Abstract
Membrane-associated guanylate kinases (MAGUKs) regulate the formation and function of molecular assemblies at specialized regions of the membrane. Allosteric regulation of an intramolecular interaction between the Src homology 3 (SH3) and guanylate kinase (GK) domains of MAGUKs is thought to play a central role in regulating MAGUK function. Here we show that a mutant of the Drosophila MAGUK Discs large (Dlg), dlgsw, encodes a form of Dlg that disrupts the intramolecular association while leaving the SH3 and GK domains intact, providing an excellent model system to assess the role of the SH3-GK intramolecular interaction in MAGUK function. Analysis of asymmetric cell division of maternal-zygotic dlgsw embryonic neuroblasts demonstrates that the intramolecular interaction is not required for Dlg localization but is necessary for cell fate determinant segregation to the basal cortex and mitotic spindle alignment with the cortical polarity axis. These defects ultimately result in improper patterning of the embryonic central nervous system. Furthermore, we demonstrate that the sw mutation of Dlg results in unregulated complex assembly as assessed by GukHolder association with the SH3-GK versus PDZ-SH3-GK modules of Dlgsw. From these studies, we conclude that allosteric regulation of the SH3-GK intramolecular interaction is required for regulation of MAGUK function in asymmetric cell division, possibly through regulation of complex assembly.
The membrane-associated guanylate kinase (MAGUK)2 superfamily consists of ubiquitous scaffolding proteins that are composed of a common core of contiguously linked modular domains (protein-protein interaction domains, PDZ and SH3 domains, and a domain with homology to the yeast guanylate kinase, GK domain). MAGUKs are concentrated at sites of cell-cell contact (1) and organize a variety of cell adhesion molecules, cytoskeletal proteins, receptors, ion channels, and associated signaling molecules at specialized regions of the membrane (2). Protein complex organization by MAGUKs has been thought to occur, at least in part, through allosteric regulation that arises from an intramolecular interaction between the SH3 and GK domains. This interaction has been shown to regulate binding of numerous MAGUK ligands in vitro (3–5). However, whereas the SH3-GK interaction has been extensively characterized biochemically (6–8), very little data exists regarding its physiological relevance. Here we investigate the role of the SH3-GK intramolecular interaction of Discs large (Dlg), a prototype of the MAGUK superfamily.
Loss of dlg activity results in overgrowth of imaginal discs and tumor formation (9). Dlg localizes to septate and neuromuscular junctions and is essential for establishing and maintaining apicobasal polarity of Drosophila epithelia (10). Dlg also plays an important role in regulating the process of asymmetric cell division (ACD) (11–13). ACD is a mechanism for generating cellular diversity via unequal mitotic divisions of progenitor cells. For instance, in wild-type Drosophila neuroblasts, cell signaling networks interact to allow for asymmetric segregation of basal cell fate determinants, followed by alignment of the mitotic spindle along the apical-basal cortical polarity axis (see Refs. 14 and 15) for review). ACD results in the formation of a self-renewing, stem-cell like neuroblast and a smaller ganglion mother cell, which has neuronal or glial fate. In dlg germline clone (dlgGLC) embryonic metaphase neuroblasts, ganglion mother cell fate determinants are not restricted to the basal cortex (11, 12) and the mitotic spindle does not reliably align with the apical-basal cortical polarity axis (13). Defects in neurogenesis have also been observed for dlgGLC embryos (16), as well as embryos treated with RNA interference against an alternatively spliced isoform of Dlg (17), providing evidence for the function of Dlg in neuronal differentiation and axon guidance. Such defects in neurogenesis are thought to be attributed to defective localization of basal cell fate determinants during ACD (17). Although Dlg function is important in a broad range of dynamic cellular processes, the role of the Dlg SH3-GK intramolecular interaction in Dlg activity is poorly understood.
One potential role for the SH3-GK intramolecular association is to regulate MAGUK complex assembly. In vitro binding assays demonstrated that mutations disrupting this intramolecular interaction allowed mutant SH3-GK modules to associate with SH3 or GK domains of various MAGUK proteins in trans, providing a mechanism of regulating oligomerization of MAGUKs in vivo (6, 8). A role in clustering of ion channels was also observed as mutations that disrupted the intramolecular association, whereas having no effect on association with the potassium channel KV1.4 or homo-oligomerization of PSD-95, resulted in loss of channel clustering in vivo (18). Furthermore, multiple in vitro studies support its regulation of binding of protein ligands with the GK domain of MAGUKs: examples include interaction of GK domains of Dlg with GukHolder (GukH) (3), SAP97 with guanylate kinase-associated protein (4), and PSD-93 with the microtubule-associated protein 1A (5). These studies suggest that allosteric modulation of the SH3-GK intramolecular interaction is important for regulation of complex assembly, yet little evidence exists that such regulation of MAGUKs is required for their function in vivo.
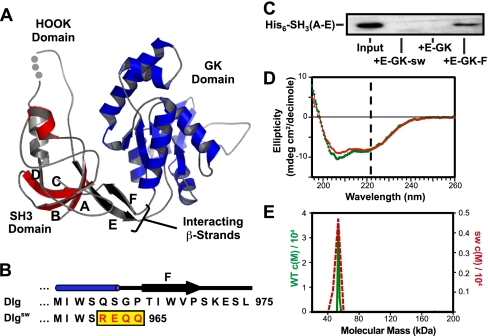
In crystal structures of the SH3-GK module of PSD-95, two β-strands that emerge from the SH3 and GK domains appear to mediate the interaction between the domains (Fig. 1A) (19, 20). Functional studies of the interaction of these β-strands demonstrate that a COOH-terminal truncation of the strand following the GK domain abrogates the intramolecular association of the SH3-GK module. This 13-amino acid truncation of PSD-95 is comparable with a dlg allele that had previously been identified in a genetic screen, dlgsw (21). Drosophila containing only the sw form of Dlg (refer to COOH-terminal sequences in Fig. 1B) die in the latter stages of embryonic development due to failure of dorsal closure and terminal defects (21). We hypothesized that this mutation disrupts the intramolecular interaction while leaving the SH3 and GK domains largely intact, making the dlgsw allele an excellent model system for assessing the role of this interaction in MAGUK function.
FIGURE 1.
The dlgsw allele disrupts the SH3-GK intramolecular interaction formed by interacting E and Fβ-strands. A, a ribbon diagram of the SH3-GK module of PSD-95 (Protein Data Bank 1KJW (19)) created using MOLSCRIPT (58) and RASTER3D (59), highlighting the SH3 (red), HOOK (gray), and GK (blue) domains. The intramolecular interaction between the SH3 and GK domains is mediated by the interaction of two β-strands (shown in black), one strand is contributed by the linker region following the SH3 domain (strand E), whereas the second strand is contributed by a COOH-terminal strand that follows the GK domain (strand F). B, sequence comparison of the COOH-terminal regions of WT Dlg versus Dlgsw. C, GST pull-downs of the Dlg SH3 domain using GST fusions of WT and mutant Dlg GK domains. Association of protein components was detected by an anti-His antibody that recognized an NH2-terminal on the SH3 domain of Dlg. D, circular dichroism wavelength scans, monitoring the change in ellipticity as a function of wavelength. Changes in α-helical content are indicated by the change in ellipticity at 222 nm (see black dashed line). Comparison of CD spectra of WT PDZ-SH3-GK (green circles) and PDZ-SH3-GK(sw) (red squares) indicate no significant change in α helical character. E, Sedfit analysis of sedimentation velocity scans of WT PDZ-SH3-GK (green solid line) and PDZ-SH3-GKsw (red dashed line). Note that the estimated molecular weights provided are based upon the assumption that the protein exists in a globular conformation.
