Abstract
The sequencing of the human genome has led to the identification of many genes whose functions remain to be determined. Because of conservation of genetic function, microbial systems have often been used for identification and characterization of human genes. We have investigated the use of the Escherichia coli SOS induction assay as a screen for yeast and human genes that might play a role in DNA metabolism and/or in genome stability. The SOS system has previously been used to analyze bacterial and viral genes that directly modify DNA. An initial screen of meiotically expressed yeast genes revealed several genes associated with chromosome metabolism (e.g., RAD51 and HHT1 as well as others). The SOS induction assay was then extended to the isolation of human genes. Several known human genes involved in DNA metabolism, such as the Ku70 end-binding protein and DNA ligase IV, were identified, as well as a large number of previously unknown genes. Thus, the SOS assay can be used to identify and characterize human genes, many of which may participate in chromosome metabolism.
Keywords: genome stability
Human genome stability requires the orchestration of a large battery of gene products that can influence cell progression, chromosome structure, replication and repair, transcription, and various responses to environmental genotoxicants. Alterations in any of these genes or their regulated activities can potentially lead to spontaneous or damage-induced genetic instability and altered chromosome metabolism, including mutation, recombination, and aneuploidy. Changes in genetic stability and chromosomal metabolism are hallmarks of many inherited disorders, including the multistage process leading to cancer.
The maintenance of genome stability is highly complex. Based on observations in microbes and evolutionary conservation with mammals, hundreds to thousands of genes are likely to be involved in human genome stability. For example, there are human cognates for several of the proteins involved in microbial DNA repair (discussed in ref. 1). The discovery of this apparent conservation between mammalian and microbial systems has led to insights into the structure and function of genes that affect genome stability and has provided impetus for bioinformatic analyses of genomes.
Whereas the number of human genes responsible for genome stability may be numerous, the opportunities to identify them have been limited; thus, many more mammalian genes remain to be identified. To date, the identification of human DNA metabolism genes has been achieved primarily via techniques that include the use of degenerate oligonucleotides from known genes, followed by PCR, bioinformatic analysis, and subsequent identification of homologous genes from DNA sequence databases, and direct complementation of a well-characterized mutant.
Although these strategies have proven invaluable, they are limited in their ability to identify a broad range of genes involved in genome stability. We have developed an alternative approach for the rapid isolation and characterization of a wide variety of human genes that may have a role in chromosome metabolism and genome stability. Our approach is based on the concept that some human genes when overexpressed in model microbial systems can phenotypically alter specific genetic endpoints as they relate to genome stability. For example, overexpression in Escherichia coli of the human mismatch repair gene homolog MSH2 (a gene whose deficiency is responsible for certain hereditary nonpolyposis colon cancers) supported its role in the mutation process (2). Additionally, mammalian DNA polymerase β mutants have been characterized by their ability to functionally complement DNA polymerase I in E. coli (3). In addition, numerous heterologous DNA repair genes have been identified and characterized via functional complementation of prokaryotic repair mutants (discussed in ref. 4).
We have investigated the use of the E. coli SOS induction assay to identify human genes that might play a role in DNA metabolism and/or in genome stability. Because it is responsive to a variety of chromosomal perturbances, as originally described by Kenyon and Walker (5), the SOS system was previously used to characterize viral and prokaryotic enzymes involved in DNA metabolism, such as the T7.3 endonuclease gene (6), DNA methylases (7), and the EcoRI and BamHI restriction endonucleases (8–10), and for the isolation of novel thermostable restriction enzymes and nucleases (11, 12). Based on these results, a broad spectrum of bacterial genes associated with DNA metabolism might be identifiable with the SOS assay (9).
Given its sensitivity to chromosomal perturbances, we asked whether the SOS response could be elicited by human genes in a RecA-dependent manner. To test the validity of this approach, an initial screen of meiotically expressed yeast genes was performed that revealed several genes associated with chromosome metabolism. In the subsequent screen of genes expressed in human testis, several chromosome stability genes were identified, including previously uncharacterized genes. Thus, the SOS assay provides a way to identify and characterize the functions of human genes involved in chromosomal metabolism.
MATERIALS AND METHODS
Bacterial Strains.
The strain JH139 for assaying the SOS response contains the dinD1∷MudI1734 (kanR lac) fusion as previously described and kindly provided by Joseph Heitman (Duke University, Durham, NC; ref. 9). A recA1 derivative of this strain was constructed with standard phage P1 transductions and confirmed by UV sensitivity to give the strain JRC139 (13). Standard YT medium contained 100 μg/ml ampicillin (Amp), 35 μg/ml 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal), and 0.1 mM phenylethyl-β-d-galactopyranoside (TPEG). Induction of the trc promoter was accomplished by using 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG).
Yeast Meiotic and Human Testis cDNA Library Construction.
