Abstract
The coupling of two nitric oxide (NO) molecules in heme active sites is an important contributor to the conversion of NO to nitrous oxide (N2O) by heme-containing enzymes. Several formulations for the presumed heme-Fe{N2O2}n− intermediates have been proposed previously, however, no crystal structures of heme-Fe{N2O2}n− systems have been reported to date. We report the first isolation and characterization of a stable bimetallic hyponitrite iron porphyrin, [(OEP)Fe]2(µ–N2O2), prepared from the reaction of the [(OEP)Fe]2(µ–O) with hyponitrous acid. Density functional calculations were performed on the model compound [(porphine)Fe]2(µ–N2O2) to characterize its electronic structure and properties.
Heme-assisted coupling of nitric oxide (NO) to form Fe{N2O2}n− intermediates plays an important part in the reduction of NO to N2O.1,2 In transition-metal chemistry, such metal{N2O2}n− moieties can be generated from attack of NO on a metal-NO group,3,4 from metal-induced coupling of NO,5 or from transfer of an intact (N2O2)n− from a diazeniumdiolate to a metal.6 It is interesting to note that only a small number of metal{N2O2}-containing compounds have been structurally characterized, and the {N2O2} binding modes determined to date are sketched below as structures A (M = Pt, Ni),5,6 B (M = M’ = Co),7 C (M = Ru)8 and D (M = Co).9
Enzymes such as the NO reductase (NOR) from Paracoccus denitrificans, cyt ba3 and caa3 from Thermus thermophilus, and cyt cbb3 from Paracoccus stutzeri catalyze the conversion of NO to N2O using bimetallic active sites.1,2 For these biological systems, intermediates resembling structure B have been proposed, where the metal{N2O2} moiety is N-bound to heme Fe (M), and where M’ (non-heme Fe or Cu) may contact both O atoms.1,10 Collman et al. have proposed, using results from the reaction of a diferrous synthetic model of NOR with NO, that a trans bis-nitrosyl intermediate forms at the active site of NOR followed by NO coupling (structure E) to give N2O and a bis-ferric product.11 Resonance Raman spectroscopy has been employed to assign a HONNO-bridged Fe-Cu species (i.e., a protonated analogue of E) during NO reduction by T. thermophilus cyt ba3.12 To the best of our knowledge, however, no adduct of heme and an N2O2 moiety has been structurally characterized.
The title compound was obtained from the reaction of [(OEP)Fe]2(µ-O) in anhydrous toluene with an in situ-generated ether solution of hyponitrous acid (Supporting Information) (eq 1). Workup gave the product [(OEP)Fe]2(µ-N2O2) (1) as dark purple microcrystals in 52% isolated yield.
| (1) |
The IR spectrum of 1 reveals a band at 982 cm−1 assigned to υas of the N-O group (υas(15NO) 973 cm−1). This band is lower than that reported for H2N2O2 at 1014/1003 cm−1 (υas(15NO) 991/980 cm−1).13 The υs(NO) and υ(NN) bands were not observed in the IR spectrum, presumably due to the symmetry of 1. The EPR spectrum of 1 as a CH2Cl2/toluene glass (77 K) shows g = 5.74 and 2.03; similar data were obtained for high-spin (H.S.) ferric hydroxo porphyrins.14
The X-ray crystal structure of 1 reveals that the N2O2 ligand is bound to each Fe via the η1-O binding mode and that the ONNO moiety is trans (Figure 1). The N–N bond length of 1.250(3) Å suggests double bond character (c.f., 1.256(2) Å in crystalline Na2N2O2).15 The distance between the two Fe centers is 6.7 Å, and is longer than the 4.4 Å distance between the Fe and Cu centers in T. thermophilus cyt ba3 that exhibits NOR activity.16 The magnitude of the Fe–N(por) bond lengths in 1 and the apical displacement of Fe from the 4N-atom porphyrin mean plane (ΔFe = 0.40 Å) are indicative of H.S. ferric Fe centers. It follows that the N2O2 ligand in 1 must have significant hyponitrite (i.e., dianionic) character.
Figure 1.
Molecular structure of [(OEP)Fe]2(µ-ONNO) (1). Hydrogen atoms and the dichloromethane solvates have been omitted for clarity. Selected bond lengths (Å) and angles (°): Fe–O = 1.889(2), O–N = 1.375(2), N–N = 1.250(3), Fe–N(por) = 2.049(2)–2.064(2), ∠FeON = 118.56(12), ∠NNO = 108.5(2).
