The nuclear envelope is a double-layered membrane that encloses the nuclear genome and transcriptional machinery. In dividing cells of metazoa, the nucleus completely disassembles during mitosis creating the need to re-establish the nuclear compartment at the end of each cell division. Given the crucial role of the nuclear envelope in gene regulation and cellular organization, it is not surprising that its biogenesis and organization have become active research areas. We will review recent insights into nuclear membrane dynamics during the cell cycle.
Introduction
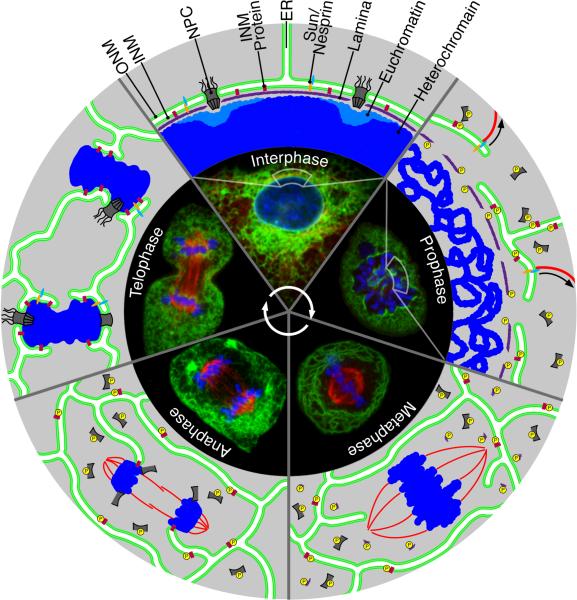
A physical membrane barrier around the nucleus was first suspected almost 100 years ago based on micromanipulation studies [1]. Later, electron microscopy (EM) images revealed that these ‘nuclear envelopes’ consist of two parallel membranes, the inner (INM) and outer nuclear membrane (ONM) [2]. Both membranes are penetrated by nuclear pore complexes (NPCs), large protein assemblies that mediate bidirectional exchange of molecules between the nucleoplasm and the cytoplasm [3]. The ONM is continuous with the endoplasmic reticulum (ER) and is studded with ribosomes. Although lipids can diffuse freely between the NE and the ER (Figure 1, Interphase), the protein composition of the NE differs dramatically from ER tubules and sheets. This indicates specific mechanisms of protein targeting and unique roles of the NE in regulating nuclear functions. Research of the last few years has revealed active roles of the NE in the organization of chromatin and the cytoskeleton as well as in cell cycle progression [4]. For instance, recent studies have demonstrated that heterochromatin tightly associates with the INM and the targeting of certain chromatin regions to the NE directly controls gene expression [5-7].
Figure 1.
NE dynamics during the cell cycle. Changes in the organization of the NE throughout open mitosis are shown in representative live-cell images and schematic illustrations. In live-cell images, ER and NE membranes are labeled with Sec61β(aa1−65)GFP (green), microtubules are labeled with α-tubulin-mcherry (red) and chromatin is stained with Hoechst 33342 (blue). Interphase: During interphase the NE completely surrounds the nucleus and is punctuated by nuclear pore complexes (NPC), which are transport channels present where the inner and outer nuclear membrane (INM, ONM) fuse. Distinct transmembrane proteins are present in the INM that bind to the nuclear lamin, a protein meshwork found within the nucleus, and chromatin. The ONM forms a continuous lipid bilayer with the ER, sharing many of the ER-associated proteins. Prophase: NE breakdown is initiated during prophase, when many on the constituents of the NE and lamina are phosphorylated to disrupt the protein-protein interactions. This, along with microtubule tearing of the NE (arrows) rapidly exposes the chromatin to the forming spindle. Metaphase: By metaphase the membranes of the NE have completely redistributed into the ER, clearing all membranes from the chromosomes. Soluble proteins of the NE and lamina are distributed mainly through the cytoplasm. Anaphase: Late in anaphase, NPC components and ER tubule tips are targeted to the segregated chromosomes, likely by dephosphorylation of the proteins involved in these targeting processes. Telophase: In telophase membranes expand around the chromosomes and NPC continues to assemble.
NE proteins, which mediate the multiple NE functions, can be categorized in essentially four classes: components of the nuclear pore, INM- and ONM-proteins and lamins. The first group is composed of ∼30 proteins, nucleoporins, which constitute the NPCs, the exclusive sites of nucleocytoplasmic transport [3]. NPCs form hollow cylinders with nucleoplasmic and cytoplasmic filamentous attachments that together form a transport channel across the lipid bilayer [8]. Although NPCs are membrane embedded, only three transmembrane nucleoporins have been identified, leaving us with the puzzle of how this ∼90 MDa complex assembles. A detailed model of the molecular architecture of the yeast NPC was recently proposed based on biophysical and proteomic parameters [9], and is likely to provide important insights into the pore structure in metazoa since the overall shape and protein folds seem to have been conserved throughout evolution. The second group of proteins, which specifically localizes to the INM [10], links the NE to chromatin organization. For instance, several integral membrane proteins such as emerin, lamin B receptor, Lap 2β and MAN1 have been shown to interact with regulators of chromatin organization (e.g. HP1 and BAF) and transcription factors [11,12]. Only recently, INM proteins have also been shown to ‘communicate’ with the third class of ONM-specific proteins, which interact with the cytoskeleton [13]. A general principle emerging from these studies is that protein ‘bridges’ are established across the perinuclear space, e.g. lamin-interacting SUN proteins and actin-binding proteins such as nesprins [14,15]. The fourth class of NE proteins constitutes the lamina, a meshwork of intermediate filaments, which is composed of A- and B- type lamins. Strikingly, mutations in lamins and INM proteins are linked to a large number of different human diseases [16,17] and aging [18], highlighting the crucial role of the NE protein network for normal cell function.
