Abstract
Introduction
Toll-like receptors play an important role in the innate immune system and are found to be crucial in severe diseases like sepsis, atherosclerosis, and arthritis. TLR2 and TLR4 expression is upregulated in the inflammatory diseases. Angiotensin II in addition to stimulating vasoconstriction also induces an increase in ROS and a proinflammatory phenotype via AT1R. Angiotensin II type-1 receptor blocker (ARB), widely used as an antihypertensive drug, has been reported to also have anti-inflammatory effects. Thus, we investigated whether an ARB exerts anti-inflammatory effects via inhibiting TLR2 and TLR4 expression.
Methods and results
Monocytes were isolated from healthy human volunteers and treated with the synthetic lipoprotein Pam3CSK4 or LPS in the absence or presence of candesartan. Pretreatment of human monocytes with candesartan significantly decreased Pam3CSK4 or LPS induced TLR2 and TLR4 expression of both mRNA and protein levels (P < 0.05 vs. control) along with decrease in the activity of NF-κB and the expression of IL-1β, IL-6, TNF-α, and MCP-1. Furthermore, candesartan treated mice show decreased TLR2 and TLR4 expression compared to vehicle control mice.
Conclusion
Pam3CSK4 and LPS induced TLR2 and TLR4 expression at mRNA and protein levels are inhibited by candesartan both in vitro and in vivo. Thus, we define a novel pathway by which candesartan could induce anti-inflammatory effects.
Keywords: Toll-like receptors, LPS, Angiotensin receptor blocker, Monocytes, Nuclear factor-kappa B, Inflammation
1. Introduction
Toll-like receptors (TLRs), a family of conserved pattern recognition receptors are important because of their diverse functions in the regulation and linking of immune and inflammatory processes [1]. TLRs are widely distributed on immune cells such as monocytes, macrophages, dendritic cells, neutrophils, B cells, as well as mucosal epithelial and endothelial cells [2]. TLRs are expressed in a plethora of inflammatory diseases such as sepsis syndrome, asthma, rheumatoid arthritis, atherosclerosis, systemic lupus erythematosus (SLE), and inflammatory bowel disease [3]. Engagement of TLRs on the cells by specific ligands leads to the increase in the expression of inflammatory mediators such as interleukin-1β, IL-6, TNF-α, MCP-1, etc. via NF-κB [4]. Both TLR2 and TLR4 are present in human atheroma, diabetes, and also have been shown in animal models to promote atherosclerosis [5–10].
Angiotensin II following engagement of the AT1 receptor promotes vasoconstriction, oxidative stress, inflammation and atherosclerosis [11–14]. Angiotensin II type 1 (AT1) receptor blockers (ARBs) like candesartan prevent cerebrovascular events and also help reduce progression of coronary heart diseases [15–17]. Candesartan (ARBs) is widely used for the treatment of high blood pressure [18], management of chronic heart failure [19], diabetic nephropathy [20], reverse endothelial dysfunction [21], and attenuate oxidative stress [22]. Candesartan has been reported to have antiatherosclerotic effects such as reducing neointimal formation in rats [23] and diminishing vascular inflammation [24–26].
Given the importance of TLR2 and TLR4 in atherosclerosis, we tested the effects of candesartan on the activity and downstream biomediators of TLR2 and TLR4 in human monocytes.
2. Materials and methods
2.1. Monocyte isolation
Mononuclear cells were isolated from fasting heparinized blood (60 ml) obtained from healthy human volunteers (>18 years of age) by Ficoll-Hypaque gradient [27]. Informed consent was obtained from the participants and the study was approved by the Institutional Review Board of UC Davis Medical Center. Monocytes were isolated by magnetic cell sorting using the depletion technique (Miltenyi Biotech, Auburn, CA). Using this technique in our laboratory, we have shown that at least 88% of cells are positive for CD14 by flow cytometry [27]. Monocytes isolated from three healthy volunteers were pooled for each experiment to minimize the variations in the response.
We monitored the endotoxin levels in the culture media, reagents (RPMI, PBS, BSA, etc.) using Limulus Amoebocyte Lysate Assay (Cambrex, Milwaukee, MI) and the average endotoxin level was less than 100 EU/ml consistently in all the experiments, as any endotoxin contamination interferes with accurate TLR measurements. Candesartan (CV-11974) was a gift from AstraZeneca (AstraZeneca R&D, Molndal, Sweden).
