Abstract
Intermedilysin (ILY) is an unusual member of the family of cholesterol-dependent cytolysins because it binds to human CD59 (hCD59) rather than directly to cholesterol-rich membranes. Binding of ILY to hCD59 initiates a series of conformational changes within the toxin that result in the conversion of the soluble monomer into an oligomeric membrane-embedded pore complex. In this study the association of ILY with its membrane receptor has been examined throughout the assembly and formation of the pore complex. Using ILY mutants trapped at various stages of pore assembly, we show ILY remains engaged with hCD59 throughout the assembly of the prepore oligomer, but it disengages from the receptor upon the conversion to the pore complex. We further show that the assembly intermediates increase the sensitivity of the host cell to lysis by its complement membrane attack complex, apparently by blocking the hCD59-binding site for complement proteins C8α and C9.
The cholesterol-dependent cytolysins (CDC)2 are a family of structurally related pore-forming toxins that are important virulence factors for a variety of Gram-positive pathogens (1–4). The CDCs are secreted by the bacterium as soluble monomers and then bind to cholesterol-rich eukaryotic cell membranes (5). Once bound, the monomers laterally diffuse and interact with one another to form a large oligomeric prepore structure comprised of 35–40 CDC monomers. One of the hallmarks of this family of toxins is the absolute requirement of their pore-forming mechanism on membrane cholesterol (1). Membrane cholesterol serves to target the CDCs to the eukaryotic cell membrane and is necessary to convert the prepore oligomer to the inserted pore complex (6). Two classes of CDCs currently exist. The first class is typified by perfringolysin O (PFO) from Clostridium perfringens that appears to bind directly to cholesterol-rich membranes, an interaction mediated by three short loops in domain 4 (7). The second group includes intermedilysin (ILY) from Streptococcus intermedius and vaginolysin from Gardnerella vaginalis (8). These CDCs bind to the glycosylphosphatidylinositol-anchored protein human CD59 (hCD59). It has been shown for ILY that it first binds hCD59 and then inserts its domain 4 loops in a cholesterol-dependent fashion (7). Why the latter two CDCs have evolved to specifically bind hCD59 and whether they remain engaged with this receptor throughout the assembly of the pore complex remains unclear. S. intermedius is a pathogen frequently associated with abscesses of the oral cavity as well as with life-threatening abscesses of the head, neck, and liver (9, 10). ILY appears to be important in establishing these deep-seated abscesses as S. intermedius isolated from these sites produces levels of ILY 6–10 times greater than strains isolated from peripheral site infections or the oropharynx (9). ILY binds only human cells, whereas other CDCs, such as PFO, bind to most cholesterol-rich eukaryotic membranes. The species selectivity of ILY is because of its specificity for human hCD59 and appears to be encoded in domain 4 of the toxin (11, 12).
CD59 is an 18–20-kDa surface-expressed glycoprotein tethered to the cell membrane via a glycosylphosphatidylinositol anchor. It is widely distributed on most human and nonhuman cell types. It is associated with a number of important cellular functions that include serving as an adaptor molecule for a candidate C1q receptor (C1qRO–2) (13, 14) and acting as a cell-signaling molecule (15). Its primary role, however, is regulating the terminal pathway of complement by inhibiting the formation of the membrane attack complex (MAC) on host cells by binding to C8α and C9, thus preventing the formation of the MAC pore (16–18). In various autoimmune diseases and inflammatory conditions, excessive complement activation can saturate the available CD59 resulting in MAC-mediated host cell injury (19). CD59 exhibits species selectivity such that it most effectively inhibits only the homologous MAC (20). ILY recognition of the same or similar structural differences in CD59 is the basis for its species selective activity (11).
ILY binding to hCD59 triggers a series of conformational changes in ILY leading to its membrane oligomerization into the prepore complex (6). This is accompanied by the cholesterol-dependent insertion of three loops at the base of domain 4 and the insertion of the undecapeptide, events that are necessary for the conversion of the prepore to a pore complex (7). It is not known, however, whether ILY remains engaged with hCD59 throughout its assembly into the pore complex. Whether ILY remains engaged during and after the assembly of the pore complex may also impact the ability of the eukaryotic cell to protect itself from the host MAC because a previous study suggested the ILY-binding site on hCD59 overlaps that for complement proteins C8α and C9 (11). To address these questions, we investigated the interaction of ILY with hCD59 during the assembly of the ILY pore complex. We further determined whether nonlytic assembly intermediates of ILY increase MAC-mediated damage to host cells by short circuiting the protective function of hCD59. These studies show ILY remains engaged during the assembly of its prepore complex and disengages from its receptor upon pore formation. In addition, we show that engagement of hCD59 by ILY prior to pore formation significantly increases the host cell sensitivity to the host MAC-mediated lysis.
EXPERIMENTAL PROCEDURES
Bacterial Strains, Plasmids, and Chemicals—The gene for ILY was cloned into pTrcHisA (Invitrogen) as described previously (6). ILYH242C (cysteine-containing derivative) is considered wild type toxin except where noted. All mutations were made in the native ILY background unless otherwise stated. All chemicals and enzymes were obtained from Sigma, VWR, and Research Organics except where noted. All fluorescent probes were obtained from Molecular Probes (Invitrogen). Anti-hCD59 H19-FITC conjugated and unconjugated was obtained from Pharmingen. Anti-hCD59 MEM43 and FITC-conjugated MEM43 were obtained from AbCAM. Anti-hCD59 10G10 expressing mouse B cell myeloma was a generous gift from Dr. Marilyn Telen (Duke University Medical Center). Purification of 10G10 antibody from tissue culture supernatants was performed using Affi-Gel Protein-A MAPS II antibody purification kit (Bio-Rad) as the manufacturer specified. Goat anti-mouse FITC-conjugated secondary antibody was obtained from AbCAM. All other secondary antibodies were obtained from Bio-Rad.