In the studies presented here, we utilized the dlgsw mutant allele to explore the role of the intramolecular interaction in regulating complex assembly and furthermore, to assess the in vivo, physiological significance of this interaction for Dlg function in neuroblast asymmetric cell division. We find that disruption of the SH3-GK intramolecular interaction results in unregulated complex assembly. Such a loss in regulated complex assembly results in loss of Dlg function in the process of asymmetric cell division as assessed by localization of cell fate determinants in a dividing neuroblast and alignment of the mitotic spindle along the apical-basal cortical polarity axis, ultimately resulting in defects in neurogenesis. Thus, these data contribute to our understanding of how the SH3-GK intramolecular interaction regulates MAGUK function in formation and regulation of membrane specializations.
EXPERIMENTAL PROCEDURES
Cloning, Overexpression, and Purification—DNA encoding the G isoform of wild-type (WT) and the sw mutant of Dlg were cloned from cDNA libraries that were created by reverse transcription of mRNAs extracted from yw and dlgsw Drosophila melanogaster, respectively. DNA encoding full-length Cript was cloned from a cDNA library created by reverse transcription of mRNAs extracted from 3T1 Mus musculus cells. For bacterial expression, WT Dlg PDZ-SH3-GK (amino acids 474–975), SH3-GK-(598–975), SH3-(581–681), E-GK-F-(771–975), E-GK-(771–962), and corresponding sw mutant fragments were subcloned and ligated into the pET-19b derivative pBH and/or pGEX vectors. Recombinant His-tagged fusions of Dlg proteins were purified using nickel-nitrilotriacetic acid (Qiagen) and Q-Sepharose anion exchange (Sigma) chromatography. In some instances, these proteins required further purification using HiLoad 16/60 Superdex 200 (GE Healthcare) chromatography. For bacterial expression, WT Cript was subcloned and ligated into the pET-19b derivative pBH containing an N-terminal green fluorescent protein fusion. Recombinant His-tagged fusions of GFP-Cript required single step purification using nickel-nitrilotriacetic acid (Qiagen) chromatography. Purity of recombinantly expressed proteins was judged to be >90% using SDS-PAGE and high pressure liquid chromatography. Protein concentrations were determined by Bradford assays and UV absorbance in the presence of 0.1 n NaOH using the extinction coefficient for Trp (22).
For S2 cell expression of a hemagglutinin (HA)-tagged GukH fragment, the sequence coding for amino acids 800–1044 of the isoform C of GukH was subcloned and ligated into a pMT-/V5-HisA vector (Invitrogen). Drosophila embryo-derived Schneider cells (S2 cells) grown in Schneider's insect media supplemented with 10% fetal bovine serum were transiently transfected using 0.8 μg of pMT-HA-(800–1044) GukH DNA and Effectene reagent (Qiagen). Protein expression was induced after 24 h with 0.5 mm CuSO4 and cells were collected 48 h post-induction. Cells were pelleted by centrifugation at 1,000 × g for 5 min, washed twice in ice-cold phosphate-buffered saline (PBS), and incubated in lysis buffer (50 mm Tris, pH 8, 150 mm NaCl, 1% Nonidet P-40, and a complete protease inhibitor mixture from Roche; 200 μl/transfection) for 30 min on ice. Following incubation, transfected S2 cells were passaged through a 23-guage needle and cell debris was removed by centrifugation at 12,000 × g for 15 min.
In Vitro Binding Assays—For qualitative in trans GST pull-downs (Fig. 1C), Escherichia coli cell lysates containing the GST fusion protein of interest were incubated with glutathione-agarose beads and washed three times with binding buffer (100 mm HEPES, pH 7.5, 100 mm NaCl, 1 mm dithiothreitol, 0.5% Triton X-100). A His-tagged fusion of the SH3 domain of Dlg was added to a concentration of 25 μm and incubated with the beads at room temperature for 15 min. The reactions were then washed three times with binding buffer to remove unassociated proteins. Bound proteins were eluted from the glutathione-agarose beads by the addition of SDS loading buffer and were screened by Western blot analyses using a mouse monoclonal anti-His antibody (1:1000; Qiagen).
For qualitative GST pull-downs to assess HA-(800–1044) GukH association with Dlg in the absence and presence of GFP-Cript (Fig. 6C), E. coli cell lysates containing GST fusions of WT PDZ-SH3-GK or PDZ-SH3-GKsw were incubated with glutathione-agarose beads and then washed three times with PBS. Subsequently, 150 μl of S2 cell extract, containing HA-(800–1044) GukH, was added in the absence or presence of 2.5 or 5 μm GFP-Cript or a GFP-peptide fusion derived from the mushroom body defect protein (GFP-MUDp) and incubated at 4 °C for 1 h. The reactions were then washed three times with 10 mm HEPES, pH 7.5, 100 mm NaCl, 1 mm dithiothreitol, and 0.002% Triton X-100. Bound proteins were eluted using SDS loading buffer and screened by Western blot analysis using a mouse monoclonal anti-HA (1:1000; Covance) and Ponceau stain.
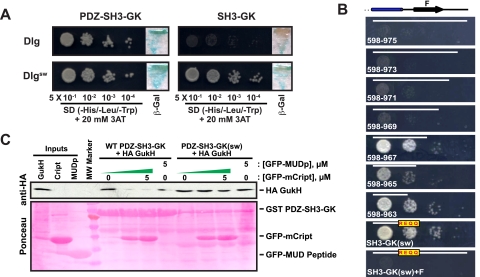
FIGURE 6.
Disruption of the SH3-GK intramolecular interaction results in unregulated complex assembly. Yeast two-hybrid results as assessed by expression of HIS3 and β-galactosidase yeast reporter constructs. For determination of HIS3 expression a serial dilution of yeast co-transformed with pGBK 749–1044 GukH and pGAD Dlg constructs were plated on SD (–His/–Leu/–Trp + 20 mm 3-amino-1,2,4-triazole (3AT)) media starting with an A600 of 0.5 and allowed to grow for 4 days at 30 °C. A, analysis of WT Dlg versus sw fragments, and B, COOH-terminal truncation of Dlg association with GukH as assessed by yeast two-hybrid analysis. COOH-terminal regions of SH3-GK Dlg constructs used are indicated by the white bars within each dilution series. C, GST pull-down of 800–1044 GukH using GST fusions of WT and mutant PDZ-SH3-GK Dlg modules in the absence or presence of increasing concentrations of a GFP-Cript or GFP-MUDp (negative control, which does not associate with Dlg). Association of protein components was detected by an anti-HA antibody that recognized an NH2-terminal epitope on the 800–1044 GukH construct and Ponceau stain.
