Abstract
Nuclear receptors (NRs) regulate gene expression in essential biological processes including differentiation and development. Here we report the systematic profiling of NRs in human and mouse embryonic stem cell (ESC) lines and during their early differentiation into embryoid bodies. Expression of the 48 human and mouse NRs was assessed by quantitative real-time PCR. In general, expression of NRs between the two human cell lines was highly concordant, whereas in contrast, expression of NRs between human and mouse ESCs differed significantly. In particular, a number of NRs that have been implicated previously as crucial regulators of mouse ESC biology, including ERRβ, DAX-1, and LRH-1, exhibited diametric patterns of expression, suggesting they may have distinct species-specific functions. Taken together, these results highlight the complexity of the transcriptional hierarchy that exists between species and governs early development. These data should provide a unique resource for further exploration of the species-specific roles of NRs in ESC self-renewal and differentiation.
An atlas of nuclear receptor expression during early mouse and human embryonic stem cell differentiation demonstrates similarities and differences between species.
Embryonic stem cells (ESCs) derived from early embryos have unique capabilities of self-renewal and pluripotency (to differentiate into cell lineages of the three germ layers within certain environments) (1). Thus, ESCs offer an unprecedented opportunity to study differentiation events in vitro that mimic events after implantation in both mice and humans. Furthermore, understanding these events has become increasingly important because of the potential use of ESCs in cell-based replacement therapies (2). Despite the keen interest in ESC biology, the signal transduction and transcriptional regulatory pathways involved in the maintenance, differentiation, and manipulation of ESCs still is not completely understood.
To date, the mouse has been the mainstay of mammalian experimental embryology because of its well-defined genetics and favorable reproductive characteristics. Indeed, much information about early human development has been generated from studies in mouse. However, several fundamental differences exist between mouse and human in early development. For example, human and mouse embryos differ in the timing of zygotic genome activation; in the formation, structure, and function of the fetal membranes and placenta; and in the formation of an embryonic disc instead of an egg cylinder (3,4,5). For this reason, it has become desirable and necessary to pursue comparative studies of mouse and human models of embryogenesis.
One strategy that has been used to characterize mouse and human ESCs and identify the unique molecular components that define each has been genome-wide transcriptome profiling (6,7,8,9,10,11). Although these studies have revealed species-specific patterns of expression and identified important biomarkers that define undifferentiated and differentiated states, gleaning further mechanistic insight into how these processes are regulated has been difficult because, by nature, the genome-wide approach results in datasets that are subjective to open-ended bioinformatic analyses.
Nuclear receptors (NRs) represent a superfamily of ligand-dependent transcription factors that govern diverse aspects of development, reproduction, basal metabolic function, and nutrient uptake and metabolism through a common mechanism of action (12,13,14). Included in this superfamily are the classic endocrine receptors that mediate the actions of steroid hormones, thyroid hormones, and the fat-soluble vitamins A and D; the lipid-sensing receptors that respond to dietary metabolites of cholesterol, fatty acids, and xenobiotics; and a number of orphan receptors whose ligands and physiological functions are still being characterized. Given the importance of NRs in controlling cell differentiation and development, comparing the expression of NRs during early ESC differentiation would be expected to provide a unique view of the early transcriptional regulatory networks involved in ESC self-renewal and differentiation. With the exception of one report evaluating the expression of receptors for estrogen, progesterone, and glucocorticoids (15), virtually nothing is known about systemic NR expression in human ESCs. Therefore, as a first step toward exploring the species-specific roles of NRs in mouse and human ESC differentiation, in the present study, we characterized the mRNA expression profile of the NR superfamily in two well-studied, human ESC lines (H1 and H9) and the mouse ESC line (CMTI-1) using a high-throughput quantitative real-time PCR (QPCR) method. Bioinformatic analysis of the resulting expression profiles revealed significant differences in the patterns of expression of mouse and human NRs in undifferentiated ESCs, underscoring the importance of species-specific studies in stem cell populations. Furthermore, NR profiling during the early differentiation of mouse and human ESCs into embryoid bodies revealed the existence of a complex, temporally regulated transcriptional network involving numerous, previously unsuspected nuclear receptors during early embryonic differentiation. Taken together, our data provide the first comprehensive study of a single family of transcriptional regulators in ESCs. This information should serve as a useful resource for exploring species-specific processes of ESC self-renewal and differentiation.
Results
Characterization of ESCs
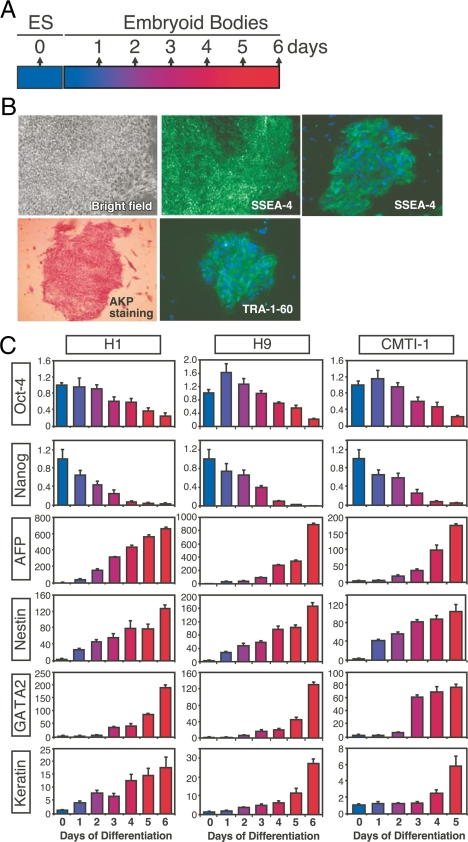
Traditional methods to induce ESC differentiation involve first culturing ESC clumps in suspension for 5–6 d, after which they spontaneously form three-dimensional embryoid bodies (16,17). Typically, the sphere-like embryoid bodies are then treated with different stimuli, including growth factors, chemical agents, and matrix factors to drive differentiation into specific cell types. Thus far, different specific cell types derived from ESCs have been broadly reported (18). However, the low differentiation efficiency and complexity of the resultant cell lineages have made it difficult to illuminate molecular events leading to cell type-specific differentiation. For this reason, we concentrated on characterizing the NR expression profiles in two human (H1 and H9) and one mouse (CMTI-1) ESC line, as well as during the first 5 (for mouse) or 6 (for human) days of spontaneous differentiation of these cells as outlined in Fig. 1A. These cell lines were chosen because of their widespread use in the stem cell field (e.g. CMTI-1 is the most widely used ESC for generating germline mouse knockouts). The undifferentiated state of human ESCs was confirmed by immunofluorescence staining for stage-specific embryonic antigen 4 (SSEA-4) and tumor rejection antigen-1-60 (TRA-1-60), and the presence of alkaline phosphatase (AKP) activity (Fig. 1B). Similar analysis was used to confirm the undifferentiated state of mouse ESCs (data not shown). In addition, decreased expression of pluripotent gene markers, Oct-4 and Nanog, were monitored by QPCR in both species of ESCs (Fig. 1C), indicating the progression of differentiation was occurring as has been observed by others (19,20). Further confirmation of the differentiated state was achieved from observing the appearance of early germ layer-specific gene expression for AFP (an endoderm marker), Nestin (a neuroectoderm marker), GATA-2 (a mesoderm and trophoblast marker), and keratin (an epidermal ectoderm marker).
