Abstract
Tissue transglutaminase (tTG) has been implicated in the pathogenesis of Parkinson disease (PD). However, exactly how tTG modulates the structural and functional properties of α-synuclein (α-syn) and contributes to the pathogenesis of PD remains unknown. Using site-directed mutagenesis combined with detailed biophysical and mass spectrometry analyses, we sought to identify the exact residues involved in tTG-catalyzed cross-linking of wild-type α-syn and α-syn mutants associated with PD. To better understand the structural consequences of each cross-linking reaction, we determined the effect of tTG-catalyzed cross-linking on the oligomerization, fibrillization, and membrane binding of α-syn in vitro. Our findings show that tTG-catalyzed cross-linking of monomeric α-syn involves multiple cross-links (specifically 2-3). We subjected tTG-catalyzed cross-linked monomeric α-syn composed of either wild-type or Gln → Asn mutants to sequential proteolysis by multiple enzymes and peptide mapping by mass spectrometry. Using this approach, we identified the glutamine and lysine residues involved in tTG-catalyzed intramolecular cross-linking of α-syn. These studies demonstrate for the first time that Gln79 and Gln109 serve as the primary tTG reactive sites. Mutating both residues to asparagine abolishes tTG-catalyzed cross-linking of α-syn and tTG-induced inhibition of α-syn fibrillization in vitro. To further elucidate the sequence and structural basis underlying these effects, we identified the lysine residues that form isopeptide bonds with Gln79 and Gln109. This study provides mechanistic insight into the sequence and structural basis of the inhibitory effects of tTG on α-syn fibrillogenesis in vivo, and it sheds light on the potential role of tTG cross-linking on modulating the physiological and pathogenic properties of α-syn.
Parkinson disease (PD)2 is a progressive movement disorder that is caused by the loss of dopaminergic neurons in the substantia nigra, the part of the brain responsible for controlling movement. Clinically, PD is manifested in symptoms that include tremors, rigidity, and difficulty in initiating movement (bradykinesia). Pathologically, PD is characterized by the presence of intraneuronal, cytoplasmic inclusions known as Lewy bodies (LB), which are composed primarily of the protein “α-synuclein” (α-syn) (1) and are seen in the post-mortem brains of PD patients with the sporadic or familial forms of the disease (2). α-Syn is a presynaptic protein of 140 residues with a “natively” unfolded structure (3). Three missense point mutations in α-syn (A30P, E46K, and A53T) are associated with the early-onset, dominant, inherited form of PD (4, 5). Moreover, duplication or triplication of the α-syn gene has been linked to the familial form of PD, suggesting that an increase in α-syn expression is sufficient to cause PD. Together, these findings suggest that α-syn plays a central role in the pathogenesis of PD.
The molecular and cellular determinants that govern α-syn oligomerization and fibrillogenesis in vivo remain poorly understood. In vitro aggregation studies have shown that the mutations associated with PD (A30P, E46K, and A53T) accelerate α-syn oligomerization, but only E46K and A53T α-syn show higher propensity to fibrillize than wild-type (WT) α-syn (6-8). This suggests that oligomerization, rather than fibrillization, is linked to early-onset familial PD (9). Our understanding of the molecular composition and biochemical state of α-syn in LBs has provided important clues about protein-protein interactions and post-translational modifications that may play a role in modulating oligomerization, fibrillogenesis, and LB formation of the protein. In addition to ubiquitination (10), phosphorylation (11, 12), nitration (13, 14), and C-terminal truncation (15, 16), analysis of post-mortem brain tissues from PD and Lewy bodies in dementia patients has confirmed the colocalization of tissue transglutaminase (tTG)-catalyzed cross-linked α-syn monomers and higher molecular aggregates in LBs within dopaminergic neurons (17, 18). Tissue transglutaminase catalyzes a calcium-dependent transamidating reaction involving glutamine and lysine residues, which results in the formation of a covalent cross-link via ε-(γ-glutamyl) lysine bonds (Fig. 2F). To date, seven different isoforms of tTGs have been reported, of which only tTG2 seems to be expressed in the human brain (19), whereas tTG1 and tTG3 are more abundantly found in stratified squamous epithelia (20). Subsequent immuno-histochemical, colocalization, and immunoprecipitation studies have shown that the levels of tTG and cross-linked α-syn species are increased in the substantia nigra of PD brains (17). These findings, combined with the known role of tTG in cross-linking and stabilizing bimolecular assemblies, led to the hypothesis that tTG plays an important role in the initiation and propagation of α-syn fibril formation and that it contributes to fibril stability in LBs. This hypothesis was initially supported by in vitro studies demonstrating that tTG catalyzes the polymerization of the α-syn-derived non-amyloid component (NAC) peptide via intermolecular covalent cross-linking of residues Gln79 and Lys80 (21) and by other studies suggesting that tTG promotes the fibrillization of amyloidogenic proteins implicated in the pathogenesis of other neurodegenerative diseases such as Alzheimer disease, supranuclear palsy, Huntington disease, and other polyglutamine diseases (22-24). However, recent in vitro studies with full-length α-syn have shown that tTG catalyzes intramolecular cross-linking of monomeric α-syn and inhibits, rather than promotes, its fibrillization in vitro (25, 26). The structural basis of this inhibitory effect and the exact residues involved in tTG-mediated cross-linking of α-syn, as well as structural and functional consequences of these modifications, remain poorly understood.
FIGURE 2.
tTG-catalyzed cross-linking of α-syn involves one to three intramolecular cross-links. A-C, MALDI-TOF/TOF analysis of native (—) and cross-linked (- - -) α-syn, showing that most tTG-catalyzed cross-linking products of WT or disease-associated mutant forms of α-syn are intramolecularly linked (predominant peak with two cross-links), and up to three intramolecular cross-links can occur (left shoulder). The abbreviations M and m/cl are used to designate native and cross-linked α-synuclein, respectively. D and E, kinetic analysis of α-syn (A30P) cross-linking monitored by MALDI-TOF and SDS-PAGE. F, schematic depiction of the tTG-catalyzed chemical reaction (isodipeptide formation) between glutamine and lysine residues.
In this study, we have identified the primary glutamine and lysine residues involved in tTG-catalyzed, intramolecularly cross-linked monomeric α-syn and investigated how cross-linking these residues affects the oligomerization, fibrillization, and membrane binding of α-syn in vitro. Using single-site mutagenesis and mass spectrometry applied to exhaustive proteolytic digests of native and cross-linked monomeric α-syn, we identified Gln109 and Gln79 as the major tTG substrates. We demonstrate that the altered electrophoretic mobility of the intramolecularly cross-linked α-syn in SDS-PAGE occurs as a result of tTG-catalyzed cross-linking of Gln109 to lysine residues in the N terminus of α-syn, which leads to the formation of more compact monomers. Consistent with previous studies, we show that intramolecularly cross-linked α-syn forms off-pathway oligomers that are distinct from those formed by the wild-type protein and that do not convert to fibrils within the time scale of our experiments (3-5 days). We also show that membrane-bound α-syn is a substrate of tTG and that intramolecular cross-linking does not interfere with the ability of monomeric α-syn to adopt an α-helical conformation upon binding to synthetic membranes. These studies provide novel mechanistic insight into the sequence and structural basis of events that allow tTG to inhibit α-syn fibrillogenesis, and they shed light on the potential role of tTG-catalyzed cross-linking in modulating the physiological and pathogenic properties of α-syn.
EXPERIMENTAL PROCEDURES
Cloning, Expression, and Purification of α-Syn Variants—Site-directed mutagenesis of α-syn was performed using PCR with the QuikChange™ site-directed mutagenesis kit (Stratagene, La Jolla, CA). A pT7 plasmid carrying the human wild-type α-syn gene was used as a template for mutagenesis. The following mutations were introduced using appropriate mutagenesis primers: Q79N, Q109N, K32R, K34R, K80R, and K96R. The double mutants Q79N/Q109N and K32R/K34R were obtained by introducing a second mutation into the gene containing the corresponding first mutation. All constructs were verified by sequencing. Wild-type α-syn protein and its mutants were expressed in Escherichia coli and purified as described previously (12).
Cross-linking Reactions—Purified human WT or mutant α-syn (35 μm) was cross-linked with 0.008 unit of guinea pig tTG for 2 h at 37 °C. Reaction solutions contained 10 mm Tris (pH 7.6), 150 mm NaCl, 10 mm dithiothreitol, 25 mm HEPES, and 5 mm CaCl2, unless otherwise stated. The enzyme was omitted from control samples because preliminary experiments showed that the omission of CaCl2 or tTG from the reaction produced similar results. Reactions were quenched by the addition of excess EDTA (25 mm), and the degree of cross-linking was assessed by native PAGE, SDS-PAGE, and mass spectrometry. Prior to gel loading, aggregates of very high molecular weight that formed during the incubation were removed using microcon filters with a molecular mass cutoff of 100 kDa (Millipore Corp., Bedford, MA). The retentate (high molecular weight aggregates) and the flow-through solution (monomeric α-syn) were analyzed by 12% native PAGE or SDS-PAGE.
