRIP, and NCOA6) is a multifunctional transcriptional coactivator with two NR boxes (3). The second NR box specifically interacts with the liver X receptors, and the first NR box binds multiple NRs, which include retinoic acid (RA) receptor (RAR) and glucocorticoid receptor (GR) (4). ASC-2 also recruits androgen receptor (AR) via the tumor suppressor retinoblastoma (5). In support of the functionality of these interactions, ASC-2 has been shown to serve as a coactivator of RAR, AR, liver X receptors, and other ASC-2-interacting NRs (4,5). In addition, ASC-2 has been demonstrated to function as a coactivator of multiple other transcription factors (3).
H3K4 trimethylation is tightly associated with promoters and early transcribed regions of active genes (6,7). Enzymes for H3K4 methylation include Set1, MLL1, MLL2, MLL3, MLL4, Ash1, and Set7/9 (8). The C termini of MLLs and Set1 contain a SET domain (8), which is associated with a histone lysine-specific methyltransferase activity. MLLs and Set1 form similar complexes, collectively named Set1-like complexes (8). ASC-2 is a component of a Set1-like H3K4-methyltransferase complex that we named ASCOM, which contains MLL3 or MLL4 (9,10,11,12). Interestingly, ASCOM has recently been shown to contain additional subunits (11,12), including a H3K27-demethyase UTX (13,14,15,16). These results suggest that ASCOM has two distinct histone modifiers linked to transcriptional activation, because H3K4 methylation counters the repressive chromatin environment imposed by H3-K9/K27 methylation (17).
Several chromatin remodeling complexes containing a member of the Swi1/Snf2 family of nuclear ATPases carry out structural modifications of chromatin (18). BRG1 and BRM, the mammalian homologs of yeast Swi2 (18), are central to the function of these Swi/Snf complexes. They are also required for transactivation by many NRs, including estrogen receptor (19,20,21,22), AR (23), GR (19,24,25,26,27,28,29), and RAR (30). Notably, mechanisms through which NRs recruit Swi/Snf to their target promoters include direct interactions with one or more components of Swi/Snf; e.g. BRM (20), BRG1 (20,22), and BAF57 (21) for estrogen receptor, BAF250 (25) or BAF60a (31) for GR, and BAF60c for peroxisome proliferator-activated receptor-γ and other NRs (32).
ASC-2 has been shown to bind to DRIP130, a component of the DRIP/TRAP/ARC complex, which links NRs and the RNA-PolII transcription machinery (33). ASC-2 has also been shown to associate with histone acetyltransferases CBP and p300 (3,33,34). Consistent with the latter results, we found that both H3K4 trimethylation and H3/H4 acetylation of a RAR target gene RAR-β2 are lost in ASC-2-null cells (10). These results raise an interesting possibility that ASCOM, through protein-protein interactions, may serve as a novel platform to dynamically integrate the function of multiple coactivators during NR transactivation.
In further support of this idea, here we provide evidence indicating that NR transactivation by ASCOM involves an unexpected cross talk with Swi/Snf. Our results further demonstrate that this interplay appears to function through mutually facilitated recruitment of ASCOM and Swi/Snf to NR target genes. Importantly, this cross talk is enabled by specific interactions between MLL3/4-SET and INI1, a core subunit of Swi/Snf (18). Overall, our results raise an interesting possibility that direct protein-protein interactions may have general importance in integrating the activity of multiple coactivators for successful transactivation.
Results
Association of Swi/Snf and ASCOM in the nucleus
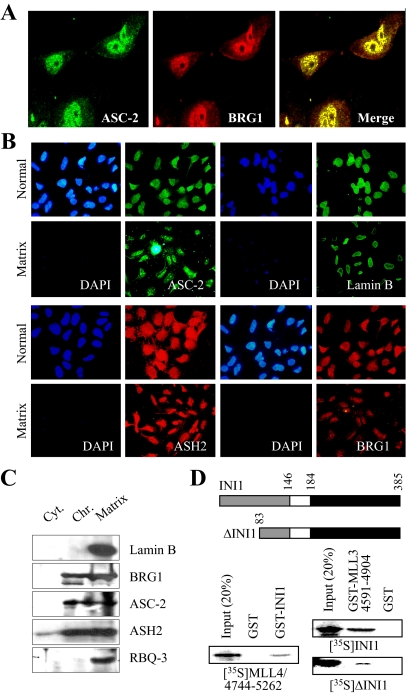
From our effort to uncover any possible cross talk of ASCOM with other coactivators, we found that ASC-2 and BRG1 are tightly colocalized in HeLa nuclei, as shown by indirect immunofluorescence confocal microscopy (Fig. 1A). Notably, Swi/Snf has been reported to be enriched in the nuclear matrix (35). The nuclear matrix, defined as the nuclear structure that remains after the salt extraction of nuclease-treated nuclei, consists of a peripheral lamina-pore complex and an internal filamentous ribonucleoprotein network that has not been well defined (36). Intriguingly, nuclease fractionation studies have demonstrated that transcriptionally active DNA is tightly associated with the nuclear matrix (36). Thus, we further examined whether ASC-2 is colocalized in the nuclear matrix using antibodies against ASC-2, BRG1, ASH2, RBQ-3, and lamin B, one of the major components of the nuclear matrix (36). When we analyzed the nuclear material remaining after in situ sequential deoxyribonuclease (DNase) I and high salt extraction (35), all the labeling patterns were similar before and after extraction, although the intensity of the signal decreased in extracted nuclei (Fig. 1B and data not shown). As expected, the 4′,6-diamidino-2-phenylindole signal disappeared completely in the nuclear matrix preparations (Fig. 1B). These data suggest that ASCOM is at least in part colocalized with Swi/Snf in the nuclear matrix. To directly test this idea, we isolated the nuclear matrix by the previously described sequential extraction procedure (35). In the first fractionation step, soluble cytoplasmic proteins were removed by extraction with Triton X-100. Chromatin proteins were then released by DNase I digestion and extraction with 0.25 m ammonium sulfate. 95% of the histones were extracted in this step (data not shown). After washing with 2 m NaCl, the last fraction consisted of structural nuclear matrix proteins and nuclear matrix-associated proteins. We analyzed these three distinct preparations by SDS-PAGE and immunoblotting. As shown in Fig. 1C, about 50% of the BRG1 was released with the chromatin proteins, whereas the remainder was tightly associated with lamin B, as previously described (35). In further support of the immunofluorescence results (Fig. 1B), BRG1 as well as three components of ASCOM (i.e. ASC-2, ASH2, and RBQ-3) were associated with the lamin B-enriched nuclear matrix (Fig. 1C). Taken together, we concluded that ASCOM and Swi/Snf are highly colocalized in the nucleus, and this colocalization appears to occur at least in part in the nuclear matrix.
Figure 1.
Association of ASCOM and Swi/Snf in the nuclear matrix. A, ASC-2 and BRG1 are highly colocalized in the nucleus. B, Like the nuclear matrix protein lamin B, ASC-2, ASH2, and BRG1 were found associated with the nuclear matrix. Similar results were obtained for RBQ-3, another component of ASCOM (data not shown). C, Fractionations of nuclear extracts into three parts revealed that lamin B, BRG1, ASC-2, ASH2, and RBQ-3 are enriched in the nuclear matrix. D, Schematic representation of wild-type INI1 and ΔINI1 is as shown. GST alone or GST fusion to INI1 or MLL3–4591-4904 were tested for binding 35S-labeled, in vitro-translated MLL4-4744-5262, INI, or ΔINI1, as indicated.
