Abstract
One hallmark of cancer cells is their compromised ability to undergo apoptosis, or programmed cell death. Strategies targeting key apoptosis regulators with the goal of overcoming resistance to apoptosis have significant therapeutic potential for the development of new classes of anticancer drugs. Smac is a pro-apoptotic protein which, by binding to the inhibitor of apoptosis proteins (IAPs), antagonizes their cellular anti-apoptotic function. It interacts with IAPs through its four N-terminal amino acid residues (AVPI). Small molecules that mimic this four residue sequence are being studied as the basis of a new therapeutic strategy for the treatment of human cancers and other diseases. In this Account, we provide an overview of the design, synthesis and evaluation on both peptidic and non-peptidic small-molecule Smac mimetics.
Conspectus
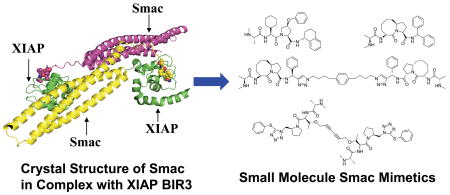
Smac/DIABLO is a protein released from mitochondria into the cytosol in response to apoptotic stimuli. Smac promotes apoptosis at least in part through antagonizing inhibitor of apoptosis proteins (IAPs), including XIAP, cIAP-1 and cIAP-2. Smac interacts with these IAPs via its N-terminal AVPI binding motif. There has been an enormous interest in academic laboratories and pharmaceutical companies in the design of small-molecule Smac mimetics as potential anticancer agents. This task is particularly challenging because it involves targeting protein-protein interactions. Nevertheless, intense research has now generated potent, specific, cell-permeable small-molecule peptido-mimetics and non-peptidic mimetics.
To date, two types of Smac mimetics have been reported, namely monovalent and bivalent Smac mimetics. The monovalent compounds are designed to mimic the binding of a single AVPI binding motif to IAP proteins, whereas the bivalent compounds contain two AVPI binding motif mimetics tethered together through a linker. Studies from several groups have clearly demonstrated that both monovalent and bivalent Smac mimetics not only enhance the antitumor activity of other anticancer agents but also can induce apoptosis as single agents in a subset of human cancer cell lines in vitro and are capable of achieving tumor regression in animal models of human cancer. In general, bivalent Smac mimetics are 100–1000 times more potent than their corresponding monovalent Smac mimetics in induction of apoptosis in tumor cells. However, properly designed monovalent Smac mimetics can achieve oral bioavailability and may have major advantages over bivalent Smac mimetics as potential drug candidates.
In-depth insights on the molecular mechanism of action of Smac mimetics have been provided by several independent studies. It was shown that Smac mimetics induce apoptosis in tumor cells by targeting cIAP-1/-2 for the rapid degradation of these proteins, which leads to activation of NF-kB, and production and secretion of TNF-α. TNF-α promotes formation of an RIPK1-dependent caspase-8-activating complex, leading to activation of caspase-8 and -3/-7, and ultimately to apoptosis. For the most efficient apoptosis induction, Smac mimetics also need to remove the inhibition of XIAP to caspase-3/-7. Hence, Smac mimetics induce apoptosis in tumor cells by targeting not only cIAP-1/2 but also XIAP. The employment of potent, cell-permeable, small-molecule Smac mimetics has yielded important insights into the regulation of apoptosis by IAP proteins. To date, at least one Smac mimetic has been advanced into clinical development. Several other Smac mimetics are in an advanced preclinical development stage and are expected to enter human clinical testing for the treatment of cancer in the near future.
Introduction
Apoptosis, or programmed cell death, is a critical cellular process in normal development and homeostasis of multicellular organisms. Inappropriate regulation of apoptosis is associated with many human diseases, including cancer,1–3 and it is now recognized that one hallmark of cancer cells is their compromised ability to undergo apoptosis.2 Targeting critical apoptosis regulators is an attractive strategy for the development of new classes of therapies for the treatment of cancer and other human diseases.1–3
The inhibitor of apoptosis proteins (IAPs) are a class of central apoptosis regulators, although their role is not limited to apoptosis (Figure 1).4,5 The X-linked inhibitor of apoptosis protein (XIAP) is perhaps the best characterized member of all the IAPs.5 It effectively suppresses apoptosis at least in part by binding to and inhibition of three members of the caspase family of enzymes, the two effectors, caspase-3 and -7, and an initiator, caspase-9.6 XIAP contains three baculoviral IAP repeat (BIR) domains. The third BIR domain (BIR3) of XIAP selectively targets caspase-9,7,8 whereas the BIR2 domain, together with the linker preceding BIR2, inhibits both caspase-3 and caspase-7.9–11 Consistent with its potent apoptosis inhibitory function, XIAP is found to be highly expressed in many human tumor cell lines and tumor samples from patients12, and plays an important role in the resistance of cancer cells to a variety of anticancer drugs.13
Figure 1.
A simplified apoptotic pathway.
Smac/DIABLO is a protein released from mitochondria into the cytosol in response to apoptotic stimuli.14,15 Smac functions as an endogenous inhibitor of XIAP and other IAP proteins (Figure 1).6 As a dimer,16 Smac targets both the BIR2 and BIR3 domains in XIAP concurrently and prevents the inhibition of XIAP not only to caspase-9 but also to caspase-3/-7.6 It blocks the inhibition of XIAP to caspase-9 by binding to the BIR3 domain in XIAP through its AVPI tetra-peptide binding motif and competing directly with a similar ATPF tetrapeptide in caspase-9.6,17,18 The mechanism with which Smac removes the inhibition of XIAP to caspase-3/-7 is not completely clear, but it has been proposed that the interaction of Smac protein through its AVPI motif to the BIR2 domain of XIAP also prevents the binding and inhibition of XIAP to caspase-3/-7.19,21
Because XIAP blocks apoptosis at the down-stream effector phase, a point where multiple signaling pathways converge, it represents a particularly attractive molecular target for the design of new classes of anticancer drugs aimed at overcoming the apoptosis resistance of cancer cells.5,13 Several strategies have been employed for the design of small-molecular inhibitors of XIAP. One approach has been to block the interaction between XIAP BIR2 and caspase-3/-7. Employing high throughput screening and parallel solid-phase synthesis, Wu and colleagues identified a series of small molecule inhibitors of the caspase-3/XIAP interaction.22 Schimmer and colleagues discovered a class of polyphenylureas by chemical library screening and showed that by antagonizing XIAP and with a mechanism of action different from that of Smac protein, these compounds can restore the activities of caspase-3 and –7, but not that of caspase-9.23 Another strategy is to design small molecules to mimic the Smac AVPI binding motif; this has attracted a great deal of attention in recent years and is the focus of this Account.
1. Structural basis for the design of Smac mimetics
The crystal structure of Smac/DIABLO protein in a complex with the XIAP BIR3 domain has been determined by Shi’s group17 and the solution structure of Smac peptide complexed with the BIR3 domain was established by Fesik and his colleagues at Abbott Laboratories.18
The crystal structure showed that Smac protein forms an elongated homodimer (Figure 2A).17 Both crystal and NMR solution structures17,18 clearly revealed that the N-terminal four residues (Ala1-Val2-Pro3-Ile4) in Smac recognize and bind to a surface groove on XIAP BIR3 (Figure 2B). The methyl group of the Ala1 residue inserts into a small hydrophobic pocket; the free amino group forms strong hydrogen bonds to the Glu314 and Gln319 residues on BIR3 and the backbone carbonyl group forms a suboptimal hydrogen bond to the indole NH group in Trp323. The amino and carbonyl groups of Val2 form optimal hydrogen bonds with the carbonyl and amino groups of Thr308, respectively, while its side chain, with no interactions with protein residues, is exposed to solvent. The 5-membered ring of Pro3 has van der Waals contacts with the side chains of Trp323 and Tyr324 and finally, the amino group of the Ile4 residue forms a hydrogen bond with the carbonyl group of Gly306, and its hydrophobic side chain inserts into a hydrophobic pocket formed by the side chains of Leu292 and Val298 and the hydrophobic portion of the side chains in Lys297 and Lys299. Such structural information at the atomic level has been the basis for the design of both peptidic and non-peptidic Smac mimetics.
Figure 2.