2.2. Animal model
To determine the in vivo pleiotropic effects of candesartan, we treated normal male C57BLJ/6 mice (8–10 weeks of age, Jackson Labs, Bar Harbor, Maine) for 10 days with candesartan dissolved in 0.9% NaCl + 50 mM Na2CO3 (2 mg/kg body wt, i.p.) for 10 days with suitable vehicle control mice (n = 4 mice/group). After 10 days peritoneal macrophages were isolated, and challenged ex vivo with Pam3CSK4 and LPS. Macrophages were analyzed for anti-mouse TLR2 (Cat#51-9021) and anti-mouse TLR4 (Cat#12-9041, eBioscience, San Diego, CA) expression using flow cytometry. 80–90% of the cells isolated were CD68 positive. All the procedures described here were approved by the Animal Care and Use Committee of UC Davis, CA.
2.3. siRNA transfection assays
A pool of small interfering RNAs (siRNA) for human AT1 receptor (Cat# sc-29750) was obtained from Santa Cruz Biotechnology (Santa cruz, CA). THP-1 cells in 12 well plates were transiently transfected with 20 µmole/l siRNAs and siPORT Amine reagent following manufacturer’s instructions, with suitable vehicle and scrambled siRNA controls (Cat# sc37007), and subsequently treated with Pam3CSK4 (170 ng/ml) and LPS (160 ng/ml) for 24 h as described previously. Cells were collected for flow cytometry and RT-PCR. Transfection rates of 70–80% of cells were accepted for all the experiments. Knock-down efficiency of the siRNAs is indicated via (i) flow cytometric analysis of TLR2 and TLR4, and (ii) real time RT-PCR of the target gene.
2.4. Fluorescence activated cell sorter analysis of TLR2 and TLR4
Monocytes were treated with the synthetic lipoprotein Pam3Cys-Ser-(Lys)4 (Pam3CSK4; 170 ng/ml; a selective TLR2 agonist; Invivogen, San Diego, CA) or LPS (160 ng/ml; TLR4 agonist; E. coli 026:B6, Sigma St. Louis, MO) in the presence or absence of candesartan (0.1 µM, 1 µM, or 5 µM) with suitable controls. Following treatment, cells were washed twice with cold PBS–BSA (0.1%), incubated with anti-human TLR2 (Cat#17-922, eBioscience) and TLR4 antibodies (Cat#12-9917; eBioscience) or isotype matched IgG controls (mouse IgG2a, K cat#14-4724; eBioscience) in accordance with manufacturer’s instructions and 5000–10,000 events were analyzed with BD FACS Array Bioanalyzer (BD Biosciences, San Jose, CA). The viability (>92%) and integrity of monocytes after stimulation with LPS/Pam3CSK4 stimulation or candesartan pretreatment was confirmed by trypan blue exclusion method and microscopic morphological examination. Results were expressed as mean fluorescence intensity (MFI)/105 cells. The intra-and inter-assay CV were determined to be <10%.
2.5. Reverse transcriptase-polymerase chain reaction (RT-PCR)
RNAwas extracted from the monocytes using TRI reagent (Invitrogen, Carlsbad, CA) reagent. The first strand of cDNA was synthesized using total RNA (1 µg/reaction). cDNA (50–100 ng) was amplified using primers (Invivogen, San Diego, CA) specific for TLR2 and TLR4 and β-actin (R&D, Minneapolis, MN) following manufacturer’s cycling parameters. TLRs and β-actin were amplified for 30 cycles. TLR2 and TLR4 yield a band between 450–700 bp and β-actin at 528 bp on 2% agarose gels and band intensities were determined using Image Quant Software (GE Healthcare Biosciences, Piscataway, NJ) as described previously [28].
2.6. Enzyme linked immuno sorbent assay (ELISA)
The release of IL-1β, IL-6, MCP-1 and TNF-α were measured in the supernatants of monocytes treated with LPS or Pam3CSK4 in the absence or presence of candesartan using high sensitive ELISA assays (R&D Systems, Minneapolis, MN), as reported previously [29]. IL-1β, IL-6, MCP-1 and TNF-α concentrations were expressed as picograms/mg cell protein. The intra- and inter-assay CV were determined to be <10%.
2.7. NF-κB transcription factor assays
The activation of NF-κB binding to the nucleus of monocytes treated with LPS or Pam3CSK4 in the absence or presence of candesartan was determined using the non-radioactive Trans AM-NF-κB p65 transcription factor assay® (Active Motif, Carlsbad, CA) as described else where in detail [30] following manufacturer’s instructions. Nuclear extracts were prepared according to the protocol of the manufacturer. The intra- and inter-assay CV for these assays was <7%.
For examining the NF-κB DNA binding activity, electrophoretic mobility shift assay was performed using a non-radioactive method (Pierce, Rockford, IL) using the consensus and mutated oligos from Santa cruz. NF-κB oligonucleotides were labeled with biotin following manufacturer’s instructions. The labeled oligonucleotides were purified using spin columns, and the binding reactions consisted of labeled oligonucleotides and the nuclear protein. The bound DNA-protein complex was separated by PAGE electrophoresis and NF-κB bands were detected as described previously [29].
