Abstract
Down-regulation of immune response in aging individuals puts this population at a potential risk against infectious agents. In-depth studies conducted in humans and mouse models have demonstrated that with increasing age, T cell immune response against pathogens is compromised and response to vaccinations is subdued. In the present study, using a mouse model, we demonstrate that older animals exhibit greater susceptibility to Encephalitozoon cuniculi infection, and their ability to evoke an antigen-specific T cell response at the gut mucosal site is reduced. The dampening of T cell immunity was due to the defective priming by the dendritic cells (DC) isolated from the mucosal tissues of aging animals. When primed with DC from younger mice, T cells from older animals were able to exhibit optimal antigen-specific response. The functional defect in DC from older mice can be attributed to large extent to reduced IL-15 message in these cells, which can be reversed by addition of exogenous IL-15 to the cultures. IL-15 treatment led to optimal expression of co-stimulatory molecules (CD80 and CD86) on the surface of older DC and restored their ability to prime a T cell response against the pathogen. To our knowledge, this is the first report, which demonstrates the inability of DC population from aging animals to prime a robust T cell response against an infectious agent. Moreover, the observation that IL-15 treatment can reverse this defect has far reaching implications in developing strategies to increase vaccination protocols for aging population.
Keywords: Dendritic cells, mucosa, microsporidia, IL-15, CTL
Introduction
Age associated decreases in immune function are thought to contribute greatly to the increased morbidity and mortality seen in elderly populations. Aging of the immune system involves both humoral and cell mediated immunity. Changes in humoral immunity consist of a decrease in the number of B lymphocytes leading to a poor antibody response (1, 2). Defects in cellular immunity include a decrease in absolute numbers of naïve CD3, CD4 and CD8 T cells and alterations in signal transduction through TCR (3). Furthermore, substantial changes in the functional and phenotypic profiles of T cells have been reported in aging humans and rodents (4, 5).
Recent studies have shown that age related defects in the immune system are not restricted to adaptive immunity but can also be extended to the innate immune response. Neutrophils, macrophages and NK, important first lines of defense against bacterial and parasitic infections, are impaired with advancing age (6–8). Moreover, studies with animal models suggest that dendritic cells, which serve as important antigen-presenting cells (9), are less able to stimulate T cells thereby contributing to changes in their functionality during advanced age (10).
Microsporidia are single celled obligate intracellular parasites that have emerged as an opportunistic infection causing diarrhea and systemic disease in persons with AIDS (11). Increased awareness and improved diagnostics of microsporidia infections has revealed a high occurrence of disease in non-HIV infected elderly population (12–14). One reason microsporidial infections are more frequent in aged human population could be attributed to downregulation of immune responses against the pathogen in these individuals.
Most of what is known about the immune response against microsporidia is based on Encephalitozoon cuniculi. The importance of T cells in controlling E. cuniculi infection was demonstrated by adoptive transfer studies using athymic or SCID mice (15, 16). Although both T cell subsets (CD4 and CD8) produce IFNγ during E. cuniculi infection (17), when challenged via i.p. (intraperitoneal) route, protective immunity is mediated exclusively by CD8+ T cells (18, 19). Mutant animals lacking CD8+ T cells or perforin gene are highly susceptible to i.p. challenge with the pathogen (19). The situation is somewhat different after oral infection, as depletion of both CD4 and CD8+ T cells, is needed to achieve mortality in animals challenged via this route (20). Apparently, IFNγ production by CD4 or CD8+ T cells is critical for survival, as knock out mice exhibit severe susceptibility to per-oral infection. Although, not as susceptible as IFNγ−/− mice, perforin−/− animals exhibit mortality to per-oral infection, suggesting the importance of host cytotoxic T lymphocytes (CTL) response against the pathogen (21).
Recent observations from our laboratory have demonstrated the importance of DC in priming mucosal T cell responses against this pathogen (21). In the present study, we evaluated the age related changes in T cell responses against E. cuniculi infection in older mice and the ability of DC to prime immune responses against this pathogen.
Material And Methods
Mice
C57BL/6 mice of different age groups were purchased from Charles Rivers (Wilmington, MA). SCID mice on C57BL/6 background, originally purchased from Jackson Laboratory were bred in our Animal Research Facility. Animals were housed and bred under IACUC approved conditions at the Animal Research Facility at LSUHSC (New Orleans, LA) and George Washington University (Washington, DC).
Parasites and infection
A rabbit isolate of E. cuniculi (genotype II), kindly provided by E. Didier (Tulane Regional Primate Research Center, Covington, LA) was maintained by continuous passage in rabbit kidney cells (RK-13), obtained from American Type Culture Collection (Manassas, VA). The RK-13 cells were maintained in RPMI-1640 (Mediatech Inc, Herndon, VA) containing 10% FCS (Hyclone Laboratories, Logan, UT). Organisms were collected from the culture medium, centrifuged at 325 × g for 10 min and washed twice with PBS. Mice were orally infected with 2×107 spores/mouse.
Detection of cytokines
Quantitation of cytokine mRNA was performed by PCR. MLN lymphocytes and splenocytes from E. cuniculi infected animals from different age group were collected on day 14 p.i. (post infection). RNA was isolated using TRIzol (Invitrogen, Carlsbad CA) according to manufacturer’s instructions. Reverse transcription was performed with Moloney murine leukemia virus reverse transcriptase (Invitrogen) and random hexamer primers (Promega, Madison, WI). Expression of mRNA for IFNγ was analyzed by quantitative PCR with the PQRS quantitative method according to published protocol (20). The lymphocytes from uninfected mice were used to establish a baseline value of 1.0 against which the cytokine message level in the test was quantitated.
CTL assay
A CTL assay was performed according to a standard procedure in our laboratory (22). Briefly, mouse peritoneal macrophages were harvested 2 days after i.p. inoculation with 1 ml of thioglycolate. The macrophages were washed three times in PBS and dispensed at a concentration of 5×104 cells/well in U-bottom 96-well plates. After overnight incubation, the cells were infected with 2×105 spores of E. cuniculi per well for 48 hours. The cells were washed extensively with PBS to remove any extracellular parasites. Macrophages were labeled with 51Cr (0.5μCi/well) for 2 hours at 37°C. Macrophages were washed 5 times with PBS and incubated with MLN and spleen cells at various E: T ratios in a final volume of 200 μl of culture medium. The microtiter plates were centrifuged at 200 × g for 3 min and incubated at 37°C for 4 hours. Samples (100μl) were removed and assayed for released cpm by scintillation counting. The percentage of lysis was calculated as follow: ((mean cpm of test sample - mean cpm of spontaneous release)/(mean cpm of maximal release – mean cpm of spontaneous release))/100.
T cell proliferation
Antigen-specific proliferation of T cell population was determined by thymidine incorporation assay according to a standard protocol in our laboratory (22). Briefly, MLN and spleen lymphocytes were isolated and cultured in 96-well flat-bottom plates in RPMI1640 at a concentration of 2×105 cells per well. The cells were stimulated with E. cuniculi spores (5×103 spores/well). After 72 hours incubation at 37°C in 5% CO2, thymidine (0.5μCi/well; Amersham, Arlington Heights, IL) was added to the wells. Cells were harvested on a glass filter using an automated multiple sample harvester (Brandel M12, Gaithersburg, MD), dried, and incorporation of radioactive thymidine determined by liquid scintillation (Beckman Coulter, Fullerton, CA).
