Abstract
OBJECTIVE: To clarify the clinical presentation and course of patients with spontaneous pneumomediastinum (SP) and to determine the usefulness of diagnostic testing in these patients.
PATIENTS AND METHODS: We conducted a retrospective review of 62 consecutive adult patients (age ≥18 years) diagnosed as having SP during an 11-year period from July 1, 1997, to June 30, 2008. The study cohort included 41 men and 21 women (median age, 30 years; interquartile range, 20-69 years).
RESULTS: Among the 62 study patients, the most common presenting symptoms were chest pain (39 patients [63%]), cough (28 [45%]), and dyspnea (27 [44%]). Preexisting lung diseases were identified in 27 patients (44%) and included interstitial lung disease, asthma, lung malignancies, bronchiolitis obliterans syndrome, chronic obstructive pulmonary disease, bronchiectasis, and cystic lung lesions. The initial diagnosis of SP was achieved by chest radiography in 52 patients (84%); the remaining cases were diagnosed by computed tomography. Forty-seven patients (76%) were hospitalized for a median duration of 2.5 days. Additional diagnostic procedures were performed in 27 patients (44%) and included contrast esophagography, bronchoscopy, and esophagogastroduodenoscopy; however, they did not yield a pathologic cause in any patient. Pneumothorax was identified in 20 patients (32%), but less than one-third of these patients underwent chest tube thoracostomy. No episodes of mediastinitis or sepsis occurred. Recurrence of SP was seen in 1 patient, and thoracoscopic surgery was performed in 1 patient for persistent air leak (pneumothorax).
CONCLUSION: Spontaneous pneumomediastinum was associated with a relatively benign clinical course; however, pneumothorax was seen in 32% of cases. Diagnostic testing to determine a pathologic cause yielded little clinically relevant information in these patients.
Spontaneous pneumomediastinum was associated with a relatively benign clinical course; however, pneumothorax was seen in 20 (32%) of the patients, but less than one-third of these patients underwent chest tube thoracostomy. Diagnostic testing to determine a pathologic cause yielded little clinically relevant information.
CT = computed tomography; ICU = intensive care unit; IQR = interquartile range; LOS = length of stay; SP = spontaneous pneumomediastinum
Spontaneous pneumomediastinum (SP) is defined by the appearance of free air in the mediastinum that is not preceded by trauma, surgery, or other medical procedures. It is an uncommon condition that often presents with sudden onset of symptoms, including chest pain, neck pain, dyspnea, or signs of subcutaneous emphysema.1-6 It may occur with or without a concomitant pneumothorax.
Although SP is generally considered to be associated with a relatively benign clinical course, published data pertaining to SP are relatively limited.1-6 Additionally, the usefulness of diagnostic testing in determining a cause for SP has not been clearly established.6,7 The aim of the current study was to investigate the presenting features and clinical course of SP as well as the results of diagnostic testing by reviewing a consecutive series of adult patients diagnosed as having this condition during an 11-year period.
PATIENTS AND METHODS
The study was conducted at Mayo Clinic's site in Rochester, MN. A computer-assisted search was performed to identify all adult cases of SP diagnosed during an 11-year period from July 1, 1997, to June 30, 2008. Medical records were reviewed to exclude cases of traumatic and iatrogenic pneumomediastinum, including those associated with chest trauma, cardiopulmonary resuscitation, mechanical ventilation, or any surgical/interventional procedures. Neonatal and pediatric patients (<18 years) were also excluded. A total of 62 adult patients with SP were identified. Approval was obtained from the Mayo Clinic Institutional Review Board before beginning the study.
Medical records of all patients confirmed as having SP were reviewed to extract data regarding demographics, clinical presentation, imaging studies, precipitating factors, comorbidities, diagnostic testing, treatment, hospitalization, hospital course, and follow-up. We defined presenting symptoms as those symptoms identified in the medical records during a clinical evaluation leading to the diagnosis of SP. Preexisting lung diseases described in the medical records for a clinical episode leading to the diagnosis of SP were extracted and identified as potential predisposing factors. Precipitating factors were defined as recent (within 1 week) activities or events described in the medical records as potential immediate causes for the development of SP. Follow-up period was defined as the interval from the last day of hospitalization or clinical evaluation leading to the diagnosis of SP to the last available clinical note documenting follow-up medical care.