2.8. Statistical analysis
All experiments were performed on at least four occasions in duplicate or triplicate. Results of the experimental studies are reported as the means ± S.D. Differences were analyzed by ANOVA with appropriate post hoc analyses. A probability value of <0.05 was considered significant. All statistical analyses were performed using GraphPad Prizm Software (San Diego, CA).
3. Results
3.1. Candesartan reduces the expression of TLR2 and TLR4 protein and mRNA expression in human monocytes
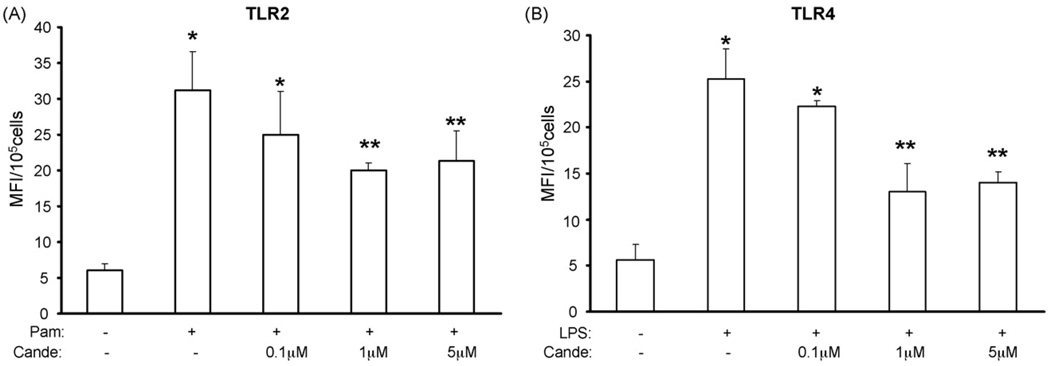
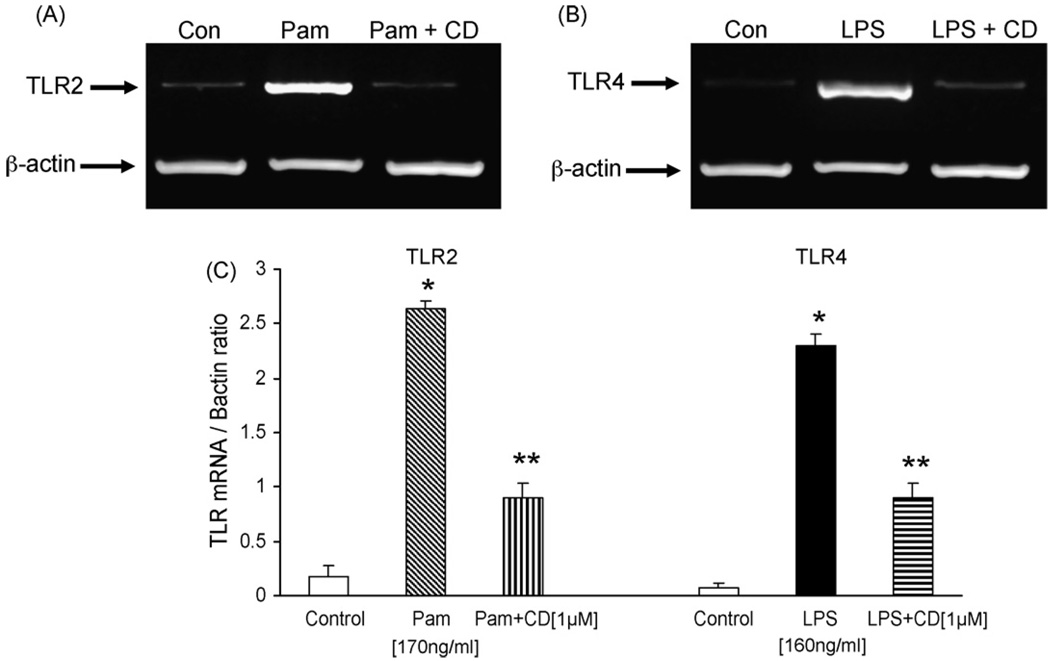
Because Pam3CSK4 and LPS are known to increase TLR2 and TLR4 protein expression, respectively, we first examined the effect of candesartan treatment on Pam3CSK4 and LPS induced TLR2 and TLR4 expression by flow cytometric analysis. Half-maximum doses of Pam3CSK4 and LPS used in the present study were determined in pilot dose response experiments (data not shown). Stimulation of human monocytes with Pam3CSK4 (170 ng/ml) or LPS (160 ng/ml) for 24 h led to a 5.2 ± 1 fold and 4.5 ± 1.04 fold increase in TLR2 and TLR4 surface expression compared with control (P < 0.05, n = 4) and, pretreatment with candesartan significantly reduced the expression of TLR2 and TLR4 protein in a concentration dependent manner with a maximal reduction at 1 µM candesartan (Fig. 1A and B). Furthermore, to determine if the decrease in monocytes TLR2 and TLR4 expression by candesartan resulted from reduced mRNA expression, we investigated the TLR2 and TLR4 mRNA levels by semi-quantitative RT-PCR followed by densitometry. Treatment with candesartan (1 µM) significantly reduced the Pam3CSK4 or LPS induced TLR2 and TLR4 mRNA expression (Fig. 2A–C). Because 1 µM candesartan showed maximal TLR2 and TLR4 inhibition, this dose was used in all subsequent experiments.
