Abstract
In mice lacking the blood coagulation regulator thrombomodulin, fibrinolytic degradation products (FDP) of fibrin induce apoptotic cell death of a specialized cell type in the placenta, polyploid trophoblast giant cells. Here, we document that this bioactivity of FDP is conserved in human FDP, is not limited to trophoblast cells, and is associated with an Aα-chain segment of fibrin fragment E (FnE). The majority of proapoptotic activity is arginine-glycine-aspartic acid (RGD)-independent and requires caveolin-1–dependent cellular internalization of FnE. Internalization through caveoli is mediated by an epitope contained within Aα52-81 that is necessary and sufficient for cellular uptake of FnE. Aα52-81 does not cause apoptosis itself, and competitively inhibits FnE internalization and apoptosis induction. Apoptotic activity per se resides within Aα17-37 and requires the N-terminal neoepitope generated by release of fibrinopeptide A. Cellular internalization of FnE elicits depression of mitochondrial function and consequent apoptosis that is strictly dependent on the activity of caspases 9 and 3. These findings describe the molecular details of a novel mechanism linking fibrin degradation to cell death in the placenta, which may also contribute to pathologic alterations in nonplacental vascular beds that are associated with fibrinolysis.
Introduction
Fibrinolysis, the breakdown of fibrin by the primary protease of the fibrinolytic system, plasmin, destabilizes and eventually decomposes the fibrin-platelet aggregates of newly formed blood clots, and in general occurs after fibrin is formed and deposited in various anatomical locations. Plasmin digestion of fibrin produces proteolytic fragments (fibrin degradation products [FDP]) of defined size and molecular composition, including fibrin fragments E (FnE) and D-dimer (DD), consisting of fibrin's central E domain and a covalently linked dimer of 2 adjacent D-domains, respectively, plus an amino-terminal peptide fragment of the fibrinogen Bβ-chain (Bβ15-42). The presence of such fragments in circulating blood reflects release of fragments after degradation of fibrin at the site of its formation. The plasma concentration of FDP is greatly augmented in patients with thrombotic disorders such as venous thromboembolism or myocardial infarction, disseminated intravascular coagulation, infection, certain types of leukemia, and other pathologic entities.1–3
In studies of the function of the blood coagulation regulator thrombomodulin (Thbd) in placental development, we noted that fibrin deposition at the surface of a specialized placental cell type (ie, polyploid trophoblast giant cells) was associated with excessive in situ DNA fragmentation, suggestive of apoptosis.4 In vivo trophoblast cell death could be prevented by treating animals with the inhibitor of fibrinolysis, tranexamic acid, and did not occur in animals devoid of fibrinogen.4 In vitro studies confirmed that murine FDP, but not fibrinogen, fibrin, or degradation products of fibrinogen, induced DNA fragmentation (as judged by terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling [TUNEL]-assay) and cell death of placental trophoblast cells.4
The goal of the current work was to identify the relevant fibrin degradation fragment(s) causing cell death, determine the structural basis of this biologic effect, and define the molecular mechanism by which FDP induces cell death.
Methods
Animals and cells
Mouse trophoblast stem cells (gift of J. Cross, University of Calgary, Calgary, AB) were maintained and differentiated as previously described.5 Human choriocarcinoma cell lines JEG3 and BeWo6,7 and HeLa cells8 were obtained though ATCC (JEG3, HTB-36; BeWo, CCL-98; HeLa, CCl-2; Manassas, VA). Primary human umbilical vein endothelial cells (HUVECs) were prepared by the BloodCenter of Wisconsin Endothelial Cell Core. Human aortic endothelial cells were obtained from Cascade Biologics (Invitrogen, Carlsbad, CA) and maintained in Medium 200 (Invitrogen, Grand Island, NY). Passage 2-4 primary mouse embryonic fibroblasts (MEF) were prepared from wild-type C57Bl/6 mice by the BloodCenter's Transgenic Core facility. Caveolin-1–deficient MEF prepared from cav1-knockout embryos9 were kindly provided by Michael Lisanti (Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA), caspase-9–deficient MEF prepared from caspase-9–knockout mice10 were kindly provided by Richard Flavell (Yale University School of Medicine, New Haven, CT), caspase-311 and caspase-7–deficient MEF11 were kindly provided by Richard Flavell and Saquib Lakhani (Yale University School of Medicine, New Haven, CT). Adult female mice of CD-1 strain were purchased from Charles River Laboratories (Wilmington, MA). All animal experiments were conducted according to standards and procedures approved by the Animal Care and Use Committee of the Medical College of Wisconsin.
Preparation of FDP and fibrinogen polypeptides
Human fibrinogen was purified from plasma as described12 and converted to fibrin by incubating with one unit human α-thrombin (3000 NIH U/mg; Enzyme Research Laboratories, West Bend, IN) per milligram fibrinogen for 30 minutes at 37°C. FDP were produced by digestion with plasmin (Enzyme Research Laboratories). The reaction was terminated by addition of aprotinin (5 U trasylol per U plasmin; Bayer, West Haven, CT), and FDP were lyophilized. Fractionation of FDP was achieved by anion-exchange chromatography on diethylaminoethyl (DEAE)-sepharose developed with a Tris-phosphate pH gradient (pH 8.65-4.1).13 Preparation of FnE and FgnE-hementin was performed as described earlier.14,15 For reduction and alkylation of sulfhydryl groups, protein was dialyzed against 0.2 M N-ethylmorpholine and 8.4 M guanidine HCl, pH 8.5, for 2 hours at room temperature. After 30 minutes' incubation with dithiothreitol (DTT), a 5-fold molar excess of iodoacetic acid was added to the reaction mixture, followed by dialysis against 5% acetic acid. Reduced/alkylated fibrinogen Aα-, Bβ-, and γ-chains were purified by anion-exchange chromatography on DEAE-sepharose developed with a nonlinear gradient from 0.01 to 0.2 M phosphate-Tris in 8 M urea, pH 6.4. Fractions containing individual chains were pooled and subjected to N-terminal sequencing via automated Edman degradation to confirm identity, purity, and intactness by the Protein Analysis Core Facility of the Medical College of Wisconsin. FDP fractions devoid of small fragments were prepared by gel filtration of total FDP on Centricon-10 and Centricon-30 spin columns (Amicon, Beverly, MA)
Peptide synthesis
Peptides were synthesized by the Blood Research Institute's Protein Core, using standard 9-fluorenylmethoxy carbonyl (FMOC) method. All peptides were purified by reverse-phase high-performance liquid chromatography (HPLC), and subjected to mass spectroscopy for validation. Sequence numbering for the Aα-chain is according to human fibrinogen (accession NP_068657): fibrinopeptide B (FPB), QGVNDNEEGFFSAR; Bβ1-42, QGVNDNEEGFFSARGHRPLDKKREEAPSLRPAPPPISGGGYR; Bβ15-42, GHRPLDKKREEAPSLRPAPPPISGGGYR; fibrinopeptide A (FPA), ADSGEGDFLAEGGGVR; Aα17-19, GPR; Aα17-22, GPRVVE; Aα17-28, GPRVVERHQSAC; Aα17-37, GPRVVERHQSACKDSDWPFCS; Aα17-37 scrambled, SGAPFRPVWVDESRDHKQASA; Aα17-37-TAT, GPRVVERHQSACKDSDWPFCS-YGRKKRRQRRR; Aα52-83, KGLIDEVNQDFTNRINKLKNSLFEYQKNNKDS; Aα83-104, SHSLT-TNIMEILRGDFSSANNR; Aα83-104(D97E), SHSLTTNIMEILRGEFSSANNR.