Interactions of MLL3/4-SET and INI1
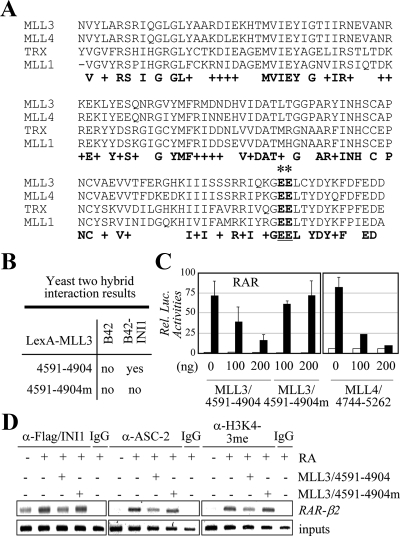
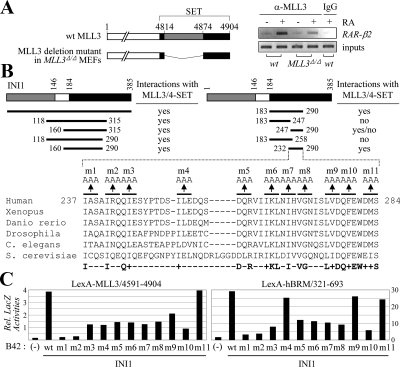
ASCOM and Swi/Snf are two distinct complexes and thus not expected to stably associate with each other in solution. Indeed, the purifications of ASCOM did not include any subunit of Swi/Snf (9,11,12). However, the colocalization in the nucleus and/or the assembly of these two complexes in common target DNA sites may still involve direct interactions between their subunits. Interestingly, the C-terminal SET domain of MLL1 has been reported to interact with INI1, a core component of the Swi/Snf complexes (37). The sequence analysis revealed that this MLL1 domain is well conserved in both MLL3 and MLL4; i.e. about 95% homology between MLL3 and MLL4 and about 67% homology between MLL3 and MLL1 (see Fig. 5A). Thus, we tested whether INI1 also interacts with MLL3/MLL4. Glutathione S-transferase (GST) fusion to full-length INI1 strongly bound 35S-labeled, in vitro-translated MLL4 fragment encompassing the SET domain (MLL4 residues 4744-5262) (Fig. 1D). GST fusion to MLL3-SET (MLL3 residues 4591-4904) also bound 35S-labeled, in vitro-translated INI1 as well as ΔINI1, an INI1 deletion mutant containing INI1 residues 83-385 (Fig. 1D). Notably, these in vitro interactions were unusually stable; i.e. robust binding in 200 mm NaCl and washing in 600 mm NaCl. Although they are apparently not sufficient to link ASCOM and Swi/Snf in solution stably, these interactions may play pivotal roles in colocalizing ASCOM and Swi/Snf in the nucleus and/or in functionally integrating both complexes onto NR target genes during NR transactivation.
Figure 5.
Suppression of RAR transactivation by MLL3/4-SET involves their interactions with INI. A, The SET domains of MLL3, MLL4, TRX, and MLL1 are as shown. Asterisks indicate two residues originally described in TRX to be involved with recognizing INI1 (47). B, The yeast two-hybrid results for LexA-MLL3/4591-4904 and LexA-MLL3/4591-4904m and B42 alone and B42-INI1. C, The impact of MLL3/4591-4904, MLL3/4591-4904m, and MLL4/4744-5262 on RAR transactivation was measured using RARE-LUC reporter in HeLa cells. D, HEK293 cells transfected with Flag-INI1, either without or with MLL3/4591-4904 or MLL3/4591-4904m, were subjected to ChIP analyses for the recruitment of INI1 and ASC-2 to RAR-β2 as well as for H3K4 trimethylation of RAR-β2. Before ChIP, cells were treated with 0.1 μm RA for 15 min (for INI1 and ASC-2) or 1 h (for H3K4 trimethylation).
Swi/Snf enables the autonomous transactivation and NR-coactivator functions of ASC-2
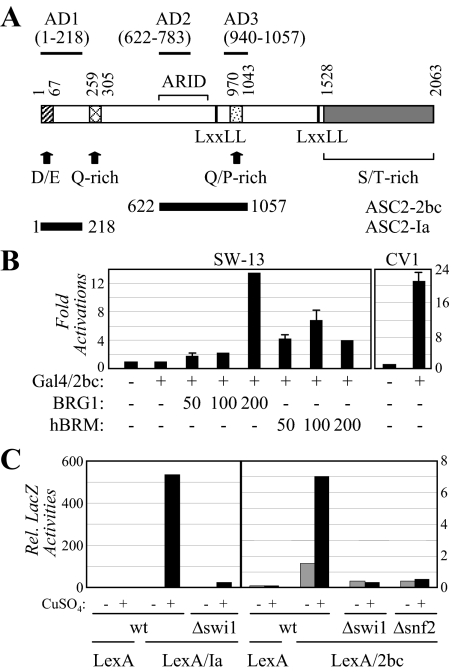
We have previously reported three separate autonomous transactivation domains: AD1, AD2, and AD3, which consist of human ASC-2 residues 1-218, 622-849, and 849-1057, respectively (3). Mahajan and Samuels (34) have also mapped two autonomous transactivation domains to ASC-2 residues 302-783 and 940-1124. Thus, the boundaries of AD2 and AD3 are likely to be refined to ASC-2 residues 622-783 and 940-1057, respectively (Fig. 2A). Interestingly, the potent activity of AD2/3 in CV1 and other cells (Fig. 2B and data not shown) was ablated in SW13 cells, which lack both BRG1 and BRM (38), unless BRG1 or BRM was reexpressed (Fig. 2B). Similarly, both AD1 and AD2/3 (i.e. ASC2-Ia and ASC2-2bc in Fig. 2A) were also inactive in mutant yeast strains defective for the yeast Swi/Snf subunits Swi1 and Snf2 (39) (Fig. 2C). These data suggest that the autonomous transactivation function of ASC-2 requires Swi/Snf in both mammalian and yeast cells.
Figure 2.
Swi/Snf is required for the autonomous transactivation function of ASC-2. A, Schematic representation of ASC-2 and its deletion constructs as well as the three autonomous transactivation domains (AD1-3). B, The autonomous transactivation by Gal4 fusion to ASC2-2bc was measured in SW13 and CV1 cells in the absence and presence of an increasing amount of BRG1 or hBRM. C, Wild-type and mutant yeast strains were transformed with pCL313, pCL313 (or 314)-ASC2-Ia, or pCL313 (or 314)-ASC2-2bc plasmids, along with pSH18-34 reporter plasmid, using available selection markers, and measured for the LacZ activity using liquid β-galactosidase assays.
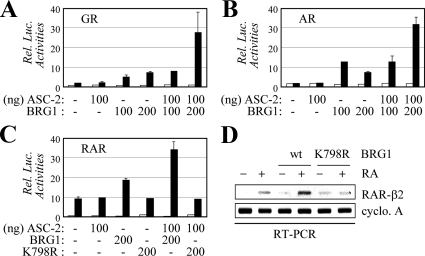
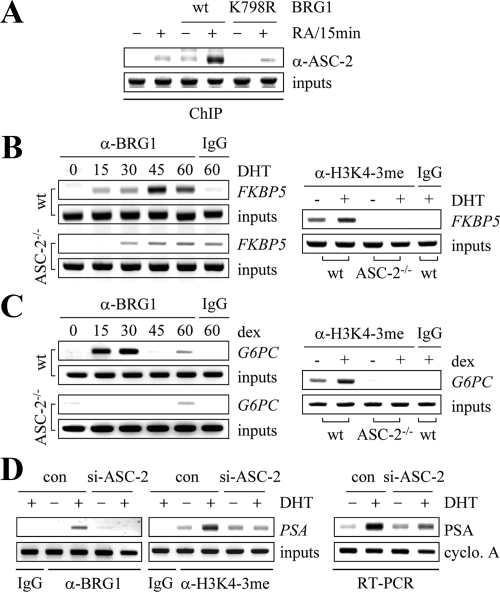
Samuels and colleagues (40) have recently proposed that AD3 is essential for NR signaling, based on their observation that RA-dependent transactivation by endogenous RAR is transduced to Gal4 fusion to AD3 but not AD2. These results, coupled with the requirement for Swi/Snf in AD3 function (Fig. 2, B and C), raise the possibility that ASC-2 may also require Swi/Snf to mediate NR transactivation. In support of this idea, GR and AR transactivation was not observed in SW13 cells without coexpressed BRG1, and moreover BRG1 was required for ASC-2 to enhance GR and AR transactivation (Fig. 3, A and B). Interestingly, RAR transactivation was still observed in SW13 cells, although it was not as potent as in other cells with intact Swi/Snf (Fig. 3C). These results suggest that the recruitment of Swi/Snf to RAR may not be a rate-limiting step for RAR transactivation and/or other chromatin remodelers may act redundantly for RAR in SW13 cells. Importantly, although BRG1 was also required for ASC-2 to further enhance RAR transactivation, BRG1-K798R, a mutant BRG1 defective in its ATPase activity lacked this effect (Fig. 3C). Similarly, AR transactivation was not rescued by this mutant BRG1 in SW13 cells (data not shown).
