Abstract
Background
Caveolae, lipid-rich microdomains of the sarcolemma, localize and enrich cardiac protective signaling molecules. Caveolin-3 (Cav-3), the dominant isoform in cardiac myocytes, is a determinant of caveolae formation. We hypothesized that cardiac myocyte-specific overexpression of Cav-3 would enhance the formation of caveolae and augment cardiac protection in vivo.
Methods and Results
Ischemic preconditioning (IPC) in vivo increased formation of caveolae. Adenovirus for Cav-3 increased caveolar formation and phosphorylation of survival kinases in cardiac myocytes. A transgenic (TG) mouse with cardiac myocyte-specific overexpression of Cav-3 (Cav-3 OE) showed enhanced formation of caveolae on the sarcolemma. Cav-3 OE mice subjected to ischemia/reperfusion injury had a significantly reduced infarct size relative to TGneg mice. Endogenous cardiac protection in Cav-3 OE mice was similar to wild-type mice undergoing IPC; no increased protection was observed in preconditioned Cav-3 OE mice. Cav-3 knockout mice did not show endogenous protection and showed no protection in response to IPC. Cav-3 OE mouse hearts had increased basal Akt and GSK3β phosphorylation comparable to wild-type mice exposed to IPC. Wortmannin, a PI3K inhibitor, attenuated basal phosphorylation of Akt and GSK3β and blocked cardiac protection in Cav-3 OE mice. Cav-3 OE mice had improved functional recovery and reduced apoptosis at 24 h of reperfusion.
Conclusion
Expression of caveolin-3 is both necessary and sufficient for cardiac protection, a conclusion that unites long-standing ultrastructural and molecular observations in the ischemic heart. The current results indicate that increased expression of caveolins, apparently via actions that depend on PI3K, has the potential to protect hearts exposed to ischemia-reperfusion injury.
Keywords: heart, cardiac protection, myocardial ischemia, caveolae, caveolin
INTRODUCTION
The concept that an organ can develop tolerance to subsequent ischemic stress was initially suggested by studies of traumatic injury.1 In 1986, Murry et al2 found that non-lethal injury, termed ischemic preconditioning (IPC), could protect the heart from lethal injury. Subsequent work has shown that IPC is a highly effective way to protect multiple organs from ischemic injury. Many studies have evaluated individual signaling molecules and pathways in IPC3 but no single, unifying intervention explains the temporal efficiency (i.e., rapid coupling of plasma membrane to intracellular signaling) and spatial 3-dimensionality (i.e., simultaneous activation of numerous parallel pathways) of IPC. An emerging idea in signal transduction emphasizes the role of multi-protein complexes organized in discrete microenvironments in cell regulation and pathophysiology;4, 5 such organization perhaps might explain the temporal/spatial conundrum of IPC.
Caveolae, cholesterol- and sphingolipid-enriched invaginations of the plasma membrane, a subset of lipid/membrane rafts, are one such microenvironment.6-8 Caveolins, structural proteins essential for caveolae formation, are present in three isoforms:9 caveolin-1 and -2 (Cav-1 and -2) are expressed in multiple cell types, while caveolin-3 (Cav-3) is found primarily in striated (skeletal and cardiac) muscle and certain smooth muscle cells.10 Caveolins have scaffolding domains that anchor and regulate the function of proteins that modulate a variety of cellular processes11 and signal transduction.4, 5 Caveolins can function as scaffolds for multiple, interacting signaling molecules, thereby providing temporal and spatial regulation of cellular signal transduction.5
Disruption of caveolae attenuates protection of adult cardiac myocytes from ischemic damage12 and Cav-3 knockout mice (Cav-3 KO), which lack cardiac myocyte caveolae, are resistant to pharmacological preconditioning.13 Such findings imply that myocyte caveolae are a prerequisite for protection from ischemia-reperfusion injury. Because Cav-3 expression is essential for the formation of caveolae in cardiac myocytes,14 we hypothesized and provide evidence that cardiac myocyte-specific overexpression of Cav-3 increases the formation of caveolae and enhances protective signaling from ischemia, thus identifying cell-specific expression of caveolins and caveolae as a novel approach to achieve such protection.
METHODS
All authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Antibodies
Antibody sources: polyclonal antibody to Cav-1, Abcam Inc. and Cell Signaling; polyclonal antibody to Cav-2, Abcam; monoclonal and polyclonal antibody to Cav-3, BD Transduction, Abcam, and Santa Cruz Biotechnology; monoclonal iNOS, polyclonal nNOS, polyclonal antibodies to phospho-eNOS (Ser1177) and (Thr495), Cell Signaling; polyclonal antibody to IAP-1, Abcam; monoclonal antibody to Fas, Upstate; monoclonal to GAPDH, IMGENEX.
Animals
Animals were treated in compliance with the Guide for the Care and Use of Laboratory Animals (National Academy of Science) and protocols were approved by the VA San Diego Healthcare System IACUC. Animals were kept on a 12 h light-dark cycle in a temperature-controlled room with ad lib access to food and water.
Cav-3 OE mice were produced in a C57BL/6 background. Full-length Cav-3 cDNA (455 bp) was cloned into a vector containing the α myosin heavy chain promoter and was used for microinjection (UCSD Transgenic Core, Supplemental Fig. 1). Cav-3 KO mice were created as reported previously.15 Transgene negative siblings served as controls for Cav-3 OE mice (TGneg) and wild-type C57BL/6 mice served as controls for Cav-3 knockout mice.
Electron microscopy (EM)
Whole hearts or cells were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer for 2 h, post-fixed in 1% OsO4 in 0.1 M cacodylate buffer (1 h) and embedded as monolayers in LX-112 (Ladd Research). Sections were stained in uranyl acetate and lead citrate and observed with an electron microscope (JEOL 1200 EX-II, JEOL USA or Philips CM-10, Philips Electronic Instruments). Random sections were taken by an EM technician blinded to the treatments.
Sucrose density membrane fractionation
Whole hearts were fractionated using sucrose density gradients as reported.16 Fractions 4-6 were buoyant membrane fractions enriched in caveolins and proteins associated with caveolins. Fractions 9-12 were defined as non-buoyant fractions.
Immunoblot
Whole tissue or cell lysates were separated by SDS-polyacrylamide gel electrophoresis using 10% polyacrylamide precast gels (Invitrogen) and transferred to polyvinylidene difluoride membranes by electroelution. Membranes were blocked in 20 mM TBS Tween (1%) containing 4% bovine serum albumin and incubated with primary antibody overnight at 4°C. Blots were visualized using secondary antibodies conjugated with horseradish peroxidase from Santa Cruz Biotech and ECL reagent from GE Healthcare.
Adult cardiac myocytes (CM)
CM were isolated from male Sprague-Dawley rats as described.12 Myocytes were plated in 4% fetal bovine serum on laminin (2 μg/cm2)-coated plates for 1 h. Plating media was changed to serum-free media (1% bovine serum albumin) to remove non-myocytes and CM were incubated at 37 °C in 5% CO2.
Plasmid and recombinant adenovirus production
A mouse Cav-3 cDNA (455bp) was generated, cloned, and co-transfected with pJM170 (containing E1 region deletion). Plaques were expanded in HEK293 cells transformed with adenovirus (Adv) E1. Adv containing LacZ (Adv.LacZ) served as control. CM were treated with Adv.LacZ or Adv.Cav-3 for 16-24 h.
