Abstract
JAK2 plays important roles in the regulation of a variety of cellular processes including cell migration, proliferation, and protection from apoptosis. Recently the L611S point mutation in JAK2 has been identified in a child with acute lymphoblastic leukemia. Here we analyzed the mechanism by which JAK2 exhibits its oncogenicity. In BaF3 murine hematopoietic cells, L611S mutant increased the expression of antiapoptotic proteins including X chromosome-linked inhibitor of apoptosis protein, inhibitor of apoptosis protein, and Bcl-XL. We also showed that JAK2 L611S mutant protects BaF3 cells from cytokine withdrawal-induced apoptotic cell death and leads to cytokine-independent cell growth. Furthermore BaF3 cells expressing JAK2 L611S mutant gained the ability to induce tumorigenesis in nude mice. The L611S mutant also exhibited malignancy, including prompt invasion and spreading into various organs, leading to rapid lethality of the mice. Finally we showed that a specific JAK2 inhibitor, AG490, potently inhibited cytokine-independent cell growth induced by JAK2 L611S mutant via the induction of apoptotic cell death. In addition, treatment with AG490 significantly inhibited the JAK2 L611S mutant-induced tumorigenesis in nude mice. Thus, our results both in vitro and in vivo strongly suggest that L611S mutant of JAK2 harbors potent oncogenic activity, and this probably requires the antiapoptotic signaling pathway.
The tyrosine kinase Janus kinase 2 (JAK2)2 is the essential component of various cytokine signal transductions. Recent studies showed that JAK2 activates the mitogen-activated protein kinase family (ERK, c-Jun NH2-terminal kinase (JNK), and p38), Akt, and the signal transducers and activators of transcription (Stat) family in various tissues and is involved in numerous biological functions such as cell growth, cell survival, and differentiation (1, 2). Therefore, it has been reported that the disruption of the regulation of JAK2 activity is associated with hematopoietic disorders and oncogenesis (3–5).
JAK2 contains seven regions with significant sequence homology between the kinases, termed Jak homology (JH) domains (1, 2). The JH1 domain is located within the carboxyl terminus of the protein and contains the tyrosine kinase domain. The adjacent JH2 domain shows close homology to the JH1 domain; however, it lacks critical residues required for tyrosine kinase activity. Under normal conditions, this JH2 domain negatively regulates kinase activity. Theoretical models of JAK2 structure suggest that the JH1 and JH2 domains are facing each other and that the activation loop of JAK2 is buried at this interface (6). Upon activation of JAK2, phosphorylation of the activation loop at Tyr1007/1008 occurs and is believed to prevent this JH1-JH2 interaction and therefore relieve inhibition (7–9).
The JAK2 deletion mutant lacking JH2 domain exhibited receptor-independent constitutive activation (8, 9). Furthermore JH2 domain mutations have been demonstrated to be involved in various myeloproliferative diseases (10–12). These facts clearly suggest that JH2 domain may harbor the ability to negatively regulate JAK2 activity. The single point mutation leading to a V617F substitution in the JH2 domain of JAK2 has been identified in the majority of patients with myeloproliferative disorders such as polycythemia vera, essential thrombocytosis, and primary myelofibrosis. It has also been demonstrated that this mutation in JAK2 leads to deregulated kinase activity and constitutive activation of the signaling pathway (10–12).
Kratz et al. (13) reported a novel JAK2 point mutation (L611S) in a patient with acute lymphoblastic leukemia that is quite close to the V617F mutation observed in various myeloproliferative diseases. It is easy to imagine that the mutation of several amino acid residues in JH2 domain would disrupt the regulation of JAK2 activity. Indeed we previously reported that the limited region in JH2 domain is critical for the regulation of JAK2 using systematically mutated JAK2 constructs. In the course of screening, we found that several point mutation in JH2 domain of JAK2, including Y613E, V617F, and L611S, exhibited the ability to induce erythroid colony formation even in the absence of erythropoietin (Epo) in JAK2-deficient fetal liver (14). Thus, it is expected that an acute lymphoblastic leukemia (ALL) patient-derived L611S mutation would induce the constitutive activation of JAK2. Here, to define the signaling mechanisms activated by JAK2 L611S mutant, we investigated the characterization of this mutant both in vitro and in vivo. Interestingly we show that JAK2 L611S mutant could act as an oncogene to transform BaF3 cells and to promote tumor formation in nude mice. Furthermore our results also suggested that treatment with the JAK2 inhibitor AG490 could be a clue for the targeted therapy against JAK2 L611S mutant-related disorders.
EXPERIMENTAL PROCEDURES
Reagents—Recombinant human Epo (ESPO®3000) was purchased from Kirin Brewery Co. (Tokyo, Japan). AG490 was purchased from Tocris Bioscience (Ellisville, MO). For administration to nude mice, AG490 was diluted in DMSO at a concentration of 25 mg/ml. The stock solution was further diluted to 2.5 mg/ml in phosphate-buffered saline (PBS). Anti-phospho-JAK2 (Tyr1007/1008) antibody, anti-phospho-Stat5 antibody (Tyr694), anti-phospho-ERK antibody (Thr202/Tyr204), anti-phospho-Akt antibody (Ser473), anti-Stat5 antibody, anti-ERK antibody, anti-Akt antibody, and anti-XIAP antibody were purchased from Cell Signaling Technology (Danvers, MA). Anti-JAK2 antibody, anti-Bcl-XL antibody, and anti-β-actin antibody were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Anti-cIAP1 antibody was purchased from R&D Systems, Inc. (Minneapolis, MN). Peroxidase-conjugated rabbit anti-mouse and goat anti-rabbit secondary antibodies were from Dako (Glostrup, Denmark).
Cell Cultures—BaF3 cells were infected with empty virus (MSCV), wild type JAK2 (WT) c-HA, or mutant JAK2 c-HA (L611S) with erythropoietin receptor c-FLAG and established as described previously (14). These cells were cultured in RPMI 1640 medium (Nissui Seiyaku, Tokyo, Japan) supplemented with 10% fetal bovine serum (BioWest), 100 μg/ml l-glutamine (Nacalai Tesque, Tokyo, Japan), 100 μg/ml penicillin (Nacalai Tesque), 100 μg/ml streptomycin (Nacalai Tesque), and 5 units/ml Epo.
BaF3 Cell Growth Assay—Transduced and exponentially growing BaF3 cells were washed twice with PBS and incubated with RPMI 1640 medium supplemented with 1% fetal bovine serum and 100 mg/ml l-glutamine in the presence or absence of Epo (5 units/ml) for the indicated times. Living cells were counted using a Beckman Coulter Vi-CELL (Beckman Coulter, Fullerton, CA). Cell viability was checked by trypan blue exclusion method.
