Abstract

The nickel-catalyzed reaction of carbonyls and dienes was accomplished in a regio- and stereo-selective fashion employing a stoichiometric amount of bis(pinacolato)diboron. This reductive coupling furnishes an allyl boronic esters as the reaction product, a compound which was readily converted to the derived allylic alcohol by oxidative work-up.
The dimetallation of unsaturated substrates is an effective tool for enriching the functional and stereochemical complexity of simple hydrocarbon substrates.1 In the particular case of diboration, reaction of alkenes,2 dienes,2b,3 allenes,4 and enones5 provides reactive intermediates that may participate in stereoselective cascade reaction sequences. In general, these reactions are energetically highly favored with the addition of bis(ethyleneglycolato)diboron to ethylene calculated to be exothermic by 41.7 kcal/mol.6 A useful extension of this methodology would arise if the reactivity inherent in diboration reactions could be used to promote multi-component coupling processes. Considering the oxophilic nature of boron, an attractive starting point for these studies is the reductive coupling of unsaturated hydrocarbons and carbonyls, a transition metal catalyzed process that is enabled by the use of metal hydrides, molecular hydrogen, or their equivalents, to facilitate C-C bond formation.7 In particular, the coupling of simple dienes and carbonyls, a Ni-promoted process first discovered by Wilke8 with subsequent pioneering studies in catalysis by Mori9 and Tamaru,10 was targeted.11 In the context of diboron-promoted coupling, we considered that this transformation might be a very effective tool for the preparation of synthetically-versatile allylboronates.12
Mechanistic studies by Ogoshi have provided the first structural evidence that the Ni-promoted reductive (and alkylative) coupling of dienes and aldehydes is initiated by cyclometallation of the substrates.13 In the presence of a metal hydride, subsequent σ-bond metathesis and by reductive elimination generate the unsaturated reaction product (eq. 1). Here, we demonstrate that diboron reagents may participate in a similar σ-bond metathesis reaction, thereby providing access to organoboronic esters as the reaction product (eq. 2). A notable aspect of this reaction is that the products are obtained with excellent levels of stereoselectivity and with regioselectivity that complements the stepwise diene diboration/carbonyl allylation reaction.12b A unique and particularly useful feature of this process is that oxidative work-up directly provides a functionally- and stereochemically-enriched product whose synthesis would otherwise require multiple steps.
 |
(1) |
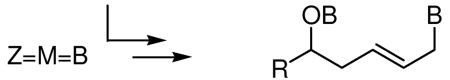 |
(2) |
Initial experiments surveyed the Ni-catalyzed, room temperature reaction of bis(pinacolato)diboron, trans-1,3-pentadiene, and benzaldehyde. After extensive optimization of reaction parameters (ligand, solvent, temperature, concentration), the optimal set of reaction conditions was found to include PCy3 as the ligand, THF as the solvent, at room temperature, and a substrate concentration of 0.2 M. With these conditions, benzaldehyde, trans-1,3-pentadiene, and B2(pin)2 were effectively coupled and, after oxidative work-up, the allylic alcohol product was obtained in good yield and stereoselectivity (Table 1, entry 1).
Table 1.
Ni-Catalyzed Coupling of Dienes, Aldehydes, and B2(pin)2.
 | |||||
|---|---|---|---|---|---|
| entry | aldehyde | diene | product | d.r.a | yieldb |
| 1 |

