Abstract
The carboxyl-terminal cholinesterase-like (ChEL) domain of thyroglobulin (Tg) has been identified as critically important in Tg export from the endoplasmic reticulum. In a number of human kindreds suffering from congenital hypothyroidism, and in the cog congenital goiter mouse and rdw rat dwarf models, thyroid hormone synthesis is inhibited because of mutations in the ChEL domain that block protein export from the endoplasmic reticulum. We hypothesize that Tg forms homodimers through noncovalent interactions involving two predicted α-helices in each ChEL domain that are homologous to the dimerization helices of acetylcholinesterase. This has been explored through selective epitope tagging of dimerization partners and by inserting an extra, unpaired Cys residue to create an opportunity for intermolecular disulfide pairing. We show that the ChEL domain is necessary and sufficient for Tg dimerization; specifically, the isolated ChEL domain can dimerize with full-length Tg or with itself. Insertion of an N-linked glycan into the putative upstream dimerization helix inhibits homodimerization of the isolated ChEL domain. However, interestingly, co-expression of upstream Tg domains, either in cis or in trans, overrides the dimerization defect of such a mutant. Thus, although the ChEL domain provides a nidus for Tg dimerization, interactions of upstream Tg regions with the ChEL domain actively stabilizes the Tg dimer complex for intracellular transport.
The synthesis of thyroid hormone in the thyroid gland requires secretion of thyroglobulin (Tg)2 to the apical luminal cavity of thyroid follicles (1). Once secreted, Tg is iodinated via the activity of thyroid peroxidase (2). A coupling reaction involving a quinol-ether linkage especially engages di-iodinated tyrosyl residues 5 and 130 to form thyroxine within the amino-terminal portion of the Tg polypeptide (3, 4). Preferential iodination of Tg hormonogenic sites is dependent not on the specificity of the peroxidase (5) but upon the native structure of Tg (6, 7). To date, no other thyroidal proteins have been shown to effectively substitute in this role for Tg.
The first 80% of the primary structure of Tg (full-length murine Tg: 2,746 amino acids) involves three regions called I-II-III comprised of disulfide-rich repeat domains held together by intradomain disulfide bonds (8, 9). The final 581 amino acids of Tg are strongly homologous to acetylcholinesterase (10–12). Rate-limiting steps in the overall process of Tg secretion involve its structural maturation within the endoplasmic reticulum (ER) (13). Interactions between regions I-II-III and the cholinesterase-like (ChEL) domain have recently been suggested to be important in this process, with ChEL functioning as an intramolecular chaperone and escort for I-II-III (14). In addition, Tg conformational maturation culminates in Tg homodimerization (15, 16) with progression to a cylindrical, and ultimately, a compact ovoid structure (17–19).
In human congenital hypothyroidism with deficient Tg, the ChEL domain is a commonly affected site of mutation, including the recently described A2215D (20, 21), R2223H (22), G2300D, R2317Q (23), G2355V, G2356R, and the skipping of exon 45 (which normally encodes 36 amino acids), as well as the Q2638stop mutant (24) (in addition to polymorphisms including P2213L, W2482R, and R2511Q that may be associated with thyroid overgrowth (25)). As best as is currently known, all of the congenital hypothyroidism-inducing Tg mutants are defective for intracellular transport (26). A homozygous G2300R mutation (equivalent to residue 2,298 of mouse Tg) in the ChEL domain is responsible for congenital hypothyroidism in rdw rats (27, 28), whereas we identified the Tg-L2263P point mutation as the cause of hypothyroidism in the cog mouse (29). Such mutations perturb intradomain structure (30), and interestingly, block homodimerization (31). Acquisition of quaternary structure has long been thought to be required for efficient export from the ER (32) as exemplified by authentic acetylcholinesterase (33, 34) in which dimerization enhances protein stability and export (35).
Tg comprised only of regions I-II-III (truncated to lack the ChEL domain) is blocked within the ER (30), whereas a secretory version of the isolated ChEL domain of Tg devoid of I-II-III undergoes rapid and efficient intracellular transport and secretion (14). A striking homology positions two predicted α-helices of the ChEL domain to the identical relative positions of the dimerization helices in acetylcholinesterase. This raises the possibility that ChEL may serve as a homodimerization domain for Tg, providing a critical function in maturation for Tg transport to the site of thyroid hormone synthesis (1).
In this study, we provide unequivocal evidence for homodimerization of the ChEL domain and “hetero”-dimerization of that domain with full-length Tg, and we provide significant evidence that the predicted ChEL dimerization helices provide a nidus for Tg assembly. On the other hand, our data also suggest that upstream Tg regions known to interact with ChEL (14) actively stabilize the Tg dimer complex. Together, I-II-III and ChEL provide unique contributions to the process of intracellular transport of Tg through the secretory pathway.
EXPERIMENTAL PROCEDURES
Materials—Lipofectamine 2000, Dulbecco's modified Eagle's medium (DMEM), Zysorbin, fetal bovine serum, penicillin, and streptomycin were from Invitrogen; Complete protease inhibitor mixture was from Roche Applied Science; brefeldin A, protein G-agarose, and protein A-agarose were from Sigma; endoglycosidase H was from New England Biolabs (Beverly, MA); Trans35S-Label was from MP Biomedicals (Irvine, CA); TransIT-LT1 transfection reagent was from Mirus (Madison, WI). Rabbit polyclonal anti-Myc and anti-GFP were from Immunology Consultants, Inc. (Newberg, OR); monoclonal anti-acetylcholinesterase (mAb303) was from Millipore (San Francisco, CA), and monoclonal anti-HA (MMS-101P) was from Covance (Princeton, NJ). Rabbit polyclonal anti-Tg has been previously described (31).