(A). Crystal structure of Smac in complex with XIAP BIR3 protein. (B). Detailed interactions between the AVPI binding motif and XIAP BIR3 residues. (C). Crystal structure of compound 18 in complex with XIAP BIR3 (Protein Data Bank access code: 2JK7), in superposition to that of Smac AVPI peptide in complex with XIAP BIR3.
2. Design of Smac-based peptides
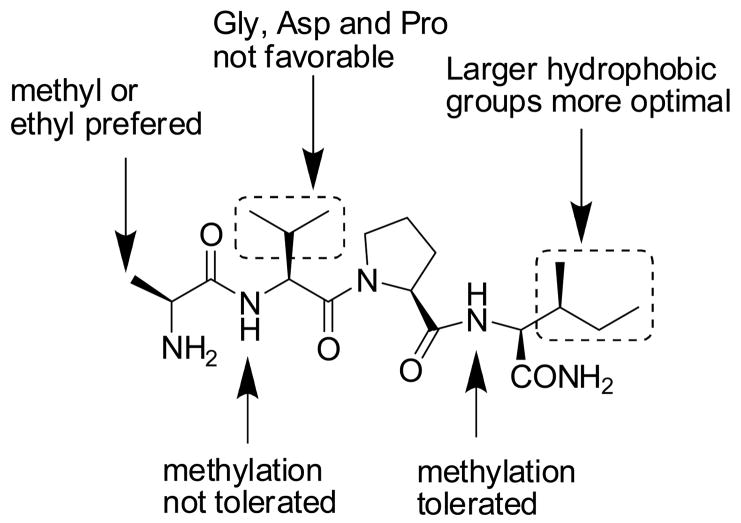
McLendon’s group has carried out extensive modifications to the AVPI tetra-peptide and obtained a comprehensive structure-activity relationship of Smac-based peptides binding to the XIAP BIR3 domain (Figure 3).24
Figure 3.
Summary of structure-activity relationship of Smac-based peptides to XIAP BIR3.
The AVPI peptide binds to XIAP BIR3 with a Kd value of 480 nM, but replacement of the Ala1 residue by a Gly or Ser residue results in a more than 20-fold loss in binding affinity, while a slight improvement in binding affinity is achieved with the unnatural amino acid, 2-aminobutyric acid. Replacement of the valine in AVPI shows that this position can tolerate many other amino acid residues without significant reduction in binding affinity. This is consistent with the experimental structural information, which shows that the side chain of valine has no close contacts with protein residues and is exposed to solvent. Replacement of the valine residue by aspartate, glycine or proline however, results in significant loss in binding affinity. Modifications of the fourth residue, isoleucine, indicate that a hydrophobic residue such as valine, phenylalanine, tryptophan or leucine is highly preferred with phenyalanine being the most preferred residue. A charged or polar residue such as lysine, arginine, glutamate, aspartate, histidine, glutamine and asparagine at this position is detrimental to binding. Modification at the peptide bond between residues 1 and 2 by methylation disrupts a structurally important hydrogen bond and has a large negative effect on binding, but N-methylation of residue 4 has a much smaller effect. The structure-activity relationship obtained from this study has provided a very useful guide to the design of peptidic- and non-peptidic mimetics of Smac.
Smac-based peptides have potent binding affinities to XIAP, but they are not cell-permeable. To address this limitation, a number of early studies employed a strategy to tether a carrier peptide to a Smac-based peptide so facilitating intracellular delivery.25–27 It was shown that these relatively cell-permeable Smac-based peptides can sensitize various tumor cells in vitro to the anti-tumor activity of Apo-2L/TRAIL, as well as chemotherapeutic agents such as paclitaxel, etoposide, SN-38, doxorubicin and cisplatin.26 Furthermore, one cell-permeable Smac-based peptide was shown to strongly enhance the anti-tumor activity of Apo-2L/TRAIL in an intracranial malignant glioma xenograft model in vivo, achieving complete eradication of established tumors.25 Similarly, another cell-permeable Smac-based peptide in combination with cisplatin was shown to regress tumor growth in vivo in H460 non-small cell lung cancer xenografts with little toxicity to the mice.27 Of note, these cell-permeable peptides were injected directly into the tumors in both in vivo studies. Nevertheless, these early studies provide the important proof-of-the-concept that cell-permeable, Smac peptido-mimetics and non-peptidic mimetics may be useful as new cancer therapies, especially when used in combination with other anticancer drugs.
3. Design of Smac peptidomimetics
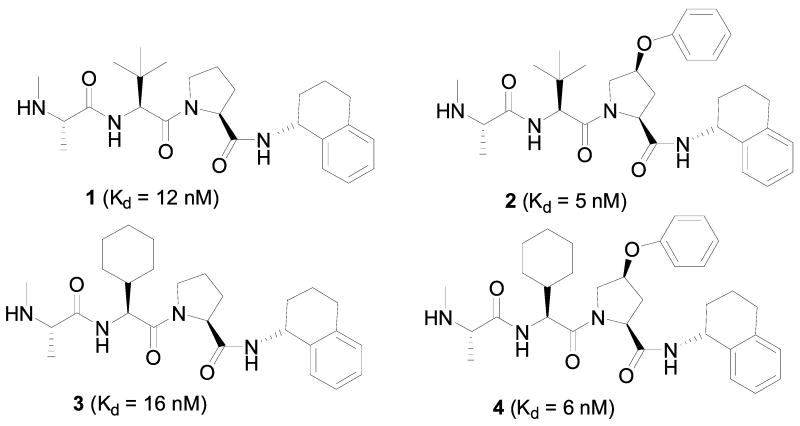
Oost and colleagues at Abbott Laboratories have carried out extensive chemical modifications of the AVPI peptide in an effort to derive potent Smac peptidomimetics.28 Chemical modifications of the Ala1 residue using different amino acids showed that the natural methyl or an ethyl side chain are most preferred for binding, consistent with the data obtained by McLendon’s group.24 Substitution of the free terminal amino group by one methyl group is well tolerated, but dimethylation decreases the binding to XIAP BIR3 by a factor of >100. Consistent with the previous report,24 modifications of the Val2 showed that this residue can be replaced by many other residues without a significant loss of the binding affinity, although replacement by Gly leads to a >30-fold loss of binding affinity. In addition, it was shown that an L-configuration of this residue is essential for binding. Replacement of the 5-membered ring in the Pro3 residue with 4- or 6-membered rings results in a 5–7-fold loss in binding affinity and a greater loss with other residues. Introduction of a hydrophobic group to the 5-membered ring in Pro3 can slightly improve the binding affinity. Modifications to the Ile4 residue showed that a variety of hydrophobic entities are tolerated, phenylanaline and phenylglycine being the most preferred. Among all the compounds designed and evaluated, compounds 1, 2, 3 and 4 have the highest binding affinities to XIAP BIR3 with Kd values of 12, 5, 16 and 6 nM, respectively (Figure 4).
Figure 4.
Representative potent Smac peptido-miemtics.
These potent Smac peptidomimetics have been shown to be effective in rescuing XIAP BIR3-mediated inhibition of caspase activity in a fully reconstituted functional assay containing Apaf-1, caspase-9 and procaspase-3, dATP and cytochrome c. Compounds 1, 2 and 3 achieve EC50 values of 0.29, 0.24 and 0.31 μM, respectively, in recovering the caspase activity. Such functional data provide direct evidence for their functional antagonism against XIAP BIR3.
These potent Smac peptidomimetics are also effective in induction of caspase-3 activation in the MDA-MB-231 human breast cancer cell line. Compounds 1 and 3 potently inhibit cell growth with IC50 values of 68 and 13 nM, respectively, in the MDA-MB-231 cell line and effectively induce cell death. Compound 3 also demonstrates modest activity in inhibition of tumor growth in the MDA-MB-231 xenografts in mice.
These in vitro and in vivo data obtained using potent and cell-permeable Smac peptidomimetics provide important evidence that Smac mimetics may have a therapeutic potential as single agents for the treatment of human cancer in a subset of human cancers.