Glutathione S-transferase (GST) fusion proteins
DNA fragments encoding Aα1-52, Aα 1-81, Aα1-104, and Aα 1-104(D97E) were subcloned into the pGEX 6p-1 expression vector (GE Healthcare, Piscataway, NJ). Sequence-verified vectors were transfected into BL21 cells (Stratagene, Cedar Creek, TX), and GST-fusion proteins were purified from isopropyl-β-D-thiogalactopyranoside (IPTG)-induced cultures using glutathione 4B sepharose (RediPack GST Purification Modules; GE Healthcare). Recombinant GST-1-104 protein was eluted with 10 mM glutathione in 50 mM Tris-HCl, pH 8.0, to obtain the intact GST-fusion protein. All recombinant proteins beginning at the thrombin-cleavage site at Aα17 were obtained by incubating column-bound GST protein with thrombin (40 U/mL packed sepharose; 3000 NIH-U/mg; Enzyme Research Laboratories) for 1 hour at room temperature, followed by addition of neutralizing amounts of the thrombin-inhibitor, hirudin (Sigma-Aldrich, St Louis, MO).
TUNEL and caspase-3 activity assay
Measurement of caspase-3 activity in cell lysates was carried out using a commercial reagent (caspase-3 substrate I, fluorogenic; Calbiochem, San Diego, CA) according to the manufacturer's instruction, and caspase-3 activity was measured and analyzed using an fmax fluorometer (Molecular Devices, Sunnyvale, CA). For analysis of DNA fragmentation in cultured cells by TUNEL assay, cells were cultured with the indicated reagents on Lab-Tek II chamber slides (Nalge Nunc International, Rochester, NY), washed twice with phosphate-buffered saline (PBS), fixed in 2% formaldehyde/PBS for 40 minutes at room temperature, and permeabilized for 2 minutes with ice-cold 0.1% Triton 100/0.1% sodium citrate. TUNEL staining of fixed cells and on histologic sections was performed with an In Situ Cell Death Detection kit (Roche, Indianapolis, IN), followed by nuclear counterstain with Hoechst 33 342 (Invitrogen). Detection of activated caspase-3 in histologic sections used phycoerythrin (PE)-conjugated anti-mouse caspase-3 (activated) antibodies (1:100 dilution; BD Pharmingen, San Jose, CA) and isotype-matched nonimmune serum as control. Data were documented by fluorescence microscopy (Nikon Eclipse TE200; Nikon, Melville, NY) and image capture/analysis with a SPOT digital camera and MetaMorph image analysis software (Molecular Devices).
Internalization studies
FnE, α2-macroglobulin (kindly provided by Dr Dudley Strickland, University of Maryland School of Medicine, Baltimore, MD) and indicated peptides were labeled with fluorescein isothiocyanate (FITC; Sigma-Aldrich). For binding assays by fluorescence activated cell-sorting (FACS), JEG3 or MEF cells were grown to approximately 80% confluence, trypsinized (0.05% trypsin/ethylenediaminetetraacetic acid [EDTA]), collected by centrifugation (270g for 5 minutes), washed twice, and resuspended at 105 cells/200 μL in PBS. Cells were incubated with 0.5 μM FITC-labeled FnE in PBS/2.5 mM CaCl2 at room temperature for 30 minutes in the presence/absence of unlabeled competitor. Cell-associated fluorescence of at least 104 cells was measured using a Becton Dickinson LSRII FACS analyzer (BD, Franklin Lakes, NJ). Cellular localization of FITC-labeled peptides was determined in adherent cells by confocal fluorescence microscopy (TCS SP2 Laser-Scanning Confocal Microscopic Imaging System; Leica Microsystems, Bannockburn, IL). Caveolin immunocytochemistry was performed using rabbit anticaveolin-1 antibody and goat anti-rabbit immunoglobulin G (IgG)-Cy3 conjugated (BD Biosciences, San Jose, CA), followed by confocal fluorescence microscopy. The role of lipoprotein receptor-related protein (LRP)-mediated endocytosis was tested by determining the sensitivity of FnE-FITC internalization to receptor-associated protein (RAP). Inclusion of a 100-fold molar excess (10 μM) of RAP (kindly provided by Dr Strickland, University of Maryland School of Medicine) inhibited internalization of 100 nM FITC-α2-macroglobulin, but did not reduce FnE-FITC internalization under identical experimental conditions. An antibody directed against the αX integrin subunit (mouse anti–human CD11c; Biosource, Camarillo, CA) that blocks αXβ2-dependent adhesion14 was included at 0.1 μg/μL. At this concentration, the antibody completely blocked αXβ2-dependent adhesion of polymorphonuclear leukocyte (PMN) to immobilized fibrinogen, reproducing earlier results.14
Analysis of mitochondrial function
Wild-type MEF were cultured to approximately 80% confluence, followed by incubation with 2 μM FnE. The potentiometric dye MitoTracker Red (CMX ROS; Invitrogen) was added to the culture medium to a final concentration of 100 nM at various time points after FnE exposure. Cells were fixed 15 minutes after Mitotracker addition in 2% paraformaldehyde, and images of cultures were captured on an inverted fluorescence microscope at a wavelength of 555 nm using a SPOT camera. The average red fluorescence intensity per cell was measured using MetaMorph software, corrected for background fluorescence in areas devoid of cells. Data are derived from 3 independent experiments and represent analysis of 5 fields per experiment and time point. Similar results were obtained using FACS analysis of cells exposed to FnE, followed by staining with the potential-sensitive dye JC-1 (Mitoprobe JC-1; Invitrogen), trypsination, and detection of fluorescence at approximately 529 nm (green) and approximately 590 nm (red), with a lower red:green ratio indicating reduction of δψm. In these FACS experiments, gates were set using the mitochondrial uncoupling reagent carbonyl cyanide m-chlorophenylhydrazone (CCCP) as a positive control, and the 51-82 peptide (no cell death–inducing activity as measured by TUNEL) as the negative control.
Microphotography
A Nikon Eclipse TE200 microscope with a 40×/0.6 numeric aperture (NA) objective (Figures 1A, 4F-I, and 7H-Q), 20×/0.45 (Figures 1B, 5F, and 6C,G,H) or 10×/0.3 (Figures 1C,D, 4K-M, 5G,H, and 6A,D-F) objective (Nikon), equipped with a CoolSnap ES camera (Roper Scientific Photometrics, Pleasanton, CA) and Metamorph acquisition software (Universal Imaging, Sunnyvale, CA) was used to acquire microphotographs at room temperature from sections mounted in Vectashield medium (Vector, Burlingame, CA). Figure 7A through H images were acquired on a Nikon Eclipse TE600 Microscope with a 20× objective/0.5 aperture, equipped with a SPOT Insight camera Model II.2 Color Mosaic and SPOT acquisition software (Diagnostic Instruments, McHenry, IL) at room temperature from slides mounted in Vectashield. Figures 4C through E and 5A through D were captured on a Leica TCS SP2 Laser-Scanning Confocal Microscope equipped with a SPOT II Digital Camera System and acquisition software (Diagnostic Instruments) at room temperature in the FITC channel (Figures 4C,D and 5A,B) or Texas Red channel (Figure 5C).
