Abstract
OBJECTIVE: To evaluate whether acute kidney injury (AKI), defined as an increase in the serum creatinine level of 0.3 mg/dL or more within 48 hours, predicts outcomes of non-critically ill patients.
PATIENTS AND METHODS: Among the adults admitted from June 1, 2005, to June 30, 2007, to the medical wards of a community teaching hospital, 735 patients with AKI and 5089 controls were identified. Demographic and health information, serum creatinine values, and outcomes were abstracted from patients' computerized medical records. Outcomes of patients with AKI were compared with those of controls. In an additional case-control analysis, more detailed clinical information was abstracted from the medical records of 282 pairs of randomly selected, age-matched AKI cases and controls. Conditional multivariate logistic regression analyses were used to adjust for potential confounders of AKI effect on outcomes.
RESULTS: Overall, patients with AKI had higher in-hospital mortality (14.8% vs 1.5%; P<.001), longer lengths of stay (median 7.9 vs 3.7 days; P<.001), and higher rates of transfer to critical care areas (28.6% vs 4.3%; P<.001); survivors were more likely to be discharged to an extended care facility (43.1% vs 20.3%; P<.001). Conditional multivariate logistic regression analyses of the 282 pairs of cases and controls showed that patients with AKI were 8 times more likely to die in hospital (odds ratio [OR], 7.9; 95% CI [confidence interval], 2.9-15.3) and were 5 times more likely to have prolonged (≥7 days) hospital stays (OR, 5.2; 95% CI, 3.5-7.9) and require intensive care (OR, 4.7; 95% CI, 2.7-8.1), after adjustment for age, comorbidities, and other potential confounders.
CONCLUSION: In this study, AKI was associated with adverse outcomes in non-critically ill patients.
Acute kidney disease (defined as an increase in the serum creatinine level of ≥0.3 mg/dL within a 48-hour period) was associated with adverse outcomes in non-critically ill patients. Overall, patients with acute kidney disease had higher in-hospital mortality, longer lengths of stay, and higher rates of transfer to critical care areas.
AKI = acute kidney injury; CI = confidence interval; CKD = chronic kidney disease; ECF = extended-care facility; GFR = glomerular filtration rate; ICU = intensive care unit; LOS = length of stay; NGAL = neutrophil gelatinase-associated lipocalin; OR = odds ratio; RR = risk ratio; RRT = renal replacement therapy
Renal dysfunction is a common cause or complication of acute illness and is associated with excess morbidity and mortality.1-11 No universally accepted definition for acute kidney dysfunction has been established. To the extent that nonuniform definitions hinder scientific inquiry, formulation of robust, scientifically valid definitions may expedite future studies of the epidemiology, pathophysiology, and treatment of renal failure. In 2005, Chertow et al2 published seminal findings suggesting that increases in the serum creatinine level as small as 0.3 mg/dL (to convert to μmol/L, multiply by 88.4) are highly associated with patients' outcomes. Later that year, heavily influenced by Chertow's observation, the Acute Kidney Injury Network ratified the following interim diagnostic criteria for acute kidney injury (AKI): “An abrupt (within 48 hours) reduction in kidney function currently defined as an absolute increase in serum creatinine of more than or equal to 0.3 mg/dL (≥26.4 μmol/L), a percentage increase in serum creatinine of more than or equal to 50% (1.5-fold from baseline), or a reduction in urine output (documented oliguria of less than 0.5 mL/kg per hour for more than six hours).”8 We found this definition—simplified to any increase in serum creatinine level of 0.3 mg/dL or more within 48 hours—to be strongly associated with outcomes of critically ill patients.1 The hypothesis for our study was that AKI similarly predicts in-hospital mortality, need for renal replacement therapy (RRT), and prolonged hospital stays of non-critically ill patients.
PATIENTS AND METHODS
This combined retrospective cohort and case-control study was conducted after approval by the institutional review board of Bridgeport Hospital, a 350-bed community teaching hospital affiliated with the Yale New Haven Health System. All adults admitted to the hospital medical wards from June 1, 2005, to June 30, 2007, were potentially eligible for inclusion. Patients were excluded if they had less than 2 creatinine measurements during their hospital stay, had undergone RRT in the 12 weeks before hospitalization, required RRT in the first 48 hours of hospitalization, or were directly admitted to critical care units. For patients with more than 1 hospitalization during the study period, only data from the first admission were analyzed.