BrdUrd Cell Proliferation Assay—Transduced and exponentially growing BaF3 cells were washed twice with PBS and incubated with RPMI 1640 medium supplemented with 1% fetal bovine serum and 100 mg/ml l-glutamine in the presence or absence of Epo (5 units/ml) for 24 h. The proliferation rate was determined using the BrdUrd labeling and detection enzyme-linked immunosorbent assay kit III (Roche Applied Science).
Cell Cycle Analysis—After treatment, cells were washed with PBS and fixed with 70% (v/v) ethanol at –20 °C overnight. Cells were then centrifuged at 5,000 rpm for 2 min and resuspended in PBS containing 10 μg/ml RNase A (Wako, Tokyo, Japan) and 100 μg/ml propidium iodide (Sigma-Aldrich). Following 30-min incubation, cell cycle parameters were determined by flow cytometry analysis using FACSCalibur as described previously (15). All data were recorded and analyzed using CellQuest software.
DNA Fragmentation Assay—Genomic DNA was prepared for gel electrophoresis as described previously (15). Electrophoresis was performed on a 1% (w/v) agarose gel in Tris-boric acid buffer. Fragmented DNA was visualized by staining with ethidium bromide after electrophoresis.
Immunoprecipitation and Western Blotting—Cells were harvested in ice-cold PBS and lysed in Nonidet P-40 lysis buffer (50 mm Tris-HCl, pH 7.4, 10% glycerol, 50 mm NaCl, 0.5% sodium deoxycholate, 1% Nonidet P-40, 20 mm NaF, 0.2 mm Na3VO4) supplemented with protease inhibitors. Cell lysates were centrifuged at 15,000 rpm for 15 min to remove debris, and the supernatants were incubated with the indicated antibody for 4 h. Immune complexes were precipitated with protein G-Sepharose (Zymed Laboratories Inc.), washed three times with lysis buffer, and then eluted with Laemmli sample buffer for SDS-PAGE. Eluted proteins were resolved by SDS-PAGE and transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA). Membranes were probed using the designated antibodies and visualized with the ECL detection system (GE Healthcare).
RNA Isolation and Reverse Transcription-PCR—RNA was prepared using an RNA purification kit (Qiagen, Hilden, Germany). Reverse transcription was performed using an oligo(dT)20 primer and 2 μg of total RNA for first strand cDNA synthesis as described previously (16). PCR was performed at an annealing temperature of 57 °C with 22 amplification cycles for XIAP, cIAP, and glyceraldehyde-3-phosphate dehydrogenase. For Bcl-XL, PCR was performed at an annealing temperature of 60 °C with 25 amplification cycles. PCR products were resolved and electrophoresed in a 1.5% agarose gel in TAE (Tris-acetic acid-EDTA) buffer. The PCR primer sequences were as follows: glyceraldehyde-3-phosphate dehydrogenase, 5′-ACTCCACTCACGGCAAATTC-3′ (upstream) and 5′-CCTTCCACAATGCCAAAGTT-3′ (downstream); XIAP, 5′-GGCCGGACTATGCTCATTTA-3′ (upstream) and 5′-TGCCCCTTCTCATCCAATAG-3′ (downstream); cIAP, 5′-TGCTGTGGCCTGATGTTGGATA-3′ (upstream) and 5′-GAAAATGTCTGCGGTGCTCTGA-3′ (downstream); Bcl-XL, 5′-TGGTGGTCGACTTTCTCTCC-3′ (upstream) and 5′-CTCCATCCCGAAAGAGTTCA-3′ (downstream).
Animal Tumorigenesis and Administration of AG490—To test the oncogenic potentials of JAK2 L611S mutant in vivo, 1 × 107 transduced BaF3 cells (MSCV, WT, or L611S BaF3 cells) were injected subcutaneously into BALB/c nude mice aged 4 weeks. After injection of BaF3 cells, the mice were treated daily for 10 days with intraperitoneal injection of AG490 (500 μg/day). Two weeks postinoculation, the animals were sacrificed, and the weights of tumor, liver, spleen, and lymph node were recorded. For intravenous inoculation, 5 × 104 transduced BaF3 cells (MSCV, WT, or L611S BaF3 cells) were injected into BALB/c nude mice aged 7 weeks.
Histological Examination—After sacrifice, liver and spleen of each nude mouse were fixed in 4% paraformaldehyde and then dehydrated gradually in alcohol. The tissues were embedded in paraffin and were sectioned at a thickness of 2 μm. Lymph node was embedded in Tissue-Tek OCT compound (Sakura Finetek, Torrance, CA) and frozen in liquid nitrogen. Cryostat sections were cut at 6 μm, air dried, and stored at –20 °C until use. The sections were stained with hematoxylin and eosin and analyzed for the presence of tumor cell infiltration using an Olympus BX50 microscope (Olympus, Tokyo, Japan) with Olympus Micro DP70 software.
Plasmid, Retrovirus Production, and Infection—MSCV-IRES-GFP-Stat5A (1*6) was a gift from Dr. James N. Ihle (St. Jude Children's Research Hospital). A constitutively active form of murine Stat5A cDNA (1*6) harbors substitutions at amino acid residue 711 from serine to phenylalanine (S711F) and at 299 from histidine to arginine (H299R) as reported previously (17). HEK293T cells were transfected with helper retrovirus plasmid together with retroviral plasmids by FuGENE 6 transfection reagent according to the manufacturer's instructions (Roche Diagnostics). Viruses were harvested 24–60 h posttransfection, pooled, and stored on ice. BaF3 cells were infected using the precoated plates by RetroNectine (Takara) according to the manufacturer's instructions.