A yeast meiotic cDNA library was developed for expression in the SOS induction system. To increase our chances of isolating yeast cDNAs affecting genome stability, we purified poly(A)+ mRNA from the diploid yeast strain MR48 (14) at various times in meiosis (0.5, 1, 2, 4 and 6 h; after 6 h, spore formation was visually confirmed). During meiosis, there is a significant induction of various DNA metabolic activities including replication and recombination, as well as increases in specific activities of enzymes associated with these processes (14). Cells were harvested and guanidium isothiocyanate-prepared meiotic RNA was isolated as described (15). mRNA was purified with a poly(A)+ mRNA isolation kit (Qiagen, Santa Clarita, CA). Pooled mRNA fractions from each time point were used for cDNA synthesis (equal amounts of mRNA were used from each time point). To ensure a greater proportion of near-full-length transcripts, first-strand cDNA synthesis was performed by using SuperScript II reverse transcriptase (Life Technologies, Gaithersberg, MD), which contains point mutations in the RNase H domain (eliminates degradation of the mRNA template) and an oligo(dT)-primer containing a NotI restriction site. SalI- or XhoI-cloning adapters were added to the resulting cDNA, and the ligation products were digested with NotI. The cDNA was size selected (>500 bp) before ligation to the expression vector. The synthesized cDNA was directionally cloned (SalI to NotI or XhoI to NotI) into the E. coli expression vector pSE380 (ref. 16; Invitrogen). This vector contains a strong trc promoter for high-level induction, the lacO operator, and lacIq repressor gene for transcriptional regulation and convenient cloning sites for directional cloning of our synthesized cDNAs adjacent to the trc promoter. A yeast meiotic cDNA library of approximately 4 × 107 primary transformants was constructed in this vector. This number of primary transformants ensures nearly complete coverage of all expressed cDNAs. Similarly, we constructed a pSE380 expression library from cDNA generated from human testis poly(A)+ mRNA (CLONTECH). A library of approximately 4 × 107 primary transformants was constructed (corresponding to nearly complete coverage of all expressed cDNAs).
Library Screening in E. coli for SOS Induction.
The cDNA libraries were transformed into the SOS detection strain JH139. This strain contains the dinD1∷lacZ reporter construct, which exhibits a low basal level of β-galactosidase but a large increase in β-galactosidase when DNA damage is introduced (i.e., SOS response). The transformants were plated on medium containing antibiotic (YT + Amp) to achieve approximately 200–250 transformants per plate. After overnight growth, plates were subsequently replica-plated to YT + Amp, YT + Amp + X-Gal + TPEG, and YT + Amp + X-Gal + TPEG + IPTG (TPEG, a competitive inhibitor of β-galactosidase, reduced the background level of the enzyme to better visualize the SOS induction) and grown overnight. Plates were then scored for colonies that exhibited the SOS/LacZ+ response when cDNAs were induced by IPTG (blue color on YT + Amp + X-Gal + TPEG + IPTG). (Occasionally SOS/LacZ+ transformants were observed independently of IPTG; these were likely because of gratuitous recognition of some yeast sequences as promoter elements in E. coli, a phenomenon that has been exploited in the cloning of yeast genes that complement certain E. coli mutations.) The SOS/LacZ+ transformants were purified, and the plasmid was isolated and transformed back into JH139. They were also transformed into an isogenic recA derivative of JH139 to establish that the SOS response was indeed RecA dependent or, alternatively, prevented growth of the recA mutant. The RecA dependence would be expected if, for example, the expressed cDNA caused a perturbation in DNA metabolism. cDNA inserts exhibiting RecA-dependent SOS induction or lack of growth were amplified with TaqPlus polymerase (Stratagene) and the primers 5′-GCGCCGACATCATAACGGTTCTGGC-3′ (PSESTRT) and 5′-CGGCGCTACGGCGTTTCACTTCTGAGTTCG-3′ (PSEEND). The PCR products were gel purified and sequenced on an ABI model 373A DNA sequencer with the ABI PRISM sequencing kit (Perkin–Elmer) and the oligonucleotide 5′-GGCTCGTATAATGTGTGGAATTGTGAGCGG-3′ (380OLIGO). Sequence analyses were performed with the BLASTX and BEAUTY algorithms of the BCM Search Launcher (Human Genome Center, Baylor College of Medicine, Houston; refs. 17–19). Finally, that the expression of the SOS response was caused by the isolated clones was confirmed by mutagenesis of the cloned cDNA inserts. Mutagenesis was performed either by λHOP insertions into the cDNA (20) or by frameshifting the amino-terminal coding sequence via restriction enzyme digestion of the cDNA with subsequent Klenow fragment fill-in reactions.
RESULTS
Isolation of Yeast Meiotic cDNAs That Induce SOS.
The SOS response in E. coli, a coordinate regulatory cascade involving approximately 20 genes, is induced by DNA-damaging agents or drugs that inhibit DNA metabolism. Furthermore, many proteins that exhibit DNA metabolic activity induce the SOS response when expressed (7–12). Therefore, we reasoned that this system might be applicable to the identification of human genes involved in DNA metabolism and genome stability. Because the efficacy and level of sensitivity of this approach for identifying eukaryotic cDNAs was not known, we initiated a screen for Saccharomyces cerevisiae genes, which, when expressed in E. coli, could induce the SOS system. Because the entire genome of S. cerevisiae has been sequenced, identification of yeast genes involved in DNA or chromosome metabolism with the SOS assay would validate the utility of the screen.