|
|

|
>20:1 | 66 |
| 2 |

|
|

|
>20:1 | 63 |
| 3 |

|
|

|
>20:1 | 63 |
| 4 |

|
|

|
>20:1 | 62 |
| 5 |

|
|

|
>20:1 | 53 |
| 6 |

|

|

|
>20:1 | 70c |
| 7 |

|

|

|
>20:1 | 65c |
| 8 |

|

|

|
N/A | 34c |
| 9 |

|

|

|
N/A | 68d |
| 10 |

|
|

|
>20:1 | 47c |
| 11 |

|
|

|
>20:1 | 24c |
| 12 |

|

|

|
>20:1 | 57 |
Determined by analysis of unpurified reaction.
Isolated yield of purified material.
10 mol% Ni(cod)2, 10 mol% PCy3, and a reaction time of 14 h.
10 mol% Ni(cod)2, 10 mol% P(OEt)3. With PCy3 this reaction provides 10% yield. Purification was aided by acetylation.
A number of other aldehydes and dienes participate in the stereoselective diboron-promoted diene-aldehyde coupling. As depicted in Table 1, aromatic aldehydes tend to be effective substrates with B2(pin)2 and trans-piperylene (Table 1, entries 1-5). With the more encumbered 3-methylpentadiene (entries 6, 7) a versatile trisubstituted alkene was furnished; within the limits of detection, this product was obtained as a single stereoisomer with respect to the stereocenters and the alkene. With butadiene (entry 8) reaction yields suffered, even with further optimization of reaction conditions. In contrast to butadiene, isoprene (entry 9) reacted with comparable efficiency as compared to 1,3-pentadiene and furnished a single alkene stereoisomer as determined by 1H NMR analysis of the oxidation product. With this substrate, the use of P(OEt)3 as the ligand was critical - with PCy3, significant amounts of a double allylation product, incorporating two equivalents of the aldehyde, were obtained. Entries 10 and 11 document that the reaction is not necessarily limited to aromatic aldehydes; both saturated and α,β-unsaturated aldehydes engaged in the reaction, although yields were diminished with these substrates (entries 10, 11). Lastly, entry 12 shows that with cis-1,3-pentadiene the same stereoisomer was favored as when the trans diene was employed (cf. entry 1). Here, it merits mention that when the cis diene was subjected to the catalyst, in the absence of the other reactants, rapid cis/trans isomerization resulted (data not shown). Thus, it is plausible that diene isomerization occurred during the course of the reaction, and the trans alkene was more rapidly incorporated into the product.
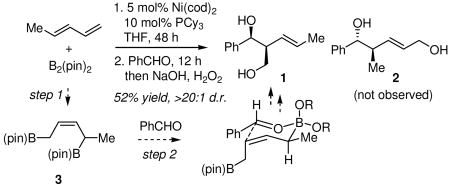
To probe features of the mechanism that might be important for further reaction design, exploratory experiments were carried out. When trans-1,3-pentadiene and B2(pin)2 were allowed to react with the catalyst for 48 hours, prior to the addition of benzaldehyde, addition product 1 was obtained (eq. 3; with 6 h reaction time for the first step, a mixture of 1 and 2 resulted). This addition product is regioisomeric with respect to the products in Table 1 and appears most likely to result from sequential Ni-catalyzed 1,4 diene diboration to give 3, followed by selective aldehyde allylation (see eq. 3). This conjecture was supported by the outcome in equation 4.14 The fact that the three-component reaction in Table 1 takes a different course than the process in equation 3, suggests that a sequential diene diboration followed by aldehyde allylation does not operate in Table 1; a more likely mechanism is related to that in Scheme 1.
 |
(3) |
 |
(4) |
In conclusion, we have demonstrated that diboron reagents can be used to facilitate stereoselective intermolecular coupling of dienes and aldehydes. The reaction products are particularly well suited for the construction of polyketide natural products and other useful chiral materials. Studies in asymmetric catalysis and in alternate transformations of the allyl boron product are underway.
