Abstract
A water-soluble triacetic acid cryptophane-A derivative (TAAC) was synthesized and determined by isothermal titration calorimetry (ITC) and fluorescence quenching assay to have a xenon association constant of 33,000 M−1 at 293 K, which is the largest value measured for any host molecule to date. Fluorescence lifetime measurements of TAAC in the presence of varying amounts of xenon indicated static quenching by the encapsulated xenon and the presence of a second non-xenon-binding conformer in solution. Acid-base titrations and aqueous NMR spectroscopy of TAAC and a previously synthesized tri-(triazole propionic acid) cryptophane-A derivative (TTPC) showed how solvation of the carboxylate anions can affect the aqueous behavior of the large, nonpolar cryptophane. Specifically, whereas only the crown-crown (CC) conformer of TTPC was observed, a crown-saddle (CS) conformer of TAAC was also detected in aqueous solution.
Introduction
Xenon-129 biosensors offer exciting potential for the simultaneous magnetic resonance imaging (MRI) of multiple frequency-resolved biomolecular targets. In addition to being a relatively abundant isotope of a non-toxic noble gas, the xenon-129 nucleus is spin-1/2 and can be laser-polarized to increase nuclear magnetic resonance (NMR) signals more than 10,000-fold. The polarizability of the xenon electron cloud imparts considerable environmental sensitivity to the chemical shift of the 129Xe nucleus, producing a nearly 300 ppm 129Xe NMR chemical shift window in common solvents.1 This sensitivity facilitates the simultaneous detection of monatomic 129Xe in different chemical environments. Biosensors exploiting this property have been generated by attaching a xenon-binding cryptophane-A moiety to protein-specific ligands such as biotin2,3 or protease-specific peptides.4 Cryptophane-based biosensors can be spectrally and spatially resolved in MR imaging5–8 and a previously synthesized tri-(triazole propionic acid) cryptophane-A derivative (TTPC, Figure 1) was shown to be a competent xenon binder, even under near-physiological conditions.9 The crystal structure of a benzenesulfonamide-functionalized cryptophane-A complexed with carbonic anhydrase II was solved10 and hyperpolarized 129Xe spectroscopy has demonstrated the ability of a series of the benzenesulfonamide-functionalized cryptophane-A derivatives to exhibit isozyme specific chemical shift changes upon binding to carbonic anhydrases I and II.11 Many potential biosensing applications motivate the design and study of new xenon-binding host molecules in both organic12–15 and aqueous phases.13–15
Figure 1.
Water-soluble cryptophanes TAAC and TTPC
The study of gas encapsulation by host molecules remains a challenge, with few suitable analytical techniques available.16 Recent studies of hydrocarbon gas binding by synthetic hosts in solution have relied on direct observation of the encapsulated 1H NMR signals.16,17 Even binding of gasses without proton resonances such as Ar, CO, O2, and N2 by self assembled oxime capsules in chloroform have been studied by 1H NMR spectroscopy.18 In the solid phase, single crystals of calix[4]arene have been shown to store gaseous guest molecules with high thermal stability.19 Elegant studies of NO, air, SO2, and xenon adsorbtion by the Ripmeester lab have demonstrated that p-tert-butylcalix[4]arene host molecules are capable of creating discreet gas binding spaces in the solid state.20,21 Xenon binding to the host cavities in amphiphilic calixarene-based solid lipid nanoparticles has also been studied through the use of continuous flow hyperpolarized 129Xe MAS NMR.22 While solution NMR has proven to be useful in the direct determination of association constants from 10-104 M−1, competition experiments are necessary to measure greater affinities.23 Fluorescence spectroscopy and isothermal titration calorimetry (ITC) allow for the direct detection of binding equilibria at micromolar concentrations of analyte. These techniques can be applied to host-gas chemistry through the controlled addition of saturated gas solutions of known concentration, as previously demonstrated for xenon.9
In this study a new water-soluble xenon-binding host, triacetic acid cryptophane-A (TAAC) was synthesized and compared to the previously synthesized TTPC (Figure 1).9 TAAC and TTPC each have a tri-substituted cyclotriveratrylene (CTV) moiety, which differs in the linkage to three carboxylates. Although the cryptophane core of TAAC and TTPC is identical, TAAC was shown by NMR and pH titration to exhibit different solution-phase behavior. TAAC also exhibited roughly 2-fold higher affinity for xenon, as measured by fluorescence quenching assay and ITC. Both TAAC and TTPC were studied by time-correlated single-photon counting (TCSPC), which confirmed static fluorescence quenching by the encapsulated xenon. These studies demonstrate that the solubilizing groups appended to otherwise identical cryptophane cores can have a significant effect on molecular conformation and xenon-binding. This provides new insight into the aqueous phase behavior of the cryptophane component of known xenon biosensors.
Experimental Procedures
Reagents
Organic reagents and solvents were used as purchased from the following commercial sources: Acros: cesium carbonate, anhydrous dimethylsulfoxide (DMSO), anhydrous dimethylformamide (DMF), d6-DMSO, CDCl3, 4-hydroxy-3-methoxybenzylalcohol, triphenylphosphine, 1,2 dibromoethane, sodium borohydride, 10% palladium on carbon, 3,4-dihydroxybenzaldhyde, ethyl bromoacetate, palladium(II) acetate. Fisher: sodium chloride, potassium phosphate, ethyl acetate, dichloromethane, chloroform, hydrochloric acid, sodium hydroxide, sodium sulfate, acetone, hexanes, sodium iodide, potassium hydroxide. Cambridge Isotope Laboratories: deuterium oxide. Airco Industrial Gases: research grade xenon gas. Aldrich: 3,4-dihydro-2H-pyran, allyl bromide, pyridinium p-toluenesulfonate, sodium deuteroxide 40 wt.% in D2O, diethylamine.
General Methods
All organic reactions were carried out under nitrogen atmosphere. 1H NMR (500.14 MHz) and 13C (125.77 MHz) spectra were obtained on a Bruker AMX 500 or DMX 600 spectrometer at the University of Pennsylvania NMR Facility. Spectra were referenced to TMS at 0.00 ppm in CDCl3 or the residual solvent peak at 2.50 ppm in d6-DMSO. Electrospray ionization (ESI) mass spectrometry was performed in low-resolution mode on a Micromass LC Platform and in high-resolution mode on a Micromass Autospec at the Mass Spectrometry Center in the Chemistry Department at the University of Pennsylvania. For fluorescence and ITC measurements in buffer, solutions were prepared with water deionized using Mar Cor Premium Grade Mixed Bed Service Deionization. Column chromatography was performed using 60 Å porosity, 40–75 µm particle size silica gel form Sorbent Technologies. Thin layer chromatography was performed using silica gel plates with UV light at 254 nm for detection.