Preparation of Large Unilamellar Vesicles, Small Unilamellar Vesicles, and α-Syn-Liposome Complexes—1-Palmitoyl-2-oleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] sodium salt (POPG, Avanti Polar Lipids Inc., Pelham, AL) was purchased as a chloroform solution, and the solvent was removed by evaporation and lyophilization. The phospholipid was resuspended to a final concentration of 10 mg/ml in a solution of 50 mm HEPES (pH 7.5), 150 mm NaCl. To increase the efficiency of large unilamellar vesicles formation, 10 cycles of freezing in dry ice and thawing in a 37 °C water bath were carried out. Small unilamellar vesicles were prepared by extrusion through a 100-nm polycarbonate membrane (Avestin, Mannheim, Germany) according to the manufacturer's instructions. The large unilamellar vesicles and small unilamellar vesicle preparations were stored at 4 °C and used within 5 days.
Cross-linking of Membrane-bound α-Syn—For vesicle-binding assays, 10 μm α-syn was incubated with freshly prepared POPG vesicles at a mass ratio of 1:18 in 20 mm sodium phosphate buffer (pH 7.5) containing 200 μm CaCl2. The CaCl2 concentration was optimized to prevent salt-induced dissociation of membrane-bound α-syn. The membrane binding of α-syn prior to and after the cross-linking reaction was analyzed by monitoring the extent of α-helical structure in membrane-bound α-syn using CD spectroscopy. Finally, the release of α-syn from the vesicles was triggered by the addition of 300 mm NaCl, and the samples were passed through filters with a molecular mass cutoff of 100 kDa to separate the released α-syn from that contained in the vesicles. The filtrate was subjected to SDS-PAGE and mass spectrometry (MS) analysis to assess the cross-linking state of α-syn.
Far-UV Circular Dichroism Spectroscopy—Far-UV CD spectra were recorded at 25 °C in a Jasco J-715 spectropolarimeter at protein concentrations of 10 or 30 μm in degassed 20 mm sodium phosphate buffer (pH 7.5) using 0.1-cm cuvettes. As many as six spectra were collected for each sample to improve the signal-to-noise ratio. All spectra were corrected for the buffer base line.
Proteolytic Digestions—α-Syn samples were digested in 10 mm Tris (pH 7.6) and 150 mm NaCl using chymotrypsin, Lys-C, and/or Glu-C at a protein-to-enzyme ratio of 100:1 (w/w) unless otherwise stated. Glu-C and chymotrypsin were obtained from Bioconcept (Switzerland), and Lys-C was purchased from Wako (Neuss, Germany). Digestions were carried out for 2 h at 37 °C or overnight at room temperature. Reactions were stopped by the addition of trifluoroacetic acid to a final concentration of 2%. Samples were digested with chymotrypsin or trypsin gold (Promega, Dübendorf, Switzerland) overnight, unless otherwise stated. The digestion was stopped with 1 μl of 10% trifluoroacetic acid, and samples were stored at 4 °C until further use.
Infusion Electrospray Ionization-Mass Spectrometry—Samples were infused at a low flow rate (250 nl/min) through a TriVersa NanoMate source (Advion BioSystems, Ithaca, NY), and MS detection was performed with an LTQ ion trap (Thermo Scientific, Wohlen, Switzerland). After proteolytic digestion, 1.5-2 μg of each sample was desalted and concentrated using C18 Stage Tips (Proxeon, Odense, Denmark) and subsequently analyzed. Specific peptide sequence information was generated by manual collision-induced dissociation fragmentation of the peaks of interest, and Ultra-Zoom single-ion monitoring mode was used when high resolution acquisitions were required. The GPMAW tool (version 7.1, Lighthouse data, Odense, Denmark) was used to generate a list of expected peptides from the different enzymatic digestions, as well as to compute their theoretical fragmentation patterns. The comparison of experimentally measured fragmentation signatures against theoretical ones was performed manually.
Matrix-assisted Laser Desorption/Ionization-Time-of-Flight-Mass Spectrometry—Aliquots (2 μl) of intact purified protein samples were used for MALDI-TOF-MS measurements. Two matrix solutions were prepared as follows: a saturated solution of sinapinic acid in methanol (matrix solution 1) and a 14 mg/ml sinapinic acid solution in 0.1% trifluoroacetic acid/acetonitrile (1:1, matrix solution 2). Depending on the sample and the initial conditions, a dried droplet or a two-layer sample preparation was selected for the molecular weight analysis. For the two-layer preparation, a thin matrix layer was first generated on the target plate using matrix solution 1. The sample (1 μl) were mixed with 1 μl of sinapinic acid/matrix solution 2 and then 0.5 μl of this mixture was deposited on top of the thin layer and allowed to air-dry. In the dried droplet method, the mixture of sample and matrix was deposited directly on the target plate. Samples were deposited twice and analyzed with an ABI 4800 MALDI-TOF/TOF instrument in linear mode (Applied Biosystems, Rotkreuz, Switzerland). Typically, 2400 laser shots were summed by random sampling of the surface to generate the spectra that were calibrated externally using the [M + H]+ peak of insulin, ubiquitin, and cytochrome c, all of which were prepared with the same matrix and deposited close to the samples.
Sample Preparation for Analysis of Protein Digests—Digested protein samples were desalted on C18 StageTips (Proxeon, Odense, Denmark) and mixed 1:1 with either 2,5-dihydroxybenzoic acid (20 mg/ml in 1% picolinic acid/acetonitrile (1:1 v/v)) or α-cyano-4-hydroxycinnamic acid (7 mg/ml in acetonitrile, 0.1% trifluoroacetic acid (1:1 v/v)). Typically, 0.5 μl of this mixture was deposited on the target plate and allowed to air-dry. Samples were analyzed on an ABI 4800 MALDI-TOF/TOF instrument in reflectron mode.
Native and SDS-PAGE and Immunoblotting—Gels were prepared using standard Laemmli techniques. Gels were stained with Simply Blue Safe Stain (Invitrogen). For Western blot analysis, the separated proteins were transferred to a nitrocellulose membrane (Omnilab, Mettmenstetten, Switzerland). The membrane was blocked for 1 h at room temperature under constant rocking using Odyssey blocking buffer (Li-COR Biosciences, Bad Homburg, Germany) and diluted 1:3 in phosphate-buffered saline (PBS). Membranes were incubated with the following primary mouse monoclonal antibodies at 4 °C with constant rocking overnight: mouse anti-α-syn (1:1000 dilution; epitope, residues 15-123 of α-syn; BD Transduction Laboratories), sc-211 (1:500 dilution; epitope, residues 21-125 of α-syn; Santa Cruz Biotechnology), Zymed LB 509 (1:500 dilution; epitope, residues 115-122 of α-syn; Zymed Laboratories Inc.), H3C (1:500 dilution; epitope, amino acids 128-140 of α-syn), and 7071 (1:1000 dilution; epitope, N terminus of α-syn). H3C and 7071 antibodies were kindly provided by Dr. Julia George (Urbana, IL) and Dr. Peter Lansbury (Cambridge, MA), respectively. For detection of γ-glutamyl-lysine cross-links, the mouse monoclonal IgM antibody 81D4 was used (1:500 dilution; Covalab, Villeurbanne, France). Membranes were washed four times with PBS-T (0.01% PBS containing Tween 20), followed by incubation with goat anti-mouse IgM conjugated to Alexa Fluor 680 or, in the case of the 81D4 antibody, to Alexa Fluor 800. Finally, membranes were washed four times with PBS-T and three times with PBS and scanned in a Li-COR scanner at a wavelength of 700 nm or, in the case of the 81D4 antibody, a wavelength of 800 nm.
Transmission Electron Microscopy (TEM)—Samples of α-syn (5-10 μl) were deposited on Formvar-coated 200 mesh copper grids (Electron Microscopy Sciences, Hatfield, PA). Grids were washed with two drops of double-distilled H2O and stained with 2 drops of freshly prepared 1% (w/v) uranyl acetate (Electron Microscopy Sciences). Specimens were viewed on a Philips CIME 12 electron microscope, operated at 80 kV. Digitized photographs were recorded with a slow scan CCD camera (Gatan, model 679).