Circular Dichroism Scans—Circular dichroism (CD) spectra were collected using a Jasco J-720 CD spectrophotometer equipped with a temperature controlled cell holder, using a 1-nm bandpass and scanning from 260 to 190 in 0.5-nm increments. Three scans in a 0.1-cm path length quartz cuvette were recorded and averaged. Spectra were collected in 10 mm NaPO4, pH 7.5, 100 mm NaCl at 22 °C. Spectra were corrected for contributions from buffer alone by subtraction (23).
Analytical Ultracentrifugation—Sedimentation velocity studies of WT PDZ-SH3-GK and PDZ-SH3-GKsw were conducted on a Beckman XL-I analytical ultracentrifuge at 50,000 × g at 20 °C. Proteins were equilibrated in 20 mm HEPES, pH 7.5, 100 mm NaCl, 1 mm Tris(2-carboxyethyl)phosphine. Sedimentation was continuously monitored for 5 h using an absorbance of 280 nm. Sedimentation velocity data were subsequently analyzed using Sedfit (24, 25).
Fly Stocks and Collection of Embryos—Flies of the genotype yw,dlgsw/Basc,y+ were kindly provided by Francois Schweisguth (Ecole Normale Supêrieure, Paris, France). Flies were raised at 18 °C and virgin homozygous yw,dlgsw females, and heterozygous yw,dlgsw/Y males were sorted from flies containing the Basc balancer. These flies were crossed to allow for collection of embryos with no maternal or zygotic contribution of WT Dlg (i.e. dlgsw,m/z).
Fixation and Antibody Staining of Drosophila Embryos and Dissociated Neuroblasts—Drosophila embryos were dechorinated by rinsing embryos in bleach solution for 1–2 min and then fixed using a biphasic solution of heptane over 4% formaldehyde in PEM buffer (100 mm Pipes, pH 6.9, 1 mm EGTA, 1 mm MgSO4) for 25 min. Upon fixation, embryos were blocked in PBS, 0.3% Triton X-100, and 1% bovine serum albumin. In vitro neuroblast cultures from yw and dlgsw,m/z embryos were prepared using previously published methods (26). Cells were settled onto a coverslip for 30 min and fixed using 4% formaldehyde in PBS for 20 min. Upon fixation, cells were blocked in PBS, 0.1% saponin, and 1% bovine serum albumin.
Embryos and dissociated neuroblasts were then stained using various primary antibodies, including rabbit anti-phospho-histone H3 (1:500–1:1000; Upstate), mouse anti-α-tubulin (1:1000; Sigma), mouse anti-γ-tubulin (1:1000; Sigma), mouse anti-Dlg 4F3E3 (1:100 (27)), rabbit anti-Scribble (1:1000 (37)), rabbit anti-aPKCζ (1:100–1:500; Santa Cruz Biotechnology Inc.), rat anti-Pins (1:500 (28)), guinea pig anti-Miranda (1:500 (29)), mouse anti-Prospero (1:100 (30)), rabbit anti-zipper (1:500 (31)), rabbit anti-Insc (1:1000; W. Chia), rabbit anti-HB9 (1:500–1:1000 (32)), guinea pig anti-Eve (1:1000; East Asian Distribution Center for Segmentation Antibodies), and mouse anti-FasII (1:100; C. Goodman) followed by treatment with fluorescent-conjugated secondary antibodies from Jackson Immunoresearch Laboratories and Invitrogen. Images were collected using a Leica TCS SP2 confocal using a ×63/1.4NA objective. ImageJ, Photoshop, and Illustrator software were used for subsequent data analysis and preparation of figures.
Yeast Two-hybrid—For yeast two-hybrid, pGADT7 and pGBKT7 vectors (Clontech, Palo Alto, CA) were used to monitor association reactions. GUKHolder (amino acids 749–1044) was cloned into the DNA-binding domain vector (pGBKT7), whereas all Dlg constructs were cloned into the Gal4 activation domain vector (pGADT7). The Saccharomyces cerevisiae AH109 strain (Clontech) was cotransformed with pGADT7 and pGBKT7 plasmids and plated on SD medium (–Leu/–Trp). Colonies were subsequently screened for interaction by screening for growth on SD medium (–His/–Leu/–Trp + 20 mm 3-amino-1,2,4-triazole) and for β-galactosidase activity using 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal) as a substrate.
RESULTS
The Dlgsw Mutant Selectively Disrupts the SH3-GK Intramolecular Interaction While Leaving the Domains Intact—As alternative splicing and multiple promoters lead to numerous Dlg isoforms (17, 21), we utilized the sw allelle that includes a frameshift mutation near the end of the coding region of the dlg locus to test for the role of the SH3-GK intramolecular interaction. Although the truncation of the sequence after the GK domain caused by the frameshift is predicted to disrupt the intramolecular interaction, this has not been confirmed. Furthermore, this mutation could cause domain unfolding. We used in trans, qualitative binding assays of an SH3 domain construct by WT or mutant GK domains to test for the ability of wild-type and mutant proteins to form an intramolecular interaction. This analysis (Fig. 1C) revealed that a GST fusion of the WT GK domain construct that includes the F strand (i.e. E-GK-F) associates with the SH3 domain construct that contains β-strands A-E (i.e. SH3(A-E)), whereas constructs that lack the F strand (i.e. E-GK or E-GK-sw) are unable to associate with the SH3 domain. These results are consistent with previous reports that indicate the role of interaction of E and F β-strands in formation of the SH3-GK intramolecular interaction (19, 20).
Although the intramolecular interaction is disrupted in Dlgsw, it is possible that the truncation encoded by this allele disrupts the integrity of the SH3 and GK domains. To ensure that the Dlgsw protein is correctly folded, we assessed the overall secondary structure of WT PDZ-SH3-GK and mutant PDZ-SH3-GKsw using circular dichroism spectroscopy. Circular dichroism-monitored wavelength scans (Fig. 1D) indicate that the Dlgsw mutations do not compromise the overall fold of the GK domain, as no significant change in α-helical content (i.e. Δ molar ellipticity at 222 nm) was observed.
Dlg is known to oligomerize through a region NH2-terminal to the PDZ1 domain (33, 34). However, intermolecular interactions have also been proposed to occur between MAGUK SH3-GK modules. To determine whether the sw mutation affects the oligomerization state of the SH3 and GK domains, we monitored the oligomerization state of WT PDZ-SH3-GK and mutant PDZ-SH3-GKsw using analytical ultracentrifugation sedimentation velocity experiments. Results from these experiments indicate that both the sw and wild-type proteins are monomeric in solution (Fig. 1E). Thus, we conclude that the oligomerization state of Dlg is unlikely to be affected in the context of full-length Dlgsw. This conclusion is also supported by analysis of a PSD-95 mutation, PSD-95ΔC, which lacks the 13 COOH-terminal residues, which does not disrupt its ability to associate with this NH2-terminal multimerization domain (18). Together, these results support the use of Dlgsw as a model for understanding the role of the SH3-GK intramolecular interaction in Dlg function, as this mutant specifically disrupts the interaction while leaving the SH3 and GK domains largely intact.