Immunofluorescence
Ventricular tissue was mounted on a cryostat (-23 °C) and 10 μm sections were cut in long axis. Sections were prepared for immunofluorescence microscopy and deconvolution images were obtained as described.17
Cholesterol and NOS activity assays
Cholesterol in fractions was measured using the Amplex Red Cholesterol Assay Kit (Invitrogen) as described by the manufacturer. Basal NOS activity was determined in TGneg and Cav-3 OE mice. Homogenized tissues were prepared and measured using a Nitric Oxide Synthase Assay Kit, Colorimetric (EMD Biosciences) as described by the manufacturer. Additionally, TGneg and Cav-3 OE hearts were fractionated on a sucrose density gradient and NOS activity was assessed in buoyant (caveolar, BF) and non-buoyant (non-caveolar, non-BF) fractions using a [3H]-arginine NOS assay (EMD Bioscience) that measures conversion of [3H]-arginine to [3H]-citrulline as described by the manufacturer.
Echocardiography
Echocardiography was performed in mice anesthetized with isoflurane using an echocardiograph and L15/6-MHz transducer (Sonos 5500, Philips Medical Systems) as described previously.18
In vivo ischemia-reperfusion experimental protocol
Pentobarbital (80mg/kg)-anesthetized mice were mechanically ventilated and ischemia was produced by occluding the left coronary artery with a 7-0 silk suture on a tapered BV-1 needle (Ethicon) for 30 min.18 After 30 min occlusion, the ligature was released and the heart was reperfused for 2 or 24 h. IPC was induced by occlusion of the left coronary artery for 5 min followed by 15 min reperfusion just prior to ischemia. Some Cav-3 OE mice were treated with 5-hydroxydecanoate (5-HD, 10 mg/kg i.v., a mitochondrial KATP channel inhibitor, Sigma) 10 min before ischemia or wortmannin (15μg/kg, a phosphoinositide 3-kinase [PI3K] inhibitor) 15 min before ischemia.
Infarct size
The area at risk (AAR) was determined by staining with 1% Evans blue (1.0 ml, Sigma).18 The heart was immediately excised and cut into 1 mm slices (McIlwain tissue chopper; Brinkmann Instruments). Left ventricle was counterstained with 1% 2,3,5,-triphenyltetrazolium chloride (Sigma). Images were analyzed by Image-Pro Plus (Media Cybernetics) and infarct size was determined by planimetry. Cardiac troponin I (TnI) levels in the serum were measured using a High Sensitivity Mouse Cardiac Troponin-I ELISA Kit (Life Diagnostics).
Cardiac function
Mice underwent surgery as described in the ischemia-reperfusion protocol and were allowed to recover for 24 h. After 24 h, mice were anesthetized with pentobarbital (80mg/kg) and cardiac catheterization was performed using a high-fidelity 1.4F Mikro-tip pressure transducer (SPR-671, Millar). The catheter was advanced via the right carotid artery into the left ventricle after measuring mean arterial pressure (MAP). Parameters were determined by an algorithm from EMKA Technologies.
Apoptosis
For TUNEL assays, AAR was removed from the mice after 24 h of reperfusion. The tissue was cut, fixed in 3.7% formalin and sections (5 μm) were used for TUNEL assays using the Apoptosis Detection Kit (R&D Systems), according to the manufacturer's instructions. Real-time PCR analysis of gene expression of pro- and anti-apoptotic genes was performed on total RNA isolated from the heart after 24 h of reperfusion using a RNeasy Mini Kit (Qiagen Inc) as described previously.18 Immunoblots for Fas and IAP-1 were performed.
Statistical Analysis
Data analysis was performed by observers blinded to experimental groups. Group size to determine the primary outcome variable of infarct size was determined by power analysis. The power analyses suggest that a sample size of 7 mice per experimental group was sufficient assuming an alpha of 0.05, two-tailed at 90% power with a hypothetical mean difference of 20%. We performed statistical analysis with Prism 4.0 (GraphPad) by the unpaired Student's t-test or one-way ANOVA followed by post-hoc test with Bonferroni correction for multiple comparisons. All data are expressed as mean ± SEM. Statistical significance was defined as P <0.05.
RESULTS
Ischemic preconditioning modulates membrane caveolae and Cav-3 expression
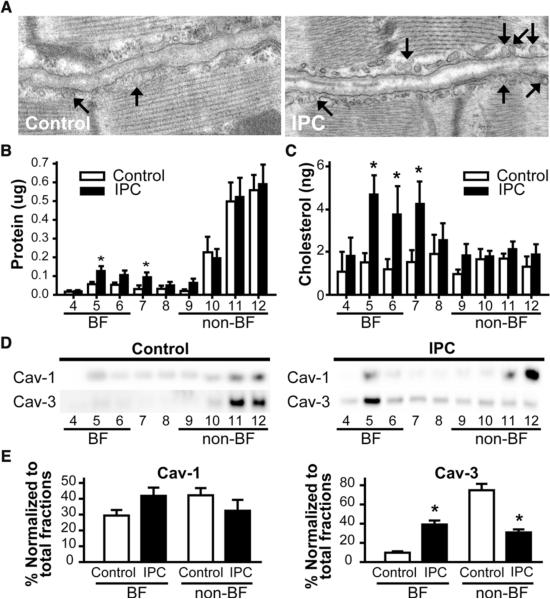
We assessed the effect of IPC on cardiac membrane caveolae by subjecting mice to IPC and performing electron microscopy (EM). Representative EM images show that IPC increases formation of caveolae (arrows in Fig. 1A). To verify these morphologic findings using biochemical techniques, hearts from IPC and control animals were fractionated on a discontinuous sucrose gradient and analyzed for protein and cholesterol content and for distribution of caveolin. IPC increases the total protein and cholesterol content in fractions 4-6 (buoyant fractions), which are enriched in caveolin17 (Fig. 1B,C) and increases the amount of Cav-3, but not Cav-1, in buoyant fractions (Fig. 1D,E). These finding are consistent with evidence that formation of cardiac myocyte caveolae is dependent on Cav-3.14, 19
Figure 1. IPC increases the expression of caveolae and the enrichment of protein and cholesterol in buoyant fractions.
Hearts were subjected to a 5 min IPC stimulus. Control wild-type animals underwent no treatment. (A) Electron microscopy showed an increase in number of caveolae compared to control hearts (arrow). (B) and (C) Excised control and hearts subjected to IPC underwent sucrose density fractionation. Protein and cholesterol assays showed increased protein and cholesterol in buoyant fractions (BF) after IPC (*P < 0.05). (D) and (E) Fractions were probed for Cav-1 and Cav-3. Cav-3, but not Cav-1, was increased in buoyant fractions after IPC (representative immunoblots are shown) and confirmed by densitometry normalized to total fraction amounts (E). *P <0.05 relative to respective control. Data are from 6 mice per group.