To characterize the electronic structure and properties of 1, we performed calculations using density functional theory (DFT) on the model compound [(porphine)Fe]2(η1,η1,µ-N2O2) (1-calc) in the trans and cis configurations. The DFT calculations were based on the B3LYP exchange-correlation functional and a triple-zeta polarization basis set. In both trans and cis configurations, the H.S. species is calculated to be lower in energy relative to the intermediate- (I.S.) and low-spin (L.S.) variants by 8 and 19 kcal/mol, respectively (Table S2). The calculated geometry of the trans H.S. species most closely reproduces the crystal structural data (Table 1). Curiously, the different spin states of the trans isomer are calculated to be ~2 kcal/mol higher in energy than the respective cis variants. This small difference lies within the typical error of 3–5 kcal/mol estimated for B3LYP calculations of transition-metal complexes. Thus, the DFT calculations are consistent with the EPR and structural data in establishing the identity of a stable H.S. trans species. The small energy gap between the trans and cis configurations suggests that isolation of the cis derivative of 1 might be attainable.
Table 1.
Selected crystal data for 1, and calculated geometry data (in Å and °) for [(porphine)Fe]2(η1,η1,µ-N2O2)(1-calc).
| Fe–O | O–N | N–N | ∠NNO | ΔFe | |
|---|---|---|---|---|---|
| 1 (crystal; trans) | 1.889 | 1.375 | 1.250 | 108.5 | 0.402 |
| 1-calc | |||||
| trans-H.S. | 1.909 | 1.376 | 1.269 | 108.9 | 0.482 |
| trans-I.S. | 1.951 | 1.364 | 1.280 | 109.3 | 0.272 |
| trans-L.S. | 1.831 | 1.398 | 1.266 | 107.4 | 0.249 |
| cis-H.S. | 1.899 | 1.390 | 1.254 | 117.0 | 0.485 |
| cis-I.S. | 1.945 | 1.379 | 1.262 | 117.9 | 0.279 |
| cis-L.S. | 1.831 | 1.403 | 1.258 | 116.5 | 0.232 |
To probe the charge distribution in 1, we used multipole-derived population analysis for the trans H.S. form of 1-calc. The atomic charges on the Fe, O and N atoms are shown in Figure 2. To further understand the charge distribution in the FeONNOFe moiety, we used the Nalewajski-Mrozek scheme to calculate the bond orders. Accordingly, the bond orders for Fe–O, O–N, and N–N in 1-calc trans-H.S. (Figure 2) are 0.79, 1.25, and 1.84, respectively, which supports the notion of an N=N double bond in the hyponitrite bridge.
Figure 2.
Calculated atomic charges and bond orders (bo) for the FeONNOFe moiety.
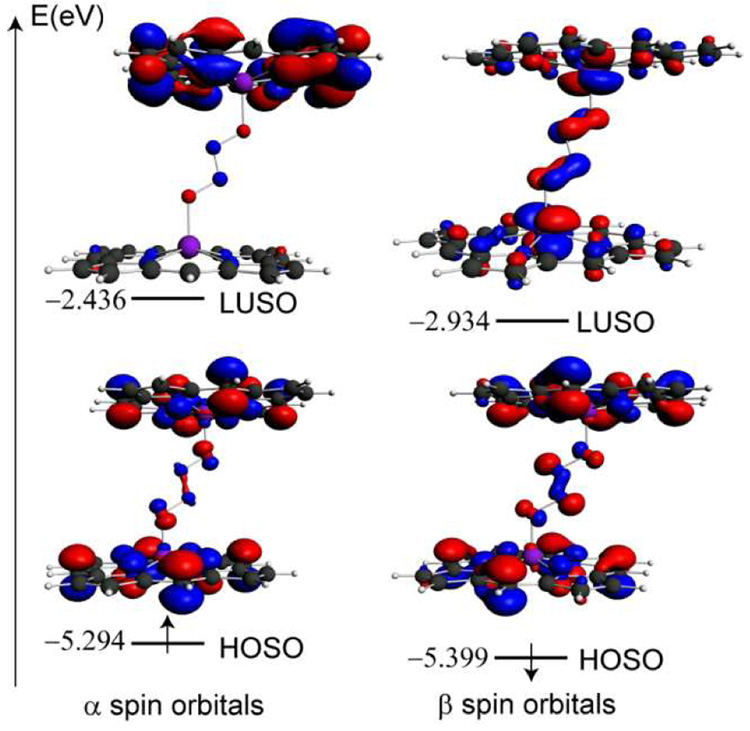
The Kohn-Sham orbitals of the trans H.S. species of 1-calc are expected to be similar to those of square pyramidal metal complexes, for which the highest occupied orbital and the lowest unoccupied orbital are the result of anti-bonding interactions between dz2 as well as d(x2-y2) orbitals of the metal and the symmetrized combinations of the ligand σ orbitals. The frontier spin orbitals from the unrestricted openshell calculation are shown in Figure 3. The highest occupied α spin orbital exhibits more porphine character while the lowest unoccupied spin β orbital displays more metal character. The N atoms of the hyponitrite bridge form a bonding interaction in both highest occupied spin orbitals.