RESULTS
Leukemia-derived JAK2 L611S Mutant Induced Constitutive Activation of ERK, Akt, and Stat5—Recently a point mutation of JAK2 (L611S), illustrated in Fig. 1A, has been found in a child with ALL (13). L611S mutation is located in JH2 domain of JAK2. To investigate the function of this L611S mutation, we generated the BaF3 cells by retroviral infection with the control virus (BaF3/MSCV), viruses including the wild type JAK2 c-HA (BaF3/WT) or the JAK2 mutant c-HA harboring L611S mutation (BaF3/L611S), and retrovirus including the cDNA of Epo receptor c-FLAG (Fig. 1A). First to test whether L611S mutation could affect JAK2 activity, the phosphorylation at Tyr1007/1008 in the activation loop was determined. Although wild type JAK2 was phosphorylated at Tyr1007/1008 in response to Epo, the phosphorylation of JAK2 L611S mutant was constitutively observed (Fig. 1B). In addition, JAK2 L611S mutant induced the constitutive phosphorylation of ERK, Akt, and Stat5 regardless of Epo stimulation, although the phosphorylation of these molecules was induced by Epo in BaF3/MSCV cells and BaF3/WT cells (Fig. 1C). Interestingly the expression of JAK2 L611S mutant conferred growth factor independence to BaF3 cells (Fig. 1D, left). On the other hand, in the presence of Epo, no difference in cell growth of each type of cells was observed (Fig. 1D, right). Furthermore the proliferation rates of these cells were analyzed by BrdUrd incorporation as shown in Fig. 1E. In the presence of Epo, active proliferation of these three cell lines was detected. In contrast, in the absence of Epo, only the BaF3 cells expressing JAK2 L611S mutant showed significant proliferation ability.
FIGURE 1.
ALL-derived L611S mutation induces constitutive activation of JAK2. A, scheme of JAK2 L611S mutant (upper). BaF3 cell lines were infected with retrovirus encoding WT c-HA or JAK2 mutant c-HA (L611S) and erythropoietin receptor c-FLAG. Cell lysates were immunoblotted (IB) with anti-HA antibody, anti-JAK2 antibody, anti-FLAG antibody, or anti-β-actin antibody. MSCV indicates empty virus. B and C, transduced BaF3 cells were washed twice with PBS and left untreated or were stimulated with Epo (5 units/ml) for 24 h. B, cell lysates were subjected to immunoprecipitation (IP) using an anti-HA antibody and immunoblotted with antibodies to anti-phospho (P)-Tyr1007/1008 JAK2 or anti-HA antibody. C, cell lysates were immunoblotted with anti-phospho-Thr202/Tyr204 ERK antibody, anti-ERK antibody, anti-phospho-Ser473 Akt antibody, anti-Akt antibody, anti-phospho-Tyr694 Stat5 antibody, or anti-Stat5 antibody. D, transduced BaF3 cells were washed twice with PBS and left untreated or were stimulated with Epo (5 units/ml). Surviving cells were counted using a Beckman Coulter Vi-CELL at the indicated times. Results represent the mean ± S.D. of three independent experiments. E, transduced BaF3 cells were washed twice with PBS and left untreated or were stimulated with Epo (5 units/ml) for 24 h. Cell proliferation was measured by determining the BrdUrd (BrdU) incorporation. Results represent the mean ± S.D. of three independent experiments.
JAK2 L611S Mutant Inhibited Cytokine Withdraw-induced Apoptotic Cell Death—Cytokine deprivation has been classically used to study molecular processes of apoptosis. Following Epo withdraw, both BaF3/MSCV cells and BaF3/WT cells underwent death. On the other hand, only BaF3/L611S cells could survive for 3 days after Epo deprivation. In the presence of Epo, cell death was not detected in these three cell lines (Fig. 2A). We next determined the different phases of cell cycle distribution in these cells following 24 h of Epo deprivation. There was a significant increase of sub-G1 phase, which is consistent with apoptotic cells, in both BaF3/MSCV cells and BaF3/WT cells (Fig. 2B). Epo deprivation also induced the activation of casepase-3 and caspase-9 in both BaF3/MSCV cells and BaF3/WT cells (Fig. 2C). Furthermore a ladder pattern of DNA internucleosomal fragmentation was clearly seen in both BaF3/MSCV cells and BaF3/WT cells following 24 h of Epo deprivation, confirming that these cells underwent apoptotic cell death (Fig. 2D). On the other hand, there were no drastic changes of cell cycle distribution, caspase activity, and DNA fragmentation in BaF3/L611S cells regardless of Epo stimulation (Fig. 2, B, C, and D). We also analyzed the property of JAK2 L611S mutant using another murine myeloid cell line, DA3, which requires the presence of growth factor for its proliferation. As shown in supplemental Fig. 1S, DA3 cells expressing JAK2 L611S mutant survived after cytokine removal and exhibited antiapoptotic effects.
FIGURE 2.
Expression of JAK2 L611S mutant significantly inhibits apoptosis induced by cytokine removal. A, transduced BaF3 cells were washed twice with PBS and left untreated or were stimulated with Epo (5 units/ml) for the indicated times. The viability of these cells was determined by the trypan blue exclusion method. Results represent the mean ± S.D. of three independent experiments. B, cells were fixed, treated with propidium iodide, and subjected to fluorescence-activated cell sorting analysis as described under “Experimental Procedures.” C, caspase activities were measured by the cleavage of substrates, Ac-DEVD-7-amino-4-methycoumarin for caspase-3 or Ac-LEHD-7-amino-4-methycoumarin for caspase-9, respectively. Results represent the mean ± S.D. of three independent experiments. D, DNA was isolated from cells and subjected to agarose gel electrophoresis. E, XIAP, cIAP, and Bcl-XL mRNAs were determined by reverse transcription-PCR analysis. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used as an internal control. F, whole cell lysates were immunoblotted (IB) with anti-XIAP antibody, anti-cIAP1 antibody, anti-Bcl-XL antibody, or anti-β-actin antibody.
XIAP and cIAP1 are prominent members of the inhibitor of apoptosis protein family that exert antiapoptotic effects by interfering with the processing and activities of the executioner caspase-3 (18, 19). Bcl-XL is also known to be an important modulator of antiapoptosis (20, 21). Thus, we investigated the effect of JAK2 L611S mutant on the expression of the antiapoptotic proteins. Interestingly Epo deprivation reduced mRNA expression of XIAP, cIAP, and Bcl-XL in both BaF3/MSCV cells and BaF3/WT cells. On the other hand, the mRNA expression level of these antiapoptotic genes was not changed in BaF3/L611S cells regardless of Epo stimulation (Fig. 2E). Consistently although Epo withdraw induced a drastic decline in the protein levels of XIAP, cIAP, and Bcl-XL in both BaF3/MSCV cells and BaF3/WT cells, constant expression of these proteins was observed in BaF3/L611S cells even in the absence of Epo stimulation (Fig. 2F). These data demonstrate that JAK2 L611S mutant strongly inhibits the cytokine deprivation-induced apoptosis.
