Abstract
Acute and chronic aortic diseases have been diagnosed and studied by physicians for centuries. Both the diagnosis and treatment of aortic diseases have been steadily improving over time, largely because of increased physician awareness and improvements in diagnostic modalities. This comprehensive review discusses the pathophysiology and risk factors, classification schemes, epidemiology, clinical presentations, diagnostic modalities, management options, and outcomes of various aortic conditions, including acute aortic dissection (and its variants intramural hematoma and penetrating aortic ulcers) and thoracic aortic aneurysms. Literature searches of the PubMed database were conducted using the following keywords: aortic dissection, intramural hematoma, aortic ulcer, and thoracic aortic aneurysm. Retrospective and prospective studies performed within the past 20 years were included in the review; however, most data are from the past 15 years.
AAD = acute aortic dissection; ACS = acute coronary syndrome; ARB = angiotensin II receptor blocker; COPD = chronic obstructive pulmonary disease; CT = computed tomography; dP/dt = force of left ventricular ejection; EDS = Ehlers-Danlos syndrome; FBN = fibrillin; ICU = intensive care unit; IMH = intramural hematoma; IRAD = International Registry of Acute Aortic Dissection; MRI = magnetic resonance imaging; TAA = thoracic aortic aneurysm; TEE = transesophageal echocardiography; TGFBR = transforming growth factor β receptor
Acute and chronic aortic diseases have been diagnosed and studied by physicians for centuries. Descriptions of such pathologic conditions of the aorta as aneurysm and dissection date back as early as the 2nd century during the time of Galen, whose discoveries were largely made through studies of apes. More “recent” reports were described by Vesalius in 1557, followed by Nichols in 1732, who detailed the process of aortic dissection. In 1761, Morgagni reported detailed pathologic features of a patient with a ruptured aorta.1 However, our true understanding of aortic pathology began with the dissertation by Shennan2 in 1934, which included a description of penetrating atheromatous plaques of the thoracic aorta. His report was followed by the first successful management of aortic dissection by DeBakey in 1955.1
Since then, our knowledge of the pathologic conditions of the aorta has grown considerably and continues to evolve with ongoing research into the pathophysiology of these conditions, technological advances in the modes of detection, and improved therapeutic options. Clinical databases, such as the International Registry of Acute Aortic Dissection (IRAD), the largest current registry for acute aortic syndromes, have also contributed tremendously to our knowledge of acute aortic pathology.
For the practicing clinician, knowledge of aortic disease is paramount because patients with aortic conditions contribute significantly to the overall mortality from cardiovascular disease. This review discusses the pathophysiology, epidemiology, presentation, diagnosis, and therapeutic options for several forms of acute and chronic aortic pathology: aortic dissection (including its variants intramural hematoma [IMH] and penetrating atheromatous aortic ulcer) and thoracic aortic aneurysm (TAA). Literature searches were performed of the PubMed database using the following key words: aortic dissection, intramural hematoma, aortic ulcer, and thoracic aortic aneurysm. Retrospective and prospective studies conducted within the past 15 years, along with some data from years prior, were included in this review.
PATHOPHYSIOLOGY AND RISK FACTORS
Acute Aortic Syndromes
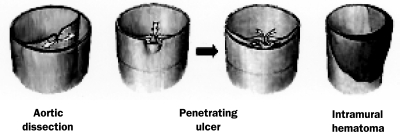
Acute aortic syndromes comprise classic aortic dissection, IMH, and penetrating atherosclerotic ulcer (Figures 1 and 2).3 The aortic wall consists of 3 layers (tunica intima, tunica media, and adventitia). Acute aortic dissection (AAD) is presumed to occur when an intimal tear develops, permitting entry of blood to a diseased underlying media characterized by elastic degeneration and smooth muscle cell loss. Chronic acquired conditions, such as systemic arterial hypertension, sometimes in combination with atherosclerosis, cause thickening and fibrosis of the intimal layer and degradation and apoptosis of smooth muscle cells in the media. These processes lead to necrosis and fibrosis of the elastic components of the arterial wall, which in turn produce wall stiffness and weakness, from which dissection and rupture may arise. Because of the high pressure of blood flow in the aorta and thus through the intimal tear, a second or “false” lumen is created, which has the potential for rapid expansion to the outer aspect of the aortic media and which may propagate proximally or distally.1,4
FIGURE 1.
Schematic of aortic dissection (left), penetrating aortic ulcer (middle), and intramural hematoma (right). Adapted from Cardiol Clin,3 with permission from Elsevier.
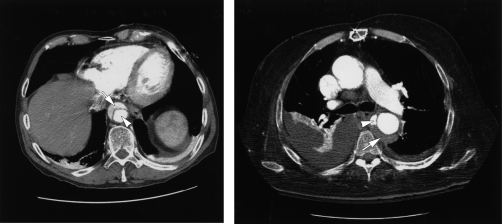
FIGURE 2.
Left, Computed tomogram showing aortic dissection with false lumen (arrow) and true lumen (arrowhead); Right, Computed tomogram showing intramural hematoma (arrow) and penetrating aortic ulcer (arrowhead).
Chronic arterial hypertension has been widely accepted as the most common acquired condition that leads to dissection of the aorta from high shear stress. Nearly 75% of patients with AAD have a history of hypertension.5,6 Other acquired conditions that have been associated with AAD include direct blunt trauma, tobacco use, hyperlipidemia, cocaine (including crack cocaine) use, and pregnancy.4 However, not all studies have shown an association between atherosclerosis and AAD. In many, the vast number of dissections occurred within the ascending aorta, where atherosclerotic disease was far less severe than in the descending aorta. In fact, some studies have suggested that the presence of widespread atherosclerosis actually limits the extent of dissection.5,7
Iatrogenic causes of AAD include cardiac procedures (eg, intra-arterial catheterization, open cardiac surgery, and intra-aortic balloon pump insertion) and appear to be more common among older patients with concomitant diabetes mellitus, chronic hypertension, and/or preexisting atherosclerosis. Using data from IRAD, Januzzi et al8 found that 5% of all cases of AAD were iatrogenic. Most of the iatrogenic type A AADs were due to cardiac surgical procedures, whereas most iatrogenic type B AADs were secondary to percutaneous cardiac procedures. Of concern is that iatrogenic aortic dissection often does not present with the classic symptoms of dissection (eg, pain), and frequently the results of imaging studies do not show “classic findings” of aortic dissection. Patients with iatrogenic aortic dissection also tend to be older, with a higher incidence of risk factors for vascular disease.8 These factors contribute to the similar mortality rates between iatrogenic and spontaneous aortic dissection.8
Rarely, pregnancy can be associated with AAD. Aortic dissections occurring in pregnant females typically occur in the third trimester but can also develop in the early postpartum period. Although their cause is still debated, such dissections are thought to occur as a result of pregnancy-related hemodynamic changes, such as increases in cardiac output and heart rate in the setting of an already weakened aortic wall from underlying connective tissue disease (discussed in next paragraph), long-standing hypertension, or pregnancy-induced hypertension. Treatment of the dissection in this patient population varies little from that of the nonpregnant population (see Management Options), with 1 exception: after 30 weeks of gestation, pregnant females with dissection involving the ascending aorta should undergo emergent Cesarean section before surgical repair of the aorta.9
The most common genetically inherited conditions that are associated with acute aortic syndromes and aneurysm formation include Marfan syndrome, Ehlers-Danlos syndrome (EDS), familial aortic dissection, and annuloaortic ectasia. Marfan syndrome is the most prevalent connective tissue disorder among the hereditary disorders, with an incidence of approximately 1 in 10,000 and an autosomal dominant mode of inheritance. Marfan syndrome, in its most common form, is caused by a sequence variation in the fibrillin (FBN) gene and has 3 main characteristics: (1) aortic aneurysms/dissections often occurring at the base of the aorta (annuloaortic ectasia), (2) impaired vision from dislocations of the lenses (ectopia lentis), and (3) long, thin extremities. As seen in all inherited forms of aortic wall disease, the deficiency in FBN results in increased breakdown of the elastic components of the aortic wall. In Marfan syndrome particularly, increased expression of metalloproteinases in vascular smooth muscle cells may further promote elastolysis (ie, the loss of elastic fibers), resulting in aortic rupture.10
Ehlers-Danlos syndrome, another connective tissue disorder that is characterized by skin hyperelasticity and hypermobile joints, can be classified into 11 types, with type IV EDS resulting from a defect in the synthesis or structure of type III procollagen. Patients with type IV EDS are at the greatest risk of aortic rupture because the normal aorta is rich in type III collagen. The incidence of EDS (1 in 5000) is greater than that of Marfan syndrome and may be slightly higher among blacks.11,12
In addition to these 3 major connective-tissue disorders, aortic dissection and aneurysm, unaccompanied by other Marfan-like properties, have been reported in family members with FBN sequence variations.13 In addition, the Loeys-Dietz syndrome, phenotypically similar to vascular EDS, was recently identified in patients with heterogeneous sequence variations in the transforming growth factor β receptors 1 and 2 (TGFBR1 and TGFBR2, respectively). Patients with Loeys-Dietz syndrome manifest TAAs and dissections in an autosomal dominant pattern of inheritance and at an early age of onset.14 Similar studies have also shown evidence for familial aortic pathology in the absence of the Marfan syndrome.15-17 Despite the subtle genetic differences between connective-tissue disorders and familial TAA/dissection syndrome, the common denominator among many of these genetic conditions is breakdown of the elastic fiber milieu, involving chromosomal loci 5q13-14 and 11q23.2-q24.18,19
Annuloaortic ectasia refers to the process in which elastolysis occurs in the aortic annulus. Such histologic changes cause weakening of the aortic wall and thus aneurysm and dissection of the ascending aorta. This pathologic process may lead to clinically important aortic regurgitation.