All data were analyzed using JMP 7.0 statistical software (SAS Institute, Cary, NC). The Wilcoxon rank sum test or 2-sample t tests were used for the comparisons of continuous data, and the χ2 test for the analysis of categorical data. We hypothesized a priori that patients with concomitant pneumothoraces would have longer hospitalizations with more intensive care unit (ICU) admissions.
RESULTS
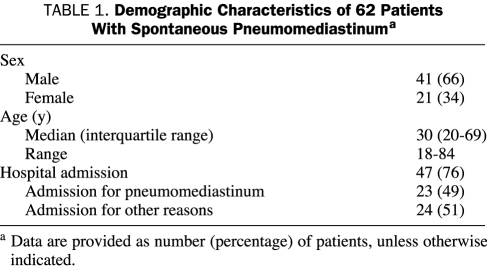
Demographic data for the 62 patients, including 41 men and 21 women, are outlined in Table 1. The median age at diagnosis of SP was 30 years (interquartile range [IQR], 20-69 years). Of the 62 study patients, 21 (34%) were active or past smokers at the time of diagnosis. Pneumomediastinum was diagnosed in these patients most often in the emergency department (49 patients [79%]) but also in the ICU (7 patients [11%]), outpatient clinic (5 patients [8%]), and hospital ward (1 patient [2%]).
TABLE 1.
Demographic Characteristics of 62 Patients With Spontaneous Pneumomediastinuma
Presenting Features
Chest pain, the most common presenting symptom, occurred in 39 patients (63%) (Table 2). Less common symptoms included dyspnea, cough, neck pain, light-headedness, dysphagia, and dysphonia. Subcutaneous emphysema was noted in 28 patients (45%), but none was documented to have Hamman crunch on examination.
TABLE 2.
Presenting Symptoms of 62 Patients With Spontaneous Pneumomediastinuma
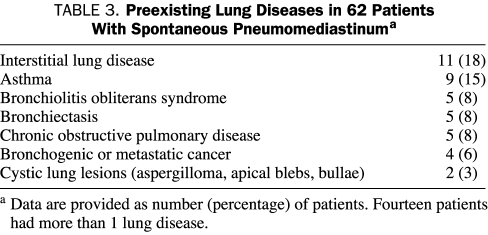
One or more preexisting lung disorders were identified in 27 patients (44%) and are outlined in Table 3. Eleven patients (18%) had evidence of interstitial lung disease that included connective tissue disease-associated interstitial lung disease (3 patients), radiation-induced lung fibrosis (2 patients), hypersensitivity pneumonitis (1 patient), idiopathic pulmonary fibrosis (1 patient), cryptogenic organizing pneumonia (1 patient), and nonspecific or post-inflammatory lung fibrosis (3 patients). Other lung conditions included asthma, bronchogenic or metastatic cancer to the lung, bronchiolitis obliterans syndrome related to allogeneic hematopoietic stem cell transplant, chronic obstructive pulmonary disease, bronchiectasis, and cystic lung lesions.
TABLE 3.
Preexisting Lung Diseases in 62 Patients With Spontaneous Pneumomediastinuma
Precipitating factors that may have led to the development of SP were identified in 21 patients (34%). These included inhalational drug abuse (marijuana, cocaine, methamphetamine) (6 patients), upper respiratory infections with coughing (5 patients) or noninfectious asthma exacerbation (4 patients) among those with chronic asthma, severe retching and vomiting related to diabetic ketoacidosis (4 patients), and preceding athletic activities (2 patients; basketball and football, respectively).