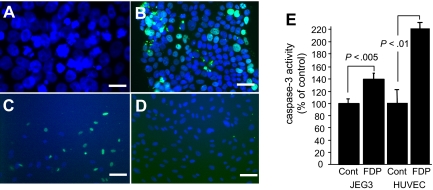
Figure 1.
Human FDP induce apoptosis in human JEG3 choriocarcinoma cells and vascular endothelial cells. (A) FDP induce in JEG3 cells nuclear condensation and fragmentation, and (B) DNA fragmentation as detected by TUNEL (green). (C,D) TUNEL stain of HUVECs cultured in the presence (C) or absence (D) of FDP. Nuclear stain with Hoechst 33 342; bar indicates 100 μm. (E) Caspase-3 amidolytic activity in cell extracts of JEG3 and HUVECs cultured for 48 hours without (cont) or with approximately 2.5 μM FDP. Bars represent mean (± standard error [SE]) from 3 independent experiments; n = 8 per experiment.
Figure 4.
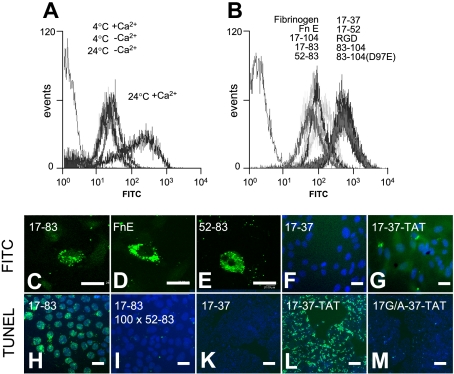
Apoptosis induction by FnE requires internalization by the Aα52-83 motif. (A) Representative FACS analysis of FITC-labeled FnE uptake (2 μM) by JEG3 cells in suspension. Uptake only occurs at 24°C in the presence of Ca2+. (B) Uptake of FITC-labeled FnE into JEG3 cells is competitively inhibited by a 100-fold excess of unlabeled FnE and Aα-chain fragments containing the 52-83 motif and by intact fibrinogen, but not by RGD-containing peptides. (C-G) JEG3 cells in suspension culture were incubated with FITC- or FAM-labeled preparations of the indicated peptides (2 μM, 24°C). Distribution of fluorescence was determined by confocal microscopy of cytospin preparations. (H-M) Representative TUNEL staining of adherent JEG3 cells cultured for 48 hours in the presence of 2 μM 17-83 peptide (H), 2 μM 17-83 peptide plus a 100-fold excess (200 μM) 52-83 peptide; 20 μM 17-37– (K), 17-37-TAT– (L), or 17G/A-37-TAT–peptides (M). Bars represent 50 μm (C-I) or 200 μm (K-M).
Figure 7.
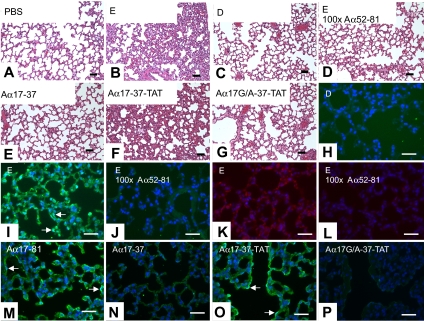
In vivo effects of FDP. (A-G) Lung morphology of mice injected with the indicated reagents; H&E. (H-J, M-P) TUNEL stain to detect in situ DNA-fragmentation on corresponding sections. Green fluorescence indicates TUNEL-positive staining. Nuclear counterstain with Hoechst dye (blue). (K,L) Immunostaining for activated caspase-3 (red). Equivalent loading concentrations of D- and E-fragments estimated from average molecular weight of fragments; synthetic peptides were injected to achieve a loading concentration in blood similar to that of D- and E-fragment (∼2 μM). Arrows denote apoptotic endothelium surrounding presumptive blood vessels filled with erythrocytes (determined by examination of phase contrast images). Bars represent 50 μm.
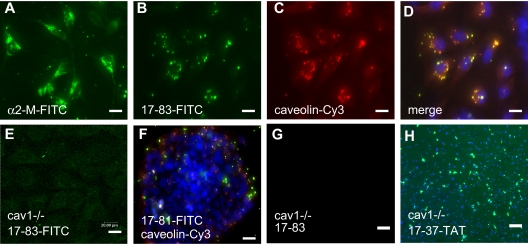
Figure 5.
Internalization of Aα17-83 requires caveolin-1. (A-F) Intracellular localization of α2-macroglobulin/α2–M with FITC-labeled α2-M–antibody, FITC-labeled Aα17-83, and caveolin-1 with Cy3-conjugated anti-cav1–antibody in MEF (A-E) and JEG3 cells (F) by confocal fluorescence microscopy. Aα17-83 colocalizes with caveolin-1. (G-H) MEF prepared from cav1−/− embryos were cultured for 48 hours in the presence of 2 μM Aα17-83 (G) or 20 μM 17-37–TAT, followed by TUNEL staining. Cav1-deficient MEFs are resistant to apoptosis induced by Aα17-83; but not Aα17-37–TAT. Bars represent 20 (A-E), 50 (F), or 200 μm (G,H).
Figure 6.
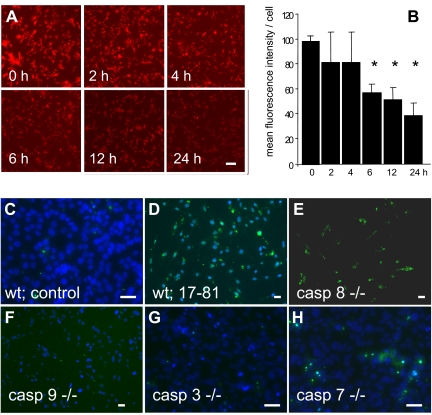
FnE activates the mitochondrial pathway of apoptosis. (A) Time course of mitochondrial membrane potential loss in response to FnE. MEF were cultured in the presence of 2 μM FnE, followed by staining with mitotracker reagent at the indicated times. Bar indicates 100 μm. (B) Images were analyzed using MetaMorph software to measure the fluorescent intensity per cell. Bars represent average plus or minus SE from 2 independent experiments; *P < .05 by Student t test. (C-H) MEF derived from wild-type mice (C,D) or knockout mice lacking the indicated caspases (E-H) were cultured for 48 hours in the presence of 2 μM Aα17-81, followed by TUNEL assay. MEF deficient in caspase-9 or -3 are resistant to FDP-induced apoptosis. Bars indicate 50 μm.
Results
Cross-species conservation of FDP-induced cell death
To determine whether the ability of murine FDP to cause cell death is conserved across species, FDP were prepared from human fibrinogen using human thrombin and plasmin. Reproducing former results obtained with murine FDP, exposure of in vitro differentiated murine trophoblast cells to 40 or 80 μg/mL unfractionated human FDP, but not at lower concentration, for 48 hours caused a significant loss of adherent cells, nuclear condensation, and DNA fragmentation as detected by TUNEL assay (not shown). Likewise, exposure of human trophoblast-derived BeWo-cells and JEG3 human choriocarcinoma cells to human FDP, but not fibrinogen, caused DNA fragmentation, nuclear condensation, and a significant increase of caspase-3 amidolytic activity in cytoplasmic extracts of FDP-treated cells (Figure 1A,B,E). Caspase amidolytic activity was blocked in the presence of the caspase 3-selective inhibitor Ac-DVED-CHO, but not the caspase-1 selective inhibitor Ac-YVAD (not shown). The in vitro apoptosis-inducing activity of FDP was further documented for MEF cells, Hela-cells, and primary human endothelial cells isolated from the umbilical vein (Figure 1C-E) or from the pulmonary aorta (not shown).