The dynamic life cycle of the NE in proliferating cells
Yeast, filamentous fungi and some protists undergo closed mitosis where either the spindle forms inside the nucleus or their microtubules are able to penetrate an intact nuclear envelope [19-21]. In contrast, the NE of metazoan cells completely disintegrates during cell division in order to allow the mitotic spindle to access chromosomes [22]. As a consequence of this open mitosis every dividing cell has to reform the NE and reestablish the identity of the nuclear compartment [23]. In the following paragraphs we will describe new findings regarding the dynamic changes of the vertibrate NE during the cell cycle and describe molecular mechanisms that regulate the life cycle of the nuclear membrane.
NE breakdown
Nuclear envelope breakdown (NEBD) marks the entry into mitosis and precedes the formation of the mitotic spindle apparatus (Figure 1, Prophase). In somatic mammalian cells, interactions of microtubules with the NE generate mechanical forces that contribute to rupture the lamina in a dynein-mediated process [24-26]. Interestingly, the small GTPase Ran has been suggested to regulate microtubule dynamics during NEBD, adding to many functions of Ran throughout the cell cycle [24]. However, nuclear disassembly can also occur in the absence of microtubules [27] indicating that NEBD is regulated at several levels. In Drosophila embryos and starfish oocytes, NPC disassembly appears to be an early event in NEBD [27,28]. In the latter system, NEBD proceeds in two defined steps, sequential release of peripheral nucleoporins followed by fenestration of the NE by large gaps [27].
Currently, it is unclear what triggers NEBD, but the phosphorylation of NE components by several kinases such as Cdk1, PKC, NIMA and Aurora A seems to be crucial [29-32]. Hyperphosphorylation of NE proteins is thought to result in disrupting protein complexes and/or in the activation of factors involved in NEBD. Recently, results from C. elegans and Xenopus egg extracts point to a critical, yet non-essential role of the trans-membrane nucleoporin gp210, which is enriched in its phosphorylated form at the NE just before NEBD occurs [33]. The mechanism by which gp210 triggers disassembly of NPCs the lamina remains to be analyzed.
Depletion of Nup153 from extracts inhibits nuclear envelope breakdown (NEBD) during mitosis. Nup153 was shown to recruit the COPI complex, which mediates retrograde transport from the Golgi to the ER, to the nuclear envelope [34]. A direct role of the COPI complex in this process was further supported by antibodies against β-COP which inhibited NEBD [34]. What roles Nup153 and the COPI complex play in this process has yet to be elucidated.
The Mitotic NE
Between prophase and early anaphase, during which time chromosomes align in the metaphase plate and segregate, chromatin is essentially free of membranes (Figure 1, Metaphase) [35,36]. Also, the majority of soluble nucleoporin subcomplexes is thought to be distributed throughout the cytoplasm, whereas all transmembrane NE proteins, including the three nucleoporins gp210, Ndc1 and POM121, have been shown to reside in the mitotic ER [35-37]. The localization of these membrane proteins suggests that the precursor membrane of the NE is the mitotic ER. This notion is further supported by recent findings using 3D modeling of electron tomography, which demonstrates that the ER remains an intact network in mitosis. Interestingly, in contrast to interphase, the ER in metaphase and anaphase is essentially free of sheets and forms a dense yet highly dynamic network of tubules [37].
NE Reformation
In late anaphase, membranes start to associate with chromatin by a poorly understood mechanism (Figure 1, anaphase). Recent studies of intact cells and cell-free nuclear assembly systems show that ER membrane tubules are targeted to chromatin via tubule ends and reorganized into flat nuclear membrane sheets by DNA-binding-NE-specific membrane proteins [38]. In contrast to previous models, which proposed vesicle fusion to be the principal mechanism of NE formation [39], these new studies suggest that the nuclear membrane forms by the chromatin-mediated reshaping of the endoplasmic reticulum [36,37]. This notion is further supported by recent high-resolution EM data of mitotic ER [37] and in vitro studies, which show that the formation of ER tubules are required for NE assembly [36].