4. Design of conformationally constrained Smac mimetics
The Wang laboratory from the University of Michigan was the first to report the design of conformationally constrained, bicylic Smac mimetics using a structure-based strategy (Figure 4).29–32
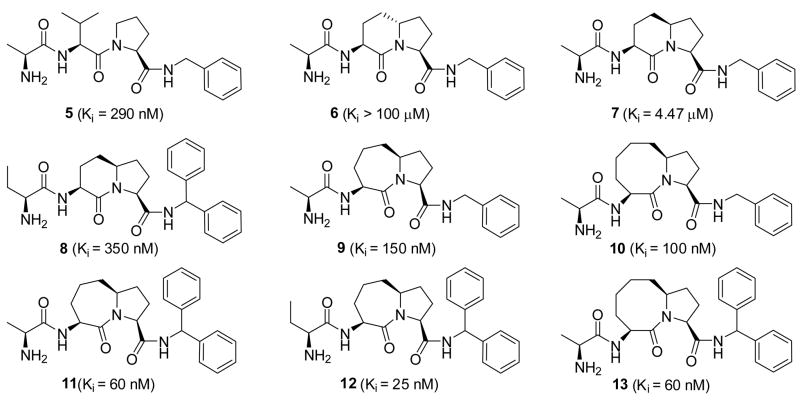
The experimental structures of Smac protein/peptide in complex with XIAP BIR3 show that the isopropyl group of Val2 is exposed to the solvent and has no specific interaction with the XIAP BIR3 protein, while the five–membered ring of Pro3 has hydrophobic contacts with Trp323 in XIAP BIR3. Based upon modeling predictions, it was proposed that these two residues can be fused together to form a bicyclic lactam structure without seriously altering the conformation of the AVPI peptide bound to XIAP BIR3. Cyclization of the side chains of Val2 and Pro3 produces a new chiral center and in order to determine which configuration of the chiral center is desirable for binding to XIAP BIR3, two stereoisomers, 6 and 7, each containing the [6,5] bicyclic ring structure were designed and synthesized.29,30 While 7 has a Ki value of 4.47 μM to XIAP BIR3 protein, 6 shows a Ki value >100 μM, indicating that the R configuration for the chiral center is much better suited to binding to XIAP BIR3. Using SAR obtained from peptidic Smac mimetics, compound 8 was designed and shown to have a Ki of 350 nM, as potent as the natural Smac AVPI peptide.
Compound 7 containing the [6,5] bicyclic lactam structure is 8 times less potent than the AVPI peptide and 15 times less than its corresponding peptidic mimetic 5. Modeling revealed that although compound 7 can largely mimic both 5 and the Smac AVPI peptide for binding to XIAP, the binding is less than optimal. Expansion of the [6,5] bicyclic ring system in compound 7 to either a [7,5] or an [8,5] ring system results in compounds that mimic the bound conformation of the AVPI peptide to XIAP more closely and show improved binding affinities.30–32 This leads to the design of compounds 9 and 10 which bind to XIAP BIR3 with Ki values of 150 and 100 nM, respectively, 30 or 45 times more potent than compound 7. Using SAR obtained for [6,5] ring contained Smac mimetics, compounds 11, 12 and 13 were designed and shown to have Ki values of 60, 25 and 14 nM, respectively.
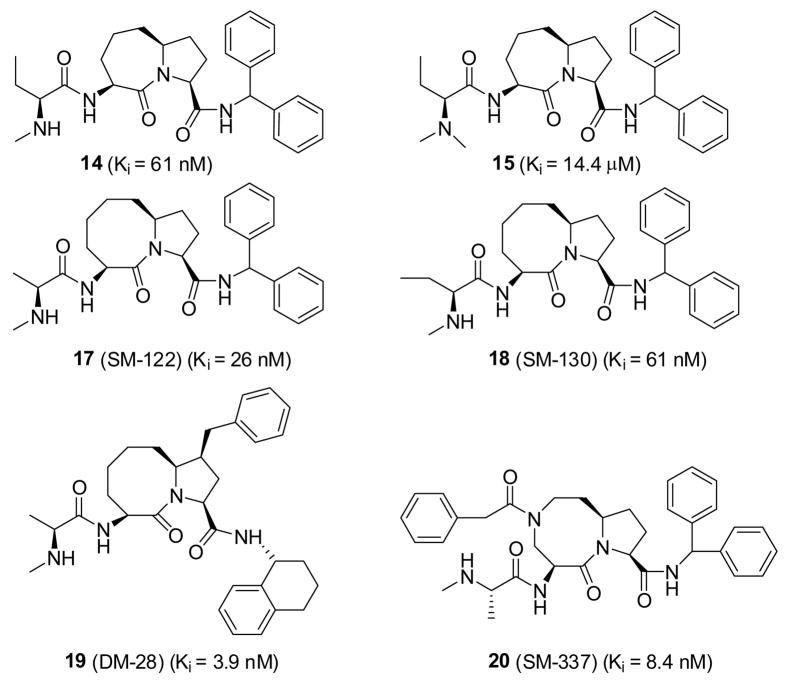
Although compounds 11, 12 and 13 achieve high binding affinities to XIAP BIR3, they were found to have very weak activities in cell-based assays in both cell growth inhibition and apoptosis induction in sensitive cancer cell lines, such as the MDA-MB-231 breast cancer cell line (Table 1). Their weak cellular activity suggests that they are not very cell-permeable, and it was hypothesized that the primary amine group in these compounds may have a detrimental effect on their cell permeability or metabolic stability. To test this idea, compound 14, 15 and 16 (Table 1) were designed by replacing the primary amino group in compound 12 with a secondary or tertiary amine or a hydroxyl group.31,32 Evaluation of their binding affinities to XIAP BIR3 indicated that replacement of the primary amine group with an N-methyl amine decreases the binding affinity by a factor of 2 (14 vs 12), but replacement of the primary amino group with a dimethylamine (15) or a hydroxyl (16) weakens the binding affinity by 600 or 1000 times, respectively. Hence, the binding data showed that the primary and secondary amino groups are highly preferred at this site for Smac mimetics to achieve high binding affinities to XIAP BIR3 protein.
Table 1.
Binding affinities of bicyclic Smac mimetics to XIAP BIR3 protein and cell growth inhibition in the MDA-MB-231 cancer cell line.
 | |||||
|---|---|---|---|---|---|
| R1 | R2 | Binding affinity to XIAP BIR3 (Ki μM) | Cell growth Inhibition (IC50, μM) | ||
| 11 (SM-104) | CH2 | NH2 | Me | 0.060 | >10 |
| 12 (SM-102) | CH2 | NH2 | Et | 0.025 | >10 |
| 13 (SM-128) | (CH2)2 | NH2 | Me | 0.014 | >10 |
| 14 (SM-131) | CH2 | CH3NH | Et | 0.061 | 0.1 |
| 15 (SM-160) | CH2 | N(CH3)2 | Et | 14.4 | 3.0 |
| 16 (SM-161) | CH2 | OH | Et | 29.0 | 70.0 |
| 17 (SM-122) | (CH2)2 | CH3NH | Me | 0.026 | 0.26 |
| 18 (SM-130) | (CH2)2 | CH3NH | Et | 0.067 | 0.41 |
These compounds were tested for their activity in inhibiting cell growth in the MDA-MB-231 human breast cancer cell line. Although 12 and 14 have comparable binding affinities to XIAP BIR3, they display drastically different cellular activities. Compound 12 has very weak activity in inhibition of cell growth with an IC50 of 50 μM (Table 1), but 14 achieves an IC50 value of 0.1 μM, and is thus 500 times more potent than 12. Interestingly, although 15 has a much weaker binding affinity than 12 to XIAP, it is more potent than 12 in the cell growth inhibition assay (IC50 = 3.0 μM). Compound 16 with a hydroxyl group, has an IC50 value of 70 μM, consistent with its very weak binding affinity to XIAP BIR3. Furthermore, replacement of the primary amino group with an N-methylamino group in compound 13 containing the [8,5] ring (compound 17, Table 1) also dramatically improves their cellular activities.
Additional modifications based upon the core structure of compound 17 yielded 19 (Figure 6).34 Compound 19 has a Ki value of 3.9 nM to XIAP BIR3 protein, 6-times more potent than compound 17.34 It also potently inhibits cell growth in the MDA-MB-231 breast cell line and has an IC50 value of 8.9 nM.34 Modifications of the core structure of compound 17 led to compound 20 (Figure 6), which contains a diazabicyclic core structure.33 Compound 19 binds to XIAP BIR3 with a Ki value of 8.9 nM and potently inhibits cancer cell growth in the MDA-MB-231 cell line with an IC50 value of 31 nM.33
Figure 6.