Fig. 1.
(A) TLR2 protein expression was measured in human monocytes following Pam3CSK4 challenge in the presence or absence of candesartan (0.1 µM to 5 µM) by flow cytometry as described in Section 2. Values are expressed as mean fluorescence intensity units (MFI)/105 cells. *P < 0.05 vs. control; **P < 0.05 vs. PAM3CSK4; n = 4 experiments; Cande indicates candesartan. (B) TLR4 protein expression was measured in human monocytes following LPS challenge in the presence or absence of candesartan (0.1 µM to 5 µM) by flow cytometry as described in Section 2. Values are expressed as mean fluorescence intensity units (MFI)/105 cells. *P < 0.05 vs. control; **P < 0.05 vs. LPS; n = 4 experiments; Cande indicates candesartan.
Fig. 2.
(A) Representative RT-PCR gel of TLR2 mRNA in human monocytes following Pam3CSK4 exposure with or without candesartan as described in Section 2. (B) Representative RT-PCR gel of TLR4 mRNA in human monocytes following LPS exposure with or without candesartan as described in Section 2. (C) Quantitative analysis of TLR2 and TLR4 mRNA by densitometry. Values are normalized to β-actin mRNA. *P < 0.05 vs. control; **P < 0.05 vs. LPS/PAM3CSK4; n = 4 experiments in duplicate.
3.2. Candesartan treatment protects against Pam3CSK4 and LPS challenge in mice using TLR2 and TLR4
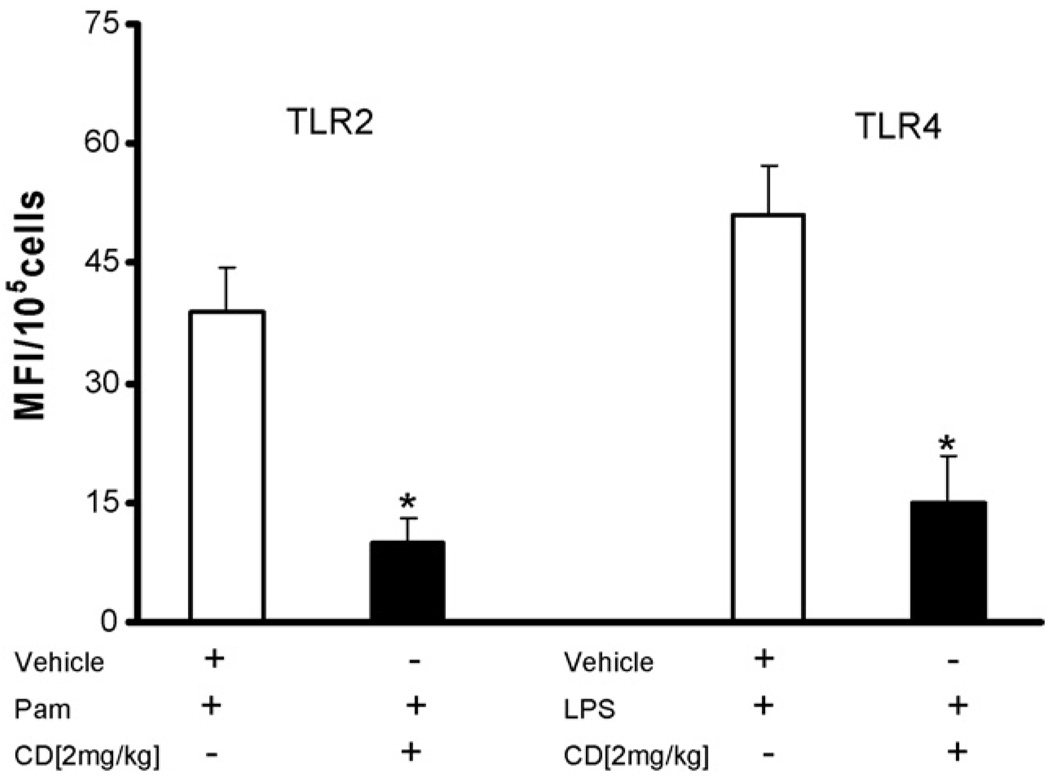
We treated male C57BLJ/6 mice for 10 days with candesartan and challenged the peritoneal macrophages with TLR2 (Pam3CSK4) and TLR4 (LPS) agonists. Candesartan treatment significantly decreased Pam induced TLR2 expression in mice by 74% (39 ± 5 to 10 ± 3 MFI/105 cells, P < 0.001) and LPS induced TLR4 expression by 70% (51 ± 6 to 15 ± 5 MFI/105 cells, P < 0.002) compared to vehicle control (Fig. 3).