Figure 3.
Swi/Snf enables the NR-coactivator function of ASCOM. A–C, SW13 cells were transfected with vectors for GR, AR, RAR, BRG1, and BRG1-K798R, treated with either vehicle alone or 0.1 μm ligand for each NR, and measured for the luciferase activity driven by the cotransfected reporters, as indicated. D, SW13 cells were treated with 0.1 μm RA for 12 h and subjected to RT-PCR using the primer set for RAR-β2 mRNA.
To test whether intact BRG1 is indeed required for transactivation of endogenous RAR target genes, we examined the level of RAR-β2 mRNA in SW13 cells expressing wild-type BRG1 or BRG1-K798R using RT-PCR. Consistent with the transfected reporter gene results (Fig. 3C), RA-induced expression of RAR-β2 was significantly enhanced by wild-type BRG1, but not by BRG1-K798R (Fig. 3D). These results suggest that the ATPase activity of Swi/Snf is clearly required for ASCOM to mediate NR transactivation. Overall, these results reveal a novel cross talk between ASCOM and Swi/Snf during NR transactivation.
BRG1 facilitates the recruitment of ASC-2 to NRs and vice versa
To elucidate the molecular basis for the functional dependence of ASCOM on Swi/Snf during NR transactivation, we carried out chromatin immunoprecipitation (ChIP) assays. In SW13 cells, ASC-2 was relatively weekly associated with the RAR-β2 promoter region even in the presence of RA (Fig. 4A). Surprisingly, this recruitment was clearly enhanced by reexpressed wild-type BRG1, whereas it was not affected by the ATPase-defective mutant BRG1 (Fig. 4A). These results are nicely correlated with the luciferase reporter assays for RAR transactivation and RT-PCR results for RAR-β2 mRNA (Fig. 3, C and D). Importantly, these results demonstrate that Swi/Snf facilitates the recruitment of ASC-2/ASCOM to RAR target genes. We also noted that expression of BRG1 in SW13 cells triggered relatively weak recruitment of ASC-2 to RAR-β2 even in the absence of RA (Fig. 4A). These results raise an intriguing possibility that Swi/Snf may elicit an ability of ASCOM to weakly bind to unliganded RAR, thereby getting ASC-2 ready to strongly bind to RAR when RA arrives. Overall, these results suggest that intact Swi/Snf is required to facilitate NRs to recruit ASCOM.
Figure 4.
ASCOM and Swi/Snf facilitate each other’s recruitment to NR target genes. A, SW13 cells were transfected with vector alone or vectors encoding wild-type BRG1 or ATPase-defective mutant BRG1-K798R, treated with 0.1 μm RA for 15 min, and subjected to ChIP analyses using anti-ASC-2 antibody and the primer set for the RAR response elements in RAR-β2. B and C, Wild-type and ASC-2-null cell lines derived from MEFs were treated with 0.1 μm dihydrotestosterone (DHT) or dexamethasone (dex) for the indicated time (0–60 min) and subjected to ChIP analyses using anti-BRG1 antibody and the primer sets for the AR response elements in FKBP5 and for the GR response elements in G6PC. Cells were also treated with 0.1 μm DHT or dex for 2 h and subjected to ChIP analyses using antibody against trimethylated H3K4. D, LNCaP cells expressing control siRNA or siASC-2 (10) were treated with 0.1 μm DHT and subjected to ChIP analyses using anti-BRG1 (after 45 min) or antibody against trimethylated H3K4 (after 2 h) and the primer sets for the AR response elements in PSA. Cells were also treated with 0.1 μm DHT for 12 h and subjected to RT-PCR using the primer set for PSA mRNA.
Isw1p ATPase has been shown to display higher affinity to chromatin when it is di- and trimethylated at H3K4 (41). Thus, we considered a possibility that recruitment of Swi/Snf to NR target genes is also facilitated by their ASCOM-mediated H3K4 methylation. Consistent with the identification of BRG1 and other Swi/Snf subunits in a novel nuclear receptor corepressor complex (42), BRG1 is expected to bind both unliganded and liganded RARs. Thus, we first tested this issue using the known target genes for AR and GR, which may provide clearer results because they lack the basal repression function and hence should not recruit Swi/Snf in the absence of ligand. As revealed in our ChIP results using a cell line derived from wild-type mouse embryo fibroblasts (MEFs) (Fig. 4B), BRG1 indeed occupied the previously described AR-responsive element in FKBP5 (43) only in the presence of ligand. Interestingly, BRG1 was recruited to this element in a time-dependent manner with a peak binding at 45 min after ligand treatment. Although this binding pattern was preserved, the binding affinity was significantly attenuated in ASC-2-null MEF cell line (Fig. 4B). Importantly, these results were nicely correlated with the impaired H3K4 trimethylation of the FKBP5 AR-responsive element in ASC-2-null MEF cell line (Fig. 4B, right). We obtained similar results for the glucocorticoid response elements in G6PC (44) (Fig. 4C). These results demonstrate that ASC-2 facilitates NRs to recruit Swi/Snf. However, it remains to be determined whether the binding affinity of Swi/Snf to NR target genes is indeed enhanced directly through ASCOM-mediated H3K4 trimethylation, because the observed correlation between H3K4 trimethylation and BRG1 recruitment to AR/GR target genes could just be a secondary event and ASCOM may use other mechanisms to facilitate the recruitment of Swi/Snf to NR target genes.
Interestingly, despite the successful ligand-dependent recruitment of BRG1 to FKBP5/G6PC and their H3K4 trimethylation (Fig. 4, B and C), our RT-PCR results revealed that ligand-dependent expression of FKBP5 and G6PC was not recapitulated even in wild-type MEF cell line (data not shown). It is possible that although the coordinated recruitment of ASCOM and Swi/Snf to NR response elements in FKBP5/G6PC occurs in these cells, they could have defects in the subsequent events for successful transcription of FKBP5/G6PC such as other essential transcription factors and coactivators. Thus, to further test whether Swi/Snf requires ASC-2 for optimal occupancy of NR target genes, we used a functionally relevant system for testing AR transactivation, i.e. ligand-dependent expression of AR target gene PSA in LNCaP human prostate cancer cells (45). Our RT-PCR experiments revealed that PSA is indeed induced in expression by ligand in LNCaP cells expressing control small interfering RNA (siRNA) (Fig. 4D). In contrast, expression of PSA was significantly attenuated in LNCaP cells expressing siRNA against ASC-2 (10) (Fig. 4D). Importantly, these results corresponded to reduced level of not only H3K4 trimethylation of the PSA AR-responsive element but also its recruitment of BRG1 (Fig. 4D). These data, along with the results in MEF cell lines (Fig. 4, B and C), indicate that ASC-2 facilitates NRs to recruit Swi/Snf. Our results also suggest that ASCOM is likely to be responsible for ligand-dependent H3K4 trimethylation of AR and GR target genes.
Taken together, our results show that AR, GR, and RAR transactivation appears to involve an unexpected cross talk between ASCOM and Swi/Snf, which functions at least in part through mutual facilitation of ASCOM and Swi/Snf recruitment to AR/GR/RAR target genes. Notably, this cross talk may also extend to other members of the NR superfamily.