Representative potent, cell-permeable, non-peptidic Smac mimetics and control compounds reported from the Wang laboratory in the University of Michigan and their collaborators.
To obtain a solid structural basis for the interaction of these designed bicyclic conformationally constrained Smac mimetics with XIAP BIR3, a crystal structure of compound 18 in a complex with XIAP BIR3 was determined (Figure 2C).32 This crystal structure showed that compound 18 closely mimics the Smac AVPI peptide for binding to XIAP BIR3 in both hydrogen bonding and hydrophobic interactions. Furthermore, the predicted binding model by computational modeling for compound 18 in complex with XIAP BIR3 is in an excellent agreement with that revealed in the crystal structure.32
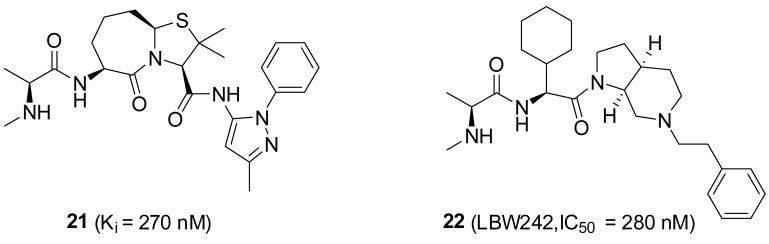
Zobel and colleagues from Genentech reported the design of a set of conformationally constrained Smac mimetics.35 The most potent compound (21) binds to XIAP BIR3 with a Ki value of 230 nM (Figure 7). Scientists from Novartis reported the design of LBW242 (compound 22, Figure 7). Compound 22 was designed by cyclization of the 3rd and 4th residues in the AVPI peptide and binds to XIAP BIR3 with an IC50 value of 280 nM.36,37
Figure 7.
Conformationally constrained Smac mimetics from Genentech and Novartis.
5. Design of bivalent Smac mimetics
It has been demonstrated that the natural Smac protein forms a dimer (Figure 2A)16,17 and binds to XIAP protein constructs containing BIR2 and BIR3 domain with a much higher affinity than the Smac AVPI peptide. Indeed, functional studies have shown that Smac protein is a much more efficient and potent antagonist than the AVPI peptide against XIAP protein containing the BIR2 and BIR3 domains in relieving the inhibition by XIAP of the activity of caspase-9 and caspase-3 and -7.19,20 The Smac AVPI binding motif binds to both BIR2 and BIR3 domains, although with a stronger affinity to BIR3.18 Thus small molecules designed to have two “AVPI” binding motifs may mimic the mode of action of Smac protein to target XIAP and be capable of achieving very high binding affinities to XIAP by concurrently targeting both the BIR2 and BIR3 domains in the protein.
The Wang and Harran laboratories from the University of Texas Southwestern Medical Center reported the discovery of such a bivalent small molecule Smac mimetic in 2004.38 In biochemical binding assays, this bivalent Smac mimetic 24 binds to recombinant XIAP BIR3 protein with an affinity comparable to that of its monovalent counterpart, compound 23, and the AVPF peptide (Figure 8). However, when the XIAP full-length protein was used, it was estimated that compound 24 may have an affinity higher than Smac protein, whose estimated Kd value is 0.3 nM. It also relieves the caspase-3 inhibition by XIAP with a potency similar to that of Smac protein but much higher than that of the corresponding monovalent Smac mimetics.38 Recently, Nikolovska-Coleska et al., using a newly established fluorescence-polarization based assay, determined that compound 24 binds to XIAP containing both the BIR2 and BIR3 domains with a Ki value < 0.7 nM.40
Figure 8.
First bivalent small-molecule Smac mimetic from the laboratories of Wang and Harran at the University of Texas Southwestern Medical Center.
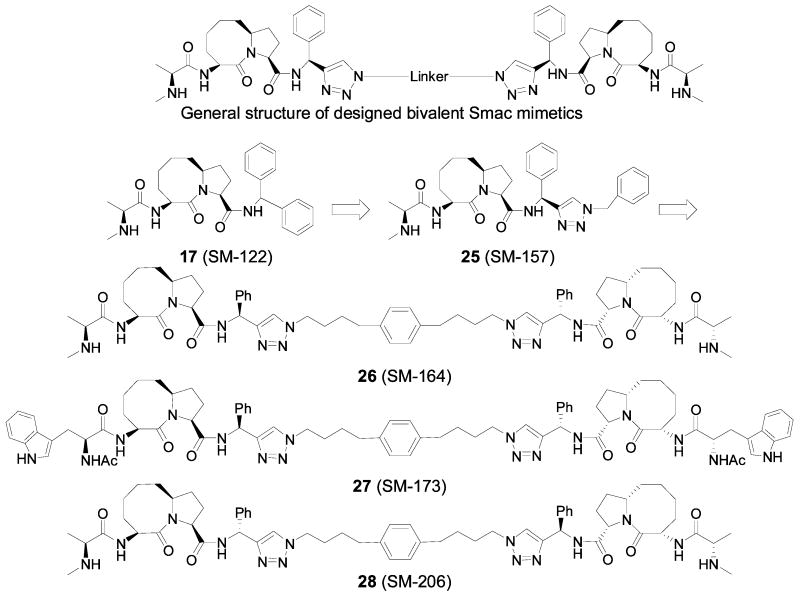
Sun et al. recently reported the structure-based design of non-peptidic, bivalent Smac mimetics based upon conformationally constrained monovalent Smac mimetics and they characterized in detail the interaction of both monovalent and bivalent Smac mimetics with different XIAP protein constructs (Figure 9).39
Figure 9.
Bivalent small-molecule Smac mimetics from the Wang laboratory at the University of Michigan.
Although compound 17 (SM-122) was initially designed to target the XIAP BIR3 domain, modeling predicted that it may also bind to XIAP BIR2. Using the surface plasmon resonance method and a biotinylated analogue of SM-122, it was determined that SM-122 indeed binds to XIAP BIR2 with an IC50 value of 5 μM. For the design of bivalent Smac mimetics, it was critical to identify sites appropriate for tethering. Computational modeling indicated that the pro-R phenyl ring in SM-122 inserts into a hydrophobic pocket in both BIR2 and BIR3 domains, while the pro-S phenyl ring is exposed to solvent and has no similar interaction with the protein. This phenyl ring is therefore a suitable site for tethering two molecules of SM-122 to one another. Modeling further suggested that the pro-S phenyl ring may be replaced with a [1,2,3]-triazole structure without no detrimental effect. Tethering two monovalent Smac mimetic molecules together can be easily accomplished with “click chemistry”. Modeling analysis showed that the linker region between BIR2 and BIR3 domains of XIAP should be quite flexible, suggesting that a bivalent Smac mimetic with a relatively short linker between the two monovalent Smac mimetics could concurrently interact with both BIR domains. Based upon these considerations, a bivalent Smac mimetic 26 (SM-164) was designed and synthesized. To test if the stereospecificity of the two triazole rings is important for binding, a stereoisomer 28 (SM-206), differing from SM-164 only in the configuration of the two chiral centers where the triazole rings are connected, was designed and synthesized. To test the specificity of SM-164, compound 27 (SM-173) was designed as an inactive control. In compound 27, the methyl group in each monovalent binding unit was replaced by a methyl-1H-indole to disrupt the hydrophobic interactions at this site and the methylamino group was replaced by an acetamido group to block hydrogen bond formation.
In order to determine the binding affinities of these designed bivalent Smac mimetics accurately, a fluorescence-polarization based assay using the XIAP protein containing both BIR2 and BIR3 domains and a fluorescently tagged bivalent Smac-based peptide tracer was developed. Compounds 17, 26, 28 and the AVPI peptide were determined to bind to XIAP containing both BIR2 and BIR3 domains with IC50 values of 438, 1.39, 71.5 and 10,396 nM, respectively, while the designed inactive control 27 shows no appreciable binding at 100 μM. Hence, the bivalent Smac mimetic 26 is 271 times more potent than the monovalent compound 17 and >7,000 times more potent than the Smac AVPI peptide, respectively. The stereoisomer 28 is 51-times less potent than compound 26, confirming the importance of the stereospecificity. The binding data show that the bivalent Smac mimetic 26 has an extremely high binding affinity for XIAP BIR2-BIR3 protein and is much more potent than monovalent 17 and the natural Smac AVPI peptide.