Apoptotic activity of FDP resides in the thrombin-cleaved Aα17-104 chain of FnE
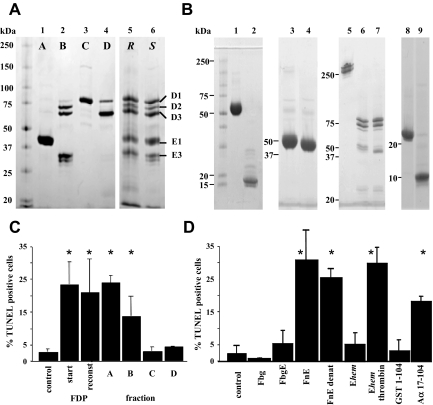
To identify the specific FDP components causing cell death, human FDP were fractionated by gel filtration to remove low molecular weight peptides. Fractions devoid of small (≤ 30 kDa) fragments, added at 80 μg/mL, retained activity in the cell death assay with human JEG3 cells, and synthetic peptides (2 μM) resembling fibrinopeptide A (Aα1-16), fibrinopeptide B (Bβ1-14), Bβ1-42, or Bβ15-42 did not induce cell death. Preparative fractionation of plasmin-treated fibrin via anion-exchange gel chromatography on DEAE-sepharose yielded FDP pools relatively enriched for specific fragments and mixtures thereof (Figure 2A). A reconstituted sample (80 μg/mL) generated by combining individual fractions to resemble by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis the starting material exhibited similar apoptotic activity as unfractionated FDP, indicating that the majority of bioactivity had been preserved during the fractionation process. Apoptotic activity was only associated with fractions containing FnE (Figure 2C).
Figure 2.
Apoptotic activity of FDP resides in Aα17-104. (A) Nonreducing SDS-PAGE analysis of total FDP (lane 6, S) fractionated by DEAE ion-exchange chromatography. Fractions A-D (lanes 1-4) were combined (lane 5, R) to reconstitute the starting material (lane 6, S); Coomassie stain. (B) Nonreducing SDS-PAGE analysis of native (FnE, lane 1) and reduced/alkylated fibrin fragment E (lane 2); fibrinogen fragment E derived by proteolysis with hementin (Ehem; lane 3); Ehem treated with thrombin (lane 4); fibrinogen (Fgn; lane 5); fibrinogen degradation products after plasmin treatment (Fgn-DP; lane 6); Fgn-DP treated with thrombin (lane 7); recombinant E coli GST-Aα1-104 (GST1-104; lane 8) and purified recombinant Aα17-104 (lane 9). (C) Apoptotic activity of fractionated FDP on JEG3 cells, corresponding to preparations characterized in panel A. (D) Apoptotic activity of fibrin(ogen)-derived fragments characterized in panel (B). Fbg, fibrinogen; FbgE, fibrinogen fragment E; FnE, fibrin fragment E; FnE denat., FnE after reduction/alkylation. Ehem, fibrinogen fragment E derived by hementin proteolysis of fibrinogen; Ehem-thrombin, Ehem treated with thrombin to release fibrinopeptide A; GST 1-104, intact recombinant E coli GST fusion protein with Aα1-104; Aα17-104, recombinant fibrin a-chain fragment derived by thrombin-cleavage of GST 1-104, followed by removal of GST 1-16. Bars in (C) and (D) represent mean plus or minus SE from 3 independent experiments. *P < .05 by t test.
Preparative fractionation of plasmin digests of either fibrin or fibrinogen yielded FnE and fibrinogen fragment E (FgnE), respectively, which differ in the presence (FgnE) or absence (FnE) of Aα1-16 (FPA) and Bβ1-14 (FPB) (Figure 2B). FgnE did not exhibit apoptotic activity, whereas thrombin-treated FgnE (resembling FnE) caused cell death. Digestion of intact fibrinogen with hementin, a protease isolated from the glands of the giant amazon leech,16,17 produces a unique fragment Ehem that differs from plasmin-generated FgnE with respect to the precise site of cleavage within the coiled-coil C-terminal region of the E fragment. Sequence analysis of the component chains of the specific Ehem fragment at hand showed that the amino-terminal end of the Aα- and γ-chain were still intact, whereas the Bβ-chain was truncated due to hementin cleavage at Arg44, Lys53, and Lys54. This particular Ehem fragment did not induce cell death, but gained activity upon treatment with thrombin to release FPA. Apoptotic activity associated with FnE and thrombin-treated Ehem was retained after reduction and alkylation of sulfhydryl groups with iodoacetic acid (Figure 2B,D). Reduction/alkylation of intact fibrinogen, followed by anion-exchange chromatography to purify individual, intact fibrinogen Aα-, Bβ-, and γ-chains, and digestion of individual chains with thrombin or plasmin alone, or with thrombin followed by plasmin showed that only thrombin- and plasmin-treated Aα-chain preparations induced apoptosis of JEG3 cells (not shown). Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) analysis of tryptic digests prepared from thrombin- and plasmin-treated Aα-chain indicated that the longest Aα-chain fragment detected comprised the Aα17-104 fragment, consistent with the known composition of FnE. To exclude the possibility that apoptotic activity was due to a contaminating component associated with FnE or isolated Aα-chains, the fibrinogen Aα-peptide 1-104 was produced as a GST fusion protein in Escherichia coli. Recombinant Aα17-104, purified after thrombin-cleavage of resin-bound GST-Aα1-104, but not GST alone or intact GST-Aα1-104 caused apoptosis of JEG3 cells (Figure 2B,D).
We conclude from this series of experiments that the cell death–inducing activity of FDP is mediated by a sequence-defined, rather than a conformational epitope in FnE contained within amino acids 17-104 of the Aα-chain. The gain of cell death-inducing bioactivity by the fibrinogen Aα-chain requires release of FPA by thrombin, and C-terminal cleavage by plasmin or hementin.
Structural determinants of apoptotic activity associated with Aα17-104
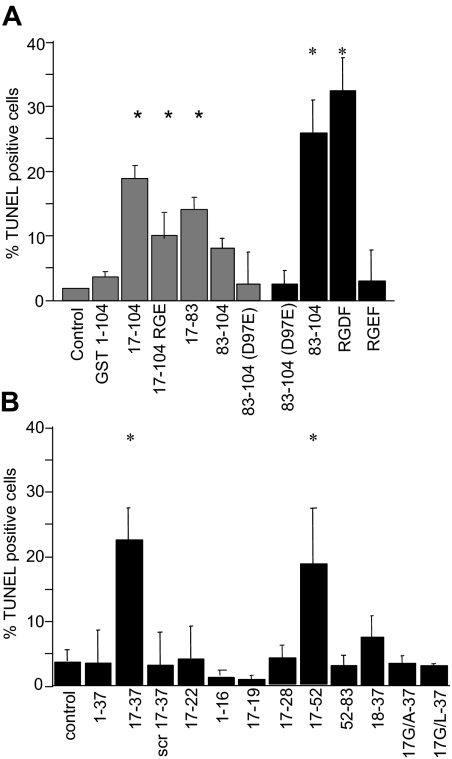
To determine the role of the RGD motif comprised by Aα95-97 of FnE, JEG3 cells were exposed to recombinant E coli protein Aα-chain variants and synthetic peptides derived from this region (Figure 3A). Arginine-glycine-aspartic acid-phenylalanine (RGDF) and Aα83-104, but not arginine-glycine-glutamic acid-phenylalamine (RGEF) peptide or Aα83-104(D97E), induced significant cell death at the micromolar concentration. The Aα17-83 truncation peptide lacking the RGD motif exhibited similar apoptotic activity as Aα17-104(D97E), and either RGD-defective variant exhibited approximately 70% of the apoptotic activity associated with the recombinant Aα17-104 protein. Accordingly, approximately one-third of the overall apoptotic activity associated with Aα17-104 is accounted for by the Aα95RGD97 motif, whereas the majority of apoptotic activity is RGD-independent and resides within the Aα17-83 fragment.
