Abstract
Chromatin can exert a regulatory effect on gene transcription by modulating the access of transcription factors to target genes. In the present study, we examined whether nuclear actions of the incretin hormones, glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1, involve modulation of β-cell chromatin structure. Stimulation of INS-1(832/13) β-cells or dispersed mouse islets with glucose-dependent insulinotropic polypeptide or glucagon-like peptide-1 resulted in the post-translational modification of core H3 histones, through acetylation and phosphorylation. Both increased histone H3 acetyltransferase and reduced histone deacetylase activities contributed. Subsequent studies demonstrated that incretin-mediated histone H3 modifications involved activation of protein kinase A, p42/44 mitogen-activated protein kinase (MAPK), and p38 MAPK signaling modules, resulting in the activation of mitogen- and stress-activated kinase-1. Additionally, modification of histone H3 increased its association with the transcription factor, phosphorylated cAMP-response element-binding protein (phospho-CREB) and with cAMP-responsive CREB coactivator 2. Incretin-activated CREB-related Bcl-2 transcription was greatly reduced by a histone acetyltransferase inhibitor, demonstrating the functional importance of histone H3 modification. This appears to be the first demonstration of β-cell chromatin modification in response to the incretins and the studies indicate that their regulatory effects involve coordinated nuclear interactions between specific signaling modules, chromatin-modifying enzymes and transcription factors.
The gastrointestinal incretin hormones, glucose-dependent insulinotropic polypeptide (GIP)2 and glucagon-like peptide-1 (GLP-1), exert pleiotropic effects on pancreatic islets that include the potentiation of glucose-stimulated insulin secretion, expansion of β-cell mass via induction of β-cell proliferation, and reduction of β-cell apoptosis (1–8). Recently, members of two classes of incretin-related compounds have been approved by the FDA for the treatment of type 2 diabetes, the incretin mimetic exenatide (Byetta™) and the DPP-IV inhibitor sitagliptin (Januvia™), resulting in a burgeoning interest in this class of hormones. Although significant progress has been made in incretin biology over the past few years, there is relatively little known about their mode of action in gene regulation. In the current study we examined the possible involvement of GIP and GLP-1 in β-cell chromatin modification.
Eukaryotic chromatin is composed of an octamer of four core histones (H2A, H2B, H3, and H4) around which 147 bp of DNA are wrapped. Chromatin can exert a regulatory effect on gene transcription by modulating accessibility of transcription factors to target genes (9). Different post-translational modifications in the N termini of the histones have been identified, including acetylation, methylation, phosphorylation, ubiquitination, sumoylation, ADP-ribosylation, deamination, and proline isomerization, and these modifications have direct effects on gene expression (10–12). Histone acetyltransferases (HATs) are involved in the acetylation of histone N termini, resulting in chromatin adopting an “open” conformation, whereas histone deacetylase (HDAC) generally promotes a “closed” conformation, by reversing the process (13–15). Histone H2B has been shown to be acetylated at lysine residues 5, 12, 15, and 20 (16–17), whereas histone H3 is acetylated at lysines 9, 14, 18, and 23 (18–19). Among these sites, acetylation of H3 at Lys-9 has been considered to play a dominant role in histone deposition and chromatin assembly (18–19). Additionally, phosphorylation at serine 10 and 28 and threonine 1 has been shown to be tightly regulated for chromosome condensation during cell cycle progression (19–21).
In the present study, we have shown that GIP and GLP-1 induce core histone H3 protein modifications through the regulation of histone H3 acetyltransferase (H3AT) and HDAC activity, and activation of protein kinase A (PKA), p42/44 mitogen-activated protein kinase (MAPK), p38 MAPK, and mitogen- and stress-activated kinase-1 (MSK-1) signaling modules are involved in this process. Additionally, modification of histone H3 increased its association with the transcription factor, phosphorylated cAMP-response element-binding protein (phospho-CREB) and with cAMP-responsive CREB coactivator 2 (TORC2) in the nucleus. Incretin-mediated post-translational modification of core histones is therefore likely to be an important component of their effects on gene transcription in β-cells.
EXPERIMENTAL PROCEDURES
Cell Culture—INS-1 β-cells (clone 832/13) were kindly provided by Dr. C. B. Newgard (Duke University Medical Center, Durham, NC). INS-1 cells were cultured in 11 mm glucose RPMI 1640 (Sigma Laboratories, Natick, MA) supplemented with 2 mm glutamine, 50 μm β-mercaptoethanol, 10 mm HEPES, 1 mm sodium pyruvate, 10% fetal bovine serum, 100 unit/ml penicillin G-sodium, and 100 μg/ml streptomycin sulfate. Cell passages 45–70 were used. In incretin stimulation experiments a peptide concentration of 100 nm was used, because previous studies have demonstrated near-maximal β-cell responses at this concentration (7, 8).
Islet Isolation—Male C57BL/6 mice (12 weeks old, Charles River) were anesthetized by intraperitoneal injection of pentobarbital (30–40 mg/kg). Islets were isolated by collagenase digestion and dispersed to single cells as described previously (22). Dispersed islets were cultured in RPMI 1640 supplemented with 5 mm glucose, 0.25% HEPES, 7.5% fetal bovine serum, 100 units/ml penicillin G-sodium, and 100 μg/ml streptomycin sulfate.
Preparation of Nuclear Extracts—Nuclear proteins were isolated as described by Schreiber et al. (23). Briefly, cells were washed with phosphate-buffered saline and disrupted with 200 μl of ice-cold buffer A (10 mm HEPES, pH 7.9, 10 mm KCl, 1.5 mm MgCl2, 1 mm EDTA, 1 mm dithiothreitol, 0.1% Nonidet P-40, and protease inhibitors). Following centrifugation, the resulting pellet was re-suspended in 20 μl of buffer B (20 mm HEPES, pH 7.9, 400 mm NaCl, 1 mm EDTA, 1 mm dithiothreitol, 20% glycerol, and protease inhibitors) and incubated on ice for 10 min. After clarification of the mixture by centrifugation, the supernatant (nuclear extract) was collected and subjected to Western blot analysis or enzyme activity assay.
Western Blot Analysis—Protein samples were separated on a 13% SDS/PAGE gel and transferred onto nitrocellulose membranes (Bio-Rad Laboratories). Probing of the membranes was performed with acetyl-histone H3 (Lys-9), acetyl-histone H3 (Lys-18), acetyl-histone H3 (Lys-23), phospho-histone H3 (Ser-10), histone 3, and HDAC1, -2, -3, -4, -5, and -7 antibodies (Cell Signaling Technology, Beverly, MA). Immunoreactive bands were visualized by enhanced chemiluminescence (Amersham Biosciences) using horseradish peroxidase-conjugated IgG secondary antibodies.