Chest Imaging Studies
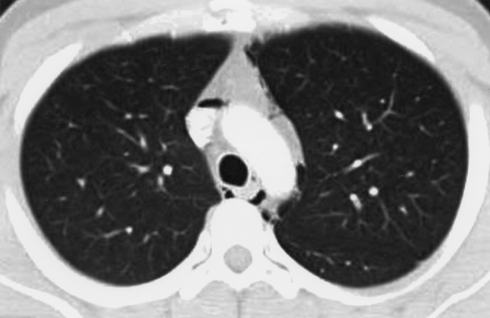
Chest radiography was the initial chest imaging study in 58 patients (94%); in 52 patients, posteroanterior and lateral views were obtained, and in the remaining 6 patients anterior-posterior portable radiography was performed. Of the 58 patients undergoing chest radiography, SP was identified in 52 (90%). For the 6 patients whose SP was not identified by chest radiography (posteroanterior and lateral views were obtained in all 6 patients), the diagnosis was achieved by computed tomography (CT) of the chest. The amount of air in the mediastinum was too small to be detected by chest radiography in 3 of these patients. In 2 other patients, chest radiography had been performed on the day before CT and the development of pneumomediastinum during the interval could not be excluded. In the 1 remaining patient, SP was detectable on chest radiography on retrospective review of the radiographs. Chest CT was performed in 55 patients (89%) within 48 hours of initial presentation and showed SP in all patients (Figure).
FIGURE.
Computed tomogram (CT) of the chest of a 22-year-old patient presenting with diabetic ketoacidosis and associated vomiting. Scattered presence of air in the mediastinum is evident on the CT. Oral contrast medium administered during the CT study revealed no evidence of esophageal rupture. Pneumomediastinum was detected via portable chest radiography performed earlier on the same day. On follow-up CT of the chest 12 days later, pneumomediastinum had completely resolved.
Other Diagnostic Procedures
Abdominal CT was performed for evaluation of abdominal symptoms in 10 patients (16%) but did not reveal clinically important abdominal pathology in any of the patients. In 1 patient with diabetic ketoacidosis, SP was initially identified on an abdominal CT that was performed for evaluation of severe vomiting and abdominal pain.
Additional diagnostic procedures were performed in 27 patients (44%) to identify the cause of the pneumomediastinum (eg, airway or esophageal lesions). Contrast-enhanced esophagography was performed in 22 patients (35%), particularly in those with recent retching, vomiting, or dysphagia, but revealed no esophageal pathology. Findings were normal for the 4 patients (6%) who underwent bronchoscopy and the 2 patients (3%) who underwent esophagogastroduodenoscopy.
Clinical Course
Of the 62 study patients, 47 (76%) were hospitalized for a median hospital length of stay (LOS) of 2.5 days (IQR, 2.0-7.0 days; range, 1.0-67.0 days). Of these hospitalized patients, 23 (49%) were admitted primarily for SP. In the remaining 24 patients (51%), hospitalization occurred primarily for another reason; these reasons included diabetic ketoacidosis (4 patients); complications related to allogeneic hematopoietic stem cell transplant (4 patients); cancer-related complications (4 patients); abdominal abscess (2 patients); progression of connective tissue disease-related interstitial lung disease (2 patients) or hypersensitivity pneumonitis (1 patient); exacerbation of chronic obstructive pulmonary disease (1 patient), asthma (1 patient), or bronchiectasis (1 patient); community-acquired pneumonia (1 patient); seizures (1 patient); cardiac failure (1 patient); and diarrhea (1 patient). Patients who were admitted primarily for SP had a shorter median hospital LOS than did those admitted primarily for another condition (2 vs 6 days; P=.001).
Concomitant pneumothorax was identified in 20 patients (32%). Of these 20 patients with pneumothorax, 1 was not hospitalized because the pneumothorax was a small apical one. Of the hospitalized patients with pneumothorax, 6 underwent placement of a chest tube, including 2 patients who had bilateral pneumothoraces requiring bilateral chest tubes. One of these patients with a chest tube subsequently underwent a video-assisted thoracic surgery for bullectomy (severe bullous emphysema) and pleurodesis for persistent air leak. The 13 remaining patients had small apical pneumothoraces that did not require chest tube drainage. Hospital LOS in patients with pneumothorax (median, 5.0 days; IQR, 1.0-7.0 days) was not significantly different than that in patients in whom pneumothorax was not identified (median, 4.5 days; IQR, 1.0-7.0 days).
