Abstract
The role of the hedgehog (HH) signaling pathway in ovarian function was examined in transgenic mice in which expression of a dominant active allele of the signal transducer smoothened (SmoM2) was directed to the ovary and Müllerian duct by cre-mediated recombination (Amhr2cre/+SmoM2). Mutant mice were infertile and had ovarian and reproductive tract defects. Ovaries contained follicles of all sizes and corpora lutea (CL), but oocytes were rarely recovered from the oviducts of superovulated mice and remained trapped in preovulatory follicles. Measures of luteinization did not differ. Cumulus expansion appeared disorganized, and in vitro analyses confirmed a reduced expansion index. Microarray analysis indicated that expression levels of genes typical of smooth muscle were reduced in mutant mice, and RT-PCR showed that levels of expression of muscle genes were reduced in the nongranulosa, theca-interstitial cell-enriched fraction. Whereas a layer of cells in the outer theca was positively stained for smooth muscle actin in control ovaries, this staining was reduced or absent in mutant ovaries. Expression of a number of genes in granulosa cells that are known to be important for ovulation did not differ in mutants and controls. Expression of components of the HH pathway was observed in both granulosa cells and in the nongranulosa, residual ovarian tissue and changed in response to treatment with equine chorionic gonadotropin/human gonadotropin. The results show that appropriate signaling through the HH pathway is required for development of muscle cells within the theca and that impaired muscle development is associated with failure to release the oocyte at ovulation.
Overactivation of signaling through the hedgehog pathway in ovarian follicles of transgenic mice impairs development of muscle cells within the theca and prevents ovulation.
The hedgehog (HH) signaling pathway plays a critical role in the development of multiple organs in the embryo and in remodeling processes in adult tissues (1,2). In addition, aberrant HH signaling is associated with the development of cancer (3). Processes regulated by HH signaling include cell fate determination, proliferation, and differentiation. HH signaling is critical for ovarian function in Drosophila (1), but its role in the mammalian ovary is not known. Expression of components of the HH signaling pathway have been reported in the mouse ovary, and granulosa and theca cells have been identified as potential targets (4,5).
In mammals, components of the HH pathway include three secreted protein ligands, sonic, Indian, and desert hedgehog (SHH, IHH, DHH), which are differentially expressed in various tissues. There are two transmembrane receptors, patched (PTCH) 1 and 2, which are thought to function similarly, and a transmembrane protein smoothened (SMO), which transduces the signal. In the absence of HH ligand, PTCH inhibits signaling through SMO. Binding of ligand to PTCH relieves inhibition of SMO and signaling occurs. Signaling through SMO is conveyed by modulation of the activity of a family of transcription factors, GLI1, GLI2, and GLI3 (6). Gli1 is a transcriptional target of HH signaling and has been used as indicator of activated HH signaling (7,8,9,10). Whereas deletion of Gli1 in the mouse is compatible with normal development (11), deletion of Gli2 and Gli3 generate phenotypes that mimic deletion of Shh, indicating that they are essential for mediating its effects on development (12). GLI2 and GLI3 proteins may act as transcriptional activators or may undergo proteolytic processing to shortened forms that function as transcriptional repressors (13,14).
Gene targeting to alter HH signaling in mice has generally resulted in embryonic lethality, indicating that conditional transgenic approaches are necessary to determine function in adult tissues. The current study analyzed the effect of conditional expression of a dominantly active allele of SMO, known as SmoM2, in the ovary. SMOM2 contains a point mutation that prevents inhibition of its activity by PTCH, thus leading to constitutive activation of HH signaling (15). The phenotype observed in mice expressing dominant active SmoM2 indicates that HH signaling is required for proper development of a layer of smooth muscle cells around follicles and for the release of oocytes from follicles at the time of ovulation.
Results
Generation of mice with conditional expression of dominant active Smo
Mice in which a dominant active allele of Smo, known as SmoM2, is conditionally expressed in the ovary were created using a cre/loxP strategy. A previously engineered transgenic mouse line was used in which expression of an inserted SmoM2/yellow fluorescent protein (Yfp) fusion gene is blocked by a loxP-flanked stop signal [SmoM2 mice (16)]. Homozygous SmoM2 mice were crossed to Amhr2cre/+ mice in which Cre recombinase sequence was inserted into the Amhr2 gene (17). The Amhr2cre/+ allele was originally shown to direct expression of Cre to the gonads and mesenchyme of the Müllerian duct beginning at embryonic d 12.5 (17). Subsequent studies demonstrated Amhr2cre/+-mediated recombination in the adult ovary in granulose cells as well as theca cells (18) and in the developing and adult reproductive tract (19,20).
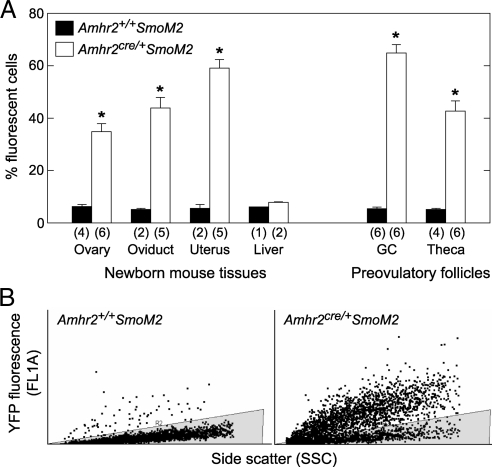
In the current study, sites of Cre-mediated recombination in Amhr2cre/+SmoM2 mice were determined by examining expression of the Yfp fusion gene. Cell suspensions for use in flow cytometry to detect YFP were prepared from whole ovary, oviduct, and uterus of newborn mice and from theca and granulosa cells of preovulatory follicles from equine chorionic gonadotropin (eCG)/human chorionic gonadotropin (hCG)-treated immature mice. Cells from genotype-matched control mice lacking Cre sequence (Amhr2+/+SmoM2) and from liver were analyzed as negative controls. In cells from Amhr2cre/+SmoM2 mice, approximately half of the cells expressed detectable levels of YFP, whereas cells from control mice and liver cells from mice of both genotypes showed background fluorescence (Fig. 1).
Figure 1.
Assessment of the efficiency of cre-mediated recombination in Amhr2cre/+ SmoM2 mice. Dispersed cells from tissues of mutant and control mice were analyzed by flow cytometry to measure fluorescence associated with expression of the SmoM2/Yfp reporter gene. A, Percent of cells from reproductive tissues expressing YFP. Signal obtained from tissues of control mice and from liver of mice of both genotypes represent background fluorescence. Data are mean ± sem of (n) replicates. *, P < 0.05 vs. cells of the same type from Amhr2+/+SmoM2 mice. B, Representative plots of FL1 fluorescence vs. side scatter in granulosa cells. The shaded area is the area representing background fluorescence; cells with fluorescence associated with YFP expression are above the shaded area. GC, Granulosa cells.
Fertility, ovulation, and luteinization
To test fertility, Amhr2cre/+SmoM2 mutant mice and Amhr2+/+SmoM2 control mice were caged continuously with CD-1 males of proven fertility. Vaginal plugs were observed in both mutant and control mice. Control mice had one or two litters within a 2-month period, averaging 9.6 ± 1.0 pups per litter, whereas mutant mice produced no litters (n = 5). In mutant females, mating caused a severe inflammatory response in the reproductive tract (described below). For this reason, subsequent studies on ovarian function were performed using virgin mice to eliminate the confounding effect of uterine pathology associated with breeding. Histological analysis of the reproductive tract and ovaries of virgin Amhr2cre/+SmoM2 mice ranging from prepubertal to 1 yr of age indicated that mice remain healthy when not bred (data not shown). Daily vaginal smears showed that estrous cycle length was longer in mutant mice (6.8 ± 0.3 d) than control mice (5.0 ± 0.4 d, P < 0.05). The percent of days spent in diestrus was greater in mutants than controls (60 ± 3% vs. 31 ± 9%), and the percent of days in proestrus was less (12 ± 2% vs. 27 ± 4%) (supplemental Fig. 1 published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend. endojournals.org).
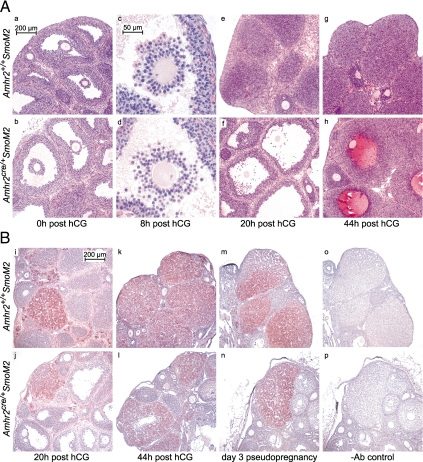
Response to superovulation was tested by collection of oocytes from the ampulla of eCG-primed prepubertal (21–23 d old) and adult mice (50–60 d old) 20 h after treatment with hCG. Oocytes were readily recovered from immature and adult Amhr2+/+SmoM2 control mice (42.7 ± 5.8 and 24.0 ± 1.0 oocytes per mouse, respectively), whereas only occasional oocytes were recovered from immature and adult Amhr2cre/+ SmoM2 mutant mice (0.3 ± 0.3 and 0.3 ± 0.3 oocytes per mouse, respectively). Histological analysis of ovaries isolated 48 h after eCG suggested that preovulatory follicles develop similarly in mutant and control mice (Fig. 2A, panels a and b). By 8 h after hCG, cumulus expansion had occurred in control mice but appeared disorganized in mutant mice (Fig. 2A, panels c and d). Oocytes within follicles of mutant and control mice resumed meiosis as evidenced by the presence of metaphase chromosomes (Fig. 2A, panels c and d). At 20 h after hCG, ovaries of control mice contained newly formed corpora lutea (CL), whereas ovaries of mutant mice lacked new CL and had preovulatory-type follicles containing trapped oocytes (Fig. 2A, panels e and f). No sign that follicle rupture had occurred was observed in histological sections from mutant mice. At 44 h after hCG, luteinization had further progressed in control mice, whereas in mutant mice follicles appeared to be luteinizing but contained blood-filled cavities with trapped oocytes (Fig. 2A, panels g and h). CL containing trapped oocytes were observed in adult mutant mice but not in controls (data not shown). These results indicate that ovulation in Amhr2cre/+SmoM2 mice is severely reduced and that the process of luteinization may be delayed or prolonged.