2,7,12-Tris(2-[2-allyloxy-4-[tetrahydro-pyran-2-yloxymethyl]-phenoxyl]-ethoxy)-3,8,13-trimethoxy-10,15-dihydro-5H-tribenzo[a,d,g]cyclononene (3)
2 (1.460 g, 3.575 mmol) and cesium carbonate (6.98 g, 21.45 mmol) were added to an oven-dried flask with stir bar and purged with nitrogen gas. Dry DMF (150 mL) was added by syringe and the mixture was allowed to stir for 30 min at rt. Linker 1 (5.981 g, 14.30 mmol) was then added and the reaction was placed in a 55 °C oil bath with stirring overnight. The reaction was poured into saturated NaCl (600 mL), and extracted 3 times with Et2O (300 mL). The combined organics were washed 5 times with saturated NaCl (300 mL), dried over Na2SO4, and evaporated under reduced pressure. The crude material was then pumped under high vacuum to remove any residual DMF and purified by column chromatography (CH2Cl2 → 90:10 CH2Cl2:acetone) to obtain 3 (2.74 g, 60% yield) as a clear glass. 1H NMR (CDCl3) δ = 6.98 (s, 3H, aryl), 6.94-6.87 (m, 9H, aryl), 6.83 (s, 3H, aryl), 6.04 (m, 3H, allyl), 5.38 (m, 3H, allyl), 5.22 (m, 3H, allyl), 4.73 (d, 3H, Hax, J = 13.6 Hz), 4.69 (d, 3H, Ph-CH2-O, J = 11.8 Hz), 4.67 (t, 3H, THP), 4.59 (d, 6H, allyl), 4.38 (d, 3H, Ph-CH2-O, J = 11.8 Hz), 4.35 (m, 12H, O-CH2-CH2-O), 3.91 (m, 3H, THP), 3.74, (s, 9H, O-CH3), 3.53 (m, 3H, THP), 3.53 (d, 3H, Heq, J = 13.5 Hz), 1.88-1.49 (m, 18H, THP). 13C NMR (CDCl3) δ = 148.62, 148.51, 148.08, 146.79, 133.44, 132.91, 131.81, 131.74, 120.87, 117.27, 116.64, 114.62, 114.50, 114.01, 97.38. 69.92, 68.40, 68.21, 67.90, 61.99, 56.12, 36.18, 30.47, 25.35, 19.30. HRMS calcd for C75H90O18 (M + Na+) 1301.6025, found 1301.6015.
Tri-Allyl Cryptophane (4)
3 (560 mg, 0.39 mmol) was dissolved in 250 mL CHCl3 and 250 mL formic acid was added with magnetic stirring. The reaction was purged with N2 before being heated to reflux for 9 h. The solvent was then evaporated under reduced pressure. Toluene was added to the crude material and evaporated under reduced pressure to remove residual formic acid. The crude material was then purified by column chromatography (CH2Cl2 → CH2Cl2:diethyl ether 90:10) to obtain 4 (152 mg, 40% yield). 1H NMR (CDCl3) δ = 6.76 (s, 3H, aryl), 6.74 (s, 3H, aryl), 6.72 (s, 3H, aryl), 6.67 (s, 3H, aryl), 6.05 (m, 3H, allyl), 5.45 (m, 3H, allyl), 5.34 (m, 3H, allyl), 4.59 (d, 3H, Hax, J = 13.9 Hz), 4.55 (d, 3H, Hax, J = 13.7 Hz), 4.49 ( m, 6H, allyl), 4.16 (m, 12 H, O-CH2-CH2-O), 3.74 (s, 9H, O-CH3), 3.40 (d, 3H, Heq, J = 13.5 Hz), 3.37 (d, 3H, Heq, J = 13.4 Hz). 13C NMR (CDCl3) δ = 149.95, 149.21, 147.53, 146.96, 134.53, 134.36, 134.02, 132.51, 131.91, 122.35, 120.94, 117.17, 116.89, 114.47, 70.27, 69.83, 69.58, 56.48, 36.43. HRMS calcd for C60H60O12 (M + MeCN + Na+) 1036.4248 found 1036.4261.
Tri-OH Cryptophane (5)
4 (303 mg, 0.31 mmol), triphenylphosphine (135 mg, 0.51 mmol), palladium acetate (7.7 mg, 0.03 mmol), diethylamine (1.5 g, 20.5 mmol), THF (4.8 mL), and water (0.95 mL) were added to a screw-capped high-pressure tube. The tube was purged with nitrogen, sealed, and placed in an 80 °C oil bath with magnetic stirring for 4 h. After cooling to rt, the solvent was removed by evaporation under reduced pressure. The crude material was dissolved in CH2Cl2 (100 mL), washed twice with 1 M HCl (50 mL) and once with saturated NaCl (50 mL). The crude material was then adsorbed onto silica gel and purified by column chromatography (CH2Cl2 → 80:20 CH2Cl2:acetone) to obtain 5 (220 mg, 84% yield) as a white solid. 1H NMR (DMSO-d6) δ = 8.50 (s, 3H, Ar-OH), 6.83 (s, 3H, aryl), 6.77 (s, 3H, aryl), 6.60 (s, 3H, aryl), 6.53 (s, 3H, aryl), 4.50 (d, 3H, Hax, J = 13 Hz) 4.40 (d, 3H, Hax, J = 13 Hz), 4.20-4.00 (m, 12H, O-CH2-CH2-O), 3.73 (s, 9H, Ar-OCH3), 3.31 (d, 3H, Heq, J = 14 Hz), 3.15 (d, 3H, Heq, J = 14 Hz). 13C NMR (DMSO-d6) δ = 148.91, 146.59, 145.81, 144.29, 133.79, 133.33, 131.15, 129.58, 120.62, 120.10, 117.45, 114.22, 68.50, 55.72, 35.03. HRMS calcd for C51H48O12 (M+Na+) 875.3043, found 875.3053.
Tri-EtOAc Cryptophane (6)
5 (189 mg, 0.22 mmol) and cesium carbonate (0.43 g, 1.3 mmol) were added to an oven-dried flask with stir bar and purged with nitrogen gas. Dry DMF (5 mL) was added by syringe and the mixture was allowed to stir for 30 min at rt. Ethyl bromoacetate (0.22 g, 1.33 mmol) was then added and the reaction was placed in a 60 °C oil bath with stirring overnight. The DMF was removed by evaporation under reduced pressure. The crude reaction mixture was dissolved in CH2Cl2 (50 mL), washed with saturated NaCl (50 mL), dried over Na2SO4, and evaporated under reduced pressure. The crude material was then pumped under high vacuum to remove any residual DMF and purified by column chromatography (CH2Cl2 → 80:20 CH2Cl2:diethyl ether) to obtain 6 (0.194 g, 79% yield) as a white solid. 1H NMR (CDCl3) δ =6.76 (s, 3H, aryl), 6.75 (s, 3H, aryl), 6.71 (s, 3H, aryl), 6.69 (s, 3H, aryl), 4.58 (d, 3H, Hax, J = 14 Hz), 4.55 (d, 3H, Ar-O-CH2-CO2, J = 16 Hz ), 4.54 (d, 3H, Hax, J = 14 Hz), 4.50 (d, 3H, Ar-O-CH2-CO2, J = 16 Hz ), 4.32-4.19 (m, 18H, O-CH2-CH2-O, CO2-CH2-CH3), 3.76 3.67 (s, 9H, Ar-OCH3), 3.40 (d, 3H, Heq, J = 14 Hz) 3.38 (d, 3H, Heq, J = 14 Hz), 1.35 (t, 9H, CH2-CH3, J = 7 Hz). 13C NMR (CDCl3) δ = 168.74, 149.73, 148.02, 147.73, 146.79, 134.02, 133.95, 133.73, 131.79, 121.88, 120.62, 118.18, 114.71, 69.56, 69.24, 67.20, 61.20, 56.21, 36.20, 36.11, 14.34 HRMS calcd for C63H66O18 (M + Na+) 1133.4147, found 1133.4134.