Adenoviral overexpression of Cav-3 in adult cardiac myocytes in vitro increases caveolae formation and phosphorylation of survival kinases
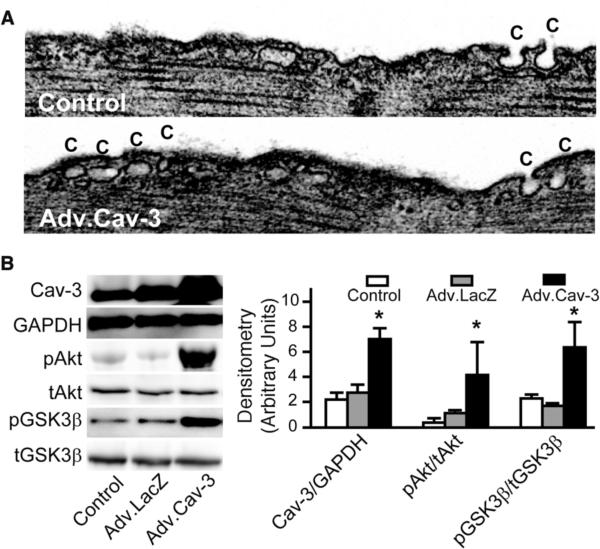
Cardiac myocytes incubated with a Cav-3-adenovirus have increased expression of caveolae (Fig. 2A) and increased levels of phosphorylated protein kinase B (Akt) and glycogen synthase kinase 3β (GSK3β), two enzymes associated with IPC-induced cardiac protection3 (Fig. 2B).
Figure 2. Cav-3 adenovirus increases caveolae expression in adult cardiac myocytes (ACM).
(A) ACM incubated with an adenovirus (Adv) encoding full-length mouse Cav-3 (Adv.Cav-3) for 72 h increased caveolae (C) number. (B) ACM exposed to no virus, Adv.LacZ or Adv.Cav-3 for 72 h. were lysed and immunoblotted (left panel). Adv.Cav-3-treated ACM have increased expression of Cav-3 protein as well as increased phosphorylated phospho Akt and GSK3β compared to control or LacZ-treated ACM (n = 4 for Akt and GSK3β and n=6 for Cav-3) (right panel). *P < 0.05 vs. Adv.LacZ.
Cardiac myocyte-specific Cav-3 overexpressing mice have increased myocyte caveolae but unaltered nitric oxide synthase (NOS) expression and activity
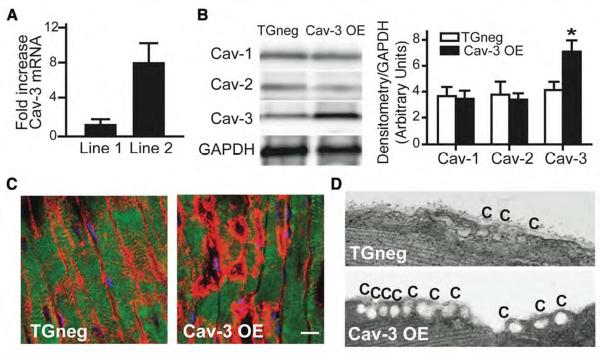
Using an α-myosin heavy chain promoter, we generated mice with cardiac myocyte-specific overexpression of Cav-3 (Supplementary Fig. 1A). Thirty-nine mouse lines were generated, 5 of which were positive for the transgene (TG) (Supplementary Fig. 1B). Two of these 5 lines were propagated, one of which had an 8-fold elevation in Cav-3 mRNA expression compared to TG-negative (TGneg) animals (Fig. 3A). We developed and characterized this line and refer to it as the Cav-3 overexpressor (Cav-3 OE). Cav-3 OE mice have increased Cav-3 protein expression without a change in Cav-1 and Cav-2 expression (Fig. 3B and red pixels, 3C). The increased protein expression results in an increased number of caveolae in cardiac myocytes (Fig. 3D); increased expression of Cav-3 was not observed in lung, brain, liver, kidney or skeletal muscle of these mice (data not shown).
Figure 3. Cardiac myocyte-specific Cav-3 OE mice: caveolin and caveolae.
(A) Real-time PCR of Cav-3 mRNA expression in two founder lines. Line 2 had 8-fold increased Cav-3 mRNA expression compared to Line 1. Data are represented relative to TGneg and normalized to GAPDH expression (n = 4). (B) Immunoblot of Cav-1, -2, and -3 in whole heart homogenates from Cav-3 OE and TGneg mice (left panel). Densitometry was normalized to expression of GAPDH and showed a significant increase in Cav-3 protein in whole heart homogenates (right panel; *P = 0.011, n=7 for TGneg and n=9 for Cav-3 OE). (C) Immunohistochemistry showed increased Cav-3 (red pixels) in sarcolemma of cardiac myocytes from Cav-3 OE vs. TGneg mice. The bar denotes 10 μm. (D) Electron microscopy shows increased caveolae (C) in cardiac myocytes from Cav-3 OE vs. TGneg mice.
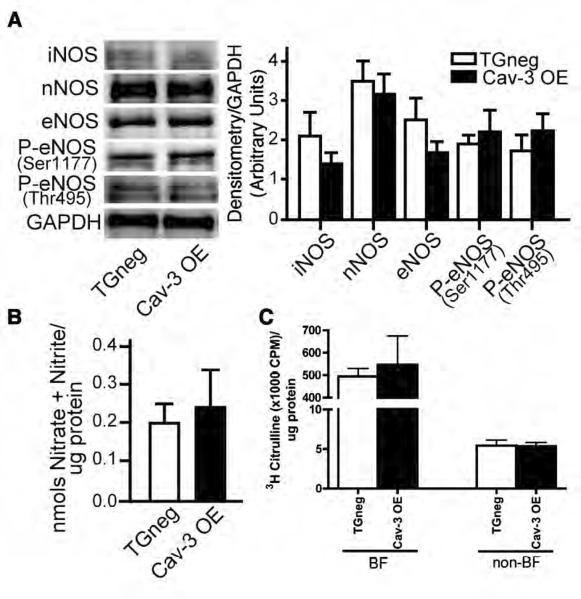
Because caveolins negatively regulate activity of NOS isoforms,11, 20 we quantitated basal NOS expression and activity in the Cav-3 OE mice. Given the well-known interaction of NOS with caveolins, we were surprised to find similar expression of NOS and phosphorylated endothelial NOS (eNOS) isoforms and similar NOS activity in the whole hearts of TGneg and Cav-3 OE mice (Fig. 4A,B). To determine if activity of NOS in caveolin-enriched membrane fractions may be altered by cardiac myocyte-specific caveolin-3 overexpression, we subjected TGneg and Cav-3 OE hearts to sucrose density fractionation and assessed NOS activity in BF and non-BF using a [3H]-arginine assay. No difference in NOS activity was observed in BF and non-BF in TGneg vs. Cav-3 OE (Fig. 4C). Rat cerebellum homogenate was used as a positive control ([3H]-citrulline, 42000 cpm/μl sample).
Figure 4. NOS expression and activity.