Cocaine use is a common cause of emergency department visits in the United States, with over 50,000 cocaine users presenting to emergency departments with symptoms of chest discomfort each year.20 Cocaine has been widely accepted as an important risk factor for cardiovascular disease through its ability to accelerate the development of atherosclerosis and to induce coronary spasm and lethal arrhythmias. Hsue et al21 reported findings indicating that more than one-third of their study patients in an inner city hospital in San Francisco, CA, with aortic dissection used cocaine minutes to hours before their presentation. However, in IRAD, the largest international registry for AAD, the percentage of patients with cocaine-related AAD is much smaller (0.5%). Most IRAD patients (as well as most patients in the study by Hsue et al) had underlying hypertension and were long-time smokers, suggesting that cocaine exacerbates the increase in shear stress that already exists from chronic hypertension, perhaps further exacerbated by repeated tobacco exposure.
After Shennan's description, Stanson et al22 further characterized penetrating atheromatous plaques of the thoracic aorta in 1986 as ulcers that destroy the immediately surrounding intima as they tunnel and dissect their way into the aortic media. A common sequela of this process includes the formation of a hematoma within the aortic wall with the potential for pseudoaneurysm formation and subsequent rupture.22,23 These lesions differ from classic aortic dissection in that nearly 90% of penetrating aortic ulcers occur in the descending thoracic aorta among patients with extensive atherosclerotic disease, whereas the degree of atherosclerotic disease in patients with dissection is variable and, in many instances, minimal.24
Intramural hematomas of the aorta result from rupture of the vaso vasorum within the medial wall, resulting in aortic infarction; in a third or more of cases, IMHs evolve into aortic dissections. Most cases of IMH occur within the descending thoracic aorta in patients with chronic systemic arterial hypertension. Like AAD, IMH may extend up or down the aorta, regress (10% of cases), and reabsorb. However, unlike classic AAD, there is no identifiable “tear” within the aortic intima; as a result, no direct communication occurs between the hematoma and the aortic lumen, unless the IMH develops into a dissection.25
Thoracic Aortic Aneurysms
Mechanisms of TAA formation overlap those of aortic dissection and largely involve the process of cystic medial necrosis, wherein focal degeneration of the elastic and muscle tissue within the tunica media of the aortic wall occurs. The aortic wall subsequently weakens and dilates as a result of the high pressure of intraluminal blood flow. Acquired and hereditary conditions can exacerbate the process of medial necrosis.
Bicuspid aortic valves are among the most common congenital heart abnormalities, occurring in approximately 2% of the population. Previous studies have established the association between congenital bicuspid aortic valves and proximal aortic dilatation, aortic aneurysm/dissection, and annuloaortic ectasia. Patients with bicuspid aortic valves may inherit a predisposition to cystic medial necrosis of the aorta. In fact, some studies report that as many as half of all patients with a congenital bicuspid aortic valve have or will develop dilatation of the ascending aorta.26-29 What is the link between congenital bicuspid aortic valves and TAAs? Although the exact mechanism has yet to be determined and is likely to be multifactorial, patients with a congenital bicuspid aortic valve may have a common genetic defect(s) that leads to weakening of the aortic wall, thus predisposing them to the development of a TAA.27,30 However, no direct correlation between thoracic aortic dilatation and aortic valve dysfunction has been established.31
Independent risk factors for rupture of a TAA include degree of maximal dilatation; rate of expansion of the aneurysm; and nondimensional characteristics, such as a history of tobacco use, presence of chronic obstructive pulmonary disease (COPD), advanced age, and the presence of hypertension. When the diameter of an aneurysm reaches 5 cm, the likelihood of rupture increases, and this risk continues to increase considerably as the aneurysm expands. In fact, the risk of rupture increases by a factor of nearly 2 for each additional centimeter of thoracoabdominal aneurysmal growth.32,33
A history of smoking is strongly associated with the development of thoracic and abdominal aortic aneurysms and is a strong predictor for aneurysmal rupture, likely secondary to the destructive effects of tobacco use on connective tissue.34 Not surprisingly, a clear link between COPD and aneurysmal rupture also exists.6
Johansson et al35 showed a marked increase in the incidence of ruptured TAAs with aging, as patients in their sixties were compared with their octogenarian counterparts. Consistent with this initial finding, subsequent studies reported a relative risk of rupture of 2.6 for each decade of age. This direct correlation between age and incidence is not sex specific; the increase is nearly identical between men and women.34
Hypertension, in particular diastolic hypertension, is widely seen among patients with aortic aneurysm, and with it comes a greater risk for rupture.34,36 Thus, aggressive management of high blood pressure in the outpatient setting is paramount, and the threshold for referral of these patients for surgical repair should be lower, especially in patients with more difficult-to-control hypertension. Finally, other causes of TAA include syphilis and infectious aortitis, both of which are extremely rare in developed countries.
CLASSIFICATION AND STAGING SYSTEMS
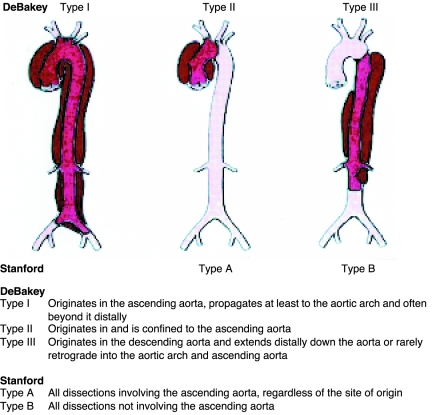
Classification schemes of aortic dissection consist of the DeBakey types and the more commonly used Stanford classification. As depicted in Figure 3, the DeBakey classification comprises 3 types of aortic dissection (I, II, and III); the Stanford classification, 2 types (A and B). Within the DeBakey system, type I denotes a dissection that involves the full span of the aorta (ascending/descending thoracic aorta and abdominal aorta); type II, a dissection from the aortic root through the level of the origin of the innominate artery; and type III, a dissection from the descending aorta (distal to the origin of the left subclavian artery) to the aortic bifurcation. Dissections in the Stanford classification are categorized based on the involvement of the ascending aorta (type A) or the lack of such involvement (type B). The latter system is more widely embraced because of its simplicity. Neither scheme provides clarity for dissections originating in or limited to the aortic arch.
FIGURE 3.
The most common classification systems of thoracic aortic dissection: Stanford and DeBakey. Adapted from Circulation,10 with permission.
EPIDEMIOLOGY
Acute Aortic Syndromes
Initial information regarding the incidence of aortic dissection and its variants was sparse and inaccurate because it was obtained from sporadic autopsy reports. Recent population-based and observational studies using international registries (most notably IRAD), however, have allowed major improvements in this regard, primarily because of improvements in diagnostic testing (see Diagnostic Modalities). Studies by Mészáros et al5 and Clouse et al37 have estimated the incidence of aortic dissection to range from 2.9 to 3.5 per 100,000 person-years among populations in Olmsted County, Minnesota, and Western Europe, respectively. Several studies from IRAD have furthered our insight into demographic characteristics of AAD. Two-thirds of patients with AAD are male, and 62% have a documented type A dissection. Furthermore, patients with a type B dissection are generally older than their type A dissection counterparts (mean of 66 vs 61 years, respectively).5,24,38 In contrast, patients with penetrating aortic ulcers and IMHs are even older, with a mean age of 77 years (reflecting the increased frequency of atherosclerosis associated with aortic ulcers) and 69 years, respectively.24,25
Nienaber et al39 evaluated differences in AAD by sex. They showed that nearly twice as many women as men older than 70 years experienced an AAD. Surprisingly, fewer women were accurately diagnosed as having an AAD within the first 24 hours of hospital presentation compared with men, despite women having a greater frequency of hypertension in the study. With the exception of age (women older than 70 years were more likely to present with AAD), few significant differences existed in the presenting signs and symptoms between men and women.
Thoracic Aortic Aneurysms
As with AAD, determining the true incidence of TAAs is challenging because many cases go undetected. In a population-based study during a 15-year period from 1980 to 1994, Clouse et al37 determined an overall age- and sex-adjusted incidence rate of 10.4 per 100,000 person-years. This value is nearly 3 times the number reported between 1951 and 1980, likely the result of improved diagnostic modalities in the past 2 decades and an aging population. Most TAAs occur in the ascending aorta or aortic arch followed by the descending thoracic aorta. The average age at the time of diagnosis is 69 years, with women being significantly older than men. The leading cause of mortality from this type of aneurysm is aortic rupture, accounting for 60% of deaths.37
CLINICAL PRESENTATION
Acute Aortic Syndromes
Patients with AAD, IMH, penetrating aortic ulcer, or even a leaking TAA present with similar signs and symptoms. Sudden onset of severe, sharp chest pain is the classic presenting symptom, occurring in 73% of patients and even more frequently among younger patients. Anterior chest pain is typical with IMH and type A dissections, whereas type B dissections are more commonly associated with back and abdominal pain, although this pattern is variable. Abruptness of onset, the most sensitive pain descriptor, is present in approximately 90% of patients. Other common presentations for type A and B dissections include syncope (13% of type A AADs) and abdominal pain (22% of type A AADs and 43% of type B AADs), which have important prognostic implications, signaling increased risks for shock, mesenteric and limb ischemia/infarction, and in-hospital mortality, partly because of delays in diagnosis. Contrary to popular thinking, the pain associated with acute dissection was most commonly described as “sharp” and instantaneous in onset rather than “tearing” or “ripping” in the largest international registry of aortic dissection.10,38,40,41
Patient demographics play an important role in how AADs present. Initially, patients younger than 40 years more commonly present without hypertension, are more likely to have a bicuspid aortic valve, and are more likely to have undergone prior aortic valve surgery.42 In younger patients, the origin of type A dissections tends to be more proximal (eg, sinuses of Valsalva and sinotubular junction).42 In contrast, elderly patients (≥70 years) are more likely to be hypertensive (≥150/90 mm Hg) at presentation, have underlying atherosclerosis, and present with a coexisting aneurysm. Furthermore, higher rates of diabetes mellitus and prior cardiac surgery occur among the elderly population.43
Physical examination findings can be helpful in making the diagnosis of AAD. Hypertension at initial hospital presentation is common; more patients with type B dissection are hypertensive (70%) than those with type A dissection (36%).38 This may be explained in part by the fact that a large number of patients with type A AAD within IRAD were younger than 40 years and had no history of hypertension.42 Many of these younger patients are thought to have a connective-tissue defect (eg, Marfan syndrome or bicuspid aortic valve) rather than hypertension as their principal underlying cause of aortic dissection. A diastolic murmur of aortic insufficiency can often be auscultated at the time of presentation or soon after and is more common in type A (44%) than in type B (12%) dissections.10,25 Pulse deficits, likewise, hold profound prognostic implications for poor outcomes (eg, neurologic deficits, altered mental status, coma, hypotension, shock, renal failure, and/or limb ischemia) but are not monitored as closely as they should be. Within IRAD, pulse deficits were detected in 30% or fewer of type A AAD cases. The most common pulse deficits were decreased or absent right brachial pulses, right femoral pulses (15%), left femoral pulses (14%), left brachial pulses (12%), and left common carotid pulses (6%). Interestingly, more than 50% of patients with pulse deficits had weak or absent pulsations in more than 1 vessel.44
A key point for clinicians is that nearly 30% of patients later found to have AAD are initially diagnosed as having other conditions. Particularly concerning is that an AAD can be misdiagnosed as an acute coronary syndrome (ACS) when the dissection extends to the coronary ostia, resulting in elevated cardiac biomarkers and dynamic electrocardiographic changes suggestive of cardiac ischemia. Still, certain symptomatic differences exist between AAD and ACS. The classic symptom of chest pain in AAD is as severe at the time of onset as it ever becomes and is often associated with a “tearing,” “ripping,” or “stabbing” sensation; chest pain associated with ACS typically has a crescendolike onset and is less severe. By taking note of these subtle differences, the clinician may avoid fatally misdiagnosing a patient's condition because the initial treatment for ACS involves anticoagulation, antiplatelet agents, and sometimes fibrinolytic agents—therapies that are contraindicated in the setting of AAD. Thus, AAD must be included in the differential diagnosis in patients presenting with unexplained syncope, chest or back pain, acute abdominal pain, sudden onset of heart failure, pulse deficits, or malperfusion syndromes.