Acute kidney injury cases were defined as patients with an absolute increase in serum creatinine of 0.3 mg/dL or more within any 48-hour period. Non-AKI control patients were defined as patients without an increase in serum creatinine of 0.3 mg/dL or more during their hospital stay. Patients in whom creatinine levels increased by 0.3 mg/dL or more after more than 48 hours were not included in the final analysis because most lacked creatinine measurements in the 48 hours before the noted increase, preventing accurate classification.
A sample of the AKI cases and controls was selected for the case-control portion of the study; for this sample, more detailed data abstraction and comprehensive analyses to adjust for potential confounders were conducted. From the total cohort of eligible AKI cases, 282 were randomly sampled using a computer-generated random-sampling process. The cases were then grouped into decades of age (eg, 20-29 years, 30-39 years). Both cases and controls were stratified into 8 age groups. A 1:1 frequency matching approach was used, whereby an equal number of cases and controls were randomly sampled in each decade group. No individual pairing (or individual matching) of cases and controls was done. In all, 282 cases and 282 controls were sampled. The sample size of 564 assured a study power of 80% to 90%, assuming a mortality rate of 1.5% in the control group and an expected odds ratio (OR) of mortality of at least 5.
Data Collection
Patients' demographic and laboratory data were downloaded from the hospital's electronic database. The following information was also ascertained: age, sex, race, RRT, transfer to critical care areas, hospital lengths of stay (LOSs), and mortality and disposition destinations (home vs extended-care facilities [ECFs]).
Potential confounders, such as the discharge diagnoses, comorbidities, and nephrotoxic medications, were not available in the electronic database. Information on these variables was manually extracted from the patients' complete medical records for the sampled cases and controls. Discharge diagnoses were categorized on the basis of the International Classification of Diseases, Ninth Revision, Clinical Modification9 groups as cardiovascular, pulmonary, gastrointestinal, related to infectious disease, and related to metabolism and nutrition, among others. Comorbidities included coronary artery disease; chronic heart failure; hypertension; diabetes mellitus; chronic kidney disease (CKD), defined as an estimated glomerular filtration rate (GFR) of less than 60 mL/min at baseline as determined by the Modification of Diet in Renal Disease formula; cirrhosis; chronic obstructive pulmonary disease; and cancer. Risk factors for acute kidney dysfunction included clinically documented volume depletion (eg, vomiting, diarrhea, hemorrhage) and use of nonsteroidal anti-inflammatory medications, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, radiologic contrast agents, thiazides, loop diuretics, trimethoprim-sulfamethoxazole, aminoglycosides, acyclovir, or cyclosporine.
Statistical Analyses
In the cohort study, median values and interquartile ranges are reported for age and hospital LOS. Wilcoxon rank sum tests were used to compare medians between patients with and without AKI. Categorical variables are reported as percentages and compared with χ2 tests. Risk ratios (RRs) and 95% confidence intervals (CIs) were used to examine the effects of AKI, old age (>65 years), and race (white vs nonwhite) on outcomes. Outcomes of interest included in-hospital mortality, prolonged LOS (≥7 days), and ECF discharge.
For the case-control component of the study, conditional logistic regression analyses were used to assess the association of AKI with outcomes, including in-hospital mortality, discharge to an ECF, transfer to an intensive care unit (ICU), hospital LOS of 3 days or more, and hospital LOS of 7 days or more, and to adjust simultaneously for age, sex, race, 2 or more comorbidities, and presence of CKD. Nephrotoxic medication and volume depletion were considered causative of AKI and were not adjusted in the model. Stratified analyses (Mantel-Haenszel approach) were used to adjust for matching age groups and to examine the association between AKI and race (white vs nonwhite), sex, use of nephrotoxic medications, volume depletion, 2 or more comorbidities, and CKD. The magnitude of association was documented with the adjusted Mantel-Haenszel OR. Two-tailed P values based on the corrected Mantel-Haenszel χ2 test statistic are reported.12,13
All analyses were performed using SAS version 8.2 software (SAS Institute, Cary, NC) and Epi Info version 3.4.1 software (Centers for Disease Control and Prevention, Atlanta, GA). A 2-tailed P value of <.05 was considered statistically significant.