(A) Immunoblot analysis of basal expression of NOS and phosphorylated eNOS in TGneg and Cav-3 OE mice (left panel). Densitometry was normalized to GAPDH (right panel). No differences were observed in expression of any NOS isoforms. Data are from 5 mice per group. (B) Basal NOS activity was measured in TGneg and Cav-3 OE murine whole heart homogenates. No differences in basal NOS activity was observed between groups. (C) Hearts from TGneg and Cav-3 OE mice were homogenized in triton-X and fractionated on a discontinuous sucrose density gradient to separate buoyant (caveolae) and non-buoyant (non-caveolar membrane) fractions (BF and non-BF, respectively). NOS activity was measured in BF and non-BF using a [3H]-arginine assay. No difference in NOS activity was observed in the two separate fractions in TGneg vs. Cav-3 OE mice. Data are from 6 mice per group.
Cav-3 OE mice are protected from ischemia-reperfusion injury
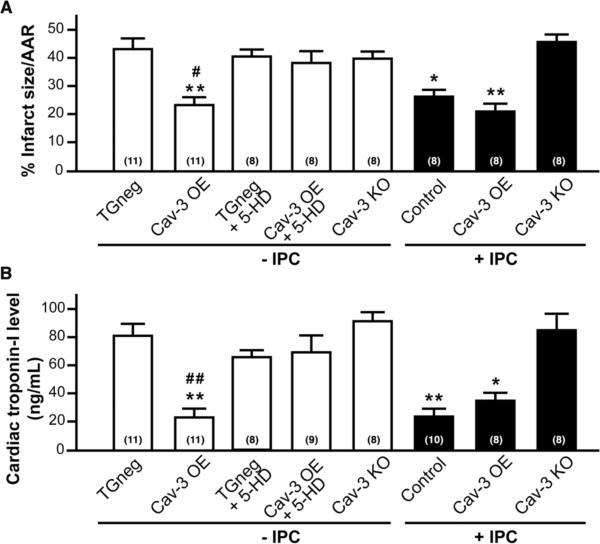
Cav-3 OE mice exposed to 30 min of cardiac ischemia and 2 h of reperfusion have a substantial reduction in infarct size compared to TGneg mice (23.4 ± 3.0% vs. 43.0 ± 3.9% risk area, n = 11, P < 0.001, Fig. 5A), even though these mice show no differences in pre-occlusion hemodynamics (heart rate, mean arterial pressure, and rate-pressure product; Supplementary Table 1) or in the cardiac AAR for ischemic damage (Supplementary Fig. 2). This “endogenous protection” in Cav-3 OE mice is similar to that produced in TGneg mice subjected to IPC (26.3 ± 2.4% risk area, n = 8 P < 0.05) and was not enhanced by IPC (21.1 ± 2.8% risk area, n = 8) but was attenuated by pretreatment with 5-HD, a mitochondrial ATP-sensitive potassium (KATP) channel inhibitor (38.1 ± 4.4% risk area, n = 8) (Fig. 5A). Akin to the results with 5-HD, IPC does not protect Cav-3 KO mice from ischemic damage (Fig 5A). Cardiac TnI confirmed infarct size measurements (Fig 5B).
Figure 5. Cardiac protection in Cav-3 OE mice.
Mice were subjected to ischemia-reperfusion injury. (A) Infarct size (percent of AAR) was reduced by IPC in control animals; however, Cav-3 OE mice were protected to similar levels with and without IPC. Cav-3 KO mice could not be protected with IPC. Treatment of Cav-3 OE mice with 5-hydroxydecanoate (5-HD; 10 mg/kg i.v.), a mitochondrial KATP channel inhibitor, abolished protection. TGneg treated with 5-HD had similar infarct size to controls. *P < 0.05, **P < 0.001 vs. TGneg mice, and #P < 0.05 vs. Cav-3 OE + 5-HD. Group sizes are indicated on the individual bars in parentheses. (B) Serum cardiac troponin-I, a marker of cardiac myocyte damage, was measured following 2 h of reperfusion. *P < 0.05, **P < 0.001 vs. TGneg mice, and ##P < 0.01 vs. Cav-3 OE + 5-HD. Group sizes are indicated on the individual bars parentheses.
Role of survival kinases in endogenous protection
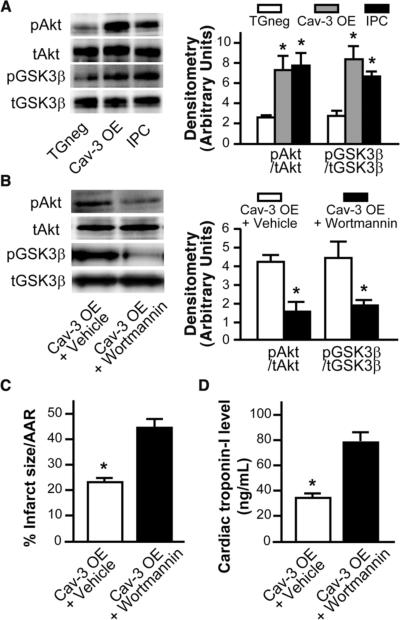
Hearts excised from Cav-3 OE mice had a ~3-fold increase in basal phosphorylation of Akt and GSK3β (P < 0.05, n = 6, Fig. 6A), signaling molecules involved in cardiac protection.21 The level of basal elevation in Akt and GSK3β in the hearts of Cav-3 OE mice was comparable to the elevation seen after IPC in vivo (Fig. 6A). To determine the role of PI3K/Akt/GSK3β in this endogenous protection, Cav-3 OE mice were treated with wortmannin, a PI3K inhibitor, or DMSO (vehicle). Wortmannin reduced the phosphorylation of both Akt and GSK3β in Cav-3 OE mice relative to vehicle control (Fig. 6B). Vehicle-treated Cav-3 OE mice showed a cardiac protected phenotype (as in Fig. 5A) with reduced infarct size and cTnI (23.3 ± 2.0% risk area, n = 7, Fig. 6C,D). Wortmannin attenuated the endogenous cardiac protection observed in vehicle Cav-3 OE mice (45.0 ± 3.0% risk area, n = 7, Fig. 6C,D).
Figure 6. Role of survival kinases in protection of Cav-3 OE mice.
(A) Whole heart homogenates showed elevated phosphorylation of Akt and GSK3β in IPC-treated mice and Cav-3 OE mice when compared to TGneg mice (n = 6 for TGneg, n=5 Cav-3 OE, and n=6 for IPC). Total Akt or GSK3β was used to assess protein loading. * P < 0.05 vs. TGneg mice. (B-D) To determine the role of PI3K/Akt/GSK3β in the endogenous protection, Cav-3 OE mice were treated with a PI3K inhibitor, wortmannin (15μg/kg), 15 minutes prior to index ischemia-reperfusion. DMSO vehicle treated Cav-3 OE served as controls (n=7). Wortmannin treatment resulted in decreased basal phosphorylation of Akt and GSK3β (B). Additionally, wortmannin treatment attenuated the endogenous protection seen in vehicle treated Cav-3 OE mice with respect to infarct size (C) and cardiac troponin-I (D), n=7 for both groups in C and D.