Cells and Transfection—Chinese hamster ovary cells (CHO) transfected with a human CD59 (CHOhCD59)-expressing plasmid were a generous gift of Dr. Stephen Tomlinson (New York University Medical Center, New York) (19). The cells were maintained in F-12 Kaighn's medium (10% v/v fetal calf serum, 1% v/v penicillin/streptomycin) (Invitrogen). Two rounds of sorting by FLOW were used to obtain cell populations uniformly expressing high levels of hCD59. For sorting, CHO cells were detached with 5 mm EDTA in phosphate-buffered saline (PBS), washed once with PBS, and resuspended in F-12, 10% fetal calf serum.
Eight rounds of QuikChange mutagenesis were performed on the hCD59 plasmid in order to replace the first nine amino acids of the malaria epitope tag (NANPNANPNALG located between residues 2 and 3 of the mature human CD59) with the HA epitope from influenza (YPYDVPDYA). CHO cells were then transfected at a 3:2 ratio using the FuGENE 6 reagent (Roche Applied Science) as per the manufacturer's instructions. Positive clones expressing the HA hCD59 (CHOHAhCD59) were selected using anti-HA-FITC antibody (Sigma) by cell sorting (Influx cell sorter, OUHSC Flow Cytometry Core Facility).
CHOHAhCD59 Membrane Preparation—CHOHAhCD59 cells (10 × 106 cells) were resuspended in 1 ml of membrane buffer (1× PBS containing 1 complete protease inhibitor mixture (Roche Applied Science) and 0.5 mg/ml DNase (Roche Applied Science)). The cells were homogenized in a Dounce homogenizer for 20 strokes, and the mixture was then subjected to one freeze/thaw cycle with liquid nitrogen. This process was repeated a total of three times. The membranes were then centrifuged 13,000 × g for 15 min at 4 °C, and the pellet was resuspended in 100 μl of 1× PBS.
Co-immunoprecipitation of ILY with Anti-HA Antibodies—CHOHAhCD59 membranes (2.5 μl) were incubated with ILYml, ILYpp, or ILYwt (171 nm) in a total volume of 100 μl of 1× PBS for 1 h at 37 °C. The samples were centrifuged 13,000 × g for 15 min to remove excess ILY. The pellet was then resuspended in 100 μl of CelLytic M Cell Lysis buffer (Sigma anti-HA immunoprecipitation kit) supplemented with 10 mm n-octyl β-d-glucopyranoside (Sigma) and allowed to incubate on ice for 10 min to solubilize the membranes. The samples were immunoprecipitated via the anti-HA immunoprecipitation kit according to the manufacturer's protocol (Sigma). Eluted samples were subjected to a 4–20% SDS-PAGE and then transferred to nitrocellulose for Western blotting analysis. Rabbit anti-ILY antibodies and mouse anti-CD59 (H19 from Pharmingen) were used for Western analysis.
Antibodies and Serum—Rabbit antisera to CHO cell membranes were a kind gift from Dr. S. Tomlinson. Normal human serum was obtained from the blood of healthy volunteers. The serum was then incubated with 5 ml of Affi-gel protein A-agarose (Bio-Rad) as per manufacturer's instructions todeplete the serum of antibodies. The depleted serum was then quick frozen with liquid nitrogen and stored at –80 °C.
Generation and Purification of ILY and Its Derivatives—The generation of amino acid substitutions in the gene for ILY was performed via PCR QuikChange mutagenesis (Stratagene). The Oklahoma Medical Research Foundation Core DNA sequencing facility performed DNA sequence analysis of each mutant toxin gene. The expression and purification of recombinant ILY and its derivatives from Escherichia coli were carried out as described previously (6, 21).
Hemolytic Activity—The hemolytic activity of each ILY mutant on human erythrocytes was determined as described previously (21). The HD50 is defined as the concentration of toxin required to lyse 50% of the human erythrocytes under standard assay conditions.
SDS-Agarose Gel Electrophoresis (SDS-AGE) and Immunoblot Analyses—SDS-AGE was carried out as described previously (6, 21, 22). Briefly, ILY (171 nm) was incubated in the presence or absence of human erythrocytes (1.5 × 106 cells) for 30 min at 37 °C. Samples were solubilized with SDS sample buffer at 37 °C for 2 min, and then the monomeric and oligomeric complexes were resolved on a 1.5% SDS-AGE gel and immunoblotted with rabbit anti-ILY antibody followed by anti-rabbit IgG horseradish peroxidase secondary antibody (Bio-Rad). Reactive species were visualized using a chemiluminescent substrate (ECL Western Blotting Detection Reagents, GE Healthcare) and autoradiography.
Receptor Availability Assay—We have previously shown specific anti-hCD59 antibodies block ILY binding to hCD59 (11). Therefore, we used these antibodies to probe the availability of the ILY-binding site on hCD59 at specific stages of its assembly process. Flow cytometry was used to monitor the ability of fluorescent derivatives of these antibodies (or fluorescently labeled secondary antibody to the primary mAb) to bind hCD59. If ILY disengaged from hCD59 at a specific stage of its assembly, then the binding sites for these antibodies would become accessible to the antibody. Therefore, the experimental approach was to use this panel of anti-CD59 monoclonal antibodies to probe the availability of hCD59 on erythrocytes preincubated with wild type ILY (ILYwt), monomer locked ILY (ILYml), or prepore locked ILY (ILYpp). PFO, a CDC that does not bind hCD59, was used as a negative control.