Figure 1.
Experimental design for expression profiling of NRs in ESCs. A, Schematic representation of ESC differentiation timeline. Undifferentiated ESC lines were expanded routinely and permitted to differentiate spontaneously into embryoid bodies with a minimum of 50 cell clumps for human H1 and H9 cells and a minimum of 106 ESCs for mouse CMTI-1 cells. Differentiated samples collected every day contained 50–100 embryoid bodies in both cases. B, Bright-field microscopy showing morphology of undifferentiated hESCs (top left). Cell colonies exhibit high AKP activity (bottom left) indicated by the intense staining pattern. Right panels represent immunofluorescence staining of human ESC colonies with anti-SSEA-4 (top middle and top right) and anti-TRA-1-60 (bottom right), respectively. As anticipated, ESCs exhibited strong immunoreactivity to these two antibodies. C, Oct-4, Nanog, AFP, Nestin, GATA-2, and Keratin expression quantitated by QPCR during spontaneous human (H1 and H9) and mouse (CMTI-1) ESC differentiation into embryoid bodies. 18S RNA was used as internal standard. The x- and y-axes represent time (day) after induced differentiation and relative expression of NRs, respectively. Relative expression levels were determined by the PCR efficiency-corrected method as described in Materials and Methods. Values are expressed as mean ± sd from triplicates of each sample.
Undifferentiated ESCs exhibit species-specific expression of a core set of NRs
Using a QPCR method developed specifically for NR expression profiling (12,21), we analyzed the expression of all 49 mouse and 48 human NRs in undifferentiated ESCs and during their early spontaneous differentiation into embryoid bodies. The raw and annotated data sets are available as part of the Nuclear Receptor Signaling Atlas (NURSA), an online open access resource for nuclear receptor research (www.NURSA.com). Names and abbreviations of NRs are listed in supplemental Table 1 (published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals. org); the human and mouse QPCR primer sequences used in this study are described (supplemental Table 2) (21) and are also available on the NURSA web site.
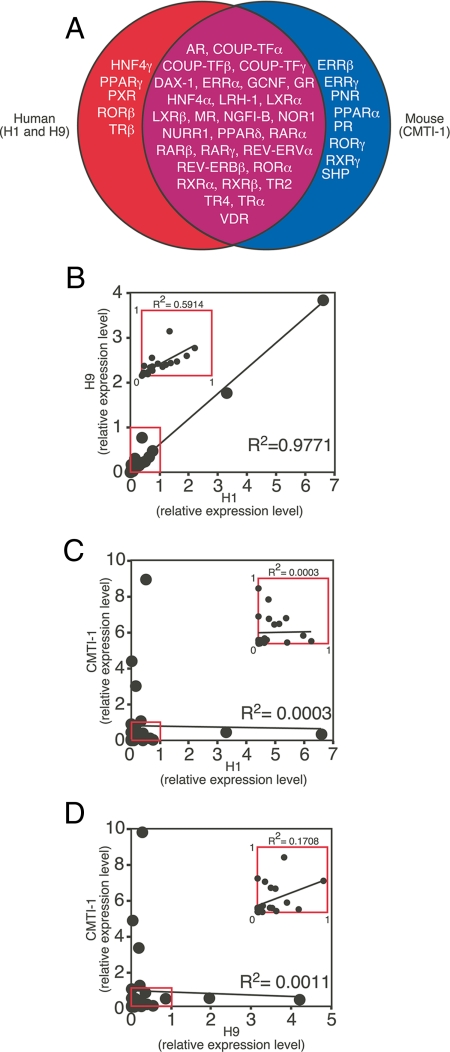
Analysis of the mRNA expression profiles revealed that although there was 100% identity in NR expression between the two human undifferentiated cell lines, only 29 NRs (59%) were coexpressed in both human and mouse ESC lines (Fig. 2A). These NRs included AR, COUP-TFα, COUP-TFβ, COUP-TFγ, DAX-1, ERRα, GCNF, GR, HNF4α, LRH-1, LXRα, LXRβ, MR, NGFI-B, NOR1, NURR1, PPARδ, RARα, RARβ, RARγ, REV-ERBα, REV-ERBβ, RORα, RXRα, RXRβ, TR2, TR4, TRα, and VDR. These receptors appear to define a common set of NRs that may play key roles in ESC self-renewal and differentiation. Surprisingly, five of the NRs (HNF4γ, PPARγ, PXR, RORβ, and TRβ) were expressed only in human ESCs, and eight (ERRβ, ERRγ, PNR, PPARα, PR, RORγ, RXRγ, and SHP) were expressed only in mouse ESCs (Fig. 2A). Seven (10%) of the NRs (CAR, ERα, ERβ, FXR, PPARγ2, SF1, and TLX) were undetectable [cycle time (Ct) ≥33] in any of the three ESC lines.
Figure 2.
