Abstract
Objectives:
The aim of the present study was to evaluate the cytotoxic effects of three different provisional restoration materials on fibroblasts. Two bis-acrylic based [Tempofit Duomix (Detax), Protemp 3 Garant (3M ESPE)] and one urethan dimethacrylate [Revotek LC (GC Corporation)] based provisional restoration materials used.
Methods:
Materials were prepared according to the manufacturers’ instructions in standard teflon disks (2×5 mm) and four samples were extracted in 7 ml of Basal Medium Eagle with 10% new born calf serum and 100 mg/ml penicillin/streptomycin for 24 hours. The L929 fibroblast cells were plated (25.000 cells/ml) in well plates, and maintained in a CO2 incubator at 37°C for 24h. After 24 hours, the incubation medium was replaced by the immersed medium in which the samples were stored and the L929 fibroblasts were incubated in contact with eluates for 24 hours at 37°C for 24h. The fibroblast cell viability was analyzed by measuring the mitochondrial activity with the methyltetrazolium test (MTT). Twelve well used for each specimen and experiment repeated for two times. The data was statistically analyzed by Mann-Whitney U tests.
Results:
The results showed that, Revotek LC and Protemp 3 Garant were not cytotoxic for fibroblast cells when compared to control group (P>.05). However, Tempofit duomix was cytotoxic for L929 fibroblasts when compared to control group and other tested materials (P<.05).
Conclusions:
Taking into consideration the limitations of an in vitro study, our study indicate that provisional restoration materials might have cytotoxic effects on fibroblasts and should be selected carefully for clinical applications.
Keywords: Provisional restoration materials, Cytotoxicity, Fibroblast, MTT
INTRODUCTION
Dental materials contain a great variety of different monomers and additives.1 Because of the complex chemical composition and the incomplete monomer–polymer conversion, several components are leached out from each resin-based restorative material into the oral environment.2,3 This in turn may cause some adverse effects.4 Previous studies have used in vitro cytotoxicity tests to evaluate the biological risks of resin composites used in dentistry.1,5 Cytotoxicity tests have primarily focused on restorative materials such as glass ionomers, dental adhesives and composite resins.6–8 However, fewer studies on prosthodontic materials have been published, and investigations regarding the cytotoxicity of provisional prosthodontic materials are even more limited.5,9
Provisional restorations are used in the interim between tooth preparation and fitting a definitive restoration. The length of time between preparation of teeth and cementation of final restorations can vary from a few days for straightforward cases, to several weeks or even, in the case of complex reconstruction, several months. Provisional restorations are generally essential to cover freshly cut dentine, stabilize the position of the prepared tooth, regain chewing function and phonation, maintain esthetic appearance and evaluate the minimal thickness of the definitive restoration. They can also help stabilize the periodontal condition prior to definitive restoration.10
Provisional materials can be classified by the type of resin. Acrylic polymethyl or polyethyl methacrylates belong to the oldest group of provisional materials. The latest class of materials is formed by bis-acryl composite resins, which are comparable to composite resins used for direct restoration therapy.10 They consist of an organic matrix and inorganic fillers. Bis-acryl composites produce less heat and shrinkage during polymerization than other resins, resulting in a better marginal fit.11 Aesthetically they are reasonable and are more color stable than polymethyl or polyethyl methacrylates.12 Most recently, visible light cured resins have been introduced based on urethane dimethacrylate. These resins have good mechanical properties, being light cured, the operator has some control over the material’s working time and colour is relatively stable but marginal fit can be poor.10,13
Acrylates and mainly methacrylates were found to cause cytotoxic effects.14 Evaluation of the cytotoxicity of dental resin materials showed a relationship between their composition and the degree of cytotoxicity.15 Continuous cell lines, like L929 mouse fibroblasts are being routinely used for the testing of cytotoxic properties of dental materials because of their reproducible growth rates and biological responses.1 The purpose of this in vitro study was to evaluate the effect of current bis-acryl and urethane dimethacrylate based provisional materials on the fibroblast cell viability.