The yeast cDNAs were derived from cells at various stages of meiosis (see Materials and Methods) and were placed under the control of a highly regulatable promoter. The clones were examined for their ability to induce the SOS response, which was detected by induction of a dinD1∷LacZ reporter cassette. Among approximately 26,000 transformants, of which one-third would be expected to be in-frame, 70 yielded an SOS response that was RecA dependent (i.e., the recA strain did not grow or there was no SOS response in the recA strain containing the expressed cDNA; discussed below).
The 5′ end of each isolate was sequenced to identify the genes in the yeast genome. As shown in Table 1, most (41 isolates) of the cDNAs corresponded to the meiosis-specific SPS4 gene which codes for a putative 38.6-kDa basic protein with a deduced pI of 9.7. Most of the SPS4 isolates were full length or nearly full length. Previously, it was shown that SPS4 is a major transcript in meiosis (21). Two of the isolates were full-length isolates of the RAD51 gene, a RecA homolog required in yeast for genome stability, meiosis, and repair after exposure to many genotoxicants (22). Furthermore, RAD51 homologs have been identified in mammalian cells and are also proposed to play a role in genome stability in higher eukaryotes (23). A homolog of the mouse and human MOV34 (POH1) gene was also isolated. The MOV34 protein is highly conserved throughout evolution and corresponds to the human 26S proteosome regulatory chain (24). The human counterpart, POH1, appears to play a role in multidrug resistance and increased resistance to UV irradiation when overexpressed (25). The Schizosaccharomyces pombe homolog appears to play a role in higher order chromosome structure (26). This gene appears to be essential for viability in fission yeast, Drosophila, and mammalian cells (26–28). Disruption of this gene in S. cerevisiae indicates that it is essential for viability as well (unpublished observation). The yeast gene corresponding to this mammalian proteasomal subunit has been termed RPN7, for regulatory particle non-ATPase (29). Mutational analysis of the SPS4, RPN7, and RAD51 cDNAs indicates that the expressed protein products of these cDNAs are responsible for the SOS/LacZ+ phenotype, because both insertion/deletions and frameshift mutations within the coding regions abolish the SOS response.
Table 1.
Yeast genes isolated in an SOS screen of 26,000 meiotic cDNA transformants
| Genes | Isolates | Description |
|---|---|---|
| SPS4 | 41 | Major meiotic transcript; basic charge |
| RAD51 | 2 | RecA homolog |
| RPN7 | 1 | Homolog to human POH1 |
| HHT1 | 1 | Histone H3 |
| SPR28 | 2 | Meiotic septin |
| SNT309 | 1 | Splicing factor |
| MET32 | 1 | Transcriptional regulator |
| HOP2 | 1 | Inhibitor of meiotic nonhomologous chromosomal synapsis |
| YCR010C | 1 | ORF on chromosome III, similar to FUN34 |
| YPL055C | 5 | ORF on chromosome XVI |
| YPR078C | 9 | ORF on chromosome XVI |
| YPL033C | 1 | ORF on chromosome XVI |
| YPR007C | 1 | ORF on chromosome XVI |
| YBR214W | 2 | ORF on chromosome II |
| YGR247W | 1 | ORF on chromosome VII |
There were 70 total isolates and 15 total unique isolates (based on sequencing).
Another DNA-associated protein was identified, histone H3 (encoded by the HHT1 gene). Two independent isolates of the SPR28 septin were identified, as well as the splicing factor SNT309 and the zinc finger DNA-binding transcriptional regulator MET32 (30, 31). We also isolated the HOP2 gene, which is meiosis specific and required for preventing synapsis between nonhomologous chromosomes (32). In addition to these cDNAs, seven unknown yeast ORFs were identified. One of the unknowns, YCR010C exhibits a high degree of homology with the FUN34 transmembrane protein. Phenotypes for disruption and overexpression of these unknown genes are currently being tested.
Isolation of Human Testis cDNAs That Induce SOS.
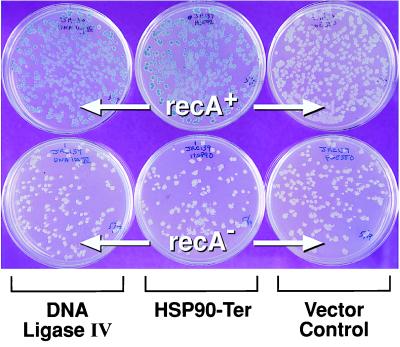
Based on our ability to detect yeast meiotic cDNAs that could induce the SOS response, we undertook a screen of cDNAs derived from human testis mRNA. Of approximately 65,000 transformants examined (one-third of the clones in our constructed library would be expected to be in-frame), we identified 58 (Table 2) that induced an RecA-dependent SOS response or required RecA for growth. As shown in Table 2, five of these corresponded to genes that had been previously identified [Ku70, DNA ligase IV, poly(A) polymerase, HSP105, and hTAFII18]. Nonspecific effects were considered unlikely if the cDNAs were unable to induce SOS in a recA strain or, alternatively, if they prevented growth of the recA strain (Table 3). The RecA specificity of the SOS response or inviability in a recA strain suggests that the cDNAs can exert their effects through perturbances in DNA metabolism and cell growth. Approximately two-thirds of the isolated human cDNAs identified in this screen exhibited RecA-dependent growth when the cDNAs were induced. An example of the recA-dependent SOS response is shown in Fig. 1. The predicted protein sizes encoded by the isolated cDNAs are presented in Table 3.