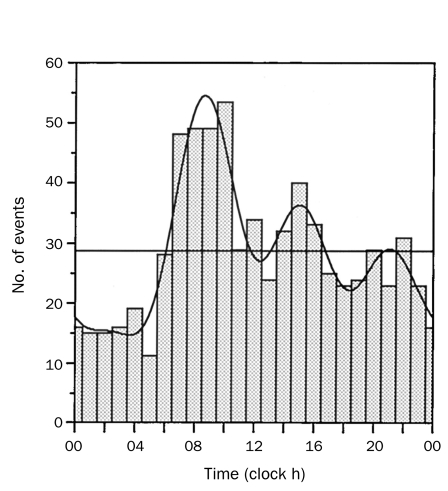
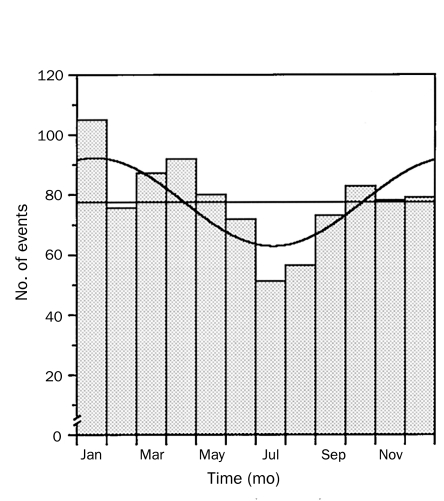
Finally, previous studies have shown that chronobiological patterns occur in a number of cardiovascular disorders, including AAD.45,46 Among 957 patients enrolled in IRAD at the time of the study, a high frequency of AAD occurred from 6:00 am to 12:00 pm, with a peak occurrence between 8:00 am and 9:00 am (Figure 4). Further analysis revealed that the frequency of AAD was higher during the winter months of October through January (most IRAD Centers are in the northern hemisphere) (Figure 5).
FIGURE 4.
Circadian variation in onset of acute aortic dissection. Histograms represent number of total events occurring in each hour of the day. Superimposed is overall best-fitting curve calculated by rhythm analysis, resulting from 4 significant harmonics with 24-, 12-, 8-, and 6-hour periods. Adapted from Circulation,45 with permission from Wolters Kluwer Health.
FIGURE 5.
Seasonal variation in onset of acute aortic dissection. Histograms represent the number of total events occurring in each month of the year. Superimposed is the overall best-fitting curve calculated by rhythm analysis resulting from a single component with a period of 8766 hours. Adapted from Circulation,45 with permission from Wolters Kluwer Health.
Thoracic Aortic Aneurysms
Thoracic aortic aneurysms often present with no symptoms and are thus typically detected on routine physical examination or on evaluations for another problem. However, severe chest or back pain is frequently reported when rupture of the aneurysm occurs. Chronic heart failure may also occur with aneurysmal dilatation of the aortic root and subsequent aortic valve regurgitation. Patients with TAAs often have a history of hypertension. Clinicians who manage patients with TAAs must be cognizant of the risk factors for TAA rupture; both the size and rate of growth of the TAA have been consistently shown to be critical in predicting rupture. Several studies have shown an increasing risk of rupture after the TAA has surpassed 5 cm in diameter.34,47,48 In fact, one study showed that the risk of rupture nearly doubles with every 1-cm increment of aneurysmal diameter.32 Subsequently, rapid rates of expansion were found to be an independent risk factor for TAA rupture.47
DIAGNOSTIC MODALITIES
In the past 2 decades, survival has improved for patients with acute aortic syndromes or TAAs as a result of technological advances in diagnostic modalities. Although often performed in patients with signs and symptoms consistent with acute aortic syndromes, more basic techniques such as electrocardiography and chest radiography can yield misleading results. Electrocardiographic findings are frequently abnormal in AAD, revealing changes suggestive of left ventricular hypertrophy, myocardial ischemia, nonspecific ST-T wave deviations, or even acute myocardial infarction. Chest radiography is helpful, with 85% of patients with acute aortic syndrome or TAA having a widened mediastinum. Other chest radiographic findings not uncommonly seen include pleural effusions and abnormal aortic/cardiac contours. It is important to note that 10% to 15% of patients with AAD have normal findings on chest radiography.39
Current diagnostic techniques for both acute aortic syndromes and TAAs center around the use of computed tomography (CT), transesophageal echocardiography (TEE), magnetic resonance imaging (MRI), and aortography. These 4 techniques provide variable information as to the site of origin, extent of dissection, classification of dissection, surrounding areas of hemorrhage if applicable, and other pathologic sequelae of the dissection.
One of the largest studies evaluating these 4 techniques found that nearly all patients with AAD (98%) undergo 1 of the 4 imaging studies, and two-thirds of patients undergo multiple imaging studies. Spiral CT, which is readily accessible in most emergency departments in the West, is less expensive, provides marked anatomic information regarding the aorta and surrounding structures, and identifies the presence of aortic rupture by revealing extravasation of blood, contrast medium, and other fluid. The sensitivity of CT scanning is 93% for both type A and type B AAD.49 Spiral CT is currently the most frequently used modality worldwide for diagnosing AAD; in nearly two-thirds of patients, spiral CT is the first diagnostic tool in making the diagnosis of AAD. Similarly, contrast-enhanced CT or CT angiography is often used when determining the degree of aneurysmal dilatation (Figure 6). Computed tomographic scanning has several limitations, particularly in diagnosing type A AAD, such as the inability to evaluate coronary artery and aortic valve involvement. Other potential problems with CT include motion artifact due to cardiac motion and streak artifact from implanted devices, making the diagnosis a greater challenge. In addition, the iodinated contrast load ranges from 80 to 120 mL per study, which can result in contrast-induced nephropathy in select patients.50
FIGURE 6.
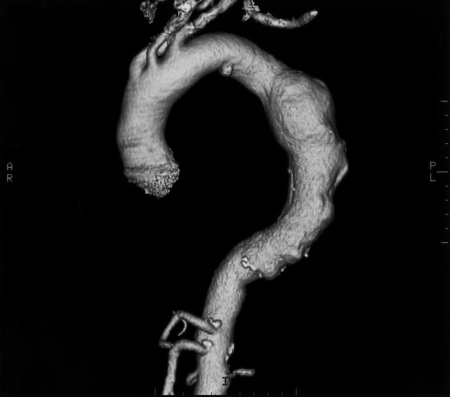
Computed tomographic angiogram showing a thoracic aortic aneurysm. AR = anterior right; PL = posterior left.
A semi-invasive procedure easily performed at the bedside, TEE is the second most commonly used initial tool in the diagnosis of AAD, with a utilization rate among patients in IRAD of nearly 33%.49 A distinct anatomic advantage of TEE over transthoracic echocardiography is the proximity of the esophagus to the thoracic aorta; this proximity allows TEE to provide clear imaging of this portion of the aorta and its surrounding structures. However, imaging of the proximal aortic arch and descending thoracic aorta with TEE is difficult because of interference from the air-filled trachea and main stem bronchus. The high sensitivity of TEE for type A AADs (90%) argues strongly for its use in patients with suspected type A AADs; with a sensitivity of only 80%, its usefulness in the diagnosis of type B AADs is less favorable. Although TEE can and often is performed quickly (10-15 minutes) in the emergent setting, it should be performed and interpreted only by a fully trained clinician (typically a cardiologist or anesthesiologist). Furthermore, TEE requires the use of conscious sedation and can cause bradycardia and elevations in systemic blood pressure as a result of patient retching and gagging. Moreover, TEE should be avoided in patients with esophageal disease (eg, varices, strictures). For these reasons, many clinicians perform transthoracic echocardiography first, followed by TEE for confirmatory purposes (28% of patients in IRAD).49 However, TEE is less useful in the diagnosis of TAAs, particularly of those in the descending thoracic aorta and proximal arch, because of the already described interpositioning of the primary airway.