JAK2 L611S Mutant Could Promote Tumorigenesis in Nude Mice—Next to address the function of JAK2 L611S mutant in vivo, we examined whether subcutaneous inoculation of transduced BaF3 cells into nude mice could induce tumor formation. Interestingly at 10 days after inoculation, significant tumor formation was observed in the nude mice receiving BaF3/L611S cells but not BaF3/MSCV cells and BaF3/WT cells (data not shown). The tumors first appeared at the injection sites after 5 days of inoculation and then dramatically grew to an average of 0.69 ± 0.15 g at 14 days (data not shown). As compared with BaF3/MSCV and BaF3/WT, the life spans of nude mice receiving BaF3/L611S cells were severely reduced, and all mice died by 22 days after inoculation (Fig. 3A). In the nude mice receiving BaF3/L611S cells, the sizes of liver, spleen, and lymph node were abnormally enlarged compared with those in the mice receiving BaF3/MSCV and BaF3/WT (Fig. 3B). Namely the weights of liver, spleen, and lymph node of BaF3/L611S cell-injected mice were significantly increased (Fig. 3C). The average weight of liver in BaF3/L611S cell-inoculated mice increased about 1.5-fold (1.74 ± 0.13 versus 1.17 ± 0.05 g for BaF3/MSCV and 1.21 ± 0.17 g for BaF3/WT), whereas the average weight of spleen in BaF3/L611S mice increased even more severely to about 9-fold (0.72 ± 0.09 versus 0.08 ± 0.02 g for BaF3/MSCV and 0.07 ± 0.01 g for BaF3/WT). Similarly the average weight of lymph node in BaF3/L611S cell-inoculated mice also increased about 7-fold (120 ± 1.6 versus 17.3 ± 0.3 mg for BaF3/MSCV and 14.3 ± 0.5 mg for BaF3/WT) (Fig. 3C). Furthermore we also examined intravenous injection of transduced BaF3 cells because intravenous inoculation is a more suitable model for leukemia. As shown in Fig. 3D, after intravenous injection of BaF3/L611S cells, nude mice died within 9 days. The intravenous injection of BaF3/L611S cells induced enlargement of liver and spleen as well as subcutaneous injection (Fig. 3E). The average weight of liver in BaF3/L611S cell-inoculated mice increased about 1.8-fold (2.56 ± 0.13 versus 1.40 ± 0.13 g for BaF3/MSCV and 1.37 ± 0.07g for BaF3/WT), whereas the average weight of spleen in BaF3/L611S mice increased about 7-fold (1.02 ± 0.08 versus 0.14 ± 0.03 g for BaF3/MSCV and 0.12 ± 0.02 g for BaF3/WT) (Fig. 3F). Therefore, the overexpression of JAK2 L611S mutant at the intracellular compartment could promote tumor formation in vivo.
FIGURE 3.
JAK2 L611S mutant induced tumor formation in nude mice. A, B, and C, 1 × 107 BaF3 cells infected with empty virus (MSCV), WT, or JAK2 mutant (L611S) were subcutaneously (s.c.) injected into nude mice. A, a total of eight nude mice were injected for each BaF3 cell line. For 30 days postinoculation, mouse survival was monitored daily. B, 14 days postinoculation, mice were sacrificed, and the morphological changes of liver, spleen, and lymph node were photographed. C, 14 days postinoculation, four mice were sacrificed, and the weights of liver, spleen, and lymph node were recorded and graphed. * and ** indicate significant difference p < 0.01 and p < 0.005, respectively. D, E, and F, nude mice were intravenously (i.v.) inoculated (tail vein) with 5 × 104 BaF3 cells infected with empty virus (MSCV), WT, or JAK2 mutant (L611S). D, a total of six nude mice were injected for each BaF3 cell line. For 14 days postinoculation, mouse survival was monitored daily. E, 5 days postinoculation, mice were sacrificed, and the morphological changes of liver and spleen were photographed. F, 5 days postinoculation, three mice were sacrificed, and the weights of liver and spleen were recorded and graphed. * and ** indicate significant difference p < 0.01 and p < 0.005, respectively.
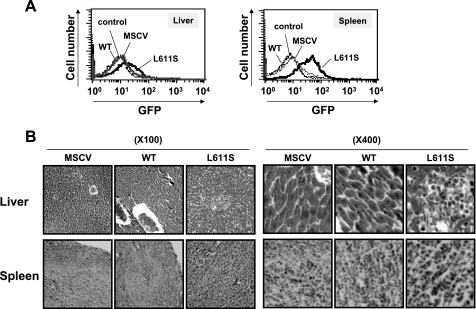
JAK2 L611S Mutant Promoted Tumor Cell Invasion—In this study, we used a retrovirus expression vector, MSCV-IRES-GFP, and sorted the infected cells based on green fluorescent protein (GFP) expression as described previously (14). To examine the possible invasion effect of BaF3/L611S cells, the hepatocytes and splenocytes were prepared from the subcutaneously BaF3 cell-injected nude mice, and GFP expression levels in these cells were monitored by flow cytometry analysis. Interestingly GFP-positive cells were observed in hepatocytes and splenocytes prepared from BaF3/L611S cell-inoculated mice (Fig. 4A). Furthermore various organ sections including liver, spleen, and lymph node were prepared and stained with hematoxylin and eosin. In the subcutaneous injection site, the BaF3/L611S cells behaved like a malignant tumor and could actively grow and penetrate into various organs. In liver, the infiltration of tumor cells disrupted the arrangement of hepatocytes in the lobules (Fig. 4B, upper). Also these BaF3/L611S cells frequently promoted the dissemination of transformed cells into spleen (Fig. 4B, bottom). Furthermore lymph node sections showed that the invasion of tumor cells was widely spread in nude mice inoculated with BaF3/L611S cells (data not shown). In contrast, there were no invasion by tumor cells and tissue distributions observed when BaF3/MSCV cells and BaF3/WT cells were inoculated. Thus, these results indicate that the oncogenic properties of JAK2 L611S mutant are not only associated with cellular transformation and tumorigenesis but also have a strong invasion activity into various organs, which leads to rapid animal death.
FIGURE 4.
JAK2 L611S mutant promoted cell penetration and invasion. 1 × 107 BaF3 cells infected with empty virus (MSCV), WT, or JAK2 mutant (L611S) were subcutaneously injected into nude mice. A, 14 days postinoculation, hepatocytes and splenocytes were prepared from injected nude mice. GFP-positive cells in hepatocytes and splenocytes were monitored by flow cytometry analysis. B, 14 days postinoculation, the sections of liver and spleen were stained with hematoxylin and eosin (magnification: ×100, left; ×400, right).