MATERIALS AND METHODS
The provisional restoration materials tested in this study are shown in Table 1. Two of the tested materials were bis-acryl based (Tempofit Duomix, Detax, Germany & Protemp 3 Garant, 3M ESPE, Germany) and one was urethane dimethacrylate based (Revotek LC, GC Corporation, Japan) provisional restoration materials. Test specimens were prepared according to the manufacturers’ instructions in standard teflon discs, 5 mm in diameter and 2 mm of height. All specimens were prepared and handled under aseptic conditions to limit the influence of biological contamination on the cell culture tests. Specimens were prepared between mylor and glass slabs to minimize the oxygen inhibition and maximize the surface smoothness. Tempofit Duomix is a two-part base/catalyst, hand-mix, self-curing and bis-acrylic composite based provisional restoration material. Base and catalyst were extruded equal amounts by pressing onto piston in the dispenser onto mixing pad. Both components mixed with spatula within 20 – 30 sec. homogeneously. Then applied into the teflon disc and after 2 min – 2 min 30 sec curing completed. Protemp 3 Garant is a two-part base/catalyst, auto-mix, self-curing and bis-acrylic composite based provisional restoration material. Using the Garant dispenser, the base and catalyst were extruded directly into the teflon disc and after 2 min 30 sec curing completed. Revotek LC is a light cure single component sculptable composite resin for temporary restorations. Using a spatula required amount of material dispensed and applied into the teflon disc. The specimen was light-cured for 6 sec by LED light curing unit (LED, Bluephase, Ivoclar Vivadent, Liechtenstein, Austria).
Table 1.
Material name, company, lot number and composition.
| Materials | Company | Lot # | Composition |
|---|---|---|---|
| Tempofit Duomix | Detax, Ettlingen, Germany | 315185 | Mixture of methacrylic resins and silane treated glass with auxiliary matters and pigments |
| Protemp 3 Garant | 3M ESPE, Seefeld, Germany | 51200 | Dimethacrylate, Silicic acid, Initiators, Diacrylate, Stabilizers, Synthetic resins, Pigments, Dyes, Strontium glass powder |
| Revotek LC | GC Corporation, Tokyo, Japan | 704091 | Urethane, Silica powder, Camphorquinone |
Four samples prepared for each group for cytotoxicity test. The samples immersed in 7 ml culture medium for 24 hours at 37°C to extract residual monomer or cytotoxic substances. The culture medium containing material extracts were sterile filtered to use on the cell cultures.
Cytotoxicity testing
L929 fibroblast cell line (ATCC CCL 1) cultured in Basal Medium Eagle (BME), Biological Industries, Israel) containing 10% new born calf serum (Biochrom AG, Berlin, Germany) and 100 mg/ml penicilin/streptomysin (Biological Industries, Israel) at 37°C in a humidified atmosphere of 95% air/5% CO2. Cell cultures between the twelve and fifteen passages were used in this study. Confluent cells were detached with 0.25% trypsin and seeded at a density of 5×103 well in 96-well plate at 37°C under 5% CO2 for 24h and. After 24 hours incubation, culture medium was replaced with 200 μl of culture medium containing material extracts of provisional restoration materials. Original culture medium was served as control in this study. Cultures were incubated for 24 hours at 37°C and 5% CO2 for 24 hours. The viability of cells exposed to material extracts was assessed using succinic dehydrogenase activity. The succinic dehydrogenase activity has been shown to be reasonably representative of mitochondrial activity in the cells and reflects both cell number and activity.16 The old medium removed and cell cultures were rinsed with phosphate buffer saline (PBS) and 200 μl aliquots of freshly prepared MTT [3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide, Sigma Aldrich, Germany] solution (0.5 mg/mL in BME) were added to each well. After a 2h incubation period (37°C, 5% CO2) the supernatant was removed and the intracellulary stored MTT formazan was solubilized in 200 μl dimethyl sulfoxide for 30 min at room temperature. The absorbance at 540 nm was spectrophotometrically measured. Twelve replicate cell cultures were exposed to a constant concentration of a single material in at least two independent experiments. The treated groups compared to cell survival in untreated controls. Differences between mean values were statistically analyzed using the Mann-Whitney U test.
RESULTS
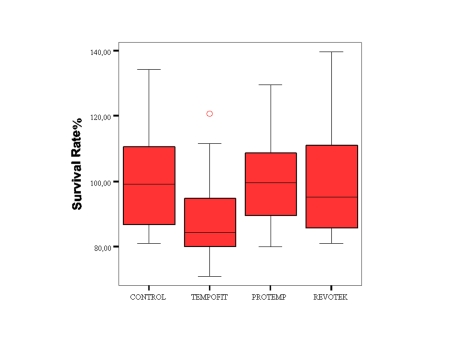
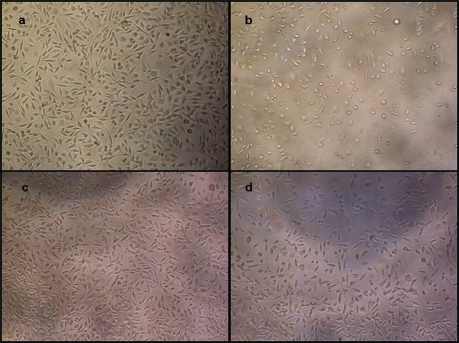
The results of cytotoxicity test with provisional restoration materials are summarized in Figure 1. Reduced cell density is shown for Tempofit in Figure 2(b). In contrast, Protemp 3 Garant group demonstrate full cell density in Figure 2(c).