RESULTS
Characteristics and Outcomes of the Overall Cohort
Of the 16,039 patients admitted to medical wards during the study period, the following were excluded: 5406 patients for whom this was not a first admission, 128 patients who received RRT before admission or within the first 48 hours of hospitalization, 1968 patients who were initially admitted to the ICU, and 2504 patients who had no or only 1 creatinine measurement. Of the 6033 remaining eligible patients, 209 (3.5%) were excluded who had increases in serum creatinine level of 0.3 mg/dL or more but at unspecified time intervals (eg, when creatinine values were not measured daily). The final cohort consisted of 5824 patients, of whom 735 (12.6%) were AKI cases and 5089 (87.4%) were controls (Figure).
FIGURE.
Flow of patients through the study. AKI = acute kidney injury; ICU = intensive care unit; RRT = renal replacement therapy.
a For patients with multiple admissions, only the first admission was included.
b Patients with no or only one creatinine value during their hospital stay.
c Patients with creatinine increase of >0.3 mg/dL (to convert to μmol/mL, multiply by 88.4) in >48 h, including those with missing creatinine data within 48 h.
d Cases were defined as patients with an absolute increase in serum creatinine of >0.3 mg/dL within 48 h.
e Controls were defined as patients with no absolute increase in serum creatinine >0.3 mg/dL during their hospital stay.
The mean ± SD age of the total cohort was 66.3±18.8 years. Of the 5824 patients in the cohort, 2723 (46.8%) were males, 3962 (68.0%) were white, 993 (17.1%) were African American, and 786 (13.5%) were Hispanic. Overall, 73 (1.3%) required new RRT and 429 (7.4%) required transfer to critical care areas during their hospital stay. In-hospital mortality was 3.2% and the mean ± SD LOS was 5.7±6.5 days. Of the survivors, 4354 (77.2%) were discharged home and 1286 (22.8%) required posthospital care in an ECF.
Patients with AKI were older (mean ± SD age, 72.9±15.0 vs 65.4±19.2 years; P<.001), and were more likely to be older than 65 years (72.4% vs 54.0%) when compared with non-AKI controls (Table 1). In univariate analysis, patients with AKI had significantly higher in-hospital mortality than controls (RR, 10.1; 95% CI, 7.6-13.4). Other factors associated with in-hospital mortality were age greater than 65 years (RR, 4.3; 95% CI, 2.9-6.4) and nonwhite race (RR, 0.4; 95% CI, 0.3-0.7), but sex was not associated with mortality. Acute kidney injury was also a significant risk factor for a hospital stay of 7 or more days (RR, 3.2; 95% CI, 3.0-3.5), ICU transfer (RR, 6.6; 95% CI, 5.6-7.9), and discharge to an ECF (RR, 2.1; 95% CI, 1.9-2.4).
TABLE 1.
Demographic Characteristics and Outcomes of Patients in the Cohorta
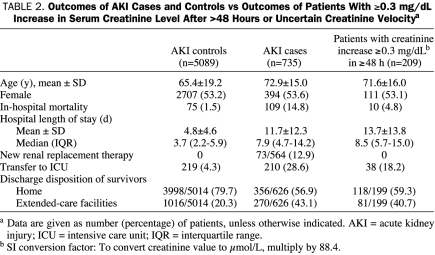
Characteristics of the 209 excluded patients who had an increase in serum creatinine level of 0.3 mg/dL or more after more than 48 hours or an unknown time horizon were also compared with AKI cases and controls (Table 2). Although in-hospital mortality rates (4.8%) and ICU transfer rates (18.2%) for these patients were midway between those of AKI cases and controls, their age distribution, LOS, and ECF placement rates were similar to those of patients with AKI.