Localization of Discs Large and Scribble Are Not Regulated by the SH3-GK Intramolecular Interaction—To assess the role of the SH3-GK intramolecular interaction in Dlg function, we examined neuroblasts from dlgsw,m/z embryos (those lacking both maternal and zygotic wild-type dlg activity) for defects in asymmetric cell division. In wild-type embryos, Dlg localizes to the neuroblast cortex with enrichment at the apical region of the cell, and is required for cortical polarization and spindle positioning (11–13). We first determined if the SH3-GK intramolecular interaction is required for Dlg targeting by examining Dlgsw protein localization. Localization of Dlg to membrane specializations such as the postsynapse requires interaction of the HOOK domain (see Fig. 1A), as well as the first two PDZ domains of Dlg with cytoskeletal-binding partners (35). Given the intervening position of the HOOK between the SH3 and GK domains of Dlg we determined whether the localization of Dlg in Drosophila neuroblasts is affected by disruption of the SH3-GK intramolecular interaction. However, as shown in Fig. 2D, localization of Dlgsw is indistinguishable from wild-type (Fig. 2A). We also assessed localization of Scribble (Scrib), which forms a tripartite complex with GukHolder and Dlg (36). Previous studies of Scrib localization in dlgm52 null mutant neuroblasts have shown that Dlg is required for cortical recruitment of Scribble (37). In dlgsw,m/z embryonic neuroblasts localization of Scrib is shown to be indistinguishable in comparison to wild-type (Fig. 2, B versus E). Based on these data, we conclude that the SH3-GK intramolecular interaction is not required for Dlg or Scrib localization.
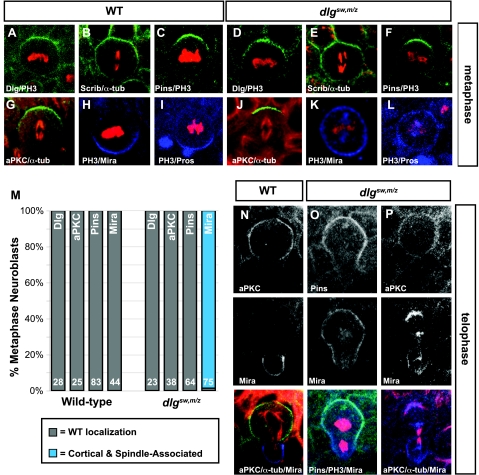
FIGURE 2.
The SH3-GK intramolecular interaction is required for restriction of cell fate determinants to the basal cortex of a dividing neuroblast. Lateral view of metaphase and telophase neuroblasts from stage 9–11 embryos. Genotypes are listed above the panels. Top row, localization of the Scribble complex components (Dlg, A and D; and Scribble, B and E) and Pins (C and F) in WT and dlgsw,m/z metaphase neuroblasts. Second row, localization of proteins to apical (green) and basal (blue) cortical domains in WT and dlgsw,m/z metaphase neuroblasts: aPKC (G and J), Miranda (H and K), and Prospero (I and L). Quantitation of apical enrichment of Dlg and Scrib, apical exclusion of Pins and aPKC, and basal exclusion of Miranda and Prospero in metaphase neuroblasts is indicated in panel M. The number of neuroblasts scored and marker of cortical polarity are indicated within each bar. Third, fourth, and fifth rows, localization of proteins to apical and basal cortical domains in corresponding telophase neuroblasts (N–P), showing individual stains, as well as merged images (aPKC or Pins in green, Miranda in blue, and α-tubulin or phospho-histone H3 (PH3) in red) to illustrate proper positioning of apical cortical domains and cortical restriction of Miranda to the ganglion mother cell by the latter stages of telophase.
Neuroblast Polarity Is Disrupted in dlgsw,m/z Embryos—Given that Dlgsw is appropriately enriched at the apical cortex during metaphase, we asked if the SH3-GK intramolecular interaction of Dlg plays a role in establishing and maintaining cell polarity in Drosophila neuroblasts. During metaphase, neuroblasts establish mutually exclusive apical and basal cortical domains that give rise to the distinct daughter cell fates (see Refs. 14 and 15 for review). The apical domain consists of the Insc and Par complexes, which direct the segregation of factors such as Miranda to the basal domain. In dlgsw,m/z embryos, components of the Insc (e.g. Pins, 100%, n = 64; Fig. 2, C versus F) and Par (e.g. aPKC, 100%, n = 38; Fig. 2, G versus J) apical complexes localize to the apical cortex indistinguishable from wild-type indicating that the SH3-GK intramolecular interaction is not required for apical polarity. In contrast, the basal component Miranda (Mira) is unpolarized, no longer partitioning exclusively to the basal cortex during metaphase (99%, n = 75; Fig. 2, H versus K). Mislocalization of Prospero, a cargo protein of Mira is also observed (Fig. 2, I versus L). Although Miranda was shown to remain mislocalized in early telophase (Fig. 2O), by late telophase Miranda became correctly polarized into the basal cortex through a process known as “telophase rescue” (see Fig. 2, P versus N). This phenotype is also observed in neuroblasts from Dlg null embryos (11, 12). Thus, whereas the SH3-GK intramolecular interaction is not required for localization, it is necessary for the function of Dlg in establishing basal polarity during asymmetric cell division.
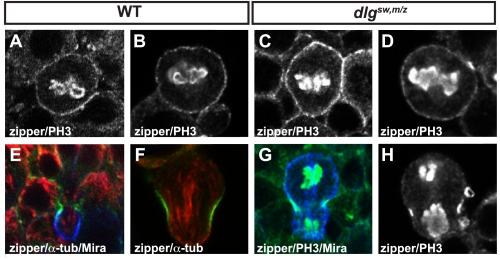
Where does the Dlg SH3-GK intramolecular interaction function in the molecular pathway that leads to neuroblast polarity? Miranda polarization is thought to occur through a complex, multistep pathway involving the Par complex protein aPKC (38). In this model, kinase activity from aPKC inactivates the tumor suppressor Lethal giant larvae (Lgl) from the apical cortex. When active, Lgl antagonizes the activity of cortical myosin II, which itself displaces Miranda from the cortex (39). The overlap of aPKC and Miranda at the apical cortex that we observe in dlgsw,m/z neuroblasts (Fig. 2) indicates that either Dlg is required for coupling of aPKC activity to Miranda displacement by myosin II, or it is necessary for apical aPKC activity. To distinguish between these possibilities, we examined zipper, the heavy chain of myosin II, localization in dlgsw,m/z neuroblasts. As shown in Fig. 3, we observed no effect on zipper localization in dlgsw,m/z metaphase (Fig. 3, A–B versus C–D) or telophase (Fig. 3, E–F versus G–H) neuroblasts in comparison to wild-type (although we observed strong cleavage furrow localization, as expected, we did not observe the apical enrichment reported in Ref. 39). These data suggest that the role of the SH3-GK intramolecular interaction of Dlg in Mira exclusion may be to regulate aPKC kinase activity rather than to couple this activity to Miranda displacement.