Critical roles for the MLL3/4-INI1 interactions in the dominant-negative action of MLL3/4-SET in RAR transactivation
Our results indicate that NR transactivation involves a cross talk between ASCOM and Swi/Snf. However, the molecular basis by which these two complexes communicate with each other during NR transactivation is unclear. Based on the strong interactions between MLL3/4-SET with INI1 (Fig. 1D), we reasoned that these interactions may play essential roles in NR transactivation by physically linking ASCOM and Swi/Snf and that this linkage could be responsible for the observed mutual facilitation of these two complexes in recruitment to NR target genes (Fig. 4). To test this idea, we turned to our previous finding that a MLL3 fragment containing the SET domain (Fig. 5A) is a potent inhibitor of RAR transactivation in HeLa cells (9). Interestingly, mutation of two neighboring glutamic acids of TRX to alanines, which are also conserved in MLL3/4 and MLL1 (Fig. 5A), has been reported to abolish the interactions of TRX with INI1 (46). Thus, we introduced these mutations into MLL3/4591-4904 (i.e. MLL3/4591-4904 with E4873A and E4874A mutations; MLL3/4591-4904m in Fig. 5, B–D). Our yeast two-hybrid tests with INI1 fused to the transactivation domain B42 revealed that INI1 indeed interacts with LexA fusion to MLL3/4591-4904 but not with LexA-MLL3/4591-4904m (Fig. 5B). Importantly, the inhibitory activity of MLL3/4591-4904 on RAR transactivation (9) was no longer observed with MLL3/4591-4904m (Fig. 5C), although the nuclear localization of INI1 was intact in the presence of MLL3/4591-4904m and the expression level of MLL3/4591-4904m was comparable to that of MLL3/4591-4904 (data not shown). The dominant-negative function of MLL3-SET appears to be also conserved in MLL4-SET, because RAR transactivation was suppressed by a similar MLL4 construct (MLL4 residues 4744-5262) (Fig. 5C). These results suggest that the dominant-negative activity of MLL3/4-SET in RAR transactivation (9) requires their ability to interact with INI1.
To test whether the dominant-negative activity of MLL3/4-SET involves any impact on the observed mutual facilitation of ASCOM and Swi/Snf in recruitment to NR target genes (Fig. 4), we carried out ChIP experiments. Consistent with the involvement of Swi/Snf in the basal repression of RAR transactivation (42), INI1 was recruited to RAR-β2 in the absence of RA but this recruitment was further enhanced by RA (Fig. 5D). Strikingly, the RA-enhanced recruitment of INI1 to RAR-β2 was blocked by MLL3/4591-4904 but not by MLL3/4591-4904m (Fig. 5D). Recruitment of ASC-2 to RAR-β2 and its H3K4 trimethylation were also impaired by MLL3/4591-4904 but not by MLL3/4591-4904m (Fig. 5D).
Taken together, these results suggest that the molecular mechanisms for the dominant-negative function of MLL3/4-SET (9) are likely to involve their ability to not only compete with wild-type MLL3/4 for binding INI1 but also disrupt the mutually facilitated recruitment of ASCOM and Swi/Snf to NR target genes.
INI1 requires its interactions with MLL3/4 to support the ASCOM-Swi/Snf cross talk during RAR transactivation
We have reported MLL3Δ/Δ mice expressing an in-frame deletion mutant of MLL3 (10). The mutant MLL3 protein was comparably expressed as wild-type MLL3 protein and incorporated into ASCOM (10). Because the deletion spans a core region of the MLL3-SET domain including the highly conserved E4873 and E4874 residues (Fig. 5A), the mutant MLL3 is expected to lack the interactions with INI1. Interestingly, we have found that RA-dependent recruitment of MLL3 to RAR-β2 is significantly attenuated in MLL3Δ/Δ MEF cell line when compared with the wild-type MEF cell line (Fig. 6A), despite the finding that MLL3 proteins are equally expressed in these two cell types (data not shown). These results are consistent with the crucial roles of MLL3/4-SET-INI1 interactions in facilitating the recruitment of ASCOM to NR target genes.
Figure 6.
Roles for the MLL3/4-SET-INI1 interactions in the cross talk between ASCOM and Swi/Snf. A, Schematic representation of wild-type and a deletion mutant of MLL3 expressed in MLL3Δ/Δ MEF cell line (10). Wild-type and MLL3Δ/Δ MEF cell lines were subjected to ChIP analyses for the recruitment of MLL3 proteins to RAR-β2. Before ChIP, cells were treated with 0.1 μm RA for 15 min. B, Schematic representation of INI1 deletion and alanine scanning mutants. The yeast two-hybrid results with the INI1 deletion mutants fused to B42 and LexA-MLL3/4591-4904 are as indicated. C, The alanine scanning mutants in full-length INI1 fused to B42 were tested for interactions with LexA-MLL3/4591-4904 and LexA-hBRM/321-693 in yeast, as determined by liquid β-galactosidase assays. B42 alone and B42-INI1 wild type were used as controls.
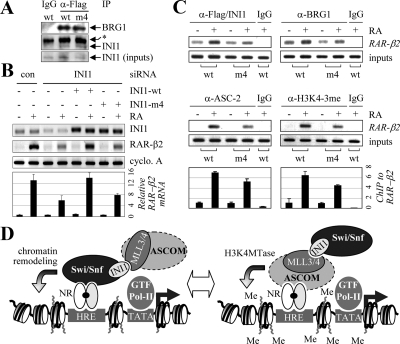
To further establish the importance of MLL3/4-SET-INI1 interactions in NR transactivation, we decided to identify specific INI1 mutants that incorporate into Swi/Snf by maintaining the previously described interactions with BRG1 and BRM (47,48) but no longer bind to MLL3/4-SET and examine whether these mutants fail to support NR transactivation fully. To map the INI1 region that is responsible for interacting with the MLL3/4-SET domain, we constructed a series of nine deletion mutants (Fig. 6B). Our yeast two-hybrid tests with these deletion mutants fused to B42 revealed that a fragment containing the INI1 residues 232-290 is sufficient to interact with LexA-MLL3/4591-4904 (Fig. 6B). Using a similar approach, we also found that the previously described interactions of hBRM and INI1 (47) are mapped to hBRM residues 321-693 (data not shown). Next, to precisely identify INI1 residues underlying the MLL3/4-INI1 interactions, we introduced a series of 11 alanine scanning mutations into the mapped MLL3/4-interacting region of INI1 in the context of full-length INI1 fused to B42 (Fig. 6B). Most mutants with impaired interactions with LexA-MLL3/4591-4904 showed reduced interactions with LexA-hBRM/321-393 (Fig. 6C). However, the mutant #4 displayed only approximately 30% of the interactions observed with B42 fused to wild-type INI1 and LexA-MLL3/4591-4904, whereas it effectively interacted with LexA-hBRM/321-693 (Fig. 6C). In addition, the mutant #4 was relatively intact in the previously described interactions between INI1 and c-Myc (49) (data not shown). Thus, the mutant #4 represents a specific INI1 mutant that appears to lack the interactions with the MLL3/4-SET domain although preserving other interactions in INI1. Consistent with these results, this full-length #4 INI1 mutant was properly localized to the nucleus (data not shown) and incorporated into Swi/Snf, as demonstrated by its ability to co-immunopurify with BRG1 from HEK293 cells (Fig. 7A). Before we tested the ability of this #4 INI1 mutant on RAR transactivation, we first tested the effect of a specific siRNA construct against INI1 (48) on RA-dependent expression of RAR-β2. siINI1 suppressed expression of RAR-β2 significantly (Fig. 7B). Interestingly, this suppressed expression of RAR-β2 was restored by wild-type INI1 but not by the full-length #4 INI1 mutant. The expression level of the INI1 mutant #4 was comparable to that of wild-type INI1, as determined by RT-PCR and Western blotting analysis (Fig. 7B and data not shown). Strikingly, our ChIP results revealed that the INI1 mutant #4 is not as responsive to RA as wild-type INI1 in recruitment to RAR-β2 (Fig. 7C). In addition, the occupancy of RAR-β2 by BRG1 and ASC-2 as well as the level of H3K4 trimethylation of RAR-β2 were significantly impaired in the presence of the INI1 mutant #4 in comparison with wild-type INI1. Thus, the inability of the INI1 mutant #4 to rescue the impaired expression of RAR-β2 by siINI1 (Fig. 7B) is nicely correlated with not only a reduction in recruitment of Swi/Snf and ASCOM to RAR-β2 but also a decreased level of H3K4 trimethylation of RAR-β2. We also obtained similar results for another RAR target gene, HoxA1 (14,15,16) (data not shown). These results clearly demonstrate that disruption of the MLL3/4-INI1 interactions results in impaired NR transactivation through interfering with efficient recruitment of ASCOM and Swi/Snf to NR target genes. Our semiquantitative RT-PCR and ChIP assays have been highly reproducible, as demonstrated by quantitative RT-PCR experiments (e.g. Fig. 7, B and C, lower panels).