Saturating levels of ILY and the ILY derivatives (ILYml (171 nm), ILYpp (85.5 nm), or ILYwt (85.5 nm)) were incubated with washed erythrocytes (1 × 106 cells) in PBS (reaction volume 100 μl) for 30 min at 4 °C. Samples were pelleted (14,000 × g for 5 min), and excess toxin was removed. The pellets were then resuspended in 100 μl of PBS containing 0.1% (w/v) BSA bovine serum albumin and kept on ice for 10 min to allow binding. The erythrocytes were washed twice with PBS and then resuspended in 100 μl of anti-hCD59 antibody (33.3 nm) and incubated on ice for 30 min. Samples were washed twice with PBS and then brought to a final volume of 500 μl of PBS and analyzed by a FACSCalibur flow cytometer (University of Oklahoma Health Sciences Center) and FLOWJO software (Tree Star). The emission wavelength was set to 530 nm, and the excitation was set at 488 nm with a bandpass of 30 nm. Samples assayed with 10G10 anti-hCD59 were resuspended in 100 μl of goat anti-mouse-FITC (33.3 nm) for 15 min on ice and washed twice with PBS. Samples assayed with either H19-FITC or MEM43-FITC anti-hCD59 were analyzed directly.
Förster Resonance Energy Transfer (FRET) Analysis—Flow cytometry was used to monitor the interaction between ILY and its receptor during pore formation on the surface of erythrocytes by FRET. FRET between donor (FITC)-labeled anti-hCD59 MEM43 monoclonal antibody (AbCAM) and acceptor (TRITC)-labeled ILY or PFO was determined as described previously (6, 12) with the following modifications. Cell-associated hCD59 was indirectly labeled with a fluorescein-labeled monoclonal antibody that did not interfere with ILY binding or oligomerization. Briefly, human erythrocytes (1 × 106) were preincubated with anti-hCD59 MEM43-FITC-conjugated antibody (66.6 nm) for 1 h on ice in a reaction volume of 100 μl to label hCD59 with the donor fluorophore. Subsequently, either unlabeled or tetramethylrhodamine-labeled PFOpp, ILYpp, or ILYwt (42.7 nm) (acceptors) were added to the samples and incubated an additional 30 min on ice. PBS was added to the samples to a final volume of 500 μl, and each sample was analyzed by FACSCalibur flow cytometer (University of Oklahoma Health Sciences Center) and FLOWJO software (Treestar). Changes in the donor (FITC-labeled hCD59) fluorescence because of FRET with the acceptor (TRITC-labeled ILYpp) were determined by comparing donor emission intensity in the presence of unlabeled ILY to the donor emission intensity in the presence of acceptor-labeled ILY. Acceptor-labeled PFO was used as a negative control.
Complement-mediated CHO Cell Lysis Assay—Assays were performed as described previously (19). Briefly, subconfluent CHO cells expressing human CD59 (CHOhCD59) were detached with 5 mm EDTA in PBS, washed once with PBS, and resuspended in F-12 Kaighn's media supplemented with 10% fetal calf serum (Invitrogen). 200 μl of cells (1 × 105 cells) were incubated in the presence or absence of the indicated amounts of monomer locked ILY (ILYml) or prepore locked ILY (ILYpp) on ice for 15 min. Cells were then sensitized with rabbit anti-CHO membrane serum (5% final concentration) for 15 min on ice. Normal human serum diluted in F-12 Kaighn's medium was then added to a final concentration of 10% (final volume of 400 μl). After 60 min at 37 °C, cell viability was determined by adding propidium iodide (5 μg/ml) and measuring the proportion of propidium iodide-stained (dead) cells to total cells by FACSCalibur flow cytometer (University of Oklahoma Health Sciences Center) and FLOWJO software (Treestar). These experiments were also performed with the monomer and prepore locked variants of PFO. Cells treated with 5 μg of PFO were used as 100% lysis controls. Cells treated without normal human serum were used as background controls.
RESULTS
Characterization of Disulfide ILY Mutants Trapped in Monomeric or Prepore Stages—As described above, receptor binding by ILY initiates a series of ordered conformational changes that lead to oligomerization of membrane-bound monomers and the formation of the pore. Mutants of ILY trapped at distinct stages of the cytolytic mechanism were generated to determine whether it remained bound to the receptor during assembly of the pore. We previously reported two nonlytic mutants in the related CDC PFO that lock the toxin in the membrane-bound monomeric and prepore oligomeric stages of the pore-forming mechanism (23, 24). Disulfide bridges were engineered into PFO to restrict critical structural transitions necessary for completion of the pore-forming mechanism. Inhibiting these structural changes trapped the toxin in either the membrane-bound monomer or prepore stage. The crystal structures of ILY and PFO are highly homologous so it was likely analogous disulfide bridges could be generated in ILY with similar results (25, 26). The appropriate cysteine substitutions were generated in ILY to form the membrane-bound monomer locked mutants (Thr-346 to cysteine and Ile-361 to cysteine) and prepore locked mutants (Gly-83 to cysteine and Ser-217 to cysteine).
ILYml and ILYpp were purified and assayed for cytolytic activity on both human erythrocytes and on Chinese hamster ovary cells expressing hCD59 (CHOhCD59). The oxidized forms of ILYml and ILYpp retained less than 0.1% of the cytolytic activity of native ILY on both human erythrocytes and the CHOhCD59 cells (Table 1). The addition of the reducing agent dithiothreitol prior to the assay reduced the disulfide bridge and restored activity to approximately wild type levels. These data show the cytolytic mechanism is reversibly inhibited by the introduction of the disulfide bridges.
TABLE 1.