Tri-Acid Cryptophane (TAAC)
6 (194 mg, 0.17 mmol), 2 M KOH (7.35 mL), and THF (8.75 mL) were added to a screw-capped high-pressure tube. The tube was purged with nitrogen, sealed, and placed in a 70 °C oil bath with magnetic stirring overnight. The THF was then removed by evaporation under reduced pressure. The aqueous solution was transferred to a centrifuge tube, acidified with 12 M HCl and centrifuged. The solid pellet was redissolved in 1 M NaOH, acidified with 12 M HCl and centrifuged. The solid pellet was then dispersed in deionized water, centrifuged, and the water decanted. After lyophilization of the resulting pellet, 0.1559 g of TAAC was obtained in 87% yield. 1H NMR (DMSO-d6) δ = 6.85 (s, 3H, aryl), 6.83 (s, 3H, aryl), 6.82 (s, 3H, aryl), 6.77 (s, 3H, aryl), 4.54 (s, 6H, Ar-O-CH2-CO2), 4.48 (d, 6H, Hax, J = 13 Hz), 4.06–4.25 (m, 12H, O-CH2-CH2-O), 3.67 (s, 9H, Ar-OCH3), 3.31 (d, 3H, Heq, J = 13 Hz), 3.28 (d, 3H, Heq, J = 13 Hz). 13C NMR (d6-DMSO) δ = 170.16, 148.75, 147.173, 145.60, 133.55, 133.45, 132.42, 131.69, 120.41, 118.95, 115.86, 114.96, 68.64, 68.17, 65.49, 55.87, 34.88. HRMS calcd for C57H54O18 (M + Na+) 1049.3208, found 1049.3211.
Isothermal Titration Calorimetry
ITC samples were prepared and experiments were performed as previously described9 using a MicroCal VP-ITC titration microcalorimeter (Northampton, MA) at 293 K. Standard protocols and data analysis were used.24,25 Control enthalpograms are given in the Supporting Information (Figure S1).
Steady-State Fluorescence
Steady-state fluorescence spectra were acquired using a Varian Cary Eclipse fluorimeter equipped with a Peltier multicell holder for temperature control. Concentration measurements necessary for fluorescence work were obtained using an Agilent 8453 UV-Vis spectrophotometer. Xenon binding determination by fluorescence quenching was performed on 15 µM solutions of TAAC or TTPC, as previously described.9 The following single-site binding model was used:
| (1) |
where the left side of Eq. 1 indicates the fraction of cryptophane (Cr) in solution that is bound by xenon, and KA is the xenon association constant.
The quantum yields of TAAC and TTPC in 0.001 M, pH 7.2 phosphate buffer were measured relative to tryptophan.26
Aqueous 1H NMR Spectroscopy
Solutions (180 mM) were made by deprotonation of 0.011 mmol TAAC and TTPC using sodium deuteroxide 40 wt.% in D2O and subsequent dissolution in 600 µL of 10% D2O/H2O. These samples were transferred to 5-mm controlled atmosphere valve sample tubes (New Era Spectroscopy) to allow for degassed- and xenon-saturated 1H NMR spectroscopy.
Time-resolved Fluorescence
Time-resolved fluorescence measurements were performed at 293 K using the TCSPC method. The TCSPC system consisted of the third harmonic of a Ti:sapphire femtosecond laser (Coherent Chameleon) generating 80 MHz output pulses at 275 nm, a subtractive double monochromator with a MCP-PMT (Hamamatsu R2809U) and a TCSPC board (Becker & Hickl, SPC-730). Emission at 310 nm was monitored. Data analysis was done using the FLUOFIT software (Picoquant GmbH). Fluorescence decays were deconvolved with an instrument response function of 35 ps and globally fit using a three exponential model.
Results
Synthesis
TAAC was synthesized (Scheme 1) by a modification of the synthesis of monoallyl-cryptophane by Darzac et al.27,28 Cyclotriveratrylene 1 in DMF was deprotonated with cesium carbonate and reacted with 4.5 equiv 2-[3-allyloxy-4-(2-iodo-ethoxy)-benzyloxy]-tetrahydropyran 2 at 55 °C in 60% yield to obtain 3.
Scheme 1.
13-step synthesis of water-soluble triacetic acid cryptophane TAAC*
*Conditions: (a) Cs2CO3, DMF, 55 °C, 12 h, 60%; (b) CHCl3, HCOOH, reflux, 9 h, 40%; (c) Pd(OAc)2, P(Ph)3, (Et)2NH, THF, H2O, 80 °C, 4 h, 84%; (d) ethylbromoacetate, Cs2CO3, DMF, 60 °C, 12 h, 79%.; (e) KOH (2 M), THF, 70 °C, 12 h, 87%.
In 50:50 chloroform:formic acid, the cyclization of 3 proved extremely slow at 55 °C and higher temperatures were necessary to push the reaction beyond a partially cyclized intermediate. It is hypothesized that the three allyl groups provided steric hindrance that allowed the observation of this intermediate. An optimum reaction time of 9 h at reflux was found (Table 1) to give triallyl-cryptophane 4 in 40% yield, compared to 2.5 h at 55 °C for monoallyl-cryptophane.28 Use of a higher boiling solvent system, 50:50 1,2-dichloroethane:formic acid, led to decomposition of 3. The cyclized product 4 is known to be of the anti isomer, as confirmed by its X-ray crystal structure with chloroform as an encapsulated guest.29
Table 1.
Reaction times and yields for formation of tri-allyl cryptophane 4.
| Solvent | Temp (°C) | Time (h) | Yield |
|---|---|---|---|
| HCOOH, CHCl3 | 55 | 2.5 | 0% |
| HCOOH, CHCl3 | reflux | 3 | 17% |
| HCOOH, CHCl3 | reflux | 6 | 32% |
| HCOOH, CHCl3 | reflux | 9 | 40% |
| HCOOH, CHCl3 | reflux | 22 | 25% |
| HCOOH, Cl(CH2)2Cl | reflux | 1 | decomp |
Deprotection of 4 to the triphenol-cryptophane 5 was accomplished in 84% yield by palladium catalysis using known procedures.30 The triphenol 5 was then triply alkylated using ethylbromoacetate and cesium carbonate in DMF to give triester-cryptophane 6 in 79% yield. Saponification of 6 in THF using potassium hydroxide produced TAAC in 87% yield. The conversion of starting 3,4-dihydroxybenzaldehyde to TAAC occurred in 13 steps in 1.7% overall yield with a longest linear sequence of 10 steps.
Binding of Xenon by TAAC
The xenon affinity of TAAC was studied by fluorescence quenching (Figure 2) and ITC (Figure 3). An extinction coefficient of 12,040 M−1cm−1 at 280 nm was determined for TAAC, which varied only slightly from the previously determined value of ε280 = 12,400 M−1cm−1 for TTPC.9 As in the previous fluorescence quenching study with TTPC, temperature-equilibrated solutions of xenon-saturated water were added sequentially by syringe to a septum-sealed fluorescence cuvette and fluorescence intensity measurements were taken.9 An association constant of 33,000 ± 2000 M−1 at 293 K was obtained for TAAC by this method. No measurable change in the absorption spectrum of either TAAC or TTPC was observed upon xenon binding.