Figure 2.
A, Ovaries from Amhr2+/+SmoM2 and Amhr2cre/+SmoM2 mice stained with hematoxylin and eosin (a–h). Immature (21–23 d old) mice were injected with eCG followed 48 h later by hCG (0 h). Preovulatory follicle formation appears similar in mutant and control mice at 0 h after hCG (a and b), but at 8 h expansion of the cumulus in mutant mice appears disorganized compared with control mice (c and d). At 20 h, ovaries of control mice contain newly formed CL, whereas ovaries of mutant mice contain unruptured preovulatory-like follicles with trapped oocytes (e and f). At 44 h, CL development had further progressed in control mice, whereas luteinizing follicles of mutant mice have blood-filled cavities with entrapped oocytes (g and h). B, Immunohistochemical detection of SCC (reddish brown) in ovaries from mutant and control mice counterstained with hematoxylin (blue). At 20 h after hCG (I and j), occasional CL stained positively for SCC, but most did not. At 44 h (k and l) and in mice on d 3 of pseudopregnancy (m and n), CL of both mutant and control mice showed distinct staining of SCC. Nonspecific staining, in which nonimmune IgG was added in place of the SCC antibody, is shown (o and p). Ab, Antibody.
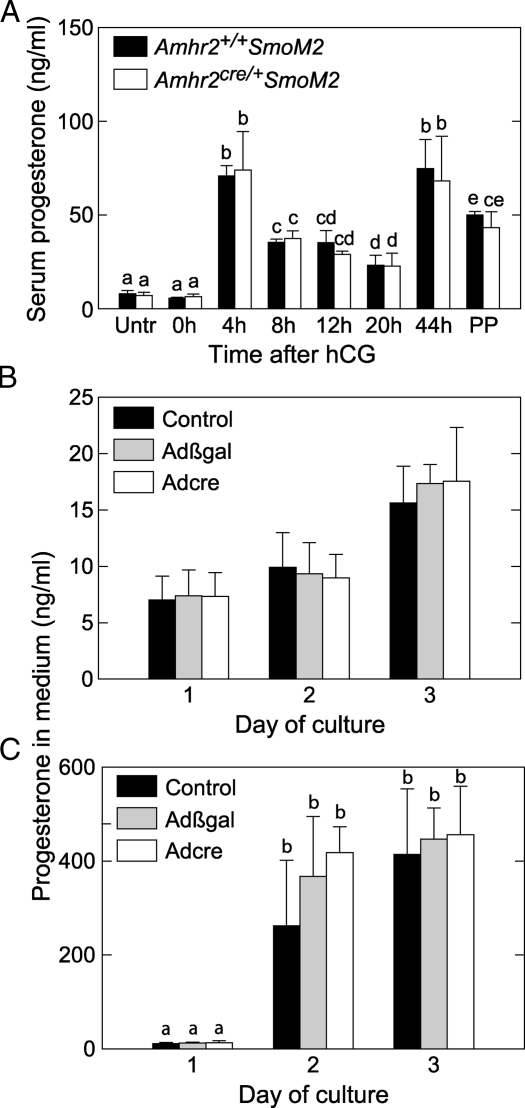
To assess the differentiation of granulosa cells into luteal cells after the LH surge, sections of ovaries were stained for the enzyme cytochrome P450, family 11, subfamily a, polypeptide 1, also known as side chain cleavage (SCC), which is essential for progesterone production. At 20 h after hCG, only occasional CL of control and mutant mice stained positively for SCC (Fig. 2B, panels i and j). At 44 h, SCC was expressed in CL of control mice and also in CL of mutant mice, which sometimes contained blood-filled cavities (Fig. 2B, panels k and l). On d 3 of pseudopregnancy, CL of mutant and control mice appeared grossly similar; both stained positively for SCC and lacked a central cavity (Fig. 2B, panels m and n). Serum concentrations of progesterone after treatment with eCG/hCG and on d 3 of pseudopregnancy were similar in mutant and control mice (Fig. 3A).
Figure 3.
Effects of dominant active SmoM2 on progesterone production. A, Serum progesterone concentrations do not differ in Amhr2cre/+SmoM2 and control mice. Immature (21–23 d old) mice were injected with eCG followed 48 h later by hCG (0 h); n = 4 mice/timepoint. Untr, No hormone treatments; PP, d 3 of pseudopregnancy. B, Activation of SmoM2 expression in cultured granulose cells does not alter progesterone production. Granulosa cells from SmoM2 mice were infected with Adcre at the time of plating to induce expression of the SmoM2/Yfp fusion gene or with no adenovirus or Adβgal as controls. ANOVA indicated no overall difference. C, Granulosa cells from SmoM2 mice were treated as described for panel B, except that 10 ng/ml oLH was added after 1 and 2 d of culture. For data shown in panels B and C, n = 3 separate granulosa cell preparations tested, each receiving all treatments. Data are mean ± sem. Bars without common superscripts are significantly different (P < 0.05).
In Amhr2cre/+SmoM2 mice, follicle cells may express SmoM2 for a prolonged period of time because recombination through Amhr2cre/+ begins during embryonic development. Therefore, effects of acutely inducing expression of SmoM2 in granulosa cells in vitro were determined. Granulosa cells were isolated from preovulatory follicles of eCG-primed homozygous SmoM2 mice and infected in vitro with a recombinant adenovirus expressing Cre (Adcre) or with a control adenovirus expressing β-galactosidase (Adβgal) as a control. The concentration of progesterone in culture medium increased over 3 d and was not altered by infection with adenoviruses (Fig. 3B). Treatment of parallel cultures with LH after the first day of culture increased the magnitude of progesterone secretion, but infection with adenoviruses had no effect (Fig. 3C). Activated expression of the SmoM2/Yfp fusion gene by Adcre was confirmed by flow cytometry of granulosa cells analyzed 16 h after infection. Whereas the majority of cells infected with Adcre were positive for YFP (71 ± 2%), only background fluorescence was observed in cells infected with Adβgal (5 ± 1%).
As indicated above, within 1 month after being caged with males, Amhr2cre/+SmoM2 female mice showed signs of distress and bleeding from the vagina. Histological analysis of the reproductive tract showed changes consistent with an extensive inflammatory response within the uterine lumen (data not shown). Aspects of reproductive tract development are altered in mutant mice, a finding consistent with Cre-mediated recombination occurring in the developing Müllerian duct, and will be reported in detail elsewhere.
Microarray analysis
Microarray analysis was performed to obtain insight into the cause of ovulatory failure in Amhr2cre/+SmoM2 mice. Single arrays were run using whole ovarian RNA prepared from three groups of immature mutant and control mice; untreated, 48 h after eCG and 4 h after hCG in eCG-primed mice. The data were sorted to identify transcripts in which expression differed between mutants and controls for all treatment groups. Six of the 15 transcripts that were most prominently down-regulated in mutants represent proteins that play a role in muscle function (Table 1, muscle genes shown in bold). Two down-regulated transcripts are for extracellular matrix proteins, periostin and tenascin C (TNC). The Amhr2 transcript is reduced in mutant mice, consistent with the presence of a single functional Amhr2 allele. Gene ontogeny analysis of microarray data using the ErmineJ program indicated that the three biological processes most significantly different between mutant and control mice were muscle system processes, smooth muscle contraction, and muscle development (P < 1 × 10−12). Subsequent quantitative RT-PCR analyses of five selected genes confirmed differences between mutants and controls (Table 1).
Table 1.