Confocal Microscopy—INS-1 cells were treated with GIP or GLP-1 (100 nm) for 24 h. Following treatment, immunocytochemical staining was performed using antibodies against acetyl-histone Lys-9, acetyl-histone Lys-18, or phospho-histone H3 Ser-10 and insulin and visualized with Texas Red® dye-conjugated anti-rabbit secondary antibody and Alexa fluor® 488-conjugated anti-mouse antibody. Stained cells were imaged using a Zeiss laser scanning confocal microscope (Axioskop2). All imaging data were analyzed using the Northern Eclipse program (version 6).
HDAC, H3AT, and MSK-1 Activity Assays—The HDAC (Biomol, Plymouth Meeting, PA), H3AT (Active motif), and MSK-1 FP Fluorescein Green assay kit, KinEASE™ (Millipore) were used to measure enzyme activity of HDAC, H3AT, and MSK-1, according to the manufacturer's protocols. Enzyme activity is presented as relative activity normalized to protein concentration.
Co-immunoprecipitation—Following treatment, nuclear extracts were isolated, and acetylated histones H3 at Lys-9, Lys-18, or phosphorylated histone H3 at Ser-10 were respectively immunoprecipitated, using Dynabead protein A (Invitrogen) and anti-acetyl histone H3 Lys-9, Lys-18, or anti-phospho histone H3 Ser-10 antibody. The precipitated products were then resolved by SDS-PAGE and probed with anti-phospho-CREB (Ser-133) or anti-TORC2 antibody.
Quantitative Real-time Reverse Transcription-PCR—Total RNA was extracted, and cDNA fragments were generated by reverse transcription. 100 ng of cDNA was used in the real-time PCR to measure Bcl-2 expression, whereas 10 ng of cDNA was used in the 18 S rRNA control PCR. The primer and probe sequences used for the amplification of Bcl-2 were as follows: forward primer, 5′-CTG AGT ACC TGA ACC GGC ATC-3′; reverse primer, 5′-TGG CCC AGG TAT GCA CCC AGA-3′; probe, 5′-6-carboxyfluorescein-CCC CAG CAT GCG ACC TCT GTT TG-3′-6-carboxytetramethylrhodamine. All reactions followed the typical sigmoidal reaction profile, and cycle threshold was used as a measurement of amplicon abundance.
Statistical Analysis—Data are expressed as means ± S.E. with the number of individual experiments presented in the figure legends. Significance was tested using analysis of variance (ANOVA) with Newman-Keuls post hoc test (p < 0.05) as indicated in the figure legends.
RESULTS
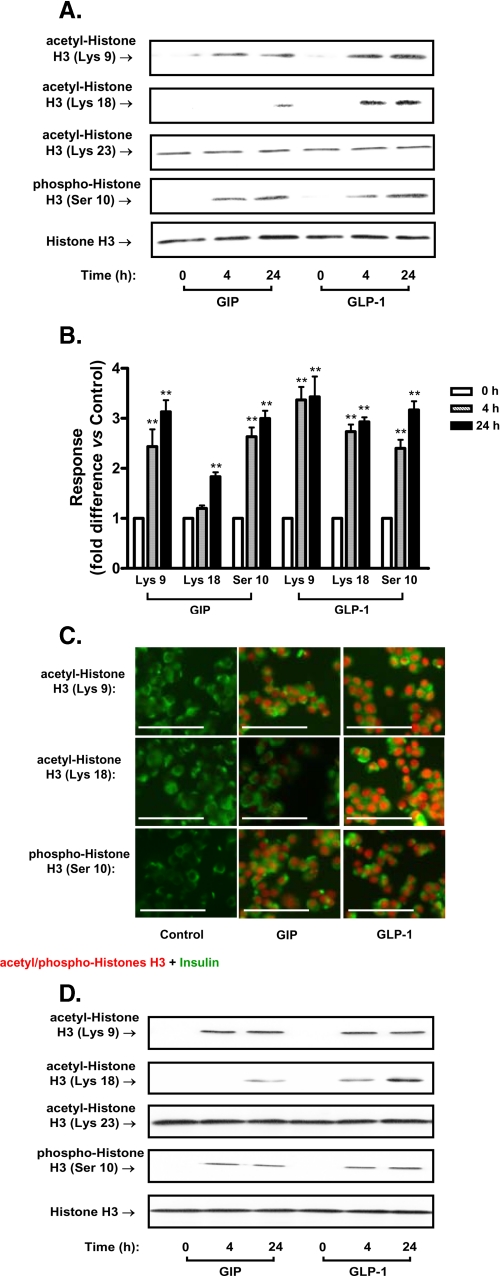
Effect of Incretins on Histone H3 Modification in Pancreatic β-Cells—The effects of GIP and GLP-1 on the post-translational modification of histone H3 in INS-1 (832/13) β-cells were first determined. As shown in Fig. 1 (A and B), both GIP and GLP-1 (100 nm) treatment increased acetylation of histone H3 at Lys-9 and Lys-18 and phosphorylation at Ser-10, whereas there were no significant changes in acetylation of histone H3 at Lys-23. Acetylation of histone H3 at Lys-9 and Lys-18 and phosphorylation at Ser-10 demonstrated concentration-dependent responses to GIP or GLP-1 (supplemental Fig. S1). Immunocytochemical staining of INS-1 (832/13) β-cells confirmed the effects of incretins on histone H3 detected by Western blotting (Fig. 1C). Similar histone modification results were observed with dispersed mouse islets. Both incretins increased acetylation of histone H3 at Lys-9 and Lys-18 and phosphorylation at Ser-10. On the other hand, there were no significant changes in acetylation of histone H3 at Lys-23 (Fig. 1D).
FIGURE 1.