Compounds 17 and 26 were evaluated for their ability to antagonize XIAP in cell-free functional assays. In these assays, XIAP containing BIR2-BIR3 domains and the linker preceding BIR2 (residues 120–356) dose-dependently inhibits the activity of caspase-9 and caspase-3/-7 and achieves complete inhibition at 50 nM. Both compounds 17 and 26 antagonize XIAP in a dose-dependent manner and are capable of restoring the activity of caspase-9, as well as that of caspase-3 and caspase-7. Consistent with their binding affinities to XIAP, compound 26 is 100 times more potent than 17. At a concentration equimolar to that of XIAP, compound 26 completely overcomes the inhibition of XIAP and fully restores the activity of caspase-9 and -3/-7, indicating its extremely high potency as an XIAP antagonist. In comparison, the Smac AVPI peptide at a concentration of 100 μM, (2,000 times the concentration of XIAP protein) is needed to completely restore the activity of caspase-9 and caspase-3/-7. The inactive control 27 has a minimal effect at 100 μM. These functional data showed that while both compounds 17 and 26 function as antagonists of XIAP the bivalent Smac mimetic 26 is 100- and 2000-times more potent than the corresponding monovalent Smac mimetic 17 and the Smac AVPI peptide, respectively, consistent with their binding affinities with XIAP.
The mode of binding of these monovalent and bivalent Smac mimetics to XIAP containing either BIR3-only or BIR2-BIR3 domains were further investigated by analytical gel filtration using wild-type and mutated XIAP proteins and heteronuclear single quantum correlation (HSQC) NMR spectroscopy. The analytical gel filtration and NMR data provide clear evidence that in the presence of XIAP BIR3-only protein, one bivalent Smac mimetic 26 molecule interacts with two BIR3-only molecules, causing dimerization. However, in the presence of XIAP protein containing both BIR2 and BIR3 domains, one bivalent Smac mimetic 26 molecule interacts concurrently with both BIR2 and BIR3 domains in XIAP.
Jiang and colleagues reported the synthesis and evaluation of compound 29, a bivalent Smac peptidic ligand.48 Using this compound as a tool, they demonstrated that binding of compound 29 with the BIR2 domain of XIAP effectively antagonizes inhibition of caspase-3 by XIAP. They showed further that binding of Smac protein with the BIR3 domain anchors the subsequent binding of Smac with the BIR2 domain, which in turn attenuates the caspase-3 inhibitory function of XIAP, suggesting that both Smac protein and bivalent Smac mimetics may bind to XIAP containing BIR2 and BIR3 domains in a sequential and cooperative manner.
Compound 30 was designed and employed as a cell-permeable, bivalent Smac mimetic by Varfolomeev and colleagues from Genentech to investigate the mechanism of action of Smac mimetics in induction of apoptosis.44 Compound 30 was determined to bind to XIAP containing both BIR2 and BIR3 domains with a Ki value of 1.30 nM.
Compound 31 is a potent and cell-permeable bivalent Smac mimetic, developed by TetraLogic Pharmaceuticals.43 Although it was indicated to bind to XIAP with a picomolar affinity, no quantitative value was provided.43
Nikolovska-Coleska and her colleagues reported the design, synthesis and evaluation of compound 32 as a cyclic, bivalent Smac mimetic.49 They showed that compound 32 binds to XIAP containing both BIR2 and BIR3 domains with a biphasic dose-response curve, revealing two binding sites with IC50 values of 0.5 and 406 nM, respectively. Compound 32 binds to XIAPs containing the BIR3-only and BIR2-only domain with Ki values of 4 nM and 4.4 μM, respectively. Gel filtration experiments using wild-type and mutated XIAPs showed that 32 forms a 1:2 stoichiometric complex with XIAP containing the BIR3-only domain. However, it forms a 1:1 stoichiometric complex with XIAP containing both BIR2 and BIR3 domains, and both BIR domains are involved in the binding. Compound 32 efficiently antagonizes inhibition of XIAP in a cell-free functional assay and is >200 times more potent than its corresponding monovalent Smac mimetic. Determination of the crystal structure of 32 in complex with the XIAP BIR3 domain confirms that it induces homodimerization of the XIAP BIR3 domain and provides a structural basis for the cooperative binding of one molecule of compound 32 to two XIAP BIR3 molecules. On the basis of this crystal structure, a binding model of XIAP containing both BIR2 and BIR3 domains and 32 was constructed, which suggested that the binding of compound 32 to XIAP blocks the binding of XIAP to caspase-3/-7.
Another recently reported cell-permeable bivalent Smac mimetic is compound 33, designed and developed by scientists from Aegera Therapeutics in Montreal.46 Compound 33 binds to XIAP BIR3 protein with an IC50 value of 100 nM.46
Smac mimetics bind not only to XIAP but also to cIAP-1/-2 and ML-IAP
The design of Smac mimetics was primarily based upon the interaction between Smac and XIAP. However, Smac also binds to cIAP-1/-2 and ML-IAP. Thus, it is not surprising that small-molecule Smac mimetics also target other IAP proteins, in addition to XIAP.
For monovalent Smac mimetics, compound 21 was shown to bind to cIAP-1, cIAP-2 and ML-IAP with Ki values of 50, 130 and 50 nM, respectively.35 Compound 17 binds to cIAP-1 and cIAP-2 BIR3 proteins with very high affinities, having Ki values of 1.0 and 1.8 nM, respectively.33 Compound 19 binds to cIAP-1 and cIAP-2 BIR3 proteins with Ki values of 1.5 and 4.2 nM, respectively.33
For bivalent Smac mimetics, compound 26 was determined to bind to cIAP-1 protein containing BIR2 and BIR3 domains with a Ki value of 0.3 nM and to cIAP-2 BIR3 protein with a Ki value of 1.1 nM, respectively.47 Compound 30 was shown to bind to cIAP-1 containing both BIR2 and BIR3 domains with a Kd value of 0.46 nM.44 It was also indicated that compound 31 binds to cIAP-1 with a picomolar affinity, but the precise value was not reported.43 Compound 33 binds to cIAP-1 and cIAP-2 BIR3 proteins with IC50 values of 17 nM and 34 nM, respectively.46 Although the precise binding affinities of the bivalent Smac mimetic 24 to cIAP-1/-2 were not reported, it was shown that its biotinylated analogue can pull down endogenous cIAP-1 and cIAP-2 in cell lysates, in addition to XIAP.38
Taken together, these biochemical data clearly show that both monovalent and bivalent Smac mimetics bind with high affinities to not only XIAP, but also other IAP proteins, including cIAP-1 and cIAP-2 proteins.
Smac mimetics as new anticancer agents
A number of studies have demonstrated that Smac-based cell-permeable peptides are effective in sensitizing cancer cells to a variety of anticancer agents.25–27 Subsequent studies showed that both monovalent and bivalent Smac mimetics as single agents can inhibit cell growth and induce apoptosis in cancer cells.28,31–39
Oost and colleagues showed that peptide-mimetic 3 inhibits cell growth in 7 different cell lines with diverse tumor types, with IC50 values ranging from 7 nM to 2 μM.28 These include breast cancer cell lines BT-549 and MDA-MB-231, leukemia cell line HL-60, melanoma cell line SK-MEL-5, renal cancer cell line RXF-393, ovarian cancer cell line SK-OV-3, non-small cell lung cancer cell lines NCI-H23 and NCI-H522. Zobel and colleagues showed that compound 21 inhibited cell growth with IC50 values of 100 nM and 2 μM in the MDA-MB-231 breast cancer cell line and A-2058 melanoma cell line, respectively. These Smac mimetics were shown to also effectively induce cell death and apoptosis in cancer cells in a caspase-dependent manner.