Figure 2.
Fluorescence quenching of TAAC (15 µM) by Xe in 1 mM, pH 7.2 phosphate buffer, 293 K. Inset: Curve fit for a single-site binding model.
Figure 3.
Enthalpogram of 5.05 mM aqueous xenon solution titrated into 0.77 mM TAAC at 293 K.
In order to confirm the association constants obtained by fluorescence quenching, ITC experiments were performed. ITC was used previously to determine the xenon binding affinity for TTPC9 and more recently for a water-soluble cucurbit[6]uril derivative.31 ITC measurements were performed at 293 K on 0.77 mM TAAC in 20 mM, pH 7.5 phosphate buffer (Figure 2), which gave a ΔH of −4.3 ± 0.7 kcal*mol−1. From these data, ΔS of 5.9 cal*mol−1*K−1 and ΔG of −6.09 kcal*mol−1 were calculated. The xenon association constant determined for TAAC at 293 K, KA = 33,000 ± 3000 M−1, was virtually identical to the value determined by steady-state fluorescence assay, and was significantly larger than that determined previously for TTPC, KA = 17,000 ± 2000 M−1.9 Thermodynamic parameters for TAAC and TTPC are compared in Table 2.
Table 2.
Thermodynamic binding parameters of Xe@TAAC obtained by ITC at 293 K
| Cryptophane | KA (M−1×104) | ΔG (kcal mol−1) | ΔH (kcal mol−1) | TΔS (kcal mol−1) |
|---|---|---|---|---|
| TAAC | 3.33 ± 0.28 | −6.06 | −4.34 ± 0.66 | 1.72 |
| TTPCa | 1.70 ± 0.17 | −5.69 | −3.14 ± 0.20 | 2.55 |
previously reported values9
Fluorescence Spectroscopy
The room-temperature fluorescence spectra of TAAC and TTPC consisted of a broad band with a maximum at 313 nm in phosphate buffer, similar to the 1,2-dialkoxybenzene chromophores that form the cryptophane.32 TAAC exhibited a higher quantum yield than TTPC (Φf = 0.06 vs. 0.01 at 293 K). The emission of both molecules was partially quenched by ambient oxygen, implying weak oxygen binding. Because the only chemical difference between TAAC and TTPC is the nature of the pendant solubilizing groups, the lower quantum yield of TTPC could be explained by excited-state quenching by the three triazole rings appended to its cryptophane core. The quantum yield of TAAC is also temperature dependent, with a linear decrease of 0.0007/K between 283 and 313 K (Figure S2, Supporting Information). This temperature dependence is attributed to the increase in molecular motions and accessibility of different cage conformations as the thermal energy of the cryptophane system is increased.
Xenon has been shown to quench fluorescence by promoting intersystem crossing from the first singlet excited state of a chromophore (S1) to the lowest triplet state (T1).33 Xenon quenching of cryptophane fluorescence is necessarily static in nature as the xenon atom is encapsulated within the chromophore assembly of the cryptophane cage, forming a complex between the fluorophores and quencher. The fluorescence quenching of TAAC and TTPC by xenon was plotted using the Stern-Volmer equation for static quenching:34
| (2) |
Where Fo is the fluorescence intensity without xenon, F is the fluorescence intensity in the presence of xenon, KS is the static quenching constant. If quenching is complete upon xenon encapsulation by the cryptophane, then the Stern-Volmer plot of xenon quenching cryptophane fluorescence is linear and the slope of the fitted line is equivalent to the association constant of xenon for the cryptophane:
| (3) |
However, in the Stern-Volmer plots for TAAC and TTPC (Figure 4), a linear correlation is observed only at low xenon concentrations, with a deviation from linearity towards the x-axis at higher xenon concentrations. At xenon saturation, maxima are achieved of Fo/F = 6 for TAAC and Fo/F = 2 for TTPC. Fits to the initial linear sections of the plots give apparent Stern-Volmer quenching constants, Ks, of 19,300 M−1 and 4,700 M−1, respectively, for encapsulated xenon quenching (Figure 5). Horizontal deviations in Stern-Volmer plots are normally observed when there is hindered access to a particular population of fluorophores in a sample, for example a buried tryptophan residue in a protein.34 While it may be conceivable that a co-encapsulated water molecule could prevent xenon from contacting one or more of the six 1,2-dialkoxybenzene chromophores of the cryptophane cores of TAAC and TTPC, aqueous NMR measurements provide no evidence of encapsulated water, even under degassed conditions (vide infra).
Figure 4.
Stern-Volmer plots of fluorescence quenching by xenon of TAAC (blue diamonds) and TTPC (pink squares).
Figure 5.
Initial slopes of steady-state Stern-Volmer plots of TAAC (blue diamonds) and TTPC (pink squares).
TCSPC measurements of the fluorescence lifetimes of TAAC and TTPC were undertaken in an effort to understand the nature of the xenon quenching and the horizontal deviation from linearity in the steady-state Stern-Volmer plots.12 The low quantum yield and short lifetime of TTPC allowed only mono-exponential tailfits to be obtained, giving lifetimes of ~300 ps without encapsulated xenon and ~200 ps when saturated with xenon (Figure S3, Supporting Information).
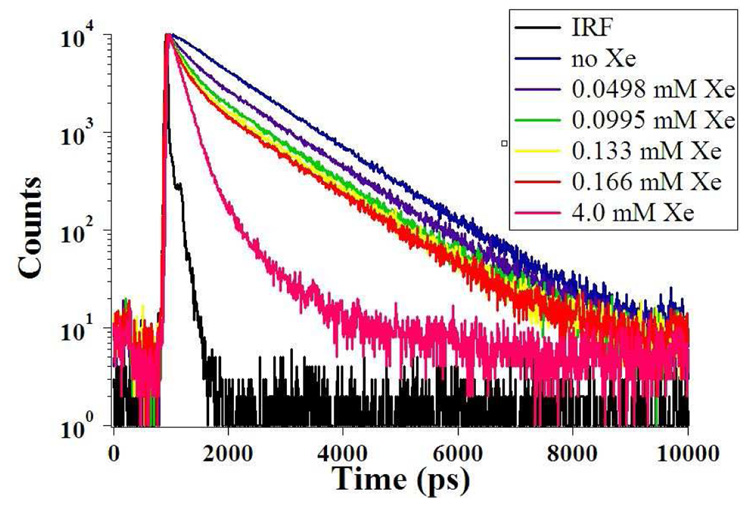
The higher quantum yield of TAAC allowed for decay fits deconvolved from the TCSPC instrument response function. Rigorously purified TAAC in aqueous solution exhibited a double exponential decay (Figure 6 and Table 3), which was fitted to two exponentials with lifetimes of 1.1 ns (95% of total intensity) and 0.3 ns (5% of total intensity). The smaller amplitude, shorter-lived component is consistent with the presence of a second, minor conformer in solution. This minor conformer is assigned to be the non-xenon-binding crown-saddle (CS) conformer of TAAC. By comparison, the lifetime of the related chromophore, 1,2-dimethoxybenzene is monoexponential with a lifetime of 1.4 ns.32 With xenon encapsulation by TAAC, a very short-lived component with lifetime of 0.13 ns replaced the 1.1 ns lifetime component.
Figure 6.