Site-directed Mutagenesis of Mouse Tg cDNA—ChEL domain mutations were introduced with the QuikChange site-directed mutagenesis kit (Stratagene) using the following mutagenic primers (paired with their complements): D2708C,G2709stop in Tg (“Tg-CD,” 5′-CCAGACTTTGAAGGATGCATGTTGAGCCAAGGATGCACAGTTAACC-3′); D2708C in ChEL (ChEL-CD, 5′-CCAGACTTTGAAGGATGCATGTGGAGCCAAGGATGCACAGTTAACC-3′); ChEL-Myc (5′-CAAGAGCTACAGCAAAGAACAGAAACTGATCTCTGAGGAGGACTTATGATTAATGCTTCG-3′); ChEL-HA (5′-CCAAGAGCTACAGCAAATACCCTTACGACGTCCCCGATTACGCGTAGGTTAATGCTTCGC-3′). Truncated Tg regions I-II-III and secretory ChEL were made as described previously (14). I-II-III-Myc and -HA were made using the following primers and their complements (5′-CCGGAAGTCTGAACAGAAGTTGATCTCAGAGGAGGACCTATAGACACCTTCTGTACGC-3′ for I-II-III-Myc; 5′-CCGGAAGTCTTACCCCTATGACGTCCCAGATTATGCATGATCCACACCTTCTGTACGC-3′ for I-II-III-HA). An N-glycosylation site (A2538N, V2540T), which is homologous to that produced in acetylcholinesterase (35), was added to the Tg-CD and ChEL constructs using the following primer and its complement oligonucleotide (5′-GGACTCAGATGCCCGCATCCTTGCTAATGCTACATGGTATTACTCCTTGGAGCACTCC-3′). Each construct was confirmed by direct DNA sequencing before expression in 293 cells.
Cell Culture and Transfection—293 cells were cultured in DMEM with 10% fetal bovine serum in 6-well plates at 37 °C in a humidified 5% CO2 incubator. Plasmids were transiently transfected using TransIT-LT1 or Lipofectamine 2000 transfection reagent according to the manufacturer's instructions.
Metabolic Labeling and Immunoprecipitation—Transfected 293 cells were starved for 30 min in Met/Cys-free DMEM and then pulse-labeled with 180 μCi/ml 35S-labeled amino acids. The labeled cells were then washed with an excess of cold Met/Cys and chased in complete DMEM. At each time point, cells were lysed in buffer containing 1% Nonidet P-40, 0.1% SDS, 0.1 m NaCl, 2 mm EDTA, 25 mm Tris, pH 7.4, and a protease inhibitor mixture (for cells expressing authentic acetylcholinesterase, the lysis buffer contained 1% Triton X-100, 0.1 m NaCl, 2 mm EDTA, 25 mm Tris, pH 7.4, and protease inhibitors). For immunoprecipitation, anti-Tg or anti-acetylcholinesterase antibodies were incubated with samples overnight at 4 °C, and the immunoprecipitate was recovered with protein A-agarose (for anti-Tg) or protein G-agarose (for anti-acetylcholinesterase). For co-immunoprecipitation studies, samples were incubated overnight at 4 °C with anti-Myc or anti-GFP antibodies and protein A-agarose. Immunoprecipitates (or co-precipitates) were washed three times, boiled in SDS sample buffer, resolved by SDS-PAGE, and analyzed by fluorography or phosphorimaging.
Endoglycosidase H Digestion—Immunoprecipitates were boiled for 10 min in a denaturing buffer containing 0.5% SDS plus 1% 2-mercaptoethanol (or, for Tg-D2708C,G2709stop known as Tg-CD, omitting mercaptoethanol), and digested with 250 units of endoglycosidase H in 50 mm sodium citrate, pH 5.5, for 1 h at 37 °C.
RESULTS
Use of Epitope Tagging to Follow Tg Transport and Dimerization—The use of sucrose-velocity gradient centrifugation has been a “gold standard” for examination of the homodimerization of wild-type endogenous Tg (36, 37). However, for the study of dimerization properties of recombinantly expressed mouse Tg and its domains, we exploited bioengineering methods to tag discrete Tg subunits. Using a (0.5-h) pulse- (5-h) chase protocol, we established that wild-type Tg tagged at the carboxyl terminus with a green fluorescence protein (GFP) moiety was efficiently secreted, being converted from an endoglycosidase H (endo H)-sensitive form (large mobility shift upon endo H digestion) to an endo H-resistant form (small mobility shift upon digestion), precisely as is seen for untagged Tg (Fig. 1A). Adding a triple Myc (3×Myc) tag at the carboxyl terminus of wild-type Tg produced a similarly well secreted protein whose release to the medium could be blocked by inclusion of the rdw (G2298R) Tg mutation (Fig. 1B). Coexpression of wild-type Tg-GFP with Tg-3×Myc offered one new means to examine Tg dimerization. When Tg-GFP was expressed in 293 cells in the presence or absence of co-expressed Tg-3×Myc, secreted Tg-GFP was positively recovered in the medium, as detected by Western blotting with anti-GFP antibody (Fig. 1C, right panel). If the medium was first immunoprecipitated with anti-Myc antibody, then Tg-GFP expressed by itself could no longer be recovered, although it had been secreted (Fig. 1C, left panel). By contrast, when co-expressed, immunoprecipitation of the medium with anti-Myc co-precipitated Tg-GFP (Fig. 1C, left).
FIGURE 1.
Association of epitope-tagged thyroglobulins. A, 293 cells untransfected (also called Control) or transfected with the constructs shown were pulse-labeled for 30 min with 35S-labeled amino acids and chased for 5 h. Both cells (C) and media (M) were immunoprecipitated with anti-Tg, divided in two equal portions, and either mock-digested (–) or digested (+) with endo H. Secreted Tg and Tg-GFP have acquired endo H resistance, although some glycans on each Tg or Tg-GFP molecule remain sensitive to endo H. B, 293 cells untransfected or transfected with the constructs shown were pulse-labeled for 30 min with 35S-labeled amino acids and chased for 4 h. Both cells and media were immunoprecipitated with anti-Tg. C, 293 cells were transfected with the constructs shown, and media were collected for 24 h. Each sample was divided in two portions and either immunoprecipitated with anti-Myc antibody (IP, left half of panel) or analyzed without immunoprecipitation (right half of panel). Success of co-precipitation was established by SDS-PAGE, electrotransfer, and immunoblotting with anti-GFP. D, cells and media, collected as in C, were divided in two portions and either immunoprecipitated with anti-GFP (IP, left half of gel) or analyzed without immunoprecipitation (right half of gel). Success of co-precipitation was established by SDS-PAGE, electrotransfer, and immunoblotting with anti-Myc. E, 293 cells were transfected with I-II-III-Myc, I-II-III-HA, both, or neither, and the cells were lysed at 48 h in a nondenaturing buffer containing 1% Nonidet P-40. Each sample was divided and either immunoprecipitated with anti-Myc (IP, right panels) or analyzed without immunoprecipitation (No IP, left panels). Lack of co-precipitation was established by SDS-PAGE, electrotransfer, and immunoblotting with anti-HA (upper panels) and anti-Myc (lower panels).