Sixteen patients (26%) required admission to the ICU and had a median ICU LOS of 2.5 days (IQR, 2.0-5.0 days; range, 1.0-40.0 days). Of the 16 admissions to the ICU, SP was the primary reason for ICU admission in only 4 instances. Other reasons included allogeneic hematopoietic stem cell transplant-related complications (4 patients), diabetic ketoacidosis (2 patients), acute respiratory distress syndrome (2 patients), cardiogenic shock (1 patient), non-small-cell lung cancer (1 patient), seizures (1 patient), and small bowel ileus (1 patient).
Antibiotics were administered in 16 patients (26%) out of concern for mediastinitis or sepsis. However, none of the study patients were documented to have sepsis or mediastinitis during their hospital course.
Patients were followed up for a median duration of 6 months (IQR, 0-24 months; range, 0-84 months). Pneumomediastinum recurred in 1 patient; these 2 episodes were likely related to inhalational drug use. Three patients had prolonged SP lasting 6 to 9 weeks. All 3 patients were allogeneic hematopoietic stem cell transplant recipients with graft-vs-host disease and bronchiolitis obliterans syndrome. Tension pneumopericardium was not observed.
Of the study patients, 5 (8%) died while in the hospital: 3 as a result of complications related to advanced lung cancer or widely metastatic disease, 1 after withdrawal of support for advanced connective tissue disease-associated interstitial lung disease, and 1 of multiorgan failure after allogeneic hematopoietic stem cell transplant. None of these deaths could be attributed directly to SP.
DISCUSSION
Spontaneous pneumomediastinum is an uncommon disorder that can be encountered in a variety of clinical settings. The pathophysiology of SP was delineated by Macklin8 in his experimental animal model. His work showed how alveolar hyperinflation causes alveolar damage with subsequent leakage of air from alveolar spaces into the interstitium followed by proximal migration of air toward the hilum and the mediastinum alongside the pulmonary vasculature. Once the air reaches the mediastinum, it travels along tissue planes and can reach the neck, face, abdomen, and even the limbs, resulting in subcutaneous emphysema. The accumulation of air between the anterior parietal pericardium and the overlying chest wall can produce a crunching sound correlated with cardiac motion that was described by Hamman9 in 1939 and is now known as the Hamman crunch.
In the current review of 62 consecutive adult cases, we found SP to be associated with a relatively benign clinical course. Although most of the study patients were hospitalized, half were admitted for reasons other than SP. Nearly all patients with concomitant pneumothorax were hospitalized, but only one-third of these patients underwent chest tube thoracostomy. No therapeutic interventions were needed for the management of SP itself.
Spontaneous pneumomediastinum has been associated with a variety of structural lung diseases that may predispose patients to the development of SP, including emphysema, asthma, interstitial lung diseases, and bronchiectasis.1,10,11 These disorders were also present in our cohort of patients. Among the less common predisposing factors encountered were bronchiolitis obliterans syndrome in allogeneic hematopoietic stem cell recipients, intrathoracic malignancies, and focal cystic or cavitary lesions.12 The presence of underlying lung disease may adversely affect the outcome of patients with SP. For example, a recent report described a patient with amyopathic dermatomyositis-associated interstitial lung disease who developed tension pneumomediastinum requiring tube drainage and died of acute respiratory distress syndrome.13 Tension pneumomediastinum or tension pneumopericardium results from persistent entry of air into the mediastinal or pericardial spaces via a 1-way valve mechanism, resulting in increasing gas pressure in these compartments. Increasing compartmental pressure, which can impair venous return and cardiac function, constitutes a medical emergency. Air should be promptly evacuated via either percutaneous methods or a surgical approach.14
Our analysis attempted to separate predisposing conditions from precipitating factors. Previous studies have reported several precipitating factors, including vomiting associated with diabetic ketoacidosis,15-17 inhalational drug abuse,18-21 coughing,1 and sports.1 In one-third of our patients with SP, we were able to identify a similar preceding event or activity that may have precipitated the SP.