How are the ER tubules targeted to chromatin? The simplest idea is that integral ER proteins with chromatin-binding capacity are recruited to chromatin. This idea is consistent with recent findings that DNA-binding activity of some INM proteins is required for NE formation in vitro [38] and that membrane sheets formed efficiently on protein-free immobilized DNA in vitro [36]. Additionally, binding of several INM proteins to chromatin constituents has been implicated in NE assembly. For example, two studies have demonstrated that the integral INM protein LBR, which binds to heterochromatin-binding protein 1 (HP1), is required for targeting and anchoring NE membranes to chromatin in vitro [40,41]. Similarly, the barrier-to-autointegration factor (BAF), a chromatin-binding protein [42] and its kinase Vrk have recently been shown to play a direct role in NE formation by recruiting LEM domain proteins to chromatin [43]. Vrk seems to be a critical regulator in this process since BAF phosphorylation reduces chromatin binding and interactions with LEM domain proteins such as emerin [12,44]. In summary, NE formation is likely to involve a complex interplay of trans-membrane NE proteins distributed into mitotic ER with the reorganizing chromatin.
Chromatin undergoes a number of conformational changes during cell division, mainly massive condensation in prophase, segragation in anaphase, and decondensation in telophase (Figure 1). Interestingly, maximal chromatin compaction is not reached in metaphase but in late anaphase, after sister chromatid segregation [45]. The chromokinesin kinesin-like DNA binding protein (Kid) has recently been show to be required for the formation of a compact chromosome cluster during anaphase and the proper enclosure of the segregated chromosomes into a single nucleus [46]. Kid targets to the space between anaphase chromosomes and is involoved in chromatin condensation. The depletion of Kid results in fenestrated nuclei and multi-nucleation in early embryonic cells. Interestingly, Kid targeting to anaphase chromosomes is an Importin-β-dependent process [47], adding to the growing number of mitotic processes regulated by this transport receptor.
Recent findings support the idea that NE formation and chromatin decondensation are mechanistically linked. It was shown that Cdc48/p97, a hexameric ATPase previously implicated in membrane fusion [48] and ubiquitin-dependent processes [49], inactivates Aurora B by extracting it from chromatin allowing chromatin decondensation. Inhibition of Cdc48/p97 blocked NE formation suggesting that chromatin decondensation is required for NE formation [50]. Therefore, Aurora B removal might be a crucial step in opening chromatin structure. Since NE membranes associate with chromosomes in late anaphase, it will be interesting to determine how these complex remodeling process regulate recruitment of ER membranes to initiated NE formation (Figure 1, Telophase).
While the above studies are consistent with the idea that NE formation occurs by chromatin-mediated reshaping of the ER, as mentioned above, there are experimental data that suggest that the NE forms by the fusion of vesicles. Results from Xenopus have shown that in vitro nuclear assembly initiated from fragmented ER vesicles is blocked by GTPγS [51,52]. However, these findings do not discriminate between fusion events that are involved in ER reconstitution or NE assembly. When the ER is allowed to preform, NE formation occurs in the presence of GTPγS and ATPγS, suggesting that membrane fusion is not required to form flat sheets [36]. Similarly, a recently observed role of SNARE proteins in NE formation could be an indirect effect of blocked ER reconstitution [53].
Simultaneously with membrane coating of chromatin, NPCs are reassembled from disassembled precursors in late anaphase/telophase. This assembly process is coordinated by the stepwise recruitment of NPC proteins to chromatin [54]. Several nucleoporins have been shown to be essential for pore assembly [23]. Most notably, depletion of the Nup107/160 complex, results in NPC-free nuclear membranes [55,56]. In vertebrates only two out of the three transmembrane nucleoporins seem to be involved in NE formation, Ndc1 and POM121 [57,58]. Their exact role is unknown, but an interesting link between POM121 and the Nup107/160 complex has been made. Nuclear membrane formation might actually be linked to pore assembly by a poorly understood checkpoint by which the Nup107/160 complex ‘senses’ nuclear membranes [59]. Interestingly, Nup133, a member of the Nup107/160 complex, has been identified as containing an ALPS-like motif, which contains an amphipathic alpha-helical domain that have been shown to act as a membrane curvature sensor in vitro [60]. It is possible that this domain is involved in targeting the Nup107/160 complex to membranes during NE formation.
While the targeting of soluble nucleoporins to membranes and chromatin during NE formation is still not fully understood, some progress has been made in determining how the Nup107/160 complex is recruited to chromatin. Mel-28/ELYS was identified in a screen in C. elegans for factors involved in pronuclear formation and in its absence the Nup107/160 complex is no longer recruited to chromatin [61,62]. Mel-28/ELYS contains an AT-hook domain [63] and the simplest model is that it binds directly to DNA and recruits the Nup107/16o complex. RanGTP stimulates Mel-28/ELYS recruitment [62], revealing yet another Ran-mediated step in NE formation [23]. Other nucleoporins have been implicated in pore assembly, but their exact role remains to be determined. For instance, a complex of Nup53 and Nup155 has recently been shown to be essential for NE formation in nematodes and vertebrates [64,65], however, how Nup53 coordinates interactions between chromatin, membranes and soluble Nup155 remains unclear.
The interphase nucleus
The NE is reformed as a closed membrane barrier at the end of cell division, reestablishing the nuclear compartment by selective nuclear transport, i.e. coordinated redistribution of mitotically dispersed cytosolic and nucleoplasmic components. Relatively little is known about this transition and questions such as when pores become active for transport remain to be analyzed. However, it is clear that even after its formation, the NE undergoes a series of changes necessary for cell cycle progression and transcription. These steps include the assembly of a lamina [66], insertion of new pores, and NE expansion [23]. A recently uncovered interaction between the nucleoporin ELYS and Mcm2−7 replication licensing proteins suggest a direct link between pore assembly and DNA metabolism [67]. The determinants of nuclear size and pore number remain to be determined.