Aggregation Studies Using the Thioflavin T (ThT) Fluorescence Assay—A sample of α-syn (35 μm, 400 μl) was incubated at 37 °C with constant rotation. Samples for ThT readings and TEM were taken after 24, 48, and 72 h of incubation, unless stated otherwise. Fibril formation was monitored by ThT fluorescence assay. Readings were carried out with both the protein and ThT concentration of 5 μm each in 100 μl of 50 mm glycine-NaOH (pH 8.5). ThT fluorescence measurements were recorded on an “Analyst AD” spectrometer (Bucher Biotec, Basel, Switzerland) at an excitation wavelength of 450 nm and an emission wavelength of 485 nm. The relative fluorescence at 485 nm was used as a measure of the amount of fibrillar aggregates formed in solution. All samples were analyzed in triplicate and corrected for the fluorescence level of freshly solubilized α-syn. Values are expressed as the percentage increase in ThT fluorescence of three measurements (means ± S.D.). Data were plotted using Prism Graph Pad (version 4.0).
RESULTS
tTG Intramolecular Cross-linking Alters the Conformational Properties of the C-terminal Region ofα-Syn—Purified human WT and mutantα-syn (30 μm) were cross-linked with tTG (0.008 unit) for 2 h at 37 °C. Prior to gel loading, the cross-linking reaction mixture was passed through a filter with a molecular mass cutoff of 100 kDa to remove larger aggregates (retentate) that formed during the incubation. The cross-linked monomeric species, which elute through this filter, were then analyzed by 12% native PAGE. Fig. 1A shows that the presence of tTG resulted in a significant shift in the migration of monomeric α-syn (lanes 4-6). Under these conditions, we observed ≥90% cross-linking of WTα-syn or the PD-associated mutants A30P and A53T. The cross-linking products of α-syn are monomeric and contain only intramolecular cross-links. Although these observations have been highlighted in previous studies (25, 26), the structural basis underlying the altered electrophoretic mobility of cross-linked monomeric α-syn has remained a mystery. The CD spectra of native and intramolecularly cross-linked monomeric α-syn were virtually identical and were consistent with a random coil structure (Fig. 8). We hypothesized that the conformational changes induced by tTG-catalyzed cross-linking should affect the ability of antibodies to recognize the monomer. To test this hypothesis, tTG cross-linked human WT, A30P, and A53T α-syn samples were analyzed by Western blotting using antibodies against various regions of α-syn as follows: N-terminal region (7071 and BD Biosciences (epitope residues 15-123)), C-terminal region (sc-211 (epitope residues 121-125)), H3C region (epitope residues 128-140), and LB 509 region (epitope residues 115-122). As expected, all the N- and C-terminal antibodies recognized the bands corresponding to native, noncross-linked WT and mutant α-syn (Fig. 1A, lanes 1-3). The intramolecularly cross-linked monomeric α-syn was recognized by antibodies against residues within the C-terminal region 121-140, but not by antibodies specific for residues in the C-terminal region of 115-122. Interestingly, the intramolecularly cross-linked monomers were only weakly detected by antibodies against the N-terminal region of α-syn (Fig. 1A, lanes 4-6). These findings suggest that tTG catalyzes the formation of intramolecular cross-links involving C-terminal and N-terminal residues in monomeric α-syn. The monoclonal antibody 81D4, which reacts with N-ε (γ-l-glutamyl)-l-lysine isopeptides, recognized full-length cross-linked α-syn but not the native monomeric protein (supplemental Fig. 1).
FIGURE 1.
tTG-mediated intramolecular cross-linking of α-syn alters its conformation, electrophoretic mobility, and accessibility to antibodies against epitopes in the N-terminal region. A, monomeric WT and mutant α-syn analyzed in the absence (lanes 1-3) and presence of tTG (lanes 4-6), with the latter showing significant changes in electrophoretic mobility. The letters M and m are used to designate native and intramolecularly cross-linked α-synuclein, respectively. For immunoblots, full sequence recognition was achieved for native, non-cross-linked WT and mutant α-syn, using N-terminal and C-terminal monoclonal antibodies (lanes 1-3); in contrast, the N termini were not detected in cross-linked samples (lanes 5 and 6). a.a., amino acids. B, SDS-PAGE analysis of chymotrypsin proteolysis of native (lanes 2-7) and cross-linked WT and mutant α-syn (lanes 8-13). Digestion of cross-linked samples gave a pattern of proteolytic fragments (lanes 11-13) that was different from that of native α-syn (lanes 5-7). C, sequence of WT α-syn showing the preferred enzymatic cleavage sites of chymotrypsin (red), Lys-C (green), and Glu-C (blue).
FIGURE 8.
tTG cross-linking does not interfere with the binding ofα-syn to lipid vesicle membranes. A, CD spectra of cross-linked (cl)α-syn in the presence of vesicles. In the presence of POPG vesicles, cross-linked A30P α-syn undergoes a change in secondary structure from random-coil (—) to α-helices (- - -). B, SDS-PAGE analysis of membrane-bound native (lane 5) and cross-linked (lane 6) monomeric α-syn from A after passage through a filter with a molecular mass cutoff of 100 kDa. Both retentate (R) and flow-through (F) were separated onto a 12% SDS-PAGE (lanes 5-8; M, monomeric; m, cross-linked monomeric; L, lipids). C and D, CD spectra of membrane-bound WT and Q mutant α-syn before (C) and after (D) cross-linking.
To further probe the conformational differences between native and intramolecularly cross-linked monomeric α-syn, we subjected both species to proteolysis by chymotrypsin. Proteolytic digestion of 30 μm native α-syn (WT, A30P, or A53T) at a protein/enzyme ratio of 100:1 produced a proteolytic fragment of ∼5 kDa, which corresponded to residues 40-94 (Fig. 1B, lanes 5-7). The corresponding tTG cross-linked monomers were partially resistant to chymotrypsin proteolysis (Fig. 1B, lanes 11-13), as evidenced by the presence of multiple bands in the range of 5-17 kDa. This result suggests significant conformational differences between the native and cross-linked α-syn monomer.
tTG-mediated Intramolecular Cross-linking of α-Syn Occurs Predominantly at Two Sites—To understand the structural basis for the change in electrophoretic mobility that occurs when α-syn becomes intramolecularly cross-linked, we sought to determine the number of tTG-induced cross-links and the reactive residues involved. tTG was incubated with WT α-syn or α-syn mutants associated with PD (A30P, A53T), and the extent of cross-linking was determined by MALDI-TOF-MS (linear mode) and SDS-PAGE. Native monomeric forms of WT, A30P, and A53T α-syn have molecular masses, respectively, of 14460.2, 14486.2, and 14490.2 Da. tTG-catalyzed formation of an isopeptide bond between a glutamine and lysine residue (transamidation) results in the loss of 17.024 Da (Fig. 2F). In the presence of tTG, we observed molecular masses corresponding to monomeric α-syn species containing one, two, or three tTG cross-links (Fig. 2, A-C). The α-syn monomer with two tTG-induced cross-links was consistently observed to be the predominant species under all cross-linking conditions tested so far in our laboratory. These results demonstrate that tTG-induced cross-linking of monomeric α-syn occurs at multiple residues. To determine whether multiple intramolecular cross-linking is required for inducing the conformational changes and altering the electrophoretic mobility of monomeric α-syn, we monitored the kinetics of cross-linking and the shift in the monomeric band by MALDI-TOF-MS and SDS-PAGE, respectively. Intramolecular cross-linking of A30P α-syn is a rapid process that takes place within only a few minutes and appears to be complete with two cross-links after only ∼45 min (Fig. 2, D and E). Within the first 5-15 min, the singly cross-linked monomer was the predominant species, whereas the doubly cross-linked species became the predominant species after 45 min. Although the shift toward the doubly and triply cross-linked species was accompanied by an increase in the fast migrating monomeric α-syn, this species was observed within the first 5 min of the reaction. Similar results were observed for WT and A53T α-syn (data not shown). These results suggest that a single specific intramolecular cross-linking reaction is responsible for the structural changes in monomeric α-syn and that this cross-link may preferentially precede other cross-links.
Residues Gln79 and Gln109 Are the Major Sites of tTG-catalyzed Cross-linking—To identify the residues involved in tTG-catalyzed cross-linking of α-syn, both native and cross-linked A30P α-synuclein (30 μm) were subjected to chymotrypsin digestion and analyzed by SDS-PAGE and MALDI-TOF-MS. Two different peptide fragments, residues 40-94 and 95-125, were found to contain a single intramolecular cross-link (Fig. 3A, upper spectrum). We consistently observed the appearance of a large peptide fragment with a mass average of m/z [6555.4 + H]+, which was due to the tTG-catalyzed cross-linking of the C-terminal fragment (residues 95-125) to the N-terminal fragment (residues 9-39) of α-syn (Fig. 3, A and C). The appearance of the N-terminal fragment of α-syn (residues 9-39) is not consistent with the enzymatic specificity of chymotrypsin, which should normally generate a fragment including residues 5-39. However, because control digestions produced the same fragment, we cannot rule out the possibility that α-syn is susceptible to fragmentation at residue Leu8.