Aortography and MRI are less commonly used in diagnosing acute aortic syndromes (22% and 19%, respectively), probably because of the limited availability of such technology in the emergent setting and specific contraindications (aortography is contraindicated in patients with renal failure, and MRI is contraindicated in patients with implantable devices, aneurysm clips, and other metallic implants and is often refused by patients with even mild claustrophobia). However, MRI has certain advantages: it is not associated with radiation exposure, has a high sensitivity for type A and type B AAD, is useful in detecting features of IMH and penetrating aortic ulcer, and is considered the test of choice for follow-up evaluations of patients with surgically and/or medically managed aortic disease. In addition, the safety profile of gadolinium is more favorable than that of the iodinated contrast medium used in CT, even though rare cases of renal fibrosis have been documented with gadolinium. Lastly, retrograde aortography, previously considered the test of choice for AAD, has largely been replaced by these less invasive but more accurate diagnostic modalities. Other disadvantages of aortography include the potential for false-negatives due to a thrombosed false lumen, simultaneous and equal opacification of the true and false lumen, or IMH.49-51 In comparison, aortography is considered by many to be the preferred diagnostic tool for its accuracy in defining the anatomy of a TAA. Magnetic resonance angiography is also extremely useful because it allows the evaluation of surrounding structures, such as the trachea and left main bronchus, as well as the status of main branch arteries.
The use of biomarkers has not yet become commonplace in the diagnostic armamentarium for AAD but is an area of active research. Previous studies evaluating D-dimer in the setting of AAD have revealed a negative predictive value of 100%; however, specificity is poor because elevations are also common in such conditions as pulmonary embolism and deep venous thrombosis.52 Myosin and actin constitute the major portion of the contractile apparatus of muscle tissue. Smooth-muscle myosin, a specific form of myosin found in cells of the aortic media, is released into the circulation at the time of aortic wall damage. Previous studies have shown that serum smooth-muscle myosin heavy-chain protein levels peak within 24 hours of AAD and then are rapidly cleared, analogous to serum assays done to detect an acute myocardial infarction.53,54 According to the findings of these preliminary studies, smooth-muscle myosin heavy-chain protein shows promise as a potential laboratory biomarker for the detection of aortic dissection. Further research in this area is currently ongoing and, it is hoped, will one day lead to the rapid and accurate screening of patients with suspected AAD.
MANAGEMENT OPTIONS
Acute Aortic Syndromes
The initial management of all acute aortic syndromes involves placement of the patient in an intensive care unit (ICU) or other highly monitored setting, if not immediate transfer of the patient to the operating room; blood pressure and heart rate should be intensively managed and urine output and cardiac rhythm closely observed. This is critical for both type A and B AADs, IMHs, and symptomatic penetrating aortic ulcers. Type A AADs are an emergent condition with extremely high mortality within the first 24 hours of onset if not surgically repaired within hours of hospital presentation. In contrast, type B AADs are commonly treated medically with antihypertensive medications. Surgical or endovascular repair is reserved for patients with type B AADs if pain is refractory, blood pressure control is not possible, or complications from the dissection occur, such as limb ischemia, extension of dissection, aortic rupture, evidence of periaortic leaking, and/or organ malperfusion.
Immediate Medical Therapy and Stabilization of Acute Aortic Syndromes
Beyond the type of AAD, blood pressure reduction and hemodynamic stabilization are of critical importance in the initial treatment. The goal systolic blood pressure in most patients should be between 100 and 120 mm Hg while maintaining sufficient organ perfusion (especially cerebral, cardiac, and renal). This is best achieved through the use of β-blockers and vasodilators to reduce the force of left ventricular ejection (dP/dt). Sodium nitroprusside is a frequently used vasodilator and is particularly effective when used concomitantly with intravenous β-blocking agents. Sodium nitroprusside should not be used as monotherapy because it can abruptly raise dP/dt, potentially worsening the dissection. Adequate β-blockade is achieved when the heart rate is at or close to 60 beats/min. Among β-blockers, labetalol is an attractive choice, with its combined α- and β-blocking properties and resulting ability to lower both left ventricular contractility and systemic arterial pressure. In patients with labile blood pressures and/or in those expected to undergo surgery soon, esmolol, with its short half-life of 0.13 hours, may be a more appropriate choice than other β-blockers, the half-lives of which range from 3 to 8 hours. A brief trial of esmolol may also be useful in patients with substantial COPD to test their ability to tolerate β-blockers without developing bronchospasm.
Although β-antagonists are extremely effective agents in the setting of AAD, they are contraindicated in the presence of certain conditions, including second or third degree atrioventricular block, fulminant heart failure, severe COPD with bronchospasm, and sinus bradycardia. In such instances, calcium channel antagonists may be a suitable substitute. Among them, intravenous diltiazem and verapamil are most effective with their combined negative inotropic and vasodilatory effects. Recently, Mochizuki et al55 showed that the addition of angiotensin II receptor blockers (ARBs) may decrease the risk of cardiovascular events, including AAD, in hypertensive patients. Blood pressure control alone seems unlikely to be the sole mechanism behind this finding. The benefit may result from a lowering of TGFBR activity levels, which has been seen with ARB administration in a Marfan syndrome animal model.56
Surgical Therapy in Type A (I or II) Acute Aortic Syndromes
Type A (I or II) AAD is a highly lethal condition with a mortality rate of 1% to 2% each hour after onset. At 24 hours after presentation, medical management alone carries a mortality rate of 20%; at 48 hours after presentation, 30%; and at 1 month, greater than 50%. Factors that contribute to mortality include the development of pericardial tamponade from aortic rupture into the pericardium, acute myocardial ischemia/infarction from involvement of the coronary arteries, extension of dissection into branch vessels compromising distal organ perfusion, aortic rupture into a pleural space, and aortic valve involvement resulting in acute onset heart failure. Thus, type A AAD is a true surgical emergency. Operative intervention for type A AAD aims to prevent these consequences. Similarly, patients with IMH of the ascending aorta should be treated surgically as for type A AAD because they have improved outcomes with surgery.57 Current surgical techniques include resuspension or replacement of the aortic valve if it has been disrupted by the dissection, coronary artery bypass grafting if these arteries have been compromised and/or if they harbor substantial obstruction, and resection of the intimal tear and replacement of the ascending aorta with a Dacron composite or interposition graft (Figure 7). When the dissection involves the aortic arch, partial or total arch replacement may be necessary with either retrograde or anterograde cerebral perfusion. Regardless of whether the arch is replaced, most surgeons today induce profound hypothermic circulatory arrest to perform the distal anastomosis. Despite effective surgical intervention, a mortality rate of 10% to 35% is seen at even the best centers.58 Some patients die before undergoing surgery. The risk of death without surgical intervention is significantly higher (50% vs 9%) among patients with type A AAD who are in shock at the time of presentation.59 For extremely ill, moribund patients, operative intervention may not be offered because mortality is highly likely with or without surgery. In some centers, patients with malperfusion are first treated endovascularly with fenestration and/or branch stenting and given an opportunity to stabilize before being offered surgery.60
FIGURE 7.
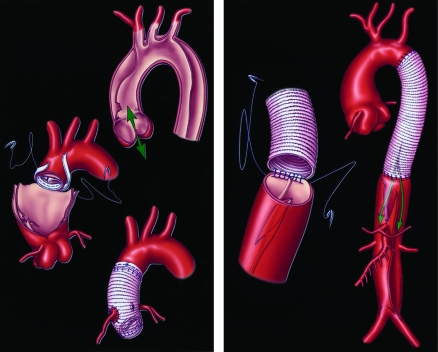
Schematic of an open surgical type aortic dissection repair for the ascending (left) and descending (right) aorta. Left, arrows represent direction of blood flow across aortic valve (anterograde or retrograde). Right, arrows represent blood flow into true and false lumens.
Surgical Therapy in Type B Acute Aortic Syndromes
Most patients with a type B (or type III) AAD and patients with IMH of the descending aorta are currently treated with aggressive medical therapy. This approach is due in equal parts to the high operative mortality rate and the relatively low mortality rate among those treated medically.24,57 Routine surgical repair has not been shown to be of substantial benefit among hemodynamically stable patients. Suzuki et al61 showed that, among the 384 patients in IRAD with type B AAD, in-hospital mortality was 13% overall and 32% for those requiring surgery. In a recent report from Estrera et al,62 the mortality rate among patients with uncomplicated type B dissection cared for in a center with particular expertise in the management of aortic disease was 1.2%. As already mentioned, aggressive medical therapy consists of β-blocking agents and other antihypertensive agents (initially, intravenous agents; later, transition to oral forms) to maintain a systolic blood pressure of less than 120 mm Hg and a low heart rate. Surgical intervention is reserved for patients with complications arising from the dissection, such as limb ischemia, malperfusion syndrome, and rupture. Other indications for surgery include refractory pain, progression of the dissection, aneurysm expansion, and refractory hypertension.
Medical management is successful in most cases of type B dissection. In a recently reported series of 476 patients with type B AAD, 82 were surgically treated. The most common reasons for surgical repair were recurrent pain and extension of dissection compromising distal arteries. Nearly one-third of patients had more than 1 indication for surgery. Replacement of the descending aorta occurred in 70% of surgical patients. Other procedures included partial or complete replacement of the aortic arch, aortic fenestration, and stenting (see Endovascular Therapy for Acute Aortic Syndromes). Normotension at the time of surgery and reduced hypothermic circulatory arrest time were associated with more favorable outcomes after surgery. Independent predictors of surgical mortality were age older than 70 years and preoperative shock/hypotension.63
Endovascular Therapy for Acute Aortic Syndromes
In the current era, endovascular therapy with percutaneous stent-graft placement and/or aortic fenestration has become more common for the treatment of type B AAD, penetrating aortic ulcer, and IMH. Endovascular repair provides patients who are not surgical candidates or who refuse surgical repair a second option that may retard the natural history of the acute aortic syndrome. Among patients with type A AAD, endovascular procedures (eg, fenestration) relieve malperfusion by decompressing the false lumen (equalizing pressures between the true and false lumens) and permitting re-expansion of the true lumen before definitive surgical repair. Stent placement in the true lumen seeks to redirect flow into the true lumen and to facilitate closure of the entry tear into the false lumen; closure of the entry tear induces thrombosis of the false lumen and, in turn, may promote long-term effective healing of the involved aortic segment.
The US Food and Drug Administration has not yet approved any endovascular stent grafts for treatment of aortic dissection in the acute or chronic phase. Such stent grafts, however, have been used in Europe and off-label in the United States. Currently, indications for percutaneous fenestration and/or stent-graft placement include the obstruction of aortic branch arteries, descending dissection with intractable pain, extravasation of blood from the aorta (periaortic hematoma) as a sign of impending rupture, or a rapidly expanding false lumen. If attempts at endovascular repair are unsuccessful, open surgical repair may then be necessary.