The identical media samples were also analyzed in reverse. Once again, when Tg-3×Myc was expressed in the presence or absence of co-expressed Tg-GFP, secreted Tg-3×Myc was positively recovered as detected by Western blotting with anti-Myc (Fig. 1D, right panel). If first immunoprecipitated with anti-GFP, then Tg-3×Myc expressed by itself could not be recovered, indicating that anti-GFP did not cross-react with Tg-3×Myc (Fig. 1D, left panel). The anti-GFP antibody was less efficient for Tg-GFP immunoprecipitation (not shown). Nevertheless, to the extent that immunoprecipitation occurred, anti-GFP specifically co-precipitated wild-type Tg-3×Myc when co-expressed with wild-type Tg-GFP (Fig. 1D, left). Together, the data in Fig. 1, C and D, provide strong evidence of dimerization between Tg subunits.
Putative Dimerization Sequences in the Tg ChEL Domain—A complete list of the constructs used to study Tg domains involved in dimerization is shown in Fig. 2A. Of the various Tg domains, the carboxyl-terminal ChEL domain (581 residues) has distinct homology to acetylcholinesterase (11, 12), which undergoes homodimerization via formation of a four-helix bundle (two helices from each monomer (33–35)). Using the PSIPRED program for prediction of protein secondary structure (38), we examined the sequence of the Tg ChEL domain. We found that sequences in the carboxyl-terminal half of the ChEL domain are predicted with high confidence to form helical segments (Fig. 2B, highlighted). Within the domain, these sequences are positioned identically to the dimerization helices of acetylcholinesterase (33). We thus hypothesized that Tg might use this carboxyl-terminal region to drive homodimerization necessary for intracellular transport.
FIGURE 2.
Relationship of the ChEL domain to the full-length Tg protein. A, a summary of the constructs used in this study, including incorporation at the carboxyl terminus of various epitope tags. SP, signal peptide. B, the 581-residue domain extends from residues 2,166 (including a SalI restriction site creating the I2,167R replacement shown in this figure) to the carboxyl-terminal residue 2,746. Based on secondary structural prediction, two α-helical segments (highlighted) are predicted to match what is referred to as the α-7/8 and α-10 helices of acetylcholinesterase (33, 35). Residues that are bolded and underlined indicate sites that have been mutagenized. Within the α-7/8 helical homology sequence, replacement of A-A-V by N-A-T introduces an N-linked glycosylation acceptor site that could potentially disrupt the four-helix bundle. The site of the D2708C,G2709stop mutation (introduced just downstream of the α-10 helical homology sequence) is also highlighted; introduction of the extra Cys residue at this site is used to test the possible formation of an intersubunit disulfide bond.
Engagement of the ChEL Domain in Tg Dimerization—Evidence has shown that Tg with a dysfunctional ChEL domain cannot homodimerize (31). To check possible homodimerization of regions I-II-III in the absence of ChEL, we engineered I-II-III-Myc- and I-II-III-HA-tagged proteins. Either individually or by co-transfection, both constructs were well expressed in 293 cells as detected by immunoblotting with anti-HA or anti-Myc (Fig. 1E, left panel). Under co-immunoprecipitation conditions, immunoprecipitation with anti-Myc recovered the I-II-III-Myc protein; however, no co-immunoprecipitation of I-II-III-HA could be detected (Fig. 1E, right panel). Co-immunoprecipitation also could not be detected when the anti-HA antibody was employed for immunoprecipitation (not shown). These results (in contrast to those obtained for the isolated ChEL domain, see below) suggest that Tg I-II-III, which is known to be defective in export from the ER (14, 30), cannot homodimerize. By contrast, in the absence of I-II-III, secretory ChEL with a carboxyl-terminal Myc tag is efficiently secreted (14), and current evidence indicates that epitope tagging itself does not block Tg dimerization (Fig. 1, C and D). To examine the dimerization potential of secretory ChEL-Myc with full-length wild-type Tg, the two proteins were co-expressed in 293 cells. With or without secretory ChEL-Myc, full-length Tg was efficiently secreted as judged by anti-Tg immunoprecipitation (Fig. 3, left panel). Despite that newly synthesized ChEL is secreted rapidly (14) in comparison with full-length Tg (39), newly synthesized Tg could nevertheless be co-precipitated from the medium with secretory ChEL-Myc (Fig. 3). Although the efficiency of co-precipitation was low (Fig. 3) (indeed, only ChEL that has not homodimerized remains available to heterodimerize with Tg, competing with Tg homodimerization), the data (Figs. 1E and 3) collectively suggest that the isolated ChEL domain is both necessary and sufficient for dimerization of Tg.
FIGURE 3.
Heterodimerization of the ChEL domain with Tg. 293 cells were either untransfected or transiently transfected to express wild-type Tg (wt Tg) or that plus secretory ChEL bearing a carboxyl-terminal Myc epitope tag (ChEL-Myc). Cells were pulse-labeled for 30 min with 35S-labeled amino acids and chased for 5 h, and the media were immunoprecipitated with either anti-Tg (which recovers both proteins, left panel) or anti-Myc (right panel). The samples were analyzed by SDS-PAGE and fluorography (which, after anti-Myc immunoprecipitation, was overexposed ∼2-fold). The positions of prestained molecular mass standards are included (at left).
Both authentic acetylcholinesterase (AChE) and the Tg ChEL domain use their 6 Cys residues for intrachain disulfide bonding, whereas a unique additional Cys residue in authentic AChE (Fig. 4A, upward arrow) falls in a peptide extension at the extreme carboxyl terminus of the monomeric protein just downstream from the ChEL homology region that includes one of the dimerization helices (33, 35). Crystallographic evidence indicates that this unpaired Cys residue can form an intersubunit disulfide bond, which covalently stabilizes AChE homodimers (40). The presence of such a bond may be exploited in an assay of dimerization potential by nonreducing SDS-PAGE. In 293 cells pulse-labeled with 35S-labeled amino acids and chased for 3 h, recombinant AChE was specifically immunoprecipitated from the chase medium bathing transfected cells, whereas only background bands were recovered from cells transfected with empty vector (Fig. 4B). Unrelated to minor heterogeneity in N-linked glycosylation (data not shown), secreted AChE could be recovered at two positions equivalent to monomer and dimer molecular masses (Fig. 4B). Because dimerization of AChE does not require, nor uniformly employ, the optional intersubunit disulfide bond (41, 42), it is likely that all secreted AChE (Fig. 4B) is homodimeric, although only a portion is stabilized by the intersubunit disulfide bridge.
FIGURE 4.