Results of microarray analysis of ovarian RNA from Amhr2cre/+SmoM2 (mutant) and Amhr2+/+SmoM2 (control) mice, and confirmation by quantitative RT-PCR
| Quantitative RT-PCR (arbitrary units)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fold difference in array (mutant vs. control)
|
Untreated
|
0 h post hCG
|
4 h post hCG
|
||||||||
| Gene symbol | Gene title | 0 h | 48 h | 4 h | Mean | Con | Mut | Con | Mut | Con | Mut |
| Cnn1 | Calponin 1 | −2.52 | −4.55 | −3.11 | −3.30 | 74 ± 4a | 24 ± 1b | 113 ± 15c | 21 ± 4b | 129 ± 15c | 43 ± 8d |
| Des | Desmin | −1.68 | −2.66 | −5.91 | −2.98 | 69 ± 5a | 35 ± 4b | 127 ± 3c | 34 ± 3b | 141 ± 35c | 39 ± 8b |
| Actg2 | Actin, γ 2, smooth muscle, enteric | −2.41 | −4.20 | −2.44 | −2.91 | 62 ± 10a | 26 ± 4b | 115 ± 8c | 30 ± 8b | 122 ± 29c | 44 ± 5a,b |
| Postn | Periostin, osteoblast- specific factor | −3.30 | −2.43 | −1.61 | −2.35 | ||||||
| Akr1c13 | Aldo-keto reductase family 1, member C13 | −1.72 | −3.82 | −1.49 | −2.14 | ||||||
| Myh11 | Myosin, heavy polypeptide 11, | ||||||||||
| Smooth muscle | −1.83 | −1.99 | −2.68 | −2.14 | |||||||
| Tnc | Tenascin C | −1.91 | −1.88 | −2.53 | −2.09 | 154 ± 11a | 69 ± 5b | 70 ± 7b | 29 ± 2c | 324 ± 44d | 115 ± 11e |
| BC005512 | cDNA sequence BC005512 | −2.03 | −1.91 | −2.04 | −1.99 | ||||||
| Amhr2 | Anti-Mullerian hormone type 2 receptor | −1.79 | −1.75 | −2.43 | −1.97 | ||||||
| Tagln | Transgelin | −2.06 | −2.27 | −1.60 | −1.95 | 87 ± 2a,b | 45 ± 4c | 109 ± 17a | 33 ± 3d | 148 ± 10e | 69 ± 11b |
| Tpm2 | Tropomyosin 2, β | −1.91 | −2.20 | −1.69 | −1.92 | ||||||
| Bdnf | Brain-derived neurotrophic factor | −1.42 | −1.79 | −2.74 | −1.91 | ||||||
| Akr1c12/13 | Aldo-keto reductase family 1, member C12/C13 | −1.56 | −3.92 | −1.12 | −1.90 | ||||||
| Slc2a4 | Solute carrier family 2 (facilitated glucose transporter), member 4 | −2.50 | −1.36 | −1.94 | −1.88 | ||||||
| — | Gene model 879 (NCBI) | −2.15 | −1.83 | −1.62 | −1.82 | ||||||
Data are presented for 15 genes that were expressed at the lowest levels in mutants compared with controls, based on the geometric mean fold difference. Each fold difference represents single arrays of RNA pooled from ovaries of three control or mutant mice. RT-PCR data are mean ± sem of RNA from each of the three control or mutant mice used in the array samples. For RT-PCR data, values without common superscripts are significantly different (P < 0.05). Muscle genes are shown in bold.
To determine the predominant cell type in which changes in muscle gene expression occurred, granulosa cells and residual ovarian tissue were isolated and assayed for smooth muscle marker genes, Cnn1, Des, Actg2, and Tagln. Expression of muscle genes was low in granulosa cells, did not differ in mutant and control mice, and did change over time after eCG/hCG treatment. Levels of expression of smooth muscle genes were much higher in residual tissue than in granulosa cells and were substantially reduced in mutant mice compared with controls (Fig. 4). Minor fluctuations in levels of muscle gene expression were observed after eCG/hCG treatments. Tnc expression increased after hCG in both granulosa cells and residual tissue; levels in residual tissue, but not in granulosa cells, were reduced in mutants compared with controls (Fig. 4). Expression of endothelin 2 (Edn2), an agonist capable of inducing contraction of follicular muscle cells (21,22), increased dramatically between 8 and 12 h after hCG in both mutant and control mice and was expressed primarily in granulosa cells (Fig. 4).
Figure 4.
Expression of genes associated with smooth muscle (Cnn1, Des, Actg2, Tagln) or extracellular matrix (Tnc) and expression of Edn2 in granulosa cells and residual ovarian tissue from Amhr2+/+SmoM2 and Amhr2cre/+SmoM2 mice. Tissues were obtained from untreated mice (UT) or from eCG-primed mice before (0 h) or after injection of hCG. Total RNA was assayed by quantitative real-time RT-PCR. Data are mean ± sem; n = 3 granulosa cell and residual tissue preparations. Bars without common superscripts are significantly different (P < 0.05).
Immunohistochemistry of smooth muscle
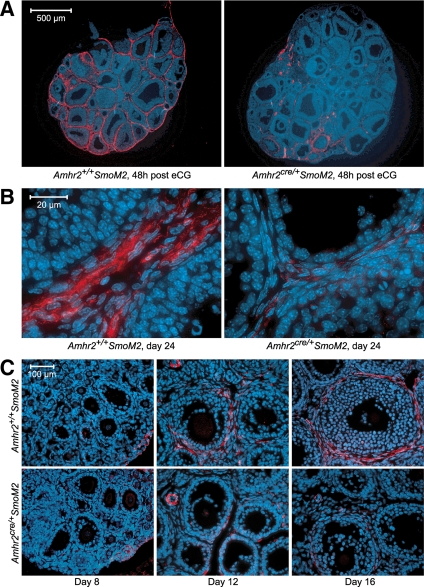
To determine the location of cells with the characteristics of smooth muscle cells, ovarian sections from immature mice were stained for smooth muscle actin α (SMA), which is considered to be a marker for smooth muscle cells, and 4,6-diamidino-2-phenylindole or Hoechst 33342, which mark nuclei. In control mice that were untreated or assessed at 48 h after eCG, a SMA-positive layer of cells was present in the outer theca, consistent with previous studies (21,23) (Fig. 5, A and B). Costaining for von Willebrand’s factor (VWB), which marks endothelial cells, showed that prominent staining for SMA did not coincide with endothelial cells within the theca layer (supplemental Fig. 2). Large blood vessels in the stroma stained positively for SMA in the outer layer and positively for VWB in the inner endothelial cell layer (supplemental Fig. 2). In untreated and eCG-treated mutant mice, SMA staining was absent or dramatically reduced in the theca of the majority of follicles whereas staining in large blood vessels and low-intensity staining in the vicinity of theca endothelial cells appeared similar to controls (Fig. 5, A and B, and supplemental Fig. 2). In ovaries from eCG-primed immature mutant and control mice that were isolated 12 h after hCG, the pattern of SMA staining was similar to that observed in untreated mice and eCG-treated mice (data not shown). When SMA staining was examined in younger mice, it was found to be undetectable in ovaries of 8-d-old control and mutant mice in which primordial and primary follicles predominated (Fig. 5C). On d 12 and 16 of age, SMA was detectable in ovaries of control mice in the outer theca layer of follicles with greater than two layers of granulosa cells (tertiary follicles) and was substantially reduced to nondetectable in follicles of mutant mice (Fig. 5C).
Figure 5.
Staining for the smooth muscle marker, SMA, in ovaries of Amhr2+/+SmoM2 and Amhr2cre/+SmoM2 mice at different ages (B and C) and in immature mice 48 h after injection of eCG (A). Sections were counterstained for nuclei with Hoechst 33342 (blue). Results were confirmed in at least three mice of each genotype and age.
Expression of components of the HH signaling pathway
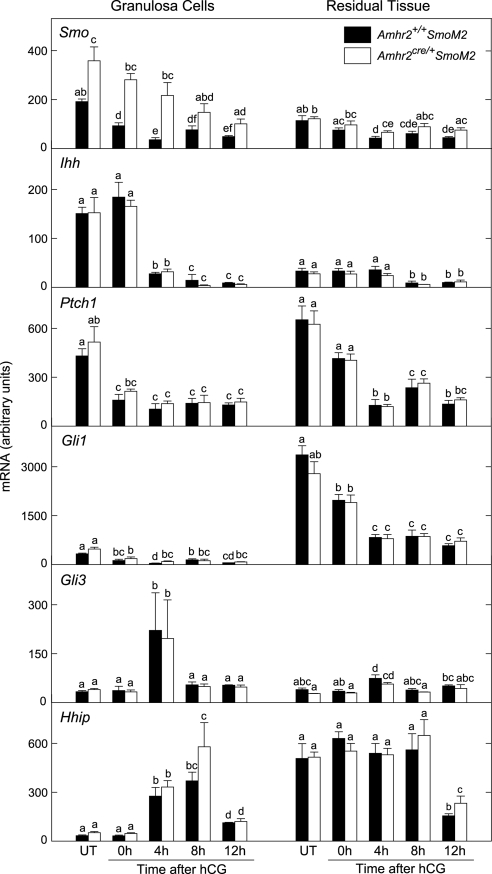
Expression of components of the HH pathway was detectable in both granulosa cells and residual ovarian tissue, and levels of expression changed in response to treatments with eCG/hCG (Fig. 6). Smo was consistently elevated in granulosa cells of mutant mice relative to controls. Smo levels were lower in residual tissue than in granulosa cells and were slightly elevated in mutants relative to controls at several time points. Ihh was expressed at substantially higher levels in granulosa cells than in residual tissue; expression in granulosa cells declined between 0 and 4 h after hCG and remained at basal levels thereafter. Levels of Ptch1, Gli3, and Hhip were within the same general range in granulosa cells and residual tissue and in mutant and control mice. However, the pattern of changes in gene expression in response to eCG/hCG differed in the two fractions of cells. Levels of Gli1 were substantially higher in residual tissue compared with granulosa cells and did not differ between mutants and controls. Gli1 in residual tissue decreased in response to eCG/hCG.