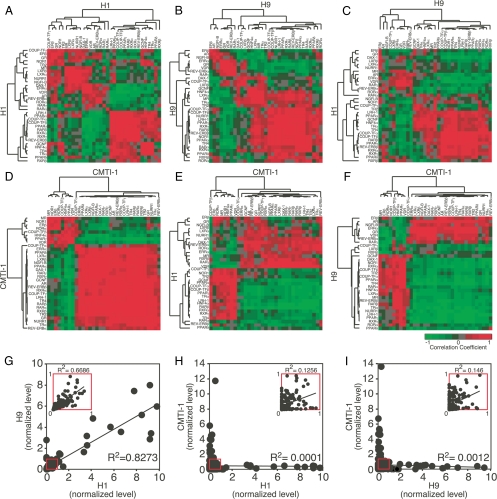
Expression of NRs in undifferentiated human and mouse ESCs. A, The Venn diagram depicts mRNA expression (Ct ≤33) of the NR superfamily in human (H1 and H9) and mouse (CMTI-1) ESCs. B–D, Correlation of the relative expression levels for 29 NRs that were commonly expressed in undifferentiated human and mouse ESC lines. Relative expression values between each cell line are compared by scatter plot. Linear regression analysis was used to obtain the overall correlation (R2) of NR expression for each cell line pair (H1 vs. H9 in B H1 vs. CMTI-1 in C and H9 vs. CMTI-1 in D). Both x- and y-axes represent normalized expression levels for each sample. Insets represent magnified scattered plots of red boxed regions. Note that expression of NR1H5 (also known as FXRβ), which is a mouse-specific NR and a pseudo-gene in humans, was undetectable in undifferentiated and differentiated ESCs and therefore was excluded from further analyses.
Although the profiling results demonstrated that an essential fraction of NR mRNAs were present in both human and mouse ESCs, there were significant differences in the relative levels of these mRNAs when their expression was compared between the two species (supplemental Table 3). Interestingly, in the two human cells, REV-ERBβ and TR4 mRNA expression was more than 10-fold higher than any of the other NRs (see Figs. 3 and 4 for comparisons of relative expression levels). In contrast, in the mouse ESCs, RARγ, NGFI-B, and LXRβ were the most abundantly expressed, and again at levels that were more than 10-fold higher than other NRs (Figs. 3 and 4). Linear regression analysis based on the relative levels of the core set of NRs expressed in the undifferentiated cell lines revealed that although the two human ESCs (H1 and H9) showed a nearly identical pattern of expression (R2 = 0.98) (Fig. 2B), the correlations of the expression pattern between either of the two human cell lines (H1 and H9) and the mouse cell line (CMTI-1) were insignificant (R2 ≤ 0.001) (Fig. 2, C and D). To exclude the possibility that the observed species differences were due to the dominant effects of the small number of highly expressed NRs in each group, similar results were obtained by repeating the analysis without including these highly expressed NRs (insets in Fig. 2, B–D). Taken together, these data imply that species-specific differences in NR profiles may contribute to distinct transcriptional programming of mouse vs. human ESCs.
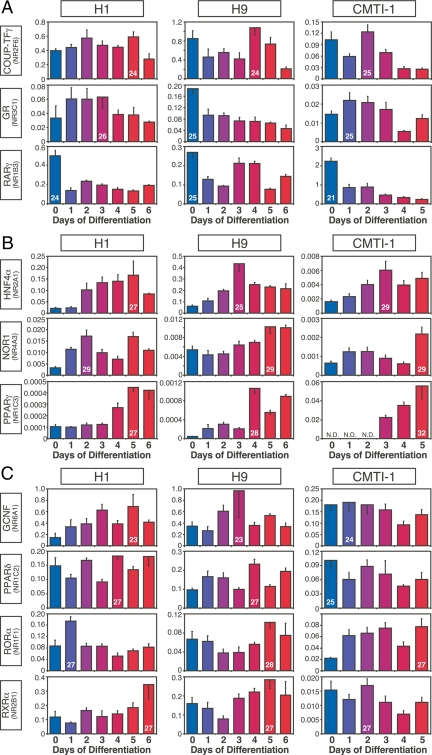
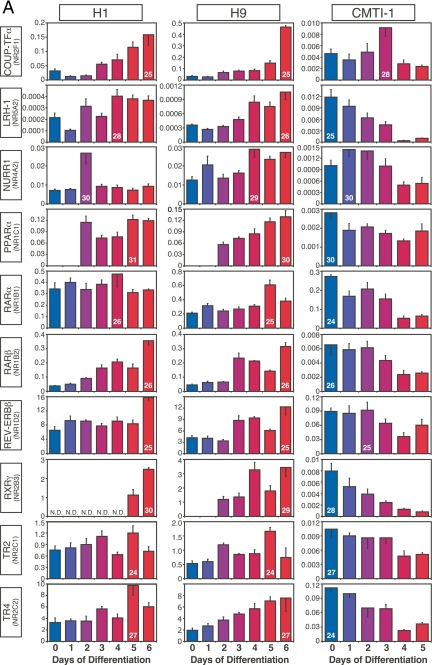
Figure 3.
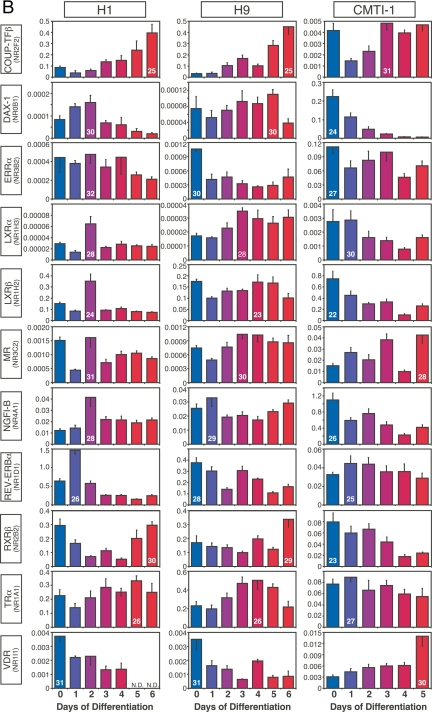
NRs with similar patterns of expression in mouse and human ESCs during embryoid body formation. A, Expression of NRs that tended to decrease upon ESC differentiation. B, Expression of NRs that tended to increase upon ESC differentiation. C, Expression of NRs that remained relatively constant during ESC differentiation. Comparisons are between human H1 and H9 and mouse CMTI-1 ESC lines. The x- and y-axrs represent time (day) after induced differentiation and relative expression of NRs, respectively. Relative expression levels were determined by the PCR efficiency-corrected method as described in Materials and Methods. Values are expressed as mean ± sd from triplicates of each sample. Ct higher than 32 is considered below the limit of detection. Ct of the highest expressing value for each NR group is indicated inside its corresponding bar.
Figure 4.