Transfer of immune T cells
C57BL/6 mice of different age groups (8 week and 9 month old) were infected per-orally as mentioned above. Animals were sacrificed at day 14 p.i, MLN and spleen were collected and T cells from the tissues isolated by magnetic purification using TCRβ antibody according to the manufacturer’s instructions (Stem Cell Technology, Vancouver, BC Canada). Sorted T cells (92–96% pure), were adoptively transferred (5×106 cells/mouse) to SCID mice via i.v. (intravenous route). Two days post transfer, recipient mice were orally challenged with 2×107 E. cuniculi spores/mouse. Morbidity and mortality was monitored daily until termination of the experiment.
In vitro priming of T cells by DC
MLN and splenic DC were isolated from naïve C57BL/6 mice of different age groups according to previously published protocol (23). Briefly, mice were sacrificed, MLN harvested and DC isolated by enzymatic digestion (DNase I and collagenase D) followed by mechanic disruption. Cells were then labeled with anti-CD11c biotin conjugated antibody (eBioscience, San Diego CA) followed by positive magnetic selection as described above. The cells were further purified on cell sorter (FACSaria, Becton Dickinson, San Jose CA) after incubation with streptavidin conjugated to PE-Cy5.5 (eBioscience).
Sorted DC were plated at 20 000 cells/well and incubated overnight with 5 E. cuniculi spores/DC. In some of the experiments, IL-15 (50–100 ng/ml, R and D systems, Minneapolis, MN) was added to the wells. The next day, plated DC were extensively washed and purified T cells added to the culture. T cells were isolated from MLN of naïve C57BL/6 mice (8 week old or 9 month old) by positive magnetic selection as mentioned above. After 72 hr incubation, antigen-specific proliferation was measured by thymidine incorporation. In separate studies, the cultures were incubated for 96 h and cytotoxic activity against E. cuniculi infected macrophages determined by radioisotope release according to protocol described above.
In vitro activation of MLN DC
MLN DC from young and older mice were isolated as described above and plated at 20000 cells/well with 1×105 E. cuniculi spores/well in presence or absence of recombinant murine IL-15 (50 ng/ml). After overnight incubation, the supernatants were harvested and assayed for IL-12p40 production using commercially available ELISA kit (Biolegend, San Diego, CA) according to manufacturer’s instructions.
IL-15 message was measured by real time PCR, using standard protocol of our laboratory (23). Briefly, cell cultures were set up as described above and MLN DC were harvested after 48 hours incubation at 37°C. RNA was isolated with RNeasy kit (Qiagen, Valencia CA) according to manufacturer instructions, followed by reverse transcription with SuperScript III first strand synthesis system (Invitrogen). Real time PCR was performed on MyiQ Single color Real Time PCR Detection System (Bio-Rad, Hercules CA) using 200nM of primers (5mIL15 5′-CATCCATCTCGTACTTGTGTT-3′, 3mIL15 5′-CATCTATCCAGTTGGCCTCTTTT-3′, 5mβ-actin 5′-AGAGGGAAATCGTGCGTGAC-3′, 3mβ-actin 5′CAATTAGTGATGACCTGGCCGT-3′) and SYBR GreenER qPCR Supermix (Invitrogen). Samples were subjected to 50 cycles of 15 sec at 95°C and 2 min at 60°C followed by melt curve analysis. Non infected DC were used to establish a baseline value of 1.0 against which the cytokine message level was quantitated.
Expression of costimulatory molecules
Purified DC (40000 cells/well) were incubated overnight in presence of E. cuniculi (5 spores/DC). Eighteen hours later, cells were harvested and labeled with anti CD40, CD80, CD86, and MHC class II antibodies conjugated to phycoerythrin (eBioscience). DC were then analyzed with FACSAria (Becton Dickinson).
Adoptive transfer of DC
DC from naïve C57BL/6 mice were purified, plated (5×104 cells/well) and pulsed with irradiated E. cuniculi (5 spores/DC). After 24 h incubation, the cells were harvested, washed and adoptively transferred to 9 month old mice (5×105 DC/mouse) via i.v. route Two week post transfer, the recipients were sacrificed, tissues (MLN and spleen) harvested and CTL assay performed.
Statistical analysis
Statistical analysis of the data was performed using a two-sampled Student t test (24).
Results
Older mice are more susceptible to E. cuniculi infection
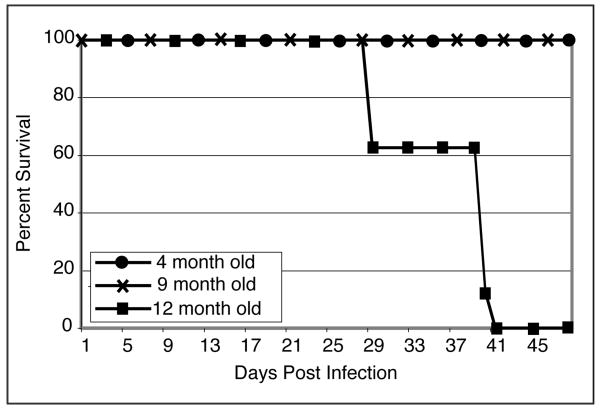
Previous studies from our laboratory have demonstrated that young (7–8 weeks old) mice are able to survive oral E. cuniculi infection without any signs of morbidity (20). To assess the ability of different aged mice to resist E. cuniculi infection, C57BL/6 mice of different age groups (4, 9 and 12 months old) were infected with 2×107 E. cuniculi spores via oral route. As shown in figure 1, while 4 and 9 month-old infected mice survived, 12 month-old infected animals succumbed to infection. Infected 12 month old mice became lethargic, lost weight and died starting as early as 28 days p.i. with all the animals from this age group succumbing to the infection by day 42 p.i. Conversely, 4 and 9 month old mice showed no signs of mortality or morbidity. These results demonstrate that with an increase in age, older animals lose their ability to survive E. cuniculi challenge.
Figure 1.
Older mice cannot survive oral E. cuniculi infection. C57BL/6 mice of different age groups (4, 9 and 12 months) were orally infected with 2×107 E. cuniculi spores (6 mice/group). Animals were monitored daily for mortality and morbidity until the end of the experiment. The experiment was repeated twice with similar results.
Sub-optimal antigen-specific response against E. cuniculi in older mice
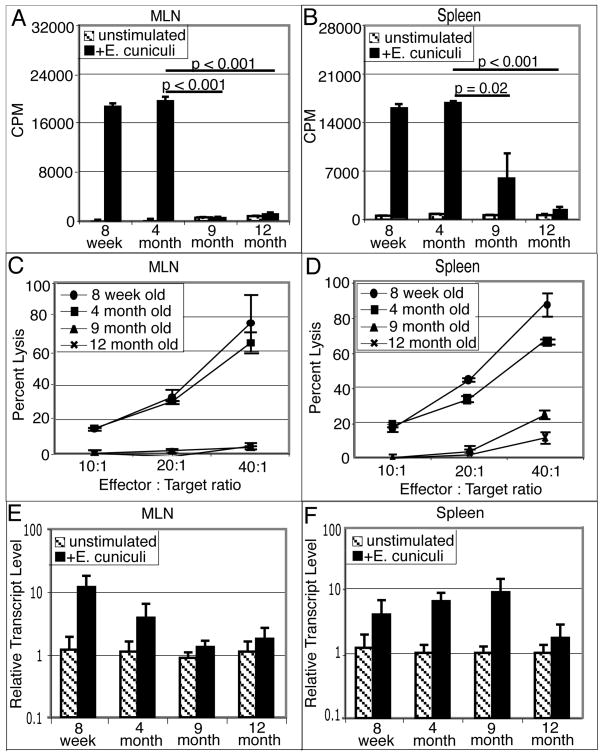
As cellular immunity has been shown to play a critical role in the protective immunity against E. cuniculi (18, 19), we studied the induction of antigen-specific T cell responses against this pathogen in older animals. At two weeks p.i, cell suspensions (spleen and MLN) from infected tissues were prepared and proliferative and cytotoxic assays performed. As shown in figure 2A and 2B, MLN and spleen cells from infected mice of younger age group (8 week and 4 months old) exhibited significant proliferation (p<0.001) in response to antigenic restimulation. Although 9 month old mice survived the infection (figure 1), the splenocytes from these mice exhibited a significant reduction in antigen specific proliferation (p=0.02) (figure 2B) and response was reduced to background levels in MLN (p ≤0.001)(figure 2A). This defect was more pronounced in 12 month old infected animals where both spleen and MLN cells failed to proliferate in response to E. cuniculi restimulation (p<0.001)(figure 2A and 2B). Spleen and MLN cells from all the groups with the exception of 12 month old animals exhibited a normal mitogenic response (data not shown).