The bivalent Smac mimetic 24 was initially shown to potentiate the activity of TRAIL and TNF-α but had no activity as a single agent in the T98G glioma cell line.38 In a subsequent study, it was demonstrated to be effective in cell growth inhibition in ~25% of human non-small carcinoma cell lung cancer cell lines and achieve IC50 values in the nanomolar range in 14% of cancer cell lines.42 Compound 24 also induces robust cell death at 100 nM in these sensitive cell lines. Furthermore, it effectively inhibits tumor growth in the HCC461 xenografts in mice and causes tumor regression in 40% of treated animals.42
Both conformationally constrained monovalent and bivalent Smac mimetics reported by the Wang laboratory at the University of Michigan and their collaborators were shown to be effective in inhibition of cell growth and induction of apoptosis in cancer cell lines.31–34,39 For example, monovalent 17 (SM-122), 19 (DM-28), and 20 (SM-337) potently inhibit cell growth and induce apoptosis in a number of cancer cell lines, including the MDA-MB-231 breast cancer cell line, SK-OV-3 ovarian cancer cell line and HL-60 leukemia cells. Compounds 17, 18 and 20 have IC50 values of 259 nM, 8.9 nM and 31 nM, respectively, in inhibition of cell growth in the MDA-MB-231 cancer cell line.33,34 Compound 26 (bivalent SM-164) achieves IC50 values of 1 nM or less in inhibition of cell growth in the MDA-MB-231, SK-OV-3 and HL-60 cell lines and also effectively induces apoptosis in these cancer cell lines at concentrations as low as 1 nM.39,47 SM-164 is capable of inducing tumor regression in the MDA-MB-231 xenografts in mice and shows no or little toxicity to animals at effective dose-schedules.47
These in vitro and in vivo data from a number of laboratories using different Smac mimetics have provided strong evidence that Smac mimetics may have a great therapeutic potential for the treatment of human cancer not only in combination with other therapeutic agents but also as single agents.
Potential advantages and disadvantages of monovalent and bivalent Smac mimetics as new anticancer agents
As potential drug candidates, there are advantages and disadvantages associated with monovalent and bivalent Smac mimetics. Monovalent Smac mimetics are less potent than their corresponding bivalent Smac mimetics.38,39 However, monovalent Smac mimetics, with a molecular weight of ~500, possess many desirable pharmacological properties as potential drug candidates. For example, pharmacokinetic studies showed that compound 20 achieves an oral bioavailability of 24% in rats33, indicating that it is possible to design monovalent Smac mimetics with good oral bioavailability. Many molecularly targeted small-molecule anticancer drugs developed in the last decade are given repeatedly in the clinic for a prolong period of time, for example daily for 3–4 weeks. An orally bioavailable Smac mimetic will provide an important advantage to its clinical development. Bivalent Smac mimetics have been shown to 100–1000 times more potent than their monovalent counterparts39 and thus could be potentially far more efficacious. However, since bivalent Smac mimetics have a molecular weight exceeding 1,000, such compounds may be expected to have very low oral bioavailability and will have to be administered by other routes of administration, such as intravenous dosing, a potential disadvantage if the drug must be given to patients frequently.
Mechanism of action of Smac mimetics in apoptosis induction
The availability of potent, specific and cell-permeable Smac mimetics has provided powerful biological tools with which to gain important insights into apoptosis regulation by IAP proteins.
Using cell-permeable Smac-based peptides, it was shown that such compounds can enhance the activity of chemotherapeutic agents and TRAIL.25–27 Such data were expected because XIAP is a potent inhibitor of apoptosis and Smac-based compounds should antagonize XIAP, thus enhancing the ability of chemotherapeutic agents and TRAIL to induce apoptosis in tumor cells. However, using a set of very potent Smac peptido-mimetics, Oost and colleagues showed that such compounds can effectively inhibit cell growth and induce apoptosis as single agents in a panel of cancer cell lines.28 The single agent activity in cancer cells has been also observed subsequently for other Smac mimetics with different chemotypes, for both monovalent and bivalent Smac mimetics.31–34 However, for a number of years, it was unclear how Smac mimetics alone can induce apoptosis in certain tumor cell lines without the need of an external apoptosis stimulus. This mystery has now been solved by several elegant and independent studies.42–46
As indicated above, Smac mimetics bind not only to XIAP but also to cIAP-1 and cIAP-2, proteins with very high affinities in biochemical assays. Several recent studies have clearly demonstrated that both monovalent and bivalent Smac mimetics cause rapid degradation of cIAP-1/-2 proteins.42–46 Binding of Smac mimetics to cIAP-1/2 induces auto-ubiquination of cIAP-1/2 proteins, followed by protein degradation in a proteasomal-dependent manner.42–46 In sensitive tumor lines, degradation of cIAP-1/-2 by Smac mimetics induces NF-kB-stimulated production of TNFα. TNFα promotes formation of an RIPK1-dependent caspase-8-activating complex upon removal of cIAP-1/-2, leading to activation of caspase-8 and -3/-7, and ultimately apoptosis.42–46 These studies established that induction of cIAP-1/2 degradation is a key early event in apoptosis induction by Smac mimetics and cIAP-1 and cIAP-2 are critical cellular targets for Smac mimetics. Furthermore, it was shown while caspase-3 plays a critical role in apoptosis induction by Smac mimetics in sensitive cancer cell lines, caspase-9 appears to play a modest role.42
Interestingly, although Smac mimetics were designed based upon the interaction of XIAP and Smac, the role of XIAP in apoptosis induction by Smac mimetics was not well defined in these studies.42–46 To address this question, Lu and colleagues have investigated the role of XIAP and cIAP-1/2 in apoptosis induction by monovalent mimetic SM-122 (compound 17) and bivalent mimetic SM-164 (compound 26). They showed that removal of cIAP-1/2 by Smac mimetics or small interfering RNA is not sufficient for robust TNFα-dependent apoptosis induction and that XIAP plays a critical role in inhibiting apoptosis induction. Although SM-164 is slightly more effective than SM-122 in induction of cIAP-1/2 degradation, SM-164 is 1000 times more potent than SM-122 as an inducer of apoptosis in tumor cells, an observation which can be attributed to its much higher potency in binding to and antagonizing XIAP. SM-164 induces rapid cIAP-1 degradation and strong apoptosis in the MDA-MB-231 xenograft tumor tissues, and achieves tumor regression, but has no toxicity in normal mouse tissues. These data provide strong evidence that Smac mimetics induce apoptosis in tumor cells by concurrently targeting cIAP-1/2 and XIAP, suggesting that XIAP and cIAP-1/2 are important cellular targets for Smac mimetics.
Summary
Since the discovery in 2000 of the Smac protein, there has been an enormous interest in academic laboratories and pharmaceutical companies in the design of small-molecule Smac mimetics. This task is particularly challenging because it involves targeting protein-protein interactions. Nevertheless, intense research has now generated potent, specific, cell-permeable small-molecule peptido-mimetics and non-peptidic mimetics. Structure-based strategy has been employed to develop monovalent Smac mimetics designed to mimic the Smac AVPI binding motif and so target the XIAP BIR3 domain. Such compounds were found to not only achieve high affinities to XIAP BIR3 but also high affinities to cIAP-1, cIAP-2 and ML-IAP proteins. Compounds that contain two “AVPI” binding motifs have also been designed and evaluated. It was found that such bivalent Smac mimetics bind to XIAP protein containing both BIR2 and BIR3 domains with an extremely high affinity, exceeding that of Smac protein. It has been clearly shown that bivalent Smac mimetics achieve such high affinities by concurrently targeting both the BIR2 and BIR3 domains in XIAP.
IAP proteins potently suppress apoptosis and Smac protein promotes apoptosis by antagonizing IAP proteins. Hence, it was originally thought that while Smac-based compounds could effectively sensitize cancer cells to other therapeutic agents in apoptosis induction by targeting IAP proteins, they may have limited activity as single agents. Subsequent studies from a number of laboratories have shown that both monovalent and bivalent Smac mimetics are capable as single agents of inducing apoptosis in some but not all human cancer cell lines. It was further shown that bivalent Smac mimetics can induce robust apoptosis in cancer cells at concentrations as low as 1 nM and are 100–1000 times more potent than the corresponding monovalent Smac mimetics. Several recent independent studies have shown that Smac mimetics induce apoptosis in tumor cells by targeting cIAP-1/-2, causing rapid degradation of these proteins. Degradation of cIAP-1/-2 activates NF-kB, which in turn induces the production of TNF-α. TNFα promotes formation of an RIPK1-dependent caspase-8-activating complex, leading to activation of caspase-8 and -3/-7, and ultimately to apoptosis. A major surprise from these recent mechanistic studies is that while caspase-3 was confirmed to play a major role in apoptosis induction by Smac mimetics, caspase-9 appears to play only a modest role. Our recent study further shows that Smac mimetics induce apoptosis in tumor cells by targeting not only cIAP-1/2 but also XIAP.47 These important insights into the regulation of apoptosis by IAP proteins were achieved, to a large extent, because of the availability of potent, cell-permeable, small-molecule Smac mimetics, highlighting the important interface between chemistry and biology to advance our understanding on the regulation of apoptosis.