Figure 3.
Frontier spin orbitals for high-spin 1-calc. HOSO and LUSO denote the highest occupied and the lowest unoccupied spin orbitals, respectively.
Protonation of a toluene solution of 1 at 0 °C using HCl results in the formation of N2O and (OEP)FeCl (eq 2). An IR
| (2) |
spectrum of the headspace reveals new bands at 2236/2213 and 1298/1266 cm−1 assigned to υas and υs of N2O, respectively. The use of the 15N-labeled 1 (labeled at hyponitrite) shifts the υas bands to 2167/2144 cm−1; the corresponding υs bands were not observed due to its occurrence outside the detection window.
We hypothesized that the protonation reaction is initiated by H+ attack on one of the O atoms of the hyponitrite bridge followed by O–N bond cleavage to give N2O. This is supported by the pronounced negative charge on the hyponitrite O atom in trans-H.S. 1-calc obtained from the DFT calculation. Further studies are underway to uncover the mechanism of the protonation reaction.
In summary, we have prepared and characterized the first isolable hyponitrite iron porphyrin complex, and describe the first established hyponitrite O-bonding mode to a heme model.
Supplementary Material
Experimental procedure, computational details and additional references, and CIF file for 1. This material is available free of charge via the internet at http://pubs.acs.org.
Acknowledgment
We are grateful to the National Institutes of Health for funding (GM076640; GBR-A).
References
- 1.Moenne-Loccoz P. Nat. Prod. Rep. 2007;24:610–620. doi: 10.1039/b604194a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeung N, Lu Y. Chem. Biodiversity. 2008;5:1437–1454. doi: 10.1002/cbdv.200890134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franz KJ, Lippard SJ. J. Am. Chem. Soc. 1999;121:10504–10512. [Google Scholar]
- 4.Schneider JL, Carrier SM, Ruggiero CE, Young VG, Tolman WB. J. Am. Chem. Soc. 1998;120:11408–11418. [Google Scholar]
- 5.Arulsamy N, Bohle DS, Imonigie JA, Moore RC. Polyhedron. 2007;26:4737–4745. [Google Scholar]
- 6.Arulsamy N, Bohle DS, Imonigie JA, Levine S. Angew. Chem. Int. Ed. 2002;41:2371–2373. doi: 10.1002/1521-3773(20020703)41:13<2371::AID-ANIE2371>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 7.Villalba MEC, Navaza A, Guida JA, Varetti EL, Aymonino PJ. Inorg. Chim. Acta. 2006;359:707–712. [Google Scholar]
- 8.Bottcher H-C, Graf M, Mereiter K, Kirchner K. Organometallics. 2004;23:1269–1273. [Google Scholar]
- 9.Bau R, Sabherwal IH, Burg AB. J. Am. Chem. Soc. 1971;93:4926–4928. [Google Scholar]
- 10.Blomberg LM, Blomberg MRA, Siegbahn PEM. Biochim. Biophys. Acta. 2006;1757:31–46. doi: 10.1016/j.bbabio.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Collman JP, Dey A, Yang Y, Decreau RA, Ohta T, Solomon EI. J. Am. Chem. Soc. 2008;130:16498–16499. doi: 10.1021/ja807700n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varotsis C, Ohta T, Kitagawa T, Soulimane T, Pinakoulaki E. Angew. Chem. Int. Ed. 2007;46:2210–2214. doi: 10.1002/anie.200602963. [DOI] [PubMed] [Google Scholar]
- 13.McGraw GE, Bernitt DL, Hisatsune IC. Spectrochim Acta. 1967;23A:25–34. [Google Scholar]
- 14.Cheng R-U, Latos-Grazynski L, Balch AL. Inorg. Chem. 1982;21:2412–2418. [Google Scholar]
- 15.Arulsamy N, Bohle DS, Imonigie JA, Sagan ES. Inorg. Chem. 1999;38:2716–2725. [Google Scholar]
- 16.Soulimane T, Buse G, Bourenkov GP, Bartunik HD, Huber R, Than ME. Embo J. 2000;19:1766–1776. doi: 10.1093/emboj/19.8.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedure, computational details and additional references, and CIF file for 1. This material is available free of charge via the internet at http://pubs.acs.org.