In appropriately selected patients, stent-graft placement for the treatment of thoracic AAD has been shown to be promising. In a small retrospective analysis, Nienaber et al64 found that, among patients with type B AAD, stent-graft recipients had lower morbidity and mortality than patients requiring surgery. Another study found that death among patients who underwent stent-graft placement was rarely directly related to the endovascular procedure but rather due to nonreversible ischemic changes compounded by patients' comorbid conditions.65 The same can be said of deaths after cardiovascular surgery; however, stent enthusiasts argue that the need to cross-clamp the aorta during open aortic replacement compounds the ischemic injury to organs that have already been malperfused. Further study with appropriate controls is needed to evaluate these treatment modalities.
Thoracic Aortic Aneurysms
The classic therapy for TAAs is surgical repair. Although the optimal timing of surgery is difficult to predict, most experts advocate surgical intervention when aneurysms of the ascending aorta reach 5.5 to 6.0 cm in diameter and those of the descending aorta reach 6.0 to 6.5 cm in diameter. Circumstances in which operative intervention may be required sooner include rapidly expanding aneurysms, associated aortic regurgitation, and/or the presence of aneurysm-associated symptoms. Patients with Marfan syndrome, familial TAA syndrome, or bicuspid aortic valve are often referred for surgical repair sooner (ie, when the aneurysm reaches 4.5-5.0 cm in diameter) because of their increased risk of aortic dissection and/or rupture at smaller aortic sizes and the relatively low risk of surgery in such patients treated in aortic centers of excellence.
Surgical repair of TAAs involves resection of the aneurysm with an appropriately sized prosthetic graft. If the root and valve are spared, a simple tube graft may suffice. If the aortic root is involved, composite root replacement with a mechanical-valve conduit, the so-called Bentall procedure, may be performed. This procedure requires reimplantation of the coronary arteries into the Dacron graft. Biological alternatives include stentless xenografts and human allografts.
Involvement of the aortic arch presents particular challenges because blood flow to the brain via the brachiocephalic vessels must be interrupted during the repair. Partial or “hemiarch” replacement will often suffice, with replacement of the underside of the arch and a single beveled distal anastomosis. If more extensive arch disease is present, total arch replacement may be required, with construction of the distal anastomosis beyond the left subclavian artery at the level of the proximal descending thoracic aorta or beyond. The brachiocephalic vessels are then reimplanted into the graft as a single button or island, or they may be individually reconstructed with a branched graft. Hypothermic circulatory arrest is required for at least a short period. Adjuncts, such as retrograde cerebral perfusion via the superior vena cava or selective antegrade perfusion of the brachiocephalic vessels, may extend the period of safe arrest or reduce the degree of hypothermia required to protect the brain.
Elective surgical repair of ascending aortic aneurysms can be accomplished in experienced centers with mortality rates less than 5%.66 The risks associated with descending thoracic aortic repair are 2-fold higher,67 and those for extensive thoracoabdominal aortic disease are double that.68 Complications from surgical repair include hemorrhage, postoperative paraplegia from spinal cord ischemia for descending thoracic repair, and stroke from cerebral ischemia during arch repairs. These potentially debilitating and/or fatal complications have prompted the exploration of less invasive therapeutic techniques, such as percutaneous stent-graft placement, especially for descending aortic disease (Figure 8). Dake et al69 studied the usefulness of stent grafts in patients with descending TAAs. Rates of stroke and paraplegia were 3% and 7%, respectively. Czerny et al70 studied graft durability, showing that overall survival among recipients of stent grafts was 96%, 86%, and 69% at 1-, 3-, and 5-year follow-up, respectively, with event-free survival rates of 90%, 82%, and 65%.
FIGURE 8.
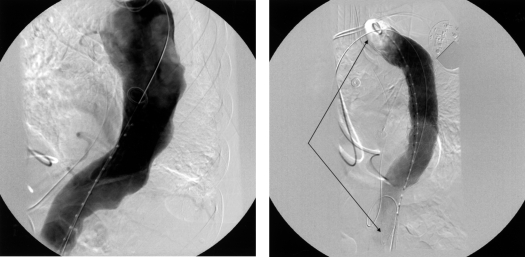
Aortogram showing a thoracic aortic aneurysm before (left) and after (right) stent-graft placement, with arrows showing the extent of the stent graft.
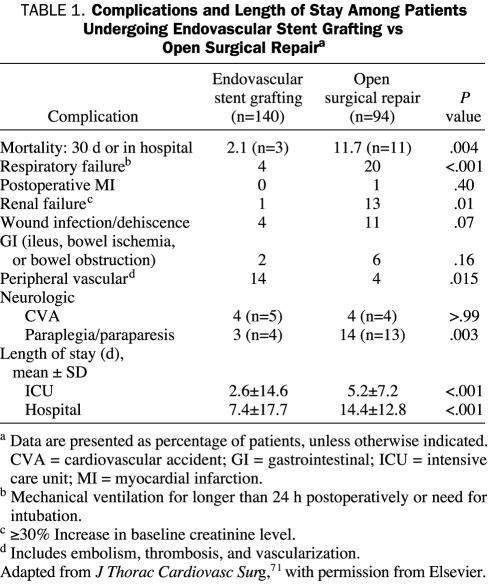
Most recently, a head-to-head multicenter phase 2 trial compared endovascular stent grafting with open surgical repair of TAAs. The study assigned the 140 patients in the endograft group to receive the TAG Thoracic Endoprosthesis (W. L. Gore and Associates, Flagstaff, AZ); the remaining 94 study patients underwent open surgical repair. Recipients of the endograft were also candidates for open surgery. Early mortality in the endograft group was 2% vs nearly 12% in the open surgery group (P<.001). Patients in the endovascular group had significantly lower rates of post-procedure spinal cord ischemia, paraplegia, respiratory failure, and renal insufficiency. Recipients of endovascular stent grafting had significantly shorter ICU and hospital stays. However, patients with endovascular therapy had significantly more peripheral vascular complications, including vascular trauma and thrombosis (Table 1).71 Two-year survival was virtually identical at 76% and 78% in the open surgical group and endovascular group, respectively; neither group had aneurysmal rupture. At 2-year follow-up, complications involving stent-graft placement included endoleaks, stent migration and fracture, and aortic reoperation; however, these complications were rare.71 Thus, endovascular repair of TAAs appears to be a viable therapeutic option and will surely improve with continuing technological advances.
TABLE 1.
Complications and Length of Stay Among Patients Undergoing Endovascular Stent Grafting vs Open Surgical Repaira
OUTCOMES, FOLLOW-UP, AND SURVEILLANCE
Acute Aortic Syndromes
In-hospital mortality rates of patients with type A AAD are high, with nearly 1 in 3 patients dying of aortic rupture or other complications. Independent predictors of in-hospital death for patients with type A AAD include age 70 years or greater, female sex, abrupt onset of pain on presentation, new Q waves and/or ST-segment deviation on electrocardiography at presentation, pulse deficit(s) on presentation, kidney failure on presentation and before surgery, and hypotension/shock/tamponade on presentation.70,72 Within IRAD, 90% of patients with type A AAD were managed surgically, with the remaining 10% managed medically. Of the 10% managed medically, 68% did not undergo surgery because they refused, were advanced in age, and/or had comorbid conditions that precluded surgical management. Mean survival of patients with type A AAD managed medically was 2.4 years. Patients who were surgically managed and survived to hospital discharge had a significantly lower follow-up mortality rate of 14% vs 37% for those managed medically.73
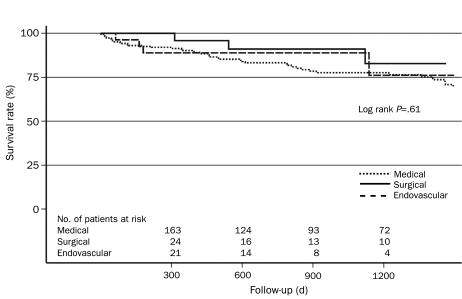
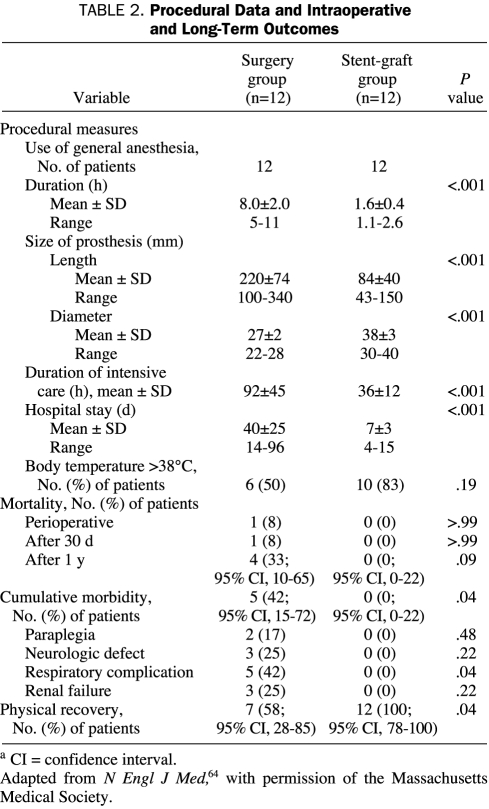
Among patients with type B AAD in IRAD, 78% of patients received aggressive medical therapy only, 11% underwent surgery, and 11% underwent endovascular repair. Recurrent pain, extension of dissection, and uncontrollable hypertension were cited as reasons for surgery or endovascular therapy in 26% of cases, whereas limb ischemia and visceral ischemia were cited in 28% and 30% of cases, respectively. In-hospital mortality was 29%, 11%, and 10% for patients undergoing surgery, endovascular therapy, and medical therapy, respectively. The unadjusted survival rate ± SD at 1 and 3 years for patients was 90.3%±4.3% and 77.6%±6.6% for medical therapy alone, 95.8%±8.0% and 82.8%±18.9% for surgery, and 88.9%±11.9% and 76.2%±25.2% for endovascular repair (Figure 9).74 Nienaber et al64 prospectively compared 24 patients with thoracic AADs who underwent either endovascular stent-graft placement or open surgery. Recipients of surgical repair had significantly longer hospital stays (mean ± SD, 40±24 days vs 7±3 days), including longer stays in the ICU, and significantly more cumulative morbidity, including paraplegia, neurologic deficits, respiratory complications, and renal failure (Table 2).