Incretin hormones modulate post-translational modification of histone H3. A, effects of GIP and GLP-1 on acetylation and phosphorylation of histone H3. INS-1 (832/13) cells were serum-starved in 3 mm glucose RPMI containing 0.1% BSA overnight and GIP (100 nm) or GLP-1 (100 nm) added for the indicated periods of time. Nuclear extracts were isolated, and Western blot analyses were performed using antibodies against acetyl-histone H3 (Lys-9), acetyl-histone H3 (Lys-18), acetyl-histone H3 (Lys-23), phospho-histone H3 (Ser-10), and histone H3. Western blots are representative of n = 3. B, densitometric analysis of A, incretin-mediated acetylation, and phosphorylation of histone H3. C, immunocytochemical analysis. INS-1 (832/13) cells were treated as described above. Immunocytochemical staining was performed using antibodies against acetyl-histone H3 (Lys-9), acetyl-histone H3 (Lys-18), or phospho-histone H3 (Ser-10) and insulin. All imaging data were analyzed using the Northern Eclipse program (version 6) and the scale bar indicates 25 μm. D, effects of GIP and GLP-1 on acetylation and phosphorylation of histone H3 in mouse islets. Islets were treated with GIP or GLP-1 (100 nm) in serum-starved 3 mm glucose RPMI containing 0.1% BSA for the indicated periods of time. Nuclear extracts were isolated and Western blot analyses were performed as described above. Western blots are representative of n = 3, and significance was tested using ANOVA with Newman-Keuls post hoc test; **, p < 0.01 versus 0 h control.
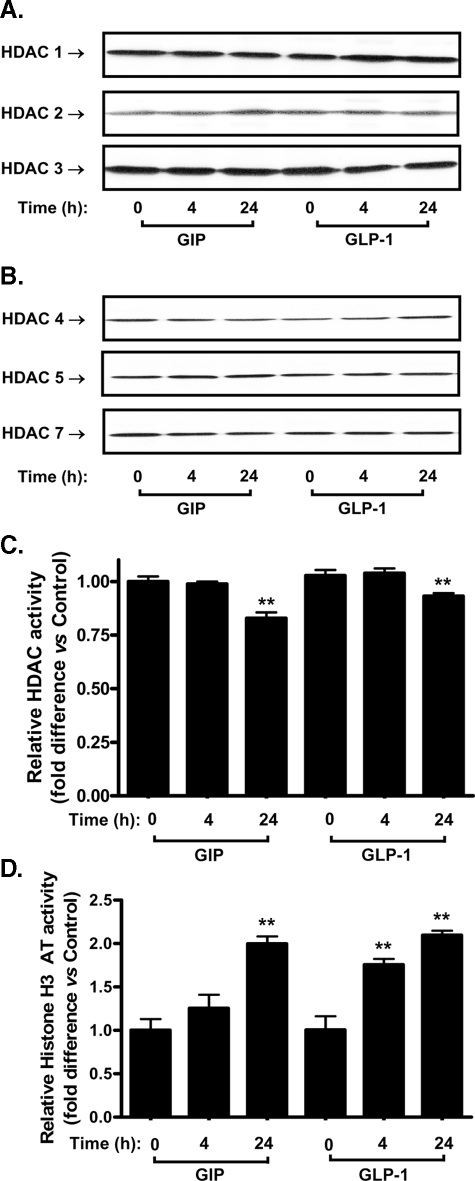
Effect of Incretins on the Activity of HDACs and H3AT—The effects of GIP and GLP-1 on protein levels or activity of chromatin-modifying enzymes were next studied. As shown in Fig. 2 (A and B), there were no significant changes in the protein levels of class 1 HDACs, HDAC1, -2, and -3 or the class 2 HDACs, HDAC4, -5, and -7 (24, 25) following treatment of INS-1 (832/13) β-cells with GIP or GLP-1 (100 nm) for 24 h. However, identical treatment conditions resulted in small, but significant (p < 0.05), decreases in HDAC activity (Fig. 2C). By contrast, incretin treatment resulted in marked increases in H3AT activity that was significant (p < 0.05) by 4 h with GLP-1 and for both peptides by 24 h (Fig. 2D). These results indicate that incretin hormones modulate β-cell chromatin structure by regulating the activity of both the HDAC and H3AT families of chromatin-modifying enzymes.
FIGURE 2.
Incretin hormones modulate the activities of HDACs and H3AT. A and B, effects of GIP and GLP-1 on Class 1 (A) and Class 2 (B) HDAC protein levels in INS-1 (832/13) β-cells. INS-1 (832/13) cells were serum-starved in 3 mm glucose RPMI containing 0.1% BSA overnight and GIP or GLP-1 (100 nm) added for the indicated periods of time. Nuclear extracts were isolated and Western blot analyses were performed using antibodies against HDAC1, -2, -3, -4, -5, and -7. C, effect of GIP and GLP-1 on HDAC activity in INS-1 (832/13) β-cells. INS-1 (832/13) cells were treated as described above. HDAC activity was determined in nuclear extracts as described under “Experimental Procedures.” D, effect of GIP and GLP-1 on histone H3 acetyltransferase (H3AT) activity in INS-1 (832/13) β-cells. INS-1 (832/13) cells were treated as described above. Nuclear extracts were isolated, and histone H3AT activity was determined from nuclear extracts as described under “Experimental Procedures.” Western blots are representative of n = 3, and all data represent three independent experiments, each carried out in duplicate. Significance was tested using ANOVA with Newman-Keuls post hoc test; **, p < 0.01 versus control.