Figure 2.
Defective mucosal immune response after E. cuniculi infection. C57BL/6 mice (8 week, 4, 9 and 12 month old) were infected per-orally with 2×107 spores. Two weeks post-infection, mice were sacrificed (3 animals/group), MLN and spleen cell suspension were prepared. Proliferative response of MLN lymphocytes (A) and splenocytes (B) was measured by thymidine incorporation after 72h incubation with E. cuniculi spores. In a separate experiment, MLN (C) and spleen cell suspensions (D) were prepared and incubated with 51Cr labeled uninfected or infected macrophages at various effector to target ratios. After 4h incubation, the cytolytic activity was determined by radioisotope release into culture supernatants. MLN (E) and splenic (F) lymphocytes were isolated 14 days post E. cuniculi infection (3 mice/group). mRNA was prepared and assayed for IFNγ message by RT-PCR. Data are representative of two separate experiments.
To further assess the development of antigen-specific responses against E. cuniculi infection in older mice, the cytolytic activity of spleen and MLN cells against parasite infected macrophages was determined. As shown in figure 2C, MLN from older mice (9 and 12 months of age) failed to lyse E. cuniculi infected targets. The cytolytic response in MLN from older animals was reduced to background lytic level at all effector: target ratios tested (figure 2C). In comparison splenic cells from 9 month old mice at an E: T ratio of 40:1 were able to lyse 18 to 20% target cells (figure 2D). Similar to our earlier observations (20), both spleen and MLN cells from younger animals (8 week and 4 month old) exhibited a strong cytotoxic response against parasite infected targets.
Since IFNγ has been reported to play an important role in immune protection against E. cuniculi infection (25), the level of cytokine message in the tissues of older animals was analyzed. Tissues (spleen and MLN) from mice orally infected with 2×107 spores of E. cuniculi were harvested 14 days p.i. and assayed for IFNγ mRNA levels by semi quantitative PCR. In agreement with our previous findings (20), IFNγ expression in both spleen and MLN of 8 week old mice increased after E. cuniculi infection (figure 2E and 2F) and a similar pattern was observed in the tissues from 4 month old infected animals. While spleen from 9 month old infected mice exhibited an increase in IFNγ mRNA, the message levels in the MLN of these mice did not rise above the background levels. However, at 12 month of age, mice infected with E. cuniculi failed to up-regulate IFNγ message in both the MLN and spleen (figure 2E–F). Based on these findings, it can be strongly suggested that age related immunosuppressive response to E. cuniculi infection occurs by 9 month and seems to be more pronounced in the MLN than spleen.
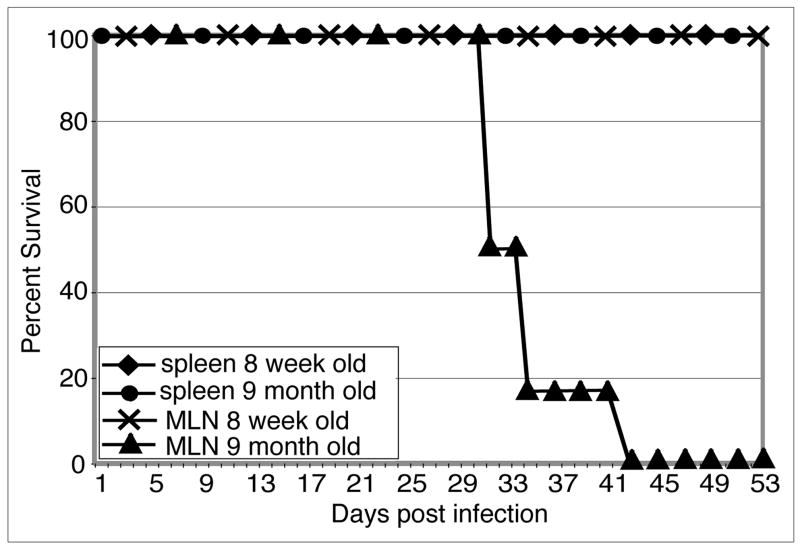
MLN T cells from older mice fail to protect SCID animals
Subsequently, adoptive transfer studies were performed to establish the defect in the mucosal immune response of the older mice. T cells from mice of different age groups (8 week and 9 month old) were isolated 2 weeks post oral infection and adoptively transferred to naïve SCID animals. As shown in figure 3, recipient mice injected with MLN T cells from older mice (9 month old), were unable to withstand E. cuniculi infection and all the animals (6/6) died by day 43 post-challenge. Interestingly, splenic T cells isolated from the same donors were able to protect SCID animals and recipients survived untill termination of the experiment (figure 3). As expected, SCID mice treated with T cells from either spleen or MLN of younger (8 week old) mice were able to protect the recipients against E. cuniculi infection. These results further emphasize that age related suppression of immune response against E. cuniculi infection occurs earlier in the gut mucosa as compared to systemic immune compartment.
Figure 3.
MLN T cells from older mice fail to protect SCID recipients: MLN lymphocytes and splenocytes from 8 week old and 9 month old mice (4 mice/group) were isolated and adoptively transferred to SCID mice (5×106 cells/mouse). Two days, post transfer, recipients were challenged per-orally with 2×107 E. cuniculi spores. Morbidity and mortality were monitored daily until the end of the experiment. There were 6 animals per group and the study was performed twice with similar results.