FIGURE 3.
MALDI-TOF/TOF analysis of chymotrypsin-digested cross-linked Q79N mutant α-syn. Proteolytic digestion of cross-linked A30P (A, upper spectrum) and cross-linked Q79N α-syn (A, lower spectrum) yields a large fragment with masses of average m/z 6558.12 [M + H]+ and 6534.47 [M + H]+, respectively, which is explained by a cross-link between fragments 9-39 and 95-125. The difference in mass observed with these cross-linked fragments accounts for the presence of a proline residue in A30P α-syn. B, proteolytic fragments of α-syn generated by chymotrypsin. C, silver-stained SDS-PAGE of chymotrypsin digests, showing a large fragment (white arrow) that appears to be resistant to further digestion, which may account for the cross-linked fragments involving residues 9-39 and 95-125.
Cross-linking at Residue Gln79—The fragment 40-94 contains two glutamine residues that may participate in tTG-catalyzed cross-linking, Gln62 and Gln79. Previous studies by Jensen et al. (21) have shown that tTG-catalyzed cross-linking of peptides corresponding to the NAC region of α-syn involves the formation of an intermolecular isopeptide bond between residues Gln79 and Lys80. To determine whether the single cross-link within the 40-94 fragment corresponds to an isopeptide bond between residues Gln79 and Lys80, we mutated glutamine 79 to an asparagine (Q79N). The Q79N mutant was subjected to tTG-catalyzed cross-linking and chymotrypsin digestion under the same conditions as those used for A30P α-syn. Consistent with the previous finding (21), we did not observe any tTG-catalyzed cross-links in the fragment comprising residues 40-94, suggesting that residue Gln79 is the primary substrate of tTG-catalyzed cross-linking within this region (Fig. 3A). Similar results were observed for WT and the A53T mutant. The Q79N mutation does not affect the formation of the two other cross-linked fragments reported for A30P α-syn, namely intramolecularly cross-linked residues 95-125 and intermolecularly cross-linked fragments (9-39) + (95-125).
Cross-linking at Residue Gln109—The C-terminal fragment 95-125 contains two glutamine residues, Gln99 and Gln109, and three lysine residues, Lys96, Lys97, and Lys102, that may be involved in the apparent tTG-catalyzed cross-linking of this fragment. To determine which of the glutamine residues is involved in tTG cross-linking, we mutated residues Gln99 and Gln109 to asparagine or glutamic acid (Q99E, Q109E, and Q109N) and subjected these mutants to tTG cross-linking and mass spectrometry analysis. Unlike the wild-type protein (Fig. 2A), cross-linking of the Gln79 and Gln109 mutants (Q79E, Q79N, Q109E, and Q109N) resulted in the formation of a singly cross-linked monomeric α-syn as the predominant species (Fig. 4A). Interestingly, the Q79E and Q79N mutants exhibited faster migration after tTG cross-linking, whereas the mutation of Gln109 to Asn or Glu inhibited the formation of the fast migrating α-syn monomer (Fig. 4A). A second minor species with two cross-links was observed in the case of all four mutants. Together, these results suggest that residues Gln79 and Gln109 are the predominant tTG cross-linking sites and demonstrate that the altered conformation and electrophoretic mobility of monomeric α-syn is because of tTG-induced intramolecular cross-linking involving residue Gln109. To test this hypothesis, we generated a double mutant in which residues Gln79 and Gln109 were both mutated to asparagine (Q79N/Q109N). The double mutant Q79N/Q109N did not undergo any cross-linking by tTG, and it exhibited, after incubation with tGT, an electrophoretic mobility in the SDS-PAGE identical to that of native non-cross-linked α-syn (Fig. 4B). These results suggest that cross-linking at residues Gln79 and/or Gln109 is required for the formation of the third cross-link observed in the case of WT α-syn and in the disease-associated mutants A30P and A53T. Interestingly, cross-linking of the Q99E mutant resulted in a shift in electrophoretic mobility and in the formation of intramolecularly cross-linked monomeric species with predominantly two tTG cross-links; these results are identical to those obtained by cross-linking of WT α-syn and the A30P mutant. This rules out the possibility that residue Gln99 contributes to the conformational changes that alter the electrophoretic mobility of cross-linked α-syn.
FIGURE 4.
Residues Gln79 and Gln109 are the primary substrates for tTG-mediated cross-links. A, SDS-PAGE and MALDI-TOF/TOF analysis of tTG-mediated cross-linking of native (—) and cross-linked (- - -) mutants of α-syn with substitutions at the putative reactive glutamine residues. B, mutating residues Gln79 and Gln109 to asparagine in the double mutant Q79N/Q109N abolishes tTG-mediated cross-linking of α-syn, as can be seen by the unchanged, singly, and doubly charged masses of full-length protein (average m/z 14431.37 [M + H]+ and 7216.18 [M + 2H]2+). Inset, SDS-PAGE analysis of tTG-incubated Q79N/Q109N double mutant and A30P α-syn. No shift in migration is observed with this double mutant as compared with A30P α-syn. The abbreviation cl denotes cross-linked α-syn.
Intramolecular Cross-linking by tTG Results in Covalent Association between the C-terminal and N-terminal Regions of α-Syn—The detection of a large peptide corresponding to the cross-linking of two peptide fragments 9-39 and 95-125 (Fig. 3A) raises the possibility that residue Gln109 may be cross-linked to lysine residues in the N-terminal region including residues 9-39. This hypothesis is consistent with NMR structural studies of monomeric α-syn that show long range interactions between the negatively charged C-terminal region and the positively charged N-terminal region of α-syn (27, 28). To determine the reactive lysine residues, we performed extensive proteolysis with multiple enzymes and mass spectrometry studies of the resulting fragments. The theoretical and preferred sites of these enzymatic cleavages in α-syn are shown in supplemental Fig. 2. In the case of the (9-39) + (95-125) cross-linked species, proteolysis by chymotrypsin followed by Lys-C digestion is expected to generate a fragment comprising the N-terminal residues 24-32 cross-linked to the C-terminal fragment 103-125. Indeed, ESI-MS analysis of peptide fragments from WT, A30P, and A53T α-syn revealed a peak with a mass of monoisotopic (mo.) m/z 1191.92 [M + 3H]3+ for WT and A53T and mo. m/z 1200.56 [M + 3H]3+ for A30P α-syn (Fig. 5A). MS/MS fragmentation of cross-linked peptides turned out to be very complex, because both peptides that were cross-linked fragment spontaneously (29), further complicating the de novo peptide sequencing. Nevertheless, a detailed comparison revealed a match between the fragmentation pattern of the candidate parent ion signal of m/z 1191.92 [M + 3H]3+ and the predicted fragments of the 103-25 cross-linked sequence, providing direct evidence of tTG-catalyzed isopeptide formation between residues Gln109 and Lys32 (Fig. 5B). To further confirm the involvement of residue Lys32, we performed the cross-linking reactions using mutants in which amino acids Lys32 and/or Lys34 were mutated to arginine (K32R, K34R, and K32R/K34R). An analysis of these lysine mutants revealed that the formation of this cross-link is likely to depend more on the structural properties of the protein than on the primary sequence, because both the K32R and the K34R mutants retained the ability to cross-link with residue Gln109 and form the cross-linked peptide containing the peptide fragments 24-34 and 103-125 (supplemental Fig. 3). This indicates that residue Lys34 constitutes an alternative site where tTG can catalyze cross-link formation. Interestingly, intramolecular cross-linking of the double mutant K32R/K34R also resulted in the formation of a fast migrating monomeric α-syn species, suggesting that these mutants alter the structure of the monomer in a way that facilitates the residue Gln109 cross-linking with other lysine residues in the N terminus (supplemental Fig. 7).
FIGURE 5.
tTG-mediated cross-linking of residues Gln109 to Lys32. A, ESI-MS analysis of the digestion products obtained after subjecting cross-linked WT, A30P, and A53T α-syn to sequential proteolysis with chymotrypsin followed by Lys-C. Lys-C fails to cleave at residue Lys32, suggesting that this residue is cross-linked to residue Gln109 (inset in A). The difference in mass observed with A30P α-syn is because of the proline residue. B, ESI-MS collision-induced dissociation fragmentation of A30Pα-syn of the parental fragment of m/z 1191.92 [M + 2H]2+, in which the C and N termini are cross-linked together. Fragmentation of C-terminal residues 103-125 appeared more evident as compared with N-terminal residues of 24-34. Nevertheless, the smallest detectable fragment with a mass of m/z 625.28 could be associated to the N-terminal residues 31-33 cross-linked to the C-terminal residues 106-109.