FIGURE 3.
Myosin II localization is not affected by disruption of the SH3-GK intramolecular interaction of Dlg. Lateral view of stage 9–11 metaphase and telophase neuroblasts. Genotypes are listed above the panels. Localization of zipper, the heavy chain of myosin II, in metaphase (A–D) and telophase (E–H) neuroblasts as observed in intact embryos (A, C, E, and G) and dissociated embryonic neuroblasts (B, D, F, and H).
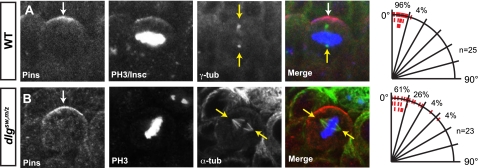
Spindle Alignment Along the Apical-Basal Cortical Polarity Axis Is Disrupted in dlgsw,m/z Embryos—Dlg has also been shown to be essential for tight coupling of the mitotic spindle with the cortical polarity axis of metaphase neuroblasts (13). Misalignment of the mitotic spindle has been shown to result in missegregation of cell fate determinants and thus an increased number of symmetric divisions (37, 40, 41). Given the role of Dlg in ensuring proper alignment of the mitotic spindle, we next asked whether the SH3-GK intramolecular interaction was required for the function of Dlg in alignment of the mitotic spindle in metaphase neuroblasts. In wild-type embryonic neuroblasts, alignment of the metaphase spindle is tightly coupled to the center of the apical Pins crescent (Fig. 4A). In contrast, only ∼60% of dlgsw,m/z mitotic spindles show tight alignment with the center of the Pins crescent (Fig. 4B). This is nearly identical to the defect previously observed for spindle alignment of dlgm52 null mutant neuroblasts (13). Thus, in addition to being critical for cortical polarity, the SH3-GK intramolecular interaction is also necessary for the function of Dlg in orienting the mitotic spindle with the apical-basal axis in metaphase neuroblasts.
FIGURE 4.
The SH3-GK intramolecular interaction is required for reliable coupling of spindle alignment with cortical polarity in metaphase neuroblasts. A, wild-type, and B, dlgsw,m/z metaphase neuroblasts scored for alignment of the mitotic spindle with the apical Pins crescent. First column, localization of Pins with the center of the crescent marked with an arrowhead. Second column, Insc and/or phospho-histone H3 (PH3) localization in the same neuroblast. Third column, localization of the mitotic spindle as shown by γ-tub or α-tub stains of the same neuroblast. Fourth column, merge of the first through the third columns, demonstrating the position of the mitotic spindle relative to the center of the Pins crescent. Last column, quantitation of spindle alignment defects, denoting the percentage of neuroblasts scored within 15° bins. Red tick marks are shown to depict the angle between the mitotic spindle and center of the Pins crescent for each neuroblast scored.
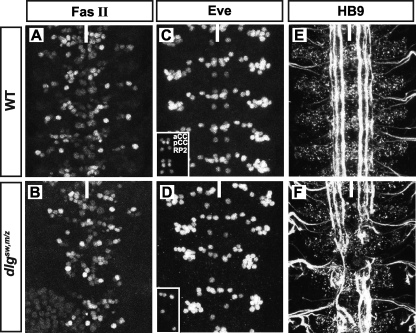
Central Nervous System Patterning and Axonal Guidance Is Disrupted in dlgsw,m/z Embryos—Given its role in segregation of cell fate determinants, is the SH3-GK intramolecular interaction also required for neurogenesis? Previous RNA interference experiments, targeting an isoform of Dlg expressed in the central nervous system of embryos, demonstrated the importance of Dlg in neuronal development as major defects in neuronal differentiation and axon guidance were observed (17). To assess the role of the SH3-GK intramolecular interaction in central nervous system patterning, subsets of motoneurons and interneurons were observed by staining for HB9 and Even-skipped (Eve). Severe distortion of HB9 and Eve patterning is observed for dlgsw,m/z versus WT stage 16 embryos (Fig. 5, B and D versus A and C). Projection of internal layers demonstrate the loss of Eve-positive neurons, as the pattern of aCC motoneuron, pCC interneuron, and RP2 motoneuron present in WT embryos (inset of Fig. 5C) is not observed in dlgsw,m/z embryos (inset of Fig. 5D). In addition, axon guidance is severely disrupted in dlgsw,m/z as observed by Fas II stains of stage 16 embryos (Fig. 5, E versus F). Thus, the SH3-GK intramolecular interaction is necessary for the function of Dlg in neurogenesis.
FIGURE 5.
The SH3-GK intramolecular interaction is required for neurogenesis in Drosophila embryos. Wild-type and dlgsw,m/z stage 16 embryos stained for markers of central nervous system patterning during embryogenesis. First and second columns, full projections of HB9 (A and B) and Eve (C and D) stains, highlighting subsets of motoneurons and interneurons. Insets in panels C and D represent internal projections, illustrating a loss of Eve+ aCC and RP2 dorsally projecting motoneurons and/or pCC intersegmental interneurons in dlgsw,m/z embryos. Third column, full projections of FasII stains (E and F) illustrating the disruption of axonal guidance in dlgsw,m/z embryos. Anterior is up and the ventral midline is depicted by a white line.
Loss of the SH3-GK Intramolecular Interaction Disrupts Regulated Complex Assembly—Why is the SH3-GK intramolecular interaction necessary for the establishment of cell polarity, spindle orientation, and neurogenesis? One established function for the MAGUK intramolecular interaction is to regulate ligand binding to the GK domain (3–5). To determine whether the defects in cell polarity observed in dlgsw,m/z embryonic neuroblasts correlate with a loss of regulated complex assembly, we examined the binding of several Dlg ligands to bind Dlgsw protein. We used the Dlg SH3-GK ligand GukH for these experiments as it has been found to be an accurate sensor of the domains functional state: GukH binds to the isolated GK domain, but not to the SH3-GK domain, but binding is restored in full-length Dlg by the third Dlg PDZ domain (PDZ3). This complex regulation appears to be a mechanism to regulate binding of PDZ3 and SH3-GK ligands as binding of the two is mutually exclusive. This leads to mutually exclusive complex formation between PDZ3 and GK ligands (3). Thus, we examined the ability of the Dlgsw protein to discriminate between PDZ3 and SH3-GK ligands to investigate possible causes for the defects observed in dlgsw,m/z embryonic neuroblasts.