Figure 7.
Importance of the MLL3/4-SET-INI1-interactions in the ASCOM-Swi/Snf cross talk supported by a specific, full-length INI1 mutant. A, Co-immunoprecipitation of BRG1 and both wild-type INI1 and the full-length INI1 mutant #4, as demonstrated using HEK293 cells expressing siINI1 and siINI1-resistant Flag-tagged wild-type or the full-length INI1 mutant #4. B, The effect of si-INI1-resistant Flag-INI1 wild-type and mutant #4 on RAR transactivation was determined by RT-PCR analyses for RA-induced expression of RAR-β2 mRNAs in HEK293 cells expressing control or anti-INI1 siRNA (39). Before RT-PCR, cells were treated with vehicle or 0.1 μm RA for 12 h. Shown below is quantitation of RAR-β2 mRNA expression by quantitative RT-PCR. C, The cells in B were also subjected to ChIP analyses for the recruitment of INI1, BRG1, and ASC-2 to RAR-β2 as well as for H3K4 trimethylation of RAR-β2. Before ChIP, cells were treated with 0.1 μm RA for 15 min (for INI1, BRG1, and ASC-2) or 1 h (for H3K4 trimethylation). Quantitation of the ChIP results for ASC-2 recruitment and H3K4 trimethylation by quantitative RT-PCR is as shown. D, The working model. ASCOM has been proposed to function as a platform to recruit other coactivators during NR transactivation (4). This study reveals that the direct protein-protein interactions between INI1 and the MLL3/4-SET domain enable a novel cross talk between ASCOM and Swi/Snf. This cross talk functions at least in part through mutually facilitating recruitment of these two complexes to NR target genes, although the exact sequence of events (for instance whether Swi/Snf functions before ASCOM) is not known.
The results with the full-length INI1 mutant #4, together with our data with MLL3/4591-4904 and MLL3/4591-4904m (Fig. 5) as well as MLL3Δ/Δ MEF cell line (Fig. 6A), strongly support the model that the MLL3/4-INI1 interactions direct the mutually facilitated recruitment of ASCOM and Swi/Snf to NR target genes, thereby playing critical roles in functionally linking ASCOM and Swi/Snf during NR transactivation.
Discussion
This report not only identifies a novel cross talk between ASCOM and Swi/Snf during NR transactivation but also highlights the general importance of direct protein-protein interactions in incorporating the function of multiple coactivators in transcription, because we demonstrated that the MLL3/4-SET-INI1 interactions play major roles in mediating the cross talk (Fig. 7D). Both ASCOM and Swi/Snf are known to be recruited to NR target genes through well defined interaction interfaces, e.g. ligand-dependent interactions of NRs and ASC-2 in recruiting ASCOM to NRs (10,50) and interactions between various subunits of Swi/Snf and NRs in tethering Swi/Snf to NR target genes (20,21,22,25,31,32). Our results suggest a model in which the MLL3/4-SET-INI1 interactions provide additional interactions that can lead to mutual facilitation of ASCOM and Swi/Snf in recruitment to NR target genes (Fig. 7D). The involvement of these interactions in the cross talk between ASCOM and Swi/Snf is supported by the stable nature of the interactions (Fig. 1D) as well as our transfection results with MLL3/4-SET (in particular with the double mutant MLL3/4591-4904m) (Fig. 5) and the ChIP results with MLL3Δ/Δ MEF cell line (Fig. 6A). However, because both the MLL3/4-SET and INI1 are known to be involved with multiple other interactions (8,46,47,48,49,50), these results need to be assessed carefully. For instance, E4873A/E4874A double mutations have been originally introduced to TRX, and this mutant lacked not only the interactions with INI1 but also an ability to form a homodimer (46). Interestingly, the latter property is shared with the SET domains of TRX and Ash1 but not with those of ySet1, E(Z), and SU(VAR)3-9 (41). However, the significance of this dimerization is unclear, and it remains to be tested whether MLL3 and MLL4 also form a homodimer. In contrast, our experiments with the full-length INI1 mutant #4 (Fig. 7) are convincing, because this mutant is defective only for the MLL3/4-SET interactions. Interestingly, despite the conserved nature of the SET domains throughout evolution, the INI1 interactions are not shared with the SET domains of Ash1, E(Z), and SU(VAR)3-9 (46). In contrast, our results expand the list of INI1-interacting SET domains to those in MLL3 and MLL4 (Fig. 1D). Given the fact that MLL1 and MLL3/4 belong to the Set1-like family of complexes, these results raise an interesting possibility that Set1 and MLL2, the remaining members of the Set1-like complexes, may also use the INI1 interactions to communicate with Swi/Snf. This possibility is supported by the results that ASH2 and RBQ-3, two common core subunits of all the Set1-like complexes (8,51), are also found associated with Swi/Snf in the nuclear matrix (Fig. 1, B and C). Thus, it is possible that Set1 and MLL2 may also interact with INI1, thereby functionally communicating with Swi/Snf, like ASCOM. In support of this idea, SNR1, a Drosophila homolog of INI1, which interacts with the C-terminal SET domain of MLL1/TRX, has been detected at discrete sites on larval salivary gland polytene chromosomes, and these sites colocalized with approximately one half of TRX binding sites (37).
The current study expands the list of coregulators functionally coupled to ASCOM, supporting the possibility that ASCOM may have a distinct platform function to recruit multiple coactivators to NRs. Because these include chromatin regulators Swi/Snf and CBP/p300 and ASCOM itself is a H3K4-methyltransferase complex (9,10,11,12), ASCOM is expected to play major roles in establishing transcriptionally active chromatin in NR target genes. In addition, these chromatin remodelers and modifiers may indirectly affect each other’s recruitment. For instance, Swi/Snf-mediated chromatin remodeling could be a prerequisite for efficient recruitment of ASCOM to NR target genes and for Gal4 fusion to ASC-2 AD2/3 to recruit factors directing their autonomous transactivation function (Fig. 2B). Similarly, ASCOM-mediated H3K4 methylation may create a direct docking site or indirectly contribute to generating proper environment for the subsequent recruitment of other complexes. This could be responsible for our observation of the impaired binding of BRG1 to FKBP5 AR- and G6PC GR-responsive elements in ASC-2-null cells as well as to PSA AR-responsive elements in LNCaP cells expressing siASC-2 (Fig. 4). However, it remains to be determined whether Swi/Snf has indeed an increased binding affinity for histones methylated at H3K4, like Isw1p (41), as our results do not exclude the possibility that H3K4 trimethylation modulates NR transactivation through a step or steps other than increasing the affinity for Swi/Snf. For instance, it is possible that ASCOM may affect the function of Swi/Snf by methylating one of its subunits. Future biochemical experiments to examine the affinity of Swi/Snf toward histone H3 methylated at K4 should help clarify these issues.