Hemolytic and cytolytic activity of disulfide-locked mutants
The hemolytic dose for 50% lysis (HD50) was determined for the disulfide locked mutants in the presence and absence of the reducing agent dithiothreitol (DTT). Toxin was incubated with human erythrocytes for 30 min at 37 °C. Shown is the relative hemolytic activity compared with wild type ILY. Likewise, toxin was added to CHO cells expressing hCD59 (CHOhCD59) to assay cytolytic activity. Cell viability was determined by adding propidium iodide (PI) (5 μg/ml) and measuring the proportion of propidium iodide-stained (dead) cells to total cells by FACSCalibur flow cytometer (University of Oklahoma Health Sciences Center) and FLOWJO software (Treestar). NA means not assayed. Data are representative of triplicate experiments.
| CDC | DTT | Hemolytic activity | Cytolytic activity |
|---|---|---|---|
| % | % | ||
| ILYwt | - | 100 | 100 |
| ILYml | - | <0.1 | <0.1 |
| ILYml | + | 80.4 | NA |
| ILYpp | - | <0.1 | <0.1 |
| ILYpp | + | 100 | NA |
SDS-AGE was used to verify the mutants were trapped at the specified stages of the pore-forming mechanism. As expected, in the presence of human erythrocytes the reduced forms of both ILYml (Fig. 1, lane 6) and ILYpp (lane 3) formed SDS-resistant oligomers similar to wild type ILY (lane 1). When the cysteines remained oxidized in a disulfide bridge ILYpp formed SDS-resistant oligomers (Fig. 1, lane 4). These oligomers are slightly less stable than those formed by the prereduced toxin, an observation consistent with what had been reported previously for PFOpp (24). As expected, the oxidized form of ILYml did not form SDS-resistant oligomers in the presence of erythrocytes, but it did form oligomers upon reduction of the disulfide bridge (Fig. 1, compare lanes 7 and 6). The results presented here are similar to those previously reported for the monomer locked PFO mutant (23).
FIGURE 1.
SDS-resistant oligomers are formed on erythrocytes by ILYpp but not ILYml toxin. SDS-AGE was used to analyze the oligomeric complexes formed by the disulfide locked mutants. ILYml (lanes 6–8) and ILYpp (lanes 3–5) were incubated in the presence or absence of human erythrocytes for 30 min at 37 °C. The ability of these mutants to form oligomers in a reduced state was also determined by the addition of 1 mm dithiothreitol to both ILYml (lane 6) and ILYpp (lane 3) samples. Wild type ILY was used as a control (lanes 1 and 2). RBC, red blood cells; DTT, dithiothreitol.
Monomer and Prepore Locked Mutants Block Antibody Binding to hCD59—We have previously shown that two anti-hCD59 monoclonal antibodies (10G10 and H19) could block binding of ILY to hCD59 (11) whereas one did not (MEM43).3 ILY binding to hCD59 should inhibit the binding of mAbs 10G10 and H19 whether by occupying their binding sites or distorting it such that the mAb could not bind. Therefore, we can determine whether ILY disengages from hCD59 during pore formation by measuring the ability of these three different anti-hCD59 monoclonal antibodies to bind to hCD59 on the surface of erythrocytes preincubated with ILY or its monomer and prepore locked mutants. The anti-hCD59 antibodies were either directly conjugated with a fluorescent dye or were detected with a fluorescently labeled secondary antibody allowing receptor bound by antibody to be detected by flow cytometry. Therefore, when ILY is bound to hCD59 we should observe a significant decrease in the ability of these mAbs to bind to CD59, because their binding site will be occupied by ILY.
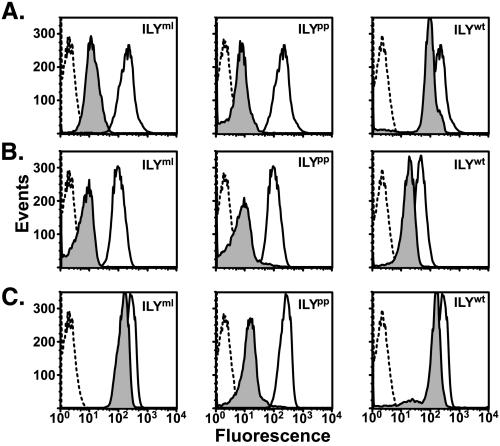
All three monoclonal antibodies were used to probe the availability of hCD59 on erythrocytes preincubated with ILYml, ILYpp, or native ILY (Fig. 2, shaded peaks). As shown in Fig. 2, the 10G10 (Fig. 2A) and H19 (Fig. 2B) binding is significantly decreased when cells are prebound with saturating levels of either ILYml or ILYpp. Conversely, when cells are incubated with saturating amounts of ILYwt, both 10G10 and H19 bound hCD59 at levels similar to that observed in the absence of ILY (Fig. 2B, solid line) suggesting that hCD59 is no longer significantly engaged by ILY when it is converted to the pore complex. A low level of receptor occupancy by ILYwt was apparent, which suggested that some of the ILY does not disengage from receptor. This can be explained by the fact that the assembly of insertion-competent oligomers is a stochastic process, and so a fraction of ILYwt monomers will be incorporated into complexes that do not achieve an insertion-competent state and therefore will not disengage the receptor.
FIGURE 2.
ILY disengagement from hCD59 occurs after establishment of the prepore complex and coincides with pore formation. Antibody accessibility was used to determine whether ILY disengaged from hCD59 during the cytolytic mechanism. Three monoclonal anti-hCD59 antibodies, 10G10 (A), H19 (B), and MEM43 (C), were tested for the ability to bind hCD59 on erythrocytes preincubated with saturating levels of ILYml, ILYpp, or ILYwt (shaded peaks). As a positive control, each antibody was tested for the ability to bind hCD59 in the absence of toxin (bold line). Background fluorescence of erythrocytes is shown (dashed line). This figure is representative of triplicate experiments.
In contrast to 10G10 and H19 mAbs, MEM43 is able to bind to hCD59 in the presence of ILYml (Fig. 2C). This result is in agreement with our previous findings that showed the application of MEM43 prior to ILY does not inhibit cytolytic activity.4 Interestingly, even though MEM43 does not interfere with pore formation, it appears the MEM43 epitope becomes occluded upon formation of the prepore complex (Fig. 2C). Once the prepore is converted to the pore, the site again becomes available, presumably because ILY dissociates from hCD59 upon the formation of the pore complex (Fig. 2C). Hence, these data suggest hCD59 is clustered around the ILY oligomer prior to formation of the pore complex. The decreased binding of 10G10 and H19 to the monomer- and prepore-engaged hCD59 could also result from conformational changes induced in the mAb epitopes by ILY binding. If true, it does not substantially change our interpretation because binding of ILY to CD59 would be responsible for deformation of the epitopes, and their structure is restored upon conversion of the prepore to the pore complex, suggesting that ILY disengages from the CD59.