We observed that, whereas the WT SH3-GK fragment is unable to associate with GukH, as previously described, the mutant SH3-GK(sw) module constitutively associates with GukH (Fig. 6A). To ensure that association of GukH was due to disruption of the interaction of the E and F strands of Dlg rather than the presence of missense mutations (i.e. REQQ, refer to Fig. 1B), COOH-terminal deletions were tested for their ability to associate with GukH. As shown in Fig. 6B, deletion of F-strand elements were sufficient to allow for GukHolder association comparable with that observed for the SH3-GK(sw) construct. In contrast, yeast two-hybrid analysis of a mutant SH3-GK construct containing the four missense mutations (i.e. SH3-GK(sw)+F) demonstrated weak association comparable with that observed for WT SH3-GK (i.e. 598–975; Fig. 6B, top panel). These data indicate that regulation of SH3-GK ligand binding is disrupted by loss of the intramolecular interaction.
We hypothesized that the SH3-GK defect could also cause loss of regulated complex assembly (i.e. simultaneous association of Dlg PDZ3 and SH3-GK ligands). To determine whether the sw mutation results in unregulated complex assembly, association of Dlg with HA GukH-(800–1044) was assessed in the absence and presence of a GFP fusion of a PDZ3 ligand, GFP-Cript. As shown in Fig. 6C, whereas addition of GFP-Cript to a concentration of 5 μm results in near complete competition of HA-GukH association with WT PDZ-SH3-GK, little competition was observed for HA-GukH association with PDZ-SH3-GKsw. These data indicate that loss of the SH3-GK intramolecular leads to simultaneous association of PDZ3 and GK ligands in contrast to the wild-type protein in which these two ligands bind in a mutually exclusive manner.
DISCUSSION
Allosteric regulation of ligand binding has been hypothesized to be essential for regulation of MAGUK function, providing a mechanism of reorganizing cellular signaling complexes in response to developmental or environmental cues. For example, association of PDZ ligands has been shown to regulate binding of GK ligands in vitro; binding of a peptide representing the COOH-terminal portion of Cript increases the affinity of the GK domain of PSD-93 for MAP1A (5), whereas it decreases the affinity of the GK domain of Dlg for GukHolder (3). Such allosteric regulation of ligand binding is proposed to occur via modulation of the SH3-GK intramolecular interaction of MAGUKs, as an SH3-GK module alone is unable to associate with GK ligands (i.e. GKAP and GukHolder), whereas full-length MAGUKs associate with an affinity comparable with the GK module alone (3, 4).
In addition to regulation of ligand binding, the SH3-GK intramolecular interaction has been proposed to play an important role in regulating the activity of ligands. Recently it was shown in vitro that whereas GAKIN can associate with full-length hDlg containing the alternatively spliced insert I3 and its corresponding SH3-GK module, only the SH3-GK module was shown to activate ATPase activity of the motor domain of GAKIN (42). Therefore, there is precedence for the importance of allosteric regulation of the SH3-GK intramolecular interaction in vitro, however, little evidence exists that such regulation is required for MAGUK function in vivo. In the studies presented here, we took advantage of the dlgsw allele to explore the role of the SH3-GK intramolecular interaction in asymmetric cell division of Drosophila neuroblasts. The sw mutant of Dlg is a good model system for understanding the role of the SH3-GK intramolecular interaction in vivo, as we provide evidence that the sw mutation disrupts the SH3-GK intramolecular interaction while leaving the two domains intact. Here we show that the SH3-GK intramolecular interaction plays an essential role for regulating the function of Dlg in asymmetric cell division of Drosophila neuroblasts and furthermore, neurogenesis. Additional experiments suggest that such loss of function is due to unregulated complex assembly.
The Role of the SH3-GK Intramolecular Interaction in Restriction of Basal Cell Fate Determinants—How does the SH3-GK intramolecular interaction of Dlg participate in partitioning cell fate determinants to the basal cortex? Previous studies demonstrate that Dlg is required for localization of Lgl to the cell cortex of embryonic neuroblasts (11, 12). Furthermore, recent studies indicate that Lgl inhibits aPKC activity (43). Such aPKC-dependent phosphorylation results in asymmetric segregation of cell fate determinants to the basal cortex of a dividing neuroblast. Like embryonic dlgGLC and lglGLC mutants (11, 12), dlgsw,m/z neuroblasts display cortical and spindle-associated localization of cell fate determinants (refer to Fig. 2K). These data suggest that the SH3-GK intramolecular interaction of Dlg plays a role in regulating the ability of aPKC to phosphorylate Lgl and thus eliminate its repression of aPKC at the apical cortex of a dividing neuroblast.
How might the SH3-GK intramolecular interaction of Dlg play a role in regulating aPKC activity? Potential models are suggested by studies of the β channel subunit of voltage-gated calcium channels (CaVβ) that contain a homologous SH3-GK module. Mutations in CaVβ that disrupt the SH3-GK intramolecular interaction have been shown to disrupt trafficking of the α subunit of CaV and ultimately control of calcium channel gating (44). Interestingly, the SH3-GK module of CaVβ has been shown to interact with small RGK-GTPases and large GTPases, such as dynamin (see Ref. 45 for review). Given that aPKC activity has recently been shown to be increased by the addition of Cdc42 (46), a small Rho-GTPase, and dynamin-associated protein 160 (47), the SH3-GK intramolecular interaction of Dlg may serve to regulate association of Cdc42 and Dap160 with aPKC. Alternatively, direct association of Dlg with aPKC may serve to regulate kinase activity, as atypical PKCs have been shown to associate with and phosphorylate MAGUKs in vivo (48).
The Role of the SH3-GK Intramolecular Interaction in Alignment of the Mitotic Spindle Along the Apical-Basal Polarity Axis—Association of Dlg with Khc-73 has been shown to play an important role in linking spindle alignment to cortical polarity (49); Khc-73 localizes to the astral microtubule plus ends, and thus is proposed to allow for clustering of Dlg at the apical pole, resulting in coupling of the mitotic spindle to the cortical polarity axis. Previous studies have demonstrated that the dlgsw mutant exhibits unreliable coupling of the mitotic spindle with the cortical polarity axis in sensory of organ precursor cells (50). Here we present evidence that such loss of reliable coupling is also observed in dlgsw,m/z Drosophila neuroblasts (Fig. 4B). How is allosteric regulation of the SH3-GK intramolecular interaction involved in coupling of the mitotic spindle with the axis of cortical polarity? Previous studies have indicated that a mutant of hDlg lacking the final 11 COOH-terminal residues, homologous to the sw mutant, had no effect on its association with GAKIN, a homologue of Khc-73 (51). Therefore, whereas loss of the SH3-GK intramolecular interaction of Dlg is not predicted to disrupt its interaction with Khc-73, consistent with apical enrichment of Dlg in dlgsw,m/z neuroblast, it is predicted to affect the ability of Khc-73 to associate with microtubules, possibly leading to the spindle alignment defect observed in dlgsw,m/z neuroblasts.