MLN DC from older mice fail to induce T cell response in vitro
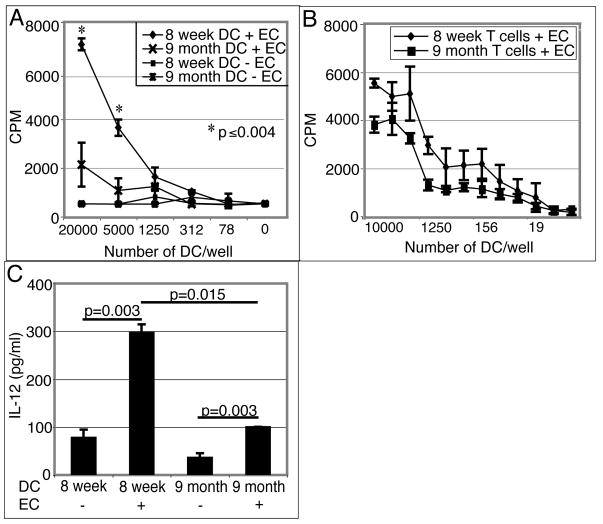
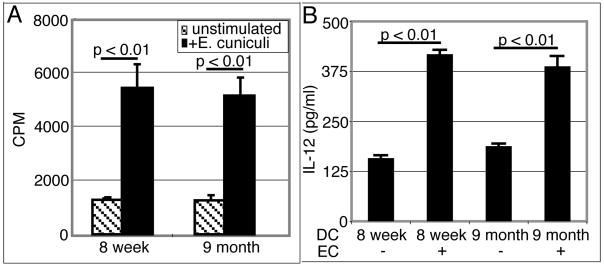
As DC are known to play a crucial role in the priming of T cells (9), we decided to evaluate the ability of these cells to induce T cell responses against E. cuniculi in older animals. Since a suboptimal immune T cell response was detected in the MLN of nine month old mice, further studies were conducted with these animals. DC from the MLN of young (8 week old) or older (9 month old) mice were isolated and cultured overnight with E. cuniculi spores. Twenty four hours later, affinity purified T cells (TCRβ+) from 8 week old mice were added to the culture. After 72 hours, the proliferative response of T cells was measured by 3H thymidine incorporation assay. As shown in figure 4A, while DC isolated from young mice induced a strong, dose dependent T cell proliferative response, T cells primed with cells from older animals showed minimal proliferation. To establish that the suboptimal proliferation is due to a defect in the ability of DC derived from older mice to prime T cells, TCRβ+ T cells were isolated from mice of different age groups (8 week old and 9 month old) and incubated with E. cuniculi pulsed DC from 8 week old mice. As shown in figure 4B, T cells from both young and older mice showed comparable proliferative responses when primed with DC isolated from younger mice. IL-12 production by DC is important for the initiation of Th1 immune response (26). Given that the role of IL-12 in the immune response against E. cuniculi has been reported (25), we analyzed the ability of DC from MLN to produce IL-12 in response to E. cuniculi stimulation. DC from young and older mice were isolated and pulsed overnight with E. cuniculi spores. The following day, the supernatants were harvested and levels of IL-12 determined by ELISA. As expected, MLN DC from younger mice produced significant amount of IL-12 when stimulated with E. cuniculi spores (figure 4C). Conversely, DC from older mice exhibited a defect in IL-12 production and yielded significantly less cytokine (p=0.0015) as compared to the cells isolated from younger animals.
Figure 4.
(A) MLN DC from older mice are unable to prime young T cells in vitro. MLN DC from 8 week old and 9 month old mice were isolated (4 mice/group), plated at different concentrations and stimulated overnight with E. cuniculi spores. The next day, T cells isolated from naïve 8 week old mice (3 mice/group) were added to the culture. After 72h incubation, proliferative response was measured by thymidine incorporation. (B) T cells from young and older mice respond equally well to in vitro priming. MLN DC from 8 week old mice (4 mice) were isolated, plated at different concentrations and stimulated with E. cuniculi. After overnight incubation, T cells isolated from MLN of naïve 8 week old or 9 month old mice (3 mice/group) were added to the culture. After 72h incubation, proliferative response was measured by thymidine incorporation. (C) Defective IL-12 production by MLN DC from older mice. Culture supernatants were assayed for IL-12 production by ELISA. Results are representative of two separate experiments.
Next, we assayed the ability of splenic DC from this age group to prime antigen-specific response. Splenic DC isolated from young (8 week old) and older (9 month old) animals were pulsed with E. cuniculi spores overnight and their ability to prime T cells to proliferate was determined by 3H thymidine incorporation assay as mentioned above. Interestingly, unlike MLN DC, splenic DC from 9 month old mice were able to prime T cell response comparable to DC from younger mice (figure 5A). Similar to the results obtained with proliferation assays the defect in IL-12 release was not observed in splenic DC from older mice. When pulsed overnight with E. cuniculi spores, they produced IL-12 levels comparable to the splenic DC isolated from younger mice (figure 5B).
Figure 5.
Splenic DC from older mice are able to prime splenocytes in vitro. Splenic DC from 8 week old and 9 month old mice were isolated, plated (20,000 DC/well) and stimulated with E. cuniculi spores. The next day, splenic T cells isolated from naïve 8 week old mice were added to the culture. (A) After 72h incubation, proliferative response was measured by thymidine incorporation. (B) Culture supernatants were assayed for IL-12 production by ELISA. Results are representative of two separate experiments.
These findings demonstrate that MLN DC from 9 month old mice are unable to prime an immune response against E. cuniculi infection as manifested by their inability to secrete IL-12 and induce T cell proliferation in vitro. Conversely, splenic DC from these animals have intact function, exhibit optimal levels of IL-12 and induce robust T cell proliferation.
Adoptive Transfer of MLN DC from older mice fail to induce CTL response ex vivo
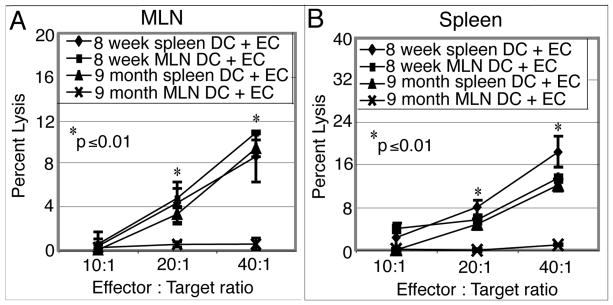
To fully determine the ability of DC from older mice to prime E. cuniculi specific immune response in vivo, adoptive transfer studies were performed. DC isolated from MLN or spleen from young (8 week old) and older (9 month old) mice were pulsed in vitro with E. cuniculi spores and subsequently transferred to naïve mice 9 months of age. Two weeks post transfer, the recipient animals were sacrificed, tissues (spleen and MLN) isolated and CTL assay performed. As shown in figure 6A and 6B, the MLN DC from older animals failed to induce cytotoxic response in mucosal or splenic compartment of recipient animals at all the E: T ratios tested. Conversely, the recipients injected with splenic DC from the same donors were able to exhibit cytotoxic activity against E. cuniculi infected targets in both mucosal and splenic sites (figure 6A and B). This response was comparable to the one observed in the recipients treated with DC isolated from younger animals (figure 6A and B). These results demonstrate that, compared to spleen, age related defect in DC starts earlier in the mucosal compartment.
Figure 6.
Adoptive transfer of MLN DC form older mice fail to prime a CTL response in vivo. DC from MLN and spleen from 8 week and 9 month old mice were isolated, plated (5×104 cells per well) and pulsed with irradiated E. cuniculi spores (2.5×105 per well). After overnight incubation, cultures were harvested and cells were adoptively transferred to 9 month old mice (5×105 per mouse) via iv route. Fourteen days later, recipient mice were sacrificed, MLN (A) and splenic (B) lymphocytes prepared and assayed for cytotoxicity. Experiment was performed twice with similar results and data are representative of one experiment.