Table 2.
Human genes isolated in an SOS screen of 65,000 transformants
| Genes | Isolates | Description |
|---|---|---|
| Ku70 | 1 | End-binding protein and putative ATP-dependent helicase II |
| Ligase | 1 | DNA ligase IV |
| RNA polymerase | 1 | Poly(A) polymerase |
| HSP90C-Ter | 2 | Terminal homology to HSP90 |
| HSP105 | 1 | Heat shock protein |
| XKLP2 homolog | 2 | Homolog of Xenopus centrosomal kinesin-like protein |
| MNS1 homolog | 1 | Homolog of mouse meiosis nuclear structural protein |
| Rabin3 homolog | 1 | Rat homolog of Ras-interacting protein |
| hTAFII18 | 1 | TFIID subunit |
| P2P-R homolog | 1 | Homolog of mouse potential proliferation protein-related |
| Kanadaptin homolog | 1 | Homolog of mouse kanadaptin |
| Annexin-binding protein | 3 | Homolog of mouse annexin-binding proteins |
| RAD50 homolog | 1 | Limited homology to yeast RAD50 |
| Unknown | 4 | Cross-reacts with antibodies to parathyroid hormone |
| Unknowns | – | 37 different cDNAs of unknown function |
There were 58 total isolates, and 51 total unique isolates (based on sequencing).
Table 3.
Growth response of strains containing human genes that induce the SOS response
| cDNA isolate | Size of human protein,* aa | Predicted size of coded protein† | Growth of recA strain‡ |
|---|---|---|---|
| Ku70 | 609 | 596 | No |
| DNA ligase IV | 844 | 192 | Yes |
| Poly(A) polymerase | 652 | 86 | Yes |
| HSP90C-Ter | NA§ | NA | Yes |
| HSP105 | 858 | 237 | No |
| XKLP2 homolog | NA | 509 | No |
| MNS1 homolog | 491 | 440 | No |
| Rabin3 homolog | 460 | 289 | Yes |
| hTAFII18 | 124 | 114 | No |
| P2P-R homolog | NA | 366 | No |
| Kanadaptin | NA | 484 | No |
| Annexin-binding protein (APB-7) | NA | 94 | No |
| RAD50 homolog | NA | NA | No |
Amino acids in reported full-length proteins.
Amino acids starting at the C-terminal end. Predicted sizes were determined by partial DNA sequencing and alignments to known homologs.
Growth of isogenic recA strain when cDNA expressed.
NA (not applicable) because these genes were first isolated in this study.
Figure 1.
Induction of the SOS response by human cDNAs is RecA dependent. Presented are two of the cDNAs, DNA ligase IV and the HSP90-terminal segment homolog HSP90C-Ter, that elicited a recA-dependent SOS response. RecA and recA strains harboring each of the indicated cDNAs were first patched to YT + Amp plates and grown overnight at 37°C. These plates were subsequently replica plated to YT + Amp, YT + Amp + X-Gal + TPEG and YT + Amp + X-Gal + TPEG + IPTG and incubated overnight at 37°C. The SOS response gives rise to blue colonies because of the induction of the dinD1∷LacZ reporter construct. The figure shows the YT + Amp + X-Gal + TPEG + IPTG plates only.
The isolation of clones corresponding to DNA ligase IV and the Ku70 end-binding protein supports and demonstrates the utility of the hypothesis that the SOS assay can be used to identify proteins with DNA metabolic activities. DNA ligase IV forms a complex with XRCC4 (via the carboxy-terminal extension of DNA ligase IV containing two tandom BRCT domains) and is responsible for DNA end joining in V(D)J recombination, as well as repair of DNA double-strand breaks in mammalian cells (33, 34). This structure–function relationship also appears to be conserved in yeast, in which the yeast DNA ligase IV homolog (LIG4) interacts with the corresponding yeast XRCC4 homolog (LIF1) to participate in nonhomologous DNA end-joining activities. Interestingly, the Ku70 protein (35) also plays a role in joining of broken molecules as part of V(D)J recombination in mammalian cells and nonhomologous end joining in yeast. In addition to DNA ligase IV and Ku70, we have identified a cDNA corresponding to the heat shock protein HSP105 (36). It is not clear how the HSP105 gene influences SOS induction; possibly, it affects folding of protein(s) associated with the SOS response or modifies genes involved with E. coli DNA metabolism. Furthermore, a role for heat shock proteins as regulators of gene transcription factors has been suggested (37, 38). Interestingly, one of the isolated cDNAs (two independent isolates) exhibited localized homology to a carboxyl-terminal 10-aa stretch of HSP90 and is referred to as HSP90C-Ter homolog (Table 4). The significance and function of this region remains to be determined. Although HSP90C-Ter and HSP105 expression resulted in the SOS response, they differed in their effects in the recA strain. Expression of HSP90C-Ter did not appear to affect growth of the recA strain, whereas HSP105 expression clearly inhibited growth. Two additional genes were identified that are involved in nucleic acid metabolism: RNA polymerase transcription factor IID subunit TAFII18 and poly(A) polymerase.
Table 4.