Cav-3 OE mice have preserved ultrastructure following ischemia-reperfusion injury
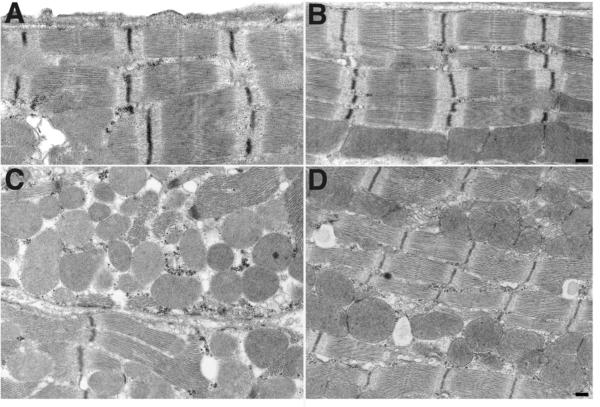
We also used EM analysis to examine the AAR after ischemia-reperfusion (30 min-2 h) in Cav-3 OE and TGneg mice (Fig. 7). Following injury, the ischemia-reperfusion TGneg groups displayed highly disorganized patterns of cardiac myocytes and their mitochondria. In addition, the sarcolemma exhibited evidence of damage, including disrupted Z-lines and myofibrillar stretching. Mitochondria were swollen and contained amorphous matrix densities, indicating that injury was in an irreversible phase22 (Fig. 7C). By contrast, hearts of Cav-3 OE mice subjected to ischemia-reperfusion showed limited ultrastructural change relative to sham and in particular, no mitochondrial swelling, myofibril stretching, or Z-line deformation (Figs. 7D).
Figure 7. Electron micrograph of area at risk from the hearts of TGneg and Cav-3 OE mice following ischemia-reperfusion.
(A) and (B) No tissue swelling or structural change were seen in sham groups from TGneg and Cav-3 OE mice. (C) After ischemia-reperfusion in TGneg mice, myofibrils were distended, Z-lines were irregular and unclear, and mitochondria were swollen and contained amorphous matrix densities. (D) Cav-3 OE mice had fewer damaged myocytes after ischemia-reperfusion: Myofibrils had well-arranged Z-lines and organized mitochondria.
Cav-3 OE mice have preserved cardiac function and reduced apoptosis following ischemia-reperfusion
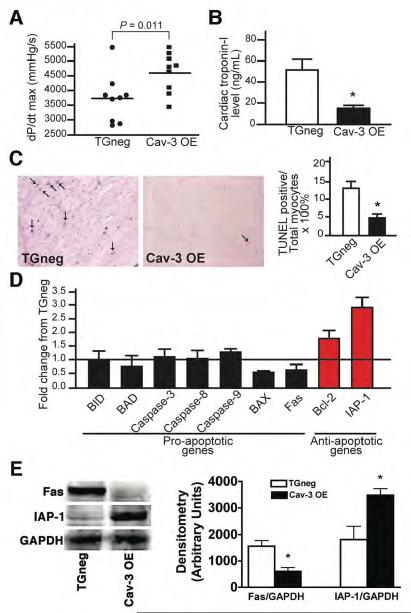
We assessed cardiac function of TGneg and Cav-3 OE mice during cardiac catheterization after 30 min ischemia and 24 h of reperfusion. Left ventricular systolic function (dP/dt max), was greater while cTnI levels were significantly lower in the Cav-3 OE mice (Fig. 8A,B).
Figure 8. Cardiac function and cell death in mice 24 h after ischemic injury.
Following 30 min of ischemia, mice were allowed to recover for 24 h. (A) Cav-3OE mice displayed better cardiac function, as measured by dP/dt max (4619 ± 226 vs. 3717 ± 275 mmHg/s, n = 9, P = 0.011). (B) Cardiac troponin-I levels were significantly decreased in Cav-3 OE mice (n = 7 of TGneg and n=8 for Cav-3 OE, *P = 0.002 vs. TGneg). (C) Apoptosis in the AAR, determined using TUNEL staining, in 24 h reperfused hearts (arrow, representative images). Nuclei staining positive for TUNEL, quantified as a percent of total nuclei, were significantly decreased in hearts from Cav-3 OE vs. TGneg mice (right panel, n=4 for TGneg and n=5 for Cav-3 OE). *P = 0.003 vs. TGneg mice. (D) Real-time PCR analysis of pro- and anti-apoptotic gene expression in AAR 24 h after reperfusion of Cav-3 OE mice and TGneg mice (n=5). (E) Immunoblot analysis shows decreased Fas (pro-apoptotic) and increased IAP-1 (anti-apoptotic) protein expression in agreement with mRNA data presented in (D) (n=4).
Cav-3 OE mice also had reduced apoptosis, assayed by terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL)-positive cells (arrows in Fig 8C, left panel), as the percent of total nuclei after 24 h of reperfusion (Fig. 8C, right panel). Hearts of Cav-3 OE animals have decreased pro-apoptotic and increased anti-apoptotic gene (Fig. 8D) and protein expression (Fig. 8E).
Total body overexpression of Cav-3 results in cardiomyopathy in 6 month old mice.23 Accordingly, we assessed morphology, echocardiography and hemodynamics in 6-9 month old cardiac myocyte-specific Cav-3 OE mice. We found that heart weight to tibia length were similar in Cav-3 OE and age-matched TGneg mice (Supplementary Table 2). In addition, echocardiographic parameters, heart rate, mean arterial pressure and rate-pressure product were similar between Cav-3 OE and TGneg mice (Supplementary Table 2).
DISCUSSION
Numerous mechanisms have been proposed to explain the ability of an organ to develop tolerance to subsequent lethal ischemia-reperfusion injury.24 IPC, an intervention that was first shown over 20 years ago to prevent such injury, has lacked a unifying hypothesis to account for the diverse pathways by which it impacts the heart and other organs. We show here that IPC alters the morphology and composition of the plasma membrane of cardiac myocytes, increasing the number of caveolae. Moreover, we find that the protein Cav-3 is both necessary and sufficient to protect the heart from ischemia-reperfusion injury. Spatial organization of signaling molecules within caveolar microdomains and the interaction of signaling molecules with caveolins thus help determine the protection of the heart from ischemia-reperfusion injury. The current data imply that expression of Cav-3 and caveolae represent a unifying mechanism for IPC.
Caveolae were first identified by electron microscopy in the 1950's by Palade and Yamada6, 7 in endothelium and epithelium, respectively, and in the sarcolemma of cardiac myocytes in 1975 by McNutt.25 Early research on myocardial ischemia focused on ischemia-induced changes in the sarcolemma26, 27 and provided evidence that caveolae in cardiac myocytes can be rapidly affected by perturbation of oxygen tension and tonicity.28, 29 In spite of such results, a role for caveolae and caveolins in the setting of ischemic preconditioning has not been explored.
An emerging concept emphasizes the organization of signaling molecules in multiprotein complexes, “signalosomes,” that form and dissociate under basal and stimulated conditions.11 Caveolins play an integral role in the dynamics of these multiprotein complexes in caveolae, by interacting with a wide range of signaling molecules that include multiple G-protein coupled receptors, Gα subunits of heterotrimeric G-proteins, Src kinases, PI3K, eNOS, protein kinase C (PKC) isoforms, extracellular signal-regulated kinase 1 and 2 (ERK 1/2), and superoxide dismutase. Many of these proteins can bind to the scaffolding domain of caveolin (CSD) and be regulated by caveolin.30 A number of proteins that bind caveolin have been overexpressed in cardiac myocytes and shown to produce tolerance to myocardial ischemia-reperfusion injury; these include adenosine receptors,31 alpha 1 adrenergic receptors,32 PKC isoforms,33 mitogen activated protein kinases,34 eNOS,35, 36 and proteins involved in the scavenging of free radicals.37 Overexpression of heat shock proteins (HSP: αB crystallin, HSP60-10 complex, and H11 kinase38-40) also confers protection from ischemic damage and conceivably caveolins contribute to such interactions.41 In silico analysis of the protein sequence of αB crystallin reveals a putative caveolin binding motif (aa167wvcyqypgy), suggesting future avenues of investigation.