Figure 6.
Expression of genes in the HH signaling pathway in granulosa cells and residual ovarian tissue from Amhr2+/+SmoM2 and Amhr2cre/+SmoM2 mice. Tissues were obtained from untreated mice (UT) or from eCG-primed mice before (0 h) or after injection of hCG. Total RNA was assayed by quantitative real-time RT-PCR. Data are mean ± sem; n = 3 granulosa cell and residual tissue preparations. Bars without common superscripts are significantly different (P < 0.05).
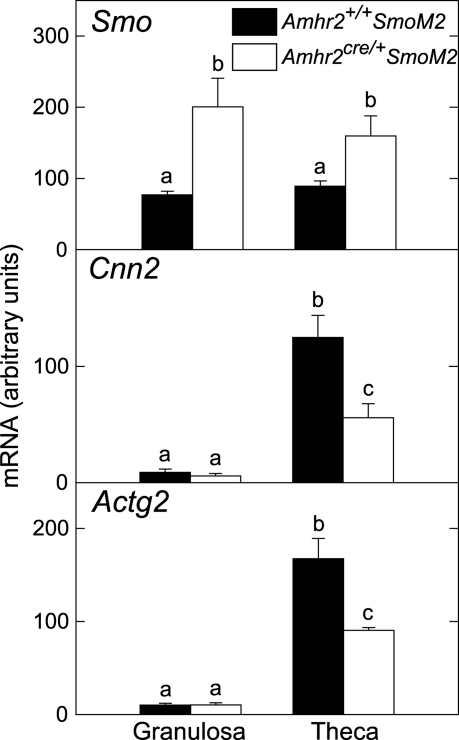
To more precisely examine localization of gene expression, theca and granulosa cells were isolated from preovulatory follicles of immature mice 24 h after injection of eCG. Smo mRNA was elevated in granulosa cells and theca of mutant mice compared with controls (Fig. 7). This result is consistent with Amhr2cre-mediated expression of the SmoM2/Yfp fusion gene in both granulosa and theca, as shown in Fig. 1. Levels of mRNA for genes typically expressed in muscle cells, Cnn1 and Actg2, were expressed predominantly in theca and were reduced in mutants compared with controls (Fig. 7).
Figure 7.
Expression of genes in isolated granulosa and theca cells of preovulatory follicles from Amhr2+/+SmoM2 and Amhr2cre/+SmoM2 mice. Cells were isolated from preovulatory follicles of immature mice 24 h after injection of eCG. Total RNA was assayed by quantitative real-time RT-PCR. Data are mean ± sem; n = 3 granulosa cell and theca preparations. Bars without common superscripts are significantly different (P < 0.05).
Expression of genes associated with differentiation of granulosa cells
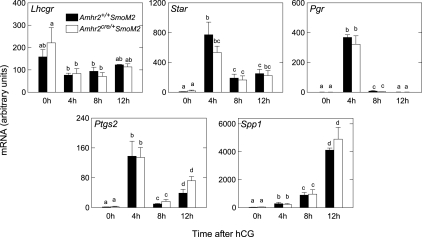
Changes in the pattern of expression of genes known to be involved in ovulation and differentiation were similar in granulosa cells of mutants and controls after injection of hCG including the following: Lhcgr, required for effects of LH on follicle differentiation and ovulation; Star, a rate-limiting enzyme required for progesterone production; Pgr, known to be required in granulosa cells for ovulation; Ptgs2 (also known as Cox-2), required for cumulus expansion and ovulation; and Spp1 (also known as osteopontin), a gene known to increase after the LH surge (24) and reported to be a target of HH signaling (25) but with unknown function in the follicle (Fig. 8).
Figure 8.
Expression of genes associated with ovulation and luteinization in granulosa cells from eCG-primed Amhr2+/+SmoM2 and Amhr2cre/+SmoM2 mice obtained after injection of hCG. Total RNA was assayed by quantitative real-time RT-PCR. Data are mean ± sem; n = 3 granulosa cell preparations. Bars without common superscripts are significantly different (P < 0.05).
Cumulus expansion
Based on the disorganized appearance of cumulus expansion in histological sections of mutant ovaries, a potential impairment of cumulus expansion was tested in isolated cumulus-oocyte complexes (COCs) in vitro. Treatment with FSH induced expansion of COCs from control mice as expected, whereas the magnitude of expansion was reduced in complexes from mutant mice (Fig. 9A). Fluorescent images of representative COCs from mutant mice showed bright YFP signal for cells in the plane of focus (Fig. 9C).
Figure 9.
Cumulus expansion is reduced in COCs expressing SmoM2. A, COCs were obtained from Amhr2+/+SmoM2 and Amhr2cre/+SmoM2 mice 48 h after injection with eCG and incubated with 0 or 100 ng/ml oFSH. Cumulus expansion was scored on a scale of 0 (no expansion) to 4 (full expansion) 20–24 h later. For each treatment, a total of 60–79 COCs from three separate COC preparations were scored. B, COCs were obtained from SmoM2 mice 48 h after injection with eCG and incubated for 6 h with Adβgal or Adcre. At 6 h, 0 or 100 ng/ml oFSH was added, and cumulus expansion was examined at 24 h. For each treatment, a total of 20–34 COCs from three separate COC preparations were scored. Data are mean ± sem. Bars without common superscripts are significantly different (P < 0.05). C, Phase contrast and fluorescent images of representative COCs from Amhr2+/+SmoM2 mice (significant expansion, no fluorescent signal observed) and Amhr2cre/+SmoM2 mice (minimal expansion, positive YFP signal observed; note cells in the plane of focus show bright signal for YFP).
To determine the effect of acute induction of SmoM2 expression on cumulus expansion in vitro, COCs were isolated from preovulatory follicles of eCG-primed mice homozygous for the SmoM2 allele and cultured with Adcre or Adβgal as control. Analysis of YFP reporter expression in COC by fluorescence microscopy confirmed that Cre-mediated activation of SmoM2 expression occurred in most cells (data not shown). Treatment with FSH increased the expansion index in complexes infected with Adβgal, whereas the response to FSH was prevented in complexes infected with Adcre (Fig. 9B). Taken together, the results show that Cre-mediated activation of SmoM2 expression in vivo (in Amhr2cre/+SmoM2 mice) and in vitro (in Adcre-infected COC from SmoM2 mice) results in a suboptimal cumulus expansion reaction.
Progression of granulosa cells through the cell cycle
Granulosa cells are reported to exit the cell cycle after exposure to the LH surge, and this is associated with their differentiation into luteal cells (26). Cell cycle distribution was similar in mutant and control mice with cells present in each stage of the cycle (G0/G1, S and G2/M) at each of the time points examined after treatment with eCG/hCG (supplemental Fig. 3). Between 8 and 12 h after hCG, the percent cells in S phase decreased and the percent cells in G2/M tended to increase in mutants and controls. Thus, exit from the cell cycle was not complete by 12 h after hCG, but movement of a wave of cells from S phase to G2/M appeared to occur between 8 and 12 h.
Discussion
Constitutive expression of dominant active SmoM2 in the ovary prevented ovulation. The major phenotypic difference identified between mutant and control mice that may contribute to ovulatory failure is the dramatic reduction in the layer of muscle cells that surround developing follicles. The presence of muscle cells or myofibroblastic-type cells in the theca layer of the follicle has been demonstrated in many mammalian species, and an extensive literature exists to suggest a potential role of contractile cells in the process of ovulation (23,27). However, a general lack of direct evidence for a requirement for muscle contraction in ovulation, as well as contradictory evidence obtained from different studies, apparently led to decreased effort in this field. Recently, treatment with endothelin-2 (END2) was shown to increase tension development in strips of rat ovarian tissue (21). End2, expressed by granulosa cells, increases immediately before ovulation in the rat (21) and mouse (22), and receptors for END2 are present within the SMA-positive muscle layer as well as in other cell types within follicles (21,22). Importantly, injection of an END2 antagonist into the rat ovary decreased the rate of ovulation in vivo, and reduced contraction of follicular smooth muscle was postulated to be responsible (21). Furthermore, systemic injection of an END2 antagonist to mice decreased ovulation rate (22). A number of genetically altered mice have been created in which ovulation is impaired, and these studies have revealed important information about the signaling pathways involved in ovulation. However, the end points affected that directly prevent ovulation have often remained undetermined. The mice generated in the current study provide a unique model with which to directly test the effects of impaired follicular muscle development on ovulation.
HH signaling regulates smooth muscle differentiation in a number of organ systems. In the developing gut, ureter, and bladder, SHH directs radial patterning of mesoderm by stimulating the proliferation of mesenchymal cell precursors and preventing their differentiation into smooth muscle cells (28,29,30). In each of these systems, mesenchymal cells located most distant from the source of SHH in the epithelium differentiate into muscle cells, whereas cells closer to the epithelium adopt nonmuscle cell fates. These findings are consistent with a well-documented aspect of HH effects on tissue patterning; a concentration gradient of HH ligand is established, and differentiation of target cells is dependent on their position within the gradient (2). Further direct evidence that expression of dominant active SmoM2 can inhibit differentiation of smooth muscle was provided by experiments in which expression of SmoM2 in Xenopus embryos increased proliferation of midgut mesenchymal cells and attenuated expression of the differentiation marker, smooth muscle actin (31).