Protein sequence* alignment between isolated cDNAs and their homologs
| Protein | Sequences examined and identified |
|---|---|
| HSP90C-Ter | Query:1 VEGDEDASRMEXVD 42 |
| +EGDEDASRME VD | |
| hHSP90:711 LEGDEDASRMEEVD 724 | |
| XKLP2-Hom | Query:10 IMKFEIDQLSRNLQNFKKENETLKSDLNNLMELLEAEKERNNKLSLQFEXDKENSSNRYL 189 |
| +MKFEIDQL + + + K ENETL+++ +NL+ELLE EKERKL+ Q E DKEN + L | |
| XKLP2:879 VMKFEIDOLKOEISDSKHENETLRAEFSNLLELLETEKERROKLTSOLEEDKENKTKELL 938 | |
| RAD50-Hom | Query:9 SRLIKNLGVDTIQMEYNASNISNSXHDSDEISGKMNTYMNSTTSSKXGYWCAN 167 |
| S LIK++ VD+ +EYN + S HDS+E++ K+ ++ + +T + N | |
| YRAD50: 424 SDLIKSITVDSONLEYNKKDRSKLIHDSEELAEKLKSFKSLSTODSLNHELEN 476 | |
| Kanadaptin-Hom | Query:23 QXDEMGCTWGMGEDAVXDDAEENPIVLXFQQEREAFYIKDPKKALQGFFDREGEE 187 |
| Q DE+GCTWGMGEDAV D+AEENPI L FQQ+REAFYIKDPKKALQGFFDREGEE | |
| Kanad:68 ODDELGCTWGMGEDAVEDEAEENPIALDFOODREAFYIKDPKKALOGFFDREGEE 122 | |
| P2P-R-Hom | Query:32 AKLNKEKVKGKVRRKVTGTEGSSSTLVDYTSTSSTGGSPVRKSEEKTDTKRTVIKTMEEY 211 |
| +K+ +EKVKGK +RKV G+EGSSSTLVDYTSTSSTGGSPVRKSEEKTDTKRTVIKTMEEY | |
| P2P-R:1038 SKIKQEKVKGKAKRKVAGSEGSSSTLVDYTSTSSTGGSPVRKSEEKTDTKRTVIKTMEEY 1097 | |
| Query:212 NNDNTAPAEXVIIMIQVPQSKWDKDDFESEEEDVKSTQPISSC∗ENLLVFIKNV∗YKALK 391 | |
| NNDNTAPAE VIIMIQVPQSKWDKDDFESEEEDVK+TQPI S + + IKNV K | |
| P2P-R:1098 NNDNTAPAEDVIIMIOVPOSKWDKDDFESEEEDVKTTOPIOSVGKPSSI-IKNVTTKPSA 1156 | |
| Annexin Vbinding protein (ABB-7) | Query:11 ERKKRDEEKAKLRKLKEKEELETGKKDQSKQKESQRKFEEETVKSKVTVDTG 66 |
| ERKKR+EEKAKLRK+KEKEELE G+K+QSKQ+E Q++ +EE + + T D G | |
| APB-7:140 ERKKREEEKAKLRKVKEKEELEKGRKEOSKOREPOKRPDEEVLVLRGTPDAG 191 |
Sequence alignments were identified by using blast/beauty searches. Alignment corresponds to amino acid alignment and position relative to the known protein.
Additional human cDNAs were isolated that exhibited significant homology to known proteins from other organisms. These include a possible human homolog of a mouse meiosis-specific nuclear structural protein MNS1 [46% protein identity over the translated sequenced region (39)], a homolog of a rat protein (Rabin3) that associates with the Ras-like GTPase Rab3A [76% protein identity over the translated sequenced region (40)], and a homolog of the Xenopus centrosomal kinesin-like protein KLP2 (41). In addition, homologs of the mouse proliferating potential-related protein (P2P-R), kanadaptin, and an annexin V-binding protein (ABP-7; three independent isolates) were identified (42–44). In the mouse, P2P-R is a member of a highly basic group of nuclear proteins that can bind to RNA and are associated with heterogeneous nuclear ribonucleoprotein particles and the RB1 tumor suppressor and may modulate differentiation (42). Although annexin V has been proposed to function as a neurotrophic factor (45), four unique proteins have been demonstrated to interact with it (44). These interacting proteins include two proteins highly homologous to helicase2 and DNA (cytosine-5) methyltransferase and two of unknown function. It is interesting that one of the clones exhibited limited relatedness to the yeast RAD50 gene required for double-strand break repair (Table 4). The functions of the remaining 38 clones (some corresponding to expressed sequence tags) are not available from current databases.
DISCUSSION
The SOS system of bacteria consists of a group of more than 20 genes that can be induced in response to DNA-damaging agents or perturbations to genome metabolism and cell progression. The recA gene, which is essential in the induction process, also plays a direct role in dealing with many kinds of lesions and genomic changes. With this in mind, we investigated the use of the E. coli SOS induction assay to identify human genes that might play a role in DNA metabolism and/or genome stability. Because of its sensitivity to a variety of chromosomal perturbances, the SOS system has previously been used to analyze bacterial and viral genes that directly modify DNA and to identify new genes from various bacterial systems. Because there is conservation of many gene functions across evolution, we anticipated that there would be genes in eukaryotes that could elicit an SOS response when expressed in E. coli. The gene products might be proteins that directly modify or interact with DNA, such as nucleases, helicases, or single-strand DNA-binding proteins, or, alternatively, they might interact with components in DNA metabolic processes. There are numerous examples of eukaryotic genes affecting growth or genetic stability in E. coli, but there have been no studies of eukaryotic genes that directly induce the SOS response.