Fig. 3.
Candesartan pretreatment (2 mg/kg body weight, 10 days) inhibits Pam3CSK4 or LPS induced TLR2 and TLR4 expression in peritoneal macrophages of C57BLJ/6 mice (n = 4/group) compared to controls (P < 0.05). Values are expressed as mean fluorescence intensity units (MFI)/105 cells. *P < 0.001 vs. CD.
3.3. Inhibition of AT1 receptor ameliorates Pam3CSK4 and LPS induced TLR2 and TLR4 expression in THP-1 cells
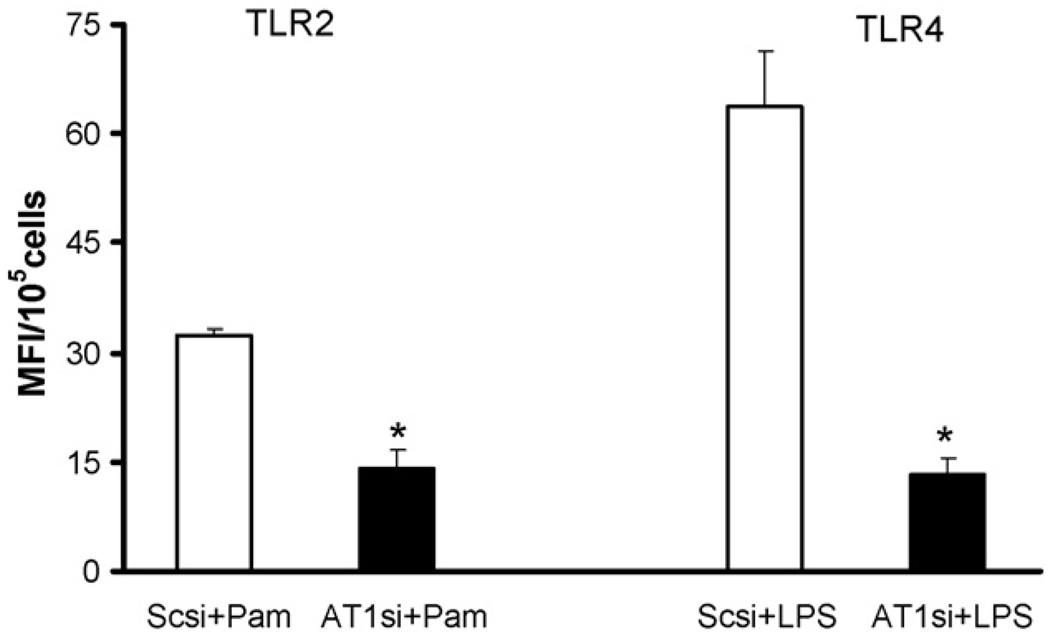
THP-1 cells were transiently transfected with AT1 receptor and scrambled control siRNAs. Transfected cells were pretreated with candesartan (1 µM) and challenged with Pam and LPS. TLR2 and TLR4 expression were measured using flow cytometry. Lack of AT1 receptor ameliorated agonist induced TLR2 expression by 55% and TLR4 by 80% compared to scrambled controls (Fig. 4). Knock down efficiency of AT1 receptor was between 70 and 80% as determined by real time RT-PCR (data not shown).
Fig. 4.
Inhibition of AT1 receptor using siRNA ameliorates Pam3CSK4 and LPS induced TLR2 and TLR4 expression in THP-1 cells. Values are expressed as mean fluorescence intensity units (MFI)/105 cells. *P < 0.001 vs. Sc + Pam and LPS; Scsi = scramble control siRNA; AT1si = AT1 receptor siRNA.