The theca is presumed to be derived from mesenchymal cells present in the newborn ovary at the time that fragmentation of ovigerous cords to form primordial follicles occurs (32), although theca progenitor cell(s) have not been definitively identified and studied (33,34). Primordial follicles are composed of a quiescent oocyte surrounded by a flattened layer of pregranulosa cells and enclosed by a basement membrane. Although the theca cell layer that will form outside the basement membrane is not yet discernable, mesenchymal cells in close association with primordial follicles include theca cell precursors (33). Factors determining the fate of cells within the theca as fibroblastic, steroidogenic, or myoid have not been determined. In growing primary follicles, a layer of dividing mesenchymal cells surrounding the basement membrane was detected in rats infused with tritiated thymidine, suggesting that an early layer of theca precursors is intimately associated with the follicle at this stage (33). Components of the HH signaling pathway are detectable by real-time RT-PCR in newborn mouse ovaries (4). During the first days of life, the ovigerous cords break down and formation of primordial follicles is complete within 4 d. In situ hybridization showed that expression of Dhh and Ihh is first detectable in granulosa cells of primary follicles, and Ptch1 and Gli1 are expressed in the theca (5). Therefore, at least as early as the primary stage, and possibly earlier, HH signaling might regulate the differentiation of mesenchymal cells and thereby influence thecal development. Results of the current study show that theca muscle cells are detectable in control mice by immunohistochemistry for SMA as early as d 12 of age, whereas staining is substantially reduced in mutant mice. Appropriate temporal and spatial signaling through the HH signaling pathway appears to be critical for cell fate determination and/or differentiation of smooth muscle cells within the theca layer of the follicle. Furthermore, development of this cell layer is likely to be essential for ovulation.
Quantitative gene expression analyses showed that levels of Smo mRNA in granulose cells of Amhr2cre/+SmoM2 mice were elevated at all time points examined after eCG/hCG treatment compared with control mice, confirming constitutive expression of SmoM2. In contrast, Smo levels in residual tissue were substantially lower than in granulosa cells and were only slightly elevated in mutants compared with controls at several time points (Fig. 6). SmoM2 was clearly expressed in the theca of mutant mice because the YFP reporter protein was detected in theca of preovulatory follicles by flow cytometry (Fig. 1), and expression of Smo mRNA was elevated in isolated theca of mutants compared with controls (Fig. 7). Failure to observe increased expression of Smo in residual tissue of mutant mice may reflect dilution of theca within the residual tissue with other cell types.
Components of the HH signaling pathway were expressed in the granulosa cells as well as the theca, in agreement with a previous report (4). The LH surge appears to trigger changes in HH signaling; Ihh decreased within 4 h after hCG and Hhip and Gli3 simultaneously increased. HHIP binds HH ligands and is thought to function as an antagonist (35), and GLI3 often acts as a transcriptional repressor. Although in a number of contexts HH signaling reduces expression of Gli3 mRNA (9,36,37), a primary means of regulation is by processing of GLI3 protein. The regulation of GLI3 processing by HH signaling is a critical determinant of many aspects of development (13,38,39,40). In the absence of HH signaling, full-length GLI3 is processed to form a truncated repressor (GLI3R) by phosphorylation at multiple sites mediated by cAMP-dependent protein kinase A, casein kinase 1, and glycogen synthase kinase 3 (13,41,42). In this regard, it is interesting that ovarian protein kinase A activity increases in response to the LH surge (43,44). Gli1 and Ptch1 are reported to be transcriptional targets of HH signaling (7,8,9,10). Whereas levels of Gli1 were much higher in residual tissue than in granulosa cells, levels of Ptch1 were within the same range in the two cell fractions. The relationship between levels of Ptch1 mRNA and HH signaling is complex; Ptch1 is a transcriptional target of HH signaling, whereas PTCH protein inhibits signaling through SMO, generating a negative feedback loop in which HH attenuates its own activity (45,46). The significance of potential changes in HH signaling in both the granulosa and residual tissue after the LH surge remains to be determined.
The disorganized appearance of cumulus expansion in Amhr2cre/+SmoM2 mice detected by histology was confirmed by in vitro assays. The fact that elevated HH signaling interfered with normal cumulus expansion is consistent with a report that expression of Ihh and Dhh decreases in COC of mice after hCG (47). A number of genetically altered mice in which ovulation is impaired were also reported to have reduced cumulus expansion, including mice null for Ptgs2 (48), Rip140 (49), Adamts1 (50), and petraxin 3 (51) and mice with defective epidermal growth factor receptor signaling (52), but the basis for this association has not been defined. Whereas impaired cumulus expansion may contribute to ovulatory failure in Amhr2cre/+SmoM2 mice, it is likely that failed development of the theca muscle cells is primarily responsible. Microarray and RT-PCR analyses showed that expression of Tnc, which encodes a protein that can contribute to hyaluronan cross-linking in the extracellular matrix, was reduced in mutant mice in the residual tissue but not in mural granulosa cells. TNC was previously identified at high levels in theca externa of rats (53). This is of potential relevance to ovulation because in a number of tissues TNC is associated with tissue remodeling and wound healing (54). TNC was also localized to the cumulus matrix of mice and women, suggesting that it may play a role in cumulus expansion (47,55,56). The transcript for periostin (Postn), which encodes an extracellular matrix protein that belongs to the same family as TNC, was also reduced in mutant mice (Table 1). The possibility that extracellular matrix components of the follicle are regulated by HH signaling requires further investigation.
In Amhr2cre/+SmoM2 mice, expression of a number of genes changed similarly after hCG to that observed in controls, including Ptgs2 and Pgr, which are essential for ovulation (57,58,59), Lhcgr, which is required for differentiation (44), and End2, which is thought to be involved in stimulating muscle contraction and follicle rupture (21). Microarray data revealed no difference between mutant and control mice in expression of Cyp17a1, encoding cytochrome P450 17α-hydroxylase/17, 20-lyase, the enzyme required for androgen synthesis in the theca (data not shown). Furthermore, oocyte maturation appeared to be initiated normally, and cell cycle analysis revealed no differences in granulosa cell proliferation. Measurements of Star mRNA, SCC, and serum progesterone indicated no alteration in luteinization in mutant mice. Furthermore, in vitro induction of SmoM2 expression by granulosa cells had no effect on progesterone production. These findings, as well as ovarian histology, suggest that many aspects of follicle development to the preovulatory stage occurred fairly normally in mutant mice, and that some of the early events in response to the LH surge were not altered. It is not yet determined why estrous cycle length is prolonged in mutant mice. Careful morphometric analysis of follicle development in mutant and control ovaries will be necessary to address this question.
The fact that blood-filled cavities were prominent in follicles of eCG/hCG-treated mutant mice suggested that development of the vasculature might be abnormal, leading to increased leakage of blood cells into the antral cavity. However, the concentration of serum progesterone did not differ in mutant and control mice, indicating that vascular development was adequate to promote the normal release of progesterone into the circulation. This reasoning is supported by the findings that impaired vascular development of CL in various strains of transgenic mice and in sheep is associated with decreased levels of progesterone in the circulation (60,61,62). In addition, immunostaining for endothelial cells indicated no obvious differences in preovulatory follicles of mutant and control mice.
In summary, dominant activation of HH signaling in developing follicles likely blocks the differentiation of precursor cells into muscle cells that normally reside in the outer theca layer of the follicle. This possibility is consistent with known effects of HH signaling on smooth muscle differentiation in other developmental systems. Although follicles develop to the preovulatory stage and undergo many changes in response to eCG/hCG normally, they fail to release the oocyte. These findings lend support to the theory that muscle cells within the theca layer are required for release of the oocyte at the time of ovulation.
Materials and Methods
Mouse strains and treatments
Amhr2cre/+ mice, provided by Dr. Richard Behringer (17) and GT(ROSA)26Sortm1(smo/YFP)Amc/J mice (16), purchased from The Jackson Laboratory (Bar Harbor, ME), were mated to obtain Amhr2cre/+SmoM2 mice (mutants) and Amhr2+/+ SmoM2 mice (controls). Mice were genotyped from tail DNA using protocols provided by The Jackson Laboratory. CD-1 mice were purchased from Charles River Laboratories (Wilmington, MA). All animals were maintained in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Studies were approved by the Cornell University Institutional Animal Care and Use Committee.
Vaginal smears were obtained daily from mice (45–49 d old) for three complete estrous cycles or 20 d, whichever came first. Fertility of mutant females was tested by caging with CD-1 males of proven fertility. In some experiments, tissues were collected from immature mice (21–23 d old) 48 h after ip injection of 5 IU eCG, or from mice treated with eCG followed 48 h later by ip injection of 5 IU hCG, or from mice made pseudopregnant by mating with a vasectomized CD-1 male. In some experiments, granulosa cells were collected from preovulatory follicles at various times after eCG/hCG treatment by puncture with a 27-gauge needle. Subsequently, granulosa cells from smaller follicles were expressed by additional puncture and discarded whereas the remaining tissue, enriched for theca and stroma, was collected (referred to as “residual tissue”). In other experiments, preovulatory follicles were isolated 24 h after eCG, and granulosa cells and theca were obtained by dissection using watchmaker’s forceps. To assess ovulation rate, immature eCG/hCG-primed mice were killed 20 h after hCG, and oocytes within the ampulla of the oviduct were flushed and counted. When mice were killed, blood was collected by cardiac puncture. Serum was stored at −20 C until assayed for progesterone using a commercial RIA kit (Siemens Medical Solutions, Los Angeles, CA).