We replicated these studies with PFO, a CDC that does not bind to hCD59. As expected the prepore locked variant of PFO did not inhibit binding of the monoclonal antibodies to hCD59 (Fig. 3).
FIGURE 3.
PFOpp does not interfere with antibody binding to hCD59. The three monoclonal antibodies were also tested for the ability to bind to hCD59 on erythrocytes preincubated with PFOpp (shaded peaks). Antibody binding in the absence of PFOpp is shown (solid line).
FRET between Donor-labeled hCD59 and Acceptor-labeled ILY—We also examined the interaction of ILY with hCD59 by measuring donor quenching of fluorescein-labeled hCD59 (donor) with acceptor-labeled ILY resulting from FRET. FRET is used to determine the close proximity of two molecules and is highly sensitive to the distance separating the donor and acceptor fluorophores (efficiency of FRET (E)∝1/r6, where r is the distance separating the donor-acceptor pair). The Förster distance for the fluorescein (donor dye)-tetramethylrhodamine (acceptor dye) pair is ≈5 nm. If donor-labeled hCD59 is bound by acceptor-labeled ILY, we should observe a decrease in donor fluorescence because of FRET. If ILY is allowed to convert to the pore complex, the quenching of the donor fluorescence should be relieved to some extent if ILY disengages from receptor during this transition.
We initially used endogenously labeled hCD59 that contained either an mCherry tag5 or the GFP fusion of CD59 (27). Although both fusions expressed well on the plasma membrane of CHO cells and could be detected with anti-HA tag or fluorescence, neither construct bound the CD59 monoclonal antibodies used herein suggesting that the CD59 itself did not fold properly. We therefore changed our system to one where hCD59 was indirectly labeled on human erythrocytes with a mAb conjugated to FITC, the donor (D) fluorescent dye. We took advantage of the fact that mAb MEM43 could bind to hCD59 without interfering with the binding and assembly of the prepore and pore complexes of ILY. In these experiments we first bound donor (D)-labeled MEM43 antibody to hCD59 followed by the addition of the various ILY constructs that were directly conjugated with the acceptor (A) dye tetramethylrhodamine. If ILY binds to hCD59, it will result in a decrease in donor emission intensity over that when unlabeled ILY is substituted for acceptor-labeled ILY. If ILY disengages from hCD59, FRET should no longer occur resulting in an increase in donor emission intensity similar to that when unlabeled ILY is present.
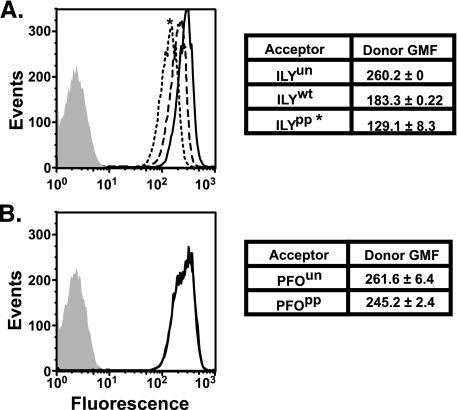
As shown in Fig. 4A, a decrease of the donor fluorescence of donor-labeled hCD59 occurs when cells are incubated with the acceptor-labeled ILYpp (dotted line) compared with the donor fluorescence in the presence of unlabeled ILYpp (solid line). By comparison, cells treated with acceptor-labeled wild type ILY (dashed line), which can convert to the pore, results in less donor quenching compared with that observed when hCD59 is engaged with the acceptor-labeled ILYpp. This is demonstrated by a shift of the fluorescent peak closer to that observed when unlabeled ILYpp is substituted for acceptor-labeled ILYpp. FRET is not completely abolished in the presence of acceptor-labeled ILYwt. As indicated above, a percentage of membrane-bound ILY will likely remain bound to the receptor because it cannot convert to a pore complex. These data show that hCD59 is associated with the ILY prepore complex, but this interaction is significantly decreased, as shown by the decreased quenching of the donor fluorescence when ILY is converted to the pore complex. It is important to note that any donor quenching observed in these experiments is solely because of FRET and not another physical process because the only difference between the negative control and the experiments with PFOpp and PFOwt is the presence of the acceptor probe on the ILY protein. When the same experiments were carried out with acceptor-labeled or unlabeled PFO, a CDC that does not bind to hCD59, there was no change in the intensity of the donor fluorescence of the hCD59 when cells were incubated with the prepore locked acceptor-labeled PFO or with unlabeled PFO (Fig. 4B).
FIGURE 4.
FRET detected association between ILY and hCD59 in prepore complexes. A cysteine was introduced at amino acid residue Asp-280 in both ILYwt and ILYpp and was labeled with tetramethylrhodamine (A, acceptor fluorophore). A, erythrocytes were preincubated with a FITC-conjugated (D, donor fluorophore) anti-hCD59 mAb (MEM43) and then incubated with either unlabeled ILYun (D + U, solid line) or acceptor-labeled ILYwt (D + A, dashed line) or ILYpp (D + A, dotted line). FRET between donor and acceptor is observed as a decrease in the fluorescence per cell (i.e. the peak will shift to the left) of the donor when the unlabeled toxin is replaced with acceptor-labeled toxin (compare D + U and D + A). No significant change in donor fluorescence was observed when acceptor-labeled PFOpp (D + A, dotted) or unlabeled PFOun (D + A, solid line) was used (B). GMF, geometric mean of fluorescence. *, p < 0.005 for the geometric mean of fluorescence of cells treated with ILYpp and ILYwt. No significant difference in the geometric mean of fluorescence of cells treated with either PFOpp or PFOwt was observed.