TABLE 2.
Outcomes of AKI Cases and Controls vs Outcomes of Patients With ≥0.3 mg/dL Increase in Serum Creatinine Level After >48 Hours or Uncertain Creatinine Velocitya
Characteristics and Outcomes of the Sampled Cases and Controls
Demographic characteristics of the randomly sampled, frequency-matched AKI cases (n=282) and controls (n=282) are shown in Table 3. The age group-adjusted ORs for AKI of selected variables are reported in Table 4. The distribution of sex was similar to that in the total (parent) population. More cardiovascular disease (30.5% vs 25.2%) and less endocrine/metabolic disease (5.0% vs 9.6%) were noted in the AKI vs the control group. Patients with AKI had a significantly higher rate of 2 or more comorbidities compared with controls (75.9% vs 49.6%; OR, 3.5; P<.001). Patients with CKD had nearly 4-fold odds of having AKI compared with those without CKD (OR, 3.9; P<.001). The percentage of patients who had been exposed to nephrotoxins or who had a history suggestive of intravascular volume depletion was also significantly higher in AKI cases than controls (72.3% vs 61.3%; OR, 1.7; P=.007 and 35.8% vs 17.4%; OR, 2.7, P<.001, respectively).
TABLE 3.
Demographic Characteristics and Outcomes of Patients Selected for the Age-Matched, Case-Control Studya
TABLE 4.
Odds Ratios for AKI of Selected Variablesa,b
Results of the conditional multivariate logistic regression analyses performed in the selected case-control samples are shown in Table 5. Acute kidney injury emerged as the most potent predictor of mortality (OR, 7.9; 95% CI, 2.9-15.3). Age, 2 or more comorbidities, sex, race, and CKD were not independently associated with mortality. After adjustment for age, sex, race, comorbidities, and CKD, AKI was also associated with increased hospital LOS, more discharges to ECFs, and more frequent transfer to critical care areas.
TABLE 5.
Predictors of Adverse Events by Conditional Multivariate Logistic Regression in Selected Age-Matched AKI Cases and Controlsa
DISCUSSION
This study confirms that AKI, defined as an increase in serum creatinine level of 0.3 mg/dL or more within 48 hours, predicts clinical outcomes of non-critically ill patients. Acute kidney injury was associated with a nearly 7-fold (adjusted OR, 7.9; 95% CI, 2.9-15.3) increased odds of death, a more than 4-fold increased odds of prolonged (≥7 days) LOS, and a nearly 4-fold increased odds of transfer to critical care units. These findings are consistent with our findings in a cohort of critically ill patients admitted to the same community hospital.1
Outcomes and interventions for renal dysfunction have been explored extensively, but most studies have examined outcomes of critically ill patients.1,8-11,14-17 Fewer studies have reported the epidemiology of renal dysfunction in non-ICU patients.18-23 Liangos et al21 used the 2001 National Hospital Discharge Survey to demonstrate that 1.9% of hospitalized patients were diagnosed as having acute renal failure. Acute renal failure was assumed to be present if it was mentioned in the discharge summary and independently predicted hospital mortality and prolonged LOS. In a retrospective analysis of hospitalized patients, Chertow et al2 first showed that a change in serum creatinine level of more than 0.3 mg/dL during the entire course of hospitalization was associated with longer LOS and higher hospital costs. Although previous studies vary as to the incidence and prevalence of acute renal dysfunction (variably defined), most have shown adverse outcomes in patients with this condition.22-24
The express purpose of the Acute Kidney Injury Network was to draft an interim evidence-based definition of AKI that would then be tested for scientific validity. The proposed definition for AKI was not intended to be final; it was expected to be revised if or when epidemiological studies were conducted to refine its precision. We published the first study to test the new definition in 496 critically ill patients. The creatinine (increase of ≥0.3 mg/dL in 48 hours) component of the AKI definition was associated with a nearly 4-fold odds of mortality and a LOS that was twice as long (14 vs 7 days). In light of the findings of Chertow et al2 and our own findings in critically ill patients, we reasoned that the same observations might apply to patients initially admitted to our hospital wards. Interestingly, patients whose creatinine levels increased by 0.3 mg/dL or more after more than 48 hours or at unclear intervals (because of insufficient creatinine data) had outcomes midway between AKI cases and controls (Table 2). This observation confirms the results of Chertow et al2 that absolute increases in creatinine level of 0.3 mg/dL or more are associated with worse outcomes irrespective of the rate of creatinine increase.