Caveolar microdomains are enriched in cholesterol. We show that IPC increases caveolar microdomains and total cholesterol primarily in buoyant membrane fractions enriched in caveolin. The mechanism by which IPC increases total cholesterol is not known. Class B scavenger receptors (CD36) localize to caveolae and regulate cholesterol homeostasis.42 Co-expression of CD36 and caveolin has been shown to enhance the uptake of cholesterol.43 It is possible that the increase in cholesterol helps drive caveolae formation or alternatively, an increase in caveolae formation may drive the influx of cholesterol. Defining this distinction will be an interesting avenue for future investigation.
Of particular importance for ischemia is the evidence that Cav-3 is an activator of PI3K/Akt/GSK3β signaling,44 a pro-survival pathway that can contribute to cardiac ischemic preconditioning.3, 24 These findings are pertinent to the current data indicating that adenoviral Cav-3-mediated overexpression in myocytes and Cav-3 OE mice show increased Cav-3 expression, formation of caveolae and phosphorylation of Akt and GSK3β. In addition, our results show that mice engineered to overexpress Cav-3 in cardiac myocytes are protected from ischemia-reperfusion injury to an extent comparable to that induced by IPC and in a manner that depends on mitochondrial KATP channel and PI3K activity, putative effectors of cardiac protection from ischemia-reperfusion injury.24, 45 Cav-3 OE mice also showed a preserved sarcomeric ultrastructure following ischemia-reperfusion injury suggesting that Cav-3 OE mice are resistant to membrane damage. This preserved ultrastructure may result from reduced injury or perhaps is a direct consequence of caveolin-3 overexpression.
The precise molecular mechanism by which Cav-3 in myocytes protects the heart and its myocytes from ischemia-reperfusion injury remains to be determined. Caveolins can inhibit activity of signaling proteins, such as eNOS and ERK1/2,46, 47 by interaction of the CSD with the binding motif of such partners. In addition, caveolins can promote signaling via enhanced receptor-effector coupling or enhanced receptor affinity.11, 48 Based on data showing that infusion of a peptide with the CSD sequence can protect the heart from ischemia-reperfusion injury, Young et al49 proposed that the CSD peptide produces ischemic tolerance by enhancing release of endothelium-derived nitric oxide.49 Additional studies show that ischemia-reperfusion injury activates a redistribution of Cav-3 and a down-regulation of Cav-1 association with ERK50 while IPC leads to a translocation of eNOS to caveolae.51 The current results, in which overexpressed Cav-3 in cardiac myocytes yields no change in basal expression of NOS isoforms or in nitric oxide (NO) generation, lead us to conclude that the ischemic tolerance produced by Cav-3 expression in myocytes does not result from changes in NO production and that cardiac myocyte eNOS is not a major source of cardiac NO. The latter idea is supported by evidence that reexpression of Cav-1 exclusively within the endothelium of Cav-1 knockout mice rescues cardiac defects.52
The phenotype of cardiac myocyte-specific Cav-3 OE mice is strikingly different from that of mice that have a total body overexpression of Cav-3 in brain, fat, liver, lung and spleen as well as smooth, skeletal and cardiac muscle.53 Such mice develop a muscular dystrophy phenotype at 3-4 weeks of age and after 6 months show cardiac degeneration, fibrosis and reduced cardiac NOS activity and cardiac function.23 In contrast, cardiac myocyte-specific overexpression of Cav-3 does not result in cardiomyopathy in 6-8 month old mice or a reduction in NOS activity. We conclude that increased cardiac myocyte expression of Cav-3 is not responsible for the late-appearing cardiac changes observed in the total body Cav-3 OE mice.
In conclusion, the current results show that IPC increases the number of plasma membrane caveolae in cardiac myocytes and that mice with cardiac myocyte-specific Cav-3 overexpression are protected from ischemic injury in a manner that mimics the cardiac protection produced by IPC. The ability to recapitulate or block the alterations of the plasma membrane produced by IPC by the respective overexpression or knockout of Cav-3 implies that Cav-3 is necessary and sufficient for IPC-induced cardiac protection. This protection may depend on PI3K and mitochondrial KATP channels. Our results also define a molecular mechanism to explain aspects of sarcolemmal ultrastructure, caveolae, and ischemic preconditioning that have been poorly understood for many years. Cardiac myocyte targeted overexpression of Cav-3 may provide a novel means to protect the heart from ischemia-reperfusion injury. More generally, our results imply that cell type-selective expression of caveolins may offer a means to augment mechanisms of preservation of the heart and perhaps other organs.
Supplementary Material
Acknowledgments
Funding Sources Supported by American Heart Association Beginning Grant in Aid 0765076Y (YMT), American Heart Association Predoctoral Fellowship 06150217Y (YTH), American Heart Association Scientist Development Grant 0630039N (HHP), NIH 1PO1 HL66941 (PAI and DMR), Senior Scholar Award from Ellison Foundation (PAI), Merit Award from the Department of Veterans Affairs (DMR), and NIH RO1 HL081400 (DMR).
Footnotes
Conflict of Interest Disclosures Patent pending, University of California, San Diego: “Molecular scaffolds for treating cardiac injury” (YMT, BPH, PMP, PAI, HHP and DMR).