AG490 Induces Apoptotic Cell Death of BaF3 Cells Expressing JAK2 L611S Mutant—Because AG490 is a specific inhibitor for JAK2 (22), we initially examined whether AG490 could inhibit the activation of JAK2 L611S mutant. The treatment with AG490 at 30 μm was found to inhibit not only Epo-induced phosphorylation of wild type JAK2 but also the constitutive phosphorylation of JAK2 L611S mutant (Fig. 5A). Furthermore constitutive activation of ERK, Akt, and Stat5 observed in BaF3/L611S cells was significantly inhibited by treatment with AG490 (Fig. 5B). To demonstrate the specific effect of AG490 as a JAK2 inhibitor, BaF3/MSCV cells, BaF3/WT cells, and BaF3/L611S cells were further infected with virus harboring a constitutively active mutant of Stat5A (1*6) or GFP as a control (Fig. 5C). As shown in Fig. 5D, treatment with AG490 inhibited Epo-induced survival of both BaF3/MSCV cells and BaF3/WT cells. The survival of BaF3/L611S cells was also inhibited by treatment with AG490 in the presence and absence of Epo stimulation. Moreover the expression of Stat5A (1*6) rescued AG490-induced cell death of MSCV/BaF3 cells, WT/BaF3 cells, and L611S/BaF3 cells regardless of Epo stimulation (Fig. 5D), suggesting that AG490 specifically inhibited JAK2 activation. Then to determine whether the antiapoptotic effect of JAK2 L611S mutant is derived from its constitutive catalytic activity, we examined the effect of AG490 on the antiapoptotic effect of BaF3/L611S cells. Interestingly treatment with AG490 increased the sub-G1 population in both Epo-stimulated BaF3/MSCV cells and BaF3/WT cells. There was also an increase of the sub-G1 phase in BaF3/L611S cells by treatment with AG490 in the absence and presence of Epo (Fig. 6A). DMSO itself had no effects on the viability and cell cycle distribution in the various conditions indicated. In addition, treatment with AG490 caused both activation of caspases and DNA fragmentation in Epo-stimulated BaF3/MSCV cells and BaF3/WT cells. Also in BaF3/L611S cells, both activation of caspase-3 and DNA fragmentation were induced by AG490 in the absence and presence of Epo stimulation (Fig. 6, B and C). In both BaF3/MSCV cells and BaF3/WT cells, AG490 potently inhibited the Epo-induced expression of antiapoptotic proteins XIAP, cIAP, and Bcl-XL at both the mRNA and protein levels (Fig. 6, D and E). Moreover the expression of XIAP, cIAP, and Bcl-XL induced by JAK2 L611S mutant was also inhibited by AG490 (Fig. 6, D and E). Thus, AG490 induced apoptosis of BaF3/L611S cells, demonstrating that the constitutive activity of JAK2 L611S mutant is required for its antiapoptotic effect.
FIGURE 5.
AG490 inhibited constitutive activation of JAK2 L611S mutant. A and B, transduced BaF3 cells were treated with AG490 (AG; 30 μm) in the absence or presence of Epo (5 units/ml) for 24 h. A, cell lysates were subjected to immunoprecipitation (IP) using an anti-HA antibody and immunoblotted (IB) with antibodies to anti-phospho (P)-Tyr1007/1008 JAK2 or anti-HA antibody (upper). B, cell lysates were immunoblotted with anti-phospho-Thr202/Tyr204 ERK antibody, anti-ERK antibody, anti-phospho-Ser473 Akt antibody, anti-Akt antibody, anti-phospho-Tyr694 Stat5 antibody, or anti-Stat5 antibody. C and D, transduced BaF3 cells with empty virus (MSCV), WT, or JAK2 mutant (L611S) were infected with GFP as a control or constitutively active mutant of Stat5A (1*6). C, cell lysates were immunoblotted with anti-Stat5 antibody or anti-β-actin antibody. D, transduced BaF3 cells were treated with AG490 (30 μm) in the absence or presence of Epo (5 units/ml) for 24 h. The viability of these cells was determined by the trypan blue exclusion method. Results represent the mean ± S.D. of three independent experiments.
FIGURE 6.
AG490 induced apoptosis in cells expressing JAK2 L611S mutant. Transduced BaF3 cells were treated with AG490 (AG; 30 μm) in the absence or presence of Epo (5 units/ml) for 24 h. A, cells were fixed, treated with propidium iodide, and subjected to fluorescence-activated cell sorting analysis as described under “Experimental Procedures.” B, caspase-3 activity was measured by the cleavage of substrate, Ac-DEVD-7-amino-4-methycoumarin. Results represent the mean ± S.D. of three independent experiments. C, DNA was isolated from cells and subjected to agarose gel electrophoresis. D, XIAP, cIAP, and Bcl-XL mRNAs were determined by reverse transcription-PCR analysis. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used as an internal control. E, whole cell lysates were immunoblotted (IB) with anti-XIAP antibody, anti-cIAP1 antibody, anti-Bcl-XL antibody, or anti-β-actin antibody.
AG490 Inhibited JAK2 L611S Mutant-induced Tumorigenesis in Nude Mice—To examine the effect of AG490 in vivo, nude mice subcutaneously injected with BaF3/MSCV cells and BaF3/L611S cells were treated with either AG490 or DMSO vehicle daily for 10 days. Interestingly at 14 days after inoculation of BaF3/L611S cells, the tumor formation at the injection sites was inhibited by AG490 treatment (Fig. 7A). Actually the weight of tumors obviously decreased as a result of treatment with AG490 (0.88 ± 0.05 g for DMSO and 0.30 ± 0.08 g for AG490) (Fig. 7D). On the other hand, there were no obvious changes observed in BaF3/MSCV cell-injected mice regardless of AG490 treatment. Interestingly the life span of nude mice receiving BaF3/L611S cells was effectively extended by AG490 treatment as compared with DMSO vehicle (Fig. 7B). During 30 days, the intraperitoneal injection of AG490 or DMSO vehicle for 10 days did not influence the survival of BaF3/MSCV-injected mice. Furthermore at 14 days after inoculation, AG490 significantly inhibited the increase of the size of liver and spleen induced by BaF3/L611S cells (Fig. 7C). The average weight of liver and spleen in BaF3/MSCV-injected mice was little changed by AG490 treatment (liver, 1.29 ± 0.11 g for DMSO and 1.32 ± 0.06 g for AG490; spleen, 0.10 ± 0.02 g for DMSO and 0.09 ± 0.02 g for AG490). However, AG490 effectively decreased the weight of liver and spleen in BaF3/L611S-injected mice (liver, 1.81 ± 0.03 g for DMSO and 1.29 ± 0.01 g for AG490; spleen, 0.55 ± 0.08 g for DMSO and 0.14 ± 0.01 g for AG490) (Fig. 7D). Compared with BaF3/MSCV-injected mice, the size of lymph node was increased in BaF3/L611S-injected mice treated with AG490 (Fig. 7C). However, the weight of lymph node in BaF3/L611S-injected mice was decreased by AG490 (lymph node, 159.8 ± 7.4 g for DMSO and 88.2 ± 6.2 g for AG490) (Fig. 7D). Therefore, AG490 significantly inhibited JAK2 L611S mutant-induced tumorigenesis in nude mice.