FIGURE 9.
Unadjusted Kaplan-Meier survival curve stratified by in-hospital management. Adapted from Circulation,74 with permission from Wolters Kluwer Health.
TABLE 2.
Procedural Data and Intraoperative and Long-Term Outcomes
Patients with IMH have early and late outcomes similar to those of patients with classic AAD. Using the largest international database for acute aortic syndromes, Evangelista et al25 showed an overall in-hospital mortality rate for IMH of nearly 21% vs 24% for classic AAD. Intramural hematoma of the ascending aorta (type A) was associated with an in-hospital mortality rate of 39%, whereas IMH not involving the ascending aorta (type B) was associated with an in-hospital mortality rate of 8%. The in-hospital mortality rate of patients who underwent surgery for a type A IMH was 43% compared with 33% for patients who underwent medical therapy alone. Thus, IMH that involves the ascending aorta is of considerable concern. The high mortality rates observed in this retrospective study can likely be explained by delays in surgical management from watchful waiting, delays in diagnosis, and the fact that patients who underwent surgery likely represented the sickest patients with lethal complications from the IMH, such as tamponade and shock. Nonetheless, because of the high mortality rates of type A IMH and the fact that nearly 20% of cases of IMH involving the ascending aorta evolve into aortic dissection, a timely surgical approach is warranted. Common reasons for in-hospital mortality among all patients with IMH included neurological sequelae, visceral ischemia, and aortic rupture. Long-term follow-up revealed that 37% of patients with type A IMH were likely to die of any cause and 25% were likely to have a new aortic aneurysm or dissection within 3 years. Long-term follow-up of patients with type B IMH showed that 21% were likely to die within 3 years of initial diagnosis. The best predictor of IMH regression without complications was a normal aortic diameter in the acute phase.25
Outcome data regarding patients with penetrating atherosclerotic ulcers of the descending aorta reflect changing patterns in how these patients are managed. Cho et al75 performed a retrospective study from 1 tertiary medical facility involving patients diagnosed as having penetrating aortic ulcer. From 1977 to 2002, a noticeable trend was seen in which patients with aortic ulcers of the descending aorta were less likely to undergo surgical therapy and were more likely to receive aggressive medical therapy. Cited reasons to undergo surgical repair included aortic rupture, rapidly increasing aortic size, and refractory pain. Early (30-day) mortality was 4% in the medically treated group and 21% in the surgical group. Surgical mortality increased during the period from 1990 to 2002 to nearly 40% owing to a more selective criterion for surgery (ie, only patients refractory to medical therapy underwent surgery). Most frequent causes of early mortality among surgical cases included bleeding, multiorgan failure, and intraoperative cardiac arrest, whereas those for recipients of medical therapy alone included aortic rupture with patient refusal of surgery and sepsis. Predictors of early surgery included a diagnosis before 1990 and aortic rupture at the time of diagnosis. Late mortality at a median follow-up of 46 months was 78% for patients who underwent surgical repair of the aorta and 42% for recipients of medical therapy alone. Causes of late mortality in both groups included primarily aorta-related and other cardiovascular disease. This single-center study substantiated the claim that a considerable number of patients with penetrating aortic ulcer can be emergently treated nonsurgically. However, because of the potential for complications, such as aortic rupture and development of a saccular aneurysm, patients receiving medical therapy should be closely monitored because they may require early or late surgical intervention.75
The clinician who manages patients with acute aortic syndromes long-term must have the following understanding: AAD is a systemic illness that involves the entire aorta and its branches, and patients with an AAD are at lifelong risk of subsequent dissection, aneurysm, and rupture and should be closely monitored for the remainder of their lives. Systemic arterial hypertension, advanced age, aortic size, and presence of a patent false lumen, in addition to genetically inherited connective-tissue disorders, all contribute to the prediction of long-term risk. Thus, such modifiable risk factors as hypertension must be aggressively managed with a goal blood pressure of less than 120/80 mm Hg. This can best be achieved with β-blockers and other potent antihypertensive agents. Currently, studies evaluating angiotensin-converting enzyme (ACE) inhibitors, calcium-channel antagonists, and diuretics for the secondary prevention of aortic disease are lacking. However, an emerging role for ARBs in the prevention of AAD was recently demonstrated in a Japanese study.55 In addition, ARBs have been shown to reduce aortic dilatation in an animal model of Marfan syndrome.56 Each patient should receive therapy with effective antihypertensive agents unless contraindications to a particular class of drugs exist. Frequently, patients with chronic aortic disease require multiple drugs for tight blood pressure control, just as in patients with uncomplicated essential hypertension.
Serial imaging is a critical aspect of follow-up care in the outpatient setting. Choice of imaging may vary on the basis of institutional availability or patient preference, but clinicians should generally use such noninvasive techniques as CT or MRI because they have similarly high sensitivity and specificity. Experts recommend follow-up imaging and examinations at 1, 3, 6, 9, and 12 months after hospital discharge and not less than annually thereafter.76 Imaging should at first span the length of the entire aorta, not just the area of initial insult, because further dissection and aneurysm can occur anywhere along the length of the vessel. Particular vigilance is required in patients with patent false lumens of the aorta, especially those with partial thrombosis.77,78 Such patients appear to be at significantly increased risk of short-term (within 1-3 years) expansion and rupture.
Thoracic Aortic Aneurysms
A retrospective study of 721 patients with nonsurgically treated TAAs was conducted during a 9-year period. Aneurysms in the descending or thoracoabdominal regions had significantly higher growth rates than those in the ascending aorta or aortic arch. Besides anatomic location, other factors associated with increased risk of rupture included initial aortic size of 6.0 cm or greater, presence of Marfan syndrome, ongoing smoking, history of stroke or coronary artery disease, and abdominal aortic aneurysm. Interestingly, female sex was associated with a higher risk of rupture.79
Long-term survival of patients with TAAs who do not undergo surgery is poor, with a 5-year survival of 56% among patients with aneurysms greater than 6 cm in diameter. Patients who undergo elective surgical repair have an approximately 85% 5-year survival rate compared with 66% and 37% survival rates for patients who undergo medical therapy alone and emergent surgery, respectively. Not surprisingly, patients with nondissecting aneurysms have better survival rates than those with dissected aortas.79 As in the case of patients with AAD, close follow-up of patients with TAAs is imperative. Because growth rates of TAAs vary among patients, there is no specific time interval for when serial imaging should be done. However, experts recommend imaging with either CT or magnetic resonance angiography every 6 months initially to assess for aneurysmal growth. Previous studies have shown the efficacy of β-blockers in retarding the rate of aortic dilatation, decreasing adverse end points (aortic dissection, chronic heart failure), and decreasing mortality.80,81 Although these studies specifically evaluated patients with Marfan syndrome, logic would argue that aggressive hypertension management would be beneficial to all patients with TAA to reduce dP/dt and systemic arterial blood pressure. In addition, because TAA is closely linked to atherosclerotic disease, outpatient medical management must also include aggressive lipid management with a goal low-density lipoprotein cholesterol level of less than 70 mg/dL (to convert to mmol/L, multiply by 0.0259).82,83 Finally, smoking cessation is essential for smokers if long-term risk is to be reduced.
CONCLUSION
Diseases of the aorta have been studied for centuries, but successful management has only been available in the past 5 decades. Studies have furthered our knowledge of acquired and inherited risk factors and pathophysiology of both acute aortic syndromes and TAAs. Substantial advances in diagnostic modalities have allowed the diagnosis of scores of patients in a timelier manner; previously, the diagnosis was not made until autopsy. However, improvements are still needed, particularly a more timely diagnosis and advances in therapeutic options with a focus on the appropriate role for percutaneous endovascular treatments. Clinicians must be aware of the clinical burden, risk factors, and signs and symptoms of aortic conditions so that proper detection of these illnesses can occur and close monitoring for both primary and secondary prevention can be performed.
Supplementary Material
On completion of this article, you should be able to (1) describe the pathophysiology and major risk factors for acute aortic syndromes and thoracic aortic aneurysm, (2) identify the common clinical presentations of patients with acute aortic syndromes and ruptured thoracic aortic aneurysm, and (3) list the major imaging modalities to diagnose acute aortic syndromes and thoracic aortic aneurysm, along with their respective advantages and disadvantages.
Footnotes
Dr Sundt receives grants for clinical or laboratory research support from Atricure, Boehringer Ingelheim, Bolton Medical, Edwards Lifesciences, Jarvik Heart, Sorin Group/Carbomedics, St Jude Medical, Thoratec Corporation, Ventracor, and W. L. Gore and Associates. The Division of Cardiovascular Diseases, Mayo Clinic, Rochester, MN, also holds technology licensing agreements with St Jude Medical and the Sorin Group. Dr Eagle received grant/research support from Biosite, Bristol-Myers Squibb, Blue Cross Blue Shield of Michigan, the Hewlett Foundation, the Mardigian Fund, Pfizer, sanofiaventis, and the Varbedian Fund. Dr Eagle is also a consultant for the National Institutes of Health National Heart, Lung, and Blood Institute; Pfizer; sanofiaventis; the Robert Wood Johnson Foundation; and Affinia.
CME Questions About Childhood and Adolescent Vaccines
This activity was designated for 1 AMA PRA Category 1 Credit(s).™
The contributions to the Symposium on Cardiovascular Diseases are now a CME activity. For CME credit, see the link on our Web site at mayoclinicproceedings.com.