REFERENCES
- 1.Noble R. The development of resistance by rats and guinea pigs to amounts of trauma usually fatal. Am J Physiol. 1943;38:346–351. [Google Scholar]
- 2.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 3.Hausenloy DJ, Tsang A, Yellon DM. The reperfusion injury salvage kinase pathway: a common target for both ischemic preconditioning and postconditioning. Trends Cardiovasc Med. 2005;15:69–75. doi: 10.1016/j.tcm.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Steinberg SF, Brunton LL. Compartmentation of g protein-coupled signaling pathways in cardiac myocytes. Annu Rev Pharmacol Toxicol. 2001;41:751–773. doi: 10.1146/annurev.pharmtox.41.1.751. [DOI] [PubMed] [Google Scholar]
- 5.Williams TM, Lisanti MP. The caveolin proteins. Genome Biol. 2004;5:214. doi: 10.1186/gb-2004-5-3-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palade G. Fine structure of blood capillaries. J Appl Phys. 1953;24:1424. Abstract. [Google Scholar]
- 7.Yamada E. The fine structure of the gall bladder epithelium of the mouse. J Biophys Biochem Cytol. 1955;1:445–458. doi: 10.1083/jcb.1.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pike LJ. Lipid rafts: bringing order to chaos. J Lip Res. 2003;44:655–667. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- 10.Song KS, Scherer PE, Tang Z, Okamoto T, Li S, Chafel M, Chu C, Kohtz DS, Lisanti MP. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J Biol Chem. 1996;271:15160–15165. doi: 10.1074/jbc.271.25.15160. [DOI] [PubMed] [Google Scholar]
- 11.Feron O, Balligand JL. Caveolins and the regulation of endothelial nitric oxide synthase in the heart. Cardiovasc Res. 2006;69:788–797. doi: 10.1016/j.cardiores.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Patel HH, Head BP, Petersen HN, Niesman IR, Huang D, Gross GJ, Insel PA, Roth DM. Protection of adult rat cardiac myocytes from ischemic cell death: role of caveolar microdomains and delta-opioid receptors. Am J Physiol Heart Circ Physiol. 2006;291:H344–H350. doi: 10.1152/ajpheart.01100.2005. [DOI] [PubMed] [Google Scholar]
- 13.Horikawa YT, Patel HH, Tsutsumi YM, Jennings MM, Kidd MW, Hagiwara Y, Ishikawa Y, Insel PA, Roth DM. Caveolin-3 expression and caveolae are required for isoflurane-induced cardiac protection from hypoxia and ischemia/reperfusion injury. J Mol Cell Cardiol. 2008;44:123–130. doi: 10.1016/j.yjmcc.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woodman SE, Park DS, Cohen AW, Cheung M, Chandra M, Shirani J, Tang B, Jelicks LA, Kitsis RN, Christ GJ, Factor SM, Tanowitz HB, Lisanti MP. Caveolin-3 knock-out mice develop a progressive cardiomyopathy and show hyperactivation of the p42/44 MAP kinase cascade. J Biol Chem. 2002;277:38988–38997. doi: 10.1074/jbc.M205511200. [DOI] [PubMed] [Google Scholar]
- 15.Hagiwara Y, Sasaoka T, Araishi K, Imamura M, Yorifuji H, Nonaka I, Ozawa E, Kikuchi T. Caveolin-3 deficiency causes muscle degeneration in mice. Hum Mol Genet. 2000;9:3047–3054. doi: 10.1093/hmg/9.20.3047. [DOI] [PubMed] [Google Scholar]
- 16.Patel HH, Tsutsumi YM, Head BP, Niesman IR, Jennings M, Horikawa Y, Huang D, Moreno AL, Patel PM, Insel PA, Roth DM. Mechanisms of cardiac protection from ischemia/reperfusion injury: a role for caveolae and caveolin-1. FASEB J. 2007;21:1565–1574. doi: 10.1096/fj.06-7719com. [DOI] [PubMed] [Google Scholar]
- 17.Head BP, Patel HH, Roth DM, Lai NC, Niesman IR, Farquhar MG, Insel PA. G-protein coupled receptor signaling components localize in both sarcolemmal and intracellular caveolin-3-associated microdomains in adult cardiac myocytes. J Biol Chem. 2005;280:31036–31044. doi: 10.1074/jbc.M502540200. [DOI] [PubMed] [Google Scholar]
- 18.Tsutsumi YM, Patel HH, Lai NC, Takahashi T, Head BP, Roth DM. Isoflurane produces sustained cardiac protection after ischemia-reperfusion injury in mice. Anesthesiology. 2006;104:495–502. doi: 10.1097/00000542-200603000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Park DS, Cohen AW, Frank PG, Razani B, Lee H, Williams TM, Chandra M, Shirani J, Souza APD, Tang B, Jelicks LA, Factor SM, Weiss LM, Tanowitz HB, Lisanti MP. Caveolin-1 null (-/-) mice show dramatic reductions in life span. Biochemistry. 2003;42:15124–15131. doi: 10.1021/bi0356348. [DOI] [PubMed] [Google Scholar]
- 20.Feron O, Kelly RA. The caveolar paradox: suppressing, inducing, and terminating eNOS signaling. Circ Res. 2001;88:129–131. doi: 10.1161/01.res.88.2.129. [DOI] [PubMed] [Google Scholar]
- 21.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murry CE, Richard VJ, Reimer KA, Jennings RB. Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circ Res. 1990;66:913–931. doi: 10.1161/01.res.66.4.913. [DOI] [PubMed] [Google Scholar]
- 23.Aravamudan B, Volonte D, Ramani R, Gursoy E, Lisanti MP, London B, Galbiati F. Transgenic overexpression of caveolin-3 in the heart induces a cardiomyopathic phenotype. Hum Mol Genet. 2003;12:2777–2788. doi: 10.1093/hmg/ddg313. [DOI] [PubMed] [Google Scholar]
- 24.Hausenloy DJ, Yellon DM. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res. 2006;70:240–253. doi: 10.1016/j.cardiores.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 25.McNutt NS. Ultrastructure of the myocardial sarcolemma. Circ Res. 1975;37:1–13. doi: 10.1161/01.res.37.1.1. [DOI] [PubMed] [Google Scholar]
- 26.Ashraf M, Halverson CA. Structural changes in the freeze-fractured sarcolemma of ischemic myocardium. Am J Pathol. 1977;88:583–594. [PMC free article] [PubMed] [Google Scholar]
- 27.Sybers HD, Maroko PR, Ashraf M, Libby P, Braunwald E. The effect of glucose-insulin-potassium on cardiac ultrastructure following acute experimental coronary occlusion. Am J Pathol. 1973;70:401–420. [PMC free article] [PubMed] [Google Scholar]
- 28.Frank JS, Beydler S, Kreman M, Rau EE. Structure of the freeze-fractured sarcolemma in the normal and anoxic rabbit myocardium. Circ Res. 1980;47:131–143. doi: 10.1161/01.res.47.1.131. [DOI] [PubMed] [Google Scholar]
- 29.Kordylewski L, Goings GE, Page E. Rat atrial myocyte plasmalemmal caveolae in situ. Reversible experimental increases in caveolar size and in surface density of caveolar necks. Circ Res. 1993;73:135–146. doi: 10.1161/01.res.73.1.135. [DOI] [PubMed] [Google Scholar]
- 30.Krajewska WM, Maslowska I. Caveolins: structure and function in signal transduction. Cell Mol Biol Lett. 2004;9:195–220. [PubMed] [Google Scholar]