Figure 3.
Apoptotic activity of Aα-chain fragments. Adherent JEG3 cells were incubated in the absence (control) or presence of 1 mM (■) or 2 μM ( ) synthetic peptides or recombinant E coli protein for 48 hours, and apoptosis was determined by TUNEL assay. (A) RGD-independent apoptotic bioactivity resides in Aα17-83. (B) Aα17-37 is sufficient to induce apoptosis at 1 mM concentration; the amino-terminal glycine residue comprising the neo-epitope generated after release of FPA from fibrinogen is necessary for apoptotic bioactivity. scr: sequence scrambled control. Bars represent the average plus or minus SE from 3 independent experiments with 3 measurements each. *P < .05 by Student t test.
) synthetic peptides or recombinant E coli protein for 48 hours, and apoptosis was determined by TUNEL assay. (A) RGD-independent apoptotic bioactivity resides in Aα17-83. (B) Aα17-37 is sufficient to induce apoptosis at 1 mM concentration; the amino-terminal glycine residue comprising the neo-epitope generated after release of FPA from fibrinogen is necessary for apoptotic bioactivity. scr: sequence scrambled control. Bars represent the average plus or minus SE from 3 independent experiments with 3 measurements each. *P < .05 by Student t test.
To delineate the epitopes mediating RGD-independent apoptotic activity within Aα17-83, additional recombinant Aα-chain fragments and synthetic peptides were tested. At 2 μM, only Aα17-83, but not Aα17-19 (GPR), 17-22, 17-28, 17-37, or 17-52 caused DNA fragmentation (not shown); indicating that Aα52-83 contained a necessary epitope. However, the latter fragment by itself did not cause cell death even at 500-fold higher concentration (ie, 1 mM). At such high concentration, Aα17-52, and Aα17-37, but not further truncated peptides (ie, Aα17-28, Aα17-22, Aα17-19 [GPR]) induced cell death (Figure 3B). Substituting the glycine residue constituting the amino-terminal neoepitope produced by thrombin cleavage of the Aα-chain for alanine or leucine or eliminating the first amino acid abolished the apoptotic activity exerted by millimolar concentrations of Aα17-37 (Figure 3B).
These observations indicated, first, that RGD-independent apoptotic activity associated with Aα17-83 requires at least 2 spatially segregated epitopes: Aα17-37, which causes apoptosis at a high concentration; and Aα52-83, which is necessary for cell death induction at micromolar concentrations, but in itself is not sufficient to cause apoptosis. Second, the N-terminal neoepitope generated by the thrombin-mediated release of fibrinopeptide A appears necessary for apoptotic function, thereby explaining why FnE, but not fibrinogen or FgnE, cause cell death.
FnE-induced apoptosis requires Aα52-82–mediated internalization of the Aα-chain N terminus
Incubation of adherent or suspended MEF or JEG3 cells in the presence of various concentrations of FITC-labeled FnE, followed by FACS analysis of cell-associated fluorescence showed a concentration-dependent, saturable acquisition of fluorescence that was calcium-dependent and only observed after prolonged (≥ 30 minutes) incubation at room temperature, but not at 4°C (Figure 4A). Acquisition of fluorescence was blocked by a 100-fold excess of unlabeled FnE, fibrinogen, Aα17-104, Aα17-83, and Aα52-83, but not by Aα83-104, RGDF, or fragment D (Figure 4B). Confocal microscopy showed that FITC-coupled FnE, Aα17-83, and Aα52-83 were localized in cytoplasmic vesicles, with little or no fluorescence associated with the cell surface (Figure 4C-E). Therefore, the acquisition of cell-associated fluorescence as measured by FACS analysis reflects uptake of labeled FnE/Aα17-83/Aα52-83 into the cell. Aα52-83, which does not induce apoptosis even at millimolar concentration (see above), competitively inhibited FnE-FITC uptake (Figure 4B) and, when added in excess, prevented the induction of apoptosis by Aα17-83 (Figure 4H,I) and FnE (not shown).
Therefore, induction of cell death requires uptake of FnE into the cytoplasm of the cell, which is mediated by a motif residing within Aα52-83. This motif is both necessary and sufficient to mediate internalization and competitively blocks cellular uptake of intact FnE, but is by itself not sufficient to induce apoptosis. This suggested that apoptotic bioactivity per se is associated with epitopes located within Aα17-52, which must be delivered into the cytoplasm via the Aα52-83 motif. Accordingly, Aα-chain fragments Aα17-19 (GPR), 17-22, 17-37, or 17-52, administered at a concentration of 2 μM, were neither internalized nor caused apoptosis at micromolar concentration (Figure 4F,K). In contrast, at millimolar concentration, these FITC-coupled peptides can be detected in the cytoplasm, likely secondary to nonspecific cellular uptake, and cause apoptosis (see above). Coupling of the Aα17-37 fragment, which was the shortest fragment to induce apoptosis at millimolar concentration, to the cell-permeabilizing protein sequence of the TAT protein enabled delivery of Aα17-37–TAT to the cytoplasm at micromolar concentration, albeit with a diffuse cytoplasmic distribution, distinct from that observed with Aα17-83, Aα52-83, or FnE (Figure 4G). Consistent with the above findings documenting a critical role of the Aα17-neoepitope generated by thrombin proteolysis at Aα17, 20 μM Aα17-37–TAT, but not Aα17G/A-37–TAT induced DNA fragmentation to a similar extent as Aα17-83 (Figure 4K-M).These results document that the apoptotic activity of FnE resides within Aα17-37, which must be delivered into the cell via the Aα52-83 motif.
Internalization of FnE requires caveolin-1
The inhibitor of LRP-mediated endocytosis, 39-kDa receptor-associated protein (RAP), did not affect internalization of FITC-FnE by MEF, and α2-macroglobulin, which is internalized in a RAP-sensitive fashion by this pathway, showed an intracellular distribution distinct from that of FITC-FnE (Figure 5A). Instead, intracellular FITC-FnE colocalized with vesicles reactive with antibodies recognizing the structural component of caveoli, caveolin-1 (Figure 5B-D). MEF prepared from caveolin-1–deficient knockout mice (cav−/− MEF) did not internalize FITC-labeled FnE, Aα17-83, or Aα52-83 (Figure 5E) and were resistant to apoptosis induced by micromolar concentrations of FnE and Aα17-83 (Figure 5G). In contrast, 20 μM Aα17-37–TAT, but not Aα17G/A-37–TAT–induced cell death in cav−/− MEF to a similar extent as in wild-type MEF (Figure 5H). Reexamination of cav1 expression in adherent JEG3 cells showed cav1 antigen only in a subpopulation of cells, overlapping with cells that internalized FITC-labeled Aα17-83 (Figure 5F). Therefore, the whole-population apoptosis assays with JEG3 cells used for previous analyses likely underestimate the apoptotic bioactivity of FnE.