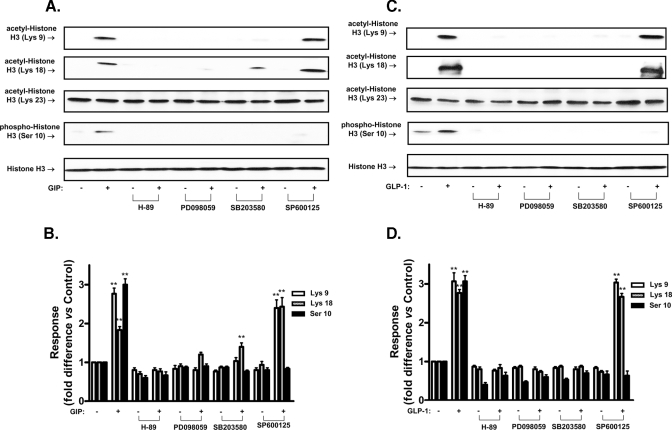
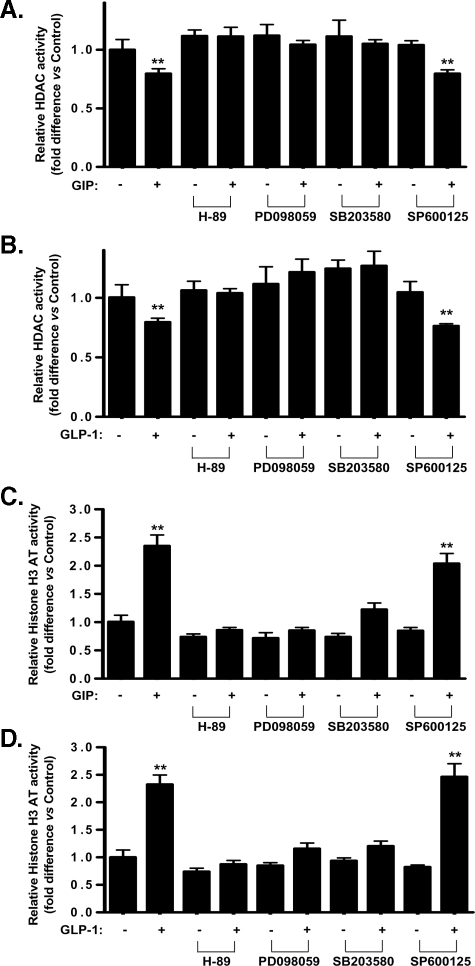
Signaling Modules Involved in Incretin-mediated Histone H3 Modification in Pancreatic β-Cells—Both GIP and GLP-1 activate a number of signal transduction pathways in β-cells, and the potential involvement in histone H3 modification of PKA, p42/44 MAPK, p38 MAPK, and stress-activated protein kinase/Jun-N-terminal kinase (SAPK/JNK) pathway activation (6, 8, 26–30) was examined. GIP- and GLP-1-stimulated acetylation of histone H3 at Lys-9 and Lys-18, but not Lys-23, was greatly reduced or ablated by inhibitors of PKA (H-89; 10 μm), MAPK kinase (MEK) 1/2 (PD098059; 75 μm), and p38 MAPK (SB203580; 10 μm), whereas the SAPK/JNK inhibitor, SP600125 (50 μm), was without effect. Phosphorylation of histone H3 at Ser-10 in response to GIP and GLP-1, was greatly reduced by all four inhibitors (Fig. 3, A–D). The observation that PKA, p42/44 MAPK, p38 MAPK, and SAPK/JNK protein kinases are involved in incretin-mediated histone H3 modification led us to examine whether they are also involved in incretin-mediated activation/inactivation of H3AT and HDAC in the nucleus. As shown in Fig. 4 (A–D), inhibition of PKA, MEK1/2, and p38 MAPK reversed the inhibitory and stimulatory effects of GIP and GLP-1 on HDAC and H3AT activities, respectively; SAPK/JNK inhibition was without effect on the modulation of either enzyme. Multiple signaling modules are therefore involved in incretin-mediated activation/inactivation of H3AT/HDAC, resulting in the post-translational modification of histone H3 core proteins.
FIGURE 3.
PKA, p42/44 MAPK, p38 MAPK, and SAPK/JNK are involved in incretin-mediated histone H3 modification. A, effect of inhibiting PKA, p42/44 MAPK, p38 MAPK, and SAPK/JNK on GIP-mediated histone H3 modifications. INS-1 (832/13) cells were serum-starved in 3 mm glucose RPMI containing 0.1% BSA overnight and stimulated for 24 h with 100 nm GIP in the presence or absence of inhibitors of PKA, p42/44 MAPK, p38 MAPK, and SAPK/JNK. H-89 (10 μm), PD98059 (75 μm), SB203580 (10 μm), and SP600125 (50 μm) were added to cells during 1-h preincubation as well as during GIP or GLP-1 stimulation. Nuclear extracts were isolated, and Western blot analyses were performed using antibodies against acetyl-histone H3 Lys-9, acetyl-histone H3 Lys-18, acetyl-histone H3 Lys-23, phospho-histone H3 Ser-10, and histone H3. B, densitometric analysis of A. C, effect of inhibiting PKA, p42/44 MAPK, p38 MAPK, and SAPK/JNK on GLP-1-mediated histone H3 modifications. INS-1 (832/13) cells were treated as described above with 100 nm GLP-1 in the presence or absence of multiple inhibitors. Nuclear extracts were isolated, and Western blot analyses were performed using antibodies against acetyl-histone H3 Lys-9, acetyl-histone H3 Lys-18), acetyl-histone H3 Lys-23, phospho-histone H3 Ser-10, and histone H3. D, densitometric analysis of C. Western blots are representative of n = 3, and significance was tested using ANOVA with Newman-Keuls post hoc test; **, p < 0.01 versus GIP or GLP-1 control.
FIGURE 4.
PKA, p42/44 MAPK, p38 MAPK, and SAPK/JNK are involved in incretin-mediated activation/inactivation of H3AT/HDAC. A and B, effect of inhibiting PKA, p42/44 MAPK, p38 MAPK, and SAPK/JNK on GIP (A) and GLP-1 (B)-mediated HDAC activity. INS-1 (832/13) cells were serum-starved in 3 mm glucose RPMI containing 0.1% BSA overnight and stimulated for 24 h with 100 nm GIP or GLP-1 in the presence or absence of inhibitors of PKA, p42/44 MAPK, p38 MAPK, and SAPK/JNK. H-89 (10 μm), PD98059 (75 μm), SB203580 (10 μm), and SP600125 (50 μm) were added to cells during 1-h preincubation as well as during GIP or GLP-1 stimulation. Nuclear extracts were isolated and HDAC activity was determined as described under “Experimental Procedures.” C and D, effect of inhibiting PKA, p42/44 MAPK, p38 MAPK, and SAPK/JNK on GIP (C)- and GLP-1 (D)-mediated histone H3 activity. INS-1 (832/13) cells were treated as described above, nuclear extracts were isolated, and H3AT activity was determined as described under “Experimental Procedures.” Western blots are representative of n = 3, and all data represent three independent experiments, each carried out in duplicate. Significance was tested using ANOVA with Newman-Keuls post hoc test; **, p < 0.05 versus control.