The definition of AKI provides a unique basis for future epidemiological and interventional outcomes studies and also describes a biochemical event that may be caused by a constellation of diverse pathophysiologic events. It does not distinguish between prerenal, renal, and postrenal mechanisms—the most common pathophysiologic construct for describing renal failure. For example, it is unknown whether prerenal azotemia is functional or actually involves kidney injury (vs functional, blood flow-related changes in creatinine clearance with normally functioning or even hyperfunctioning kidneys). Regarding intrinsic “renal” mechanisms of injury, AKI has been best studied with ischemic/toxic injuries (ie, acute tubular necrosis). Patient prognosis can vary dramatically with other causes of AKI, such as interstitial disease, glomerular injuries, and obstructive causes, depending on the underlying etiology. In reality, serum creatinine level is likely a relatively late and imprecise biomarker of kidney dysfunction. Other biomarkers, including IL-8, urinary cystatin C, and urinary neutrophil gelatinase-associated lipocalin (NGAL), are being investigated as potential earlier and more accurate markers of clinically important renal dysfunction.24
A recent article highlights the problem of not having a unique diagnosis of renal failure. Nickolas et al25 examined the sensitivity and specificity of urinary NGAL for diagnosing AKI in the emergency department. Remarkably, AKI was the outcome of interest, defined as “a new-onset 1.5-fold increase in serum creatinine level or a 25% decrease in estimated GFR from baseline values that satisfied minimal RIFLE [Risk, Injury, Failure, Loss of function, and End-stage kidney disease] criteria for serum creatinine and GFR that was sustained for at least 3 days despite volume resuscitation.” No reference was given for the origin or validity of this definition, but the authors concluded that “a single measurement of urinary NGAL helped distinguish acute injury from normal function, prerenal azotemia, and chronic kidney disease.”25 The Acute Dialysis Quality Initiative found 44 studies published before 2004 using more than 20 definitions of renal failure.5 Until investigators can agree on a unique definition, research in this area will be impeded, and replicability, at the basis of most scientific inquiry, will be hindered if not rendered impossible.
Our study had several limitations. First, some data were missing because of the retrospective study design. Sufficient laboratory data were unavailable for about 15% of patients admitted during the study period. Second, we excluded 209 patients whose creatinine level increased by 0.3 mg/dL or more during an uncertain time interval. Some of those patients would likely have been categorized as AKI cases (if creatinine levels had been measured daily). Third, age was associated with outcomes in the univariate analysis, prompting us to perform age-matched comparisons to adjust for the potentially confounding effect of age on outcomes. As a result, we could not ascertain the potential interacting effect of age and AKI on the selected outcomes. Finally, these data do not demarcate prognoses on the basis of underlying causative pathophysiology and so cannot be used to determine precise mortality estimates for subgroups of patients (eg, prerenal vs renal, glomerulonephritis vs acute tubular necrosis vs obstructive uropathy).
CONCLUSION
An abbreviated definition of AKI (ie, an increase in serum creatinine level of 0.3 mg/dL or more within 48 hours) is associated with adverse clinical outcomes, including in-hospital mortality, prolonged hospital LOS, need for intensive care, and discharge to an ECF. If our findings can be replicated at other centers, then the Acute Kidney Injury Network criteria for AKI provide a simple, scientifically valid definition that could foster research in this field.
Acknowledgments
The authors are grateful to Ryan O'Connell, MD, for his assistance coordinating with hospital informatics and Ms. Joan Huff for data acquisition from hospital information systems.