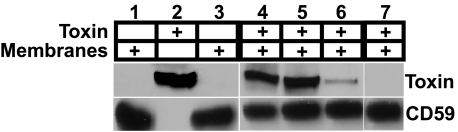
Co-immunoprecipitation Analysis of the hCD59-ILY Interaction—To directly measure the physical interaction of ILY with hCD59 during pore formation, we performed pulldown assays by immunoprecipitation of hemagglutinin-tagged CD59 from the solubilized membranes of CHO cells expressing hCD59. We determined the extent ILY co-immunoprecipitates with hCD59 in its monomer, prepore, and pore states. CHOhCD59 cell membranes preincubated with either ILYml, ILYpp, or ILYwt were solubilized and immunoprecipitated with anti-HA-coupled agarose beads (Sigma, anti-HA immunoprecipitation kit). ILYml and ILYpp efficiently co-immunoprecipitated (co-IP) with hCD59 (Fig. 5), whereas ILYwt did not significantly co-IP with hCD59. The small amount of ILYwt that co-IP with hCD59 (Fig. 5, lane 6) also indicates that not all membrane-bound ILY is incorporated into a pore complex and therefore remains bound to the receptor. When PFOpp was substituted for ILYpp, it did not co-IP with CD59. These results confirm the above studies and show by direct means ILY remains engaged with the receptor until it makes the transition to the pore complex.
FIGURE 5.
ILYml and ILYpp co-immunoprecipitate with HA-tagged hCD59. Co-immunoprecipitation experiments were performed on hCD59-transfected CHO cells (CHOhCD59) that had been preincubated with the various ILY locked mutants. Both ILYml (lane 4) and ILYpp (lane 5) co-immunoprecipitated with hCD59 more efficiently than ILYwt (lane 6). PFOpp did not co-immunoprecipitate with CD59 (lane 7). The same experiment was performed in the absence of ILY as a negative control (lane 3). CD59 (lane 1) and ILYwt (lane 2) standards were included.
ILY Binding to hCD59 Increases Host Cell Susceptibility to MAC-mediated Cell Lysis—The above studies demonstrate ILY remains engaged with hCD59 prior to its conversion to the pore complex. CD59 plays a key role in protecting host cells from complement by inhibiting MAC formation on their membrane. The engagement of hCD59 by ILY during the assembly of its prepore complex may increase the susceptibility of the host cell to lysis by MAC. We have previously shown the binding sites for ILY, C8α, and C9 are located in the same region on hCD59 (11). CHO cells that express hCD59 exhibit an increased level of protection against MAC-mediated cell lysis with human serum (19, 28). Based on our above analyses, nonlytic monomers and prepore complexes of ILY remain engaged with hCD59 and therefore may increase host cell susceptibility to MAC-mediated lysis by blocking the C8α- and C9-binding site on CD59.
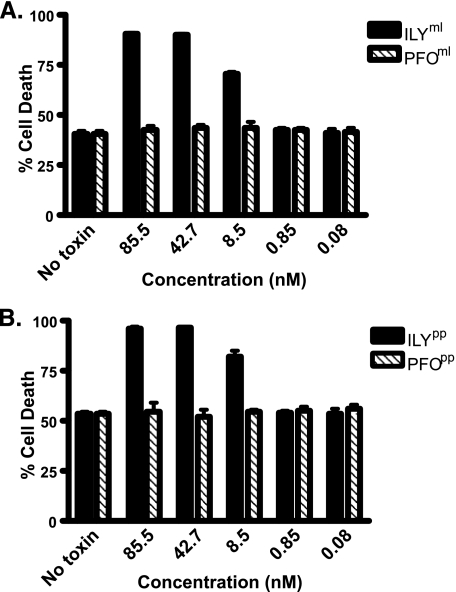
We examined the susceptibility of hCD59-expressing CHO cells to MAC-mediated lysis when cells were preincubated with ILYml or ILYpp. The cells were sensitized with anti-CHO cell antibody and then incubated with human serum. Cell death was determined by propidium iodine staining and quantified by flow cytometry. Not surprisingly, CHO cells not expressing hCD59 were completely killed in the presence of human serum (data not shown), whereas partial protection of CHO cells was observed when they were stably transfected with hCD59 (Fig. 6, A and B, no toxin control). Preincubating the CHOhCD59 cells with either ILYml (Fig. 6A) or ILYpp (Fig. 6B) increased cell death by MAC lysis in a dose-dependent manner. Cytolysis was exclusively because of MAC formation, as the locked toxins alone do not lyse the cells (Table 1). In addition, increased cell death was specific to the occupation of hCD59 by ILY, and no increase in cell death occurred when the same experiment was performed with either PFOml or PFOpp mutants (Fig. 6, A and B). These results show that nonlytic complexes of ILY specifically block the ability of hCD59 to prevent MAC-mediated cell lysis and are consistent with the above studies that show ILY remains bound to the receptor through the prepore stage of the cytolytic mechanism.
FIGURE 6.
Nonlytic ILY mutants increase the susceptibility of hCD59-expressing CHO cells to MAC-dependent lysis. hCD59-transfected CHO cells (CHOhCD59) were assayed for susceptibility to MAC-mediated lysis in the presence of both ILYml (A) and ILYpp (B) mutants. Cells were treated for 15 min on ice with various amounts of ILY and then incubated with rabbit anti-CHO membrane IgG. Human serum was added as the source of C8 and C9 to a final concentration of 10%. Cells death was determined by propidium iodide and FLOW cytometry. Assays were performed in triplicate. Background levels of cell death are shown as no toxin control.
DISCUSSION
The studies herein show that ILY remains engaged with its receptor, hCD59, during assembly of the oligomeric prepore complex and disengages from the receptor upon prepore to pore conversion. Furthermore, we showed that engagement of hCD59 by ILY limits the ability of hCD59 to protect host cells from complement-mediated lysis. Therefore, ILY not only uses hCD59 to initiate the assembly of its pore complex on the membrane of human cells, but during this process bound ILY monomers and incomplete oligomeric complexes may increase the susceptibility of the host cell to lysis by the MAC.