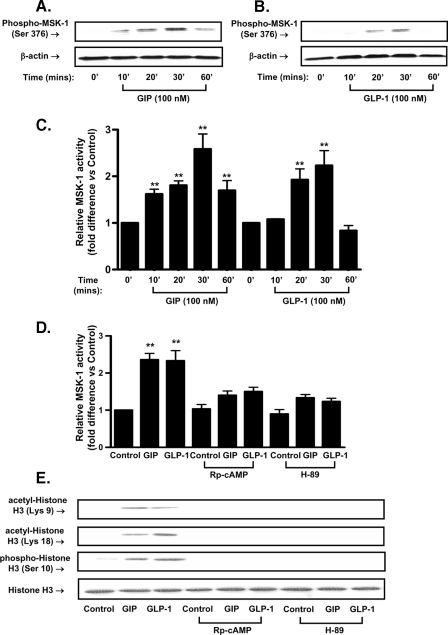
Potential Involvement of MSK-1 in Incretin-mediated Histone H3 Modification in Pancreatic β-Cells—The serine/threonine protein kinase MSK-1 has been shown to be activated by PKA, P42/44 MAPK, p38MAPK, or SAPK2 in responses to stress or mitogenic extracellular stimuli (31, 32). Its potential involvement in incretin-mediated histone H3 modification in pancreatic β-cells was therefore studied. INS-1 (832/13) β-cells were treated with GIP or GLP-1 (100 nm), and Western blot analyses were performed using antibodies against phospho-MSK-1 (Ser-376). As shown in Fig. 5A, GIP stimulated phosphorylation of MSK-1 at Ser-376, with phosphorylation apparent 10 min after initiation of GIP treatment, and thereafter sustained until the end of 1-h incubation. Slightly weaker GLP-1 stimulated phosphorylation of Ser376 MSK-1 was observed, with phosphorylation apparent 20 min after initiation of GLP-1 treatment, and thereafter sustained to 30-min incubation (Fig. 5B). The phosphorylation of Ser-376 in MSK-1 correlated well with MSK-1 enzymatic activity: Treatment of INS-1 cells with GIP or GLP-1 for 30 min resulted in 2.5- and 2.2-fold increases in MSK-1 activity, respectively (Fig. 5C). MSK-1 Ser-376 is a PKA consensus site, and the inhibitor H-89 was initially employed to examine the potential involvement of the kinase in incretin stimulation of MSK-1 activity in INS-1 (832/13) β-cells. As shown in Fig. 5D, MSK-1 activity in response to GIP or GLP-1 was greatly reduced by H-89. Because H-89 has also been reported to act as a direct inhibitor of MSK-1, Rp-cAMPs, a specific competitive inhibitor of cAMP binding to the regulatory subunit of PKA, was additionally employed. Rp-cAMPs also inhibited GIP- or GLP-1-induced stimulation of MSK-1 activity (Fig. 5D), and both inhibitors ablated incretin-stimulated acetylation of histone H3 at Lys-9 and Lys-18, and phosphorylation of histone H3 at Ser-10 (Fig. 5E). Taken together, these results strongly suggest that both PKA and MSK-1 are involved in incretin-mediated histone H3 modification.
FIGURE 5.
MSK-1 is involved in incretin-mediated histone H3 modification. A and B, time course of incretin-mediated MSK-1 phosphorylation. INS-1 cells were serum-starved in 3 mm glucose RPMI containing 0.1% BSA overnight and treated for the indicated periods of time with 100 nm GIP (A) or GLP-1 (B). Total cellular extracts were isolated, and Western blot analyses were performed using antibodies against phospho-MSK-1 and β-actin. C, time course of incretin-mediated MSK-1 activity. INS-1 cells were treated as described above, and MSK-1 activity was determined as described under “Experimental Procedures.” D, effect of H-89 or Rp-cAMPs on incretin-stimulated MSK-1 activity. INS-1 cells were treated as described above, stimulated for 30 min with GIP or GLP-1 (100 nm) in the presence or absence of H-89 (10 μm), and MSK-1 activity was determined as described under “Experimental Procedures.” E, effects of H-89 or Rp-cAMPs on incretin-stimulated histone H3 modification. INS-1 cells were treated as described above and stimulated with 100 nm GIP or GLP-1 in the presence or absence of H-89 (10 μm) or Rp-cAMPs (200 μm). Western blot analyses were performed using antibodies against acetyl-histone H3 (Lys-9), acetyl-histone H3 (Lys-18), phospho-histone H3 (Ser-10), and histone H3. Significance was tested using ANOVA with Newman-Keuls post hoc test; **, p < 0.05 versus control.
Direct Protein-Protein Interactions between Acetylated/Phosphorylated Histone H3 and Phospho-CREB or TORC2 in the Nucleus—The functional implications of incretin-mediated post-translational modification of histone H3 were next determined. It was previously demonstrated that GIP increases protein-protein interactions between phospho-CREB and TORC2 (8). In the current study, INS-1 (832/13) β-cells were treated with GIP or GLP-1 (100 nm) for 24 h, and nuclear extracts were immunoprecipitated with antibodies against histone H3 acetylated at Lys-9 or Lys-18, or phosphorylated at Ser-10, followed by immunoblotting for CREB or TORC2. Both GIP and GLP-1 increased direct protein-protein interactions between phospho-CREB (Ser-133) and TORC2 with histone H3 acetylated at Lys-9 or Lys-18, or phosphorylated at Ser-10 (Fig. 6, A–F). To define functional consequences of incretin-mediated histone H3 acetylation on CREB/TORC2-responsive target gene expression, incretin-mediated Bcl-2 gene transcription was measured in the presence or absence of HAT inhibitor. Treatment of INS-1 cells with GIP or GLP-1 (100 nm) for 24 h resulted in 3- to 3.5-fold increases in Bcl-2 mRNA transcript levels, compared with controls, and incretin-mediated Bcl-2 gene transcription was greatly reduced by HAT inhibitor (Fig. 6G). Incretin-mediated histone H3 modification is therefore likely to be an important regulator of CREB-related transcription in pancreatic β-cells.
FIGURE 6.
Incretins increase protein-protein interaction between acetyl-histone H3 and phospho-CREB or TORC2 in the nucleus. A–C, co-immunoprecipitation between acetylated histone H3 and phospho-CREB. INS-1 (832/13) cells were serum-starved in 3 mm glucose RPMI containing 0.1% BSA overnight and stimulated with 100 nm GIP or GLP-1. Nuclear extracts were isolated from each sample and immunoprecipitated (IP) with antibodies against acetyl-histone H3 at Lys-9 (A), Lys-18 (B), or phospho-histone H3 at Ser-10 (C) followed by immunoblotting (IB) for phospho-CREB (Ser-133). D–F, co-immunoprecipitation between acetylated Histone H3 and TORC2. INS-1 (832/13) cells were treated as described above. Nuclear extracts were isolated and IP with acetyl-histone H3 at Lys-9 (D), Lys-18 (E), or phospho-histone H3 at Ser-10 (F) followed by IB for TORC2. Input represents one-tenth of total nuclear extract used in the co-immunoprecipitation assay. G, effect of histone H3 modulation on incretin-mediated Bcl-2 gene transcription. INS-1 (832/13) cells were serum-starved in 3 mm glucose RPMI containing 0.1% BSA overnight, and GIP or GLP-1 (100 nm) was added in the presence or absence of HAT inhibitor II (40 μm) for 24 h. Total RNA was isolated from each sample, and real-time reverse transcription-PCR was performed to quantify Bcl-2 mRNA expression levels; shown as the -fold difference versus control normalized to 18 S rRNA levels. Significance was tested using ANOVA with a Newman-Keuls post hoc test; **, p < 0.05 versus control; ##, p < 0.05 versus GIP; and §§, p < 0.05 versus GLP-1.