Treatment of DC from older mice with IL-15 restores their ability to prime T cell responses
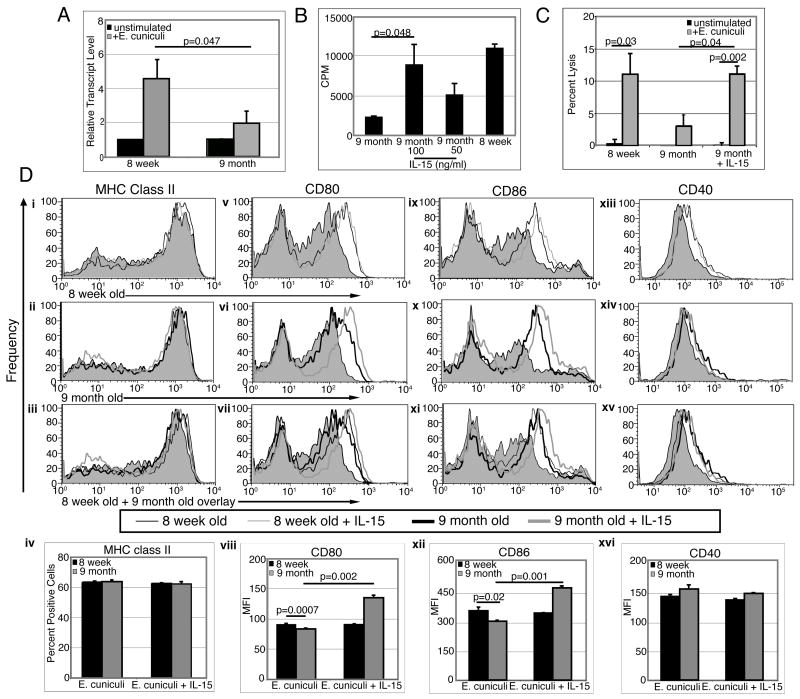
Previous studies from our laboratory have reported the essential role of IL-15 in the priming of T cell response by DC (27). Moreover, a recent study by Ohteki et al has shown that DC are the primary source of IL-15 (28). We analyzed IL-15 message expression in MLN DC from 8 week and 9 month old mice after in vitro activation with E. cuniculi. Forty eight hours post E. cuniculi stimulation, MLN DC isolated from 8 week old mice exhibited a 4.5 fold increase in IL-15 expression while those from older mice showed only a 2 fold increase over their respective non stimulated controls (p=0.047) (figure 7A). To determine if IL-15 treatment would restore DC function of older mice, cultures of purified DC pulsed with E. cuniculi spores were treated overnight with IL-15. Twenty four hours later, cells were washed and purified T cells were added to the cultures. Proliferation was measured after 4 days of incubation by thymidine incorporation assay. As shown in figure 7B, IL-15 treatment significantly improved the proliferation of T cells cultured with DC from older mice (p=0.048), suggesting that this cytokine can reverse the inability of older DC to prime T cell response.
Figure 7.
IL-15 improves T cell priming by older DC and their costimulatory molecules expression. (A) MLN DC from older mice are unable to upregulate IL-15 message after E. cuniculi stimulation. MLN DC from 8 week-old and 9 month old mice were isolated and plated (20,000 DC/well). Cells were incubated 48 h with E. cuniculi spores and analyze for IL-15 mRNA expression normalized to β actin mRNA levels. Relative expression was measured by using the mean from each group and the formula RTL (relative transcript level) = 2−ΔCt × 1000. (B) IL-15 treatment restores ability of older DC to prime young T cells in vitro. MLN DC from 8 week old and 9 month old mice were isolated and plated (20,000 DC/well). DC were incubated overnight with E. cuniculi spores and exogenous IL-15 (100 ng/ml and 50 ng/ml). The next day, cell cultures were washed and T cells isolated from MLN of naïve 8 week old mice were added. After 72h incubation, proliferative response was measured by thymidine incorporation. (C) Treatment with IL-15 restores the ability of MLN DC from older mice to prime a CTL response. MLN DC from 8 week and 9 month old mice (10–15 mice/group) were isolated, plated (5×104 DC/well) and stimulated overnight with E. cuniculi (2.5×105 spores/well). The following day, purified T cells from naïve 8 week old mice (3 mice/group) were added to the culture and subsequently after 4 day incubation CTL assay performed. (D) IL-15 increases costimulatory molecules expression by older DC. MLN DC from 8 week and 9 month old mice were isolated, plated at 40,000 DC/well and incubated overnight with E. cuniculi spores. The next day, cells were harvested and labeled for MHC class II (i–iv), CD80 (v–vii), CD86 (ix–xii) and CD40 (xiii–xvi) (—8 week old DC + E. cuniculi, —8 week old DC + E. cuniculi + IL-15,  9 month old DC + E. cuniculi,
9 month old DC + E. cuniculi,  9 month old DC + E. cuniculi + IL-15). Results are presented as mean ± SD of triplicates and experiment was performed twice with similar results.
9 month old DC + E. cuniculi + IL-15). Results are presented as mean ± SD of triplicates and experiment was performed twice with similar results.
Next, we determined if IL-15 can restore the ability of MLN DC from older mice to prime a CTL response against E. cuniculi. MLN DC from mice of different age groups (8 week and 9 month old), were pulsed overnight with E. cuniculi spores and after 24 h incubation, purified T cells isolated from younger animals (8 week old) were added to the cultures. Four days later, the cultures were harvested, incubated with radiolabeled infected macrophages and lytic activity determined by 51Cr release. As shown in figure 7C, unlike DC from 8 week old mice, cells isolated from animals of 9 month of age failed to induce an in vitro CTL response. Addition of exogenous recombinant IL-15 to DC cultures from older mice restored their ability to prime cytotoxic response (figure 7C). However, IL-15 treatment was not able to bring the IL-12 production of older MLN DC to those observed with cells from younger mice (data not shown).
To further determine the mechanism of IL-15 mediated up-regulation of T cell priming by older DC, the cells were analyzed for MHC class II expression which is pivotal for antigen presentation to CD4+ T cells and CD80 and CD86, co-stimulatory molecules important for T cell stimulation (29). While no difference in MHC class II expression between E. cuniculi primed DC from older and young mice was observed (Figure 7C i-iv), significantly lower levels of both CD80 and CD86 expression (p=0.0007 and p=0.02 respectively) was noted in the DC from older animals (figure 5C v–xii). In vitro IL-15 treatment restored the expression of CD80 and CD86 molecules on DC from older animals. However, no difference in the expression of CD40 molecule in response to E. cuniculi infection between DC isolated from young or older animals was observed (Figure 7, xiii–xvi). Also, addition of exogenous IL-15 to the cultures had no effect on the expression of this molecule.
Discussion
Our results demonstrate that with increasing age, the ability of animals to launch an optimal immune response against E. cuniculi infection is reduced, which may prove to be a risk for their survival. Down-regulation of the immune response in the older animals is manifested by the inability of T cells from the MLN of these mice, to exhibit antigen-specific proliferation or cytotoxic response against pathogen infected targets. Furthermore, our data for the first time demonstrates that generation of ineffective antigen-specific T cell response in the MLN of older animals is due to the sub-optimal priming by DC in this tissue. When primed with DC from younger animals, T cells from older mice exhibit a normal proliferative and cytotoxic response. The defect in DC population from older animals appears to be restricted to the cells from MLN as their ability to secrete IL-12 and prime T cell response against E. cuniculi infection is severely compromised. In comparison, the splenic DC isolated from the older animals, produce normal IL-12 levels and induce an optimal T cell response to this pathogen. Moreover, while transfer of DC isolated from the tissues (MLN and spleen) of younger mice led to generation of antigen-specific cytotoxic activity in the recipient animals, treatment with MLN DC from older animals failed to generate detectable CTL response. Similar to the cells isolated from young animals, DC from splenic compartment of older donors were able to induce cytotoxic response in the tissues of recipient mice. Down-regulation of DC response against E. cuniculi in older animals appears to be to large extent due to reduced IL-15 message levels in MLN, which leads to decreased expression of co-stimulatory molecules like CD80 and CD86 on these cells. However, treatment with IL-15 reverses this downregulation, leading to optimal expression of these molecules and restores their ability to prime a T cell response against the pathogen.