ASC-2 has been originally identified as a gene amplified in several human cancers (51), and the recent results indicate that it may function as a tumor suppressor. First, haploid inactivation of ASC-2 accelerates polyoma middle-T antigen-induced mammary tumorigenesis (52). Second, a candidate tumor suppressor PTIP has been identified as an additional component of ASCOM (11,12). The tumor suppressor p53 plays a key role countering the adverse effects of DNA damage (53), which otherwise can be lethal or lead to oncogenic transformation. DNA damage is known to activate damage sensors such as ATM, which in turn induces the transcriptional activity of p53 (53). Interestingly, PTIP appears to be required for ATM-mediated phosphorylation of p53 at Ser-15 and for DNA damage-induced up-regulation of the cyclin-dependent kinase inhibitor p21 (54). Consistent with these results, the loss-of-function studies in mice indicated that PTIP is essential for the maintenance of genomic stability (55). In further support of a tumor suppressive function of ASCOM, we found that it functions as a coactivator of the tumor suppressor p53; deletion of the MLL3-SET domain in mouse leads to kidney tumor development; and this kidney tumorigenic phenotype of MLL3 mutant mice becomes exacerbated in the p53+/− background (our unpublished results). Importantly, at least two subunits of Swi/Snf, BRG1 and INI1, have been linked to tumor suppression pathways and mice heterozygous for mutations at SNF5/INI1 and BRG1 are cancer prone (56,57). Taken together, one of the most interesting future challenges will be to test whether the MLL3/4-INI1 interactions defined in this report play any roles in integrating the two tumor-suppressive pathways directed by ASCOM and Swi/Snf, respectively.
Materials and Methods
Plasmids and antibodies
Vectors encoding BRG1, BRG1-K798R, BRM, INI1, ASC-2, ASC2-2bc, ASC2-Ia, Gal4-ASC2-2bc, GR, AR, and RARα, the transfection indicator construct pActin-β-gal, and the reporter constructs β-RARE-LUC, ARE-LUC, MMTV-LUC, UAS-LUC, and LexA-LacZ were as described (3,5,9,10,19). PCR fragments encoding ΔINI1 (INI1 residues 83-385), INI1 deletion mutants and alanine scanning mutants m1–m11 (Fig. 6A), hBRM residues 321-693, MLL3 residues 4591-4904 (wild-type and E4873A/E4874A double mutant), and MLL4 residues 4744-5262 were cloned into the GST fusion vector pGEX4T (Pharmacia), Gal4-fusion vector pCMX1, pcDNA3 (Invitrogen), the B42-fusion vector pJG4-5, and the LexA fusion vector pEG202. To construct cupper-inducible yeast expression vectors for lexA fusions, XhoI-HindIII fragment containing CUP1 promoter and HindIII-XbaI fragment from pEG202 were cloned into XhoI-XbaI sites of pRS to generate pCL. Finally, PCR fragments encoding ASC2-Ia and ASC2-2bc were cloned into pCL. Antibodies against ASC-2, Flag-epitope, INI1, BRG1, and Lamin B were purchased from Bethyl (Montgomery, TX), Santa Cruz Biotechnology (Santa Cruz, CA), and Boehringer Mannheim (Mannheim, Germany). Monoclonal ASC-2 antibody (A3C1) and RBQ-3, MLL4 and ASH2 antibodies were as described (9).
Indirect immunofluorescence microscopy
HeLa cells, grown on coverslips, were fixed in 3.5% freshly prepared paraformaldehyde in PBS for 10 min at room temperature and then permeabilized with 0.05% Triton X-100 in PBS for 30 min at room temperature. For in situ isolation of nuclear matrix, cells were extracted directly on the coverslips with the high-salt method (35) and fixed with 4% paraformaldehyde in PBS. The coverslips were incubated overnight with the appropriate antibodies in PBS-10% horse serum-0.1% Tween 20. Fluorescein- or Texas red-linked antirabbit or antimouse antibodies (Amersham, Piscataway, NJ) were used for detection. The cellular DNA was labeled with 0.05% 4′,6-diamidino-2-phenylindole. The preparations were observed with an Olympus Fluoview 300 laser scanning confocal microscope.
Nuclear matrix isolation
High-salt isolation of nuclear matrix was done as described (35). After washing in PBS, HeLa cells were extracted in cytoskeleton (CSK) buffer: 10 mm Pipes (pH 6.8), 100 mm NaCl, 300 mm sucrose, 3 mm MgCl2, 1 mm EGTA, supplemented with leupeptin, aprotinin, and pepstatin (1 μg/ml each), 1 mm phenylmethylsulfonyl fluoride, 1 mm dithiothreitol, and 0.5% (vol/ vol) Triton X-100. After 3 min at 4 C, the cytoskeletal frameworks were separated from soluble proteins by centrifugation at 5000 × g for 3 min. Chromatin was solubilized by DNA digestion with 1 mg/ml ribonuclease-free DNase I in CSK buffer plus protease inhibitors for 15 min at 37 C. Then ammonium sulfate was added from a 1 m stock solution in CSK buffer to a final concentration of 0.25 m, and after 5 min at 4 C, samples were pelleted. The pellet was further extracted with 2 m NaCl in CSK buffer for 5 min at 4 C and then centrifuged. This treatment removed all the DNA and histones from the nucleus, as described (35). The remaining pellet was solubilized in urea buffer to obtain the nuclear matrix-containing fraction (35).
Transfections and GST pull-down assays
SW13 (38), HeLa, and CV1 cells were grown in 24-well plates with medium supplemented with 10% fetal calf serum for 24 h. Transfections and luciferase assays were done as described (3,9), and the results were normalized to the LacZ expression. Similar results were obtained in more than two similar experiments. GST pull-down assays were carried out as described (9).
Yeast strains and β-galactosidase assay
For the analysis of reporter gene expression in yeast cells, effector plasmids, and a reporter plasmid (pSH18-34) were introduced into wild-type (YPH500) and mutant yeast strains for swi1Δ (FY1209) and snf2Δ (FY1307) (39). These cells were grown to the mid-log phase in selective synthetic complete medium and cultured for an additional 16 h in a medium with or without 0.5 mm cupper to express proteins. Liquid β-galactosidase assay of cultured cells was done in triplicates using the permeabilized-cell method or the yeast whole-cell extracts prepared by glass bead disruption. Expression of each protein was examined by the immunoblot analysis of whole-cell extracts using anti-LexA monoclonal antibody (data not shown).
ChIP
SW13, HEK293, and LNCaP cells or cell lines established from wild-type, ASC-2−/− and MLL3Δ/Δ MEFs were grown in 10-cm dishes with medium supplemented with 10% fetal bovine serum for 24 h and transfected with mammalian expression vectors for BRG1 and BRG1-K798R, GR, AR, or Flag-tagged INI1 that is redesigned to be resistant to siINI1 (58), along with control siRNA and siASC-2 (10) or siINI1 (58), as indicated. Total amounts of expression vectors were kept constant by adding pcDNA3. ChIP assays were carried out as described (9). The primers for RAR-β2 were as described (10). The primers for FKBP5 were 5′-GCA GCC TCT TCC TCA GTT TTG CTT-3′ and 5′-ACA TCA AGT GAG TCT GGT CAC TGC-3′, which generate a 267-bp PCR product spanning the ARE region. The primers for G6PC were 5′-CAG CCT CTA GCA CTG TCA AGC AGT-3′ and 5′-GAT TCA GTC TGT AGG TCA ATC CAG-3′, which generate a 217-bp PCR product spanning the GRE region. The primers for PSA were 5′-GTA TGA AGA ATC GGG GAT CGT ACC-3′ and 5′-CCT GCC CTG CTG GCA CCC AGA GGC-3′, which generate a 250-bp PCR product spanning the ARE region.
RT-PCR
Total RNA was isolated from indicated cell lines after lysis in TRIzol reagent according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA), and RT-PCRs were performed as described previously (10). The primers for RAR-β2 were 5′-GGT CCT CTG ACT GAC CTT GT-3′ and 5′-GGA AAC ATG TGA GGC TTG CT-3′, which generate a 217-bp PCR product. The primers for INI1 were 5′-TAC GCC TTC AGC GAG AAC-3′ and 5′-CAT CCG CCT CGT GTT CCT-3′, which generate a 150-bp PCR product. The primers for PSA were 5′-GGC AGC ATT GAA CCA GAG GAG-3′ and 5′-GCA TGA ACT TGG TCA CCT TCT G-3′, which generate a 118-bp PCR product.
Footnotes
This work was supported by National Institutes of Health Grant DK064678 (to J.W.L.).
Disclosure Summary: The authors have nothing to declare.