REFERENCES
- 1.Svensson LG, Crawford ES. Cardiovascular and Vascular Disease of the Aorta Philadelphia, PA: WB Saunders Co; 1997:1-6 [Google Scholar]
- 2.Shennan T. Dissecting aneurysms. Medical Research Council Special Report Series, No. 193 London, England: His Majesty's Stationery Office; 1934. [Google Scholar]
- 3.Coady MA, Rizzo JA, Elefteriades JA. Pathologic variants of thoracic aortic dissections: penetrating atherosclerotic ulcers and intramural hematomas. Cardiol Clin. 1999;17(4):637-657 [DOI] [PubMed] [Google Scholar]
- 4.Nienaber CA. Pathophysiology of acute aortic syndromes. In: Baliga RR, Nienaber CA, Isselbacher EM, Eagle KA, eds. Aortic Dissection and Related Syndromes New York, NY: Springer; 2007:17-43 [Google Scholar]
- 5.Mészáros I, Mórocz J, Szlávi J, et al. Epidemiology and clinicopathology of aortic dissection: a population-based longitudinal study over 27 years. Chest 2000;117:1271-1278 [DOI] [PubMed] [Google Scholar]
- 6.Cronenwett JL, Murphy TF, Zelenock GB, et al. Actuarial analysis of variables associated with rupture of small abdominal aortic aneurysm. Surgery 1985;98(3):472-483 [PubMed] [Google Scholar]
- 7.Roberts WC. Aortic dissection: anatomy, consequences and causes. Am Heart J. 1981;101(2):195-214 [DOI] [PubMed] [Google Scholar]
- 8.Januzzi JL, Sabatine MS, Eagle KA, et al. International Registry of Aortic Dissection Investigators Iatrogenic aortic dissection. Am J Cardiol. 2002;89:623-626 [DOI] [PubMed] [Google Scholar]
- 9.Abbas AE, Lester SJ, Connolly H. Pregnancy and the cardiovascular system. Int J Cardiol. 2005;98(2):179-189 [DOI] [PubMed] [Google Scholar]
- 10.Nienaber CA, Eagle KA. Aortic dissection: new frontiers in diagnosis and management, part I: from etiology to diagnostic strategies. Circulation 2003;108(5):628-635 [DOI] [PubMed] [Google Scholar]
- 11.Prockop DJ, Kuivaniemi H, Tromp G. Inherited disorders of connective tissue. In: Fauci AS, Braunwald E, Isselbacher KJ, et al., eds. Harrison's Principles of Internal Medicine 14th ed.New York, NY; McGraw-Hill; 1998:2183-2191 [Google Scholar]
- 12.Steinmann B, Royce P, Superti-Furga A. The Ehlers-Danlos syndrome. In: Royce PM, Steinmann B, eds. Connective Tissue and its Heritable Disorders: Molecular, Genetic, and Medical Aspects 2nd ed.New York, NY: Wiley-Liss; 1993:351-407 [Google Scholar]
- 13.Milewicz DM, Chen H, Park ES, et al. Reduced penetrance and variable expressivity of familial thoracic aortic aneurysms/dissections. Am J Cardiol. 1998;82(4):474-479 [DOI] [PubMed] [Google Scholar]
- 14.Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF- β receptor. N Engl J Med. 2006;355(8):788-798 [DOI] [PubMed] [Google Scholar]
- 15.Nicod P, Bloor C, Godfrey M, et al. Familial aortic dissecting aneurysm. J Am Coll Cardiol. 1989;13(4):811-819 [DOI] [PubMed] [Google Scholar]
- 16.Hanley WB, Jones NB. Familial dissecting aortic aneurysm: a report of three cases within two generations. Br Heart J. 1967;29(6):852-858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis RA, Merin LM. Iris flocculi and familial aortic dissection. Arch Ophthalmol 1995;113(10):1330-1331 [DOI] [PubMed] [Google Scholar]
- 18.Guo D, Hasham S, Kuang SO, et al. Familial thoracic aortic aneurysms and dissections: genetic heterogeneity with a major locus mapping to 5q13-14. Circulation 2001;103(20):2461-2468 [DOI] [PubMed] [Google Scholar]
- 19.Vaughan CJ, Casey M, He J, et al. Identification of a chromosome 11q23.2-q24 locus for familial aortic aneurysm disease, a genetically heterogeneous disorder. Circulation 2001;103(20):2469-2475 [DOI] [PubMed] [Google Scholar]
- 20.Eagle KA, Isselbacher EM, DeSanctis RW, International Registry for Aortic Dissection (IRAD) Investigators Cocaine-related aortic dissection in perspective [editorial]. Circulation 2002;105(13):1529-1530 [DOI] [PubMed] [Google Scholar]
- 21.Hsue PY, Salinas CL, Bolger AF, Benowitz NL, Waters DD. Acute aortic dissection related to crack cocaine. Circulation 2002;105(13):1592-1595 [DOI] [PubMed] [Google Scholar]
- 22.Stanson AW, Kazmier FJ, Hollier LH, et al. Penetrating atherosclerotic ulcers of the thoracic aorta: natural history and clinicopathologic correlations. Ann Vasc Surg. 1986;1(1):15-23 [DOI] [PubMed] [Google Scholar]
- 23.Cooke JP, Kazmier FJ, Orszulak TA. The penetrating aortic ulcer: pathologic manifestations, diagnosis, and management. Mayo Clin Proc. 1988;63(7):718-725 [DOI] [PubMed] [Google Scholar]
- 24.Coady MA, Rizzo JA, Hammond GL, Pierce JG, Kopf GS, Elefteriades JA. Penetrating ulcer of the thoracic aorta: what is it? How do we recognize it? How do we manage it? J Vasc Surg 1998;27(6):1006-1015 [DOI] [PubMed] [Google Scholar]
- 25.Evangelista A, Mukherjee D, Mehta RH, et al. International Registry of Aortic Dissection (IRAD) Investigators Acute intramural hematoma of the aorta: a mystery in evolution. Circulation 2005March1;111(8):1063-1070 Epub 2005 Feb 14 [DOI] [PubMed] [Google Scholar]
- 26.Gore I. Dissecting aneurysms of the aorta in persons under forty years of age. AMA Arch Pathol. 1953;55(1):1-13 [PubMed] [Google Scholar]
- 27.Pachulski RT, Weinberg AL, Chan KL. Aortic aneurysm in patients with functionally normal or minimally stenotic and bicuspid aortic valve. Am J Cardiol. 1991;67(8):781-782 [DOI] [PubMed] [Google Scholar]
- 28.McKusick VA. Association of congenital bicuspid aortic valve and Erdheim's cystic medial necrosis. Lancet 1972;1(7758):1026-1027 [DOI] [PubMed] [Google Scholar]
- 29.Nistri S, Sorbo MD, Marin M, Palisi M, Scognamiglio R, Thiene Gl. Aortic root dilatation in young men with normally functioning bicuspid aortic valves. Heart 1999;82(1):19-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huntington K, Hunter AG, Chan KL. A prospective study to assess the frequency of familial clustering of congenital bicuspid aortic valve. J Am Coll Cardiol. 1997;30:1809-1812 [DOI] [PubMed] [Google Scholar]
- 31.Hahn RT, Roman MJ, Mogtader AH, Devereux RB. Association of aortic dilatation with regurgitant, stenotic and functionally normal bicuspid aortic valves. J Am Coll Cardiol. 1992;19(2):283-288 [DOI] [PubMed] [Google Scholar]
- 32.Juvonen T, Ergin MA, Galla JD, et al. Prospective study of the natural history of thoracic aortic aneurysms [published correction appears in Ann Thorac Surg. 1997;64(2):594]. Ann Thorac Surg 1997;63(6):1533-1545 [DOI] [PubMed] [Google Scholar]
- 33.Masuda Y, Takanashi K, Takasu J, Morooka N, Inagaki Y. Expansion rate of thoracic aortic aneurysms and influencing factors. Chest 1992;102(2):461-466 [DOI] [PubMed] [Google Scholar]
- 34.Griepp RB, Ergin MA, Galla JD, et al. Natural history of descending thoracic and thoracoabdominal aneurysms. Ann Thorac Surg. 1999;67(6):1927-1930 [DOI] [PubMed] [Google Scholar]
- 35.Johansson G, Markström U, Swedenborg J. Ruptured thoracic aortic aneurysms: a study of incidence and mortality rates. J Vasc Surg. 1995;21(6):985-988 [DOI] [PubMed] [Google Scholar]
- 36.Dapunt OE, Galla JD, Sadeghi AM, et al. The natural history of thoracic aortic aneurysms. J Thorac Cardiovasc Surg. 1994;107(5):1323-1333 [PubMed] [Google Scholar]
- 37.Clouse WD, Hallett JW, Jr, Schaff HV, et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc. 2004;79(2):176-180 [DOI] [PubMed] [Google Scholar]
- 38.Hagan PG, Nienaber CA, Isselbacher EM, et al. The international registry of acute aortic dissection (IRAD): new insights into an old disease. JAMA 2000;283(7):897-903 [DOI] [PubMed] [Google Scholar]
- 39.Nienaber CA, Fattori R, Mehta RH, et al. International Registry of Acute Aortic Dissection Gender-related differences in acute aortic dissection. Circulation 2004June22;109(24):3014-3021 Epub 2004 Jun 14 [DOI] [PubMed] [Google Scholar]
- 40.Henke PK, Williams DM, Upchurch GR, et al. Acute limb ischemia associated with type B aortic dissection: clinical relevance and therapy. Surgery 2006October;140(4):532-539 Epub 2006 Sep 1 [DOI] [PubMed] [Google Scholar]
- 41.Nallamothu BK, Mehta RH, Saint S, et al. Syncope in acute aortic dissection: diagnostic, prognostic, and clinical implications. Am J Med. 2002;113(6):468-471 [DOI] [PubMed] [Google Scholar]
- 42.Januzzi JL, Isselbacher EM, Fattori R, et al. Characterizing the young patient with aortic dissection: results from the international registry of aortic dissection (IRAD). J Am Coll Cardiol. 2004;43(4):665-669 [DOI] [PubMed] [Google Scholar]
- 43.Mehta RH, O'Gara PT, Bossone E, et al. International Registry of Acute Aortic Dissection (IRAD) Investigators Acute type A aortic dissection in the elderly: clinical characteristics, management, and outcomes in the current era. J Am Coll Cardiol 2002;40(4):685-692 [DOI] [PubMed] [Google Scholar]
- 44.Bossone E, Rampoldi V, Nienaber CA, et al. International Registry of Acute Aortic Dissection (IRAD) Investigators Usefulness of pulse deficit to predict in-hospital complications and mortality in patients with acute type A aortic dissection. Am J Cardiol. 2002;89(7):851-855 [DOI] [PubMed] [Google Scholar]