As cells grow and prepare to divide, the number of NPCs doubles and the NE surface increases substantially [68]. New pore insertion into an intact NE occurs by a de novo mechanism and requires RanGTP-mediated release of Importin β from a subset of nucleoporins [69]. One of the key questions in this poorly understood process is how the INM and ONM fuse in order to generate a membrane hole. Since membrane pore formation and NPC assembly are likely to be mechanistically coordinated, it is possible that nucleoporins are directly involved in both events. In analogy to vesicle or viral fusion with target membranes, transmembrane proteins residing on either one or both sides of the nuclear envelope could be involved in the fusion of ONM and INM, making transmembrane nucleoporins ideal candidates to fulfill this function. However, none of the three metazoan transmembrane nucleoporins are found in all species, suggesting that they can act redundantly or that a yet unidentified membrane component mediates INM/ONM fusion.
Nuclear expansion requires the supply of additional nuclear membrane and proteins. In vitro nuclear expansion is blocked by disrupting the connection of nuclei with the peripheral ER [70], suggesting that membranes feed into the ONM via connections with ER tubules [36]. In growing Xenopus oocyte nuclei the presence of vesicles on the ONM has been interpreted as evidence for vesicle fusion. However, an alternative explanation is that these vesicles were generated during EM sample preparation by rupturing of ER tubules [71].
Growth of the INM requires passage of membrane components through the fusion sites with the ONM at the NPCs. The current view is that INM proteins are retained once they reach the INM by interactions with either the lamina or chromatin. How INM proteins are targeted in metazoa is less clear. One model suggested that ATP-driven changes in nucleoporin interactions might allow membrane proteins to travel across the NPC [72]. In yeast, integral INM proteins have been shown to directly interact with specific nucleoporins and transport receptors to promote their movement past the NPC [73]. By the end of interphase, the NE has undergone major changes in protein composition and is ready to break down at the onset of mitosis to start a new life cycle in the two daughter cells.
Conclusions
The NE has emerged as a critical interface between chromatin and the cytoskeleton. Many questions remain in regard to the regulation of NPC assembly and membrane targeting and flattening during NE formation. A related developing topic of study is addressing how the interaction of chromatin and the nuclear envelope regulates gene expression. It is possible that the early connection points made between NE-membrane proteins and chromatin during NE formation establish a level of chromatin regulation that has lasting effects on gene expression. This idea is supported by the recent observation that repositioning of genes to the nuclear periphery, which resulted in gene silencing, required NE break down and reformation. It will also be interesting to study how the major classes of NE proteins are regulated during the cell cycle and differentiation. Cell-type and development-specific expression of INM proteins [74] certainly suggests that there is still a lot to be learned about the crucial role of the NE in eukaryotic cell function.
Acknowledgments
We thank Maximiliano D'Angelo, Maya Capelson, Robbie Schulte, Jessica Talamas, and Jesse Vergas for critically reading the manuscript.
References
- 1.Kite GL. The Relative Permeability of the Surface and Interior Portions of the Cytoplasm of Animal and Plant Cells. Biological Bulletin. 1913;25:1–7. [Google Scholar]
- 2.Watson ML. The nuclear envelope; its structure and relation to cytoplasmic membranes. J Biophys Biochem Cytol. 1955;1:257–270. doi: 10.1083/jcb.1.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terry LJ, Shows EB, Wente SR. Crossing the nuclear envelope: hierarchical regulation of nucleocytoplasmic transport. Science. 2007;318:1412–1416. doi: 10.1126/science.1142204. [DOI] [PubMed] [Google Scholar]
- 4.D'Angelo MA, Hetzer MW. The role of the nuclear envelope in cellular organization. Cell Mol Life Sci. 2006;63:316–332. doi: 10.1007/s00018-005-5361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akhtar A, Gasser SM. The nuclear envelope and transcriptional control. Nat Rev Genet. 2007;8:507–517. doi: 10.1038/nrg2122. [DOI] [PubMed] [Google Scholar]
- 6.Shaklai S, Amariglio N, Rechavi G, Simon AJ. Gene silencing at the nuclear periphery. Febs J. 2007;274:1383–1392. doi: 10.1111/j.1742-4658.2007.05697.x. [DOI] [PubMed] [Google Scholar]