These data suggest that caveolin-1–dependent endocytosis is the predominant pathway of FnE internalization, but that caveolin-1 or caveoli per se are not required for the intracellular mechanism mediating apoptosis. The αXβ2-integrin (CD11c/CD18; p150/95) is a known receptor for the amino terminus of the Aα-chain and recognizes the Aα17-19 (GPR) motif.18 Whereas all reagents produced for use in the current study that comprise the Aα17-19 sequence blocked the previously reported αXβ2-mediated adhesion of human peripheral blood mononuclear cells to immobilized fibrinogen (data not shown), antibody-mediated inhibition of αXβ2-integrin did not alter the binding or internalization of FnE or Aα17-81 to JEG3 cells or MEF and did not block apoptosis (data not shown). This rules out that αXβ2-intergrin plays an accessory role in the caveolin-1–mediated internalization of FnE.
The Aα52-83 sequence epitope also overlaps with a high-affinity binding site for the inhibitor of fibrinolysis, plasminogen-activator inhibitor 1 (PAI-1), and PAI-1 interaction with this motif requires thrombin-mediated removal of fibrinopeptide A, as well as plasmin cleavage,19 dovetailing the requirements described here for the apoptotic activity of FnE. However, both internalization and apoptotic bioactivity of FnE in MEF prepared from PAI-1–deficient knockout mice were identical to that of wild-type MEF (data not shown).
FnE activates the mitochondrial pathway of apoptosis
Analysis of the time course of apoptosis induction by FnE in JEG3 cells or MEF showed that a significant increase in the number of TUNEL-positive cells was not detected before approximately 12 hours after addition of FnE to the culture medium. The intensity of in situ labeling of adherent wild-type MEF cells with the mitochondrial potential-sensitive “mitotracker red CMX ROS” reagent was significantly reduced as early as 6 hours after FnE exposure (Figure 6A,B), whereas FnE did not alter the fluorescence intensity of cav1−/− MEF (not shown). At the 6-hour time point, only sporadic TUNEL-positive staining was observed (not shown). This indicates that a reduction of the electrochemical potential difference across the inner mitochondrial membrane, and a reduction of mitochondrial activity, precedes apoptotic cell death as detected by DNA fragmentation after prolonged (ie, 48 hours) incubation of cells with FnE. MEF prepared from knockout mice lacking caspase-3 or caspase-9 were resistant to apoptosis induced by FnE or Aα17-81, whereas both reagents still induced apoptosis in MEF lacking caspase-7 or caspase-8 (Figure 6C-H).
These observations suggest that FnE induces cell death by activating the mitochondrial apoptosis pathway upstream, or at the level of caspase-9, likely secondary to altered mitochondrial function.
In vivo mechanism of FnE-induced apoptosis
To validate our in vitro observations in an in vivo setting, wild-type mice received 2 consecutive intravenous bolus injections of FITC-labeled FnE (180 μg/bolus; second infusion 10 hours after initial bolus). The labeled fragment accumulated preferentially in the lung and to a lesser extent in the endothelial lining and subendothelial vascular wall of larger arteries and veins throughout the vascular tree (data not shown), consistent with the disproportionate abundance of caveoli in alveolar epithelium and pulmonary capillary endothelial cells.20–22 Analysis of histologic sections prepared 24 hours after the first bolus showed marked constriction of alveolar spaces and alveolar wall swelling in the absence of inflammatory infiltrates or pronounced hemorrhage (Figure 7A,B). Infusion of equivalent amounts of D-fragment did not elicit such changes (Figure 7C). Within areas of altered morphology, apoptosis of endothelial cells lining erythrocyte-filled blood vessels and of alveolar epithelium was detected by ex vivo BrdU-incorparation/TUNEL stain (Figure 7I) and by antibodies specific for activated caspase-3 (Figure 7L). DNA fragmentation and caspase-3 activation induced by FnE were blocked by coinjection of a 100-fold molar excess of peptide Aα52-81 (Figure 7D,K,M). Intravenous administration of Aα17-81 or the cell-permeable Aα17-37–TAT fusion peptide likewise elicited apoptosis and altered lung morphology, whereas Aα17-37 and Aα17G/A-TAT did not (Figure 7E,N-Q).
Taken together, these in vivo findings are entirely congruent with the mechanism of RGD-independent apoptosis provoked by FnE as determined through in vitro studies.
Discussion
The above findings describe a novel mechanism by which FDP may induce cell death. This biologic activity is conserved in human FDP and is detectable in a variety of cell types, including endothelial cells. The majority of in vitro and in vivo cell death-inducing bioactivity of FDP is associated with Aα17-83 of FnE, with a minor contribution of the RGD motif comprised by Aα95-97. Induction of RGD-independent apoptosis requires 2 spatially segregated epitopes within Aα17-81 (ie, a motif contained within Aα52-81) and a second motif within the amino-terminal Aα17-37 fragment of FnE that includes the amino-terminal glycine residue constituting a neoepitope generated by the thrombin-mediated release of FPA from fibrinogen. Aα52-83 mediates the caveolin-1–dependent internalization of FnE, in itself does not promote apoptosis, and—by preventing caveolin-1–dependent internalization of FnE—can competitively inhibit cell death elicited by micromolar concentrations of FnE. The cell death-inducing activity of FnE per se resides in Aα17-37, delivered into the cell via Aα52-83. Internalization requires expression of caveolin-1 and caveolin-1–deficient cells are resistant to FnE-induced apoptosis. Localization of the Aα17-37 motif to caveoli or caveolin-1 containing intracellular vesicles is not essential for the intracellular mechanisms of apoptosis induction, as bypassing this route of internalization by coupling the Aα17-37 fragment to the cell permeable TAT protein motif does not abolish its apoptotic activity. We further show that uptake of FnE into the cytoplasm elicits a depression of mitochondrial function over time. Subsequent DNA fragmentation is strictly dependent on the activity of caspases 9 and 3, whereas FnE still induces apoptosis in the complete absence of caspases 7 and 8. Because the depression of mitochondrial function precedes by several hours the onset of significant DNA fragmentation, we suspect that caspase-9 activation is the result of prolonged mitochondrial dysfunction, and that the primary target of Aα17-37 is a yet-to-be-identified component that is associated with mitochondria and contributes to the maintenance of mitochondrial function. Overall, these findings indicate that the proapoptotic effect of FnE is due to activation of the mitochondrial cell death pathway.
The caveolin-1 dependence of FnE internalization suggests that FnE is a caveoli-specific cargo, akin to the predominant pathway of internalization described for albumin, cholera, and tetanus toxin, as well as certain bacteria and viruses.21 Such a selectivity of cargo molecules for caveoli could in theory derive from posttranslational modifications such as addition of glycosylphosphatidylinositol (GPI) anchors, which target certain receptors to caveolar membrane microdomains. However, covalent coupling of the Aα52-83 sequence to unrelated molecules, such as green fluorescent protein, results in caveolin-1–dependent internalization of the fusion protein (H.W., unpublished observation, May 2007), and both the recombinant E coli protein, as well as the synthetic Aα52-83 peptide are internalized in a cav1-dependent manner. One may, therefore, rule out that lipid or sugar modifications of native FnE contribute to the observed specificity of internalization. The Aα52-83 sequence also does not contain a putative caveolin-binding domain,23 and pilot experiments to detect via coimmune precipitation a direct physical interaction between Aα52-81 and the scaffolding domain of caveolin-1 were inconclusive. FnE internalization is therefore most likely secondary to interactions of the Aα52-83 motif with an as yet unidentified constituent of caveoli.