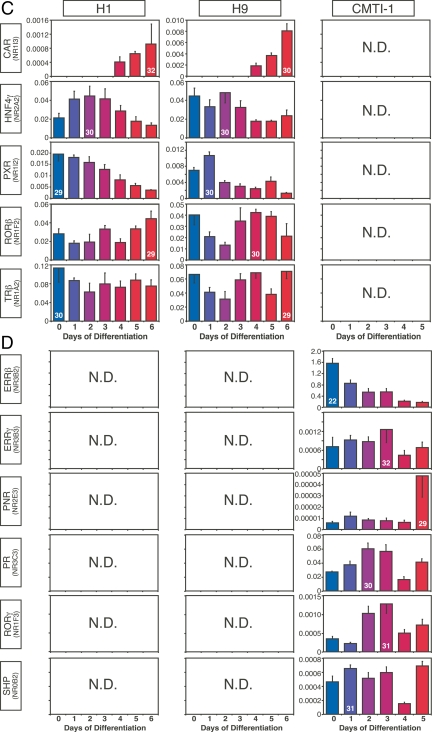
NRs with dissimilar patterns of expression in mouse and human ESCs during embryoid body formation. A, NRs with expression patterns that trended in opposite directions during human and mouse ESC differentiation. B, NRs with no concordant expression pattern between species. C and D, NRs expressed only in human (C) or only in mouse (D) during ESC differentiation. N.D., Not detected (Ct >32). The x- and y-axes represent time (day) after induced differentiation and relative expression of NRs, respectively. Values are expressed as mean ± sd from triplicates of each sample. Ct of the highest expressing value for each NR group is indicated inside its corresponding bar.
Dynamic expression of NRs during ESC differentiation
ESCs are a useful model for studying early events in development as the generation of ESC-derived embryoid bodies recapitulates early embryo development (22,23). To begin to understand the physiological relevance of NRs during the earliest stages of ESC differentiation, we profiled their expression during the first 6 d of human and 5 d of mouse ESC differentiation into embryoid bodies. The dynamic patterns of expression observed for each of the NRs during embryoid body differentiation could be divided broadly and arbitrarily into two distinguishing categories: NRs with expression patterns that were similar between human and mouse (Fig. 3) and NRs with expression patterns that were different between human and mouse (Fig. 4). NRs with similar expression patterns could be further divided into three groups: NRs (COUP-TFγ, GR, and RARγ) with decreasing expression throughout differentiation (Fig. 3A), NRs (HNF4α, NOR1, and PPARγ) with increasing expression throughout differentiation (Fig. 3B), and NRs (GCNF, PPARδ, RORα, and RXRα) with essentially unchanged expression (Fig. 3C).
Twenty-one NRs showed expression patterns that were different between species during early development (Fig. 4, A and B). Expression of NRs that tended to increase during differentiation of human but decrease during differentiation of mouse ESCs included COUP-TFα, LRH-1, NURR1, PPARα, RARα, RARβ, REV-ERBβ, RXRγ, TR2, and TR4 (Fig. 4A). Likewise, expression of NRs that were shared during human and mouse ESC differentiation, but whose patterns were not correlated, included COUP-TFβ, DAX-1, ERRα, LXRα, LXRβ, MR, NGFI-B, REV-ERBα, RXRβ, TRα, and VDR (Fig. 4B). Perhaps even more importantly, NRs that were expressed exclusively in either human and mouse undifferentiated ESCs (see Fig. 2A) maintained this exclusive expression pattern throughout embryoid body formation. Thus, CAR, HNF4γ, PXR, RORβ, and TRβ were expressed only in human ESC lines [albeit at low levels (Ct 29–32)] (Fig. 4C), whereas ERRβ, ERRγ, PNR, PR, RORγ, and SHP were detected only in mouse ESCs (Fig. 4D). Perhaps the most conspicuous difference was the expression of ERRβ, which was highly expressed (Ct 22) in mouse ESCs but undetectable in human ESCs. In addition, it is of interest to note that the relative expression levels of a number of commonly expressed NRs varied substantially (>10-fold) between human and mouse (supplemental Table 3). These NRs included DAX-1 (Ct 31 in human vs. Ct 25 in mouse), LRH-1 (Ct 29 in human vs. Ct 25 in mouse), NGFI-B (Ct 30 in human vs. Ct 26 in mouse), and RARγ (Ct 25 in human vs. Ct 21 in mouse). These data further support the notion that a select, small set of NRs may have species-specific functions that are crucial for ESC biology.
Bioinformatics analysis reveals species-specific differences in NR expression during ESC differentiation
To further evaluate the essential features that define NR expression during embryoid body formation, and further delineate those NRs that share the most similarity between the two species, we performed unsupervised cluster analysis of the pair-wise Pearson correlation values for the 33 NRs that were commonly expressed (albeit in unique patterns) throughout the differentiation process. Consistent with their expression in undifferentiated cells, heat-map patterns from self-comparisons and cell line to cell line comparisons showed that the two human ESC lines shared highly concordant expression patterns during the first 6 d of differentiation into embryoid bodies (Fig. 5, compare A and B with C). These data suggest that differentiation of the two human cell lines is progressing through a similar phenotypic pathway.
Figure 5.
Bioinformatic analysis of the NR superfamily expression profile in human vs. mouse ESCs. A–F, Heat maps representing cell line self-comparisons (H1 in A, H9 in B, CMTI-1 in D) or cell line to cell line comparisons (H1 with H9 in C, H1 with CMTI-1 in E, H9 with CMTI-1 in F) of NR expression during ESC embryoid body formation. The heat maps show unsupervised cluster analysis for pair-wise Pearson correlation values of the 33 NRs that were commonly expressed during differentiation in all three cell lines. Samples were compared pair-wise for each day of differentiation (d 0 through d 5 for CMTI-1 and through d 6 for H1 and H9). Increased color brightness indicates NR pairs whose expression was more positively (red) or negatively (green) correlated between samples. G–I, Correlation of the relative expression levels for 33 NRs that were commonly expressed during differentiation of human and mouse ESCs into embryoid bodies (d 0 through d 5). Relative expression values between each cell line are compared by scatter plot. Linear regression analysis was used to obtain the overall correlation (R2) of NR expression for each cell line pair (H1 vs. H9 in G, H1 vs. CMTI-1 in H, and H9 vs. CMTI-1 in I). Both x- and y-axes represent normalized expression levels for each sample. Insets represent magnified scattered plots of red boxed regions.
In marked contrast, there were diametric differences in the correlations between human and mouse ESCs (Fig. 5, compare D with E and F). Indeed, inspection of the human vs. mouse ESC differentiation patterns revealed a striking, almost mirror-image relationship. NRs that exhibited one correlation pattern when compared in the mouse cells exhibited a notably opposite pattern when compared between the mouse and either of the two human cells. Linear regression analysis of these data confirmed that the dynamics of NR expression between the two human ESCs during embryoid body formation were highly correlative (R2 = 0.83), whereas between the human and mouse ESCs, this correlation was insignificant (R2 < 0.001) (Fig. 5, G–I). Taken together, these marked differences in NR expression between species imply that during early differentiation, human and mouse ESCs may use distinct transcriptional programs.