Figure 1.
Cell survival of L929 cells in a methyltetrazolium test after exposure to provisional restoration materials. Data are expressed as percentage of the control cultures. Cell survival rates were calculated from independent experimental cultures: Control (n=24), Tempofit Duomix (n=24), Protemp 3 Garant (n=24), Revotek LC (n=24).
Figure 2.
Effects of provisional materials on L-929 fibroblasts: (a) control group (Original culture medium), (b) culture medium containing material extracts of Tempofit, (c) culture medium containing material extracts of Protemp 3 Garant and, (d) culture medium containing material extracts of Revotek. Cells were incubated with these mediums for 24 hours (×10).
The results showed that, eluates of the Revotek LC and Protemp 3 Garant lead to 99% and 101% cell survival. Statistically Revotek LC and Protemp 3 Garant were not cytotoxic for cells when compared to control group (P>.05). Eluates from Tempofit duomix lead to 88% cell survival. Tempofit duomix was cytotoxic for cells when compared to control group and other tested materials (P<.05).
DISCUSSION
The literature contains descriptions of cell-culture tests with various cell types to establish cell damage caused by dental materials.17 In the present study the effect of two bis-acryl and one urethane dimethacrylate based commercially available provisional restoration materials on fibroblast cells were investigated by MTT test. Fibroblasts are the targets of any chemical components that may be released from the dental restorative materials. L929 fibroblast cells were selected due to its availability, popularity and efficiency to grow in vitro.18 MTT assay is a well-established method for analyzing cell viability.16 The viability and proliferation of the cells are assessed by means of the functional state of the cell mitochondria.19 Mitochondrial dehydrogenases in living cells reduce the yellow tetrazolium salt, MTT (3-(4,5-dimethyl) thiazol-2-yl) 2,5 diphenyltetrazolium bromide) to blue MTT formazan, which is then retained in the cell. Formation of the formazan product has been found to correlate well with number of viable cells.8,19,20
Today, bis-acryl composites possess considerable amount of the market share for tooth colored provisional material. Main advantages of bis-acryl provisional materials include a lower curing temperature, reduced polymerization shrinkage (5%) with improved marginal fit, and minimal odour and taste.13,21 The low setting temperature of these materials allows them to be used directly with decreased risk of pulpal injury.22 In addition, bis-acryls are gaining in popularity, in part because of their cartridge delivery system. This dispensary method not only is convenient but also may allow for a more accurate and consistent mix.21 Dental practitioners have clearly welcomed these products and very limited data can be available about their cytotoxicity and biocompatibility.
In present study, two of the tested provisional restoration material was bis-acryl based which are chemically very similar to bisphenol-A-glycidyl methacrylate (Bis-GMA) composites. According to our results, eluates from Tempofit duomix lead to 88% cell survival and when compared to control group and other tested materials it was cytotoxic for cells (Figure 2a–b). On the hand Protemp 3 Garant, the other bis-acryl based provisional material, was not cytotoxic for L929 fibroblast cells (Figure 2c). Interestingly slightly increased cell vitality was observed with Protemp 3 Garant (101%). Differences in cytotoxicity can be partly attributed to differences in chemical composition. Protemp Garant has been modified and marketed as Protemp 3 Garant. The modifications include a newly developed monomer system, not with the rigid intermediate chain characteristic of some bis-GMA homologues, but with a somewhat flexible chain in comparison to other synthetic resins (ESPE Technical Product Profile). This modification in the monomer system may limit the cytotoxic potential of the material.
However, manufacturer of Tempofit duomix do not state any difference in monomer formulation. Probably as most other bis-acryl based provisional materials, the organic polymer matrix of Tempofit duomix is composed of traditional monomers such as Bis-GMA, triethylene glycol dimethacrylate (TEGDMA) or similar monomer systems. But one must keep in mind that resin materials may contain rather ‘unknown’ monomers and generally these monomers protect by patents. Patents may also hinder objective research.23 Only available composition of the resin cements tested in this study. They may also contain such unknown monomers.