Fluorescence decays of TAAC with increasing amounts of xenon and the deconvolved instrument response function (IRF).
Table 3.
Results of a three-exponential global fit of the lifetime data of TAAC with increasing concentrations of xenon
| [Xe] mM | Avg τ | I1.13 ns | I0.344 ns | I0.134 ns |
|---|---|---|---|---|
| 0.000 | 1.09 | 0.95 | 0.05 | 0.0 |
| 0.05 | 0.98 | 0.83 | 0.08 | 0.10 |
| 0.10 | 0.88 | 0.73 | 0.10 | 0.17 |
| 0.13 | 0.84 | 0.68 | 0.12 | 0.20 |
| 0.17 | 0.80 | 0.65 | 0.13 | 0.23 |
| 4.00 | 0.24 | 0.05 | 0.24 | 0.71 |
Stern-Volmer analysis of the average fluorescence lifetimes of TAAC with encapsulated xenon was performed using the following relationship:35
| (4) |
where a fit to the initial linear section of the plot gives an apparent time–resolved Stern-Volmer quenching constant, Kτsv, of 2,200 M−1 for encapsulated xenon quenching (Figure 7). As in the steady-state Stern-Volmer plot for TAAC, a linear correlation is observed at low xenon concentrations with a deviation from linearity towards the x-axis at higher xenon concentrations approaching a maximum at τo/τ = 4.6 for TAAC upon xenon saturation. Because Kτsv < Ks, it is apparent that analysis of the shorter 0.13 ns decay component associated with xenon encapsulation is necessary.
Figure 7.
Initial slope of time-resolved Stern-Volmer plot of TAAC.
A three-exponential global fit of the lifetime data with increasing xenon occupancy was performed and the relative intensities of the three observed lifetimes are given in Table 3. The amplitude of the 0.34 ns component remained constant at 12–14% of the total initial amplitude in the fit, indicating that this species, assigned to the CS conformer, did not take part in the xenon-binding equilibrium. At the concentrations involved, the contribution of collisional quenching between non-encapsulated xenon and the S1 state of TAAC is expected to be negligible. The Einstein-Smoluchowski equation for diffusion:
| (5) |
using the diffusion coefficient (D) of xenon in water (2.2 ± 0.4 * 10−5 cm2 s−1)36 and the short timescale of the fluorescence decays (τ = 1.1 ns) predicts a root-mean-square distance of 20 Å that aqueous xenon can travel during the TAAC S1 excited state. Even at the highest Xe concentrations (5 mM) investigated in these fluorescence experiments, collisional quenching from non-encapsulated xenon is not expected to contribute to the observed fluorescence quenching. From the fluorescence lifetime and quantum yield measurements of TAAC with no encapsulated xenon, a natural lifetime (τn) of 18 ns and its reciprocal emissive rate (Γ) of 5.5*107 s−1 were calculated from the following relationship:
| (6) |
A nonradiative decay rate (knr) of 8.5*108 s−1 was obtained from Eq. 6:
| (7) |
From the lifetime of TAAC in aqueous solution saturated with xenon (τXe = 130 ps), an apparent first-order quenching rate constant for the encapsulated xenon atom quenching the fluorescence of TAAC, kXe = 6.8*109 s−1, was then computed from Eq. 7:
| (8) |
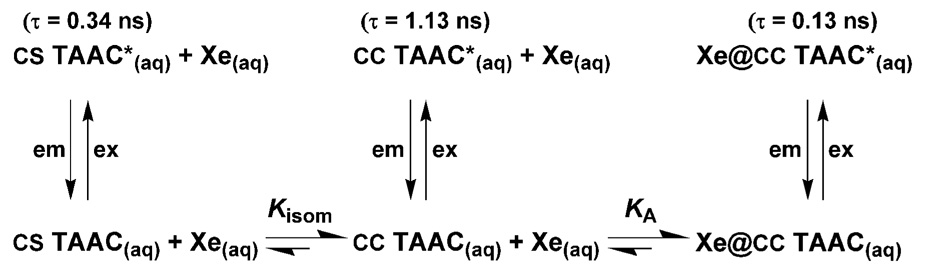
As discussed in the work of Webber,35 static quenching is a formalism in which the fluorophore-quencher complex is assumed to be an entirely dark state. In this instance, ultrafast techniques allow for the deconvolution of not only the lifetime of the Xe@TAAC complex, but also of the contribution to observed fluorescence intensity of the non-xenon-binding CS conformer of TAAC. TCSPC data show a minor population of TAAC (~5%) with a shorter lifetime than the dominant CC conformer. These observations are summarized in a modified static quenching scheme (Scheme 2) in which the effect of adding xenon to a solution of TAAC converts the population from the more emissive “empty” TAAC* excited state to a population of less emissive Xe@TAAC*, as governed by the xenon binding affinity of TAAC.
Scheme 2.
Proposed model of fluorescence quenching of TAAC by encapsulated xenon. The excitation (ex) and emission (em) wavelengths are 280 nm and 313 nm, respectively.
Acid-Base Titration
Acid-base titrations of TAAC and TTPC (Figures S4, S5, Supporting Information) were performed in order to understand the solubility requirements and anion stabilities of the two cryptophanes. TAAC exhibited a pKa of 4.1, compared to the reported pKa of 3.23 for the analagous 2-(2-methoxyphenoxy)acetic acid.37 Previous studies of sterically congested acetic acid derivatives have shown pKa differences of similar magnitude, up to 1.5 pKa units.38 Steric congestion near the carboxylic acid group decreases acidity by preventing efficient solvation of the carboxylate anion. For compound TAAC, this is due to the nonpolar surface of the ~1 nm diameter cryptophane interfering with the solvation of the three acetate anions. The nonpolar cryptophane surface must order surrounding water molecules and carboxylate solvation must compete directly with this ordered solvation sphere. In addition, based on visual observations of precipitation during the titrations, it was found that TAAC required all three acids to be deprotonated for aqueous solubility whereas TTPC required only two deprotonations for solubility to 200 µM.
The carboxylate groups of TTPC are ~5 Å farther from the cryptophane core and more easily stabilized through solvation. Titration of TTPC under the same conditions as TAAC yielded a pKa of 5.3. The pKa difference between TTPC and the analogous propanoic acid (pKa = 4.87)39 is less than one-half of a pKa unit, indicative that the carboxylates on TTPC experience less effect from the bulk of the nonpolar cryptophane. Propanoic acid is relevant for comparison to TTPC as the triazoles on TTPC are greater than two methylenes from the acid groups and not expected to contribute electrostatically to their acidity. However, the dipole moments and hydrogen-bond accepting abilities of the three triazoles appended to TTPC are expected to aid water solubility.
Aqueous NMR Spectroscopy
In order to bind xenon, the cryptophanes in this study must exist in a C3-symmetric crown-crown (CC) conformation, in which both CTV units adopt a concave form to create a molecular cavity. However, it was shown in the work of Huber et al40 that water-soluble cryptophanes are capable of existing in multiple conformations in aqueous solution. Specifically, a crown-saddle (CS) conformation was proposed, in which one of the two CTV units of the cryptophane is in an asymmetric “saddle” conformer. The CTV unit possessing the saddle conformer has been proposed to fill the cavity of the cryptophane, preventing xenon binding.