The ChEL domain and authentic AChE share capabilities in protein dimerization. A, primary sequence alignment at the carboxyl-terminal end of Tg and acetylcholinesterase indicates a nonhomologous peptide extension that bears an extra, unpaired cysteine (upward arrow) immediately following the α-10 helix (denoted Helix) known to be engaged in the homodimerization of AChE. Mouse Tg engineered to terminate with an extra, unpaired cysteine at residue 2,708 (called Tg-CD) is shown with a downward arrow on the primary sequence. B, 293 cells transiently transfected to express human AChE (hAChE) or empty vector were labeled with 35S-labeled amino acids for 2 h and chased further for 3 h in complete medium before immunoprecipitation of AChE from cell lysate (C) and medium (M). The samples were analyzed by nonreducing SDS 8%-PAGE and fluorography. Bands recovered from cells transfected with empty vector (lanes marked vector) represent nonspecific background. The position of monomeric AChE in the medium is indicated with an unlabeled arrow. C, 293 cells were either untransfected controls (C) or transiently transfected to express secretory ChEL (wt) or secretory ChEL-CD (“CD”). Cells were pulse-labeled for 30 min with 35S-labeled amino acids and chased for 4 h, at which time the cell lysates and media immunoprecipitated with a rabbit polyclonal anti-Tg. Immunoprecipitates were analyzed by SDS-PAGE under reducing or nonreducing conditions, as indicated. The position of covalent ChEL-CD dimer is shown. The position of monomeric ChEL-CD in the medium (comprised of monomers and/or noncovalent dimers) is indicated with an unlabeled arrow. The positions of prestained molecular mass standards are included (at left).
We wished to exploit this assay to examine dimerization potential of the Tg ChEL domain in the absence of co-expressed full-length wild-type Tg. With this in mind, we mutagenized secretory ChEL to introduce a Cys residue at the carboxyl terminus of mature murine Tg (Fig. 4A, downward arrow equivalent to D2708C,G2709stop mutation) immediately following the helical segment expected to participate in formation of the four-helix bundle for dimerization. The construct was denoted as ChEL-CD to signify the intent to create a construct with “covalent dimerization” potential. With or without the CD mutation, secretory ChEL proteins exhibited efficient intracellular transport (Fig. 4C), acquiring endo H resistance within a few hours of synthesis (not shown). However, by nonreducing SDS-PAGE, a fraction of secretory ChEL-CD was recovered as covalent homodimers (Fig. 4C, right). As all of the upstream 6 Cys residues are engaged in evolutionarily conserved intrachain disulfide bonds and thus unavailable for interchain pairing, the acquisition of a new intersubunit covalent bond in ChEL-CD requires engagement of the new unique Cys residue from both monomeric partners, indicating tail-to-tail dimerization of the ChEL domain like that for AChE.
To determine whether full-length Tg also engages in tail-to-tail homodimerization, we examined Tg-CD (Fig. 4A) in which the unpaired D2708C was incorporated into the larger Tg context (Fig. 5). A D2708S mutant was also prepared as a negative control. First, 293 cells transfected to express Tg-CD were pulse-labeled with 35S-labeled amino acids and chased for various times in the presence of brefeldin A (BFA) to block intracellular transport in the secretory pathway. Tg immunoprecipitates at each chase time were analyzed by nonreducing SDS-PAGE. At the zero chase time, a series of high molecular weight intermediates termed the “A, B, and C” bands (Fig. 5A, left) were identified as Tg adducts with resident oxidoreductases of the ER lumen (43). (These adducts appear as a smear when the cells are lysed in the absence of N-ethylmaleimide or a similar alkylating treatment (Fig. 5A) (14).) The “D” isoform (Fig. 5A, left), which has been shown to be a partially oxidized Tg folding intermediate (14), was also pronounced at the zero chase time. As reported for wild-type Tg (14), at 1 h of chase, there was further maturation of monomers to the fully oxidized “E” isoform (Fig. 5A, left). However, unlike for wild-type Tg, there was the new appearance of Tg-CD homodimers (Fig. 5A, left panel). In cells in which secretion was blocked by BFA treatment, intracellular Tg-CD homodimers increased in intensity during the second chase hour (Fig. 5A, left panel). From BFA-treated cells, all of these isoforms of Tg migrated as a single band upon reducing SDS-PAGE (not shown).
FIGURE 5.
Covalent assembly of Tg-CD indicates tail-to-tail engagement of Tg homodimers. A, 293 cells were either mock-transfected or transiently transfected (as indicated at the bottom) with a vector encoding a Tg-CD construct containing D2708C,G2709stop. Left panel, cells were pulse-labeled for 30 min with 35S-labeled amino acids and chased in the presence of BFA (5 μg/ml) for the times indicated. Cell lysates were immunoprecipitated with anti-Tg and analyzed by nonreducing SDS 4%-PAGE and fluorography. A smear of high molecular weight bands termed A, B, and C corresponds to those previously found to be Tg adducts with ER oxidoreductases (43), as well as a newly described Tg oxidative folding intermediate D band and the mature E band (14). Right panel, the same experiment but without BFA, in which both cell lysates and chase media were collected at 1 and 2 h of chase. Immunoprecipitated Tg from each cell lysate was divided into equal portions and either mock-digested or digested with endoglycosidase H before nonreducing SDS-PAGE and fluorography. Intracellular Tg-CD monomers are mostly endo H-sensitive. Intracellular covalent dimers are subdivided into a faster migrating endo H-sensitive population and a slower migrating population of endo H-resistant Tg-CD. In the media, the positions of covalent Tg-CD dimer and noncovalent dimer (indistinguishable from monomer by SDS-PAGE but proven to be dimer in the sucrose gradients of Fig. 6) are shown. B, as a control for the Tg-CD mutation, a D2708S,G2709stop mutant is efficiently secreted, but none of the secreted molecules form a covalent dimer.
In the absence of BFA, it was clear that homodimerization of Tg-CD occurred intracellularly even before arrival in the Golgi complex because a portion of the covalent Tg-CD homodimer had not yet acquired resistance to endo H digestion (Fig. 5A, right). Such results are consistent with the long held notion that Tg dimerization occurs in the ER and represents the last structural maturation step required for Tg export to the Golgi complex (15, 16). Over time, increasing quantities of homodimeric, endo H-resistant Tg-CD were delivered to the medium (Fig. 5A, right).