Potential Pathways of Regulation of the SH3-GK Intramolecular Interaction—What mechanisms are used to regulate the SH3-GK intramolecular interaction, and thus function of Dlg in vivo? As previously mentioned, in vitro studies have shown that association of ligands with the third PDZ domain of MAGUKs regulates GK ligand binding via modulation of the SH3-GK intramolecular interaction (3, 5). We have found that the defects observed in dlgsw,m/z neuroblasts correlate with loss of this activity as PDZ3 and SH3-GK ligands bind simultaneously to the Dlgsw protein, whereas they are mutually exclusive in the wild-type protein. The identities of the ligands that are relevant for regulation of neuroblast asymmetric cell division are unknown, however. Although the SH3-GK ligand we used (GukHolder) as a sensor for the SH3-GK functional state is apically enriched in Drosophila neuroblasts (37), its function during asymmetric cell division, if any, is unknown. Future identification of neuroblast-specific PDZ3 and SH3-GK ligands will be essential to determine the relevance of regulated complex assembly in ACD.
In addition to association of ligands with PDZ3 of MAGUKs (see Fig. 6C), interactions with the HOOK domain and posttranslational modifications are proposed to regulate MAGUK function in vivo by regulating the SH3-GK intramolecular interaction. The HOOK domain was shown to be necessary for membrane targeting of Dlg (52) and in vitro studies suggest that interactions with the HOOK domain may provide a mechanism of toggling between intra- and intermolecular states, as mutations within the HOOK domain of PSD-95 result in protein dimerization (19). Two proteins that associate with adjacent regions of the HOOK domain are Ca2+-saturated calmodulin (7, 53, 54) and members of the protein 4.1 family (55). Association of these proteins with the HOOK domain is proposed to allow for allosteric modulation of the SH3-GK intramolecular interaction, regulating self-association and interaction with SH3 and GK ligands.
An additional mode of regulation of the SH3-GK intramolecular interaction is proposed to occur by post-translational modifications, such as phosphorylation. For instance, phosphorylation by p38γ results in loss of interaction of SAP97 with GKAP (56). Interestingly, other studies have implicated phosphorylation by PKCζ in regulating MAGUK localization and function; Dlg1 localization in migrating astrocytes is Cdc42/PKCζ-dependent (57) and phosphorylation of the PDZ-SH3-GK module of ZO-2 by PKCζ allows for regulation of tight junction assembly (48). Future studies will be essential to determine the protein-protein interactions and/or post-translational modifications required for allosteric regulation of the SH3-GK intramolecular interaction of Dlg in asymmetric cell division.
Acknowledgments
We thank F. Schweisguth and C. Doe for providing fly stocks and/or antibodies, C. Hong for assistance in making reagents, and K. Tran, J. Jacobson, S. Atwood, S. Siegrist, M. Rolls, K. Siller, S. Weitzel, W. Baase, and J. Boone for helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grant GM068032 (to K. E. P.). This work was also supported by a Ruth L. Kirschstein National Research Service Award Postdoctoral Fellowship National Institutes of Health Grant F32 GM080018 (to R. A. N.) and American Heart Association Grant 0850140Z (to K. E. P.).
Footnotes
The abbreviations used are: MAGUK, membrane-associated guanylate kinase; ACD, asymmetric cell division; aPKC, atypical protein kinase C; CaVβ, β subunit of voltage-gated calcium channels; Cript, cysteine-rich interactor of PDZ; Dlg, discs large tumor suppressor protein; dlgsw,m/z, dlgsw mutant in the absence of maternal and zygotic contributions of WT Dlg; Eve, Even-skipped; GFP-MUDp, a green fluorescent protein-peptide fusion derived from the mushroom body defect protein; GLC, germline clone; GukH, GukHolder, isoform C; Lgl, lethal giant larvae; Mira, Miranda; PDZ, PSD-95/Dlg/ZO-1; PDZ1, the first PDZ domain of Dlg; PDZ3, the third PDZ domain of Dlg; Pins, Partner of Inscutable; Scrib, Scribble; SH3, Src homology 3; WT, wild type; GFP, green fluorescent protein; HA, hemagglutinin; PBS, phosphate-buffered saline; GST, glutathione S-transferase; Pipes, 1,4-piperazinediethanesulfonic acid.
References
- 1.Dimitratos, S. D., Woods, D. F., Stathakis, D. G., and Bryant, P. J. (1999) Bioessays 21 912-921 [DOI] [PubMed] [Google Scholar]
- 2.Funke, L., Dakoji, S., and Bredt, D. S. (2005) Annu. Rev. Biochem. 74 219-245 [DOI] [PubMed] [Google Scholar]
- 3.Qian, Y., and Prehoda, K. E. (2006) J. Biol. Chem. 281 35757-35763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu, H., Reissner, C., Kuhlendahl, S., Coblentz, B., Reuver, S., Kindler, S., Gundelfinger, E. D., and Garner, C. C. (2000) EMBO J. 19 5740-5751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenman, J. E., Topinka, J. R., Cooper, E. C., McGee, A. W., Rosen, J., Milroy, T., Ralston, H. J., and Bredt, D. S. (1998) J. Neurosci. 18 8805-8813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGee, A. W., and Bredt, D. S. (1999) J. Biol. Chem. 274 17431-17436 [DOI] [PubMed] [Google Scholar]
- 7.Paarmann, I., Spangenberg, O., Lavie, A., and Konrad, M. (2002) J. Biol. Chem. 277 40832-40838 [DOI] [PubMed] [Google Scholar]
- 8.Nix, S. L., Chishti, A. H., Anderson, J. M., and Walther, Z. (2000) J. Biol. Chem. 275 41192-41200 [DOI] [PubMed] [Google Scholar]
- 9.Woods, D. F., and Bryant, P. J. (1991) Cell 66 451-464 [DOI] [PubMed] [Google Scholar]
- 10.De Lorenzo, C., Mechler, B. M., and Bryant, P. J. (1999) Cancer Metastasis Rev. 18 295-311 [DOI] [PubMed] [Google Scholar]
- 11.Peng, C. Y., Manning, L., Albertson, R., and Doe, C. Q. (2000) Nature 408 596-600 [DOI] [PubMed] [Google Scholar]
- 12.Ohshiro, T., Yagami, T., Zhang, C., and Matsuzaki, F. (2000) Nature 408 593-596 [DOI] [PubMed] [Google Scholar]
- 13.Siegrist, S. E., and Doe, C. Q. (2005) Cell 123 1323-1335 [DOI] [PubMed] [Google Scholar]
- 14.Gonczy, P. (2008) Nat. Rev. Mol. Cell Biol. 9 355-366 [DOI] [PubMed] [Google Scholar]