Assessment of Cre-mediated recombination
Single-cell suspensions of uteri, oviducts, and whole ovaries from young mutant and control mice were obtained by digestion with 0.08% trypsin plus 10 μg/ml deoxyribonuclease I in DMEM Ham’s F-12 (F12) at 37 C for up to 3 h, with periodic triteration using a Pasteur pipet. Theca was obtained from preovulatory follicles of immature mice 24 h after injection of eCG by dissection, and granulosa cells were obtained by needle puncture. Cell suspensions were fixed in 80% ethanol and, within 24 h of storage at 4 C, cells were centrifuged, reconstituted in PBS, and analyzed for YFP using a FACScan flow cytometer (BD Biosciences, San Jose, CA). Excitation was at 488 nm and emission was detected by FL1 at 530 ± 30 nm. Single cells were selected based on a plot of side scatter vs. forward scatter. Plots of side scatter vs. FL1A were produced, and an area gate was constructed using matching tissues from control animals that do not express YFP, such that approximately 95% of the cells were within the area. This gate was applied to plots from mutant animals, and the proportion of cells with higher FL1A fluorescence, which represents those cells expressing YFP, was determined.
Histology and immunohistochemistry
Tissues were fixed in Bouins for histology or 2% paraformaldehyde for immunohistochemistry and embedded in paraffin, after which 5-μm sections were processed for staining with hematoxylin and eosin or for immunohistochemistry. For SCC, sections were deparaffinized, rehydrated, and treated for antigen retrieval by boiling in 10 mm citrate buffer for 10 min. Endogenous peroxidase activity was quenched with 0.3% hydrogen peroxide in PBS. Nonspecific binding was blocked with 2% normal goat serum. Sections were incubated with rabbit antirat SCC antibody (AB1244; Chemicon, Temecula, CA), or normal rabbit serum, diluted 1:2000 in PBS-1% BSA at 37 C for 1 h, washed with PBS, and then incubated with 0.4 μg/ml goat antirabbit IgG conjugated to horseradish peroxidase (Jackson ImmunoResearch, West Grove, PA) in PBS-1% BSA for 1 h at 37 C. Slides were exposed to Nova Red substrate (Vector Laboratories, Burlingame, CA) and counterstained with Gills hematoxylin.
For staining for SMA, sections were deparaffinized, rehydrated, and blocked with 2% normal goat serum. Sections were incubated with rabbit antihuman SMA (ab15267, prediluted; Abcam, Inc., Cambridge, MA) or with 1 μg/ml rabbit IgG for 20 min at room temperature, followed by incubation with 0.5 μg/ml Alexa 555 goat antirabbit IgG for 30 min at room temperature. Cell nuclei were counterstained with 5 μg/ml Hoechst 33342 dye. For dual staining for SMA and VWB, deparaffinized sections were hydrated, treated with pepsin, and blocked with a Mouse-on-Mouse reagent (Vector Laboratories) followed by 10% normal goat serum. Sections were incubated with mouse antihuman α-SMA (M0851, clone IA4; DAKO, Carpinteria, CA) diluted 1:20 and rabbit anti-VWB (A0082; DAKO) diluted 1:50 at room temperature for 25 min, followed by Alexa 488-conjugated goat antirabbit IgG and Alexa 595 goat antimouse IgG for 40 min. Cell nuclei were counterstained with 10 μg/ml 4,6-diamidino-2-phenylindole.
Progesterone production by granulosa cells expressing SmoM2
Granulosa cells from eCG-treated homozygous SmoM2 mice were plated in 96-well plates (5 × 104 cells per well) in DMEM-F12 supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 0.25 μg/ml fungizone, 1 mm pyruvate, and 2 mm glutamine and containing 10% FBS (all from Invitrogen, Carlsbad, CA). At the time of plating, cells were infected with recombinant adenovirus expressing Cre (Adcre; Microbix Biosystems, Ontario, Toronto, Canada) or a control adenovirus expressing β-galactosidase (Adβgal; provided by Dr. Wafik El-Deiry) at a dose of 1 × 106 pfu/well (total vol 50 μl). Additional media containing testosterone (final concentration 1 μm) were added 4 h after infection. Media were collected 1 d after plating, and 0 or 10 ng/ml ovine LH (NIDDK-oLH-26; from Dr. A. F. Parlow, Harbor-UCLA Medical Center) was added in serum-free media [DMEM-F12 with additives as above plus 100 ng/ml insulin (Sigma-Aldrich), 5 μg/ml transferrin (Invitrogen), 20 nm sodium selenite (Invitrogen), 0.1% BSA, and 1 μm testosterone]. Media were collected on the second and third day after plating and stored at −20 C until assayed for progesterone. To determine the efficiency of Adcre-mediated recombination, Adcre- or Adβgal-infected cells were harvested on d 3 and used for flow cytometry to detect YFP.
Microarray analysis
RNA from whole ovaries of untreated, 48-h eCG-treated and 4-h hCG-treated control and mutant mice was prepared using a RNeasy Mini Kit (QIAGEN, Valencia CA). RNA was tested before equal amounts of RNA from three mice of each genotype were pooled for microarray analysis; expression of Lhcgr mRNA was confirmed to increase after eCG, and Ptgs2 was shown to increase after hCG as expected. Microarray analyses were performed by the Microarray Core Facility of the Cornell University Life Sciences Core Laboratories Center. Labeled cRNA samples were hybridized on mouse genome 430 2.0 GeneChips and scanned by a GeneChip Scanner 3000 according to standard protocol provided by the manufacturer (Affymetrix, Santa Clara, CA). The raw array data were processed by Affymetrix GCOS software to obtain signal values, which were scaled to the default target of 500 and summarized in a pivot table. The signals were log2 transformed after being offset by 64, and log ratios were calculated as the difference between mutant and control samples at each of the three time points (see supplemental Table 1). Gene filtering was applied to include only 30,588 probe sets having at least one Present call. Gene ontogeny analysis of log2 differences between transcript levels in mutants and controls was performed using ErmineJ software (63).
Analysis of gene expression
RNA was prepared from granulosa cells, theca, residual ovarian tissue, and whole ovaries using a RNeasy Mini Kit. Reverse transcription was performed using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City CA). Real-time RT-PCR was performed on an ABI Prism 7000 using the mouse-specific assays shown in Table 2. A standard curve, used in each assay, was constructed from cDNA prepared from RNA of pooled granulosa cells or, for smooth muscle and extracellular matrix genes, from RNA of pooled whole ovaries. Results were standardized by dividing by 18S rRNA concentration and multiplying by 100. Samples from each tissue type were analyzed on the same plate. For each gene, a between-plate coefficient of variation was determined by including two common samples on each plate. The between-plate coefficients of variation ranged from 1% to 16%; the mean was 10.6 ± 1.4%.
Table 2.
Quantitative real-time RT-PCR assays
| Gene symbol | Gene name | Assay identificationa | Exonsb |
|---|---|---|---|
| Smo | Smoothened homolog | Mm01162710_m1 | 8–9 |
| Ihh | Indian hedgehog | Mm00439613_m1 | 2–3 |
| Ptch1 | Patched homolog 1 | Mm00436026_m1 | 17–18 |
| Gli1 | GLI-Kruppel family member GLI1 | Mm00494645_m1 | 2–3 |
| Gli3 | GLI-Kruppel family member GLI3 | Mm00492333_m1 | 1–2 |
| Hhip | Hedgehog-interacting protein | Mm00469580_m1 | 12–13 |
| Spp | Secreted phosphoprotein 1 | Mm00436767_m1 | 2–3 |
| Ptgs2 | Prostaglandin-endoperoxidase synthase 2 | Mm00478374_m1 | 5–6 |
| Pgr | Progesterone receptor | Mm00435625_m1 | 4–5 |
| Star | Steroidogenic acute regulatory protein | Mm00441558_m1 | 6–7 |
| Lhcgr | LH/CG receptor | Mm00442931_m1 | 6–7 |
| Actg2 | Actin, γ 2, smooth muscle, enteric | Mm00656102_m1 | 7–8 |
| Cnn1 | Calponin 1 | Mm00487032_m1 | 1–2 |
| Des | Desmin | Mm00802455_m1 | 1–2 |
| Tagln | Transgelin | Mm00441660_m1 | 1–2 |
| Tnc | Tenascin C | Mm00495662_m1 | 5–6 |
| Edn2 | Endothelin 2 | Mm00432983_m1 | 3–4 |
| 18S rRNA | 4319413E |
Taqman Gene Expression Assays (Applied Biosystems).
Exons in which forward and reverse primers anneal.