FIGURE 7.
AG490 inhibited JAK2 L611S mutant-induced tumor formation in nude mice. 1 × 107 BaF3 cells infected with empty virus (MSCV) or JAK2 mutant (L611S) were subcutaneously injected into nude mice, and the mice were treated with intraperitoneal injection of DMSO or AG490 (AG; 500 μg/day) daily for 10 days. A, nude mice were photographed 14 days postinoculation with BaF3 cells. The arrows indicate tumors in nude mice. B, a total of eight nude mice were injected with transduced BaF3 cells and treated with DMSO or AG490 (500 μg/day) daily for 10 days. For 30 days after inoculation, mouse survival was monitored daily. C, 14 days postinoculation with BaF3 cells, mice were sacrificed, and the morphological changes of liver, spleen, and lymph node were photographed. D, 14 days postinoculation, tumor, liver, spleen, and lymph node were weighed and graphed. * and ** indicate significant difference p < 0.01 and p < 0.005, respectively.
AG490 Inhibited Tumor Cell Invasion Induced by JAK2 L611S Mutant—To further examine the effect of AG490 on tumor invasion caused by JAK2 L611S mutant, GFP expression levels in hepatocytes and splenocytes at 14 days after inoculation were monitored by flow cytometry analysis. Interestingly GFP positive-cells in hepatocytes and splenocytes from BaF3/L611S cell-injected mice were reduced by AG490 treatment (Fig. 8A). These data suggest that AG490 treatment effectively inhibited the invasion of BaF3/L611S cells into liver and spleen. Furthermore the various organ sections such as liver, spleen, and lymph node were prepared at 14 days after inoculation. The infiltration of tumor cells in liver was observed in BaF3/L611S cell-injected mice with DMSO vehicle treatment. On the other hand, there was little infiltration of tumor cells in BaF3/L611S cell-injected mice with AG490 treatment similar to that in BaF3/MSCV-injected mice. Also the dissemination of transformed cells into spleen and lymph node induced by BaF3/L611S cells was clearly inhibited by AG490 (data not shown). Thus, all these data indicate that AG490 potently inhibited cellular transformation and tumorigenesis induced by JAK2 L611S mutant.
FIGURE 8.
AG490 inhibited JAK2 L611S mutant-induced cell penetration and invasion. 1 × 107 BaF3 cells infected with empty virus (MSCV) or JAK2 mutant (L611S) were subcutaneously injected into nude mice and treated with intraperitoneal injection of DMSO or AG490 (AG; 500 μg/day) daily for 10 days. A, 14 days postinoculation, hepatocytes and splenocytes were prepared from injected nude mice. GFP-positive cells in hepatocytes and splenocytes were monitored by flow cytometry analysis. B, 14 days postinoculation with BaF3 cells, the sections of liver and spleen were stained with hematoxylin and eosin (magnification: ×100, left; ×400, right).
DISCUSSION
In the case of several tyrosine kinase family members, aberrant expression, point mutation, and chromosomal translocation are reported to cause their disorderly activity. Indeed such alterations of tyrosine kinases have been found in various tumors, and these variations of tyrosine kinases have been frequently reported to result in the promotion of cellular transformation and tumorigenesis (4, 5, 23). Our current study demonstrated that JAK2 harboring a novel point mutation, L611S, shows a potent oncogenic activity stimulating a series of cancer-related processes not only for cellular transformation and tumorigenesis but also for tumor cell invasion.
Kratz et al. (13) screened the mutation of JAK2 in 286 patients with ALL and discovered the L611S mutation. They detected L611S mutation in a bone marrow DNA specimen from a 3-year-old girl with precursor B-ALL. However, the biochemical analyses of JAK2 L611S mutant have not been performed, and the effect of L611S mutation on the function of JAK2 has not been reported previously. Recently it has been reported that the majority of polycythemia vera patients harbor a unique somatic mutation (V617F) in JAK2 (10–12). Because L611S mutation is quite close to the V617F mutation, we speculated that L611S mutation might disrupt equilibrium of regulation of JAK2 and exhibit a constitutively active direction. Both V617F and L611S are located in JH2 domain, which is required to inactivate the JAK2 activity in the absence of cytokines (8, 9). Once JAK2 gains V617F mutation, it exhibits a constitutively active property by disrupting the suppressive action of JH2 domain of JAK2 (6). Strikingly we observed that L611S mutation also caused the constitutive activation of JAK2 (Fig. 1). Now we have no additional data indicating how L611S mutation drives JAK2 activity aberrantly. Probably like the V617F mutation, L611S mutation might stabilize an opened conformation of the kinase domain of JAK2 and abolish the suppressive action of JH2 to mask the activation loop in JH1 and block its kinase activity.
Previously we also reported that JAK2 L611S mutant restored formation of colony forming-unit-erythroid in the absence of Epo when JAK2 L611S mutant was used to infect JAK2-deficient fetal liver cells (14). In addition, JAK2 L611S mutant-expressing BaF3 cells gained overproliferating potential to undergo growth factor-independent cell proliferation (Figs. 1 and 2). Furthermore in the tumorigenesis analysis using nude mice, JAK2 L611S mutant-expressing cells exhibited highly transforming activity and also the ability to promote cell invasion activity in a variety of distant organs. Consequently the life spans of these tumor-carrying animals were significantly reduced (Figs. 3 and 4). Interestingly the polycythemia vera-associated mutant, JAK2 V617F, also has a similar ability to induce tumorigenesis in nude mice; however, it was apparently more malignant than JAK2 L611S. We observed that the life span of mice subcutaneously injected with JAK2 V617F mutant-expressing cells was shorter than that of mice injected with JAK2 V617F mutant-expressing cells. The average life span of mice injected with V617F-expressing cells and mice injected with L611S-expressing cells was 15.7 and 18.3 days, respectively (data not shown). Furthermore compared with the organs in BaF3/L611S cell-injected mice, the high level of tumor cell invasion was frequently observed in various organs in BaF3/V617F cell-injected mice (data not shown). Although the expression levels of each JAK2 mutant in cells should be strictly considered, it might be possible to cause a difference in the conformation change by the difference in amino acid substitution between V617F and L611S.