- 15.Knoblich, J. A. (2008) Cell 132 583-597 [DOI] [PubMed] [Google Scholar]
- 16.Perrimon, N. (1988) Dev. Biol. 127 392-407 [DOI] [PubMed] [Google Scholar]
- 17.Mendoza, C., Olguin, P., Lafferte, G., Thomas, U., Ebitsch, S., Gundelfinger, E. D., Kukuljan, M., and Sierralta, J. (2003) J. Neurosci. 23 2093-2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin, H., Hsueh, Y. P., Yang, F. C., Kim, E., and Sheng, M. (2000) J. Neurosci. 20 3580-3587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGee, A. W., Dakoji, S. R., Olsen, O., Bredt, D. S., Lim, W. A., and Prehoda, K. E. (2001) Mol. Cell 8 1291-1301 [DOI] [PubMed] [Google Scholar]
- 20.Tavares, G. A., Panepucci, E. H., and Brunger, A. T. (2001) Mol. Cell 8 1313-1325 [DOI] [PubMed] [Google Scholar]
- 21.Woods, D. F., Hough, C., Peel, D., Callaini, G., and Bryant, P. J. (1996) J. Cell Biol. 134 1469-1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beaven, G. H., and Holiday, E. R. (1952) Adv. Protein Chem. 7 319-386 [DOI] [PubMed] [Google Scholar]
- 23.Andrade, M. A., Chacon, P., Merelo, J. J., and Moran, F. (1993) Protein Eng. 6 383-390 [DOI] [PubMed] [Google Scholar]
- 24.Schuck, P. (2000) Biophys. J. 78 1606-1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuck, P., Perugini, M. A., Gonzales, N. R., Howlett, G. J., and Schubert, D. (2002) Biophys. J. 82 1096-1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grosskortenhaus, R., Pearson, B. J., Marusich, A., and Doe, C. Q. (2005) Dev. Cell 8 193-202 [DOI] [PubMed] [Google Scholar]
- 27.Parnas, D., Haghighi, A. P., Fetter, R. D., Kim, S. W., and Goodman, C. S. (2001) Neuron 32 415-424 [DOI] [PubMed] [Google Scholar]
- 28.Yu, F., Morin, X., Cai, Y., Yang, X., and Chia, W. (2000) Cell 100 399-409 [DOI] [PubMed] [Google Scholar]
- 29.Lee, C. Y., Wilkinson, B. D., Siegrist, S. E., Wharton, R. P., and Doe, C. Q. (2006) Dev. Cell 10 441-449 [DOI] [PubMed] [Google Scholar]
- 30.Spana, E. P., and Doe, C. Q. (1995) Development 121 3187-3195 [DOI] [PubMed] [Google Scholar]
- 31.Liu, S. L., Fewkes, N., Ricketson, D., Penkert, R. R., and Prehoda, K. E. (2008) J. Biol. Chem. 283 380-387 [DOI] [PubMed] [Google Scholar]
- 32.Odden, J. P., Holbrook, S., and Doe, C. Q. (2002) J. Neurosci. 22 9143-9149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsueh, Y. P., Kim, E., and Sheng, M. (1997) Neuron 18 803-814 [DOI] [PubMed] [Google Scholar]
- 34.Marfatia, S. M., Byron, O., Campbell, G., Liu, S. C., and Chishti, A. H. (2000) J. Biol. Chem. 275 13759-13770 [DOI] [PubMed] [Google Scholar]
- 35.Thomas, U., Ebitsch, S., Gorczyca, M., Koh, Y. H., Hough, C. D., Woods, D., Gundelfinger, E. D., and Budnik, V. (2000) Curr. Biol. 10 1108-1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathew, D., Gramates, L. S., Packard, M., Thomas, U., Bilder, D., Perrimon, N., Gorczyca, M., and Budnik, V. (2002) Curr. Biol. 12 531-539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albertson, R., and Doe, C. Q. (2003) Nat. Cell Biol. 5 166-170 [DOI] [PubMed] [Google Scholar]
- 38.Rolls, M. M., Albertson, R., Shih, H. P., Lee, C. Y., and Doe, C. Q. (2003) J. Cell Biol. 163 1089-1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barros, C. S., Phelps, C. B., and Brand, A. H. (2003) Dev. Cell 5 829-840 [DOI] [PubMed] [Google Scholar]
- 40.Lee, C. Y., Andersen, R. O., Cabernard, C., Manning, L., Tran, K. D., Lanskey, M. J., Bashirullah, A., and Doe, C. Q. (2006) Genes Dev. 20 3464-3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siller, K. H., Cabernard, C., and Doe, C. Q. (2006) Nat. Cell Biol. 8 594-600 [DOI] [PubMed] [Google Scholar]
- 42.Yamada, K. H., Hanada, T., and Chishti, A. H. (2007) Biochemistry 46 10039-10045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee, C. Y., Robinson, K. J., and Doe, C. Q. (2006) Nature 439 594-598 [DOI] [PubMed] [Google Scholar]
- 44.Takahashi, S. X., Miriyala, J., Tay, L. H., Yue, D. T., and Colecraft, H. M. (2005) J. Gen. Physiol. 126 365-377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hidalgo, P., and Neely, A. (2007) Cell Calcium 42 389-396 [DOI] [PubMed] [Google Scholar]
- 46.Atwood, S. X., Chabu, C., Penkert, R. R., Doe, C. Q., and Prehoda, K. E. (2007) J. Cell Sci. 120 3200-3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chabu, C., and Doe, C. Q. (2008) Development 135 2739-2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Avila-Flores, A., Rendon-Huerta, E., Moreno, J., Islas, S., Betanzos, A., Robles-Flores, M., and Gonzalez-Mariscal, L. (2001) Biochem. J. 360 295-304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siegrist, S. E., and Doe, C. Q. (2006) Development 133 529-536 [DOI] [PubMed] [Google Scholar]
- 50.Bellaiche, Y., Radovic, A., Woods, D. F., Hough, C. D., Parmentier, M. L., O'Kane, C. J., Bryant, P. J., and Schweisguth, F. (2001) Cell 106 355-366 [DOI] [PubMed] [Google Scholar]
- 51.Hanada, T., Lin, L., Tibaldi, E. V., Reinherz, E. L., and Chishti, A. H. (2000) J. Biol. Chem. 275 28774-28784 [DOI] [PubMed] [Google Scholar]
- 52.Hough, C. D., Woods, D. F., Park, S., and Bryant, P. J. (1997) Genes Dev. 11 3242-3253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fukunaga, Y., Matsubara, M., Nagai, R., and Miyazawa, A. (2005) J. Biochem. (Tokyo) 138 177-182 [DOI] [PubMed] [Google Scholar]
- 54.Paarmann, I., Lye, M. F., Lavie, A., and Konrad, M. (2008) Protein Sci. 17 1946-1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marfatia, S. M., Lue, R. A., Branton, D., and Chishti, A. H. (1994) J. Biol. Chem. 269 8631-8634 [PubMed] [Google Scholar]
- 56.Sabio, G., Arthur, J. S., Kuma, Y., Peggie, M., Carr, J., Murray-Tait, V., Centeno, F., Goedert, M., Morrice, N. A., and Cuenda, A. (2005) EMBO J. 24 1134-1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Etienne-Manneville, S., Manneville, J. B., Nicholls, S., Ferenczi, M. A., and Hall, A. (2005) J. Cell Biol. 170 895-901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kraulis, P. J. (1991) J. Appl. Crystallogr. 24 946-950 [Google Scholar]
- 59.Merritt, E. A., and Bacon, D. J. (1997) Methods Enzymol. 277 505-524 [DOI] [PubMed] [Google Scholar]