It is known that neither authentic AChE (Fig. 4B) nor endogenous Tg (43) requires an intersubunit disulfide bond for dimer formation. To examine the relationship of the intersubunit disulfide bond of Tg-CD with its dimerization, we employed sucrose velocity gradient centrifugation. Gradients were loaded from the top and finally collected from the bottom as described previously (43). In the first three gradients shown in Fig. 6, the secretion of endogenous Tg from PC Cl3 thyrocytes was compared with that of recombinant wild-type Tg expressed in 293 cells. Although 293 cells transfected with empty vector secreted no protein that could be immunoprecipitated with anti-Tg, 293 cells transfected to express wild-type recombinant mouse Tg secreted Tg protein that was recovered in the identical dimer fractions as those of PC Cl3 thyrocytes, all running as the 330-kDa band by nonreducing SDS-PAGE. When transfected to express Tg-CD, 293 cells secreted species that ran at both the 660-kDa (covalent dimer) and the 330-kDa monomer positions (Fig. 6, right). Importantly, both bands were recovered exclusively in the dimer peak, demonstrating that Tg-CD has a preserved ability to homodimerize without requiring the presence of the covalent intersubunit linkage. Altogether, the data strongly support that 1) Tg-CD proceeds through the normal Tg folding pathway, achieving homodimerization (and normal intracellular transport); and 2) within the context of the full Tg molecule, the extra unpaired cysteine engineered at position 2,708 engages in an interchain disulfide bond. We also found that Tg-D2708S,G2709stop was secreted with equally high efficiency to that of wild-type Tg or Tg-CD, excluding any detrimental effects of the Gly-2709-stop codon, but this negative control exhibited no covalent dimerization (Fig. 5B). Thus, given that secretory ChEL-CD (bearing the identical mutation) itself makes covalent homodimers (Fig. 4C), the evidence strongly indicates that tail-to-tail dimerization of Tg-CD monomers creates a juxtaposition that allows the extra unpaired Cys residue of one monomer to partner with the identical Cys residue in the other monomer.
FIGURE 6.
Dimerization of secreted Tg as measured by sucrose velocity gradient centrifugation. In each gradient, fractions (collected from the bottom) were immunoprecipitated with anti-Tg antibodies and analyzed by nonreducing SDS-PAGE. First gradient, endogenous Tg secreted by the metabolically labeled PC Cl3 thyrocyte cell line. Second gradient, media from metabolically labeled 293 cells transfected with empty vector. Third gradient, recombinant Tg secreted from metabolically labeled 293 cells that had been transfected to express wild-type mouse Tg (wt Tg). Fourth gradient, media from metabolically labeled 293 cells that had been transfected to express Tg-CD. With or without the intersubunit disulfide bond, endogenous and recombinant Tg and Tg-CD are recovered in the dimer (DIM) peak, with little or no secreted protein recovered in the monomer (MON) peak.
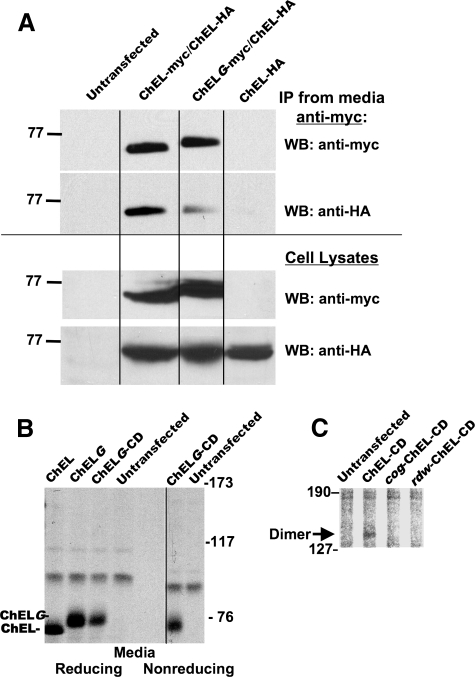
Perturbation of Dimer Stability and Suppression of That Perturbation—Previous studies have suggested that introduction of an N-linked glycosylation site into the “α-7/8” helix of authentic AChE can decrease stability of the four-helix bundle required for dimerization (35). The predicted comparable helix in the ChEL domain of Tg is highlighted in Fig. 2B. Therefore, using a secretory ChEL-Myc cDNA template, the A-A-V sequence shown in Fig. 2B was mutagenized to N-A-T within the helical region (this mutant termed “ChELG-Myc” to indicate the intent to create a new N-linked glycosylation site, Fig. 2A). First, a double epitope tag strategy was employed similar to that used successfully for full-length Tg. Specifically, secretory ChEL tagged with a carboxyl-terminal HA epitope was coexpressed in 293 cells either with secretory ChEL-Myc or with secretory ChELG-Myc (Fig. 2A). Using these constructs, both ChEL-Myc and ChELG-Myc were well expressed along with ChEL-HA (Fig. 7A, bottom panels). Anti-Myc immunoprecipitation from media was specific and comparably efficient for Myc-tagged secretory ChEL or ChELG, but the mobility of the ChELG protein was slower (Fig. 7A). (Deglycosylation experiments (not shown) confirmed that the engineered ChELG glycosylation site was utilized.) Although comparable total amounts of each ChEL protein were secreted (not shown), in comparison with ChEL-Myc, ChELG-Myc was much less efficient in co-precipitating ChEL-HA (Fig. 7A, upper panels).
FIGURE 7.
A-A-V to N-A-T mutagenesis to create an N-linked glycosylation site within the α-7/8 helical sequence (Fig. 2B) perturbs subunit contact (35). A, 293 cells were either untransfected or transiently transfected to express either secretory ChEL-Myc or ChEL-Myc bearing the extra glycosylation site (ChELG-Myc), in conjunction with an equal amount of plasmid DNA encoding secretory ChEL-HA. Cells expressing secretory ChEL-HA alone were included as a negative control. Secretion was collected for 24 h. The media were either immunoprecipitated (IP) with anti-Myc before SDS-PAGE (upper two panels) or analyzed directly without immunoprecipitation (lower two panels). Samples underwent Western blotting (WB) with either anti-Myc (to demonstrate recovery of ChEL-Myc or ChELG-Myc) or anti-HA (to examine the extent of co-precipitation of the dimerization partner). Introduction of an N-glycan slowed the electrophoretic mobility of the ChELG-Myc band and decreased co-precipitation of ChEL-HA by 72% (in three such experiments, co-precipitation decreased 52 ± 18%). B, the G mutation was introduced into secretory ChELG-CD. The listed constructs were transiently expressed in 293 cells; secretory ChEL and secretory ChELG (lacking potential for intersubunit covalent bonding) were included as controls. Cells were metabolically labeled and chased for 4 h, and the media were immunoprecipitated with anti-Tg. The samples were analyzed by SDS 5.5%-PAGE under nonreducing (and reducing) conditions as indicated, with no covalent ChELG-CD dimer detected. C, covalent homodimer synthesized in cells expressing ChEL-CD but not in cells expressing cog-ChEL-CD or rdw-ChEL-CD.