DISCUSSION
Modulation of chromatin structure plays an important role in the regulation of gene transcription in eukaryotes. Diverse post-translational modifications of core histone nuclear proteins have been found to be associated with both the active and inactive transcriptional status of target genes. These modifications, including acetylation, phosphorylation, and methylation, are thought to result from promoter binding of transcriptional co-activator or co-repressor complexes possessing enzyme-modifying activity (33–34). Such complexes do not generally have direct DNA-binding activity, but are recruited to promoters by interaction with other sequence-specific transcription factors. The resulting modifications of the core histone proteins, in turn, modulate the access of further regulatory complexes to the promoter. This complicated interplay among regulatory complexes, promoter, and core histone proteins would be missed when transcriptional activation and transcription factor binding to target promoters are studied in vitro using naked DNA templates. In the present study, we examined the effects of the incretin hormones on post-translational modifications of histone proteins. Both GIP and GLP-1 decreased HDAC activity and increased H3AT activity in the nucleus (Fig. 2, C and D), resulting in increased acetylation of histone H3 at Lys-9 and Lys-18 (Fig. 1, A and D). Effects on the deacetylase activity were small compared with those on acetylation. The deacetylase HDAC1 has been shown to form HDAC1/protein phosphatase 1 complexes that are recruited by phospho-CREB, resulting in histone deacetylation and CREB dephosphorylation (35). It is therefore likely that the small changes in activity observed were due to selective effects on HDAC1. It is not known whether the reduction in activity resulted from phosphorylation, dephosphorylation, or some other modification of the enzyme. Several of the histone deacetylase enzymes have been shown to undergo post-translational phosphorylation but, in the case of HDAC1 for example, phosphorylation does not appear to have a major effect on enzyme activity (36–37). Neither GIP nor GLP-1 was found to impact on protein levels of a number of HDAC enzymes (Fig. 2, A and B); H3AT proteins were not studied due to the large number of potential enzymes with histone acetylase activity.
Although treatment of INS-1 (832/13) β-cells with both GIP and GLP-1 (100 nm) for 24 h resulted in a substantial increase in histone H3 acetylation at Lys-18, only GLP-1, and not GIP, increased histone H3 acetylation at Lys-18 at 4 h treatment (Fig. 1A). Although the actions of GIP on pancreatic β-cells have been reported to be analogous to those of GLP-1, these results suggest the presence of complicated and selective mechanisms for the regulation of incretin-mediated histone H3 modification and target gene expression. Several kinases have been proposed to be responsible for phosphorylation of histone 3, including RSK2, a member of the p90rsk (ribosomal S6 kinase (RSK)) family (38), MSK-1, and MSK-2 (39). There now appears to be a consensus that MSK-1 and MSK-2 are the major kinases phosphorylating Ser-10 (40, 41). Both p38 MAPK and MEK/ERK pathways have been shown to activate MSK-1 in different cell types, and the current inhibitor studies provided support for the involvement of MEK1/2 and p38 MAPK in incretin-mediated phosphorylation of Ser-10 (Fig. 3, A–D) in INS-1 (832/13) β-cells. Incretin-stimulated histone H3 acetylation at Lys-9 and Lys-18, as well as the modulation of H3AT and HDAC activity (Fig. 4, A–D), were also blocked by PKA, MEK1/2, and p38 MAPK inhibition. There are a number of ways by which these signaling modules may regulate H3AT and HDAC activity. One possibility is that H3AT and HDAC activities are modulated by direct phosphorylation of the enzymes. There is growing evidence that the acetyltransferase activity of p300 is increased through phosphorylation pathways involving p38 MAPK (42), protein kinase B (43), and ERK2 (44), and it is possible that MSK-1 is the final mediator (41). Both GIP and GLP-1 increased MSK-1 activity (Fig. 5C), and H-89 ablated their ability to stimulate MSK-1 activity and histone H3 modification (Fig. 5E), in agreement with their ability to stimulate PKA activity. However, because H89 is also an inhibitor of MSK-1, its use did not allow discrimination between PKA and MSK-1 as the distal kinase. Application of Rp-cAMPs, a competitive inhibitor of cAMP binding to the regulatory subunit of PKA, also blocked incretin-mediated stimulation of MSK-1 activity, as well as histone H3 acetylation and phosphorylation in response to GIP or GLP-1. We therefore propose a model in which GIP and GLP-1 activate MSK-1 via PKA, with MSK-1 as the final mediator responsible for histone H3 modification (Fig. 7).
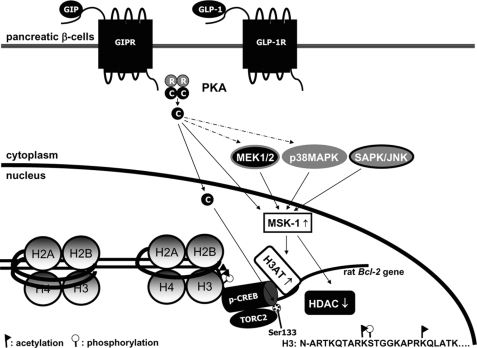
FIGURE 7.
Proposed pathways by which incretins regulateβ-cell chromatin structure and gene transcription. GIP and GLP-1 binding to the GIP receptor (GIPR) or GLP-1 receptor (GLP-1R), respectively, activate PKA, MEK1/2, and p38 MAPK signaling modules, resulting in increased MSK-1 activity, increased H3AT, and decreased HDAC activity. It is not clear as to the pathways involved in activation of the MEK1/2 and p38 MAPK signaling modules (broken arrows), because PKA is capable of performing this function in some cell types. Active PKA catalytic subunits (C), released following cAMP binding to the PKA regulatory subunits, enter the nucleus and directly phosphorylate CREB. Incretin-mediated activation/inactivation of H3AT/HDAC leads to the post-translational modification of histone H3 core protein, allowing increased accessibility and recruitment of phospho-CREB and TORC2 to target DNA.