Residue Gln10 Is Also Cross-linked to Residue Lys96—C-terminal chymotryptic digestion of A30P and Q79N (Fig. 3), but not Q109N α-syn (data not shown), yielded a fragment containing residues 95-125 and a single tTG-catalyzed cross-link. Using exhaustive proteolysis by chymotrypsin and Lys-C on Q79N α-syn, followed by ESI-MS fragmentation, we were able to narrow down the residues involved in the cross-link between residues Gln109 and Lys96 (supplemental Fig. 4A). Exhaustive proteolysis of cross-linked Q79N α-syn resulted in a peptide containing a mis-cleaved C-terminal fragment (residues 95-97) cross-linked to a peptide containing residues 103-125 (mo.) m/z 963.33 [M + 3H]3+. The involvement of residue Lys96 in the cross-link was confirmed by mutating amino acid Lys96 to arginine. Analysis of the peptide fragments obtained after tTG-catalyzed cross-linking and proteolysis by chymotrypsin revealed the absence of the intramolecular cross-link in the 95-125 fragment of K96R α-syn (supplemental Fig. 4B).
tTG-catalyzed Cross-link in the α-Syn NAC Region Involves Residues Gln79 and Lys60—Although previous findings suggest that tTG-catalyzed cross-linking of the NAC peptide (residues 65-95) involves residue Gln79, the lysine residue(s) that react with residue Gln79 have remained unknown. To determine which lysine residue(s) is involved, A30P and Q79E α-syn proteins were subjected to tTG-catalyzed cross-linking, followed by exhaustive digestion with chymotrypsin and Glu-C. Given that tTG cross-linking involving residue Gln79 occurs mainly within the region 40-94, we focused on the chymotryptic fragments generated from this peptide. Proteolysis with Glu-C was expected to yield a proteolytic fragment comprising residues Glu62-Glu83. However, MS analysis of exhaustive digests of cross-linked A30P α-syn samples showed a predominant species with one missed cleavage at residue Glu61, resulting in a fragment comprising residues Lys58-Glu83 with a mass of mo. m/z 2597.40 [M + H]+ (Fig. 6A). The theoretical monoisotopic mass of this fragment is 2614.43 [M + H]+, indicating that this fragment retains a single tTG-catalyzed cross-link (Fig. 6A, top left inset). Nevertheless, a small population of the same cross-linked Lys58-Glu83 fragment with a monoisotopic mass of m/z 2615.42 [M + H]+ was found, clearly indicating cleavage at residue Glu61 by Glu-C (Fig. 6A, top right of inset). This latter fragment was the result of cross-linking between the Lys58-Glu61 tetrapeptide (mo. 504.29 Da) and the Gln62-Glu83 fragment (mo. 2127.15 Da): (504.29 + 2127.15 Da) - 17.024 Da = mo. m/z 2615.41 [M + H]+. These findings suggest that residue Gln79 cross-links to residue Lys60 instead of residue Lys80. To test this hypothesis, we carried out similar studies with the double mutant Q79E/Q79N. Analysis of the cross-linked Q79E α-syn digests revealed the absence of any cross-links within the Lys58-Glu83 fragment, despite the presence of residue Gln62 within this sequence, which could act as a tTG substrate (Fig. 6A and below). To rule out the possibility that residue Lys80 is involved in this cross-link, we mapped the lysine residues involved in tTG-catalyzed cross-linking of residue Gln79 in the Q109E mutant. We chose this mutant to allow examination of intramolecular cross-linking within the N-terminal region in the absence of conformational changes induced by cross-links involving the C terminus. ESI-MS analysis of cross-linked Q109E α-syn subjected to sequential proteolysis with chymotrypsin followed by Lys-C clearly resulted in cleavage at residue Lys80 and generation of a cross-linked proteolytic fragment (Fig. 6B) with a monoisotopic mass of m/z 1070.56 [M + 2H]2+, corresponding to residues Thr59-Lys80. Control Lys-C digestions of native Q109E α-syn did not result in the formation of any proteolytic fragments with missed cleavage sites, further indicating that the mis-cleavage at residue Lys60 in tTG-cross-linked Q109E α-syn is due only to a cross-link present at this residue. To further confirm the involvement of residue Lys60 in this cross-link, we mutated Lys80 to arginine. If our hypothesis was correct, then the K80R mutation should not influence the number of tTG-catalyzed cross-links in monomeric α-syn, and we should still see the 59-80 fragment containing a single tTG-catalyzed cross-link. Indeed, no changes in intramolecular cross-links were found in full-length K80R mutant α-syn, and MS analysis of chymotrypsin digests still showed the presence of a cross-link on a peptide fragment corresponding to residues 40-94 (Fig. 6C). Therefore, our results rule out the possibility that residue Lys80 serves as a substrate in forming an intramolecular cross-link within the NAC region.
FIGURE 6.
Residue Gln79 is cross-linked to residue Lys60 within the NAC region of α-syn. A, MALDI-TOF/TOF (reflectron mode) analysis of cross-linked and digested A30P and Q79E α-syn. Sequential digestion of cross-linked A30P and Q79E α-syn with chymotrypsin followed by Glu-C generates a fragment of residues 58-83, with a failure to cleave at residue Glu61. Only fragments derived from A30P α-syn appear to bear a cross-link within this region, whereas fragments from Q79E α-syn no longer bear this cross-link. A small population of A30P α-syn was completely digested by Glu-C, and in this case two fragments, Lys58-Glu61 and Gln62-Glu83, were found to be cross-linked. B, ESI-MS analysis of cross-linked Q109E α-syn followed by chymotrypsin and Lys-C digestion reveals a fragment containing residues Thr59-Lys80 and an internal cross-link (inset). The incomplete cleavage at residue Lys60 suggests a possible site of cross-linking. C, MALDI-TOF/TOF analysis (reflectron mode) of chymotrypsin-digested native K80R α-syn (upper panel) and cross-linked K80R α-syn (lower panel) demonstrates that cross-linking within the proteolytic fragment comprising residues 40-94 is detected, providing further evidence that residue Lys60 serves as the primary reactive lysine residue in this particular cross-link.
Intramolecular Cross-linking of the C- and N-terminal Region of α-Syn Induces a Conformational State That Does Not Form upon Intermolecular Cross-linking of α-Syn-(1-102) and -(103-140)—To determine whether the formation of the compact monomeric structure, rather than simple cross-linking of the C- and N-terminal regions, is responsible for the change in electrophoretic mobility and tTG inhibition of α-syn fibrillization, we investigated the cross-linking of a truncated mutant of α-syn-(1-102), which lacks the C-terminal region containing 103-140. Incubation of truncated α-syn-(1-102) with tTG resulted in the formation of two intramolecular cross-links (supplemental Fig. 5A) but did not lead to a shift in the mobility of α-syn in SDS-PAGE (supplemental Fig. 5C, lanes 4 and 5). We reasoned that if the interactions between the C- and N-terminal regions of α-syn facilitate tTG-catalyzed cross-linking of Gln109 with N-terminal lysine residues, then coincubation of α-syn-(1-102) with a purified C-terminal fragment comprising residues 103-140 in the presence of tTG should result in the formation of an intermolecular cross-link. Indeed, coincubation of both fragments in the presence of tTG resulted in the formation of an α-syn species with a molecular mass and SDS-PAGE mobility similar to those of full-length α-syn (supplemental Fig. 5, B and C). Mass spectrometry analysis of the cross-linking product revealed a new peak (supplemental Fig. 5B, right inset), with the mass of m/z 14428.9375 [M + H]+, which is consistent with a transamidation reaction between intramolecularly cross-linked α-syn-(1-102) (average m/z 10156.32 [M + H]+) and a C-terminal fragment (average m/z 4289.43 [M + H]+). Intermolecular cross-linking of α-syn-(1-102) and -(103-140) resulted in the appearance of a new band with mobility identical to that of the native α-syn monomer, suggesting that changes in electrophoretic mobility require cross-linking of the full-length α-syn monomer and that such mobility shifts reflect conformational changes induced by intramolecular cross-linking of the C- and N-terminal regions of α-syn.