First Published Online February 12, 2009
Abbreviations: AR, Androgen receptor; ASC-2, activating signal cointegrator-2; ASCOM, ASC-2 complex; ChIP, chromatin immunoprecipitation; GST, glutathione S-transferase; GR, glucocorticoid receptor; H3K4, histone H3-lysine-4; LBD, ligand-binding domain; MEF, mouse embryo fibroblast; NR, nuclear receptor; RAR, retinoic acid receptor.
References
- Ribeiro RC, Kushner PJ, Baxter JD 1995 The nuclear hormone receptor gene superfamily. Annu Rev Med 46:443–453 [DOI] [PubMed] [Google Scholar]
- Smith CL, O'Malley BW 2004 Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev 25:45–71 [DOI] [PubMed] [Google Scholar]
- Lee SK, Jung SY, Kim YS, Na SY, Lee YC, Lee JW 2001 Two distinct nuclear receptor-interaction domains and CREB-binding protein-dependent transactivation function of activating signal cointegrator-2. Mol Endocrinol 15:241–254 [DOI] [PubMed] [Google Scholar]
- Mahajan MA, Samuels HH 2005 Nuclear hormone receptor coregulator: role in hormone action, metabolism, growth, and development. Endocr Rev 26:583–597 [DOI] [PubMed] [Google Scholar]
- Goo YH, Na SY, Zhang H, Xu J, Hong S, Cheong J, Lee SK, Lee JW 2004 Interactions between activating signal cointegrator-2 and the tumor suppressor retinoblastoma in androgen receptor transactivation. J Biol Chem 279:7131–7135 [DOI] [PubMed] [Google Scholar]
- Santos-Rosa H, Schneider R, Bannister AJ, Sherriff J, Bernstein BE, Emre NC, Schreiber SL, Mellor J, Kouzarides T 2002 Active genes are tri-methylated at K4 of histone H3. Nature 419:407–411 [DOI] [PubMed] [Google Scholar]
- Schneider R, Bannister AJ, Myers FA, Thorne AW, Crane-Robinson C, Kouzarides T 2004 Histone H3 lysine 4 methylation patterns in higher eukaryotic genes. Nat Cell Biol 6:73–77 [DOI] [PubMed] [Google Scholar]
- Ruthenburg AJ, Allis CD, Wysocka J 2007 Methylation of lysine 4 on histone H3: intricacy of writing and reading a single epigenetic mark. Mol Cell 25:15–30 [DOI] [PubMed] [Google Scholar]
- Goo YH, Sohn YC, Kim DH, Kim SW, Kang MJ, Jung DJ, Kwak E, Barlev NA, Berger SL, Chow VT, Roeder RG, Azorsa DO, Meltzer PS, Suh PG, Song EJ, Lee KJ, Lee YC, Lee JW 2003 Activating signal cointegrator 2 belongs to a novel steady-state complex that contains a subset of trithorax group proteins. Mol Cell Biol 23:140–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Lee DK, Dou Y, Lee J, Lee B, Kwak E, Kong YY, Lee SK, Roeder RG, Lee JW 2006 Coactivator as a target gene specificity determinant for histone H3 lysine 4 methyltransferases. Proc Natl Acad Sci USA 103:15392–15397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YW, Hong T, Hong S, Guo H, Yu H, Kim D, Guszczynski T, Dressler GR, Copeland TD, Kalkum M, Ge K 2007 PTIP associates with MLL3- and MLL4-containing histone H3 lysine 4 methyltransferase complex. J Biol Chem 282:20395–20406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issaeva I, Zonis Y, Rozovskaia T, Orlovsky K, Croce CM, Nakamura T, Mazo A, Eisenbach L, Canaani E 2007 Knockdown of ALR (MLL2) reveals ALR target genes and leads to alterations in cell adhesion and growth. Mol Cell Biol 27:1889–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Cho YW, Yu LR, Yu H, Veenstra TD, Ge K 2007 Identification of JmjC domain-containing UTX and JMJD3 as histone H3 lysine 27 demethylases. Proc Natl Acad Sci USA 104:18439–18444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan F, Bayliss PE, Rinn JL, Whetstine JR, Wang JK, Chen S, Iwase S, Alpatov R, Issaeva I, Canaani E, Roberts TM, Chang HY, Shi Y 2007 A histone H3 lysine 27 demethylase regulates animal posterior development. Nature 449:689–694 [DOI] [PubMed] [Google Scholar]
- Lee MG, Villa R, Trojer P, Norman J, Yan KP, Reinberg D, Di Croce L, Shiekhattar R 2007 Demethylation of H3K27 regulates polycomb recruitment and H2A ubiquitination. Science 318:447–450 [DOI] [PubMed] [Google Scholar]
- Agger K, Cloos PA, Christensen J, Pasini D, Rose S, Rappsilber J, Issaeva I, Canaani E, Salcini AE, Helin K 2007 UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature 449:731–734 [DOI] [PubMed] [Google Scholar]
- Shilatifard A 2006 Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annu Rev Biochem 75:243–269 [DOI] [PubMed] [Google Scholar]
- Martens JA, Winston F 2003 Recent advances in understanding chromatin remodeling by Swi/Snf complexes. Curr Opin Genet Dev 13:136–142 [DOI] [PubMed] [Google Scholar]
- Inoue H, Furukawa T, Giannakopoulos S, Zhou S, King DS, Tanese N 2002 Largest subunits of the human SWI/SNF chromatin-remodeling complex promote transcriptional activation by steroid hormone receptors. J Biol Chem 277:41674–41685 [DOI] [PubMed] [Google Scholar]
- Ichinose H, Garnier JM, Chambon P, Losson R 1997 Ligand-dependent interaction between the estrogen receptor and the human homologues of SWI2/SNF2. Gene 188:95–100 [DOI] [PubMed] [Google Scholar]
- Belandia B, Orford RL, Hurst HC, Parker MG 2002 Targeting of SWI/SNF chromatin remodelling complexes to estrogen-responsive genes. EMBO J 21:4094–4103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRenzo J, Shang Y, Phelan M, Sif S, Myers M, Kingston R, Brown M 2000 BRG-1 is recruited to estrogen-responsive promoters and cooperates with factors involved in histone acetylation. Mol Cell Biol 20:7541–7549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall TW, Link KA, Petre-Draviam CE, Knudsen KE 2003 Differential requirement of SWI/SNF for androgen receptor activity. J Biol Chem 278:30605–30613 [DOI] [PubMed] [Google Scholar]
- Cairns BR, Levinson RS, Yamamoto KR, Kornberg RD 1996 Essential role of Swp73p in the function of yeast Swi/Snf complex. Genes Dev 10:2131–2144 [DOI] [PubMed] [Google Scholar]
- Nie Z, Xue Y, Yang D, Zhou S, Deroo BJ, Archer TK, Wang W 2000 A specificity and targeting subunit of a human SWI/SNF family-related chromatin-remodeling complex. Mol Cell Biol 20:8879–8888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallberg AE, Neely KE, Hassan AH, Gustafsson JA, Workman JL, Wright AP 2000 Recruitment of the SWI-SNF chromatin remodeling complex as a mechanism of gene activation by the glucocorticoid receptor tau1 activation domain. Mol Cell Biol 20:2004–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher TM, Xiao N, Mautino G, Baumann CT, Wolford R, Warren BS, Hager GL 2002 ATP-dependent mobilization of the glucocorticoid receptor during chromatin remodeling. Mol Cell Biol 22:3255–3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer CJ, Archer TK 1998 Chromatin remodelling by the glucocorticoid receptor requires the BRG1 complex. Nature 393:88-91 [DOI] [PubMed] [Google Scholar]
- Sheldon LA, Smith CL, Bodwell JE, Munck AU, Hager GL 1999 A ligand binding domain mutation in the mouse glucocorticoid receptor functionally links chromatin remodeling and transcription initiation. Mol Cell Biol 19:8146–8157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth FJ, Fromental-Ramain C, Yamamoto K, Chambon P 2000 ATP-driven chromatin remodeling activity and histone acetyltransferases act sequentially during transactivation by RAR/RXR in vitro. Mol Cell 6:1049–1058 [DOI] [PubMed] [Google Scholar]