- 45.Mehta RH, Manfredini R, Hassan F, et al. International Registry of Acute Aortic Dissection (IRAD) Investigators Chronobiological patterns of acute aortic dissection. Circulation 2002;106(9):1110-1115 [DOI] [PubMed] [Google Scholar]
- 46.Manfredini R, Portaluppi F, Zamboni P, Salmi R, Gallerani M. Circadian variation in spontaneous rupture of abdominal aorta [letter]. Lancet 1999;353(9153):643-644 [DOI] [PubMed] [Google Scholar]
- 47.Cambria RA, Glovicki P, Stanson AW, et al. Outcome and expansion rate of 57 thoracoabdominal aortic aneurysms managed nonoperatively. Am J Surg. 1995;170(2):213-217 [DOI] [PubMed] [Google Scholar]
- 48.Lobato AC, Puech-LeLeão P. Predictive factors for rupture of thoracoabdominal aortic aneurysm. J Vasc Surg. 1998;27(3):446-453 [DOI] [PubMed] [Google Scholar]
- 49.Moore AG, Eagle KA, Bruckman D, et al. Choice of computed tomography, transesophageal echocardiography, magnetic resonance imaging, and aortography in acute aortic dissection: International Registry of Acute Aortic Dissection (IRAD). Am J Cardiol. 2002;89(10):1235-1238 [DOI] [PubMed] [Google Scholar]
- 50.Mügge A, Daniel WG, Laas J, Grote R, Lichtlen PR. False-negative diagnosis of proximal aortic dissection by computed tomography or angiography and possible explanations based on transesophageal echocardiographic findings. Am J Cardiol 1990;65(7):527-529 [DOI] [PubMed] [Google Scholar]
- 51.Muller G, Kazerooni E. CT evaluation of aortic dissection. In: Baliga RR, Nienaber CA, Isselbacher EM, Eagle KA, eds. Aortic Dissection and Related Syndromes New York, NY: Springer; 2007:87-121 [Google Scholar]
- 52.Weber T, Auer J, Eber B, Nienaber CA, Eagle KA. Value of D-dimer testing in acute aortic dissection [letter and response]. Circulation 2004;109(3):E24 [PubMed] [Google Scholar]
- 53.Katoh H, Suzuki T, Yokomori K, et al. A novel immunoassay of smooth muscle myosin heavy chain in serum. J Immunol Methods 1995;185(1):57-63 [DOI] [PubMed] [Google Scholar]
- 54.Katoh H, Suzuki T, Hiroi Y, et al. Diagnosis of aortic dissection by immunoassay for circulating smooth-muscle myosin [letter]. Lancet 1995;345(8943):191-192 [DOI] [PubMed] [Google Scholar]
- 55.Mochizuki S, Dahlöf B, Shimizu M, et al. Jikei Heart Study Group Valsartan in a Japanese population with hypertension and other cardiovascular disease (Jikei Heart Study): a randomised, open-label, blinded endpoint morbidity-mortality study. Lancet 2007;369(9571):1431-1439 [DOI] [PubMed] [Google Scholar]
- 56.Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 2006;312(5770):117-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maraj R, Rerkpattanapipat P, Jacobs LE, et al. Meta-analysis of 143 reported cases of aortic intramural hematoma. Am J Cardiol 2000;86(6):664-668 [DOI] [PubMed] [Google Scholar]
- 58.Nienaber CA, Eagle KA. Aortic dissection: new frontiers in diagnosis and management, Part II: therapeutic management and follow-up. Circulation 2003;108(6):772-778 [DOI] [PubMed] [Google Scholar]
- 59.Long SM, Tribble CG, Raymond DP, et al. Preoperative shock determines outcome for acute type A aortic dissection. Ann Thorac Surg 2003;75(2):520-524 [DOI] [PubMed] [Google Scholar]
- 60.Deeb GM, Williams DM, Bolling SF, et al. Surgical delay for acute type A dissection with malperfusion. Ann Thorac Surg. 1997;64(6):1669-1675 [DOI] [PubMed] [Google Scholar]
- 61.Suzuki T, Mehta RH, Ince H, et al. Clinical profiles and outcomes of acute type B aortic dissection in the current era: lessons from the International Registry of Aortic Dissection (IRAD). Circulation 2003;108(suppl 1):II312-II317 [DOI] [PubMed] [Google Scholar]
- 62.Estrera AL, Miller CC, Goodrick J, et al. Update on outcomes of acute type B aortic dissection. Ann Thorac Surg. 2007;83(2):S842-S845 [DOI] [PubMed] [Google Scholar]
- 63.Trimarchi S, Nienaber CA, Rampoldi V, et al. IRAD Investigators Role and results of surgery in acute type B aortic dissection: insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2006;114(1)(suppl):I357-I364 [DOI] [PubMed] [Google Scholar]
- 64.Nienaber CA, Fattori R, Lund G, et al. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med. 1999;340(20):1539-1545 [DOI] [PubMed] [Google Scholar]
- 65.Dake MD, Kato N, Mitchell RS, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med. 1999;340(20):1546-1552 [DOI] [PubMed] [Google Scholar]
- 66.Zehr KJ, Orszulak TA, Mullany CJ, et al. Surgery for aneurysms of the aortic root: a 30-year experience. Circulation 2004September14;110(11):1364-1371 Epub 2004 Aug 16 [DOI] [PubMed] [Google Scholar]
- 67.Estrera AL, Miller CC, III, Chen EP, et al. Descending thoracic aortic aneurysm repair: 12-year experience using distal aortic perfusion and cerebrospinal fluid drainage. Ann Thorac Surg. 2005;80(4):1290-1296 [DOI] [PubMed] [Google Scholar]
- 68.LeMaire SA, Miller CC, III, Conklin LD, Schmittling ZC, Coselli JS. Estimating group mortality and paraplegia rates after thoracoabdominal aortic aneurysm repair. Ann Thorac Surg. 2003;75(2):508-513 [DOI] [PubMed] [Google Scholar]
- 69.Dake MD, Miller DC, Mitchell RS, Semba CP, Moore KA, Sakai T. The “first generation” of endovascular stent-grafts for patients with aneurysms of the descending thoracic aorta. J Thorac Cardiovasc Surg. 1998;116(5):689-703 [DOI] [PubMed] [Google Scholar]
- 70.Czerny M, Grimm M, Zimpfer D, et al. Results after endovascular stent graft placement in atherosclerotic aneurysms involving the descending aorta. Ann Thorac Surg. 2007;83(2):450-455 [DOI] [PubMed] [Google Scholar]
- 71.Bavaria JE, Appoo JJ, Makaroun MS, et al. Gore TAG Investigators Endovascular stent grafting versus open surgical repair of descending thoracic aortic aneurysms in low-risk patients: a multicenter comparative trial. J Thorac Cardiovasc Surg. 2007February;133(2):369-377 Epub 2007 Jan 8 [DOI] [PubMed] [Google Scholar]
- 72.Mehta RH, Suzuki T, Hagan PG, et al. International Registry of Acute Aortic Dissection (IRAD) Investigators Predicting death in patients with acute type A aortic dissection. Circulation 2002;105(2):200-206 [DOI] [PubMed] [Google Scholar]
- 73.Tsai TT, Evangelista A, Nienaber CA, et al. International Registry of Acute Aortic Dissection (IRAD) Investigators Long-term survival in patients presenting with type A acute aortic dissection: insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2006;114(1)(suppl):I350-I356 [DOI] [PubMed] [Google Scholar]
- 74.Tsai TT, Fattori R, Trimarchi S, et al. International Registry of Acute Aortic Dissection (IRAD) Investigators Long-term survival in patients presenting with type B acute aortic dissection: insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2006November21;114(21):2226-2231 Epub 2006 Nov 13 [DOI] [PubMed] [Google Scholar]
- 75.Cho KR, Stanson AW, Potter DD, Cherry KJ, Schaff HV, Sundt TM., III Penetrating atherosclerotic ulcer of the descending thoracic aorta and arch. J Thorac Cardiovasc Surg. 2004;127(5):1393-1399 [DOI] [PubMed] [Google Scholar]
- 76.Erbel R, Alfonso F, Boileau C, et al. Diagnosis and management of aortic dissection: recommendations of the Task Force on Aortic Dissection, European Society of Cardiology. Eur Heart J. 2001;22(18):1642-1681 [DOI] [PubMed] [Google Scholar]
- 77.Bernard Y, Zimmermann H, Chocron S, et al. False lumen patency as a predictor of late outcome in aortic dissection. Am J Cardiol. 2001;87(12):1378-1382 [DOI] [PubMed] [Google Scholar]
- 78.Tsai TT, Evangelista A, Nienaber CA, et al. International Registry of Acute Aortic Dissection (IRAD) Investigators Partial thrombosis of the false lumen in patients with acute type B aortic dissection. N Engl J Med. 2007;357(4):349-359 [DOI] [PubMed] [Google Scholar]
- 79.Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg. 2002;73(1):17-27 [DOI] [PubMed] [Google Scholar]
- 80.Shores J, Berger KR, Murphy EA, Pyeritz RE. Progression of aortic dilatation and the benefit of long-term β-adrenergic blockade in Marfan's syndrome. N Engl J Med. 1994;330(19):1335-1341 [DOI] [PubMed] [Google Scholar]
- 81.Finkbohner R, Johnston D, Crawford ES, Coselli J, Milewicz DM. Marfan syndrome: long-term survival and complications after aortic aneurysm repair. Circulation 1995;91(3):728-733 [DOI] [PubMed] [Google Scholar]
- 82.Kertai MD, Boersma E, Westerhout CM, et al. Association between long-term statin use and mortality after successful abdominal aortic aneurysm surgery. Am J Med. 2004;116(2):96-103 [DOI] [PubMed] [Google Scholar]
- 83.Grundy SM, Cleeman JI, Merz CN, et al. Coordinating Committee of the National Cholesterol Education Program Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines [published correction appears in Circulation. 2004;110(6):763] Circulation 2004;110(2):227-239 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.