Discussion
This study provides the first system-based approach to understanding the role of the NR superfamily in the maintenance and differentiation of ESCs. Using a high-throughput QPCR approach, we found NRs exhibit a highly dynamic, complex pattern of expression in both undifferentiated ESCs and during early cell lineage differentiation. Given their well-known roles as mediators of hierarchical transcriptional networks in postembryonic tissues (12), this work suggests a previously unanticipated role for many of the NRs at the very earliest stages of development. Perhaps even more intriguing, we found that although human and mouse ESCs express a common set of NRs, there were substantial interspecies differences in the relative levels of the majority of NRs found in both species. Moreover, during the process of early ESC differentiation into embryoid bodies, the dynamic patterns of NR expression varied between the two species in an almost diametric fashion. Although we cannot exclude the possibility that the major differences we observed in NR expression between human and mouse undifferentiated ESCs are due to cell-specific growth rates and/or culture requirements, we note that a study comparing human ESC lines in different culture conditions found the profile of gene expression to be similar in all cases (8). This suggests that the differences observed here between NR expression in undifferentiated human and mouse ESCs are likely to be species specific rather than arising from culture conditions.
Although previous studies have highlighted the differences in gene expression that exist between mouse and human ESCs (8,10,11), the interpretation of these studies has been made difficult by the lack of mechanistic insight that can be derived from global gene array analyses. Nevertheless, one common feature of all these studies has been the finding that only a small core set of genes is likely to exist that are shared and important for ESC biology in both species (11). Reinforcing this notion, in our study, we found a surprisingly small number of NRs commonly expressed between species that also have been implicated as crucial to ESC viability and pluripotency. NRs that have been reported to be required for mouse ESC maintenance and early differentiation include COUP-TFα, ERRβ, DAX-1, GCNF, LRH-1, RARs and RXRs, and TR2 (24,25,26). However, of these NRs, only GCNF and a subset of the retinoid receptors exhibited similar patterns of expression in undifferentiated ESCs and during early embryoid body formation in both mouse and human. During mouse embryogenesis, GCNF has been shown to be essential for the repression of pluripotency genes such as Oct-4 and Nanog, and also in the initiation of differentiation (25,27). Numerous reports have shown that disruption of various members of the retinoid receptor subfamily (both RARs and RXRs) markedly impair early mouse development (28,29,30,31), and it is well known that retinoic acid is an important regulator for ESC differentiation and early development in both mice and humans (32,33,34,35,36). Therefore, it is likely a similar role for GCNF and the retinoid receptors exists in humans.
Given the importance of ERRβ, DAX-1, and LRH-1 in maintaining mouse ESC pluripotency (37,38,39), the finding that these NRs exhibited markedly different patterns of expression in human ESCs clearly warrants further investigation. ERRβ is believed to maintain the pluripotent state by interacting with Nanog and Oct-4 in mouse ESCs (38,40,41). In addition, both genetic and pharmacological mouse models have revealed a prominent role for ERRβ in trophoblast differentiation and placenta formation (42,43,44). Surprisingly, however, although we found ERRβ to be expressed abundantly in mouse ESCs, it was undetectable in human ESCs and remained so throughout their early differentiation (Fig. 4D). Thus, our observation that ERRβ is exclusively expressed in mouse ESCs might underlie a fundamental, species-specific difference that governs the formation, structure, and function of early embryonic and placental development (3,4,5).
DAX-1 is another orphan NR that appears to be crucial in early mouse embryogenesis (45). It has been implicated in other profiling and experimental studies in the maintenance of pluripotency, in part by also interacting with Nanog (39). Further evidence has shown DAX-1 is controlled by STAT3 and Oct-3/4 to maintain the self-renewal of ESCs (46). Consistent with these findings, DAX-1 expression was notably abundant in undifferentiated mouse ESCs, and gradually declined during early differentiation (Fig. 4B). However, in marked contrast to mouse, DAX-1 mRNA levels were several orders of magnitude lower in human ESCs, suggesting that its role in human ESCs may be quite different (Fig. 4B and supplemental Table 3). Likewise, LRH-1, which is believed to be a key factor in the maintenance of Oct-4 expression and stem cell proliferation (37,47), was expressed at relatively high levels in undifferentiated mouse ESCs but was nearly undetectable in differentiated embryoid bodies (Fig. 4A). Again, a diametric pattern of expression was observed in human ESCs, where LRH-1 mRNA was expressed at relatively low levels in undifferentiated cells, and increased during differentiation (Fig. 4A and supplemental Table 3).
The COUP-TFs are another subfamily of orphan NRs that have been shown to have important roles in governing neurogenesis and organogenesis in mice (48,49). Overexpression of COUP-TFα in mouse ESCs can reduce retinoic acid-associated growth arrest and increase extraembryonic endoderm gene expression, suggesting that COUP-TFα modulates the earliest stages of retinoic acid-mediated embryonic development (26). In the present study, the mRNA expression patterns of mouse COUP-TFα and β were expressed at relatively low levels during differentiation, whereas their human orthologs showed striking dynamic increases in expression during differentiation. Taken together, these studies call into question the function of these prominent factors in human ESC biology and highlight the need for further analysis.
The results from the comparison of mouse and human NR expression during ESC differentiation further strengthen the notion that the overall regulatory blueprint is more species dependent than previously appreciated. One interpretation of these data is that, as observed in the undifferentiated cells, only a fraction of NRs forms the principle component set that is needed to drive a common transcriptional programming during differentiation. However, if this were the case, one would not have expected that the overall correlation would be so diametrically different. Another plausible explanation is that the environmental niche occupied by each ESC population contributes substantially and in a species-specific way to the differentiation process. For this reason, we cannot rule out the important concern inherent with these studies that spontaneous differentiation to more than one cell type may have occurred between the different species-specific cell lines, which could clearly affect which NR is expressed temporally.
At present, the functional consequences of the expression for the majority of the human NRs that are differentially expressed during embryoid body formation are unknown, and it is not clear that they are crucial for ESC biology or species-specific functions. This notion is supported by the finding that the germline knockouts of many of these receptors have no embryonic phenotype. Nevertheless, the observation that two thirds of the NRs in this category displayed different expression patterns between species supports the possibility that innate species specificity is encoded in part by NR expression.