3.4. Candesartan inhibits the activation of NF-κB p65 in human monocytes
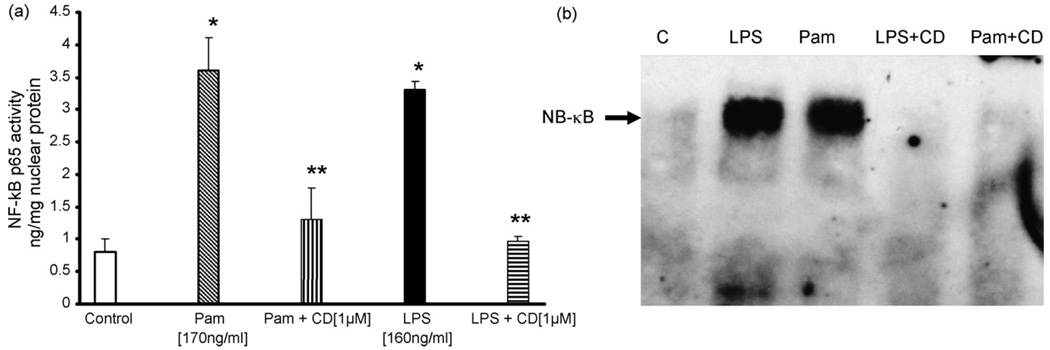
Pam3CSK4 or LPS induced TLR2 and TLR4 activity leads to increased NF-κB activation via p65. To examine, if candesartan (1 µM) may effect this activation, we measured nuclear NF-κB p65 dependent DNA binding activity using a non-radioactive transcriptional factor assay. As shown in Fig. 5a, candesartan significantly inhibited the LPS or Pam3CSK4 induced activation of NF-κB p65 activity compared to control (P < 0.05, n = 4). Gel shift analysis further confirms the inhibitory effects of candesartan (Fig. 5b).
Fig. 5.
(a) The DNA binding activity of nuclear NF-κB p65 in human monocytes following Pam3CSK4 or LPS exposure with or without candesartan was assessed by ELISA method as detailed in Section 2. Values are normalized to mg nuclear protein and expressed as mean ± S.D. *P < 0.05 vs. control; **P < 0.05 vs. LPS/PAM3CSK4; n = 4 experiments in duplicate. (b) Electrophoretic mobility gel shift assay of nuclear NF-κB in human monocytic cells following Pam3CSK4 or LPS exposure with or without candesartan as detailed in Section 2. Blot shown here is a representative of n = 4 experiments.
3.5. Candesartan mediated reduction of TLR2 and TLR4 expression decreases proinflammatory effects in human monocytes
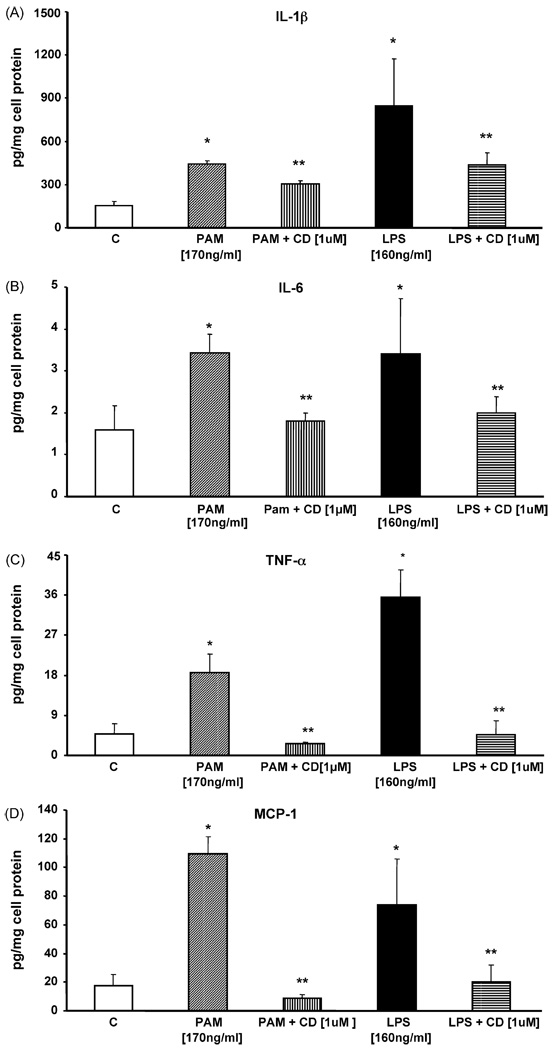
In human cell culture studies, activation of TLR2 and TLR4 by their agonists has previously been shown to upregulate expression of proinflammatory mediators such as IL-1β, IL-6, TNF-α, and MCP-1 [4,31,32]. To assess the functional relevance of reduced monocyte TLR2 and TLR4 expression, human monocytes were challenged with Pam3CSK4 (170 ng/ml) or LPS (160 ng/ml) in the presence or absence of candesartan (1 µM) for 24 h before measuring IL-1β, IL-6, TNF-α, and MCP-1 protein concentrations in the cell supernatants. Pretreatment of cells with candesartan significantly reduced Pam3CSK4 induced IL-1 β (32%), IL-6 (47%), TNF-α (86%), and MCP-1 (92%) compared to control (P < 0.05, n = 4). Also, candesartan significantly decreased LPS induced IL-1β (48%), IL-6 (41%), TNF-α (87%), and MCP-1 (73%) compared to control (P < 0.05, n = 4, Fig. 6A–D).