- Hsiao PW, Fryer CJ, Trotter KW, Wang W, Archer TK 2003 BAF60a mediates critical interactions between nuclear receptors and the BRG1 chromatin-remodeling complex for transactivation. Mol Cell Biol 23:6210–6220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debril MB, Gelman L, Fayard E, Annicotte JS, Rocchi S, Auwerx J 2004 Transcription factors and nuclear receptors interact with the SWI/SNF complex through the BAF60c subunit. J Biol Chem 279:16677–16686 [DOI] [PubMed] [Google Scholar]
- Ko L, Cardona GR, Chin WW 2000 Thyroid hormone receptor-binding protein, an LXXLL motif-containing protein, functions as a general coactivator. Proc Natl Acad Sci USA 97:6212–6217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan MA, Samuels HH 2000 A new family of nuclear receptor coregulators that integrate nuclear receptor signaling through CREB-binding protein. Mol Cell Biol 20:5048–5063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes JC, Muchardt C, Yaniv M 1997 Components of the human SWI/SNF complex are enriched in active chromatin and are associated with the nuclear matrix. J Cell Biol 137:263–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin SV, Iarovaia OV, Sjakste N, Sjakste T, Bagdoniene L, Rynditch AV, Eivazova ER, Lipinski M, Vassetzky YS 2007 Chromatin domains and regulation of transcription. J Mol Biol 369:597–607 [DOI] [PubMed] [Google Scholar]
- Rozenblatt-Rosen O, Rozovskaia T, Burakov D, Sedkov Y, Tillib S, Blechman J, Nakamura T, Croce CM, Mazo A, Canaani E 1998 The C-terminal SET domains of ALL-1 and TRITHORAX interact with the INI1 and SNR1 proteins, components of the SWI/SNF complex. Proc Natl Acad Sci USA 95:4152–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovitz A, McCombs 3rd WM, Johnston D, McCoy CE, Stinson JC 1973 New human cancer cell culture lines. I. SW-13, small-cell carcinoma of the adrenal cortex. J Natl Cancer Inst 51:691–697 [PubMed] [Google Scholar]
- Roberts SM, Winston F 1997 Essential functional interactions of SAGA, a Saccharomyces cerevisiae complex of Spt, Ada, and Gcn5 proteins, with the Snf/Swi and Srb/mediator complexes. Genetics 147:451–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan MA, Murray A, Levy D, Samuels HH 2007 Nuclear receptor coregulator (NRC): mapping of the dimerization domain, activation of p53 and STAT-2, and identification of the activation domain AD2 necessary for nuclear receptor signaling. Mol Endocrinol 21:1822–1834 [DOI] [PubMed] [Google Scholar]
- Santos-Rosa H, Schneider R, Bernstein BE, Karabetsou N, Morillon A, Weise C, Schreiber SL, Mellor J, Kouzarides T 2003 Methylation of histone H3 K4 mediates association of the Isw1p ATPase with chromatin. Mol Cell 12:1325–1332 [DOI] [PubMed] [Google Scholar]
- Underhill C, Qutob MS, Yee SP, Torchia J 2000 A novel nuclear receptor corepressor complex, N-CoR, contains components of the mammalian SWI/SNF complex and the corepressor KAP-1. J Biol Chem 275:40463–40470 [DOI] [PubMed] [Google Scholar]
- Magee JA, Chang LW, Stormo GD, Milbrandt J 2006 Direct, androgen receptor-mediated regulation of the FKBP5 gene via a distal enhancer element. Endocrinology 147:590–598 [DOI] [PubMed] [Google Scholar]
- Vander Kooi BT, Onuma H, Oeser JK, Svitek CA, Allen SR, Vander Kooi CW, Chazin WJ, O'Brien RM 2005 The glucose-6-phosphatase catalytic subunit gene promoter contains both positive and negative glucocorticoid response elements. Mol Endocrinol 19:3001–3022 [DOI] [PubMed] [Google Scholar]
- Léotoing L, Meunier L, Manin M, Mauduit C, Decaussin M, Verrijdt G, Claessens F, Benahmed M, Veyssière G, Morel L, Beaudoin C 2008 Influence of nucleophosmin/B23 on DNA binding and transcriptional activity of the androgen receptor in prostate cancer cell. Oncogene 27:2858–2867 [DOI] [PubMed] [Google Scholar]
- Rozovskaia T, Rozenblatt-Rosen O, Sedkov Y, Burakov D, Yano T, Nakamura T, Petruck S, Ben-Simchon L, Croce CM, Mazo A, Canaani E 2000 Self-association of the SET domains of human ALL-1 and of Drosophila TRITHORAX and ASH1 proteins. Oncogene 19:351–357 [DOI] [PubMed] [Google Scholar]
- Muchardt C, Sardet C, Bourachot B, Onufryk C, Yaniv M 1995 A human protein with homology to Saccharomyces cerevisiae SNF5 interacts with the potential helicase hbrm. Nucleic Acids Res 23:1127–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalpana GV, Marmon S, Wang W, Crabtree GR, Goff SP 1994 Binding and stimulation of HIV-1 integrase by a human homolog of yeast transcription factor SNF5. Science 266:2002–2006 [DOI] [PubMed] [Google Scholar]
- Cheng SW, Davies KP, Yung E, Beltran RJ, Yu J, Kalpana GV 1999 c-MYC interacts with INI1/hSNF5 and requires the SWI/SNF complex for transactivation function. Nat Genet 22:102–105 [DOI] [PubMed] [Google Scholar]
- Lee S, Lee J, Lee SK, Lee JW 2008 Activating signal cointegrator-2 is an essential adaptor to recruit histone H3 lysine 4 methyltransferases MLL3 and MLL4 to the liver X receptors. Mol Endocrinol 22:1312–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Anzick SL, Choi JE, Bubendorf L, Guan XY, Jung YK, Kallioniemi OP, Kononen J, Trent JM, Azorsa D, Jhun BH, Cheong JH, Lee YC, Meltzer PS, Lee JW 1999 A nuclear factor, ASC-2, as a cancer-amplified transcriptional coactivator essential for ligand-dependent transactivation by nuclear receptors in vivo. J Biol Chem 274:34283-34293 [DOI] [PubMed] [Google Scholar]
- Zhang H, Kuang SQ, Liao L, Zhou S, Xu J 2004 Haploid inactivation of the amplified-in-breast cancer 3 coactivator reduces the inhibitory effect of peroxisome proliferator-activated receptor γ and retinoid X receptor on cell proliferation and accelerates polyoma middle-T antigen-induced mammary tumorigenesis in mice. Cancer Res 64:7169–7177 [DOI] [PubMed] [Google Scholar]
- Efeyan A, Serrano M 2007 p53: guardian of the genome and policeman of the oncogenes. Cell Cycle 6:1006–1010 [DOI] [PubMed] [Google Scholar]
- Jowsey PA, Doherty AJ, Rouse J 2004 Human PTIP facilitates ATM-mediated activation of p53 and promotes cellular resistance to ionizing radiation. J Biol Chem 279:55562–55569 [DOI] [PubMed] [Google Scholar]
- Cho EA, Prindle MJ, Dressler GR 2003 BRCT domain-containing protein PTIP is essential for progression through mitosis. Mol Cell Biol 23:1666–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CW, Orkin SH 2004 The SWI/SNF complex: chromatin and cancer. Nat Rev Cancer 4:133–142 [DOI] [PubMed] [Google Scholar]
- Medina PP, Sanchez-Cespedes M 2008 Involvement of the chromatin-remodeling factor BRG1/SMARCA4 in human cancer. Epigenetics 3:64–68 [DOI] [PubMed] [Google Scholar]
- Cui K, Tailor P, Liu H, Chen X, Ozato K, Zhao K 2004 The chromatin-remodeling BAF complex mediates cellular antiviral activities by promoter priming. Mol Cell Biol 24:4476–4486 [DOI] [PMC free article] [PubMed] [Google Scholar]