Our approach with the SOS system has led to the identification of many eukaryotic genes that induce the SOS response in a RecA-dependent fashion. Approximately two-thirds of the identified cDNAs exhibited RecA-dependent growth as well. These results are consistent with perturbation of E. coli chromosome metabolism either directly or indirectly. This is certainly true for the isolated cDNAs that corresponded to known genes (Tables 1 and 2); 30–50% of the cDNAs code for genes that are obviously related to some aspect of chromosome metabolism or that interact with factors that can affect metabolism. These results also strongly support the view that the assay provides selectivity, because it is unlikely that, in a random sample of cDNAs, 30–50% are related to chromosome metabolism. It remains to be determined why the other known genes were isolated in the SOS assay. For example, replication might be interrupted simply by the DNA binding of an induced heterologous protein, such as histone 3A, or other DNA-binding proteins, thus eliciting the SOS response. Alternatively, the other genes could affect some aspect of normal DNA metabolism, possibly by changing protein structure (as for heat shock proteins), or they could alter transcription by components involved in SOS induction [as for poly(A) polymerase, RNA polymerase transcription factor TAFII18, or the human homolog of the mouse proliferating potential-related protein P2P-R]. A transcription factor might interact with factors involved in transcription-coupled DNA repair (46). Unexpectedly, this screen yielded a few factors that appear to be involved with the cell membrane (such as the yeast homolog of the FUN34 integral membrane protein, the meiotic septin SPR28) and targeting secretory membrane vesicles (Rabin3 and kanadaptin homologs). Although an explanation of this will require further analysis, it is possible that cell membrane perturbances may affect the E. coli SOS response. For example, Garvey et al. demonstrated that the activated RecA protein can associate with the cell membrane and affect levels of major outer membrane proteins (47).
Many of the cDNAs isolated in the SOS induction assay encoded partial fragments of the corresponding proteins. This result was not surprising given the manner in which the cDNA libraries were constructed and the properties of the expression vector. The size of the final cDNA product is greatly influenced by the efficiency of the cDNA synthesis reaction as well as the quality of the mRNA template. Furthermore, the expression vector pSE380 contains translation initiation sequences and a start codon. cDNAs containing large upstream untranslated regions would generally not produce a protein product corresponding to the ORF of the cDNA. In addition, only one-third of the clones would be expected to be in-frame. This was the case for all the cDNAs corresponding to known yeast or human genes.
Even though a significant number of the isolated clones were partial fragments, our results indicate that these expressed peptides can still induce the SOS response. The use of expressed peptides to generate selectable phenotypes is not unprecedented. For example, dominant negative mutants can result from the truncation of protein regions involved in specific functional interactions (48). This approach has been modified and expanded to screen for dominant negative mutants resulting from the expression of random DNA fragments across a number of model systems including bacteriophage λ, yeast, and mammalian cells (49–52).
It will be interesting to learn the functions of the genes corresponding to the nearly 50 previously unidentified yeast and human cDNAs. Because there were many unique genes identified, it is likely that, if this assay were expanded, a considerably larger number of genes would be identified that can induce the SOS response. This screen could be applied to various tissues, growth conditions, and cell types. In addition, by using subtracted populations of cDNAs, this assay could provide an initial functional characterization of differentially expressed genes. The present approach could be combined with other mutants to further categorize or subcategorize human genes in functionality assays. For example, many of the genes that induced the SOS response also prevented growth of a recA strain. Thus, it would be interesting to determine the impact of these genes in other mutants defective in DNA metabolic activities. Finally, our constructed yeast and human cDNA libraries could be used to complement various E. coli mutants to identify functional homologs. For example, our human cDNA library has been used to isolate expressed cDNAs that complement the spontaneous mutator phenotype that results from mutation of genes required for oxidative repair of DNA (M. Volkert, personal communication).
Recently, we described the isolation of expressed human cDNAs that specifically prevented the growth of a DNA replication-defective yeast mutant, a method we refer to as phenotypic disruption of a genetically-sensitized strain (data not shown). These and other assays could illuminate gene functions based on the phenotypic disruption responses in the various fungal and/or bacterial mutants and systems. Especially important will be the opportunity to study cDNAs that correspond to newly identified positionally cloned genes of unknown function. Since many of these genes correspond to disease genes, assays such as the SOS response or any genetically sensitized microbial assay system will provide immediate opportunities for investigations of gene functions.
Acknowledgments
The authors gratefully acknowledge the helpful comments from the various members of the Chromosome Stability Group at National Institute on Environmental Health Sciences. In particular, we would like to thank Drs. A. Greene, K. Lewis, and J. Kirschner for their helpful suggestions and expertise. Additional acknowledgements go to Drs. D. Sun and M. Dresser for their comments and advice. We are gratefully indebted to Dr. M. Kricker for help with the cDNA homology searches. This work was partially supported by a Department of Energy Interagency Agreement DE-A105-94ER61940 to M.A.R.