In order to investigate the possible presence of a CS isomer, NMR studies of TAAC and TTPC were undertaken at 300 K in aqueous 10% D2O solutions. The spectra of both compounds before degassing exhibited broad linewidths, particularly in the aromatic and ethylene linker resonances. This is attributed to the cryptophane being able to bind weakly gasses such as oxygen and nitrogen. While it is expected that both TAAC and TTPC are capable of binding water, no resonance was observed for either compound that could be attributed to encapsulated water (Figure 8 and Figure 9). Bound water molecules have been observed in cucurbiturils41 and the entropic penalty of encapsulating water has been suggested to explain the observation of CS conformers of other water-soluble cryptophanes.40 Degassing the solution with several cycles of pumping under static vacuum sharpened the aromatic and ethylene linker resonances. Saturation of the solution with xenon further sharpened all of the cryptophane core proton signals for the compounds. Similar sharpening of proton resonances upon aqueous guest encapsulation has been observed for a hexa-acid cryptophane-A derivative upon addition of chloroform.42
Figure 8.
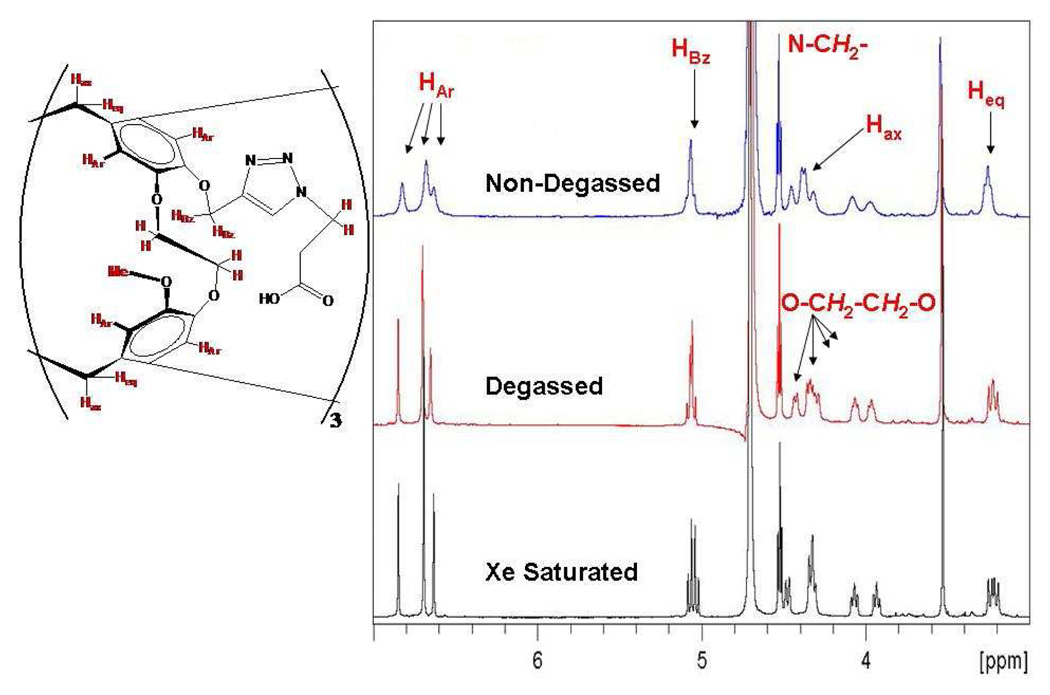
600 MHz 1H NMR spectrum of TTPC in 10% D2O.
Figure 9.
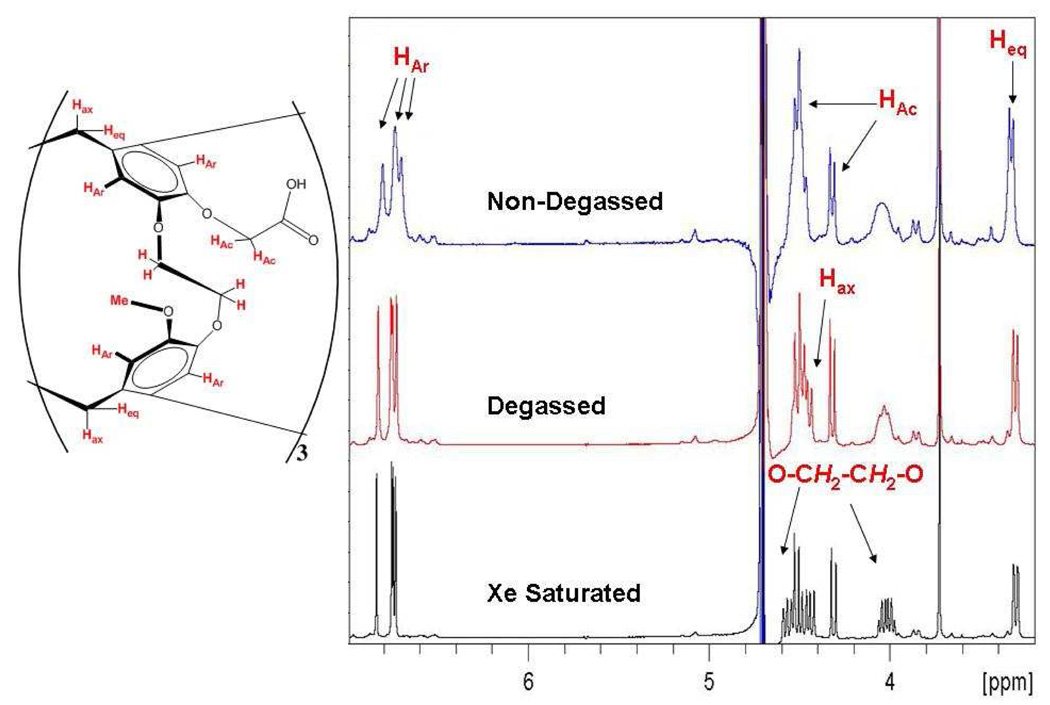
600 MHz 1H NMR spectrum of TAAC in 10% D2O.
It should be noted that in TTPC the protons farthest from the cryptophane core exhibit sharp linewidths (N-CH2 protons FWHM = 3 Hz for all three conditions) that are not affected by the nature of the various gaseous guest molecules (Figure 8). Because the cryptophane core is chiral (a mixture of Mo and Po enantiomers)43 the protons on the methylene connecting the cryptophane to the triazole (“HBz” in Figure 8) are diastereotopic, their resonances separated by 0.04 ppm and geminally splitting each other by 12 Hz upon xenon saturation. Most notably, however, no population of conformers other than the canonical CC form was detected for TTPC.