Age related changes in the immune response are known to render individuals more susceptible to infection and decreases their ability to generate responses against tumors (30–32). The immune dysfunction due to aging which contributes to morbidity and mortality is not restricted only to the very elderly, but in all likelihood can be observed during middle ages (33). Vaccination of elderly population with the influenza virus fails to generate a strong effector response in these individuals (34, 35). Although, both primary and secondary antibody response to vaccinations are subdued, the highest degree of impairment is seen in the T cell arm of the immune system (35). A substantial decrease in the number of naïve lymphocytes as a result of thymic output and expansion of oligoclonally and functionally incompetent memory lymphocytes as a result of age associated changes have been reported (36). In a murine model, it has been demonstrated that the frequency and number of lymphocyte precursors are considerably reduced by 7 month of age (37). Although the total number of T cells in secondary lymphoid organs and the CD4/CD8 ratio are relatively unaffected by aging, there is a considerable decrease in effector response of both T cell subsets (38, 39). Also, naïve CD4 T cells from aged animals have been reported to secrete less IL-2, leading to a decrease in the expression of CD25. These cells are unable to exhibit optimal proliferation to antigenic stimulation and cannot undergo complete differentiation to T helper type 1 or type 2 effector cells (40).
Although there is an abundance of information related to observed defects in effector T cell response in aging population, the status of the innate immune response in these individuals has not been studied. Most of the above mentioned reports have emphasized the defect in development of effector T cell response in these individuals. In the present study, we demonstrate that age related T cell defect in the older animals can be restored if DC from younger mice are involved in the priming of antigen specific T cell response (both in vitro and in vivo). Whenan be restored if DC from younger mice are involved in the priming of antigen specific T cell response (both in vitro and in vivo). When cultured with E. cuniculi pulsed DC from younger animals, effector function of the T cells from older mice was restored. Moreover, transfer of DC from younger animals led to induction of antigen-specific CTL response in the tissues of older animals. In addition, while transfer of MLN T cells from older mice failed to protect SCID animals from E. cuniculi challenge, one hundred percent survival was observed amongst the groups treated with immune T cells isolated from tissues of younger mice. This study strongly suggests that poor priming by DC from mucosal site is an important factor in T cell defect during early aging.
As stated above, although T cell response in aged animals has been well studied (39, 41, 42), the information regarding the effect of aging on DC function is very limited and not well defined. In one of the recent reports, it has been demonstrated that aging has no effect on the in vitro generation of monocyte derived DC and cells isolated from both young or old individuals were capable of stimulating the T cell response (43). However, studies by Maletto et al have shown that immune dysfunction in aged animals infected with Trypanosoma cruzi is due to a defect in APC function (44). Similarly, poor APC function has also been linked to a sub-optimal immune response against pneumococcal vaccines in old mice (45). A study involving monocyte derived DC from healthy volunteers demonstrated that, while infection with influenza virus was able to evoke an immune response comparable to cells isolated from young individuals, RSV infected cells from old volunteers triggered a significantly reduced response (46). However, GM-CSF as well as M-CSF production is reduced with age and this could explain the difference between in vitro and in vivo observations (47, 48).
Although the effect of aging on DC function remains somewhat enigmatic, available information suggests that down-regulation of DC function can be an important cause for a sub-optimal immune response against certain diseases in the elderly population. Our studies demonstrate that oral E. cuniculi infection can be one such situation where older animals are unable to evoke a robust T cell response against the pathogen due to poor DC priming. This defect was especially apparent in the mucosal sites as cells isolated from the MLN failed to prime an effector T cell response against the pathogen. Since IL-15, which is primarily produced by DC, has recently been shown to play a major role in induction of CD8 T cell response (49), we decided to focus our attention on this cytokine. Our current observations, for the first time demonstrate the inability of MLN DC from aging animals to express optimal IL-15 message. Interestingly, in vitro treatment of DC with IL-15 restored their ability to prime T cell immunity against this pathogen, suggesting that age related immune defect in the older mice may be significantly linked to this cytokine. Recently, importance of IL-15 in the activation of DC response has been shown by several laboratories (50, 51). In one of these studies, in vivo and in vitro exposure of splenic DC to IL-15 led to up-regulation of co-stimulatory molecules, marked increase in the IFNγ release by these cells and enhanced their ability to stimulate antigen specific CD8+ T cell proliferation (51). Similarly, our laboratory has reported that a lack of IL-15 impairs the DC response which results in poor CD4+ T cell immunity against T. gondii infection (27). Moreover, recent studies have shown that IL-15 is involved in the cross-talk between different DC sub-populations which is essential for the activation of these cells (52). In the current study, increase in IL-12 production after IL-15 treatment was not observed, suggesting that IL-15 mediated restoration of E. cuniculi specific T cell priming by DC may be independent of IL-12 production. These findings are similar to earlier report by Paryath et al in which IL-15 stimulation failed to increase pathogen related IL-12 production by PBMC from HIV infected individuals (53).
The mechanism involved in the restoration of DC function by IL-15 appeared to be dependent on the ability of the cytokine to up-regulate surface costimulatory molecules like CD80 and CD86. Both of these molecules, which are known to play an important role in T cell priming (54), were down-regulated in E. cuniculi pulsed DC from older mice. However, IL-15 treatment of DC from older animals increased their expression to the levels observed in the cells from younger mice. Recent studies have shown that transduction of IL-15 gene causes a significant increase in the expression of CD83, CD86 and CD40 molecules on these cells (51, 55). Although down-regulation in CD80 and CD86 expression on the DC of older mice was observed, the expression of MHC class II and CD40 molecule on these cells was comparable to cells from younger animals. Our observations are in agreement with recent findings by Sharma et al who demonstrated that adequate co-stimulation can generate normal CD8+ T cell response against tumors in old mice treated with DC vaccines (56). The novelty of our findings is that we convincingly demonstrate for the first time that DC isolated from mucosal sites of older mice are functionally defective against an oral pathogen. These observations strongly suggest that the decreased ability of the aging population to combat oral infections may begin with a defect in DC capacity to prime antigen-specific response, especially in the gut mucosa.
As conventional vaccines are unable to protect elderly population, new strategies need to be developed to protect these individuals against infectious agents. Our data demonstrate that mucosal DC from older animals have a defect in IL-15 induction and addition of this cytokine can to great extent restore the function of these cells. When treated with IL-15, mucosal DC from older mice are able to generate CTL response against E. cuniculi infection, which as reported earlier by our laboratory (19), is critical for protective immune response against the pathogen. These findings have strong implications in developing therapeutic agents which will enable elderly population to combat those intracellular pathogens against which CTL immunity is essential for host protection.
Acknowledgments
We thank Teresa Hawley for her help with flow cytometry.