Supplementary Material
Characterization and Procedures. This information is available free of charge through the internet at http://pubs.acs.org.
Acknowledgments
Support by the NIGMS (GM-59417) and Merck is gratefully acknowledged, as is the NSF for support of the BC Mass Spectrometry Center (grant # DBI-0619576).
References
- 1.a Marder TB, Norman NC. Topics in Catalysis. 1998;5:63. [Google Scholar]; b Ishiyama T, Miyaura N. J Organomet Chem. 2000;611:392. [Google Scholar]; c Ishiyama T, Miyaura N. Chem Record. 2004;3:271. doi: 10.1002/tcr.10068. [DOI] [PubMed] [Google Scholar]; d Beletskaya I, Moberg C. Chem Rev. 2006;106:2320. doi: 10.1021/cr050530j. [DOI] [PubMed] [Google Scholar]; e Burks HE, Morken JP. Chem Commun. 2007:4717. doi: 10.1039/b707779c. [DOI] [PubMed] [Google Scholar]; f Ramirez J, Lillo V, Segarra AM, Fernandez E. Comp Rend Chim. 2007;10:138. [Google Scholar]
- 2.a Baker RT, Nguyen P, Marder TB, Westcott SA. Angew Chem Int Ed Engl. 1995;34:1336. [Google Scholar]; b Ishiyama T, Yamamoto M, Miyaura N. Chem Commun. 1997:689. [Google Scholar]; c Iverson CN, Smith MR., III Organometallics. 1997;16:2757. [Google Scholar]; d Dai C, Robins EG, Scott AJ, Clegg W, Yufit DS, Howard JAK, Marder TB. Chem Commun. 1998:1983. [Google Scholar]; e Nguyen P, Coapes RB, Woodward AD, Taylor NJ, Burke JM, Howard JAK, Marder TB. J Organomet Chem. 2002;652:77. [Google Scholar]; f Morgan JB, Miller SP, Morken JP. J Am Chem Soc. 2003;125:8702. doi: 10.1021/ja035851w. [DOI] [PubMed] [Google Scholar]; g Trudeau S, Morgan JB, Shrestha M, Morken JP. J Org Chem. 2005;70:9538. doi: 10.1021/jo051651m. [DOI] [PubMed] [Google Scholar]; h Ramirez J, Segarra AM, Ferandez E. Tetrahedron: Asymmetry. 2005;16:1289. [Google Scholar]; i Ramírez J, Corberán R, Sanaú M, Peris E, Fernandez E. Chem Commun. 2005:3056. doi: 10.1039/b503239c. [DOI] [PubMed] [Google Scholar]; j Lillo V, Mata J, Ramirez J, Peris E, Fernandez E. Organometallics. 2006;25:5829. [Google Scholar]; k Lillo V, Mas-Marzá E, Segarra AM, Carbó JJ, Bo C, Peris E, Fernandez E. Chem Commun. 2007:3380. doi: 10.1039/b705197b. [DOI] [PubMed] [Google Scholar]
- 3.a Ishiyama T, Yamamoto M, Miyaura N. Chem Commun. 1996:2073. [Google Scholar]; b Clegg W, Thorsten J, Marder TB, Norman NC, Orpen AG, Peakman TM, Quayle MJ, Rice CR, Scott AJ. J Chem Soc Dalton Trans. 1998:1431. [Google Scholar]; c Morgan JB, Morken JP. Org Lett. 2003;5:2573. doi: 10.1021/ol034936z. [DOI] [PubMed] [Google Scholar]
- 4.a Ishiyama T, Kitano T, Miyaura N. Tetrahedron Lett. 1998;39:2357. [Google Scholar]; b Yang FY, Cheng CH. J Am Chem Soc. 2001;123:761. doi: 10.1021/ja005589g. [DOI] [PubMed] [Google Scholar]; c Pelz NF, Woodward AR, Burks HE, Sieber JD, Morken JP. J Am Chem Soc. 2004;126:16328. doi: 10.1021/ja044167u. [DOI] [PubMed] [Google Scholar]; d Burks HE, Liu S, Morken JP. J Am Chem Soc. 2007;129:8766. doi: 10.1021/ja070572k. [DOI] [PubMed] [Google Scholar]
- 5.a Lawson YG, Lesley MJG, Norman NC, Rice CR, Marder TB. Chem Commun. 1997:2051. [Google Scholar]; b Takahashi K, Ishiyama T, Miyaura N. Chem Lett. 2000:982. [Google Scholar]; c Ito H, Yamanaka H, Tateiwa J, Hosomi A. Tetrahedron Lett. 2000;47:6821. [Google Scholar]; d Takahashi K, Ishiyama T, Miyaura N. J Organomet Chem. 2001;625:47. [Google Scholar]; e Kabalka GW, Das BC, Das S. Tetrahedron Lett. 2002;43:2323. [Google Scholar]; f Bell NJ, Cox AJ, Cameron NR, Evans JSO, Marder TB, Duin MA, Elsevier CJ, Baucherel X, Tulloch AAD, Tooze RP. Chem Commun. 2004:1854. doi: 10.1039/b406052k. [DOI] [PubMed] [Google Scholar]; g Ito H, Kawakami C, Sawamura M. J Am Chem Soc. 2005;127:16034. doi: 10.1021/ja056099x. [DOI] [PubMed] [Google Scholar]; h Mun S, Lee JE, Yun J. Org Lett. 2006;8:4887. doi: 10.1021/ol061955a. [DOI] [PubMed] [Google Scholar]; i Hirano K, Yorimitsu H, Oshima K. Org Lett. 2007;9:5031. doi: 10.1021/ol702254g. [DOI] [PubMed] [Google Scholar]