In this study, we utilized the specific inhibitor for JAK2, AG490, in vitro and in vivo. Importantly AG490 inhibited the constitutive activation of JAK2 L611S mutant and induced apoptotic cell death of BaF3 cells expressing JAK2 L611S (Figs. 5 and 6). Tumorigenesis induced by BaF3/L611S cells could not be completely inhibited by temporary administration of AG490; however, one important observation is that the life span was effectively extended by AG490 as shown in Fig. 7. In addition, pathological analysis of tissue sections revealed that AG490 inhibited the penetration and invasion of tumor cells into distinct organs, such as the liver, spleen, and lymph node, within a short period of time (Fig. 8). Through a series of experiments using AG490 both in vitro and in vivo, it was demonstrated that constitutive JAK2 activity by L611S mutation was a critical cause of transformation and tumorigenesis.
Moreover it would be interesting to define the detailed signal transduction induced by JAK2 L611S mutant. As shown in Fig. 2, L611S mutant exhibited antiapoptotic activity. Interestingly our data also demonstrated that JAK2 L611S induced the constitutive expression of antiapoptotic proteins XIAP, cIAP-1, and Bcl-XL in the absence of Epo stimulation (Fig. 2), suggesting that L611S mutant might result in an antiapoptotic phenotype via the expression of these proteins. Indeed AG490 affected both cell survival induced by L611S mutation and the expression of these anti-apoptotic gene products. Now we do not have additional data indicating how L611S mutant could regulate the expression of the antiapoptotic proteins. The expression of these antiapoptotic proteins is known to be modulated by the transcriptional activity of NF-κB (24–26). Strikingly we observed the constitutive DNA binding activity of NF-κBin BaF3 cells expressing JAK2 L611S, whereas NF-κB activation was induced by Epo stimulation in both BaF3/MSCV cells and BaF3/WT cells (data not shown); however, the detailed mechanism of NF-κB activation induced by constitutively active JAK2 mutant is still uncertain. Recently we also reported that Stat3 plays a crucial role in the transforming action of TEL (translocation-Ets-leukemia)-JAK2, another type of active mutant of JAK2 (15). When the activation of Stat3 is inhibited, TEL-JAK2 failed to exhibit the transforming activity. It has also been reported that the Stat family can regulate the expression of Bcl family proteins (27, 28). This could be a clue to understanding the entire signaling pathway caused by JAK2 L611S mutant for the development of targeted therapies.
Not only JAK2 inhibitor but also other inhibitors of downstream molecules are expected to be effective for the treatment of JAK2 L611S-related disease. Our data indicated that overexpression of JAK2 L611S mutant might be the trigger for the activation of not only Stat5 but also Akt and ERK (Fig. 1). Furthermore the constitutive activation of Stat5, Akt, and ERK was clearly inhibited by treatment with AG490 (Fig. 5). Previous studies have shown that tumor-related and mitogenic signaling pathways, such as phosphatidylinositol 3-kinase-Akt and MEK-ERK, are predominantly active in tumor cells with invasive and metastatic characteristics (29–32). Thus, it is easily speculated that the phosphatidylinositol 3-kinase-Akt and MEK-ERK pathways may play certain roles in cancer development caused by JAK2 L611S mutant. Using LY294002 and U0126, specific inhibitors of phosphatidylinositol 3-kinase and MEK, respectively, we investigated the roles of the phosphatidylinositol 3-kinase-Akt and MEK-ERK pathways in JAK2 L611S mutant-induced transformation. When BaF3/L611S cells were treated with LY294002, the cells underwent apoptotic cell death. Additionally the sub-G1 population in BaF3/L611S cells was increased by LY294002 both in the absence and presence of Epo (data not shown). On the other hand, treatment with U0126 decreased the S phase but was not able to increase the sub-G1 population in BaF3/L611S cells (data not shown). Although further detailed analyses are required, these observations indicate that the phosphatidylinositol 3-kinase-Akt pathway contributes to the survival of BaF3/L611S cells and that the MEK-ERK pathway is important for the proliferation of BaF3/L611S cells, respectively.
In conclusion, the functional studies revealed that JAK2 L611S mutant is capable of eliciting tumor cell invasion. This analysis additionally highlighted the aggressive character of JAK2 L611S during cancer development. These results will be a critical step for understanding the transition from benignity to cancer. So far, this L611S mutation has been reported only in one ALL patient (13). Because the importance of the L611S mutation was clarified in this study, it might be important in the future to examine whether the L611S mutation can be detected in a number of patients with various diseases. More interestingly, it has also been reported that this L611S mutation was not found in remission marrow (13). Therefore, because the L611S mutation could be an index that demonstrates the disease condition, it may be useful for the prognostication of leukemia patients.
Supplementary Material
Acknowledgments
We thank I. Michikawa for technical assistance. We also thank Dr. J. N. Ihle for retrovirus expression vector of constitutive Stat5A mutant (1*6).
This work was supported in part by Ministry of Education, Culture, Sports, Science and Technology Grant G19790071 and grants from the Research Foundation for Pharmaceutical Sciences and the Hi-Tech Research Center Project for Private Universities in Japan.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1S.
Footnotes
The abbreviations used are: JAK, Janus kinase; cIAP-1, inhibitor of apoptosis protein-1; DMSO, dimethyl sulfoxide; Epo, erythropoietin; ERK, extracellular signal-regulated kinase; PBS, phosphate-buffered saline; Stat, signal transducers and activators of transcription; XIAP, X chromosome-linked inhibitor of apoptosis protein; MEK, mitogen-activated protein kinase/extracellular signal-regulated kinase kinase; JH, Jak homology; ALL, acute lymphoblastic leukemia; MSCV, murine stem cell virus; WT, wild type JAK2; GFP, green fluorescent protein; IRES, internal ribosome entry site; HA, hemagglutinin.