We next engineered the same N-linked glycosylation site into the covalent dimerizing ChEL-CD construct to create ChELG-CD (Fig. 2A). Based on band mobility, the glycosylation site was indeed utilized in all copies of ChELG-CD as was the case for ChELG (Fig. 7B, Reducing conditions). In this case, when all ChEL-CD was glycosylated within the presumptive four-helix bundle, covalent dimerization could no longer be detected by nonreducing SDS-PAGE (Fig. 7B; compare with Fig. 4C). Similarly, introduction of either the cog or the rdw mutations blocked formation of covalent ChEL-CD dimers (Fig. 7C).
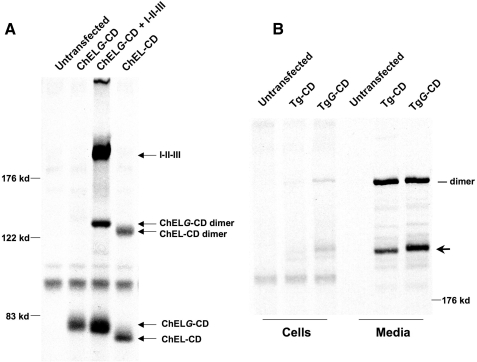
We noted that the fraction of secretory ChEL-CD that undergoes formation of the optional intersubunit covalent bond (30% ± 13%, n = 3 independent experiments) was less than that observed for full-length Tg-CD (60% ± 10%, n = 6 independent experiments). This suggested the intriguing possibility that additional information provided by upstream Tg regions I-II-III might improve tail-to-tail dimer alignment or stability. Indeed, ChEL exhibits direct physical interactions with I-II-III (14). To examine the role of I-II-III in stabilizing homodimers, we expressed the ChELG-CD construct with or without co-expression of Tg regions I-II-III. As before (Fig. 4C), covalent homodimers of ChEL-CD could be recovered without assistance from other Tg domains (Fig. 8A, last lane). In addition, as before (Fig. 7B), ChELG-CD, expressed by itself, exhibited diminished dimer stability as demonstrated by its inability to form the intersubunit covalent bond (Fig. 8A, second lane). However, upon co-expression of I-II-III, which was co-secreted with ChELG-CD, the carboxyl-terminal intersubunit disulfide bond in ChELG-CD homodimers was restored (Fig. 8A, third lane). The data indicate that in trans, Tg regions I-II-III provide added stability to the homodimerization of the Tg ChEL domain.
FIGURE 8.
Effect of Tg regions I-II-III on the dimer stability of glycosylated ChELG domain. A, 293 cells were either transiently transfected with the secretory ChELG-CD construct alone or co-transfected in the presence of Tg I-II-III. Cells expressing the covalent dimerizing ChEL-CD construct were analyzed in parallel, as a control. Cells were pulse-labeled for 20 min with 35S-labeled amino acids and chased for 4 h; media were collected and immunoprecipitated with anti-Tg followed by nonreducing SDS 5.5%-PAGE and fluorography. B, 293 cells were transiently transfected to express full-length Tg-CD or TgG-CD bearing the extra N-linked glycosylation site in the ChEL domain. Cells were pulse-labeled for 30 min with 35S-labeled amino acids and chased for 6 h before analysis by Tg immunoprecipitation and nonreducing SDS 4%-PAGE and fluorography. Mobility of the TgG-CD band establishes that the extra glycosylation site is actually used (nevertheless, covalent intersubunit interaction proceeds).
To explore the significance of these findings, we introduced the same helix glycosylation site within the context of full-length Tg-CD. As expected, TgG-CD utilized the additional glycosylation site (seen as a slower Tg monomer mobility by SDS-PAGE, Fig. 8B). Although the Tg-CD control was rapidly secreted with a majority of the protein containing the covalent intersubunit bond, TgG-CD disulfide cross-linking and secretion was only modestly inhibited (Fig. 8B). Altogether, the data indicate that although the ChEL domain is necessary and sufficient for Tg dimerization, upstream Tg regions I-II-III, either in cis or in trans, assist in Tg dimer stability.
DISCUSSION
Tg transport through the secretory pathway is essential to make Tg available for iodination in the process of thyroid hormone biosynthesis. The structural features in Tg required for its intracellular transport are beginning to be elucidated. For one thing, the Tg ChEL domain must make physical contact with upstream regions I-II-III in a manner not requiring that ChEL be contained within the contiguous Tg polypeptide (14). For another, Tg homodimerization has been suggested to be required for export from the ER (15, 16). In this report, we show that secretory ChEL and Tg are both homodimeric proteins (Figs. 1 and 4, 5, 6, 7, 8) that share predicted α-helical sequences that closely align with the helices critical for homodimerization of AChE (Fig. 2B). Moreover, the isolated ChEL domain can cross-dimerize with intact Tg (Fig. 3), suggesting that the ChEL domain encodes the minimal information necessary for Tg dimerization. To be effective, these sequences do not need to reside at the extreme carboxyl terminus of Tg because introduction of downstream GFP, Myc, or HA tags still allows dimerization via the ChEL domain to take place (Figs. 1, 3, and 7A). Although the form of GFP we employed may have oligomerization potential (44), the fact that Tg-GFP cross-dimerized with Tg-3×Myc indicates that dimerization was triggered by Tg sequences rather than by tag sequences.
For AChE, homodimers brought together via its carboxyl-terminal four-helix bundle (33) may be further stabilized by an intermolecular disulfide bridge (Fig. 4A). Introduction of an unpaired Cys residue immediately after the predicted carboxyl-terminal helical sequence in the ChEL domain also allows for intersubunit covalent bonding of secretory ChEL-CD (Fig. 4C) and full-length Tg-CD (Fig. 5). The simplicity of the one-dimensional nonreducing SDS-PAGE assay of Tg-CD dimerization makes the analysis easy. With this approach, Tg dimerization can clearly be shown to occur before acquisition of endo H resistance and also in cells in which intracellular transport is blocked by BFA treatment (Fig. 5A). The data are consistent with the longstanding hypotheses that Tg dimerization occurs before its export from the ER (15, 16) and that dimerization increases the efficiency of intracellular Tg transport (43). With or without the intersubunit disulfide bond, Tg-CD has fully preserved dimerization (Fig. 6) and exportability (Fig. 8B), but the covalent intersubunit bond presumably reflects the stability of the dimer, which yields proper tail-to-tail alignment of the monomer partners.