tTG-catalyzed Intramolecular Cross-linking of WT and Mutant α-Syn Inhibits α-Syn Fibrillization in Vitro—Previous studies by Konno et al. (25) and Segers-Nolten et al. (26) have shown that tTG-catalyzed cross-linking blocks the fibrillization of α-syn. To determine whether the intramolecular cross-linked monomeric species produced under our conditions are similar to those reported previously, we investigated their ability to aggregate and form amyloid fibrils in vitro. Purified monomeric human WT α-syn and the disease-associated α-syn mutants A53T and A30P were cross-linked as described above. Following cross-linking, monomeric α-syn was separated from both tTG-catalyzed and intermolecularly cross-linked higher molecular weight aggregates using filters with a molecular mass cutoff of 100 kDa, as described under “Experimental Procedures.” Triplicate samples (400 μl) of native and cross-linked monomeric WT and mutant α-syn (35 μm) were incubated at 37 °C with agitation, and fibril formation was monitored by ThT fluorescence and TEM. Under these conditions, the majority of native A53T α-syn was converted to fibrils within 72 h (Fig. 7, top), whereas the ThT signal of the cross-linked monomer remained at base line even after extended incubation. Similar results were observed for both WT and A30P α-syn (data not shown). TEM of the samples after 72 h revealed predominantly fibrillar assemblies in the samples containing native A53T α-syn, whereas cross-linked A53T α-syn failed to undergo fibrillogenesis and instead formed predominantly spherical aggregates (Fig. 7). To determine which cross-link is responsible for blocking the ability of cross-linked α-syn to undergo fibrillogenesis, we investigated the ability of the Q79N, Q109N, and Q79N/Q109N mutants to undergo fibrillogenesis before and after cross-linking with tTG. tTG-catalyzed cross-linking of Q79N and Q109N α-syn reduces their ability to form fibrils (Fig. 7). Spherical aggregates similar to those formed by the WT protein were observed to be the predominant species. However, mutating both residues to Q79N and Q109N blocked tTG-induced cross-linking and inhibition of amyloid formation (see TEM in Fig. 7, clQ79N/Q109N), suggesting that tTG-catalyzed cross-linking of residues Gln79 and Gln109 is essential for inhibiting the fibrillization of α-syn. To further investigate the structural properties of the oligomeric species formed by cross-linked α-syn monomers, we compared their structure and amyloidogenic properties by TEM and ThT. Following incubation of monomeric α-syn with tTG, oligomeric aggregates were recovered and separated from monomers by passing through filters with a molecular mass cutoff of 100 kDa. Oligomeric species formed by non-cross-linked WT α-syn monomers showed substantial ability to bind ThT, whereas oligomeric species formed by tTG-catalyzed cross-linking of monomers showed minimal ThT binding (supplemental Fig. 6). These results suggest that these oligomers do not possess amyloid-like structure, a finding that is consistent with their inability to convert to amyloid fibrils (supplemental Fig. 6).
FIGURE 7.
tTG inhibition of α-syn fibrillization is mediated by intramolecular cross-linking that involves residues Gln79 and Gln109. Negatively stained TEM images (scale bar, 0.2 μm) and the corresponding ThT fluorescence of native and cross-linked A53T (upper panels) and Gln to Asn α-syn mutants (Q79N, Q109N, and Q79N/Q109N; lower panels) after 3-4 days of incubation under fibril-forming conditions. The ThT values are the mean ± S.D. of three independent measurements. cl, cross-linked.
Cross-linked α-Syn Binds to Membranes of Lipid Vesicles and Undergoes a Random Coil to α-Helix Transition—To determine whether the tTG-catalyzed cross-linking of monomeric α-syn influences its membrane binding, we investigated the ability of cross-linked α-syn to bind synthetic membranes by CD and filtration assays. Cross-linked WT, A30P, or A53T α-syn (17 μm) was incubated with POPG vesicles at a protein/vesicle ratio of 1:7 for 2 h at room temperature to allow sufficient time for membrane binding. In the presence of POPG vesicles, the secondary structure of both native and cross-linked α-syn monomers shifted from random coil to α-helices (Fig. 8A). To determine the extent of membrane binding, we separated the vesicle-bound α-syn from the free α-syn by ultracentrifugation and filtration of samples through a membrane with a molecular mass cutoff of 100 kDa, and then the retentate and flow-through was analyzed using SDS-PAGE. Fig. 8B (lanes 4 and 7) shows that both native and tTG cross-linked α-syn monomers bound completely to POPG vesicles. It should be noted that 100% POPG containing vesicles are highly charged and provide much more driving force for membrane interaction, which may not be the case for membranes with a physiologically relevant lipid composition.
To further probe the effect of tTG-catalyzed cross-linking on the membrane binding properties of α-syn, we sought to determine whether cross-linking membrane-bound monomeric α-syn disrupts its secondary structure and membrane binding. Native and tTG cross-linked WT α-syn and the glutamine mutants (Q79N, Q109N, and Q79N/Q109N) were incubated with vesicles, and membrane binding was monitored by CD. Fig. 8, C and D, shows that the secondary structure of the membrane-bound form of all four proteins was unaffected by tTG-catalyzed cross-linking. In the membrane-bound state, the N-terminal region (1-85) of α-syn adopts an α-helical conformation. However, it is likely that the membrane-bound structure of the cross-linked α-syn may differ from that adopted by the native state because of conformational strains induced by the intramolecular cross-linking, particularly those involving the C and N terminus of α-syn.
To determine whether intramolecular cross-linking of the C- and N-terminal regions can still take place in the membrane-bound monomeric form of α-syn, tTG was incubated with α-syn bound to POPG vesicles and analyzed by mass spectrometry. Dissociation of the membrane-bound α-syn was achieved by the addition of 300 mm NaCl, followed by passage through filters with a molecular mass cutoff of 100 kDa to separate released α-syn from that contained in vesicles. We observed that tTG-catalyzed cross-linking of membrane-bound α-syn occurs at only one position (Fig. 9), as evidenced by the change in monomeric mass from average m/z 14486.22 H+ to 14469.88 H+. This cross-link does not cause a shift in the migration of α-syn, consistent with NMR studies demonstrating that the C- and N-terminal regions do not interact in the membrane-bound state (30).
FIGURE 9.
Limited tTG-mediated cross-linking of membrane-bound A30P α-syn. Vesicle-bound α-syn (lane 2) was dissociated from membranes by increasing the NaCl solution concentration to 300 mm. Dissociated α-syn was subjected to filtration using filters with a molecular mass cutoff of 100 kDa, and the filtrate (lane 4) was analyzed by MALDI-TOF-MS. MS analysis of vesicle-bound and cross-linked α-syn shows limited (only 1) cross-linking by tTG. This single cross-link does not induce the above observed shift in gel migration. The abbreviations R and F denote retentate and flow-through, respectively.
DISCUSSION
Tissue transglutaminase cross-links a wide range of protein substrates in vitro, including several proteins implicated in the pathogenesis of neurodegenerative diseases, such as Aβ (31), Tau (32, 33), and amyloid precursor protein (34), which are linked to Alzheimer disease; α-syn (21, 25, 26), which is linked to Parkinson disease; and polyglutamine domains (35-37), which are linked to Huntington disease and related polyglutamine disorders. Interestingly, despite the presence of multiple glutamine and lysine reactive sites in these proteins, tTG-mediated cross-linking appears to be specific and involves a small subset of glutamine and lysine residues (Ref. 38 and this study), suggesting that tTG-catalyzed cross-linking of these substrates depends on their primary sequence and/or their conformation.
This study provides mechanistic insight into the sequence and structural basis of events through which tTG inhibits α-syn fibrillogenesis in vivo, and the study sheds light on the potential role of tTG-catalyzed cross-linking in modulating the physiological and pathogenic properties of α-syn. We have shown that tTG-catalyzed cross-linking of monomeric α-syn involves multiple (2 to 3) cross-links. By subjecting tTG cross-linked monomeric α-syn to sequential proteolysis by multiple enzymes and subsequent analysis of the digests by mass spectrometry, we have succeeded in identifying the key glutamine and lysine residues involved in tTG-catalyzed intramolecular cross-linking of α-syn. These studies show that the residues Gln79 and Gln109 serve as the primary sites of tTG-catalyzed cross-links. Mutating both residues to asparagines abolishes tTG-catalyzed cross-linking of α-syn and tTG-mediated inhibition of α-syn fibrillization in vitro. To further understand the sequence and structural basis of these effects, we sought to identify the lysine residues that participate in the formation of isopeptide bonds with residues Gln79 and Gln109.
Residue Gln109 participates in multiple cross-links involving predominantly residue Lys32 and, to some extent, Lys96. Previous studies suggested that the change in electrophoretic mobility is associated with the formation of more compact α-syn monomers, but they did not provide further insight into the structural basis underlying the conformational changes induced by tTG. Using single-site mutagenesis to change each of the putative glutamine (Gln79, Gln99, and Gln109) and lysine (Lys32, Lys34, Lys60, Lys80, and Lys96) residues to asparagine and arginine, we demonstrated that a single intramolecular cross-link involving residues Gln109 and Lys32 is responsible for the observed change in electrophoretic mobility of monomers intramolecularly cross-linked using tTG. Cross-linking between residue Gln109 and the N-terminal lysine residue Lys32 is consistent with the long range interactions previously reported between the C- and N-terminal regions of α-syn (27, 28). Formation of this cross-link occurs independently of cross-links involving residue Gln79, and it appears to facilitate the formation of a third isopeptide between residues Gln99 and Lys96, resulting in further compaction of the cross-linked monomer. Consistent with these conclusions, mutation of residue Gln109 to asparagine (Q109N) or to glutamic acid (Q109E) abolished the change in electrophoretic mobility observed for WT, Q79N, and Q99N α-syn proteins cross-linked in the presence of tTG. The rapid cross-link formation within the first minutes of the reaction and the concomitant increase in the amount of protein migrating faster on the gel suggest that cross-linking of the C and N termini precedes cross-linking of the NAC region. The conformational changes caused by tTG intramolecular cross-linking of monomeric α-syn explain our failure to detect cross-linked α-syn using antibodies against the N-terminal residues 15-120, whereas C-terminal detection is readily achieved using antibodies against residues distant from Gln109 (i.e. antibodies Sc211-(121-125) and H3C-(128-140)). This observation highlights the potential impact of post-translational modifications on the immunoreactivity of α-syn and underscores the importance of using multiple antibodies against different regions in α-syn to analyze post-mortem tissues and biological samples.