The degassed aqueous NMR spectrum of TAAC indicated less than 5% population, by NMR integration, of a second species that is consistent with the CS conformer (Figure 9). In addition to the aromatic peaks that are a result of the C1 symmetry of the CS conformer, the bridging methylene group peaks at 0.0 and 2.0 ppm (Figure S6, Supporting Information) that correspond to the CH2 protons pointing into the cavity are in agreement with the observations of Huber et al.40 These peaks are not impurities as the 1H NMR of TAAC in d6-DMSO (molecular charge = 0, Figure S7, Supporting Information) shows only CC conformer without the need for degassing or xenon saturation. Another striking feature of TAAC in aqueous solution is the magnitude of diastereotopic chemical shift difference evident in the protons on the methylenes connecting the cryptophane to the carboxyl groups (“HAc” in Figure 9). These protons, as confirmed by 2-D COSY (Figure S8, Supporting Information), had resonances separated by 0.20 ppm with 15 Hz geminal splitting. This large difference in chemical shift between the two methylene protons is indicative of two very different chemical environments. When the free acid of TAAC was dissolved in d6-DMSO, the HAc methylenes appeared as a singlet (Figure S7), with no apparent difference in their chemical environment. This dependence of chemical shift on the solvent and protonation state of TAAC supports the hypothesis that the diastereotopism observed in aqueous solution is a result of the carboxylate anions being unable to access many conformations that could produce magnetic equivalence of the protons. Adopting such conformations would lead to a loss in solvating water molecules and destabilize the three anions. It is hypothesized that the more compact CS conformer allows for more efficient solvation of the carboxylates, thermodynamically stabilizing this structure and allowing for its observation. We have not identified conditions in which the CS conformation predominates in aqueous solution.
The NMR and titration data lead us to hypothesize that the CS conformer population of TAAC is caused by the three deprotonated carboxylates being poorly solvated due to their close proximity to the cryptophane core. This poor anion solvation destabilizes the CC conformer. The greater salvation gained through adoption of the CS conformer allows for the observation of both conformers in aqueous solution. The carboxylates of TTPC, being farther away from the cryptophane core, are more efficiently solvated, which eliminates the gain in solvation that appears to be associated with adopting the more compact CS conformation.
Discussion
Given the low millimolar solubility of xenon in blood and tissue at physiological temperature,44 host molecules capable of binding and localizing dissolved xenon at micromolar concentrations are necessary for in vivo studies. A xenon-binding host molecule in biological media must outcompete xenon partitioning into nonpolar spaces such as cell membranes and fatty tissue. For these reasons, high-affinity xenon-binding molecules will be important for the development of functional xenon NMR biosensors and other xenon-based imaging agents. Finally, the construction of target-specific xenon biosensors requires the appendage of one or more bioactive moieties to a xenon-binding host molecule. This work demonstrates that the nature of the groups appended to a cryptophane can have a significant effect on cryptophane aqueous behavior and xenon binding affinity.
Time-correlated single photon counting confirmed static quenching by the encapsulated xenon and a quenching model has been proposed to explain why only partial fluorescence quenching is observed at xenon saturation. Xenon was found to be a better fluorescence quencher of TAAC than TTPC, based on the longer fluorescence lifetime of TAAC. The encapsulated xenon atom occupies almost half the volume of the cryptophane cavity and presumably collides hundreds of times with each chromophore before the S1→S0 electronic transition occurs from the excited 1,2-dialkoxybenzene units of the cryptophane; and yet, the xenon does not completely quench the cryptophane fluorescence. Fluorescence quenching experiments provided a useful method for determining xenon binding constants at low micromolar concentrations (15 µM) of TAAC or TTPC in buffer solution, and gave excellent agreement with ITC measurements, which were typically performed at much higher concentrations (770 µM). Despite this discrepancy, both techniques are more sensitive than typical NMR measurements, and ITC offers advantages for obtaining thermodynamic parameters and making measurements in optically dense media such as plasma.9 The ability to prepare and handle saturated xenon solutions enabled the titration of specific quantities of xenon for the determination of xenon binding constants by both fluorescence and ITC methods.
NMR experiments indicated that TTPC exists only as the CC conformer in aqueous solution. Although TAAC exists primarily as the CC conformer in aqueous solution, resonances were also observed that correspond to a minor population (< 5%) of the CS conformer. This was consistent with TCSPC data showing a minor population of TAAC (~5%) with a shorter lifetime than the dominant CC conformer. Acid-base titration data showed destabilization of the carboxylate anions of both compounds by the nonpolar cryptophane core in aqueous solution. This destabilization is hypothesized to be the driving force for the assumption of the CS conformer by TAAC in aqueous solution.
Conclusion
We conclude that TAAC exhibits the highest affinity for xenon of any known host molecule, KA = 33,000 M−1 at 293 K. The agreement between the fluorescence quenching and ITC data further validates the use of both techniques for the determination of xenon binding constants. Although the exact origin of the −0.37 kcal/mol additional stabilization of the Xe@TAAC complex relative to Xe@TTPC at 293 K is not known, this observation demonstrates that differently functionalized cryptophane cores can lead to different xenon binding affinities. All other effects being equal, we hypothesize that introducing ionizable groups close to the cryptophane that create a molecular dipole moment within the cavity can lead to improvements in xenon binding. Solvation effects may also serve to modulate more subtly the size of the CC conformer, leading to changes in binding affinity.
Supplementary Material
ITC buffer controls, full 1H NMR spectra of TAAC and TTPC in 10% D2O/H2O, 1H-1H COSY NMR spectrum of TAAC, full 1H NMR spectra of TAAC in d6-DMSO, temperature dependence of TAAC quantum yield, TCSPC decays of TTPC, acid-base titration details and titration curves.. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgment
I.J.D. appreciates support from the DOD (W81XWH-04-1-0657), NIH (1R21CA110104, 1R33CA110104), a Camille and Henry Dreyfus Teacher-Scholar Award, and UPenn Chemistry Department. We remember Jack Leigh and thank him for providing the support of the MMRRCC. We thank George Furst, Sangrama Sahoo, Jun Gu, Roderic Eckenhoff, Jeffery Saven, and Chris Lanci for discussions and access to instrumentation; the UPenn NIH Regional Laser and Biomedical Technology Laboratories which are supported by NIH grant P41RR001348; Patrick Carroll, Jin Xi, Nick Kuzma, and One-Sun Lee for their insight and expertise.