Chest radiography appeared to be a relatively sensitive tool in diagnosing SP.22 In the 58 patients who underwent chest radiography, 3 (5%) had a pneumomediastinum that was too small to be detected by chest radiography but was revealed by CT performed on the same day. Our results are similar to those of Kaneki et al,3 who reported SP to be undetectable in 3 (9%) of their 33 patients with SP; all 3 patients had “mild” pneumomediastinum. Aside from being more accurate in detecting small amounts of air in the mediastinum, CT of the chest can occasionally reveal other findings that may provide insights into the underlying cause or associated disease.3,23
The role of additional diagnostic testing, including esophagography, bronchoscopy, and esophagogastroduodenoscopy, appears to be limited in the evaluation of patients with SP. In our cohort, 25 patients (40%) underwent bronchoscopy, esophagography, or esophagogastroduodenoscopy. None of these procedures yielded relevant findings. Our results on this issue are consistent with those of previously published reports.2,4-6,24,25
The frequency of concomitant pneumothorax (32%) seen in our cohort of patients was higher than the 6% to 11% reported in previous studies.1,26,27 This higher rate of pneumothorax in the current study vs previous studies is probably due to a higher prevalence in our patients of preexisting lung diseases, particularly interstitial lung disease and bronchiolitis obliterans syndrome. The patients in our study were also older than those in previous studies, in which the mean or median age of the study participants was in the range of 17 to 25 years.1,3-5
Although our study describes a relatively large cohort of patients with SP, it has limitations typical of retrospective studies, including limited and incomplete follow-up data. Because of these limitations in the data, we cannot accurately assess recurrence rates of SP. However, our data and recent reports suggest that recurrence of SP during hospitalization and afterwards is relatively rare. Patients referred to our tertiary medical center likely had more severe illness and a higher prevalence of comorbidities than patients seen at community-based primary care centers.
CONCLUSION
Spontaneous pneumomediastinum is associated with a relatively benign clinical course but may be complicated by the occurrence of pneumothorax in 32% of patients, a frequency higher than has been previously reported. In the absence of a concomitant pneumothorax or severe illness requiring inpatient care, patients with SP could probably be treated on an outpatient basis. The clinical course of patients with SP is influenced more by the severity of the underlying disorder (eg, advanced interstitial lung disease or graft-vs-host disease) than by SP itself. Diagnostic procedures performed to identify an underlying anatomic cause (eg, esophageal or tracheobronchial rupture) have a low yield and are unnecessary in most cases.
REFERENCES
- 1.Newcomb AE. Spontaneous pneumomediastinum: a benign curiosity or a significant problem? Chest 2005;128(5):3298-3302 [DOI] [PubMed] [Google Scholar]
- 2.Macia I, et al. Spontaneous pneumomediastinum: 41 cases. Eur J Cardiothorac Surg. 2007June;31(6):1110-1114 Epub 2007 Apr 8 [DOI] [PubMed] [Google Scholar]
- 3.Kaneki T. Spontaneous pneumomediastinum in 33 patients: yield of chest computed tomography for the diagnosis of the mild type. Respiration 2000;67(4):408-411 [DOI] [PubMed] [Google Scholar]
- 4.Koullias GJ. Current assessment and management of spontaneous pneumomediastinum: experience in 24 adult patients. Eur J Cardiothorac Surg. 2004;25(5):852-855 [DOI] [PubMed] [Google Scholar]