The involvement of residue Gln79 in tTG-catalyzed cross-linking was reported earlier by Jensen et al. (21), who showed that tTG catalyzed intermolecular cross-linking and dimerization of the NAC peptide. This is mediated by isopeptide formation between residue Gln79 of one subunit and residue Lys80 of a neighboring subunit. The presence of Lys80 adjacent to Gln79 suggests that these two residues may be cross-linked by tTG. This hypothesis was supported by a report by Ikura et al. (39) showing that tTG-catalyzed cross-linking of synthetic Aβ-(1-28) results in the formation of an isopeptide bond between residues Gln15 and Lys16 and a shift in the SDS-PAGE mobility of Aβ-(1-28). However, in this study, we ruled out the possibility that residue Lys80 serves as a tTG substrate during intramolecular cross-linking. Instead, we show that within the NAC region, tTG-catalyzed cross-linking involves the formation of an isopeptide bond between residues Gln79 and Lys60. Under the conditions used here, intramolecular cross-linking appears to precede intermolecular cross-linking of monomericα-syn, although the latter is favored at higher protein concentrations. Formation of cross-linked α-syn dimers was observed only after longer incubations and/or at higher concentrations of protein and tTG.
Implications for the Mechanism of α-Syn Aggregation and Its Function in Health and Disease—Consistent with previous reports (25, 26), we observed that intramolecular cross-linking of α-syn blocks its fibrillization and results in the accumulation of spherical aggregates with structural morphologies and dye-binding properties that are distinct from those of protofibrillar aggregates formed by native, non-cross-linked α-syn; these results were observed for WT, A30P, and A53T proteins. The non-fibrillar aggregates formed by intramolecularly cross-linked α-syn bind weakly to ThT and do not convert into amyloid fibrils even after extended incubation at 37 °C. The inhibition of α-syn fibrillization may be explained by structural distortions induced by the cross-linking of residues Gln79 to Lys60 and/or of Gln109 to Lys32/Lys34; both of these cross-links are expected to alter the conformational properties of the NAC region, which plays a crucial role in the initiation and propagation of α-syn fibrillization. Interestingly, similar observations have been reported for other unfolded and amyloid-forming substrates of tTG, including Huntingtin, Tau, human Tau proteins (τ4RD), and the N-terminal region of the yeast prion Sup35 (SupNM). This means that tTG-mediated intramolecular cross-linking of these proteins inhibits their fibrillization and results in the formation of soluble, non-amyloidogenic aggregates (25, 40, 41). These observations are also consistent with pathological findings showing the absence of tTG in Lewy bodies and of polyglutamine inclusions containing elevated levels of ε-(γ-glutamyl)-lysine cross-links, suggesting that the tTG-catalyzed formation of intra- and/or intermolecular cross-linked species occurs in the cytosol, and these species subsequently aggregate or become sequestered into inclusion bodies (18, 42, 43).
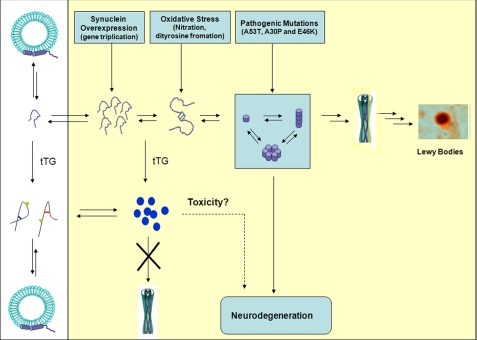
Our studies do not address the functional consequences of tTG cross-linking of α-syn in vivo. However, the results presented here, in combination with published data, point to a mechanistic model that can be tested in vivo and in cellular models of synucleinopathies. Fig. 10 presents a schematic depiction summarizing our current understanding of α-syn aggregation and membrane binding and how cross-linking may affect these processes. α-Syn exists in the cytosol in a disordered conformation and undergoes a reversible shift from random coil to α-helix upon binding to membranes. Increased expression, post-translational modifications (truncations), and/or oxidative stress can trigger α-syn oligomerization and fibril formation. Protofibrillar α-syn has been shown to induce toxicity via several mechanisms, including disruption of membranes and the formation of amyloid pores. tTG-induced intramolecular cross-linking of α-syn blocks the formation of fibrils without significantly interfering with the interaction of α-syn with membranes. Although intramolecularly cross-linked α-syn remains capable of forming high molecular weight aggregates, these aggregates appear to be off-pathway or dead-end products. These findings suggest that tTG may protect against α-syn toxicity by increasing its solubility and inhibiting its ability to form toxic aggregates (i.e. protofibrils and fibrils). Therefore, the increased levels of tTG measured in the cerebrospinal fluid (44), as well as the presence of cross-linked α-syn in post-mortem brain tissue of PD patients (18) and during normal aging (45), may reflect the activation of natural defense mechanisms to prevent amyloid formation or promote clearance of α-syn aggregates. This hypothesis assumes that intramolecular cross-linking of α-syn does not disrupt the normal function(s) of α-syn and that the aggregates formed by the cross-linked monomer are not toxic. Given that, several protein-protein and protein-ligand interactions and post-translational modifications of α-syn have been mapped to the C-terminal region (residues 110-140). Covalent cross-linking of the C- and N-terminal regions is expected to affect these interactions and the accessibility of key residues that are modified post-translationally. Furthermore, the effect of tTG-catalyzed intra- and intermolecular cross-linking of α-syn on the subcellular localization of the protein has not been investigated. It is likely that only a fraction of α-syn is cross-linked by tTG, which may be sufficient to maintain α-syn below the critical concentration required for oligomerization without significantly affecting its physiological functions. Further studies to elucidate the molecular and functional consequences of tTG-induced cross-linking of α-syn in vivo are required before the exploration of therapeutic strategies based on modulating tTG levels or activity.
FIGURE 10.
Schematic depiction summarizing our current understanding of α-syn aggregation and membrane binding and how cross-linking may affect these processes. Further studies to elucidate the molecular and functional consequences of tTG-induced cross-linking of α-syn in vivo are required before the exploration of therapeutic strategies based on modulating tTG levels or activity.
Acknowledgments
The Laboratory of Molecular Neurobiology and Neuroproteomics was the recipient of Grant 310000-110027 from the Swiss National Science Foundation.
This work was supported by the Swiss Federal Institute of Technology Lausanne. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1-7.
Footnotes
The abbreviations used are: PD, Parkinson disease; tTG, tissue transglutaminase; WT, wild type; α-syn, α-synuclein; LB, Lewy body; ESI-MS, electrospray ionization-mass spectrometry; MALDI-TOF-MS, matrix-assisted laser desorption/ionization-time-of-flight-mass spectrometry; TEM, transmission electron microscopy; POPG, 1-palmitoyl-2-oleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)]; PBS, phosphate-buffered saline; mo., monoisotopic; NAC, non-amyloid component; ThT, thioflavin T.