References
- 1.Raftery D. Annu. Rep. NMR Spectros. 2006;57:205–270. [Google Scholar]
- 2.Spence MM, Rubin SM, Dimitrov IE, Ruiz EJ, Wemmer DE, Pines A, Yao SQ, Tian F, Schultz PG. Proc. Natl. Acad. Sci., U.S.A. 2001;98:10654–10657. doi: 10.1073/pnas.191368398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spence MM, Ruiz EJ, Rubin SM, Lowery TJ, Winssinger N, Schultz PG, Wemmer DE, Pines A. J. Am. Chem. Soc. 2004;126:15287–15294. doi: 10.1021/ja0483037. [DOI] [PubMed] [Google Scholar]
- 4.Wei Q, Seward GK, Hill PA, Patton B, Dmitrov IE, Kuzma NN, Dmochowski IJ. J. Am. Chem. Soc. 2006;128:13274–13283. doi: 10.1021/ja0640501. [DOI] [PubMed] [Google Scholar]
- 5.Hilty C, Lowery TJ, Wemmer DE, Pines A. Angew. Chem. Int. Ed. 2006;45:70–73. doi: 10.1002/anie.200502693. [DOI] [PubMed] [Google Scholar]
- 6.Schroder L, Lowery TJ, Hilty C, Wemmer DE, Pines A. Science. 2006;314:446–449. doi: 10.1126/science.1131847. [DOI] [PubMed] [Google Scholar]
- 7.Garcia S, Chavez L, Lowery TJ, Han S-I, Wemmer DE, Pines A. J. Mag. Res. 2007;184:72–77. doi: 10.1016/j.jmr.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Berthault P, Bogaert-Buchmann A, Desvaux H, Huber G, Boulard Y. J. Am. Chem. Soc. 2008;130:16456–16457. doi: 10.1021/ja805274u. [DOI] [PubMed] [Google Scholar]
- 9.Hill PA, Wei Q, Eckenhoff RG, Dmochowski IJ. J. Am. Chem. Soc. 2007;129:9262–9263. doi: 10.1021/ja072965p. [DOI] [PubMed] [Google Scholar]
- 10.Aaron JA, Chambers JM, Jude KM, Costanzo LD, Dmochowski IJ, Christianson DW. J. Am. Chem. Soc. 2008;130:6942–6943. doi: 10.1021/ja802214x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambers JM, Hill PA, Aaron JA, Han Z, Christianson DW, Kuzma NN, Dmochowski IJ. J. Am. Chem. Soc. 2008 doi: 10.1021/ja806092w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Branda N, Grotzfeld RM, Valdés C, Jr, J R. J. Am. Chem. Soc. 1995;117:85–88. [Google Scholar]
- 13.Brotin T, Dutasta JP. Eur. J. Org. Chem. 2003;6:973–984. [Google Scholar]
- 14.Fogarty HA, Berthault P, Brotin T, Huber G, Desvaux H, Dutasta JP. J. Am. Chem. Soc. 2007;129 doi: 10.1021/ja073771c. 10332-+ [DOI] [PubMed] [Google Scholar]
- 15.Huber G, Beguin L, Desvaux H, Brotin T, Fogarty HA, Dutasta J-P, Berthault P. J. Phys. Chem. A. 2008;112:11363–11372. doi: 10.1021/jp807425t. [DOI] [PubMed] [Google Scholar]
- 16.Leontiev AV, Saleh AW, Rudkevich DM. Org. Lett. 2007;9:1753–1755. doi: 10.1021/ol070465v. [DOI] [PubMed] [Google Scholar]
- 17.Nakazawa J, Hagiwara J, Mizuki M, Shimazaki Y, Tani F, Naruta Y. Angew. Chem. Int. Ed. 2005;44:3744–3746. doi: 10.1002/anie.200500732. [DOI] [PubMed] [Google Scholar]
- 18.Scarso A, Pellizzaro L, Lucchi OD, Linden A, Fabris F. Angew. Chem. Int. Ed. 2007;46:4972–4975. doi: 10.1002/anie.200701123. [DOI] [PubMed] [Google Scholar]
- 19.Atwood JL, Barbour LJ, Jerga A. Science. 2002;296:2367–2369. doi: 10.1126/science.1072252. [DOI] [PubMed] [Google Scholar]
- 20.Enright GD, Udachin KA, Moudrakovski IL, Ripmeester JA. J. Am. Chem. Soc. 2003;125:9896–9897. doi: 10.1021/ja0351701. [DOI] [PubMed] [Google Scholar]
- 21.Brouwer DH, Moudrakovski IL, Udachin KA, Enright GD, Ripmeester JA. Crys. Growth Des. 2008;8:1878–1885. [Google Scholar]
- 22.Dubes A, Moudrakovski IL, Shahgaldian P, Coleman AW, Ratcliffe CI, Ripmeester JA. J. Am. Chem. Soc. 2004;126:6236–6237. doi: 10.1021/ja038653d. [DOI] [PubMed] [Google Scholar]
- 23.Fielding L. Tetrahedron. 2000;56:6151. [Google Scholar]
- 24.Wiseman T, Williston S, Brandts JF, Lin LN. Anal. Biochem. 1989;179:131–137. doi: 10.1016/0003-2697(89)90213-3. [DOI] [PubMed] [Google Scholar]
- 25.Fisher HF, Singh N. Meth. Enzymol. 1995;259:194–221. doi: 10.1016/0076-6879(95)59045-5. [DOI] [PubMed] [Google Scholar]
- 26.Kirby EP, Steiner RF. J. Phys. Chem. 1970;74:4480–4490. [Google Scholar]
- 27.Darzac M, Brotin T, Bouchu D, Dutasta JP. Chem. Commun. 2002:48–49. doi: 10.1039/b109301k. [DOI] [PubMed] [Google Scholar]
- 28.Darzac M, Brotin T, Rousset-Arzel L, Bouchu D, Dutasta JP. New J. Chem. 2004;28:502–512. [Google Scholar]
- 29.Brotin T, Dutasta J-P. Chem. Rev. 2008 doi: 10.1021/cr0680437. [DOI] [PubMed] [Google Scholar]
- 30.Brotin T, Roy V, Dutasta JP. J. Org. Chem. 2005;70:6187–6195. doi: 10.1021/jo050495g. [DOI] [PubMed] [Google Scholar]
- 31.Kim BS, Ko YH, Kim Y, Li HJ, Selvpalam N, Lee HC, Kim K. Chem. Commun. 2008:2756–2758. doi: 10.1039/b805724a. [DOI] [PubMed] [Google Scholar]
- 32.Grabner G, Monti S, Marconi G, Mayer B, Klein C, Kohler G. J. Phys. Chem. 1996;100:20068–20075. [Google Scholar]
- 33.Horrocks AR, Kearvell A, Tickle K, Wilkinso F. Trans. Faraday Soc. 1966;62:3393–3399. [Google Scholar]
- 34.Lacowicz JR. Principles of Fluorescence Spectroscopy. New York: Plenum Press; 1983. [Google Scholar]
- 35.Webber SE. Photochem. Photobio. 1997;65:33–36. [Google Scholar]
- 36.Wolber J, Doran SJ, Leach MO, Bifone A. Chem. Phys. Lett. 1998;296:391–396. [Google Scholar]
- 37.Hayes NV, Branch GEK. J. Am. Chem. Soc. 1943;65:1555–1564. [Google Scholar]
- 38.Newman MS, Fukunaga T. J. Am. Chem. Soc. 1963;85:1176–1178. [Google Scholar]
- 39.Harris DC. Quantitative Chemical Analysis. New York: W. H. Freeman and Company; 1998. [Google Scholar]
- 40.Huber G, Brotin T, Dubois L, Desvaux H, Dutasta JP, Berthault P. J. Am. Chem. Soc. 2006;128:6239–6246. doi: 10.1021/ja060266r. [DOI] [PubMed] [Google Scholar]
- 41.Germain P, Letoffe JM, Merlin MP, Buschmann HJ. Thermochim. Acta. 1998;315:87–92. [Google Scholar]
- 42.Canceill J, Lacombe L, Collet A. J. Chem. Soc. Chem. Commun. 1987:219–221. [Google Scholar]
- 43.Collet A. In: Comprehensive Supramolecular Chemistry. Atwood JL, MacNicol DD, Davis JED, Vogtle F, editors. Vol. 2. New York: Pergamon; 1996. pp. 325–365. [Google Scholar]
- 44.Clever HL. Solubility Data Series. Vol. 2. New York: Pergamon Press; 1979. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ITC buffer controls, full 1H NMR spectra of TAAC and TTPC in 10% D2O/H2O, 1H-1H COSY NMR spectrum of TAAC, full 1H NMR spectra of TAAC in d6-DMSO, temperature dependence of TAAC quantum yield, TCSPC decays of TTPC, acid-base titration details and titration curves.. This material is available free of charge via the Internet at http://pubs.acs.org.