In summary, our results show that NRs may be involved in self-renewal and maintenance of undifferentiated ESCs and early cell lineage differentiation as reflected by their complex patterns of variation during this process. At the same time, we found that major differences may exist in NR expression between mouse and human ESC populations and during early differentiation, further stressing the need to account for species-specific differences in this field of research. Future work may be directed toward understanding the exact function of each NR in maintaining ESCs in undifferentiated status and during differentiation with the final goal of generating lineage-restricted progenitor and mature cells suitable for therapeutic applications. Thus, given their importance as transcriptional sensors for endocrine hormones and other lipophilic signaling molecules that govern broad aspects of developmental, metabolic, and immune response programs, the characterization of the NR superfamily in undifferentiated and early differentiated ESCs should provide a useful resource for both basic and clinical studies.
Materials and Methods
ESC culture and differentiation
A schematic of the in vitro differentiation procedure for ESCs are shown in Fig. 1A. In brief, human ESCs (H1 and H9, NIH-designated WA01 and WA09; passage 32–40) (50) were expanded as previously described (51) on an irradiated mouse embryonic fibroblast feeder layer in growth medium consisting of knockout Dulbecco’s modified Eagle’s medium, knockout serum replacement (KO-SR), l-glutamine, β-mercaptoethanol, nonessential amino acids, and human basic fibroblast growth factor (Invitrogen, Carlsbad, CA). Cultures were split once a week by incubation in 1 mg/ml collagenase IV (Invitrogen) for 10 min at 37 C and seeded on freshly prepared inactivated mouse embryonic fibroblasts. For formation of embryoid bodies, human ES colonies were digested by using 1 mg/ml collagenase type IV and transferred into ultra-low-attachment six-well plates (Corning Inc., Corning, NY) to allow their aggregation in suspension. Human embryoid bodies were grown in the same culture medium without human basic fibroblast growth factor and with 20% fetal bovine serum (FBS) (Invitrogen) to replace the KO-SR. Medium was changed every day. To test the undifferentiated state of human ESCs, colonies were fixed to analyze AKP activity and ESC-specific surface markers, SSEA-4 and TRA-1-60, through immunofluorescence using an ESC characterization kit following the manufacturer’s recommendations (Chemicon, Temecula, CA). ESC differentiation in mouse and human ESCs was characterized by QPCR using primers (see supplemental Table 4) to OCT4 and Nanog (pluripotency primers) and three germ layer-specific proteins (AFP, nestin, GATA2, and keratin).
The murine ESC line (CMTI-1) was derived from the 129/SvEv/Tac strain of mice and is widely used in generating germline transmission in mice (Specialty Media, Flanders, NJ). These cells were routinely cultured on tissue culture plates coated with 0.1% gelatin (Sigma-Aldrich, St. Louis, MO) in DMEM/F12 (Invitrogen) in the presence of 15% ES cell-qualified FBS (Hyclone, Logan, UT), 0.1 mm 2-mercaptoethanol, 1 mm glutamine, 0.1 mm nonessential amino acids (Invitrogen) and 1000 U/ml murine leukemia inhibitory factor (Chemicon). Cells were trypsinized and replated or re-fed every second day. Murine embryoid bodies were formed from single mouse ESCs grown in suspension in DMEM plus 10% ES cell-qualified FBS without leukemia inhibitory factor on ultra-low-attachment six-well plates. Samples in the undifferentiated stage consisted of a minimum of 50 ESC clumps for human and a minimum of 106 ESCs in suspension for mouse. Differentiated samples contained 50–100 embryoid bodies that were collected every day in both cases.
RNA isolation and QPCR
Total cellular RNA was isolated from undifferentiated ESCs and different time embryoid bodies using RNA Stat-60 (Tel-Test, Friendswood, TX) as previously described (21). The mRNA levels in each sample were measured using the TaqMan-based standard curve assay with an ABI 7900HT Sequence Detection System as described previously (52). The primer/probe sets for the 48 (human) and 49 (mouse) NRs were validated as described (supplemental Table 2) (21) and online at www.NURSA.org. PCR efficiencies were calculated according to the previous report (21). PCR efficiencies were evaluated from the slope of the standard curves using the formula E = 10−1/slope, where E is efficiency. The generated efficiency was used to convert Ct from log to linear scale using E−Ct. Normalized mRNA levels were obtained by dividing the averaged, efficiency-corrected nuclear receptor values by that of 18S for each sample [(ENHR)−CtNHR/(E18S)−Ct18S]. The resulting values were multiplied by 106 and plotted ± sd from triplicates of each sample. Ct for each NR was used to assess relative changes in mRNA levels between two samples (52).
Bioinformatics analysis
Unsupervised cluster analysis was performed on the normalized RNA levels by calculating Pearson’s centered correlation coefficients followed by average linkage analysis using Eisen software (http://rana. lbl.gov/eisen/). In brief, 1) pair-wise correlation values were calculated for each receptor-to-receptor pair based on the tissue distribution profile, given the formula 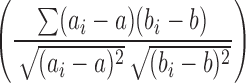 , where ai and bi are data points being compared and a and b are their respective averages. Then, the correlation values were input into the Eisen software, which then analyzed the data as follows. This calculation centers the data, meaning that the scale of the y-axis is, in essence, ignored. 2) The resulting Pearson coefficients were used to calculate the distance metric that is illustrated by the lines connecting each member of a cluster on the heat map. The NR pairs with the highest correlation coefficient segregated together to form a node. The lengths of the lines between the nodes are relative to the strength of their correlations.
, where ai and bi are data points being compared and a and b are their respective averages. Then, the correlation values were input into the Eisen software, which then analyzed the data as follows. This calculation centers the data, meaning that the scale of the y-axis is, in essence, ignored. 2) The resulting Pearson coefficients were used to calculate the distance metric that is illustrated by the lines connecting each member of a cluster on the heat map. The NR pairs with the highest correlation coefficient segregated together to form a node. The lengths of the lines between the nodes are relative to the strength of their correlations.
Supplementary Material
Acknowledgments
We thank Steven Kliewer for critically evaluating and reading the manuscript.
Footnotes
This work was supported by the Howard Hughes Medical Institute (D.J.M.), Robert A. Welch Foundation (Grant I-1275 to D.J.M.), the National Institutes of Health (NURSA Grant U19DK62434 to D.J.M.; HL068878, HL075397, and HL89544 to Y.E.C.), the American Diabetes Association (7-03-JF-18 to M.F.), and the American Heart Association Southeast Affiliate (0525510B to C.-Q.X.). D.J.M. is an investigator of the Howard Hughes Medical Institute. Y.E.C. is an established investigator of American Heart Association (0840025N).