- 5.Takada K, et al. Management of spontaneous pneumomediastinum based on clinical experience of 25 cases. Respir Med. 2008September;102(9):1329-1334 Epub 2008 Jun 26 [DOI] [PubMed] [Google Scholar]
- 6.Abolnik I. Spontaneous pneumomediastinum: a report of 25 cases. Chest 1991;100(1):93-95 [DOI] [PubMed] [Google Scholar]
- 7.Esayag Y. Spontaneous pneumomediastinum: is a chest x-ray enough? A single-center case series. Isr Med Assoc J 2008;10(8-9):575-578 [PubMed] [Google Scholar]
- 8.Macklin CC. Transport of air along sheaths of pulmonic blood vessels from alveoli to mediastinum: clinical implications. Arch Intern Med. 1939;64(5):913-926 [Google Scholar]
- 9.Hamman L. Spontaneous mediastinal emphysema. Bull Johns Hopkins Hosp. 1939;64:1-21 [Google Scholar]
- 10.Patel A. Persistent pneumomediastinum in interstitial fibrosis associated with rheumatoid arthritis: treatment with high-concentration oxygen. Chest 2000;117(6):1809-1813 [DOI] [PubMed] [Google Scholar]
- 11.Franquet T. Spontaneous pneumothorax and pneumomediastinum in IPF. Eur Radiol. 2000;10(1):108-113 [DOI] [PubMed] [Google Scholar]
- 12.Franquet T, et al. Air-leak syndromes in hematopoietic stem cell transplant recipients with chronic GVHD: high-resolution CT findings. J Thorac Imaging 2007;22(4):335-340 [DOI] [PubMed] [Google Scholar]
- 13.Powell C. A 34-year-old man with amyopathic dermatomyositis and rapidly progressive dyspnea with facial swelling. Chest 2007;132(5):1710-1713 [DOI] [PubMed] [Google Scholar]
- 14.Gabor SE. Tension pneumomediastinum after severe vomiting in a 21-year-old female. Eur J Cardiothorac Surg. 2005;28(3):502-503 [DOI] [PubMed] [Google Scholar]
- 15.Pauw RG. Mediastinal emphysema complicating diabetic ketoacidosis: plea for conservative diagnostic approach. Neth J Med. 2007;65(10):368-371 [PubMed] [Google Scholar]
- 16.Nessan VJ. Recurrent pneumomediastinum in diabetic ketoacidosis. Postgrad Med. 1974;55(6):139-140 [DOI] [PubMed] [Google Scholar]
- 17.Weathers LS. Spontaneous pneumomediastinum in a patient with diabetic ketoacidosis: a potentially hidden complication. South Med J 1995;88(4):483-484 [DOI] [PubMed] [Google Scholar]
- 18.Brody SL. Pneumomediastinum as a complication of “crack” smoking. Am J Emerg Med. 1988;6(3):241-243 [DOI] [PubMed] [Google Scholar]
- 19.Aroesty DJ. Pneumomediastinum and cervical emphysema from the inhalation of “free based” cocaine: report of three cases. Otolaryngol Head Neck Surg. 1986;94(3):372-374 [DOI] [PubMed] [Google Scholar]
- 20.Miller WE. Pneumomediastinum resulting from performing Valsalva maneuvers during marihuana smoking. Chest 1972;62(2):233-234 [DOI] [PubMed] [Google Scholar]
- 21.Mattox KL. Pneumomediastinum in heroin and marijuana users. JACEP 1976;5(1):26-28 [DOI] [PubMed] [Google Scholar]
- 22.Ba-Ssalamah A. Spontaneous pneumomediastinum. Eur Radiol. 1999;9(4):724-727 [DOI] [PubMed] [Google Scholar]
- 23.Oberholzer K. Pneumomediastinum and pneumopericardium due to malignant subcarinal lymphadenopathy: CT demonstration. Eur Radiol. 1997;7(4):583-585 [DOI] [PubMed] [Google Scholar]
- 24.Panacek EA. Spontaneous pneumomediastinum: clinical and natural history. Ann Emerg Med. 1992;21(10):1222-1227 [DOI] [PubMed] [Google Scholar]
- 25.Freixinet J. Spontaneous pneumomediastinum long-term follow-up. Respir Med. 2005September;99(9):1160-1163 Epub 2005 Apr 1 [DOI] [PubMed] [Google Scholar]
- 26.Mondello B. Spontaneous pneumomediastinum: experience in 18 adult patients. Lung 2007Jan–Feb;185(1):9-14 Epub 2007 Feb 15 [DOI] [PubMed] [Google Scholar]
- 27.Caceres M. Spontaneous pneumomediastinum: a comparative study and review of the literature. Ann Thorac Surg. 2008;86(3):962-966 [DOI] [PubMed] [Google Scholar]