- ..7.Reddy KL, Zullo JM, Bertolino E, Singh H. Transcriptional repression mediated by repositioning of genes to the nuclear lamina. Nature. 2008 doi: 10.1038/nature06727. [This is the first study providing evidence that positioning of genes to the NE depends on NE break down and reformation of the NE] [DOI] [PubMed] [Google Scholar]
- .8.Beck M, Forster F, Ecke M, Plitzko JM, Melchior F, Gerisch G, Baumeister W, Medalia O. Nuclear pore complex structure and dynamics revealed by cryoelectron tomography. Science. 2004;306:1387–1390. doi: 10.1126/science.1104808. [This study uses state of the art cryo-electron tomography of NPCs at a 6 nm resolution. This improved resolution revealed a luminal connector element that spans the space between ONM and INM that is attached to opposite sides of the membrane, where the cytoplamsic and nuclear rings make contact] [DOI] [PubMed] [Google Scholar]
- .9.Alber F, Dokudovskaya S, Veenhoff LM, Zhang W, Kipper J, Devos D, Suprapto A, Karni-Schmidt O, Williams R, Chait BT, et al. The molecular architecture of the nuclear pore complex. Nature. 2007;450:695–701. doi: 10.1038/nature06405. [A new approach was used to analyze the NPC based on biophysical and proteomic parameters, which modeled th ebest fit to place each of the 30 nucleoporins in theframe of the known dimensions of the yeast NPC] [DOI] [PubMed] [Google Scholar]
- 10.Schirmer EC, Gerace L. The nuclear membrane proteome: extending the envelope. Trends Biochem Sci. 2005;30:551–558. doi: 10.1016/j.tibs.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Stewart CL, Roux KJ, Burke B. Blurring the boundary: the nuclear envelope extends its reach. Science. 2007;318:1408–1412. doi: 10.1126/science.1142034. [DOI] [PubMed] [Google Scholar]
- 12.Bengtsson L, Wilson KL. Multiple and surprising new functions for emerin, a nuclear membrane protein. Curr Opin Cell Biol. 2004;16:73–79. doi: 10.1016/j.ceb.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Starr DA, Han M. ANChors away: an actin based mechanism of nuclear positioning. J Cell Sci. 2003;116:211–216. doi: 10.1242/jcs.00248. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Q, Skepper JN, Yang F, Davies JD, Hegyi L, Roberts RG, Weissberg PL, Ellis JA, Shanahan CM. Nesprins: a novel family of spectrin-repeat-containing proteins that localize to the nuclear membrane in multiple tissues. J Cell Sci. 2001;114:4485–4498. doi: 10.1242/jcs.114.24.4485. [DOI] [PubMed] [Google Scholar]
- 15.Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, Stahl PD, Hodzic D. Coupling of the nucleus and cytoplasm: role of the LINC complex. J Cell Biol. 2006;172:41–53. doi: 10.1083/jcb.200509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muchir A, Worman HJ. The nuclear envelope and human disease. Physiology (Bethesda) 2004;19:309–314. doi: 10.1152/physiol.00022.2004. [DOI] [PubMed] [Google Scholar]
- 17.Mounkes L, Kozlov S, Burke B, Stewart CL. The laminopathies: nuclear structure meets disease. Curr Opin Genet Dev. 2003;13:223–230. doi: 10.1016/s0959-437x(03)00058-3. [DOI] [PubMed] [Google Scholar]
- 18.Scaffidi P, Misteli T. Lamin A-dependent nuclear defects in human aging. Science. 2006;312:1059–1063. doi: 10.1126/science.1127168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heywood P. Ultrastructure of mitosis in the chloromonadophycean alga Vacuolaria virescens. J Cell Sci. 1978;31:37–51. doi: 10.1242/jcs.31.1.37. [DOI] [PubMed] [Google Scholar]
- 20.Ribeiro KC, Pereira-Neves A, Benchimol M. The mitotic spindle and associated membranes in the closed mitosis of trichomonads. Biol Cell. 2002;94:157–172. doi: 10.1016/s0248-4900(02)01191-7. [DOI] [PubMed] [Google Scholar]
- 21.Byers B. Cytology of the yeast life cycle. In Molecular Biology of the Yeast Saccharomyces. In: Strathern JN, editor. Life Cycle and Inheritance. Cold Spring Harbor Laboratory Press; EWJaJRB: 1981. pp. 59–96. [Google Scholar]
- 22.Lippincott-Schwartz J. Cell biology: ripping up the nuclear envelope. Nature. 2002;416:31–32. doi: 10.1038/416031a. [DOI] [PubMed] [Google Scholar]
- 23.Hetzer M, Walther TC, Mattaj IW. Pushing the Envelope: Structure, Function, and Dynamics of the Nuclear Periphery. Annu Rev Cell Dev Biol. 2005 doi: 10.1146/annurev.cellbio.21.090704.151152. [DOI] [PubMed] [Google Scholar]
- .24.Muhlhausser P, Kutay U. An in vitro nuclear disassembly system reveals a role for the RanGTPase system and microtubule-dependent steps in nuclear envelope breakdown. J Cell Biol. 2007;178:595–610. doi: 10.1083/jcb.200703002. [This is the first repport that the GTPase Ran is involved in NEBD, which extends the functional repertoir of Ran in the cell cycle] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beaudouin J, Gerlich D, Daigle N, Eils R, Ellenberg J. Nuclear envelope breakdown proceeds by microtubule-induced tearing of the lamina. Cell. 2002;108:83–96. doi: 10.1016/s0092-8674(01)00627-4. [DOI] [PubMed] [Google Scholar]