References
- 1.Spillantini, M. G., Schmidt, M. L., Lee, V. M., Trojanowski, J. Q., Jakes, R., and Goedert, M. (1997) Nature 388 839-840 [DOI] [PubMed] [Google Scholar]
- 2.Pollanen, M. S., Bergeron, C., and Weyer, L. (1993) Brain Res. 603 121-124 [DOI] [PubMed] [Google Scholar]
- 3.Riess, O., Jakes, R., and Kruger, R. (1998) Mol. Med. Today 4 438-444 [DOI] [PubMed] [Google Scholar]
- 4.Polymeropoulos, M. H., Lavedan, C., Leroy, E., Ide, S. E., Dehejia, A., Dutra, A., Pike, B., Root, H., Rubenstein, J., Boyer, R., Stenroos, E. S., Chandrasekharappa, S., Athanassiadou, A., Papapetropoulos, T., Johnson, W. G., Lazzarini, A. M., Duvoisin, R. C., Di Iorio, G., Golbe, L. I., and Nussbaum, R. L. (1997) Science 276 2045-2047 [DOI] [PubMed] [Google Scholar]
- 5.Kruger, R., Kuhn, W., Muller, T., Woitalla, D., Graeber, M., Kosel, S., Przuntek, H., Epplen, J. T., Schols, L., and Riess, O. (1998) Nat. Genet. 18 106-108 [DOI] [PubMed] [Google Scholar]
- 6.Conway, K. A., Harper, J. D., and Lansbury, P. T. (1998) Nat. Med. 4 1318-1320 [DOI] [PubMed] [Google Scholar]
- 7.Conway, K. A., Lee, S. J., Rochet, J. C., Ding, T. T., Williamson, R. E., and Lansbury, P. T., Jr. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 571-576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fredenburg, R. A., Rospigliosi, C., Meray, R. K., Kessler, J. C., Lashuel, H. A., Eliezer, D., and Lansbury, P. T., Jr. (2007) Biochemistry 46 7107-7118 [DOI] [PubMed] [Google Scholar]
- 9.Goedert, M. (1997) Nature 388 232-233 [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa, M., Fujiwara, H., Nonaka, T., Wakabayashi, K., Takahashi, H., Lee, V. M., Trojanowski, J. Q., Mann, D., and Iwatsubo, T. (2002) J. Biol. Chem. 277 49071-49076 [DOI] [PubMed] [Google Scholar]
- 11.Fujiwara, H., Hasegawa, M., Dohmae, N., Kawashima, A., Masliah, E., Goldberg, M. S., Shen, J., Takio, K., and Iwatsubo, T. (2002) Nat. Cell Biol. 4 160-164 [DOI] [PubMed] [Google Scholar]
- 12.Paleologou, K. E., Schmid, A. W., Rospigliosi, C. C., Kim, H. Y., Lamberto, G. R., Fredenburg, R. A., Lansbury, P. T., Jr., Fernandez, C. O., Eliezer, D., Zweckstetter, M., and Lashuel, H. A. (2008) J. Biol. Chem. 283 16895-16905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duda, J. E., Giasson, B. I., Chen, Q., Gur, T. L., Hurtig, H. I., Stern, M. B., Gollomp, S. M., Ischiropoulos, H., Lee, V. M., and Trojanowski, J. Q. (2000) Am. J. Pathol. 157 1439-1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamin, G., Uversky, V. N., and Fink, A. L. (2003) FEBS Lett. 542 147-152 [DOI] [PubMed] [Google Scholar]
- 15.Li, W., West, N., Colla, E., Pletnikova, O., Troncoso, J. C., Marsh, L., Dawson, T. M., Jakala, P., Hartmann, T., Price, D. L., and Lee, M. K. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 2162-2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crowther, R. A., Jakes, R., Spillantini, M. G., and Goedert, M. (1998) FEBS Lett. 436 309-312 [DOI] [PubMed] [Google Scholar]
- 17.Junn, E., Ronchetti, R. D., Quezado, M. M., Kim, S. Y., and Mouradian, M. M. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 2047-2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andringa, G., Lam, K. Y., Chegary, M., Wang, X., Chase, T. N., and Bennett, M. C. (2004) FASEB J. 18 932-934 [DOI] [PubMed] [Google Scholar]
- 19.Kim, S. Y., Marekov, L., Bubber, P., Browne, S. E., Stavrovskaya, I., Lee, J., Steinert, P. M., Blass, J. P., Beal, M. F., Gibson, G. E., and Cooper, A. J. (2005) Neurochem. Res. 30 1245-1255 [DOI] [PubMed] [Google Scholar]
- 20.Selkoe, D. J., Abraham, C., and Ihara, Y. (1982) Proc. Natl. Acad. Sci. U. S. A. 79 6070-6074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen, P. H., Sorensen, E. S., Petersen, T. E., Gliemann, J., and Rasmussen, L. K. (1995) Biochem. J. 310 91-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang, D. S., Dickson, D. W., and Malter, J. S. (2008) Int. J. Clin. Exp. Pathol. 1 5-18 [PMC free article] [PubMed] [Google Scholar]
- 23.Zemaitaitis, M. O., Lee, J. M., Troncoso, J. C., and Muma, N. A. (2000) J. Neuropathol. Exp. Neurol. 59 983-989 [DOI] [PubMed] [Google Scholar]
- 24.De Vivo, G., and Gentile, V. (2008) CNS Neurol. Disord. Drug Targets 7 370-375 [DOI] [PubMed] [Google Scholar]
- 25.Konno, T., Morii, T., Hirata, A., Sato, S., Oiki, S., and Ikura, K. (2005) Biochemistry 44 2072-2079 [DOI] [PubMed] [Google Scholar]
- 26.Segers-Nolten, I. M., Wilhelmus, M. M., Veldhuis, G., van Rooijen, B. D., Drukarch, B., and Subramaniam, V. (2008) Protein Sci. 17 1395-1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertoncini, C. W., Jung, Y. S., Fernandez, C. O., Hoyer, W., Griesinger, C., Jovin, T. M., and Zweckstetter, M. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 1430-1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dedmon, M. M., Lindorff-Larsen, K., Christodoulou, J., Vendruscolo, M., and Dobson, C. M. (2005) J. Am. Chem. Soc. 127 476-477 [DOI] [PubMed] [Google Scholar]
- 29.Emanuelsson, C. S., Boros, S., Hjernoe, K., Boelens, W. C., and Hojrup, P. (2005) J. Biomol. Tech. 16 197-208 [PMC free article] [PubMed] [Google Scholar]
- 30.Bussell, R., Jr., and Eliezer, D. (2003) J. Mol. Biol. 329 763-778 [DOI] [PubMed] [Google Scholar]
- 31.Zhang, J., Guttmann, R. P., and Johnson, G. V. (1998). J. Neurochem. 71 240-247 [DOI] [PubMed] [Google Scholar]
- 32.Dudek, S. M., and Johnson, G. V. (1993) J. Neurochem. 61 1159-1162 [DOI] [PubMed] [Google Scholar]
- 33.Tucholski, J., Kuret, J., and Johnson, G. V. (1999) J. Neurochem. 73 1871-1880 [PubMed] [Google Scholar]
- 34.Ho, G. J., Gregory, E. J., Smirnova, I. V., Zoubine, M. N., and Festoff, B. W. (1994) FEBS Lett. 349 151-154 [DOI] [PubMed] [Google Scholar]
- 35.Cooper, A. J., Jeitner, T. M., Gentile, V., and Blass, J. P. (2002) Neurochem. Int. 40 53-67 [DOI] [PubMed] [Google Scholar]
- 36.Violante, V., Luongo, A., Pepe, I., Annunziata, S., and Gentile, V. (2001) Brain Res. Bull. 56 169-172 [DOI] [PubMed] [Google Scholar]
- 37.Gentile, V., Sepe, C., Calvani, M., Melone, M. A., Cotrufo, R., Cooper, A. J., Blass, J. P., and Peluso, G. (1998) Arch. Biochem. Biophys. 352 314-321 [DOI] [PubMed] [Google Scholar]
- 38.Miller, M. L., and Johnson, G. V. (1995) J. Neurochem. 65 1760-1770 [DOI] [PubMed] [Google Scholar]
- 39.Ikura, K., Takahata, K., and Sasaki, R. (1993) FEBS Lett. 326 109-111 [DOI] [PubMed] [Google Scholar]
- 40.Lai, T. S., Tucker, T., Burke, J. R., Strittmatter, W. J., and Greenberg, C. S. (2004) J. Neurochem. 88 1253-1260 [DOI] [PubMed] [Google Scholar]
- 41.Karpuj, M. V., Becher, M. W., Springer, J. E., Chabas, D., Youssef, S., Pedotti, R., Mitchell, D., and Steinman, L. (2002) Nat. Med. 8 143-149 [DOI] [PubMed] [Google Scholar]
- 42.Chun, W., Lesort, M., Tucholski, J., Ross, C. A., and Johnson, G. V. (2001) J. Cell Biol. 153 25-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lesort, M., Chun, W., Tucholski, J., and Johnson, G. V. (2002) Neurochem. Int. 40 37-52 [DOI] [PubMed] [Google Scholar]
- 44.Bonelli, R. M., Aschoff, A., Niederwieser, G., Heuberger, C., and Jirikowski, G. (2002) Neurobiol. Dis. 11 106-110 [DOI] [PubMed] [Google Scholar]
- 45.Park, S. C., Yeo, E. J., Han, J. A., Hwang, Y. C., Choi, J. Y., Park, J. S., Park, Y. H., Kim, K. O., Kim, I. G., Seong, S. C., and Kwak, S. J. (1999) J. Gerontol. A. Biol. Sci. Med. Sci. 54 B78-B83 [DOI] [PubMed] [Google Scholar]