ABBREVIATIONS
- Amp
ampicillin
- X-Gal
5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside
- TPEG
phenylethyl-β-d-galactopyranoside
- IPTG
isopropyl-β-d-thiogalactopyranoside
References
- 1.Friedberg E C, Walker G C, Siede W. DNA Repair and Mutagenesis. Washington, DC: Am. Soc. Microbiol.; 1995. pp. 317–366. [Google Scholar]
- 2.Fishel R, Lescoe M, Rao M, Copeland N, Jenkins N, Garber J, Kane M, Kolodner R. Cell. 1993;75:1027–1038. doi: 10.1016/0092-8674(93)90546-3. [DOI] [PubMed] [Google Scholar]
- 3.Sweasy J B, Loeb L A. Proc Natl Acad Sci USA. 1993;90:4626–4630. doi: 10.1073/pnas.90.10.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Memisoglu A, Samson L. CRC Crit Rev Biochem Mol Biol. 1996;31:405–447. doi: 10.3109/10409239609108724. [DOI] [PubMed] [Google Scholar]
- 5.Kenyon C J, Walker G C. Proc Natl Acad Sci USA. 1980;77:2819–2823. doi: 10.1073/pnas.77.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panayotatos N, Fontaine A. J Biol Chem. 1985;260:3173–3177. [PubMed] [Google Scholar]
- 7.Heitman J, Model P. J Bacteriol. 1987;169:3243–3250. doi: 10.1128/jb.169.7.3243-3250.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heitman J, Zinder N, Model P. Proc Nat Acad Sci USA. 1989;86:2281–2285. doi: 10.1073/pnas.86.7.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heitman J, Model P. Gene. 1991;103:1–9. doi: 10.1016/0378-1119(91)90383-m. [DOI] [PubMed] [Google Scholar]
- 10.Xu S-Y, Schildkraut I. J Biol Chem. 1991;266:4425–4429. [PubMed] [Google Scholar]
- 11.Fomenkov A, Xiao J, Dila D, Raleigh E, Xu S. Nucleic Acids Res. 1994;22:2399–2403. doi: 10.1093/nar/22.12.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fomenkov A, Xu S-Y. Gene. 1995;163:109–113. doi: 10.1016/0378-1119(95)00426-7. [DOI] [PubMed] [Google Scholar]
- 13.Miller J H. Experiments in Molecular Genetics. Plainview, NY: Cold Spring Harbor Lab. Press; 1972. [Google Scholar]
- 14.Resnick M A, Sugino A, Nitiss J, Chow T. Mol Cell Biol. 1984;12:2811–2817. doi: 10.1128/mcb.4.12.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wise J A. In: Guide to Yeast Genetics and Molecular Biology. Guthrie C, Fink G R, editors. Vol. 194. San Diego, CA: Academic; 1991. pp. 405–415. [Google Scholar]
- 16.Brosius J. DNA. 1989;8:759–777. doi: 10.1089/dna.1989.8.759. [DOI] [PubMed] [Google Scholar]
- 17.Gish W, States D J. Nat Genet. 1993;3:266–272. doi: 10.1038/ng0393-266. [DOI] [PubMed] [Google Scholar]
- 18.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 19.Worley K C, Wiese B A, Smith R F. Genome Res. 1995;5:173–184. doi: 10.1101/gr.5.2.173. [DOI] [PubMed] [Google Scholar]
- 20.Kleckner N, Bender J, Gottesman S. Methods Enzymol. 1991;204:139–180. doi: 10.1016/0076-6879(91)04009-d. [DOI] [PubMed] [Google Scholar]
- 21.Garber A T, Segall J. Mol Cell Biol. 1986;6:4478–4485. doi: 10.1128/mcb.6.12.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shinohara A, Ogawa H, Ogawa T. Cell. 1992;69:457–470. doi: 10.1016/0092-8674(92)90447-k. [DOI] [PubMed] [Google Scholar]
- 23.Shinohara A, Ogawa H, Matsuda Y, Ushio N, Ikeo K, Ogawa T. Nat Genet. 1993;4:239–243. doi: 10.1038/ng0793-239. [DOI] [PubMed] [Google Scholar]
- 24.Dubiel W, Ferrell K, Dumdey R, Standera S, Prehn S, Rechsteiner M. FEBS Lett. 1995;363:97–100. doi: 10.1016/0014-5793(95)00288-k. [DOI] [PubMed] [Google Scholar]
- 25.Spataro V, Toda T, Craig R, Seeger M, Dubiel W, Harris A, Norbury C. J Biol Chem. 1997;272:30470–30475. doi: 10.1074/jbc.272.48.30470. [DOI] [PubMed] [Google Scholar]
- 26.Shimanuki M, Saka Y, Yanagida M, Toda T. J Cell Sci. 1995;108:569–579. doi: 10.1242/jcs.108.2.569. [DOI] [PubMed] [Google Scholar]
- 27.Gridley T, Gray D A, Orr-Weaver T, Soriano P, Barton E E, Francke U, Jaenisch R. Development (Cambridge, UK) 1990;109:235–242. doi: 10.1242/dev.109.1.235. [DOI] [PubMed] [Google Scholar]