- 26.Salina D, Bodoor K, Eckley DM, Schroer TA, Rattner JB, Burke B. Cytoplasmic dynein as a facilitator of nuclear envelope breakdown. Cell. 2002;108:97–107. doi: 10.1016/s0092-8674(01)00628-6. [DOI] [PubMed] [Google Scholar]
- 27.Lenart P, Rabut G, Daigle N, Hand AR, Terasaki M, Ellenberg J. Nuclear envelope breakdown in starfish oocytes proceeds by partial NPC disassembly followed by a rapidly spreading fenestration of nuclear membranes. J Cell Biol. 2003;160:1055–1068. doi: 10.1083/jcb.200211076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiseleva E, Rutherford S, Cotter LM, Allen TD, Goldberg MW. Steps of nuclear pore complex disassembly and reassembly during mitosis in early Drosophila embryos. J Cell Sci. 2001;114:3607–3618. doi: 10.1242/jcs.114.20.3607. [DOI] [PubMed] [Google Scholar]
- 29.Collas P. Sequential PKC- and Cdc2-mediated phosphorylation events elicit zebrafish nuclear envelope disassembly. J Cell Sci. 1999;112(Pt 6):977–987. doi: 10.1242/jcs.112.6.977. [DOI] [PubMed] [Google Scholar]
- 30.Portier N, Audhya A, Maddox PS, Green RA, Dammermann A, Desai A, Oegema K. A microtubule-independent role for centrosomes and aurora a in nuclear envelope breakdown. Dev Cell. 2007;12:515–529. doi: 10.1016/j.devcel.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burke B, Ellenberg J. Remodelling the walls of the nucleus. Nat Rev Mol Cell Biol. 2002;3:487–497. doi: 10.1038/nrm860. [DOI] [PubMed] [Google Scholar]
- 32.Gong D, Pomerening JR, Myers JW, Gustavsson C, Jones JT, Hahn AT, Meyer T, Ferrell JE., Jr. Cyclin A2 regulates nuclear-envelope breakdown and the nuclear accumulation of cyclin B1. Curr Biol. 2007;17:85–91. doi: 10.1016/j.cub.2006.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galy V, Antonin W, Jaedicke A, Sachse M, Santarella R, Haselmann U, Mattaj I. A role for gp210 in mitotic nuclear-envelope breakdown. J Cell Sci. 2008;121:317–328. doi: 10.1242/jcs.022525. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Prunuske AJ, Fager AM, Ullman KS. The COPI complex functions in nuclear envelope breakdown and is recruited by the nucleoporin Nup153. Dev Cell. 2003;5:487–498. doi: 10.1016/s1534-5807(03)00262-4. [DOI] [PubMed] [Google Scholar]
- 35.Ellenberg J, Siggia ED, Moreira JE, Smith CL, Presley JF, Worman HJ, Lippincott-Schwartz J. Nuclear membrane dynamics and reassembly in living cells: targeting of an inner nuclear membrane protein in interphase and mitosis. J Cell Biol. 1997;138:1193–1206. doi: 10.1083/jcb.138.6.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson DJ, Hetzer MW. Nuclear Envelope Formation by Chromatin-mediated Reorganization of the Endoplasmic Reticulum. Nature Cell Biology. 2007 doi: 10.1038/ncb1636. In Press. [DOI] [PubMed] [Google Scholar]
- .37.Puhka M, Vihinen H, Joensuu M, Jokitalo E. Endoplasmic reticulum remains continuous and undergoes sheet-to-tubule transformation during cell division in mammalian cells. J Cell Biol. 2007;179:895–909. doi: 10.1083/jcb.200705112. [A quantitative confocal and EM approach was used to show that the ER undergoes a dramatic reorganization during cell division. 3D modeling by EM tomograph revealed that membrane sheets are lost and transform into a branched tubular network] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ulbert S, Platani M, Boue S, Mattaj IW. Direct membrane protein-DNA interactions required early in nuclear envelope assembly. J Cell Biol. 2006;173:469–476. doi: 10.1083/jcb.200512078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collas P, Poccia D. Membrane fusion events during nuclear envelope assembly. Subcell Biochem. 2000;34:273–302. doi: 10.1007/0-306-46824-7_7. [DOI] [PubMed] [Google Scholar]
- 40.Pyrpasopoulou A, Meier J, Maison C, Simos G, Georgatos SD. The lamin B receptor (LBR) provides essential chromatin docking sites at the nuclear envelope. Embo J. 1996;15:7108–7119. [PMC free article] [PubMed] [Google Scholar]