The fact ILY disengages from hCD59 upon formation of the pore complex suggests there are prevailing structural requirements for receptor disengagement. We have shown previously receptor binding by domain 4 of ILY is sufficient to trigger structural changes in domain 3 that lead to oligomerization of ILY into the prepore complex (6). Domains 3 and 4 have also been shown to be conformationally coupled in PFO (6, 29). During prepore to pore conversion, a major disruption of the structure of domain 2, which is in contact with domain 4, occurs as the β-barrel inserts into the membrane (30, 31). Because domains 3 and 4 are conformationally coupled, it is possible the membrane insertion of the domain 3 transmembrane hairpins requires and/or causes conformational changes in the structure of domain 4 that break essential contacts with hCD59 and lead to its dissociation from the pore complex. These results are reminiscent of anthrax protective antigen where receptor binding is important to the formation of the pore complex, but similar to ILY, it disengages from its receptor upon prepore to pore conversion (32). Diphtheria toxin also apparently dissociates from its receptor, heparin-binding epidermal growth factor-like growth factor, at low pH prior to translocation (33). Hence, in all cases receptor disengagement occurs immediately prior to or during membrane penetration, suggesting this may be a common prerequisite for several unrelated membrane-penetrating toxins.
These data also demonstrate the necessity for the membrane insertion of the domain 4 loops of ILY. We had previously shown the direct binding to cholesterol-rich membranes for the PFO-like CDCs is mediated by three short loops at the base of domain 4 (6, 7, 11, 12, 34). Although ILY binds to hCD59 and not directly to cholesterol-rich membranes, the insertion of these loops is required for the final step where the ILY prepore is converted to the pore (6, 7, 12). The reason the membrane insertion of these loops remained critical to this stage of the ILY pore-forming mechanism was not clear from these previous studies. It was apparent that receptor binding triggered all of the necessary structural transitions for pore formation, yet the pore did not form if loop insertion was disrupted. The current studies now show that ILY disengages from the receptor upon the critical transition from prepore to pore, and all contact with the cell surface would be lost if these loops did not insert into the membrane. Hence, the domain 4 loops firmly anchor ILY to the surface as it disengages from receptor and inserts the domain 3 transmembrane β-hairpins and forms the β-barrel pore.
Monomer and prepore locked ILY mutants, which mimic assembly intermediates of ILY, and increased the susceptibility of eukaryotic cells to complement-mediated lysis. This is consistent with the fact that ILY and complement proteins C8α and C9 appear to bind similar or overlapping sites on hCD59 (11). Because ILY will be in various states of assembly on the cell surface, a fraction of the hCD59 will be engaged with ILY. Consequently, in addition to its own pore forming activity, during the assembly of its pore it has the potential to increase the probability of MAC pore formation in the host cell membrane. This may be especially critical during infection where elevated levels of MAC can overwhelm the protective effects of CD59 (19). Could this happen in vivo during abscess formation? Although no data currently exist that provide the levels of ILY in abscesses, there are data for the in vitro expression from abscess isolates of S. intermedius. S. intermedius isolates from abscesses expressed, in culture, ∼103–104 hemolytic units/mg dry weight cells (≈109–1010 bacterial cells) (9). Based on the specific activity of ILY (40 ng/5 hemolytic units (35)), the concentration of ILY at a conservative cell density of 109–1010 cells/ml is ∼140–1400 nm. This level is 16–160 times the level of monomer and prepore locked ILY (8.5 nm) that significantly inhibited the protective function of hCD59 to complement-mediated lysis. The levels of ILY in an abscess may be much higher than in vitro culture because ILY will be concentrated within the walled off abscess, and recently it has been shown that ILY is under control of the quorum-sensing LuxS system (36).
In conclusion, monomeric and prepore ILY remain bound to the hCD59 receptor, and dissociation of the receptor occurs upon prepore to pore conversion. The interaction of ILY with hCD59 prior to pore formation can interfere with the protective function of hCD59, thus increasing the probability of host cell lysis by the MAC.
Acknowledgments
The excellent technical assistance of P. Parrish is acknowledged.
This work was supported, in whole or in part, by National Institutes of Health Grants P20 RR015564-08 (to E. M. H.) and AI063444 (to R. K. T.) from NIAID.
Footnotes
The abbreviations used are: CDC, cholesterol-dependent cytolysin; ILY, intermedilysin; MAC, membrane attack complex; hCD59, human CD59; CHO, Chinese hamster ovary; PBS, phosphate-buffered saline; FITC, fluorescein isothiocyanate; HA, hemagglutinin; FRET, Förster resonance energy transfer; PFO, perfringolysin O; TRITC, tetramethylrhodamine isothiocyanate; mAb, monoclonal antibody; co-IP, co-immunoprecipitated; AGE, agarose gel electrophoresis.