References
- 1.Baker, S. J., Rane, S. G., and Reddy, E. P. (2007) Oncogene 26 6724–6737 [DOI] [PubMed] [Google Scholar]
- 2.Schindler, C., Levy, D. E., and Decker, T. (2007) J. Biol. Chem. 282 20059–20063 [DOI] [PubMed] [Google Scholar]
- 3.O'Sullivan, L. A., Liongue, C., Lewis, R. S., Stephenson, S. E., and Ward, A. C. (2007) Mol. Immunol. 44 2497–2506 [DOI] [PubMed] [Google Scholar]
- 4.Ihle, J. N., and Gilliland, D. G. (2007) Curr. Opin. Genet. Dev. 17 8–14 [DOI] [PubMed] [Google Scholar]
- 5.Lacronique, V., Boureux, A., Valle, V. D., Poirel, H., Quang, C. T., Mauchauffé, M., Berthou, C., Lessard, M., Berger, R., Ghysdael, J., and Bernard, O. A. (1997) Science 278 1309–1312 [DOI] [PubMed] [Google Scholar]
- 6.Lindauer, K., Loerting, T., Liedl, K. R., and Kroemer, R. T. (2001) Protein Eng. 14 27–37 [DOI] [PubMed] [Google Scholar]
- 7.Feng, J., Witthuhn, B. A., Matsuda, T., Kohlhuber, F., Kerr, I. M., and Ihle, J. N. (1997) Mol. Cell. Biol. 17 2497–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saharinen, P., and Silvennoinen, O. (2002) J. Biol. Chem. 277 47954–47963 [DOI] [PubMed] [Google Scholar]
- 9.Saharinen, P., Vihinen, M., and Silvennoinen, O. (2003) Mol. Biol. Cell 14 1448–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James, C., Ugo, V., Le Couédic, J. P., Staerk, J., Delhommeau, F., Lacout, C., Garçon, L., Raslova, H., Berger, R., Bennaceur-Griscelli, A., Villeval, J. L., Constantinescu, S. N., Casadevall, N., and Vainchenker, W. (2005) Nature 434 1144–1148 [DOI] [PubMed] [Google Scholar]
- 11.Levine, R. L., Wadleigh, M., Cools, J., Ebert, B. L., Wernig, G., Huntly, B. J., Boggon, T. J., Wlodarska, I., Clark, J. J., Moore, S., Adelsperger, J., Koo, S., Lee, J. C., Gabriel, S., Mercher, T., D'Andrea, A., Fröhling, S., Döhner, K., Marynen, P., Vandenberghe, P., Mesa, R. A., Tefferi, A., Griffin, J. D., Eck, M. J., Sellers, W. R., Meyerson, M., Golub, T. R., Lee, S. J., and Gilliland, D. G. (2005) Cancer Cell 7 387–397 [DOI] [PubMed] [Google Scholar]
- 12.Kralovics, R., Passamonti, F., Buser, A. S., Teo, S. S., Tiedt, R., Passweg, J. R., Tichelli, A., Cazzola, M., and Skoda, R. C. (2005) N. Engl. J. Med. 352 1779–1790 [DOI] [PubMed] [Google Scholar]
- 13.Kratz, C. P., Böll, S., Kontny, U., Schrappe, M., Niemeyer, C. M., and Stanulla, M. (2006) Leukemia 20 381–383 [DOI] [PubMed] [Google Scholar]
- 14.Funakoshi-Tago, M., Pelletier, S., Moritake, H., Parganas, E., and Ihle, J. N. (2008) Mol. Cell. Biol. 28 1792–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Funakoshi-Tago, M., Tago, K., Nishizawa, C., Takahashi, K., Mashino, T., Iwata, S., Inoue, H., Sonoda, Y., and Kasahara, T. (2008) Biochem. Pharmacol. 76 1681–1693 [DOI] [PubMed] [Google Scholar]
- 16.Funakoshi-Tago, M., Sonoda, Y., Tanaka, S., Hashimoto, K., Tago, K., Tominaga, S., and Kasahara, T. (2003) J. Biol. Chem. 278 29359–29365 [DOI] [PubMed] [Google Scholar]
- 17.Onishi, M., Nosaka, T., Misawa, K., Mui, A. L., McMahon, G. D., Miyajima, A., and Kitamura, T. (1998) Mol. Cell. Biol. 18 3871–3879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deveraux, Q. L., Takahashi, R., Salvesen, G. S., and Reed, J. C. (1997) Nature 388 300–304 [DOI] [PubMed] [Google Scholar]
- 19.Roy, N., Deveraux, Q. L., Takahashi, R., Salvesen, G. S., and Reed, J. C. (1997) EMBO J. 16 6914–6925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boise, L. H., González-García, M., Postema, C. E., Ding, L., Lindsten, T., Turka, L. A., Mao, X., Nuñez, G., and Thompson, C. B. (1993) Cell 74 597–608 [DOI] [PubMed] [Google Scholar]
- 21.Gottschalk, A. R., Boise, L. H., Thompson, C. B., and Quintáns, J. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 7350–7354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meydan, N., Grunberger, T., Dadi, H., Shahar, M. A., Arpaia, E., Lapidot, Z., Leeder, J. S., Freedman, M., Cohen, A., Gazit, A., Levitzki, A., and Roifman, C. M. (1996) Nature 379 645–648 [DOI] [PubMed] [Google Scholar]
- 23.Blume-Jensen, P., and Hunter, T. (2001) Nature 411 355–365 [DOI] [PubMed] [Google Scholar]
- 24.You, M., Ku, P. T., Hrdlicková, R., and Bose, H. R., Jr. (1997) Mol. Cell. Biol. 17 7328–7341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stehlik, C., de Martin, R., Kumabashiri, I., Schmid, J. A., Binder, B. R., and Lipp, J. (1998) J. Exp. Med. 188 211–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khoshnan, A., Tindell, C., Laux, I., Bae, D., Bennett, B., and Nel, A. E. (2000) J. Immunol. 165 1743–1754 [DOI] [PubMed] [Google Scholar]
- 27.Feldman, G. M., Rosenthal, L. A., Liu, X., Hayes, M. P., Wynshaw-Boris, A., Leonard, W. J., Hennighausen, L., and Finbloom, D. S. (1997) Blood 90 1768–1776 [PubMed] [Google Scholar]
- 28.Catlett-Falcone, R., Landowski, T. H., Oshiro, M. M., Turkson, J., Levitzki, A., Savino, R., Ciliberto, G., Moscinski, L., Fernández-Luna, J. L., Nuñez, G., Dalton, W. S., and Jove, R. (1999) Immunity 10 105–115 [DOI] [PubMed] [Google Scholar]
- 29.Danilkovitch-Miagkova, A., and Zbar, B. (2002) J. Clin. Investig. 109 863–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fidler, I. J. (2002) Semin. Cancer Biol. 12 89–96 [DOI] [PubMed] [Google Scholar]
- 31.Grant, S. (2008) J. Clin. Investig. 118 3003–3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinkade, C. W., Castillo-Martin, M., Puzio-Kuter, A., Yan, J., Foster, T. H., Gao, H., Sun, Y., Ouyang, X., Gerald, W. L., Cordon-Cardo, C., and Abate-Shen, C. (2008) J. Clin. Investig. 118 3051–3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.