The events involved in linking upstream stimulation of PKA and MSK-1 activity to the processes involved in histone modification, and their timing, are currently unknown. The changes in H3AT/HDAC activity, and increased histone H3 acetylation, may also be partially secondary to increased phosphorylation of the transcription machinery. The transcription coactivator paralogs, CREB-binding protein (CBP)/p300, possess HAT activity, and they have been shown to specifically bind to CREB phosphorylated at Ser-133. This phosphorylation-dependent interaction with CBP/p300 is critical for stimulus-induced activation of CREB (46–48), and, in turn, CBP/p300 increase histone acetylation, during the burst phase of gene transcription (49). On the other hand, HDAC1 has been shown to promote Ser-133 dephosphorylation via a stable interaction with protein phosphatase 1 (35). In the present study, GIP and GLP-1 were found to increase direct nuclear protein-protein interactions between phospho-CREB (Ser-133) (Fig. 6, A–C) or TORC2 (Fig. 6, D–F) and histone H3 acetylated at Lys-9 and Lys-18 or phosphorylated at Ser-10, suggesting that incretin-mediated histone H3 modification contributes significantly to the recruitment of transcription-related proteins.
Although the level of histone acetylation is of clear importance for the regulation of gene expression, changes in the relative balance between acetylation and deacetylation have also been shown to significantly impact on pancreatic β-cell death. Inhibition of HDACs was demonstrated to prevent cytokine-induced β-cell apoptosis and impaired β-cell function through a down-regulation of NFκB trans-activating activity (45). Previously we showed that GIP up-regulates expression of the anti-apoptotic protein Bcl-2 in β-cells (8), and administration of an HAT inhibitor has now been shown to reduce GIP or GLP-1 stimulation of Bcl-2 gene expression, indicating involvement of histone modification. Because the present studies also indicate that incretin-stimulated pathways ultimately result in decreased HDAC activity and increased H3AT activity, as well as increased interaction between acetyl-histone H3 and phospho-CREB/TORC2 (Fig. 4), HDAC inhibitors may potentiate their protective effect on pancreatic β-cells. Finally, in view of the increasing interest in long acting incretins and DPP-IV inhibitors for the treatment of diabetes, it will be important to establish how the signaling cascades, chromatin-modifying enzymes, and transcriptional machinery act coordinately to translate incretin-mediated effects on target gene expression in pancreatic β-cells.
Acknowledgments
We thank Dr. C. B. Newgard (Duke University Medical Center, Durham, NC) for kindly providing us with INS-1 β-cells (clone 832/13).
This work was supported by the Canadian Institutes of Health Research (to C. H. S. M.).
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
Footnotes
The abbreviations used are: GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide-1; HAT, histone acetyltransferase; HDAC, histone deacetylase; H3AT, histone H3 acetyltransferase; PKA, protein kinase A; MSK-1, mitogen- and stress-activated kinase-1; CREB, cAMP-response element-binding protein; phospho-CREB, phosphorylated cAMP-response element-binding protein; CBP, CREB-binding protein; ANOVA, analysis of variance; SAPK, stress-activated protein kinase; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; ERK, extracellular signal-regulated kinase; MEK, MAPK/ERK kinase; BSA, bovine serum albumin; TORC2, cAMP-responsive CREB coactivator 2.
References
- 1.Brubaker, P. L., and Drucker, D. J. (2004) Endocrinology 145 2653-2659 [DOI] [PubMed] [Google Scholar]
- 2.Drucker, D. J. (2007) J. Clin. Invest. 117 24-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yusta, B., Baggio, L. L., Estall, J. L., Koehler, J. A., Holland, D. P., Li, H., Pipeleers, D., Ling, Z., and Drucker, D. J. (2006) Cell Metab. 4 391-406 [DOI] [PubMed] [Google Scholar]
- 4.Baggio, L. L., and Drucker, D. J. (2007) Gastroenterology 132 2131-2157 [DOI] [PubMed] [Google Scholar]
- 5.Kim, S. J., Choi, W. S., Han, J. S., Warnock, G., Fedida, D., and McIntosh, C. H. S. (2005) J. Biol. Chem. 280 28692-28700 [DOI] [PubMed] [Google Scholar]
- 6.Ehses, J. A., Casilla, V. R., Doty, T., Pospisilik, J. A., Winter, K. D., Demuth, H. U., Pederson, R. A., and McIntosh, C. H. S. (2003) Endocrinology 144 4433-4445 [DOI] [PubMed] [Google Scholar]
- 7.Kim, S. J., Winter, K., Nian, C., Tsuneoka, M., Koda, Y., and McIntosh, C. H. S. (2005) J. Biol. Chem. 280 22297-22307 [DOI] [PubMed] [Google Scholar]
- 8.Kim, S. J., Nian, C., Widenmaier, S., and McIntosh, C. H. S. (2008) Mol. Cell Biol. 28 1644-1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Workman, J. L., and Kingston, R. E. (1998) Annu. Rev. Biochem. 67 545-579 [DOI] [PubMed] [Google Scholar]
- 10.Strahl, B. D., and Allis, C. D. (2000) Nature 403 41-45 [DOI] [PubMed] [Google Scholar]
- 11.Berger, S. L. (2002) Curr. Opin. Genet. Dev. 2 142-148 [DOI] [PubMed] [Google Scholar]
- 12.Bernstein, B. E., and Schreiber, S. L. (2002) Chem. Biol. 9 1167-1173 [DOI] [PubMed] [Google Scholar]