- 41.Collas P, Courvalin JC, Poccia D. Targeting of membranes to sea urchin sperm chromatin is mediated by a lamin B receptor-like integral membrane protein. J Cell Biol. 1996;135:1715–1725. doi: 10.1083/jcb.135.6.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Segura-Totten M, Kowalski AK, Craigie R, Wilson KL. Barrier-to-autointegration factor: major roles in chromatin decondensation and nuclear assembly. J Cell Biol. 2002;158:475–485. doi: 10.1083/jcb.200202019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gorjanacz M, Klerkx EP, Galy V, Santarella R, Lopez-Iglesias C, Askjaer P, Mattaj IW. Caenorhabditis elegans BAF-1 and its kinase VRK-1 participate directly in post-mitotic nuclear envelope assembly. Embo J. 2007;26:132–143. doi: 10.1038/sj.emboj.7601470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirano Y, Segawa M, Ouchi FS, Yamakawa Y, Furukawa K, Takeyasu K, Horigome T. Dissociation of emerin from barrier-to-autointegration factor is regulated through mitotic phosphorylation of emerin in a xenopus egg cell-free system. J Biol Chem. 2005;280:39925–39933. doi: 10.1074/jbc.M503214200. [DOI] [PubMed] [Google Scholar]
- .45.Mora-Bermudez F, Gerlich D, Ellenberg J. Maximal chromosome compaction occurs by axial shortening in anaphase and depends on Aurora kinase. Nat Cell Biol. 2007;9:822–831. doi: 10.1038/ncb1606. [This is the first study that addresses the dynamics of mitotic condensation of single chromosomes in live cells, which revealed that maximal compaction is reached in late anaphase and not as previously thought in metaphase] [DOI] [PubMed] [Google Scholar]
- ..46.Ohsugi M, Adachi K, Horai R, Kakuta S, Sudo K, Kotaki H, Tokai-Nishizumi N, Sagara H, Iwakura Y, Yamamoto T. Kid-mediated chromosome compaction ensures proper nuclear envelope formation. Cell. 2008;132:771–782. doi: 10.1016/j.cell.2008.01.029. [This study provides evidence that the chromokinesin Kid/kinesin-10 is involved in the shortening of the anaphase chromosome mass along the spindle axis. The data suggests that Kid-mediated chromosome compaction is required for NE formation around the entire chromosome mass] [DOI] [PubMed] [Google Scholar]
- 47.Tahara K, Takagi M, Ohsugi M, Sone T, Nishiumi F, Maeshima K, Horiuchi Y, Tokai-Nishizumi N, Imamoto F, Yamamoto T, et al. Importin-beta and the small guanosine triphosphatase Ran mediate chromosome loading of the human chromokinesin Kid. J Cell Biol. 2008;180:493–506. doi: 10.1083/jcb.200708003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kondo H, Rabouille C, Newman R, Levine TP, Pappin D, Freemont P, Warren G. p47 is a cofactor for p97-mediated membrane fusion. Nature. 1997;388:75–78. doi: 10.1038/40411. [DOI] [PubMed] [Google Scholar]
- 49.Ye Y, Meyer HH, Rapoport TA. Function of the p97-Ufd1-Npl4 complex in retrotranslocation from the ER to the cytosol: dual recognition of nonubiquitinated polypeptide segments and polyubiquitin chains. J Cell Biol. 2003;162:71–84. doi: 10.1083/jcb.200302169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ..50.Ramadan K, Bruderer R, Spiga FM, Popp O, Baur T, Gotta M, Meyer HH. Cdc48/p97 promotes reformation of the nucleus by extracting the kinase Aurora B from chromatin. Nature. 2007;450:1258–1262. doi: 10.1038/nature06388. [This study demonstrates a role of the AAA-ATPase Cdc48/p97 in nuclear assembly by inactivating the chromatin-associated kinase Aurora B. The inactivation of Aurora B allows chromatin decondensation and NE reformation] [DOI] [PubMed] [Google Scholar]
- 51.Boman AL, Delannoy MR, Wilson KL. GTP hydrolysis is required for vesicle fusion during nuclear envelope assembly in vitro. J Cell Biol. 1992;116:281–294. doi: 10.1083/jcb.116.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dreier L, Rapoport TA. In vitro formation of the endoplasmic reticulum occurs independently of microtubules by a controlled fusion reaction. J Cell Biol. 2000;148:883–898. doi: 10.1083/jcb.148.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baur T, Ramadan K, Schlundt A, Kartenbeck J, Meyer HH. NSF- and SNARE-mediated membrane fusion is required for nuclear envelope formation and completion of nuclear pore complex assembly in Xenopus laevis egg extracts. J Cell Sci. 2007;120:2895–2903. doi: 10.1242/jcs.010181. [DOI] [PubMed] [Google Scholar]
- 54.Dultz E, Zanin E, Wurzenberger C, Braun M, Rabut G, Sironi L, Ellenberg J. Systematic kinetic analysis of mitotic dis- and reassembly of the nuclear pore in living cells. J Cell Biol. 2008;180:857–865. doi: 10.1083/jcb.200707026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walther TC, Alves A, Pickersgill H, Loiodice I, Hetzer M, Galy V, Hulsmann BB, Kocher T, Wilm M, Allen T, et al. The conserved Nup107−160 complex is critical for nuclear pore complex assembly. Cell. 2003;113:195–206. doi: 10.1016/s0092-8674(03)00235-6. [DOI] [PubMed] [Google Scholar]
- 56.Harel A, Orjalo AV, Vincent T, Lachish-Zalait A, Vasu S, Shah S, Zimmerman E, Elbaum M, Forbes DJ. Removal of a single pore subcomplex results in vertebrate nuclei devoid of nuclear pores. Mol Cell. 2003;11:853–864. doi: 10.1016/s1097-2765(03)00116-3. [DOI] [PubMed] [Google Scholar]