Current investigations reported the cytotoxic effects of some resin monomers, such as BIS-GMA, TEGDMA and urethane dimethacrylate (UDMA).24,25 These resin monomers are able to deplete intracellular glutathione as well as interfere with the expression of some proteins, such as collagen I, osteonectin, and dentin sialoprotein, which play a fundamental role in the pulp repair.26,27
Among the tested materials, Revotek LC is the only UDMA based and light cure provisional material. Geurtsen et al1 reported that UDMA is as cytotoxic as BIS-GMA and TEGDMA. Elution of residual monomers from resin materials related to degree of their polymerization, properties of resin composition, and chemistry of organic solvents in vitro situation.28 Altıntas et al29 demonstrated that leaching of UDMA was lower than BIS-GMA and TEGDMA from a resin cement. Consequently, in present study, eluates of the Revotek LC showed similar cytotoxicity with control group.
CONCLUSIONS
The results of this study demonstrated that cytotoxic potential may vary among provisional materials. Taking into consideration the limitations of this in vitro study, provisional restoration materials may have cytotoxic effects and should be selected carefully for clinical applications.
REFERENCES
- 1.Geurtsen W, Lehmann F, Spahl W, Leyhausen G. Cytotoxicity of 35 dental resin composite monomers/additives in permanent 3T3 and three human primary fibroblast cultures. J Biomed Mater Res. 1998;41:474–480. doi: 10.1002/(sici)1097-4636(19980905)41:3<474::aid-jbm18>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 2.Spahl W, Budzikiewicz H, Geurtsen W. Determination of leachable components from four commercial dental composites by gas and liquid chromatography/mass spectrometry. J Dent. 1998;26:137–145. doi: 10.1016/s0300-5712(96)00086-3. [DOI] [PubMed] [Google Scholar]
- 3.Szep S, Kunkel A, Ronge K, Heidemann D. Cytotoxicity of modern dentin adhesives—in vitro testing on gingival fibroblasts. J Biomed Mater Res. 2002;63:53–60. doi: 10.1002/jbm.10083. [DOI] [PubMed] [Google Scholar]
- 4.Caughman WF, Caughman GB, Shifflett RA, Rueggeberg F, Schuster GS. Correlation of cytotoxicity, filler loading and curing time of dental composites. Biomaterials. 1991;12:737–740. doi: 10.1016/0142-9612(91)90022-3. [DOI] [PubMed] [Google Scholar]
- 5.Ergün G, Mutlu-Sagesen L, Karaoglu T, Dogan A. Cytotoxicity of provisional crown and bridge restoration materials: an in vitro study. J Oral Sci. 2001;43:123–128. doi: 10.2334/josnusd.43.123. [DOI] [PubMed] [Google Scholar]
- 6.Schedle A, Franz A, Rausch-Fan X, Spitler A, Lucas T, Samorapoompichit P, Sperr V, Boltz-Nitulescu G. Cytotoxic effects of dental composites, adhesive subtances, compomers and cements. Dent Mater. 1998;14:429–440. doi: 10.1016/s0300-5712(99)00018-4. [DOI] [PubMed] [Google Scholar]
- 7.Huang F, Chang Y. Cytotoxicity of resin-based restorative materials on human pulp cell cultures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:361–365. doi: 10.1067/moe.2002.126341. [DOI] [PubMed] [Google Scholar]
- 8.Nalçaci A, Oztan MD, Yilmaz S. Cytotoxicity of composite resins polymerized with different curing methods. Int Endod J. 2004;37:151–156. doi: 10.1111/j.0143-2885.2004.00779.x. [DOI] [PubMed] [Google Scholar]
- 9.Ye RR, Shen QP, Qiao GY.Cytotoxicity of three kinds of temporary crown and bridge materials on mouse fibroblasts in vitro Shanghai Kou Qiang Yi Xue 200817308–312.(Abstract). [PubMed] [Google Scholar]
- 10.Wassell RW, St George G, Ingledew RP, Steele JG. Crowns and other extra-coronal restorations: provisional restorations. Br Dent J. 2002;15:619–630. doi: 10.1038/sj.bdj.4801443. [DOI] [PubMed] [Google Scholar]
- 11.Tjan AH, Castelnuovo J, Shiotsu G. Marginal fidelity of crowns fabricated from six proprietary provisional materials. J Prosthet Dent. 1997;77:482–485. doi: 10.1016/s0022-3913(97)70140-9. [DOI] [PubMed] [Google Scholar]
- 12.Lang R, Rosentritt M, Leibrock A, Behr M, Handel G. Colour stability of provisional crown and bridge restoration materials. Br Dent J. 1998;185:468–471. doi: 10.1038/sj.bdj.4809839. [DOI] [PubMed] [Google Scholar]