R. K. Tweten unpublished data.
R. K. Tweten and K. S. Giddings, unpublished data.
E. M. Hotze and S. LaChapelle, unpublished data.
References
- 1.Heuck, A. P., Tweten, R. K., and Johnson, A. E. (2001) Biochemistry 40 9065–9073 [DOI] [PubMed] [Google Scholar]
- 2.Portnoy, D., Jacks, P. S., and Hinrichs, D. (1988) J. Exp. Med. 167 1459–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bricker, A. L., Cywes, C., Ashbaugh, C. D., and Wessels, M. R. (2002) Mol. Microbiol. 44 257–269 [DOI] [PubMed] [Google Scholar]
- 4.Boulnois, G. J. (1992) J. Gen. Microbiol. 138 249–259 [DOI] [PubMed] [Google Scholar]
- 5.Waheed, A. A., Shimada, Y., Heijnen, H. F., Nakamura, M., Inomata, M., Hayashi, M., Iwashita, S., Slot, J. W., and Ohno-Iwashita, Y. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 4926–4931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soltani, C. E., Hotze, E. M., Johnson, A. E., and Tweten, R. K. (2007) J. Biol. Chem. 282 15709–15716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soltani, C. E., Hotze, E. M., Johnson, A. E., and Tweten, R. K. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 20226–20231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gelber, S. E., Aguilar, J. L., Lewis, K. L., and Ratner, A. J. (2008) J. Bacteriol. 190 3896–3903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagamune, H., Whiley, R. A., Goto, T., Inai, Y., Maeda, T., Hardie, J. M., and Kourai, H. (2000) J. Clin. Microbiol. 38 220–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagamune, H., Ohnishi, C., Katsuura, A., Fushitani, K., Whiley, R. A., Tsuji, A., and Matsuda, Y. (1996) Infect. Immun. 64 3093–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giddings, K. S., Zhao, J., Sims, P. J., and Tweten, R. K. (2004) Nat. Struct. Mol. Biol. 12 1173–1178 [DOI] [PubMed] [Google Scholar]
- 12.Giddings, K. S., Johnson, A. E., and Tweten, R. K. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 11315–11320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otabor, I., Tyagi, S., Beurskens, F. J., Ghiran, I., Schwab, P., Nicholson-Weller, A., and Klickstein, L. B. (2004) Mol. Immunol. 41 185–190 [DOI] [PubMed] [Google Scholar]
- 14.Ghiran, I., Klickstein, L. B., and Nicholson-Weller, A. (2003) J. Biol. Chem. 278 21024–21031 [DOI] [PubMed] [Google Scholar]
- 15.Kimberley, F. C., Sivasankar, B., and Morgan, B. P. (2007) Mol. Immunol. 44 73–81 [DOI] [PubMed] [Google Scholar]
- 16.Rollins, S. A., and Sims, P. J. (1990) J. Immunol. 144 3478–3483 [PubMed] [Google Scholar]
- 17.Hamilton, K. K., Ji, Z., Rollins, S., Stewart, B. H., and Sims, P. J. (1990) Blood 76 2572–2577 [PubMed] [Google Scholar]
- 18.Farkas, I., Baranyi, L., Ishikawa, Y., Okada, N., Bohata, C., Budai, D., Fukuda, A., Imai, M., and Okada, H. (2002) J. Physiol. (Lond.) 539 537–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang, Y., Smith, C. A., Song, H., Morgan, B. P., Abagyan, R., and Tomlinson, S. (2005) J. Biol. Chem. 280 34073–34079 [DOI] [PubMed] [Google Scholar]
- 20.Rollins, S. A., Zhao, J., Ninomiya, H., and Sims, P. J. (1991) J. Immunol. 146 2345–2351 [PubMed] [Google Scholar]
- 21.Shepard, L. A., Heuck, A. P., Hamman, B. D., Rossjohn, J., Parker, M. W., Ryan, K. R., Johnson, A. E., and Tweten, R. K. (1998) Biochemistry 37 14563–14574 [DOI] [PubMed] [Google Scholar]
- 22.Hotze, E., and Tweten, R. K. (2002) in Perspectives in Molecular Toxicology (Ménez, A., ed) pp. 23–37, John Wiley & Sons Ltd., Chichester, UK
- 23.Ramachandran, R., Tweten, R. K., and Johnson, A. E. (2004) Nat. Struct. Mol. Biol. 11 697–705 [DOI] [PubMed] [Google Scholar]
- 24.Hotze, E. M., Wilson-Kubalek, E. M., Rossjohn, J., Parker, M. W., Johnson, A. E., and Tweten, R. K. (2001) J. Biol. Chem. 276 8261–8268 [DOI] [PubMed] [Google Scholar]
- 25.Rossjohn, J., Feil, S. C., McKinstry, W. J., Tweten, R. K., and Parker, M. W. (1997) Cell 89 685–692 [DOI] [PubMed] [Google Scholar]
- 26.Polekhina, G., Giddings, K. S., Tweten, R. K., and Parker, M. W. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 600–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nichols, B. J., Kenworthy, A. K., Polishchuk, R. S., Lodge, R., Roberts, T. H., Hirschberg, K., Phair, R. D., and Lippincott-Schwartz, J. (2001) J. Cell Biol. 153 529–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao, J., Rollins, S. A., Maher, S. E., Bothwell, A. L., and Sims, P. J. (1991) J. Biol. Chem. 266 13418–13422 [PubMed] [Google Scholar]
- 29.Heuck, A. P., Hotze, E., Tweten, R. K., and Johnson, A. E. (2000) Mol. Cell 6 1233–1242 [DOI] [PubMed] [Google Scholar]
- 30.Czajkowsky, D. M., Hotze, E. M., Shao, Z., and Tweten, R. K. (2004) EMBO J. 23 3206–3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tilley, S. J., Orlova, E. V., Gilbert, R. J., Andrew, P. W., and Saibil, H. R. (2005) Cell 121 247–256 [DOI] [PubMed] [Google Scholar]
- 32.Rainey, G. J., Wigelsworth, D. J., Ryan, P. L., Scobie, H. M., Collier, R. J., and Young, J. A. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 13278–13283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brooke, J. S., Cha, J. H., and Eidels, L. (1998) Biochem. Biophys. Res. Commun. 248 297–302 [DOI] [PubMed] [Google Scholar]
- 34.Ramachandran, R., Heuck, A. P., Tweten, R. K., and Johnson, A. E. (2002) Nat. Struct. Biol. 9 823–827 [DOI] [PubMed] [Google Scholar]
- 35.Macey, M. G., Whiley, R. A., Miller, L., and Nagamune, H. (2001) Infect. Immun. 69 6102–6109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pecharki, D., Petersen, F. C., and Scheie, A. A. (2008) Oral Microbiol. Immunol. 23 79–83 [DOI] [PubMed] [Google Scholar]