- 57.Stavru F, Hulsmann BB, Spang A, Hartmann E, Cordes VC, Gorlich D. NDC1: a crucial membrane-integral nucleoporin of metazoan nuclear pore complexes. J Cell Biol. 2006;173:509–519. doi: 10.1083/jcb.200601001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mansfeld J, Guttinger S, Hawryluk-Gara LA, Pante N, Mall M, Galy V, Haselmann U, Muhlhausser P, Wozniak RW, Mattaj IW, et al. The conserved transmembrane nucleoporin NDC1 is required for nuclear pore complex assembly in vertebrate cells. Mol Cell. 2006;22:93–103. doi: 10.1016/j.molcel.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 59.Antonin W, Franz C, Haselmann U, Antony C, Mattaj IW. The Integral Membrane Nucleoporin pom121 Functionally Links Nuclear Pore Complex Assembly and Nuclear Envelope Formation. Mol Cell. 2005;17:83–92. doi: 10.1016/j.molcel.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 60.Drin G, Casella JF, Gautier R, Boehmer T, Schwartz TU, Antonny B. A general amphipathic alpha-helical motif for sensing membrane curvature. Nat Struct Mol Biol. 2007;14:138–146. doi: 10.1038/nsmb1194. [DOI] [PubMed] [Google Scholar]
- 61.Rasala BA, Orjalo AV, Shen Z, Briggs S, Forbes DJ. ELYS is a dual nucleoporin/kinetochore protein required for nuclear pore assembly and proper cell division. Proc Natl Acad Sci U S A. 2006;103:17801–17806. doi: 10.1073/pnas.0608484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Franz C, Walczak R, Yavuz S, Santarella R, Gentzel M, Askjaer P, Galy V, Hetzer M, Mattaj IW, Antonin W. MEL-28/ELYS is required for the recruitment of nucleoporins to chromatin and postmitotic nuclear pore complex assembly. EMBO Rep. 2007;8:165–172. doi: 10.1038/sj.embor.7400889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kimura N, Takizawa M, Okita K, Natori O, Igarashi K, Ueno M, Nakashima K, Nobuhisa I, Taga T. Identification of a novel transcription factor, ELYS, expressed predominantly in mouse foetal haematopoietic tissues. Genes Cells. 2002;7:435–446. doi: 10.1046/j.1365-2443.2002.00529.x. [DOI] [PubMed] [Google Scholar]
- 64.Hawryluk-Gara LA, Platani M, Santarella R, Wozniak RW, Mattaj IW. Nup53 is Required for Nuclear Envelope and Nuclear Pore Complex Assembly. Mol Biol Cell. 2008 doi: 10.1091/mbc.E07-08-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Franz C, Askjaer P, Antonin W, Iglesias CL, Haselmann U, Schelder M, de Marco A, Wilm M, Antony C, Mattaj IW. Nup155 regulates nuclear envelope and nuclear pore complex formation in nematodes and vertebrates. Embo J. 2005 doi: 10.1038/sj.emboj.7600825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gruenbaum Y, Margalit A, Goldman RD, Shumaker DK, Wilson KL. The nuclear lamina comes of age. Nat Rev Mol Cell Biol. 2005;6:21–31. doi: 10.1038/nrm1550. [DOI] [PubMed] [Google Scholar]
- 67.Gillespie PJ, Khoudoli GA, Stewart G, Swedlow JR, Blow JJ. ELYS/MEL-28 chromatin association coordinates nuclear pore complex assembly and replication licensing. Curr Biol. 2007;17:1657–1662. doi: 10.1016/j.cub.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maul GG, Maul HM, Scogna JE, Lieberman MW, Stein GS, Hsu BY, Borun TW. Time sequence of nuclear pore formation in phytohemagglutinin-stimulated lymphocytes and in HeLa cells during the cell cycle. J Cell Biol. 1972;55:433–447. doi: 10.1083/jcb.55.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ryan KJ, McCaffery JM, Wente SR. The Ran GTPase cycle is required for yeast nuclear pore complex assembly. J Cell Biol. 2003;160:1041–1053. doi: 10.1083/jcb.200209116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.D'Angelo MA, Anderson DJ, Richard E, Hetzer MW. Nuclear pores form de novo from both sides of the nuclear envelope. Science. 2006;312:440–443. doi: 10.1126/science.1124196. [DOI] [PubMed] [Google Scholar]
- 71.Salpingidou G, Rzepecki R, Kiseleva E, Lyon C, Lane B, Fusiek K, Golebiewska A, Drummond S, Allen T, Ellis JA, et al. NEP-A and NEP-B both contribute to nuclear pore formation in Xenopus eggs and oocytes. J Cell Sci. 2008 doi: 10.1242/jcs.019968. [DOI] [PubMed] [Google Scholar]
- 72.Ohba T, Schirmer EC, Nishimoto T, Gerace L. Energy- and temperature-dependent transport of integral proteins to the inner nuclear membrane via the nuclear pore. J Cell Biol. 2004;167:1051–1062. doi: 10.1083/jcb.200409149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.King MC, Lusk CP, Blobel G. Karyopherin-mediated import of integral inner nuclear membrane proteins. Nature. 2006;442:1003–1007. doi: 10.1038/nature05075. [DOI] [PubMed] [Google Scholar]
- 74.Chen IH, Huber M, Guan T, Bubeck A, Gerace L. Nuclear envelope transmembrane proteins (NETs) that are up-regulated during myogenesis. BMC Cell Biol. 2006;7:38. doi: 10.1186/1471-2121-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]