A deleterious effect of high concentrations of FnE on endothelial cells and vascular smooth muscle cells has previously been noted as a side observation in studies of the mitogenic- and angiogenesis-promoting effects of FnE in a concentration range of 10 to 100 nM.24–26 Consistent with the present findings, these studies describe a cytotoxic effect of FnE at concentrations exceeding 1 μM. An as yet unexplored aspect of our findings is how the described mechanisms mediating apoptosis are related to the mitogenic and angiogenic activity of FnE, and how these bioactivities are integrated with effects elicited by the adhesive interaction of the Aα-N terminus with the neutrophil αXβ2 integrin,18 and of the Bβ-chain 1-42 moiety with VE-cadherin.27–30 The structural and mechanistic insights gained from the current work should facilitate experimental approaches addressing these questions.
The physiologic and pathologic context, in which the described mechanism coupling fibrinolysis to the regulation of cell survival may be operating, is largely unexplored. Our initial observation that inhibition of fibrinolysis or depletion of fibrinogen prevents in situ cell death of placental trophoblast cells suggests that fibrinolytic degradation products of fibrin can indeed induce apoptosis in vivo. The plasma concentration of FnE used in the current infusion experiments to elicit apoptosis of lung tissue was in the range of 1 to 2 μM. Circulating levels of FnE will, even in patients with severe trauma or disseminated intravascular coagulation, rarely reach or exceed a concentration of 200 to 300 nM.31 However, the local concentration of FDP generated in vivo in the vicinity of the trophoblast cell surface of thrombomodulin-deficient embryos apparently surpasses the threshold required to induce apoptosis, even in the presence of normal plasma fibrinogen levels. Similar conditions of localized fibrin deposition and fibrinolysis are in all likelihood established during the vascular remodeling that accompanies thrombotic injury to blood vessels, and in general are associated with wound healing and tissue remodeling after traumatic or inflammatory injury. One possible biologic context, in which the described mechanisms could be beneficial, may be inferred from our previous finding that FDP-induced apoptosis of placental trophoblast cells accelerates the resorption of abortion-prone embryos. Activation of apoptotic pathways is associated with alterations in the lipid composition of the plasma membrane, which serves as a phagocytosis-inducing signal for macrophages.32–34 In this manner, FnE-induced apoptosis might support the clearance of damaged cells from the field of injury, akin to the accelerated clearance of developmentally arrested thrombomodulin-null embryos. In a pathologic context, FDP-mediated cytotoxic effects may be expected to prevail under conditions of sustained and excessive FDP generation, as it occurs in patients with disseminated intravascular coagulation, acute lung injury, or acute respiratory distress syndrome.35,36 Alveolar epithelial injury and apoptosis are early features of idiopathic pulmonary fibrosis and of bleomycin-induced pulmonary fibrosis in mice,37 in acute lung injury secondary to ischemia-reperfusion,38 and increased numbers of apoptotic cells are found in the lungs of patients with chronic lung disease.33 The identification of the structural and cellular mechanisms mediating FnE-induced suppression of cellular viability constitutes a first step toward experimentally establishing the physiologic role of this mechanism.
In summary, our observations provide novel mechanistic insight how plasmin degradation products of fibrin may cause in vivo cell death of placental trophoblast cells. The described mechanisms are conserved between humans and mouse and are likely relevant for other cell types, including vascular endothelium. These findings raise new questions about the molecular details of FnE interaction with caveoli, about the intracellular target of Aα17-37 linking fibrinolysis to mitochondrial function and cell death, and about the physiologic and pathologic context in which the described mechanisms may bear relevance.
Acknowledgments
The authors gratefully acknowledge Dr M. Lisanti for providing cav1-knockout MEF and recombinant Cav1 scaffolding domain; Dr D. Strickland for helpful discussions and providing RAP protein and α2 macroglobulin; Dr D. Wallach for enabling the collaboration with Dr Kang; Dr R. Flavell and S. Lakhani for providing caspase 3/7 knockout MEF; J. Foeckler and S. Kalloway for assistance with in vivo studies in mice; and Drs F. Castellino and R.D. Balsara for providing PAI-1 knockout mice.
This work was supported by National Heart, Lung, and Blood Institute (NHLBI) grants HL60655 (to H.W.) and HL70627 (to M.W.M.), and the Ziegler Family Chair for Research (to H.W.).
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: Y.G. performed the majority of experiments and cowrote the manuscript; T.K. conducted apoptosis studies on caspase-deficient MEF; I.H. prepared native FDP and conducted functional studies; L.M. characterized the structure of FnE-hementin; B.I., E.K., and R.S. contributed apoptosis studies in endothelial cells; T.H. designed, synthesized, and analyzed peptides; M.M. oversaw the preparation of FDP and cowrote the manuscript; and H.W. designed and coordinated experiments, contributed data analysis, and cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hartmut Weiler, Blood Research Institute, BloodCenter of Wisconsin, 8727 West Watertown Plank Road, Milwaukee, WI 53226-3548; e-mail: hartmut.weiler@bcw.edu.
References
- 1.Cesarman-Maus G, Hajjar KA. Molecular mechanisms of fibrinolysis. Br J Haematol. 2005;129:307–321. doi: 10.1111/j.1365-2141.2005.05444.x. [DOI] [PubMed] [Google Scholar]
- 2.Francis CW, Marder VJ. Physiologic regulation and pathologic disorders of fibrinolysis. In: Colman RW, Hirsch J, Marder VJ, Clowes AW, George JN, editors. Hemostasis and Thrombosis: Basic Principles and Clinical Practice. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. pp. 975–1002. [Google Scholar]
- 3.Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3:1894–1904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 4.Isermann B, Sood R, Pawlinski R, et al. The thrombomodulin-protein C system is essential for the maintenance of pregnancy. Nat Med. 2003;9:331–337. doi: 10.1038/nm825. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282:2072–2075. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
- 6.Pattillo RA, Gey GO. The establishment of a cell line of human hormone-synthesizing trophoblastic cells in vitro. Cancer Res. 1968;28:1231–1236. [PubMed] [Google Scholar]
- 7.Pattillo RA, Gey GO, Delfs E, Mattingly RF. In vitro identification of the trophoblastic stem cell of the human villous placenta. Am J Obstet Gynecol. 1968;100:582–588. doi: 10.1016/s0002-9378(15)33497-9. [DOI] [PubMed] [Google Scholar]