- 31.Matherne GP, Linden J, Byford AM, Gauthier NS, Headrick JP. Transgenic A1 adenosine receptor overexpression increases myocardial resistance to ischemia. Proc Natl Acad Sci U S A. 1997;94:6541–6546. doi: 10.1073/pnas.94.12.6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rorabaugh BR, Ross SA, Gaivin RJ, Papay RS, McCune DF, Simpson PC, Perez DM. alpha1A- but not alpha1B-adrenergic receptors precondition the ischemic heart by a staurosporine-sensitive, chelerythrine-insensitive mechanism. Cardiovasc Res. 2005;65:436–445. doi: 10.1016/j.cardiores.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 33.Ping P, Song C, Zhang J, Guo Y, Cao X, Li RC, Wu W, Vondriska TM, Pass JM, Tang XL, Pierce WM, Bolli R. Formation of protein kinase C(epsilon)-Lck signaling modules confers cardioprotection. J Clin Invest. 2002;109:499–507. doi: 10.1172/JCI13200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wall JA, Wei J, Ly M, Belmont P, Martindale JJ, Tran D, Sun J, Chen WJ, Yu W, Oeller P, Briggs S, Gustafsson AB, Sayen MR, Gottlieb RA, Glembotski CC. Alterations in oxidative phosphorylation complex proteins in the hearts of transgenic mice that overexpress the p38 MAP kinase activator, MAP kinase kinase 6. Am J Physiol Heart Circ Physiol. 2006;291:H2462–H2472. doi: 10.1152/ajpheart.01311.2005. [DOI] [PubMed] [Google Scholar]
- 35.Brunner F, Maier R, Andrew P, Wolkart G, Zechner R, Mayer B. Attenuation of myocardial ischemia/reperfusion injury in mice with myocyte-specific overexpression of endothelial nitric oxide synthase. Cardiovasc Res. 2003;57:55–62. doi: 10.1016/s0008-6363(02)00649-1. [DOI] [PubMed] [Google Scholar]
- 36.Jones SP, Greer JJ, Kakkar AK, Ware PD, Turnage RH, Hicks M, van Haperen R, de Crom R, Kawashima S, Yokoyama M, Lefer DJ. Endothelial nitric oxide synthase overexpression attenuates myocardial reperfusion injury. Am J Physiol Heart Circ Physiol. 2004;286:H276–H282. doi: 10.1152/ajpheart.00129.2003. [DOI] [PubMed] [Google Scholar]
- 37.Li G, Chen Y, Saari JT, Kang YJ. Catalase-overexpressing transgenic mouse heart is resistant to ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 1997;273:H1090–H1095. doi: 10.1152/ajpheart.1997.273.3.H1090. [DOI] [PubMed] [Google Scholar]
- 38.Depre C, Wang L, Sui X, Qiu H, Hong C, Hedhli N, Ginion A, Shah A, Pelat M, Bertrand L, Wagner T, Gaussin V, Vatner SF. H11 kinase prevents myocardial infarction by preemptive preconditioning of the heart. Circ Res. 2006;98:280–288. doi: 10.1161/01.RES.0000201284.45482.e8. [DOI] [PubMed] [Google Scholar]
- 39.Lau S, Patnaik N, Sayen R, Mestril R. Simultaneous overexpression of two stress proteins in rat cardiomyocytes and myogenic cells confers protection against ischemia-induced injury. Circulation. 1997;96:2287–2294. doi: 10.1161/01.cir.96.7.2287. [DOI] [PubMed] [Google Scholar]
- 40.Ray PS, Martin JL, Swanson EA, Otani H, Dillmann WH, Das DK. Transgene overexpression of alphaB crystallin confers simultaneous protection against cardiomyocyte apoptosis and necrosis during myocardial ischemia and reperfusion. FASEB J. 2001;15:393–402. doi: 10.1096/fj.00-0199com. [DOI] [PubMed] [Google Scholar]
- 41.Gupta S, Knowlton AA. HSP60 trafficking in adult cardiac myocytes: role of the exosomal pathway. Am J Physiol Heart Circ Physiol. 2007;292:H3052–H3056. doi: 10.1152/ajpheart.01355.2006. [DOI] [PubMed] [Google Scholar]
- 42.Graf GA, Matveev SV, Smart EJ. Class B scavenger receptors, caveolae and cholesterol homeostasis. Trends Cardiovasc Med. 1999;9:221–225. doi: 10.1016/s1050-1738(00)00031-1. [DOI] [PubMed] [Google Scholar]
- 43.Matveev S, van der Westhuyzen DR, Smart EJ. Co-expression of scavenger receptor-BI and caveolin-1 is associated with enhanced selective cholesteryl ester uptake in THP-1 macrophages. J Lip Res. 1999;40:1647–1654. [PubMed] [Google Scholar]
- 44.Fecchi K, Volonte D, Hezel MP, Schmeck K, Galbiati F. Spatial and temporal regulation of GLUT4 translocation by flotillin-1 and caveolin-3 in skeletal muscle cells. FASEB J. 2006;20:705–707. doi: 10.1096/fj.05-4661fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y, Sato T, O'Rourke B, Marban E. Mitochondrial ATP-dependent potassium channels: novel effectors of cardioprotection? Circulation. 1998;97:2463–2469. doi: 10.1161/01.cir.97.24.2463. [DOI] [PubMed] [Google Scholar]
- 46.Engelman JA, Chu C, Lin A, Jo H, Ikezu T, Okamoto T, Kohtz DS, Lisanti MP. Caveolin-mediated regulation of signaling along the p42/44 MAP kinase cascade in vivo. A role for the caveolin-scaffolding domain. FEBS Lett. 1998;428:205–211. doi: 10.1016/s0014-5793(98)00470-0. [DOI] [PubMed] [Google Scholar]
- 47.Feron O, Dessy C, Opel DJ, Arstall MA, Kelly RA, Michel T. Modulation of the endothelial nitric-oxide synthase-caveolin interaction in cardiac myocytes. Implications for the autonomic regulation of heart rate. J Biol Chem. 1998;273:30249–30254. doi: 10.1074/jbc.273.46.30249. [DOI] [PubMed] [Google Scholar]
- 48.Raikar LS, Vallejo J, Lloyd PG, Hardin CD. Overexpression of caveolin-1 results in increased plasma membrane targeting of glycolytic enzymes: The structural basis for a membrane associated metabolic compartment. J Cell Biochem. 2006;98:861–871. doi: 10.1002/jcb.20732. [DOI] [PubMed] [Google Scholar]
- 49.Young LH, Ikeda Y, Lefer AM. Caveolin-1 peptide exerts cardioprotective effects in myocardial ischemia-reperfusion via nitric oxide mechanism. Am J Physiol Heart Circ Physiol. 2001;280:H2489–H2495. doi: 10.1152/ajpheart.2001.280.6.H2489. [DOI] [PubMed] [Google Scholar]
- 50.Ballard-Croft C, Locklar AC, Kristo G, Lasley RD. Regional myocardial ischemia induced activation of MAPKs is associated with subcellular redistribution of caveolin and cholesterol. Am J Physiol Heart Circ Physiol. 2006;291:H658–H667. doi: 10.1152/ajpheart.01354.2005. [DOI] [PubMed] [Google Scholar]
- 51.Koneru S, Penumathsa SV, Thirunavukkarasu M, Samuel SM, Zhan L, Han Z, Maulik G, Das DK, Maulik N. Redox regulation of ischemic preconditioning is mediated by the differential activation of caveolins and their association with eNOS and GLUT-4. Am J Physiol Heart Circ Physiol. 2007;292:H2060–H2072. doi: 10.1152/ajpheart.01169.2006. [DOI] [PubMed] [Google Scholar]
- 52.Murata T, Lin MI, Huang Y, Yu J, Bauer PM, Giordano FJ, Sessa WC. Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J Exp Med. 2007;204:2373–2382. doi: 10.1084/jem.20062340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galbiati F, Volonte D, Chu JB, Li M, Fine SW, Fu M, Bermudez J, Pedemonte M, Weidenheim KM, Pestell RG, Minetti C, Lisanti MP. Transgenic overexpression of caveolin-3 in skeletal muscle fibers induces a Duchenne-like muscular dystrophy phenotype. Proc Natl Acad Sci U S A. 2000;97:9689–9694. doi: 10.1073/pnas.160249097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.