In vitro cumulus expansion assays
Immature Amhr2cre/+SmoM2 mutant and Amhr2+/+SmoM2 control mice were primed with eCG, and COCs were isolated at 48 h by needle puncture of large antral follicles. COCs were distributed to 24-well plates (∼20/well) containing DMEMα (Invitrogen) plus antibiotics and 5% FBS and treated with 0 or 100 ng/ml ovine FSH (NIDDK oFSH-20, from Dr. A. F. Parlow). After 18 h of treatment, COCs were scored for the degree of expansion as described previously (64) using a scale of 0 indicating no expansion to a score of 4 indicating full expansion.
To test the effect of acute induction of SmoM2 expression on cumulus expansion, COC were collected from homozygous SmoM2 mice and infected with 1 × 107 pfu/ml of Adcre or Adβgal. Cultures were rocked gently for 12 h to prevent attachment of COCs to the plate during infection. Cultures were then treated with 0 or 100 ng/ml FSH and scored for cumulus expansion 18 h later. COC were examined by fluorescence microscopy for expression of YFP.
Cell cycle analysis
Granulosa cells isolated at different times after eCG/hCG treatment of immature mice were analyzed for DNA content by flow cytometry as described previously (65).
Statistical analysis
Serum progesterone concentrations, tissue mRNA concentrations, and cell cycle data were analyzed by randomized (simple) two-way ANOVA. Serum progesterone and tissue mRNA concentrations were log transformed to normalize se. Progesterone production by cultured granulosa cells and in vitro cumulus expansion experiments were analyzed by randomized complete block ANOVA, with experimental replicates as blocks; progesterone data were log transformed. Student-Newman-Keuls test was used to compare individual means if overall significance was indicated (66). All other data were analyzed by unpaired t test.
Supplementary Material
Acknowledgments
We thank Dr. Richard R. Behringer, University of Texas M.D. Anderson Cancer, Center, Houston Texas, for providing Amhr2cre/+ mice, and Patricia Fisher, New York State College of Veterinary Medicine, Cornell University, for performing SMA/VWB immunohistochemistry.
Footnotes
This work was supported by the following grants (to S.M.Q.): a Research Initiation Award from the National Science Foundation ADVANCE Institutional Transformation Grant 0547373 to Cornell University; R03HD057648 from the National Institute of Child Health and Human Development; and a grant from the Center for Vertebrate Genomics, Cornell University.
Disclosure Summary: The authors have nothing to disclose.
First Published Online February 5, 2009
Abbreviations: Adcre, Adenovirus expressing Cre; Adβgal, adenovirus expressing β-galactosidase; CG, chorionic gonadotropin; CL, corpora lutea; COC, cumulus-oocyte complex; DHH, desert hedgehog; eCG, equine CG; hCG, human CG; HH, hedgehog; IHH, Indian hedgehog; PTCH, patched; SCC, side chain cleavage; SHH, sonic hedgehog; SMA, smooth muscle actin α; SMO, smoothened; TNC, tenascin C; VWB, von Willebrand’s factor; YFP, yellow fluorescent protein.
References
- McMahon AP, Ingham PW, Tabin CJ 2003 Developmental roles and clinical significance of Hedgehog signaling. Dev Biol 53:1–114 [DOI] [PubMed] [Google Scholar]
- King PJ, Guasti L, Laufer E 2008 Hedehog signaling in endocrine development and disease. J Endocrinol 198:439–450 [DOI] [PubMed] [Google Scholar]
- Rubin LL, de Sauvage FJ 2006 Targeting the Hedgehog pathway in cancer. Nat Rev Drug Disc 5:1026–1033 [DOI] [PubMed] [Google Scholar]
- Russell MC, Cowan RG, Harman RM, Walker AL, Quirk SM 2007 The hedgehog signaling pathway in the mouse ovary. Biol Reprod 77:226–236 [DOI] [PubMed] [Google Scholar]
- Wijgerde M, Ooms M, Hoogerbrugge JW, Grootegoed JA 2005 Hedgehog signaling in mouse ovary: Indian hedgehog and desert hedgehog induce target gene expression in developing theca cells. Endocrinology 146:3558–3566 [DOI] [PubMed] [Google Scholar]
- Huangfu D, Anderson KV 2006 Signaling from Smo to Ci/Gli: conservation and divergence of Hedgehog pathways from Drosophila to vertebrates. Development 133:3–14 [DOI] [PubMed] [Google Scholar]
- Lee J, Platt KA, Censullo P, Ruiz i Altaba A 1997 Gli1 is a target of Sonic hedgehog that induces ventral neural tube development. Development 124:2537–2552 [DOI] [PubMed] [Google Scholar]
- Ikram MS, Neill GW, Regl G, Eichberger T, Frischauf AM, Aberger F, Quinn A, Philpott M 2004 Gli2 is expressed in normal human epidermis and BCC and induces Gli1 expression by binding to its promoter. J Invest Dermatol 122:1503–1509 [DOI] [PubMed] [Google Scholar]
- Marigo V, Johnson RL, Vortkamp A, Tabin CJ 1996 Sonic hedgehog differentially regulates expression of Gli and Gli3 during limb development. Dev Biol 180:273–283 [DOI] [PubMed] [Google Scholar]
- Ahn S, Joyner AL 2004 Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell 118:505–516 [DOI] [PubMed] [Google Scholar]
- Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL 2002 Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development 129:4753–4761 [DOI] [PubMed] [Google Scholar]
- Mo R, Freer AM, Zinyk DL, Crackower MA, Michaud J, Heng HH, Chik KW, Shi XM, Tsui LC, Cheng SH, Joyner AL, Hui C 1997 Specific and redundant functions of Gli2 and Gli3 zinc finger genes in skeletal patterning and development. Development 124:113–123 [DOI] [PubMed] [Google Scholar]
- Wang B, Fallon JF, Beachy PA 2000 Hedgehog-regulated processing of Gli3 produces an anterioir/posterior repressor gradient in the developing vertebrate limb. Cell 100:423–434 [DOI] [PubMed] [Google Scholar]
- Pan Y, Bai CB, Joyner AL, Wang B 2006 Sonic hedgehog signaling regulates Gli2 transcriptional activity by suppressing its processing and degradation. Mol Cell Biol 26:3365–3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, Bonifas JM, Lam CW, Hynes M, Goddard A, Rosenthal A, Epstein Jr EH, de Sauvage FJ 1998 Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature 391:90–92 [DOI] [PubMed] [Google Scholar]
- Jeong J, Mao J, Tenzen T, Kottmann AH, McMahon AP 2004 Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev 18:937–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR 2002 Requirement of Bmpr1a for Mullerian duct regression during male sexual development. Nat Genet 32:408–410 [DOI] [PubMed] [Google Scholar]
- Jorgez CJ, Klysik M, Jamin SP, Behringer RR, Matzuk MM 2004 Granulosa cell-specific inactivation of follistatin causes female fertility defects. Mol Endocrinol 18:953–967 [DOI] [PubMed] [Google Scholar]
- Arango NA, Szotek PP, Manganaro TF, Oliva E, Donahoe PK, Teixeira J 2005 Conditional deletion of β-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev Biol 288:276–283 [DOI] [PubMed] [Google Scholar]
- Deutcher E, Yao HH-C 2007 Essential roles of mesenchyme-derived β-catenin in mouse Müllerian duct morphogenesis. Dev Biol 307:227–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko C, Gieske MC, Al-Alem L, Hahn Y, Su W, Gong MC, Iglarz M, Koo Y 2006 Endothelin-2 in ovarian follicle rupture. Endocrinology 147:1770–1779 [DOI] [PubMed] [Google Scholar]
- Palanisamy GS, Cheon YP, Kim J, Kannan A, Li Q, Sato M, Mantena SR, Sitruk-Ware RL, Bagchi MK, Bagchi IC 2006 A novel pathway involving progesterone receptor, endothelin-2, and endothelin receptor B controls ovulation in mice. Mol Endocrinol 20:2784–2795 [DOI] [PubMed] [Google Scholar]
- Amsterdam A, Lindner HR, Gröschel-Stewert U 1977 Localization of actin and myosin in the rat oocyte and follicular wall by immunofluorescence. Anat Rec 187:311–328 [DOI] [PubMed] [Google Scholar]
- McRae RS, Johnston HM, Mihm M, O'Shaughnessy PJ 2005 Changes in mouse granulosa cell gene expression during early luteinization. Endocrinology 146:309–317 [DOI] [PubMed] [Google Scholar]
- Yoon JW, Kita Y, Frank DJ, Majewski RR, Konicek BA, Nobrega MA, Jacob H, Walterhouse D, Iannaccone P 2002 Gene expression profiling leads to identification of GLI1-binding elements in target genes and a role for multiple downstream pathways in GLI1-induced cell transformation. J Biol Chem 277:5548–5555 [DOI] [PubMed] [Google Scholar]
- Robker RL, Richards JS 1998 Hormone-induced proliferation and differentiation of granulosa cells: a coordinated balance of the cell cycle regulators cyclin D2 and p27 Kip1. Mol Endocrinol 12:924–940 [DOI] [PubMed] [Google Scholar]
- Espey LL 1978 Ovarian contractility and its relationship to ovulation: a review. Biol Reprod 19:540–551 [DOI] [PubMed] [Google Scholar]