- 6.Cui Q, Musaev DG, Morokuma K. Organometallics. 1997;16:1355. [Google Scholar]
- 7.Reviews: Montgomery J. Angew Chem Int Ed. 2004;43:3890. doi: 10.1002/anie.200300634.Kimura M, Tamaru Y. Top Curr Chem. 2007;279:173.Ngai MY, Kong JR, Krische MJ. J Org Chem. 2007;72:1063. doi: 10.1021/jo061895m.Moslin RM, Miller-Moslin K, Jamison TF. Chem Commun. 2007:4441. doi: 10.1039/b707737h.
- 8.Wilke G. Angew Chem Int Ed Engl. 1988;27:185. [Google Scholar]
- 9.a Sato Y, Takimoto M, Hayashi K, Katsuhara T, Takagi K, Mori M. J Am Chem Soc. 1994;116:9771. [Google Scholar]; b Sato Y, Takimoto M, Mori M. Tetrahedron Lett. 1996;37:887. [Google Scholar]; c Takimoto M, Hiraga Y, Sato Y, Mori M. Tetrahedron Lett. 1998;39:4543. [Google Scholar]; d Sato Y, Takanashi T, Hoshiba M, Mori M. Tetrahedron Lett. 1998;39:5579. [Google Scholar]; e Sato Y, Saito N, Mori M. Tetrahedron. 1998;54:1153. [Google Scholar]; f Sato Y, Takimoto M, Mori M. J Am Chem Soc. 2000;122:1624. [Google Scholar]; g Sato Y, Saito N, Mori M. J Am Chem Soc. 2000;122:2371. [Google Scholar]; h Sato Y, Sawaki R, Mori M. Organometallics. 2001;27:5510. [Google Scholar]; i Sato Y, Saito N, Mori M. J Org Chem. 2002;67:9310. doi: 10.1021/jo020438c. [DOI] [PubMed] [Google Scholar]; j Sato Y, Sawaki R, Saito N, Mori M. J Org Chem. 2002;73:656. doi: 10.1021/jo0106086. [DOI] [PubMed] [Google Scholar]
- 10.a Kimura M, Ezoe A, Shibata K, Tamaru Y. J Am Chem Soc. 1998;120:4033. [Google Scholar]; b Kimura M, Fujimatsu H, Ezoe A, Shibata K, Shimizu M, Matsumoto S, Tamaru Y. Angew Chem Int Ed. 1999;38:397. doi: 10.1002/(SICI)1521-3773(19990201)38:3<397::AID-ANIE397>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]; c Shibata K, Kimura M, Shimizu M, Tamaru Y. Org Lett. 2001;3:2181. doi: 10.1021/ol0100879. [DOI] [PubMed] [Google Scholar]; d Kimura M, Ezoe A, Mori M, Iwata K, Tamaru Y. J Am Chem Soc. 2006;128:8559. doi: 10.1021/ja0608904. [DOI] [PubMed] [Google Scholar]
- 11.For aligned work involving the coupling of dienes and carbonyls under Ir and Ru catalysis, see: Bower JF, Patman RL, Krische MJ. Org Lett. 2008;10:1033. doi: 10.1021/ol800159w.Shibahara F, Bower JF, Krische MJ. J Am Chem Soc. 2008;130:6338. doi: 10.1021/ja801213x.
- 12.For intermolecular coupling of dienes, aldehydes, and silylstannanes, see: Sato Y, Saito N, Mori M. Chem Lett. 2002:18. For a related process with dienes, aldehydes, and diborons: Morgan JB, Morken JP. Org Lett. 2003;5:2573. doi: 10.1021/ol034936z. For intramolecular couplings, see: Yu CM, Youn J, Yoon SK, Hong YT. Org Lett. 2005;7:4507. doi: 10.1021/ol051806c.Ballard CE, Morken JP. Synthesis. 2004;9:1321.
- 13.Ogoshi S, Tonomori K, Oka M, Kurosawa H. J Am Chem Soc. 2006;128:7077. doi: 10.1021/ja060580l. [DOI] [PubMed] [Google Scholar]
- 14.For Ni-catalyzed silylboration of dienes, see: Suginome M, Matsuda T, Yoshimoto T, Ito T. Org Lett. 1999;1:1567.Gerdin M, Moberg C. Adv Synth Catal. 2005:347–749.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characterization and Procedures. This information is available free of charge through the internet at http://pubs.acs.org.