- 13.Rosenstiel SF, Land MF, Fujimoto J. Contemporary fixed prosthodontics. 3rd Ed. St. Louis: CV Mosby; 2001. pp. 380–416. [Google Scholar]
- 14.Yoshii E. Cytotoxic effects of acrylates and methacrylates: Relationships of monomer structures and cytotoxicity. Biomed Mater Res. 1997;37:517–524. doi: 10.1002/(sici)1097-4636(19971215)37:4<517::aid-jbm10>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 15.Sideridou ID, Achilias DS. Elution study of unreacted bis-GMA. TEGDMA, UDMA, and bis-EMA from light-cured dental resins and resin composites using HPLC. J Biomed Mater Res B Appl Biomater. 2005;74:617–626. doi: 10.1002/jbm.b.30252. [DOI] [PubMed] [Google Scholar]
- 16.Wataha JC, Craig RG, Hanks CT. Precision of and new methods for testing in vitro alloy toxicity. Dent Mater. 1992;8:65–71. doi: 10.1016/0109-5641(92)90056-i. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg M. In vitro and in vivo studies on the toxicity of dental resin components: a review. Clin Oral Invest. 2008;12:1–8. doi: 10.1007/s00784-007-0162-8. [DOI] [PubMed] [Google Scholar]
- 18.Saw TY, Cao T, Yap AUJ, Lee Ng MM. Tooth slice organ culture and established cell line culture models for cytotoxicity assessment of dental materials. Toxicol In Vitro. 2005;19:145–154. doi: 10.1016/j.tiv.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Edmondson JM, Armstrong LS, Martinez AO. A rapid and simple MTT-based spetrophotometric assay for determining drug sensitivity in monolayer cultures. J Tissue Cult Meth. 1988;11:15–17. [Google Scholar]
- 20.Lefebvre CA, Knoernschild KL, Schuster GS. Cytotoxicity of eluates from light-polymerized denture base resins. J Prosthet Dent. 1994;72:644–650. doi: 10.1016/0022-3913(94)90298-4. [DOI] [PubMed] [Google Scholar]
- 21.Young HM, Smith CT, Morton D. Comparative evaluation of two provisional restorative materials. J Prosthet Dent. 2001;85:129–132. doi: 10.1067/mpr.2001.112797. [DOI] [PubMed] [Google Scholar]
- 22.Driscoll CF, Woolsey C, Fergiison WM. Comparison of exothermic release during polymerization of four materials used to fabricate interim restorations. J Prosthet Dent. 1991;6:504–506. doi: 10.1016/0022-3913(91)90289-9. [DOI] [PubMed] [Google Scholar]
- 23.Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, Yoshida Y, Poitevin A, Coutinho E, Suzuki K, Lambrechts P, Van Meerbeek B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials. 2007;28:3757–3785. doi: 10.1016/j.biomaterials.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 24.Schweikl H, Spagnuolo G, Schmalz G. Genetic and celluler toxicology of dental resin monomers. J Dent Res. 2006;85:870–877. doi: 10.1177/154405910608501001. [DOI] [PubMed] [Google Scholar]
- 25.Schweikl H, Hartmann A, Hiller KA, Spagnuolo G, Bolay C, Brockhoff G, et al. Inhibition of TEGDMA and HEMA-induced genotoxicity and cell cycle arrest by N-acetylcysteine. Dent Mater. 2007;23:688–695. doi: 10.1016/j.dental.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 26.de Mendonça AA, Souza PP, Costa CA. Cytotoxic effects of hard-setting cements applied on the odontoblast cell line MDPC-23. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:102–108. doi: 10.1016/j.tripleo.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 27.Engelmann J, Janke V, Volk J, Leyhausen G, von Neuhoff N, Schlegelberger B, Geurtsen W. Effects of BisGMA on glutathione metabolism and apoptosis in human gingival fibroblasts in vitro. Biomaterials. 2004;25:4573–4580. doi: 10.1016/j.biomaterials.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 28.Ferracane JL. Elution of leachable components from composites. J Oral Rehabil. 1994;21:441–452. doi: 10.1111/j.1365-2842.1994.tb01158.x. [DOI] [PubMed] [Google Scholar]
- 29.Altintas SH, Usumez A. Evaluation of monomer leaching from a dual cured resin cement. J Biomed Mater Res Part B: Appl Biomater. 2008;86B:523–529. doi: 10.1002/jbm.b.31052. [DOI] [PubMed] [Google Scholar]