This work was supported by National Institute of Health Grant AI 071778 and AI 043693 awarded to IAK
Abbreviations
- DC
Dendritic Cells
- MLN
Mesenteric Lymph Nodes
- p.i
post-infection
- i.p
intraperitoneal
- CTL
cytolytic T lymphocytes
References
- 1.Zheng B, Han S, Takahashi Y, Kelsoe G. Immunosenescence and germinal center reaction. Immunol Rev. 1997;160:63–77. doi: 10.1111/j.1600-065x.1997.tb01028.x. [DOI] [PubMed] [Google Scholar]
- 2.Song H, Price PW, Cerny J. Age-related changes in antibody repertoire: contribution from T cells. Immunol Rev. 1997;160:55–62. doi: 10.1111/j.1600-065x.1997.tb01027.x. [DOI] [PubMed] [Google Scholar]
- 3.Garcia GG, Miller RA. Differential tyrosine phosphorylation of zeta chain dimers in mouse CD4 T lymphocytes: effect of age. Cell Immunol. 1997;175:51–57. doi: 10.1006/cimm.1996.1040. [DOI] [PubMed] [Google Scholar]
- 4.Haynes L, Linton PJ, Eaton SM, Tonkonogy SL, Swain SL. Interleukin 2, but not other common gamma chain-binding cytokines, can reverse the defect in generation of CD4 effector T cells from naive T cells of aged mice. J Exp Med. 1999;190:1013–1024. doi: 10.1084/jem.190.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat Med. 2001;7:913–919. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- 6.Fortin CF, Lesur O, Fulop T., Jr Effects of aging on triggering receptor expressed on myeloid cells (TREM)-1-induced PMN functions. FEBS Lett. 2007;581:1173–1178. doi: 10.1016/j.febslet.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 7.Mocchegiani E, Malavolta M. NK and NKT cell functions in immunosenescence. Aging Cell. 2004;3:177–184. doi: 10.1111/j.1474-9728.2004.00107.x. [DOI] [PubMed] [Google Scholar]
- 8.Plowden J, Renshaw-Hoelscher M, Gangappa S, Engleman C, Katz JM, Sambhara S. Impaired antigen-induced CD8+ T cell clonal expansion in aging is due to defects in antigen presenting cell function. Cell Immunol. 2004;229:86–92. doi: 10.1016/j.cellimm.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Moser M. Regulation of Th1/Th2 development by antigen-presenting cells in vivo. Immunobiology. 2001;204:551–557. doi: 10.1078/0171-2985-00092. [DOI] [PubMed] [Google Scholar]
- 10.Agrawal A, Agrawal S, Cao JN, Su H, Osann K, Gupta S. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway. J Immunol. 2007;178:6912–6922. doi: 10.4049/jimmunol.178.11.6912. [DOI] [PubMed] [Google Scholar]
- 11.Weber R, Deplazes P, Schwartz D. Diagnosis and clinical aspects of human microsporidiosis. Contrib Microbiol. 2000;6:166–192. doi: 10.1159/000060360. [DOI] [PubMed] [Google Scholar]
- 12.Lores B, Lopez-Miragaya I, Arias C, Fenoy S, Torres J, del Aguila C. Intestinal microsporidiosis due to Enterocytozoon bieneusi in elderly human immunodeficiency virus--negative patients from Vigo, Spain. Clin Infect Dis. 2002;34:918–921. doi: 10.1086/339205. [DOI] [PubMed] [Google Scholar]
- 13.Fogla R, Padmanabhan P, Therese KL, Biswas J, Madhavan HN. Chronic microsporidial stromal keratitis in an immunocompetent, non-contact lens wearer. Indian J Ophthalmol. 2005;53:123–125. doi: 10.4103/0301-4738.16177. [DOI] [PubMed] [Google Scholar]
- 14.Abreu-Acosta N, Lorenzo-Morales J, Leal-Guio Y, Coronado-Alvarez N, Foronda P, Alcoba-Florez J, Izquierdo F, Batista-Diaz N, Del Aguila C, Valladares B. Enterocytozoon bieneusi (microsporidia) in clinical samples from immunocompetent individuals in Tenerife, Canary Islands, Spain. Trans R Soc Trop Med Hyg. 2005;99:848–855. doi: 10.1016/j.trstmh.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt EC, Shadduck JA. Murine encephalitozoonosis model for studying the host-parasite relationship of a chronic infection. Infect Immun. 1983;40:936–942. doi: 10.1128/iai.40.3.936-942.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hermanek J, Koudela B, Kucerova Z, Ditrich O, Travnicek J. Prophylactic and therapeutic immune reconstitution of SCID mice infected with Encephalitozoon cuniculi. Folia Parasitol (Praha) 1993;40:287–291. [PubMed] [Google Scholar]
- 17.Moretto M, Casciotti L, Durell B, Khan IA. Lack of CD4(+) T cells does not affect induction of CD8(+) T-cell immunity against Encephalitozoon cuniculi infection. Infect Immun. 2000;68:6223–6232. doi: 10.1128/iai.68.11.6223-6232.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braunfuchsova P, Salat J, Kopecky J. CD8+ T lymphocytes protect SCID mice against Encephalitozoon cuniculi infection. Int J Parasitol. 2001;31:681–686. doi: 10.1016/s0020-7519(01)00134-5. [DOI] [PubMed] [Google Scholar]
- 19.Khan IA, Schwartzman JD, Kasper LH, Moretto M. CD8+ CTLs are essential for protective immunity against Encephalitozoon cuniculi infection. J Immunol. 1999;162:6086–6091. [PubMed] [Google Scholar]
- 20.Moretto M, Weiss LM, Khan IA. Induction of a rapid and strong antigen-specific intraepithelial lymphocyte response during oral Encephalitozoon cuniculi infection. J Immunol. 2004;172:4402–4409. doi: 10.4049/jimmunol.172.7.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moretto MM, Weiss LM, Combe CL, Khan IA. IFN-gamma-producing dendritic cells are important for priming of gut intraepithelial lymphocyte response against intracellular parasitic infection. J Immunol. 2007;179:2485–2492. doi: 10.4049/jimmunol.179.4.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moretto M, Durell B, Schwartzman JD, Khan IA. Gamma delta T cell-deficient mice have a down-regulated CD8+ T cell immune response against Encephalitozoon cuniculi infection. J Immunol. 2001;166:7389–7397. doi: 10.4049/jimmunol.166.12.7389. [DOI] [PubMed] [Google Scholar]
- 23.Guan H, Moretto M, Bzik DJ, Gigley J, Khan IA. NK cells enhance dendritic cell response against parasite antigens via NKG2D pathway. J Immunol. 2007;179:590–596. doi: 10.4049/jimmunol.179.1.590. [DOI] [PubMed] [Google Scholar]
- 24.Neter J, Wasserman W, Kuter MH. Applied Linear Statistical Models. Irwin; Homeweed, IL: 1985. [Google Scholar]
- 25.Salat J, Sak B, Le T, Kopecky J. Susceptibility of IFN-gamma or IL-12 knock-out and SCID mice to infection with two microsporidian species, Encephalitozoon cuniculi and E. intestinalis. Folia Parasitol (Praha) 2004;51:275–282. [PubMed] [Google Scholar]
- 26.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 27.Combe CL, Moretto MM, Schwartzman JD, Gigley JP, Bzik DJ, Khan IA. Lack of IL-15 results in the suboptimal priming of CD4+ T cell response against an intracellular parasite. Proc Natl Acad Sci U S A. 2006;103:6635–6640. doi: 10.1073/pnas.0506180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohteki T, Tada H, Ishida K, Sato T, Maki C, Yamada T, Hamuro J, Koyasu S. Essential roles of DC-derived IL-15 as a mediator of inflammatory responses in vivo. J Exp Med. 2006;203:2329–2338. doi: 10.1084/jem.20061297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chai JG, Vendetti S, Bartok I, Schoendorf D, Takacs K, Elliott J, Lechler R, Dyson J. Critical role of costimulation in the activation of naive antigen-specific TCR transgenic CD8+ T cells in vitro. J Immunol. 1999;163:1298–1305. [PubMed] [Google Scholar]