Fig. 6.
(A) IL-1β concentration in supernatants of human monocytes following Pam3CSK4 or LPS treatment in the presence or absence of candesartan was measured using ELISA assay. Values are normalized to mg cell protein. *P < 0.05 vs. control; **P < 0.05 vs. LPS/PAM3CSK4; n = 4 experiments in duplicate. (B) IL-6 concentration in supernatants of human monocytes following Pam3CSK4 or LPS treatment in the presence or absence of candesartan was measured using ELISA assay. Values are normalized to mg cell protein. *P < 0.05 vs. control; **P < 0.05 vs. LPS/PAM3CSK4; n = 4 experiments in duplicate. (C) TNF-α concentration in supernatants of human monocytes following Pam3CSK4 or LPS treatment with or without candesartan was measured using ELISA assay. Values are normalized to mg cell protein. *P < 0.05 vs. control; **P < 0.05 vs. LPS/PAM3CSK4; n = 4 experiments in duplicate. (D) MCP-1 concentration in supernatants of human monocytes following Pam3CSK4 or LPS treatment with or without candesartan was measured using ELISA assay. Values are normalized to mg cell protein. *P < 0.05 vs. control; **P < 0.05 vs. LPS/PAM3CSK4; n = 4 experiments in duplicate.
Since it has previously been shown that HMG-CoA reductase inhibitors (statins) decrease TLR2 and TLR4 expression [33,34] we determined whether the combination of ARB and statin will have a greater effect. Whilst, we confirm that atorvastatin decreases TLR2 and TLR4 expression, there was no additive effect of the combination treatment (Table 1).
Table 1.
Flow cytometric analysis of TLR2 and TLR4 expression following LPS or Pam3CSK4 challenge in the presence or absence of atorvastatin, candesartan and their combination in human monocytes
| Treatment | TLR2 (MFI/105 cells) | Treatment | TLR4 (MFI/105 cells) |
|---|---|---|---|
| Control | 6 ± 0.9 | Control | 5.6 ± 1 |
| Pam (170 ng/ml) | 31 ± 5* | LPS (160 ng/ml) | 25 ± 3* |
| Pam + AT (1 µM) | 16 ± 3** | LPS + AT (1 µM) | 12 ± 2** |
| Pam + CD (1 µM) | 20 ± 4** | LPS + CD (1 µM) | 13 ± 2** |
| Pam + AT (0.5 µM) + CD (0.5 µM) | 14 ± 4** | LPS + AT (0.5 µM) + CD (0.5 µM) | 10 ± 1** |
P < 0.05 vs. control
P < 0.05 vs. Pam/LPS
Pam = Pam3CSK4; AT = atorvastatin; CD = candesartan; n = 4 experiments.
4. Discussion
The Toll-like receptors (TLRs) recognize self versus non-self molecular patterns and control several aspects of immunity and inflammation, including innate immune responses, antigen presentation, acquired immune responses, and most importantly cytokine gene expression [35]. More than 10 TLRs have been discovered and all possess an ectodomain of leucine-rich repeats involved in ligand binding and cytoplasmic Toll/IL-1 receptor domain (TIR) that interacts with TIR domain containing adaptor molecules. The activation of these receptors on cells of the innate immune system leads to the production of cytokines, chemokines, and the up-regulation of cell surface molecules. After recognizing specific ligands, TLRs use two distinctive pathways: the myeloid differentiation factor (MyD88)-dependent and independent pathways. In the MyD88 dependent pathway the signal from TLR is transduced via MyD88 and IRAK, finally activating NF-κB [36,37]. The activated NF-κB translocates to the nucleus and induces the expression of inflammatory cytokines such as IL-1, IL-6, TNF-α and MCP-1 [4,37,38]. MyD88-independent pathway is executed in TLR3 activation leading to IRF3 instead of NF-κB and induces interferon inducible genes [38]. TLR2 and TLR4 expression is upregulated in the atherosclerotic plaque and animals models of atherosclerosis [5–9,39]. Interestingly, total loss of TLR4 gene is associated with reduction in lesion size, lipid content, and macrophage infiltration in hypercholesterolemic apoE−/− mice [40]. In addition, double knock out mice models of TLR2/LDLR−/−, and TLR2/apoE−/− showed reduced development of atherosclerosis [41,42]. Accordingly, in this study we focused on the TLR2 and TLR4 interaction and their role in inflammatory processes and we showed here that Pam3CSK4 and LPS increased the TLR2 and TLR4 expression in protein and mRNA levels in human monocytes, along with activation of NF-κB and the subsequent increase in inflammation.