- 8.Lieblich JM, Weintraub BD, Rosen SW, Chou JY, Robinson JC. HeLa cells secrete alpha subunit of glycoprotein tropic hormones. Nature. 1976;260:530–532. doi: 10.1038/260530a0. [DOI] [PubMed] [Google Scholar]
- 9.Razani B, Engelman JA, Wang XB, et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 10.Zheng TS, Hunot S, Kuida K, et al. Deficiency in caspase-9 or caspase-3 induces compensatory caspase activation. Nat Med. 2000;6:1241–1247. doi: 10.1038/81343. [DOI] [PubMed] [Google Scholar]
- 11.Lakhani SA, Masud A, Kuida K, et al. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science. 2006;311:847–851. doi: 10.1126/science.1115035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mosesson MW, Sherry S. The preparation and properties of human fibrinogen of relatively high solubility. Biochemistry. 1966;5:2829–2835. doi: 10.1021/bi00873a008. [DOI] [PubMed] [Google Scholar]
- 13.Siebenlist KR, Meh DA, Mosesson MW. Plasma factor XIII binds specifically to fibrinogen molecules containing gamma chains. Biochemistry. 1996;35:10448–10453. doi: 10.1021/bi9606206. [DOI] [PubMed] [Google Scholar]
- 14.Mosesson MW, Siebenlist KR, Hernandez I, Wall JS, Hainfeld JF. Fibrinogen assembly and crosslinking on a fibrin fragment E template. Thromb Haemost. 2002;87:651–658. [PubMed] [Google Scholar]
- 15.Pechik I, Madrazo J, Mosesson MW, Hernandez I, Gilliland GL, Medved L. Crystal structure of the complex between thrombin and the central “E” region of fibrin. Proc Natl Acad Sci U S A. 2004;101:2718–2723. doi: 10.1073/pnas.0303440101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Budzynski AZ, Olexa SA, Brizuela BS, Sawyer RT, Stent GS. Anticoagulant and fibrinolytic properties of salivary proteins from the leech Haementeria ghilianii. Proc Soc Exp Biol Med. 1981;168:266–275. doi: 10.3181/00379727-168-41271. [DOI] [PubMed] [Google Scholar]
- 17.Kirschbaum NE, Budzynski AZ. A unique proteolytic fragment of human fibrinogen containing the A alpha COOH-terminal domain of the native molecule. J Biol Chem. 1990;265:13669–13676. [PubMed] [Google Scholar]
- 18.Loike JD, Sodeik B, Cao L, et al. CD11c/CD18 on neutrophils recognizes a domain at the N terminus of the A α chain of fibrinogen. Proc Natl Acad Sci U S A. 1991;88:1044–1048. doi: 10.1073/pnas.88.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smolarczyk K, Boncela J, Szymanski J, Gils A, Cierniewski CS. Fibrinogen contains cryptic PAI-1 binding sites that are exposed on binding to solid surfaces or limited proteolysis. Arterioscler Thromb Vasc Biol. 2005;25:2679–2684. doi: 10.1161/01.ATV.0000189305.84297.8b. [DOI] [PubMed] [Google Scholar]
- 20.Lisanti MP, Scherer PE, Vidugiriene J, et al. Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: implications for human disease. J Cell Biol. 1994;126:111–126. doi: 10.1083/jcb.126.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Razani B, Woodman SE, Lisanti MP. Caveolae: from cell biology to animal physiology. Pharmacol Rev. 2002;54:431–467. doi: 10.1124/pr.54.3.431. [DOI] [PubMed] [Google Scholar]
- 22.Scherer PE, Lewis RY, Volonte D, et al. Cell-type and tissue-specific expression of caveolin-2. Caveolins 1 and 2 co-localize and form a stable hetero-oligomeric complex in vivo. J Biol Chem. 1997;272:29337–29346. doi: 10.1074/jbc.272.46.29337. [DOI] [PubMed] [Google Scholar]
- 23.Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J Biol Chem. 1997;272:6525–6533. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]
- 24.Bootle-Wilbraham CA, Tazzyman S, Thompson WD, Stirk CM, Lewis CE. Fibrin fragment E stimulates the proliferation, migration and differentiation of human microvascular endothelial cells in vitro. Angiogenesis. 2001;4:269–275. doi: 10.1023/a:1016076121918. [DOI] [PubMed] [Google Scholar]
- 25.Naito M, Stirk CM, Smith EB, Thompson WD. Smooth muscle cell outgrowth stimulated by fibrin degradation products. The potential role of fibrin fragment E in restenosis and atherogenesis. Thromb Res. 2000;98:165–174. doi: 10.1016/s0049-3848(99)00202-9. [DOI] [PubMed] [Google Scholar]
- 26.Thompson WD, Smith EB, Stirk CM, Marshall FI, Stout AJ, Kocchar A. Angiogenic activity of fibrin degradation products is located in fibrin fragment E. J Pathol. 1992;168:47–53. doi: 10.1002/path.1711680109. [DOI] [PubMed] [Google Scholar]
- 27.Bach TL, Barsigian C, Chalupowicz DG, et al. VE-Cadherin mediates endothelial cell capillary tube formation in fibrin and collagen gels. Exp Cell Res. 1998;238:324–334. doi: 10.1006/excr.1997.3844. [DOI] [PubMed] [Google Scholar]
- 28.Bach TL, Barsigian C, Yaen CH, Martinez J. Endothelial cell VE-cadherin functions as a receptor for the β15-42 sequence of fibrin. J Biol Chem. 1998;273:30719–30728. doi: 10.1074/jbc.273.46.30719. [DOI] [PubMed] [Google Scholar]
- 29.Gorlatov S, Medved L. Interaction of fibrin(ogen) with the endothelial cell receptor VE-cadherin: mapping of the receptor-binding site in the NH2-terminal portions of the fibrin beta chains. Biochemistry. 2002;41:4107–4116. doi: 10.1021/bi0160314. [DOI] [PubMed] [Google Scholar]
- 30.Petzelbauer P, Zacharowski PA, Miyazaki Y, et al. The fibrin-derived peptide Bβ15-42 protects the myocardium against ischemia-reperfusion injury. Nat Med. 2005;11:298–304. doi: 10.1038/nm1198. [DOI] [PubMed] [Google Scholar]
- 31.Gando S, Tedo I, Kubota M. Posttrauma coagulation and fibrinolysis. Crit Care Med. 1992;20:594–600. doi: 10.1097/00003246-199205000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Krysko DV, D'Herde K, Vandenabeele P. Clearance of apoptotic and necrotic cells and its immunological consequences. Apoptosis. 2006;11:1709–1726. doi: 10.1007/s10495-006-9527-8. [DOI] [PubMed] [Google Scholar]
- 33.Vandivier RW, Henson PM, Douglas IS. Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest. 2006;129:1673–1682. doi: 10.1378/chest.129.6.1673. [DOI] [PubMed] [Google Scholar]
- 34.Wu Y, Tibrewal N, Birge RB. Phosphatidylserine recognition by phagocytes: a view to a kill. Trends Cell Biol. 2006;16:189–197. doi: 10.1016/j.tcb.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 35.Gando S, Kameue T, Matsuda N, Sawamura A, Hayakawa M, Kato H. Systemic inflammation and disseminated intravascular coagulation in early stage of ALI and ARDS: role of neutrophil and endothelial activation. Inflammation. 2004;28:237–244. doi: 10.1023/b:ifla.0000049049.81688.fe. [DOI] [PubMed] [Google Scholar]
- 36.Idell S. Coagulation, fibrinolysis, and fibrin deposition in acute lung injury. Crit Care Med. 2003;31:S213–220. doi: 10.1097/01.CCM.0000057846.21303.AB. [DOI] [PubMed] [Google Scholar]
- 37.Fridlender ZG, Cohen PY, Golan O, Arish N, Wallach-Dayan S, Breuer R. Telomerase activity in bleomycin-induced epithelial cell apoptosis and lung fibrosis. Eur Respir J. 2007;30:205–213. doi: 10.1183/09031936.00009407. [DOI] [PubMed] [Google Scholar]
- 38.Mura M, Andrade CF, Han B, et al. Intestinal ischemia-reperfusion–induced acute lung injury and oncotic cell death in multiple organs. Shock. 2007;28:227–238. doi: 10.1097/01.shk.0000278497.47041.e3. [DOI] [PubMed] [Google Scholar]