Disclosure Summary: The authors have nothing to disclose.
First Published Online February 5, 2009
Abbreviations: AKP, Alkaline phosphatase; Ct, cycle time; ESC, embryonic stem cell; FBS, fetal bovine serum; KO-SR, knockout serum replacement; NR, nuclear receptor; QPCR, quantitative real-time PCR; SSEA-4, stage-specific embryonic antigen 4; TRA-1-60, tumor rejection antigen-1-60.
References
- Hoffman LM, Carpenter MK 2005 Characterization and culture of human embryonic stem cells. Nat Biotechnol 23:699–708 [DOI] [PubMed] [Google Scholar]
- Donovan PJ, Gearhart J 2001 The end of the beginning for pluripotent stem cells. Nature 414:92–97 [DOI] [PubMed] [Google Scholar]
- Carter AM 2007 Animal models of human placentation: a review. Placenta 28(Suppl A):S41–S47 [DOI] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Costantini F, Lacy E 1994 Manipulating the mouse embryo: a laboratory manual. New York: Cold Spring Harbor Laboratory Press [Google Scholar]
- O'Rahilly R, Bossy J, Muller F 1981 [Introduction to the study of embryonic stages in man]. Bull Assoc Anat (Nancy) 65:141–236 (French) [PubMed] [Google Scholar]
- Brandenberger R, Wei H, Zhang S, Lei S, Murage J, Fisk GJ, Li Y, Xu C, Fang R, Guegler K, Rao MS, Mandalam R, Lebkowski J, Stanton LW 2004 Transcriptome characterization elucidates signaling networks that control human ES cell growth and differentiation. Nat Biotechnol 22:707–716 [DOI] [PubMed] [Google Scholar]
- Cloonan N, Forrest AR, Kolle G, Gardiner BB, Faulkner GJ, Brown MK, Taylor DF, Steptoe AL, Wani S, Bethel G, Robertson AJ, Perkins AC, Bruce SJ, Lee CC, Ranade SS, Peckham HE, Manning JM, McKernan KJ, Grimmond SM 2008 Stem cell transcriptome profiling via massive-scale mRNA sequencing. Nat Methods 5:613–619 [DOI] [PubMed] [Google Scholar]
- Ginis I, Luo Y, Miura T, Thies S, Brandenberger R, Gerecht-Nir S, Amit M, Hoke A, Carpenter MK, Itskovitz-Eldor J, Rao MS 2004 Differences between human and mouse embryonic stem cells. Dev Biol 269:360–380 [DOI] [PubMed] [Google Scholar]
- Richards M, Tan SP, Tan JH, Chan WK, Bongso A 2004 The transcriptome profile of human embryonic stem cells as defined by SAGE. Stem Cells 22:51–64 [DOI] [PubMed] [Google Scholar]
- Sato N, Sanjuan IM, Heke M, Uchida M, Naef F, Brivanlou AH 2003 Molecular signature of human embryonic stem cells and its comparison with the mouse. Dev Biol 260:404–413 [DOI] [PubMed] [Google Scholar]
- Wei CL, Miura T, Robson P, Lim SK, Xu XQ, Lee MY, Gupta S, Stanton L, Luo Y, Schmitt J, Thies S, Wang W, Khrebtukova I, Zhou D, Liu ET, Ruan YJ, Rao M, Lim B 2005 Transcriptome profiling of human and murine ESCs identifies divergent paths required to maintain the stem cell state. Stem Cells 23:166–185 [DOI] [PubMed] [Google Scholar]
- Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ 2006 Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell 126:789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ 2001 Nuclear receptors and lipid physiology: opening the X-files. Science 294:1866–1870 [DOI] [PubMed] [Google Scholar]
- Germain P, Staels B, Dacquet C, Spedding M, Laudet V 2006 Overview of nomenclature of nuclear receptors. Pharmacol Rev 58:685–704 [DOI] [PubMed] [Google Scholar]
- Hong SH, Nah HY, Lee YJ, Lee JW, Park JH, Kim SJ, Lee JB, Yoon HS, Kim CH 2004 Expression of estrogen receptor-α and -β, glucocorticoid receptor, and progesterone receptor genes in human embryonic stem cells and embryoid bodies. Mol Cell 18:320–325 [PubMed] [Google Scholar]
- Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R 1985 The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol 87:27–45 [PubMed] [Google Scholar]
- Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A 2000 Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol 18:399–404 [DOI] [PubMed] [Google Scholar]
- Murry CE, Keller G 2008 Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 132:661–680 [DOI] [PubMed] [Google Scholar]
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A 2003 Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 113:643–655 [DOI] [PubMed] [Google Scholar]
- Kuroda T, Tada M, Kubota H, Kimura H, Hatano SY, Suemori H, Nakatsuji N, Tada T 2005 Octamer and Sox elements are required for transcriptional cis regulation of Nanog gene expression. Mol Cell Biol 25:2475–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, Sun T, Bookout AL, Downes M, Yu RT, Evans RM, Mangelsdorf DJ 2005 A nuclear receptor atlas: 3T3-L1 adipogenesis. Mol Endocrinol 19:2437–2450 [DOI] [PubMed] [Google Scholar]
- Conley BJ, Denham M, Gulluyan L, Olsson F, Cole TJ, Mollard R 2005 Mouse embryonic stem cell derivation, and mouse and human embryonic stem cell culture and differentiation as embryoid bodies. Curr Protoc Cell Biol 23:23.2.1–23.2.22 [DOI] [PubMed] [Google Scholar]
- Rao M 2004 Conserved and divergent paths that regulate self-renewal in mouse and human embryonic stem cells. Dev Biol 275:269–286 [DOI] [PubMed] [Google Scholar]
- Gupta P, Ho PC, Huq MM, Ha SG, Park SW, Khan AA, Tsai NP, Wei LN 2008 Retinoic acid-stimulated sequential phosphorylation, PML recruitment, and SUMOylation of nuclear receptor TR2 to suppress Oct4 expression. Proc Natl Acad Sci USA 105:11424–11429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen EM, Gu P, Cooney AJ 2007 Nuclear receptors in regulation of mouse ES cell pluripotency and differentiation. PPAR Res 2007:61563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Y, Gudas LJ 2008 Overexpression of COUP-TF1 in murine embryonic stem cells reduces retinoic acid-associated growth arrest and increases extraembryonic endoderm gene expression. Differentiation 76:760–771 [DOI] [PubMed] [Google Scholar]