Although structural biology studies of the Tg ChEL domain have not yet begun, based on the foregoing results, we propose that the predicted α-helical segments within the ChEL domain of Tg are utilized in forming the contact zone engaged at the dimer interface. To weaken this putative contact, we introduced a mutation converting the A-A-V sequence of the first α-helix (Fig. 2B) to an N-A-T glycosylation acceptor site. The presence of the ChELG mutation in one of the two subunits significantly decreased co-immunoprecipitation efficiency between the dimerization partners (Fig. 7A), whereas the presence of the same mutation in both partners completely eliminated the intersubunit disulfide bond in ChELG-CD (Fig. 7B). Although these data support a dimerization mechanism involving helical interactions similar to that for AChE, there are reasons to think that Tg homodimer stability involves more than merely the four-helix bundle. First, the fraction of Tg-CD that makes an intersubunit disulfide bond (Fig. 5A) is higher than the fraction of secretory ChEL-CD making the same bond (Fig. 4C). Second, although Tg region I-II-III itself cannot dimerize (Fig. 1E), when ChELG-CD is co-expressed with I-II-III, not only is I-II-III secretion rescued, but the intersubunit disulfide bond is re-established between ChELG-CD partners (Fig. 8A), suggesting that I-II-III contributes to ChEL dimer stability. Third, mutation to add the same glycosylation site in full-length TgG-CD does not block efficient Tg secretion and only slightly decreases the fraction of TgG-CD making the intersubunit disulfide link (Fig. 8B). Altogether, the evidence points to a reciprocal relationship, i.e. even as the ChEL domain functions as an intramolecular chaperone and escort for Tg regions I-II-III (14), I-II-III also assists in the stability of homodimerization of the ChEL domain.
Multiple small Tg mutations causing congenital hypothyroidism have been identified within the ChEL domain (Refs. 20, 28, and 29 with others reviewed in Ref. 24). Both defective intramolecular chaperone function (14) and defective Tg dimerization (31) are expected consequences of such mutations. Additionally, it seems likely that many mutations in Tg regions I-II-III may fail to provide adequate homodimer stability, which could account for why many if not all cases of hypothyroidism with mutant Tg derive from a failure of intracellular transport through the secretory pathway (26).
Conversely, the present studies provide reason to believe that some of these mutants might not be totally dysfunctional, with some Tg ChEL domain mutations perhaps being intramolecularly suppressed in part by upstream Tg domains (Fig. 8B), whereas other I-II-III mutations might still be able to heterodimerize with full-length Tg (Fig. 3). In summary, intracellular Tg transport for thyroid hormone synthesis engages the ChEL domain not only as an intramolecular chaperone and escort (14), but herein we show that ChEL is also a nidus for Tg homodimerization. Although the ChEL domain is both necessary and sufficient for Tg transport through the secretory pathway, ChEL interactions with upstream Tg regions stabilize the homodimer. Structural studies will be needed to elaborate the contact sites between monomeric subunits, whereas additional molecular dissection is needed to identify the site(s) in ChEL that functionally interact with I-II-III and the site(s) in I-II-III that functionally interact with ChEL.
This work was supported, in whole or in part, by National Institutes of Health Grant DK40344 (to P. A.). This work was also supported in part by the Ministero dell'Università e della Ricerca PRIN 2006069102_004 (to B. D. J.).
Footnotes
The abbreviations used are: Tg, thyroglobulin; ChEL, cholinsterase-like; ER, endoplasmic reticulum; DMEM, Dulbecco's modified Eagle's medium; GFP, green fluorescent protein; HA, hemagglutinin; endo H, endoglycosidase H; BFA, brefeldin A; AChE, acetylcholinesterase.
References
- 1.Di Jeso, B., and Arvan, P. (2004) in The Thyroid (Braverman, L. E., and Utiger, R., eds), 9th Ed., pp. 77–95, Lippincott Williams & Wilkins, Philadelphia, PA
- 2.Taurog, A. (1999) Biochimie (Paris) 81 557–562 [DOI] [PubMed] [Google Scholar]
- 3.Marriq, C., Lejeune, P. J., Venot, N., and Vinet, L. (1991) Mol. Cell. Endocrinol. 81 155–164 [DOI] [PubMed] [Google Scholar]
- 4.Dunn, A. D., Corsi, C. M., Myers, H. E., and Dunn, J. T. (1998) J. Biol. Chem. 273 25223–25229 [DOI] [PubMed] [Google Scholar]
- 5.Lamas, L., and Taurog, A. (1977) Endocrinology 100 1129–1136 [DOI] [PubMed] [Google Scholar]
- 6.Turakulov, I., Saatov, T., Babaev, T. A., Rasuleva, G., and Makhmudov, V. (1976) Biokhimiya 41 1004–1007 [PubMed] [Google Scholar]
- 7.Xiao, S., Dorris, M. L., Rawitch, A. B., and Taurog, A. (1996) Arch. Biochem. Biophys. 334 284–294 [DOI] [PubMed] [Google Scholar]
- 8.Veneziani, B. M., Giallauria, F., and Gentile, F. (1999) Biochimie (Paris) 81 517–525 [DOI] [PubMed] [Google Scholar]
- 9.van de Graaf, S. A., Ris-Stalpers, C., Pauws, E., Mendive, F. M., Targovnik, H. M., and de Vijlder, J. J. (2001) J. Endocrinol. 170 307–321 [DOI] [PubMed] [Google Scholar]