- 30.Castle SC. Clinical relevance of age-related immune dysfunction. Clin Infect Dis. 2000;31:578–585. doi: 10.1086/313947. [DOI] [PubMed] [Google Scholar]
- 31.Laupland KB, Church DL, Mucenski M, Sutherland LR, Davies HD. Population-based study of the epidemiology of and the risk factors for invasive Staphylococcus aureus infections. J Infect Dis. 2003;187:1452–1459. doi: 10.1086/374621. [DOI] [PubMed] [Google Scholar]
- 32.Vasto S, Colonna-Romano G, Larbi A, Wikby A, Caruso C, Pawelec G. Role of persistent CMV infection in configuring T cell immunity in the elderly. Immun Ageing. 2007;4:2. doi: 10.1186/1742-4933-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pawelec G, Koch S, Griesemann H, Rehbein A, Hahnel K, Gouttefangeas C. Immunosenescence, suppression and tumour progression. Cancer Immunol Immunother. 2006;55:981–986. doi: 10.1007/s00262-005-0109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vu T, Farish S, Jenkins M, Kelly H. A meta-analysis of effectiveness of influenza vaccine in persons aged 65 years and over living in the community. Vaccine. 2002;20:1831–1836. doi: 10.1016/s0264-410x(02)00041-5. [DOI] [PubMed] [Google Scholar]
- 35.Deng Y, Jing Y, Campbell AE, Gravenstein S. Age-related impaired type 1 T cell responses to influenza: reduced activation ex vivo, decreased expansion in CTL culture in vitro, and blunted response to influenza vaccination in vivo in the elderly. J Immunol. 2004;172:3437–3446. doi: 10.4049/jimmunol.172.6.3437. [DOI] [PubMed] [Google Scholar]
- 36.Min H, Montecino-Rodriguez E, Dorshkind K. Reduction in the developmental potential of intrathymic T cell progenitors with age. J Immunol. 2004;173:245–250. doi: 10.4049/jimmunol.173.1.245. [DOI] [PubMed] [Google Scholar]
- 37.Miller JP, Allman D. The decline in B lymphopoiesis in aged mice reflects loss of very early B-lineage precursors. J Immunol. 2003;171:2326–2330. doi: 10.4049/jimmunol.171.5.2326. [DOI] [PubMed] [Google Scholar]
- 38.Effros RB, Cai Z, Linton PJ. CD8 T cells and aging. Crit Rev Immunol. 2003;23:45–64. doi: 10.1615/critrevimmunol.v23.i12.30. [DOI] [PubMed] [Google Scholar]
- 39.Grubeck-Loebenstein B, Wick G. The aging of the immune system. Adv Immunol. 2002;80:243–284. doi: 10.1016/s0065-2776(02)80017-7. [DOI] [PubMed] [Google Scholar]
- 40.Linton PJ, Haynes L, Klinman NR, Swain SL. Antigen-independent changes in naive CD4 T cells with aging. J Exp Med. 1996;184:1891–1900. doi: 10.1084/jem.184.5.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haynes L. The effect of aging on cognate function and development of immune memory. Curr Opin Immunol. 2005;17:476–479. doi: 10.1016/j.coi.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hakim FT, Flomerfelt FA, Boyiadzis M, Gress RE. Aging, immunity and cancer. Curr Opin Immunol. 2004;16:151–156. doi: 10.1016/j.coi.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 43.Steger MM, Maczek C, Grubeck-Loebenstein B. Peripheral blood dendritic cells reinduce proliferation in in vitro aged T cell populations. Mech Ageing Dev. 1997;93:125–130. doi: 10.1016/s0047-6374(96)01835-0. [DOI] [PubMed] [Google Scholar]
- 44.Maletto BA, Gruppi A, Moron G, Pistoresi-Palencia MC. Age-associated changes in lymphoid and antigen-presenting cell functions in mice immunized with Trypanosoma cruzi antigens. Mech Ageing Dev. 1996;88:39–47. doi: 10.1016/0047-6374(96)01719-8. [DOI] [PubMed] [Google Scholar]
- 45.Sen G, Chen Q, Snapper CM. Immunization of aged mice with a pneumococcal conjugate vaccine combined with an unmethylated CpG-containing oligodeoxynucleotide restores defective immunoglobulin G antipolysaccharide responses and specific CD4+-T-cell priming to young adult levels. Infect Immun. 2006;74:2177–2186. doi: 10.1128/IAI.74.4.2177-2186.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Looney RJ, Falsey AR, Walsh E, Campbell D. Effect of aging on cytokine production in response to respiratory syncytial virus infection. J Infect Dis. 2002;185:682–685. doi: 10.1086/339008. [DOI] [PubMed] [Google Scholar]
- 47.Cai NS, Li DD, Cheung HT, Richardson A. The expression of granulocyte/macrophage colony-stimulating factor in activated mouse lymphocytes declines with age. Cell Immunol. 1990;130:311–319. doi: 10.1016/0008-8749(90)90274-u. [DOI] [PubMed] [Google Scholar]
- 48.Gon Y, Hashimoto S, Hayashi S, Koura T, Matsumoto K, Horie T. Lower serum concentrations of cytokines in elderly patients with pneumonia and the impaired production of cytokines by peripheral blood monocytes in the elderly. Clin Exp Immunol. 1996;106:120–126. [PubMed] [Google Scholar]
- 49.Dubsky P, Saito H, Leogier M, Dantin C, Connolly JE, Banchereau J, Palucka AK. IL-15-induced human DC efficiently prime melanoma-specific naive CD8+ T cells to differentiate into CTL. Eur J Immunol. 2007;37:1678–1690. doi: 10.1002/eji.200636329. [DOI] [PubMed] [Google Scholar]
- 50.Regamey N, Obregon C, Ferrari-Lacraz S, van Leer C, Chanson M, Nicod LP, Geiser T. Airway epithelial IL-15 transforms monocytes into dendritic cells. Am J Respir Cell Mol Biol. 2007;37:75–84. doi: 10.1165/rcmb.2006-0235OC. [DOI] [PubMed] [Google Scholar]
- 51.Pulendran B, Dillon S, Joseph C, Curiel T, Banchereau J, Mohamadzadeh M. Dendritic cells generated in the presence of GM-CSF plus IL-15 prime potent CD8+ Tc1 responses in vivo. Eur J Immunol. 2004;34:66–73. doi: 10.1002/eji.200324567. [DOI] [PubMed] [Google Scholar]
- 52.Kuwajima S, Sato T, Ishida K, Tada H, Tezuka H, Ohteki T. Interleukin 15-dependent crosstalk between conventional and plasmacytoid dendritic cells is essential for CpG-induced immune activation. Nat Immunol. 2006;7:740–746. doi: 10.1038/ni1348. [DOI] [PubMed] [Google Scholar]
- 53.Parayath KE, Harrison TS, Levitz SM. Effect of interleukin (IL)-15 priming on IL-12 and interferon-gamma production by pathogen-stimulated peripheral blood mononuclear cells from human immunodeficiency virus-seropositive and -seronegative donors. J Infect Dis. 2000;181:733–736. doi: 10.1086/315280. [DOI] [PubMed] [Google Scholar]
- 54.Medzhitov R, Janeway CA., Jr Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- 55.Tourkova IL, Yurkovetsky ZR, Gambotto A, Makarenkova VP, Perez L, Balkir L, Robbins PD, Shurin MR, Shurin GV. Increased function and survival of IL-15-transduced human dendritic cells are mediated by up-regulation of IL-15Ralpha and Bcl-2. J Leukoc Biol. 2002;72:1037–1045. [PubMed] [Google Scholar]
- 56.Sharma S, Dominguez AL, Lustgarten J. Aging affect the anti-tumor potential of dendritic cell vaccination, but it can be overcome by co-stimulation with anti-OX40 or anti-4-1BB. Exp Gerontol. 2006;41:78–84. doi: 10.1016/j.exger.2005.10.002. [DOI] [PubMed] [Google Scholar]