- 28.Gridley T, Jaenisch R, Gendron-Maguire M. Genomics. 1991;11:501–507. doi: 10.1016/0888-7543(91)90056-k. [DOI] [PubMed] [Google Scholar]
- 29.Glickman M H, Rubin D, Freid V A, Finley D. Mol Cell Biol. 1998;18:3149–3162. doi: 10.1128/mcb.18.6.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Virgilio C, DeMarini D J, Pringle J R. Microbiology. 1996;142:2897–2905. doi: 10.1099/13500872-142-10-2897. [DOI] [PubMed] [Google Scholar]
- 31.Chen H R, Jan S P, Tsao T Y, Sheu Y J, Banroques J, Cheng S C. Mol Cell Biol. 1998;18:2196–2204. doi: 10.1128/mcb.18.4.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leu J-Y, Chua P R, Roeder G S. Cell. 1998;94:375–386. doi: 10.1016/s0092-8674(00)81480-4. [DOI] [PubMed] [Google Scholar]
- 33.Critchlow S, Bowater R, Jackson S. Curr Biol. 1997;7:588–598. doi: 10.1016/s0960-9822(06)00258-2. [DOI] [PubMed] [Google Scholar]
- 34.Grawunder U, Wilm M, Wu X, Kulesza P, Wilson T, Mann M, Lieber M. Nature (London) 1997;388:492–495. doi: 10.1038/41358. [DOI] [PubMed] [Google Scholar]
- 35.Tuteja N, Tuteja R, Ochem A, Taneja P, Huang N W, Simoncsits A, Susic S, Rahman K, Marusic L, Chen J. EMBO J. 1994;13:4991–5001. doi: 10.1002/j.1460-2075.1994.tb06826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yasuda K, Nakai A, Hatayama T, Nagata K. J Biol Chem. 1995;270:29718–29723. doi: 10.1074/jbc.270.50.29718. [DOI] [PubMed] [Google Scholar]
- 37.Frydman J, Hohfeld J. Trends Biochem Sci. 1997;22:87–92. doi: 10.1016/s0968-0004(97)01005-0. [DOI] [PubMed] [Google Scholar]
- 38.Hallstrom T C, Katzmann D J, Torres R J, Sharp W J, Moye-Rowley W S. Mol Cell Biol. 1988;18:1147–1155. doi: 10.1128/mcb.18.3.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furukawa K, Inagaki H, Naruge T, Tabata S, Tomida T, Yamaguchi A, Yoshikuni M, Nagahama Y, Hotta Y. Chromosome Res. 1994;2:99–113. doi: 10.1007/BF01553489. [DOI] [PubMed] [Google Scholar]
- 40.Brondyk W H, McKiernan C J, Fortner K A, Stabila P, Holz R W, Macara I G. Mol Cell Biol. 1995;15:1137–1143. doi: 10.1128/mcb.15.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boleti H, Karsenti E, Vernos I. Cell. 1996;84:49–59. doi: 10.1016/s0092-8674(00)80992-7. [DOI] [PubMed] [Google Scholar]
- 42.Witte M M, Scott R E. Proc Natl Acad Sci USA. 1997;94:1212–1217. doi: 10.1073/pnas.94.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen J, Vijayakumar S, Li X, Al-Awqati Q. J Biol Chem. 1998;273:1038–1043. doi: 10.1074/jbc.273.2.1038. [DOI] [PubMed] [Google Scholar]
- 44.Ohsawa K, Imai Y, Ito D, Kohsaka S. J Neurochem. 1996;67:89–97. doi: 10.1046/j.1471-4159.1996.67010089.x. [DOI] [PubMed] [Google Scholar]
- 45.Takei N, Ohsawa K, Imai Y, Nakao H, Iwasaki A, Kohsaka S. Neurosci Lett. 1994;171:59–62. doi: 10.1016/0304-3940(94)90604-1. [DOI] [PubMed] [Google Scholar]
- 46.Maxon M E, Goodrich J A, Tjian R. Genes Dev. 1994;8:515–524. doi: 10.1101/gad.8.5.515. [DOI] [PubMed] [Google Scholar]
- 47.Garvey N, St. John A C, Witkin E M. J Bacteriol. 1985;163:870–876. doi: 10.1128/jb.163.3.870-876.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herskowitz I. Nature (London) 1987;329:219–222. doi: 10.1038/329219a0. [DOI] [PubMed] [Google Scholar]
- 49.Holzmayer T, Pestov D G, Ronninson I B. Nucleic Acids Res. 1992;20:711–717. doi: 10.1093/nar/20.4.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whiteway M, Dignard D, Thomas D Y. Proc Natl Acad Sci USA. 1992;90:9410–9414. doi: 10.1073/pnas.89.20.9410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gudkov A V, Kazarov A R, Thimmapaya R, Axenovich S A, Mazo I A, Roninson I B. Proc Natl Acad Sci USA. 1994;91:3744–3748. doi: 10.1073/pnas.91.9.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caponigro G, Abedi M R, Hurlburt A P, Maxfield A, Judd W, Kamb A. Proc Natl Acad Sci USA. 1998;95:7508–7513. doi: 10.1073/pnas.95.13.7508. [DOI] [PMC free article] [PubMed] [Google Scholar]