To date, at least one Smac mimetic has been advanced into clinical development, although the chemical structure of this compound has not been disclosed. Several other Smac mimetics are in an advanced preclinical development stage and are expected to enter human clinical testing for the treatment of cancer.
Figure 5.
Conformationally constrained non-peptidic Smac mimetics
Figure 10.
Other recently published bivalent Smac peptididomimetics
Acknowledgments
We are grateful for the financial support from the Breast Cancer Research Foundation, the Prostate Cancer Foundation, the Department of Defense Prostate Cancer Program (W81XWH-04-1-0213), Ascenta Therapeutics, and the National Cancer Institute, NIH (R01CA109025). We like to thank Dr. G.W.A. Milne for his critical reading of the manuscript.
References
- 1.Ponder BA. Cancer genetics. Nature. 2001;411:336–341. doi: 10.1038/35077207. [DOI] [PubMed] [Google Scholar]
- 2.Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000;21:485–495. doi: 10.1093/carcin/21.3.485. [DOI] [PubMed] [Google Scholar]
- 3.Nicholson DW. From bench to clinic with apoptosis-based therapeutic agents. Nature. 2000;407:810–816. doi: 10.1038/35037747. [DOI] [PubMed] [Google Scholar]
- 4.Deveraux QL, Reed JC. IAP family proteins suppressors of apoptosis. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. 1999. [DOI] [PubMed] [Google Scholar]
- 5.Salvesen GS, Duckett CS. Apoptosis: IAP proteins: blocking the road to death’s door. Nat Rev Mol Cell Biol. 2002;3:401. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- 6.Shiozaki EN, Shi Y. Caspases, IAPs and Smac/DIABLO: mechanisms from structural biology. Trends Biochem Sci. 2004;29:486–94. doi: 10.1016/j.tibs.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Shiozaki EN, Chai J, Rigotti DJ, Riedl SJ, Li P, Srinivasula SM, Alnemri ES, Fairman R, Shi Y. Mechanism of XIAP-mediated inhibition of caspase-9. Mol Cell. 2003;11:519–27. doi: 10.1016/s1097-2765(03)00054-6. [DOI] [PubMed] [Google Scholar]
- 8.Srinivasula SM, Hegde R, Saleh A, Datta P, Shiozaki E, Chai J, Lee RA, Robbins PD, Fernandes-Alnemri T, Shi Y, Alnemri ES. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature. 2001;410:112–6. doi: 10.1038/35065125. [DOI] [PubMed] [Google Scholar]
- 9.Chai J, Shiozaki E, Srinivasula SM, Wu Q, Dataa P, Alnemri ES, Shi Y. Structural basis of caspase-7 inhibition by XIAP. Cell. 2001;104:769–780. doi: 10.1016/s0092-8674(01)00272-0. [DOI] [PubMed] [Google Scholar]
- 10.Huang Y, Park YC, Rich RL, Segal D, Myszka DG, Wu H. Structural basis of caspase inhibition by XIAP: differential roles of the linker versus the BIR domain. Cell. 2001;104:781–90. [PubMed] [Google Scholar]
- 11.Riedl SJ, Renatus M, Schwarzenbacher R, Zhou Q, Sun C, Fesik SW, Liddington RC, Salvesen GS. Structural basis for the inhibition of caspase-3 by XIAP. Cell. 2001;104:791–800. doi: 10.1016/s0092-8674(01)00274-4. [DOI] [PubMed] [Google Scholar]
- 12.Tamm I, Kornblau SM, Segall H, Krajewski S, Welsh K, Kitada S, Scudiero DA, Tudor G, Qui YH, Monks A, Andreeff M, Reed JC. Expression and Prognostic Significance of IAP-Family Genes in Human Cancers and Myeloid Leukemias. Clin Cancer Res. 2000;6:1796–1803. [PubMed] [Google Scholar]
- 13.Holcik M, Gibson H, Korneluk RG. XIAP: Apoptotic brake and promising therapeutic target. Apoptosis. 2001;6:253–261. doi: 10.1023/a:1011379307472. [DOI] [PubMed] [Google Scholar]
- 14.Du C, Fang M, Li Y, Wang X. Smac, a Mitochondrial Protein that Promotes Cytochrome c–Dependent Caspase Activation by Eliminating IAP Inhibition. Cell. 2000;102:33. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 15.Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, Moritz RL, Simpson RJ, Vaux DL. Identification of DIABLO, a Mammalian Protein that Promotes Apoptosis by Binding to and Antagonizing IAP Proteins. Cell. 2000;102:43. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 16.Chai J, Du C, Wu JW, Kyin S, Wang X, Shi Y. Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature. 2000;406:855–62. doi: 10.1038/35022514. [DOI] [PubMed] [Google Scholar]
- 17.Wu G, Chai J, Suber TL, Wu JW, Du C, Wang X, Shi Y. Structural basis of IAP recognition by Smac/DIABLO. Nature. 2000;408:1008. doi: 10.1038/35050012. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z, Sun C, Olejniczak ET, Meadows RP, Betz SF, Oost T, Herrmann J, Wu JC, Fesik SW. Structural basis for binding of Smac/DIABLO to the XIAP BIR3 domain. Nature. 2000;408:1004. doi: 10.1038/35050006. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y, Rich RL, Myszka DG, Wu H. Requirement of both the second and third BIR domains for the relief of X-linked inhibitor of apoptosis protein (XIAP)-mediated caspase inhibition by Smac. J Biol Chem. 2003;278:49517. doi: 10.1074/jbc.M310061200. [DOI] [PubMed] [Google Scholar]
- 20.Shiozaki EN, Shi Y. Caspases, IAPs and Smac/DIABLO: mechanisms from structural biology. Trends Biochem Sci. 2004;29:486–494. doi: 10.1016/j.tibs.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Scott FL, Denault JB, Riedl SJ, Shin H, Renatus M, Salvesen GS. XIAP inhibits caspase-3 and -7 using two binding sites: evolutionarily conserved mechanism of IAPs. EMBO J. 2005;24:645–655. doi: 10.1038/sj.emboj.7600544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu TY, Wagner KW, Bursulaya B, Schultz PG, Deveraux QL. Development and Characterization of Nonpeptidic Small Molecule Inhibitors of the XIAP/Caspase-3 Interaction. Chem Biol. 2003;10:759–67. doi: 10.1016/s1074-5521(03)00157-1. [DOI] [PubMed] [Google Scholar]
- 23.Schimmer AD, Welsh K, Pinilla C, Wang Z, Krajewska M, Bonneau MJ, Pedersen IM, Kitada S, Scott FL, Bailly-Maitre B, Glinsky G, Scudiero D, Sausville E, Salvesen G, Nefzi A, Ostresh JM, Houghten RA, Reed JC. Small-molecule antagonists of apoptosis suppressor XIAP exhibit broad antitumor activity. Cancer Cell. 2004;5:25–35. doi: 10.1016/s1535-6108(03)00332-5. [DOI] [PubMed] [Google Scholar]
- 24.Kipp RA, Case MA, Wist AD, Cresson CM, Carrell M, Griner E, Wiita A, Albiniak PA, Chai J, Shi Y, Semmelhack MF, McLendon GL. Molecular targeting of inhibitors of apoptosis proteins based on small molecule mimics of natural binding partners. Biochemistry. 2002;41:7344–7349. doi: 10.1021/bi0121454. [DOI] [PubMed] [Google Scholar]
- 25.Fulda S, Wick W, Weller M, Debatin KM. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nature Med. 2002;8:808–15. doi: 10.1038/nm735. [DOI] [PubMed] [Google Scholar]
- 26.Arnt CR, Chiorean MV, Heldebrant MP, Gores GJ, Kaufmann SH. Synthetic Smac/DIABLO peptides enhance the effects of chemotherapeutic agents by binding XIAP and cIAP1 in situ. Journal of Biological Chemistry. 2002;277:44236–44243. doi: 10.1074/jbc.M207578200. [DOI] [PubMed] [Google Scholar]