- Sukegawa A, Narita T, Kameda T, Saitoh K, Nohno T, Iba H, Yasugi S, Fukuda K 2000 The concentric structure of the developing gut is regulated by Sonic hedgehog derived from endodermal epithelium. Development 127:1971–1980 [DOI] [PubMed] [Google Scholar]
- Yu J, Carroll TJ, McMahon AP 2002 Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development 129:5301–5312 [DOI] [PubMed] [Google Scholar]
- Shiroyanagi Y, Liu B, Cao M, Agras K, Li J, Hsieh MH, Willingham EJ, Baskin LS 2007 Urothelial sonic hedgehog signaling plays an important role in bladder smooth muscle formation. Differentiation 75:968–977 [DOI] [PubMed] [Google Scholar]
- Zhang J, Rosenthal A, de Sauvage FJ, Shivdasani RA 2001 Downregulation of hedgehog signaling is required for organogenesis of the small intestine in Xenopus. Dev Biol 229:188–202 [DOI] [PubMed] [Google Scholar]
- Mazaud S, Guyot R, Guigon CJ, Coudouel N, Le Magueresse-Battistoni B, Magre S 2005 Basal membrane remodeling during follicle histogenesis in the rat ovary: contribution of proteinases of the MMP and PA families. Dev Biol 277:403–416 [DOI] [PubMed] [Google Scholar]
- Hirshfield AN 1991 Development of follicles in the mammalian ovary. Int Rev Cytol 124:43–101 [DOI] [PubMed] [Google Scholar]
- Magoffin DA 2005 Cells in focus: ovarian theca cell. Int J Biochem Cell Biol 37:1344–1349 [DOI] [PubMed] [Google Scholar]
- Chuang PT, McMahon AP 1999 Vertebrate Hedgehog signalling modulated by induction of a Hedgehog-binding protein. Nature 397:617–621 [DOI] [PubMed] [Google Scholar]
- Bastida MF, Delgado MD, Wang B, Fallon JF, Fernandez-Teran M, Ros MA 2004 Levels of Gli3 repressor correlate with Bmp4 expression and apoptosis during limb development. Dev Dyn 231:148–160 [DOI] [PubMed] [Google Scholar]
- Ohba S, Kawaguchi H, Kugimiya F, Ogasawara T, Kawamura N, Saito T, Ikeda T, Fujii K, Miyajima T, Kuramochi A, Miyashita T, Oda H, Nakamura K, Takato T, Chung UI 2008 Patched1 haploinsufficiency increases adult bone mass and modulates Gli3 repressor activity. Dev Cell 14:689–699 [DOI] [PubMed] [Google Scholar]
- Litingtung Y, Dahn RD, Li Y, Fallon JF, Chiang C 2002 Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature 418:979–983 [DOI] [PubMed] [Google Scholar]
- Wang C, Rüther U, Wang B 2007 The Shh-independent activator function of the full length Gli3 protein and its role in vertebrate limb digit patterning. Dev Biol 305:460–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziel L, Wuelling M, Schneider S, Vortkamp A 2005 Gli3 acts as a repressor downstream of Ihh in regulating two distinct steps of chondrocyte differentiation. Development 132:5249–5260 [DOI] [PubMed] [Google Scholar]
- Wang B, Li Y 2006 Evidence for the direct involvement of βTrCP in Gli3 protein processing. Proc Natl Acad Sci USA 103:33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempé D, Casas M, Karaz S, Blanchet-Tournier MF, Concordet JP 2006 Multisite protein kinase A and glycogen synthase kinase 3β phosphorylation leads to Gli3 ubiquitination by SCFβTrCP. Mol Cell Biol 26:4316–4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Robayna IJ, Alliston TN, Buse P, Firestone GL, Richards JS 1999 Functional and subcellular changes in the A-kinase-signaling pathway: relation to aromatase and Sgk expression during the transition of granulosa cells to luteal cells. Mol Endocrinol 13:1318–1337 [DOI] [PubMed] [Google Scholar]
- Richards JS 1994 Hormonal control of gene expression in the ovary. Endocr Rev 15:725–751 [DOI] [PubMed] [Google Scholar]
- Chen Y, Struhl G 1996 Dual roles for Patched in sequestering and transducing hedgehog. Cell 87:553–563 [DOI] [PubMed] [Google Scholar]
- Jeong J, McMahon AP 2005 Growth and pattern of the mammalian neural tube are governed by partially overlapping feedback activities of the hedgehog antagonists patched1 and Hhip1. Development 132:143–154 [DOI] [PubMed] [Google Scholar]
- Hernandez-Gonzalez I, Gonzalez-Robayna I, Shimada M, Wayne CM, Ochsner SA, White L, Richards JS 2006 Gene expression profiles of cumulus cell oocyte complexes during ovulation reveal cumulus cells express neuronal and immune-related genes: does this expand their role in the ovulation process? Mol Endocrinol 20:1300–1321 [DOI] [PubMed] [Google Scholar]
- Davis BJ, Lennard DE, Lee CA, Tiano HF, Morham SG, Wetsel WC, Langenbach R 1999 Anovulation in cyclooxygenase-2-deficient mice is restored by prostaglandin E2 and interleukin-1β. Endocrinology 140:2685–2695 [DOI] [PubMed] [Google Scholar]
- Tullet JM, Pocock V, Steel JH, White R, Milligan S, Parker MG 2005 Multiple signaling defects in the absence of RIP140 impair both cumulus expansion and follicle rupture. Endocrinology 146:4127–4137 [DOI] [PubMed] [Google Scholar]
- Mittaz L, Russell DL, Wilson T, Brasted M, Tkalcevic J, Salamonsen LA, Hertzog PJ, Pritchard MA 2004 Adamts-1 is essential for the development and function of the urogenital system. Biol Reprod 70:1096–1105 [DOI] [PubMed] [Google Scholar]
- Varani S, Elvin JA, Yan C, DeMayo J, DeMayo FJ, Horton HF, Byrne MC, Matzuk MM 2002 Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol Endocrinol 16:1154–1167 [DOI] [PubMed] [Google Scholar]
- Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M 2007 Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol 27:1914–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda K, Hagiwara E, Takeuchi A, Mukai C, Matsui C, Sakai A, Tamotsu S 2005 Changes in the distribution of tenascin and fibronectin in the mouse ovary during folliculogenesis, atresia, corpus luteum formation and lutolysis. Zool Sci 22:237–245 [DOI] [PubMed] [Google Scholar]
- Schellings MW, Pinto YM, Heymans S 2004 Matricellular proteins in the heart: possible role during stress and remodeling. Cardiovasc Res 64:24–31 [DOI] [PubMed] [Google Scholar]
- Familiari G, Verlangia C, Nottola SA, Renda T, Micara G, Aragona C, Zardi L, Motta PM1996 Heterogeneous distribution of fibronectin, tenascin-C, and laminin immunoreactive material in the cumulus-corona cells surrounding mature human oocytes from IVF-ET protocols—evidence that they are composed of different subpopulations: an immunohistochemical study using scanning confocal laser and fluorescence microscopy. Mol Reprod Dev 43:392–402 [DOI] [PubMed] [Google Scholar]
- Relucenti M, Heyn R, Correr S, Familiari G 2005 Cumulus oophorus extracellular matrix in the human oocyte: a role for adhesive proteins. Ital J Anat Embryol 110:219–224 [PubMed] [Google Scholar]
- Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK 1997 Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell 91:197–208 [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery Jr CA, Shyamala G, Conneely OM, O'Malley BW 1995 Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9:2266–2278 [DOI] [PubMed] [Google Scholar]
- Jefferson W, Newbold R, Padilla-Banks E, Pepling M2006 Neonatal genistein treatment alters ovarian differentiation in the mouse: inhibition of oocyte nest breakdown and increased oocyte survival. Biophys J 74:161–168 [DOI] [PubMed] [Google Scholar]
- Hsieh M, Boerboom D, Shimada M, Lo Y, Parlow AF, Luhmann UF, Berger W, Richards JS 2005 Mice null for frizzled4 (fzd4−/−) are infertile and exhibit impaired corpora lutea formation and function. Biol Reprod 73:1135–1146 [DOI] [PubMed] [Google Scholar]
- Otsuka M, Zheng M, Hayashi M, Lee JD, Yoshino O, Lin S, Han J 2008 Impaired microRNA processing causes corpus luteum insufficiency and infertility in mice. J Clin Invest 118:1944–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottsch ML, Van Kirk EA, Murdoch WJ 2002 Role of matrix metalloproteinase 2 in the ovulatory folliculo-luteal transition of ewes. Reproduction 124:347–352 [DOI] [PubMed] [Google Scholar]
- Lee HK, Braynen W, Keshav K, Pavlidis P 2005 ErmineJ: tool for functional analysis of gene expression data sets. BMC Bioinformatics 6:269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs SM 1989 Specificity of epidermal growth factor action on maturation of the murine oocyte and cumulus oophorus in vitro. Biol Reprod 41:371–379 [DOI] [PubMed] [Google Scholar]
- Quirk SM, Cowan RG, Harman RM 2006 The susceptibility of granulosa cells to apoptosis is influenced by oestradiol and the cell cycle. J Endocrinol 189:441–453 [DOI] [PubMed] [Google Scholar]
- Ott RL 2001 An introduction to statistical methods and data analysis. 5th ed. Belmont, CA: Duxbury Press; 427–459 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.