- 13.Marmorstein, R. (2001) Cell Mol. Life Sci. 58 693-703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregory, P. D., Wagner, K., and Hörz, W. (2001) Exp. Cell Res. 265 195-202 [DOI] [PubMed] [Google Scholar]
- 15.Cress, W. D., and Seto, E. (2000) J. Cell Physiol. 184 1-16 [DOI] [PubMed] [Google Scholar]
- 16.Cheung, P., Allis, C. D., and Sassone-Corsi, P. (2000) Cell 103 263-271 [DOI] [PubMed] [Google Scholar]
- 17.Thorne, A. W., Kmiciek, D., Mitchelson, K., Sautiere, P., and Crane-Robinson, C. (1990) Eur. J. Biochem. 193 701-713 [DOI] [PubMed] [Google Scholar]
- 18.Hansen, J. C., Tse, C., and Wolffe, A. P. (1998) Biochemistry 37 17637-17641 [DOI] [PubMed] [Google Scholar]
- 19.Hendzel, M. J., Wei, Y., Mancini, M. A., Van Hooser, A., Ranalli, T., Brinkley, B. R., Bazett-Jones, D. P., and Allis, C. D. (1997) Chromosoma 106 348-360 [DOI] [PubMed] [Google Scholar]
- 20.Goto, H., Tomono, Y., Ajiro, K., Kosako, H., Fujita, M., Sakurai, M., Okawa, K., Iwamatsu, A., Okigaki, T., Takahashi, T., and Inagaki, M. (1999) J. Biol. Chem. 274 25543-25549 [DOI] [PubMed] [Google Scholar]
- 21.Preuss, U., Landsberg, G., and Scheidtmann, K. H. (2003) Nucleic Acids Res. 31 878-885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacDonald, P. E., Ha, X. F., Wang, J., Smukler, S. R., Sun, A. M., Gaisano, H. Y., Salapatek, A. M., Backx, P. H., and Wheeler, M. B. (2001) Mol. Endocrinol. 15 1423-1435 [DOI] [PubMed] [Google Scholar]
- 23.Schreiber, E., Matthias, P., Muller, M. M., and Schaffner, W. (1989) Nucleic Acids Res. 17 6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gray, S. G., and Ekström, T. J. (2001) Exp. Cell Res. 262 75-83 [DOI] [PubMed] [Google Scholar]
- 25.Thiagalingam, S., Cheng, K. H., Lee, H. J., Mineva, N., Thiagalingam, A., and Ponte, J. F. (2003) Ann. N. Y. Acad. Sci. 983 84-100 [DOI] [PubMed] [Google Scholar]
- 26.Ehses, J. A., Pelech, S. L., Pederson, R. A., and McIntosh, C. H. S. (2002) J. Biol. Chem. 277 37088-37097 [DOI] [PubMed] [Google Scholar]
- 27.Klinger, S., Poussin, C., Debril, M. B., Dolci, W., Halban, P. A., and Thorens, B. (2008) Diabetes 57 584-593 [DOI] [PubMed] [Google Scholar]
- 28.Hui, H., Nourparvar, A., Zhao, X., and Perfetti, R. (2003) Endocrinology 144 1444-1455 [DOI] [PubMed] [Google Scholar]
- 29.Kemp, D. M., and Habener, J. F. (2001) Endocrinology 142 1179-1187 [DOI] [PubMed] [Google Scholar]
- 30.Ferdaoussi, M., Abdelli, S., Yang, J. Y., Cornu, M., Niederhauser, G., Favre, D., Widmann, C., Regazzi, R., Thorens, B., Waeber, G., and Abderrahmani, A. (2008) Diabetes 57 1205-1215 [DOI] [PubMed] [Google Scholar]
- 31.Deak, M., Clifton, A. D., Lucocq, L. M., and Alessi, D. R. (1998) EMBO J. 17 4426-4441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delghandi, M. P., Johannessen, M., and Moens, U. (2005) Cell. Signal. 17 1343-1351 [DOI] [PubMed] [Google Scholar]
- 33.Kouzarides, T. (2007) Cell 128 693-705 [DOI] [PubMed] [Google Scholar]
- 34.Berger, S. L. (2007) Nature 447 407-412 [DOI] [PubMed] [Google Scholar]
- 35.Canettieri, G., Morantte, I., Guzmán, E., Asahara, H., Herzig, S., Anderson, S. D., Yates, JR 3rd., and Montminy, M. (2003) Nat. Struct. Biol. 10 175-181 [DOI] [PubMed] [Google Scholar]
- 36.Cai, R., Kwon, P., Yan, Y.-N., Sambuccetti, L., Fischer, D., and Cohen, D. (2001) Biochem. Biophys. Res. Commun. 283 445-453 [DOI] [PubMed] [Google Scholar]
- 37.Karnwowska-Desaulniers, P., Ketko, A., Kamath, N., and Pflum, K. H. (2007) Biochem. Biophys. Res. Commun. 361 349-355 [DOI] [PubMed] [Google Scholar]
- 38.Merienne, K., Pannetier, S., Harel-Bellan, A., and Sassone-Corsi, P. (2001) Mol. Cell Biol. 21 7089-7096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soloaga, A., Thomson, S., Wiggin, G. R., Rampersaud, N., Dyson, M. H., Hazzalin, C. A., Mahadevan, L. C., and Arthur, J. S. C. (2003) EMBO J. 22 2788-2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davie, J. R. (2003) Sci. STKE 2003, PE33. [DOI] [PubMed]
- 41.Chwang, W. B., Arthur, J. S., Schumacher, A., and Sweatt, J. D. (2007. J. Neurosci. 27 12732-12742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saha, N. H., Jana, M., and Pahan, K. (2007) J. Immunol. 179 7101-7109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang, W. C., and Chen, C. C. (2005) Mol. Cell Biol. 25 6592-6602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen, Y. J., Wang, V. N., and Chang, E. C. (2007) J. Biol. Chem. 282 27215-27228 [DOI] [PubMed] [Google Scholar]
- 45.Larsen, L., Tonnesen, M., Ronn, S. G., Størling, J., Jørgensen, S., Mascagni, P., Dinarello, C. A., Billestrup, N., and Mandrup-Poulsen, T. (2007) Diabetologia 50 779-789 [DOI] [PubMed] [Google Scholar]
- 46.Chrivia, J. C., Kwok, R. P., Lamb, N., Hagiwara, M., Montminy, M. R., and Goodman, R. H. (1993) Nature 365 855-859 [DOI] [PubMed] [Google Scholar]
- 47.De Cesare, D., Fimia, G. M., and Sassone-Corsi, P. (1999) Trends Biochem. Sci. 24 281-285 [DOI] [PubMed] [Google Scholar]
- 48.Shaywitz, A. J., and Greenberg, M. E. (1999) Annu. Rev. Biochem. 68 821-861 [DOI] [PubMed] [Google Scholar]
- 49.Michael, L. F., Asahara, H., Shulman, A. I., Kraus, W. L., and Montminy, M. (2000) Mol. Cell Biol. 20 1596-1603 [DOI] [PMC free article] [PubMed] [Google Scholar]