- Fuhrmann G, Chung AC, Jackson KJ, Hummelke G, Baniahmad A, Sutter J, Sylvester I, Schöler HR, Cooney AJ 2001 Mouse germline restriction of Oct4 expression by germ cell nuclear factor. Dev Cell 1:377–387 [DOI] [PubMed] [Google Scholar]
- Honda M, Hamazaki TS, Komazaki S, Kagechika H, Shudo K, Asashima M 2005 RXR agonist enhances the differentiation of cardiomyocytes derived from embryonic stem cells in serum-free conditions. Biochem Biophys Res Commun 333:1334–1340 [DOI] [PubMed] [Google Scholar]
- Kastner P, Grondona JM, Mark M, Gansmuller A, LeMeur M, Decimo D, Vonesch JL, Dollé P, Chambon P 1994 Genetic analysis of RXRα developmental function: convergence of RXR and RAR signaling pathways in heart and eye morphogenesis. Cell 78:987–1003 [DOI] [PubMed] [Google Scholar]
- Luo J, Sucov HM, Bader JA, Evans RM, Giguère V 1996 Compound mutants for retinoic acid receptor (RAR)β and RARα1 reveal developmental functions for multiple RARβ isoforms. Mech Dev 55:33–44 [DOI] [PubMed] [Google Scholar]
- Zechel C 2005 Requirement of retinoic acid receptor isotypes α, β, and γ during the initial steps of neural differentiation of PCC7 cells. Mol Endocrinol 19:1629–1645 [DOI] [PubMed] [Google Scholar]
- Duester G 2008 Retinoic acid synthesis and signaling during early organogenesis. Cell 134:921–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajović S, Chowdhury K, Gruss P 1998 Genes expressed after retinoic acid-mediated differentiation of embryoid bodies are likely to be expressed during embryo development. Exp Cell Res 242:138–143 [DOI] [PubMed] [Google Scholar]
- Lammer EJ, Chen DT, Hoar RM, Agnish ND, Benke PJ, Braun JT, Curry CJ, Fernhoff PM, Grix Jr AW, Lott IT, Richard JM, Sun SC 1985 Retinoic acid embryopathy. N Engl J Med 313:837–841 [DOI] [PubMed] [Google Scholar]
- Thaller C, Eichele G 1987 Identification and spatial distribution of retinoids in the developing chick limb bud. Nature 327:625–628 [DOI] [PubMed] [Google Scholar]
- Wichterle H, Lieberam I, Porter JA, Jessell TM 2002 Directed differentiation of embryonic stem cells into motor neurons. Cell 110:385–397 [DOI] [PubMed] [Google Scholar]
- Gu P, Goodwin B, Chung AC, Xu X, Wheeler DA, Price RR, Galardi C, Peng L, Latour AM, Koller BH, Gossen J, Kliewer SA, Cooney AJ 2005 Orphan nuclear receptor LRH-1 is required to maintain Oct4 expression at the epiblast stage of embryonic development. Mol Cell Biol 25:3492–3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova N, Dobrin R, Lu R, Kotenko I, Levorse J, DeCoste C, Schafer X, Lun Y, Lemischka IR 2006 Dissecting self-renewal in stem cells with RNA interference. Nature 442:533–538 [DOI] [PubMed] [Google Scholar]
- Wang J, Rao S, Chu J, Shen X, Levasseur DN, Theunissen TW, Orkin SH 2006 A protein interaction network for pluripotency of embryonic stem cells. Nature 444:364–368 [DOI] [PubMed] [Google Scholar]
- van den Berg DL, Zhang W, Yates A, Engelen E, Takacs K, Bezstarosti K, Demmers J, Chambers I, Poot RA 2008 Estrogen-related receptor β interacts with Oct4 to positively regulate Nanog gene expression. Mol Cell Biol 28:5986–5995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang J, Wang T, Esteban MA, Pei D 2008 Esrrb activates Oct4 transcription and sustains self renewal and pluripotency in embryonic stem cells. J Biol Chem 283:35825–35833 [DOI] [PubMed] [Google Scholar]
- Chen J, Nathans J 2007 Estrogen-related receptor beta/NR3B2 controls epithelial cell fate and endolymph production by the stria vascularis. Dev Cell 13:325–337 [DOI] [PubMed] [Google Scholar]
- Luo J, Sladek R, Bader JA, Matthyssen A, Rossant J, Giguère V 1997 Placental abnormalities in mouse embryos lacking the orphan nuclear receptor ERR-β. Nature 388:778–782 [DOI] [PubMed] [Google Scholar]
- Tremblay GB, Kunath T, Bergeron D, Lapointe L, Champigny C, Bader JA, Rossant J, Giguère V 2001 Diethylstilbestrol regulates trophoblast stem cell differentiation as a ligand of orphan nuclear receptor ERRβ. Genes Dev 15:833–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris TP, Guo W, McCabe ER 1996 The gene responsible for adrenal hypoplasia congenita, DAX-1, encodes a nuclear hormone receptor that defines a new class within the superfamily. Recent Prog Horm Res 51:241–259; discussion 259–260 [PubMed] [Google Scholar]
- Sun C, Nakatake Y, Ura H, Akagi T, Niwa H, Koide H, Yokota T 2008 Stem cell-specific expression of Dax1 is conferred by STAT3 and Oct3/4 in embryonic stem cells. Biochem Biophys Res Commun 372:91–96 [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W 2000 Requirement for β-catenin in anterior-posterior axis formation in mice. J Cell Biol 148:567–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JI, Tsai SY, Tsai MJ 2003 Molecular mechanism of chicken ovalbumin upstream promoter-transcription factor (COUP-TF) actions. Keio J Med 52:174–181 [DOI] [PubMed] [Google Scholar]
- Warnecke M, Oster H, Revelli JP, Alvarez-Bolado G, Eichele G 2005 Abnormal development of the locus coeruleus in Ear2(Nr2f6)-deficient mice impairs the functionality of the forebrain clock and affects nociception. Genes Dev 19:614–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assady S, Maor G, Amit M, Itskovitz-Eldor J, Skorecki KL, Tzukerman M 2001 Insulin production by human embryonic stem cells. Diabetes 50:1691–1697 [DOI] [PubMed] [Google Scholar]
- Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, Itskovitz-Eldor J, Thomson JA 2000 Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol 227:271–278 [DOI] [PubMed] [Google Scholar]
- Bookout AL, Mangelsdorf DJ 2003 Quantitative real-time PCR protocol for analysis of nuclear receptor signaling pathways. Nucl Recept Signal 1:e012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.