- 27.Yang L, Mashima T, Sato S, Mochizuki M, Sakamoto H, Yamori T, Oh-Hara T, Tsuruo T. Predominant suppression of apoptosome by inhibitor of apoptosis protein in non-small cell lung cancer H460 cells: therapeutic effect of a novel polyarginine-conjugated Smac peptide. Cancer Res. 2003;63:831–7. [PubMed] [Google Scholar]
- 28.Oost TK, Sun C, Armstrong RC, Al-assaad AS, Bentz SF, Deckwerth TL, Ding H, Elmore SW, Meadows RP, Olejniczak ET, Oleksijew A, Oltersdorf T, Rosenberg SH, Shoemaker AR, Tomaselli KJ, Zou H, Fesik SW. Discovery of Potent Antagonists of the Antiapoptotic Protein XIAP for the Treatment of Cancer. J Med Chem. 2004;47:4417. doi: 10.1021/jm040037k. [DOI] [PubMed] [Google Scholar]
- 29.Sun H, Nikolovska-Coleska Z, Yang CY, Xu L, Liu M, Tomita Y, Pan H, Yoshioka Y, Krajewski K, Roller PP, Wang S. Structure-Based Design of Potent, Conformationally Constrained Smac Mimetics. J Am Chem Soc. 2004;126:16686. doi: 10.1021/ja047438+. [DOI] [PubMed] [Google Scholar]
- 30.Sun H, Nikolovska-Coleska Z, Yang CY, Xu L, Tomita Y, Krajewski K, Roller PP, Wang S. Structure-Based Design, Synthesis, and Evaluation of Conformationally Constrained Mimetics of the Second Mitochondria-Derived Activator of Caspase That Target the X-Linked Inhibitor of Apoptosis Protein/Caspase-9 Interaction Site. J Med Chem. 2004;47:4147–4150. doi: 10.1021/jm0499108. [DOI] [PubMed] [Google Scholar]
- 31.Sun H, Nikolovska-Coleska Z, Lu J, Qiu S, Yang CY, Gao W, Meagher J, Stuckey J, Wang S. Design, Synthesis, and Evaluation of a Potent, Cell-Permeable, Conformationally Constrained Second Mitochondria Derived Activator of Caspase (Smac) Mimetic. J Med Chem. 2006;49:7916–7920. doi: 10.1021/jm061108d. [DOI] [PubMed] [Google Scholar]
- 32.Sun H, Stuckey JA, Nikolovska-Coleska Z, Qin D, Meagher JL, Qiu S, Lu J, Yang C-Y, Saito NG, Wang S. Structure-Based Design, Synthesis, Evaluation and Crystallographic Studies of Conformationally Constrained Smac Mimetics as Inhibitors of the X-linked Inhibitor of Apoptosis Protein (XIAP) Journal of Medicinal Chemistry. doi: 10.1021/jm8006849. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng Y, Sun H, Nikolovska-Coleska Z, Qiu S, Yang C-Y, Lu J, Cai Q, Yi H, Wang S. Design, Synthesis and Evaluation of Potent and Orally Bioavailable Diazabicyclic Smac Mimetics. Journal of Medicinal Chemistry. doi: 10.1021/jm801254r. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun W, Nikolovska-Coleska Z, Qin D, Sun H, Yang C-Y, Bai L, Qiu S, Ma D, Wang S. Design, Synthesis and Evaluation of Potent, Non-Peptidic Smac Mimetics. Journal of Medicinal Chemistry. doi: 10.1021/jm801101z. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zobel K, Wang L, Varfolomeev E, Franklin MC, Elliott LO, Wallweber HJ, Okawa DC, Flygare JA, Vucic D, Fairbrother WJ, Deshayes K. Design, Synthesis, and Biological Activity of a Potent Smac Mimetic That Sensitizes Cancer Cells to Apoptosis by Antagonizing IAPs. ACS Chem Biol. 2006;1:525–533. doi: 10.1021/cb600276q. [DOI] [PubMed] [Google Scholar]
- 36.Chauhan D, Neri P, Velankar M, Podar K, Hideshima T, Fulciniti M, Tassone P, Raje N, Mitsiades C, Mitsiades N, Richardson P, Zawel L, Tran M, Munshi N, Anderson KC. Targeting mitochondrial factor Smac/DIABLO as therapy for multiple myeloma (MM) Blood. 2007;109:1220–7. doi: 10.1182/blood-2006-04-015149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaither A, Porter D, Yao Y, Borawski J, Yang G, Donovan J, Sage D, Slisz J, Tran M, Straub C, Ramsey T, Iourgenko V, Huang A, Chen Y, Schlegel R, Labow M, Fawell S, Sellers WR, Zawel L. A Smac mimetic rescue screen reveals roles for inhibitor of apoptosis proteins in tumor necrosis factor-alpha signaling. Cancer Res. 2007;67:11493–8. doi: 10.1158/0008-5472.CAN-07-5173. [DOI] [PubMed] [Google Scholar]
- 38.Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A Small Molecule Smac Mimic Potentiates TRAIL- and TNFα-Mediated Cell Death. Science. 2004;305:1471–4. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 39.Sun H, Nikolovska-Coleska Z, Lu J, Meagher JL, Yang CY, Qiu S, Tomita Y, Ueda Y, Jiang S, Krajewski K, Roller PP, Stuckey JA, Wang S. Design, Synthesis, and Characterization of a Potent, Nonpeptide, Cell-Permeable, Bivalent Smac Mimetic That Concurrently Targets Both the BIR2 and BIR3 Domains in XIAP. J Am Chem Soc. 2007;129:15279–15294. doi: 10.1021/ja074725f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nikolovska-Coleska Z, Meagher JL, Jiang S, Kawamoto SA, Gao W, Yi H, Qin D, Roller PP, Stuckey JA, Wang S. Design and characterization of bivalent Smac-based peptides as antagonists of XIAP and development and validation of a fluorescence polarization assay for XIAP containing both BIR2 and BIR3 domains. Anal Biochem. 2008;374:87–98. doi: 10.1016/j.ab.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 41.Nikolovska-Coleska Z, Wang R, Fang X, Pan H, Tomita Y, Li P, Roller PP, Krajewski K, Saito NG, Stuckeys JA, Wang S. Development and optimization of a binding assay for the XIAP BIR3 domain using fluorescence polarization. Anal Biochem. 2004;332:261–73. doi: 10.1016/j.ab.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 42.Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J, Harran P, Wang X. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12:445–56. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, Benetatos CA, Chunduru SK, Condon SM, McKinlay M, Brink R, Leverkus M, Tergaonkar V, Schneider P, Callus BA, Koentgen F, Vaux DL, Silke J. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–93. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 44.Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, Zobel K, Dynek JN, Elliott LO, Wallweber HJ, Flygare JA, Fairbrother WJ, Deshayes K, Dixit VM, Vucic D. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–81. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 45.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 46.Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Barker PA. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 47.Lu J, Bai L, Sun H, Nikolovska-Coleska Z, McEachern D, Qiu S, Miller RS, Yi H, Shangary S, Sun Y, Meagher JL, Stuckey JA, Wang S. SM-164: A novel, bivalent Smac mimetic induces apoptosis and tumor regression by concurrent removal of the blockade of cIAP-1/2 and XIAP. Cancer Research. 2008 doi: 10.1158/0008-5472.CAN-08-2655. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao Z, Tian Y, Wang J, Yin Q, Wu H, Li YM, Jiang X. A dimeric Smac/diablo peptide directly relieves caspase-3 inhibition by XIAP. Dynamic and cooperative regulation of XIAP by Smac/Diablo. J Biol Chem. 2007;282:30718–27. doi: 10.1074/jbc.M705258200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nikolovska-Coleska Z, Meagher JL, Jiang S, Yang C-Y, Qiu S, Roller PP, Stuckey JA, Wang S. Interaction of a Cyclic, Bivalent Smac Mimetic with the X-Linked Inhibitor of Apoptosis Protein. Biochemistry. 2008 doi: 10.1021/bi800785y. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]