REFERENCES
- 1.Barrantes F. Acute kidney injury criteria predict outcomes in critically ill patients. Crit Care Med. 2008;36(5):1397-1403 [DOI] [PubMed] [Google Scholar]
- 2.Chertow GM. Acute kidney injury, mortality, length of stay and costs in hospitalized patients. J Am Soc Nephrol. 2005November;16(11):3365-3370 Epub 2005 Sep 21 [DOI] [PubMed] [Google Scholar]
- 3.Lassnigg A, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15(6):1597-1605 [DOI] [PubMed] [Google Scholar]
- 4.Uchino S, et al. Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 2005;294(7):813-818 [DOI] [PubMed] [Google Scholar]
- 5.Bouman C. Definition for acute renal failure. ADQI Acute Dialysis Quality Initiative Website; http://www.ccm.upmc.edu/adqi/ADQI2/ADQI2g1.pdf Accessed March 20, 2009
- 6.Liaño F, The Madrid Acute Renal Failure Study Group Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Kidney Int. 1996;50(3):811-818 [DOI] [PubMed] [Google Scholar]
- 7.Hoste EA. Acute kidney injury: epidemiology and diagnostic criteria. Curr Opin Crit Care 2006;12(6):531-537 [DOI] [PubMed] [Google Scholar]
- 8.Mehta RL, et al. Acute Kidney Injury Network Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11(2):R31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Center for Health Statistics International classification of diseases, ninth revision, clinical modification (ICD-9-CM) Hyattsville, MD: US Department of Health and Human Services; Centers for Disease Control and Prevention; 2004. http://www.cdc.gov/nchs/about/otheract/icd9/abticd9.htm Accessed March 20, 2009 [Google Scholar]
- 10.Mangano CM. Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilization. Ann Intern Med. 1998;128(3):194-203 [DOI] [PubMed] [Google Scholar]
- 11.Chertow GM. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104(4):343-348 [DOI] [PubMed] [Google Scholar]
- 12.Kleinbaum DG. A Pocket Guide to Epidemiology New York, NY: Springer Science; 2007. [Google Scholar]
- 13.Selvin S. Epidemiologic Aanalysis: A Case-Oriented Approach New York NY: Oxford University Press; 2001. [Google Scholar]
- 14.Uchino S. An assessment of RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34(7):1913-1917 [DOI] [PubMed] [Google Scholar]
- 15.Thakar CV, et al. ARF after open-heart surgery: influence of gender and race. Am J Kidney Dis. 2003;41(4):742-751 [DOI] [PubMed] [Google Scholar]
- 16.Bellomo R, et al. ADQI workgroup Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the Acute Dialysis Quality Initiative (ADQI) group. Crit Care 2004August;8(4):R204-R212 Epub 2004 May 24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellomo R. The epidemiology of acute renal failure: 1975 versus 2005. Curr Opin Crit Care 2006;12(6):557-560 [DOI] [PubMed] [Google Scholar]
- 18.Fischer MJ. Uncomplicated Acute renal failure and post-hospital care: a not so uncomplicated illness. Am J Nephrol. 2008;28(3):523-530 Epub 2008 Jan 25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y. Hospital-acquired and community-acquired acute real failure in hospitalized patients: a ten year review. Ren Fail 2007;29(2):163-168 [DOI] [PubMed] [Google Scholar]
- 20.Hsu CY. Community-based incidence of acute renal failure. Kidney Int. 2007July;72(2):208-212 Epub 2007 May 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liangos O. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006January;1(1):43-51 Epub 2005 Oct 26 [DOI] [PubMed] [Google Scholar]
- 22.Ali T, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol. 2007April;18(4):1292-1298 Epub 2007 Feb 21 [DOI] [PubMed] [Google Scholar]
- 23.Waikar SS. Race and mortality after acute renal failure. J Am Soc Nephrol. 2007October;18(10):2740-2748 Epub 2007 Sep 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coca SG. Urinary biomarkers for acute kidney injury: perspectives on translation. Clin J Am Soc Nephrol. 2008March;3(2):481-490 Epub 2008 Feb 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nickolas TL, et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalcin for diagnosing acute kidney injury. Ann Intern Med. 2008;148(11):810-819 [DOI] [PMC free article] [PubMed] [Google Scholar]