- 10.Schumacher, M., Camp, S., Maulet, Y., Newton, M., MacPhee-Quigley, K., Taylor, S. S., Friedmann, T., and Taylor, P. (1986) Nature 319 407–409 [DOI] [PubMed] [Google Scholar]
- 11.Swillens, S., Ludgate, M., Mercken, L., Dumont, J. E., and Vassart, G. (1986) Biochem. Biophys. Res. Comm. 137 142–148 [DOI] [PubMed] [Google Scholar]
- 12.Mori, N., Itoh, N., and Salvaterra, P. M. (1987) Proc. Natl. Acad. Sci. U. S. A. 84 2813–2817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arvan, P., Kim, P. S., Kuliawat, R., Prabakaran, D., Muresan, Z., Yoo, S. E., and Hossain, S. A. (1997) Thyroid 7 89–105 [DOI] [PubMed] [Google Scholar]
- 14.Lee, J., Di Jeso, B., and Arvan, P. (2008) J. Clin. Investig. 118 2950–2958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim, P. S., and Arvan, P. (1991) J. Biol. Chem. 266 12412–12418 [PubMed] [Google Scholar]
- 16.Di Jeso, B., Pereira, R., Consiglio, E., Formisano, S., Satrustegui, J., and Sandoval, I. V. (1998) Eur. J. Biochem. 252 583–590 [DOI] [PubMed] [Google Scholar]
- 17.Berg, G., and Ekholm, R. (1975) Biochim. Biophys. Acta 386 422–431 [DOI] [PubMed] [Google Scholar]
- 18.Berg, G., Bjorkman, U., and Ekholm, R. (1980) Mol. Cell. Endocrinol. 20 87–98 [DOI] [PubMed] [Google Scholar]
- 19.Berg, G., Bjorkman, U., and Ekholm, R. (1980) Mol. Cell. Endocrinol. 17 139–144 [DOI] [PubMed] [Google Scholar]
- 20.Caputo, M., Rivolta, C. M., Esperante, S. A., Gruneiro-Papendieck, L., Chiesa, A., Pellizas, C. G., Gonzalez-Sarmiento, R., and Targovnik, H. M. (2007) Clin. Endocrinol. (Oxf.) 67 351–357 [DOI] [PubMed] [Google Scholar]
- 21.Pardo, V., Rubio, I. G., Knobel, M., Aguiar-Oliveira, M. H., Santos, M. M., Gomes, S. A., Oliveira, C. R., Targovnik, H. M., and Medeiros-Neto, G. (2008) Thyroid 18 783–786 [DOI] [PubMed] [Google Scholar]
- 22.Caron, P., Moya, C. M., Malet, D., Gutnisky, V. J., Chabardes, B., Rivolta, C. M., and Targovnik, H. M. (2003) J. Clin. Endocrinol. Metab. 88 3546–3553 [DOI] [PubMed] [Google Scholar]
- 23.Kitanaka, S., Takeda, A., Sato, U., Miki, Y., Hishinuma, A., Ieiri, T., and Igarashi, T. (2006) J. Hum. Genet. 51 379–382 [DOI] [PubMed] [Google Scholar]
- 24.Rivolta, C. M., and Targovnik, H. M. (2006) Clin. Chim. Acta 374 8–24 [DOI] [PubMed] [Google Scholar]
- 25.Matakidou, A., Hamel, N., Popat, S., Henderson, K., Kantemiroff, T., Harmer, C., Clarke, S. E., Houlston, R. S., and Foulkes, W. D. (2004) Carcinogenesis 25 369–373 [DOI] [PubMed] [Google Scholar]
- 26.Vono-Toniolo, J., Rivolta, C. M., Targovnik, H. M., Medeiros-Neto, G., and Kopp, P. (2005) Thyroid 15 1021–1033 [DOI] [PubMed] [Google Scholar]
- 27.Kim, P. S., Ding, M., Menon, S., Jung, C. G., Cheng, J. M., Miyamoto, T., Li, B., Furudate, S., and Agui, T. (2000) Mol. Endocrinol. 14 1944–1953 [DOI] [PubMed] [Google Scholar]
- 28.Hishinuma, A., Furudate, S., Oh-Ishi, M., Nagakubo, N., Namatame, T., and Ieiri, T. (2000) Endocrinology 141 4050–4055 [DOI] [PubMed] [Google Scholar]
- 29.Kim, P. S., Hossain, S. A., Park, Y.-N., Lee, I., Yoo, S.-E., and Arvan, P. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 9909–9913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park, Y. N., and Arvan, P. (2004) J. Biol. Chem. 279 17085–17089 [DOI] [PubMed] [Google Scholar]
- 31.Kim, P. S., Kwon, O.-Y., and Arvan, P. (1996) J. Cell Biol. 133 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddy, P. S., and Corley, R. B. (1998) BioEssays 20 546–554 [DOI] [PubMed] [Google Scholar]
- 33.Sussman, J. L., Harel, M., Frolow, F., Oefner, C., Goldman, A., Toker, L., and Silman, I. (1991) Science 253 872–879 [DOI] [PubMed] [Google Scholar]
- 34.Bourne, Y., Taylor, P., and Marchot, P. (1995) Cell 83 503–512 [DOI] [PubMed] [Google Scholar]
- 35.Morel, N., Leroy, J., Ayon, A., Massoulie, J., and Bon, S. (2001) J. Biol. Chem. 276 37379–37389 [DOI] [PubMed] [Google Scholar]
- 36.Seed, R. W., and Goldberg, I. H. (1965) Science 149 1380–1382 [DOI] [PubMed] [Google Scholar]
- 37.Seed, R. W., and Goldberg, I. H. (1965) J. Biol. Chem. 240 764–773 [PubMed] [Google Scholar]
- 38.Bryson, K., McGuffin, L. J., Marsden, R. L., Ward, J. J., Sodhi, J. S., and Jones, D. T. (2005) Nucleic Acids Res. 33 W36–W38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim, P. S., Lee, J., Jongsamak, P., Menon, S., Li, B., Hossain, S. A., Bae, J. H., Panijpan, B., and Arvan, P. (2008) Mol. Endocrinol. 22 477–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Velan, B., Grosfeld, H., Kronman, C., Leitner, M., Gozes, Y., Lazar, A., Flashner, Y., Marcus, D., Cohen, S., and Shafferman, A. (1991) J. Biol. Chem. 266 23977–23984 [PubMed] [Google Scholar]
- 41.Kerem, A., Kronman, C., Bar-Nun, S., Shafferman, A., and Velan, B. (1993) J. Biol. Chem. 268 180–184 [PubMed] [Google Scholar]
- 42.Gough, N. R., and Randall, W. R. (1995) J. Neurochem. 65 2734–2741 [DOI] [PubMed] [Google Scholar]
- 43.Di Jeso, B., Park, Y.-N., Ulianich, L., Treglia, A. S., Urbanas, M. L., High, S., and Arvan, P. (2005) Mol. Cell. Biol. 25 9793–9805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jain, R. K., Joyce, P. B., Molinete, M., Halban, P. A., and Gorr, S. U. (2001) Biochem. J. 360 645–649 [DOI] [PMC free article] [PubMed] [Google Scholar]