Angiotensin II type 1 (AT1) receptor blockers (ARBs) were reported to reduce inflammation in diabetic rat cardiac myocytes, reduce neointimal formation, decrease smooth cell proliferation via a variety of mechanisms including reduced oxidative stress, improving endothelial dysfunction, etc. [20–23]. Clinical studies have suggested that treatment with candesartan lead to decreased plasma levels of Creactive protein (CRP), TNF-α, IL-6, MCP-1, sICAM-1, and sVAM-1 in patients with mild to moderate chronic heart failure and hypertension [19,22,24–26]. There is a paucity of data examining the effects of ARBs on TLR expression and activity. In the present report we show that candesartan decreases TLR2 and TLR4 protein level and mRNA expression, reduces NF-κB p65 dependent activation with concomitant reduction in key inflammatory mediator production in vitro. Furthermore, administration of candesartan to mice resulted in significant reduction in TLR2 and TLR4 expression compared to vehicle control mice. Thus, we confirm our in vitro findings in human monocytes in C57BLJ/6 mice. Also, we did not find additive effect of statin and ARB combination treatment on TLR2 and TLR4 expression. Thus, we suggest that the novel mechanisms of candesartan are attributable to decreasing inflammation by inhibiting TLR2 and TLR4 expression and also indicate that the reduction in TLR2 and TLR4 expression by candesartan may represent a novel strategy to limit TLR mediated inflammatory processes. These novel findings need to be confirmed in patients.
4.1. Clinical implications
Our findings may have pathophysiological and clinical implications for patients with chronic inflammatory diseases such as diabetes [43] and atherosclerosis [5,44] because these patients have enhanced TLR2 and TLR4 expression leading to increased inflammation via expression of inflammatory mediators such as IL-1, IL-6,TNF, and MCP-1. Although further investigation is needed to clarify the precise mechanisms by which candesartan inhibit TLR2 and TLR4 expression, we submit that the documented pleiotropic anti-inflammatory effects of ARBs such as candesartan is mediated, in part, via the TLR pathway.
Acknowledgments
Grant Support: NIH K24 AT 00596, NIH DK69801, ADA 7-07-JF-16.
References
- 1.Miggin SM, O’Neill LA. New insights into the regulation of TLR signaling. J Leukoc Biol. 2006;80(2):220–226. doi: 10.1189/jlb.1105672. [DOI] [PubMed] [Google Scholar]
- 2.Verstak B, Hertzog P, Mansell A. Toll-like receptor signaling and the clinical benefits that lie within. Inflamm Res. 2007;56(1):1–10. doi: 10.1007/s00011-007-6093-7. [DOI] [PubMed] [Google Scholar]
- 3.O’Neill LA. Therapeutic targeting of Toll-like receptors for inflammatory and infectious diseases. Curr Opin Pharmacol. 2003;3(4):396–403. doi: 10.1016/s1471-4892(03)00080-8. [DOI] [PubMed] [Google Scholar]
- 4.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7(3):179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 5.Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002;105:1158–1161. [PubMed] [Google Scholar]
- 6.Xu XH, Shah PK, Faure E, et al. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation. 2001;104:3103–3108. doi: 10.1161/hc5001.100631. [DOI] [PubMed] [Google Scholar]
- 7.Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest. 2005;115(11):3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li H, Sun B. Toll-like receptor 4 in atherosclerosis. J Cell Mol Med. 2007;11(1):88–95. doi: 10.1111/j.1582-4934.2007.00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shinohara M, Hirata KI, Yamashita T, et al. Local overexpression of Toll-like receptors at the vessel wall induces atherosclerotic lesion formation. Synergism of TLR2 and TLR4. Arterioscler Thromb Vasc Biol. 2007 September 13; doi: 10.1161/ATVBAHA.106.139253. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Devaraj S, Dasu MR, Rockwood J, et al. Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: further evidence of a proinflammatory state. J Clin Endocrinol Metab. 2008;93(2):578–583. doi: 10.1210/jc.2007-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dzau VJ. Theodore Cooper Lecture. Tissue angiotensin and pathobiology of vascular disease: a unifying hypothesis. Hypertension. 2001;37:1047–1052. doi: 10.1161/01.hyp.37.4.1047. [DOI] [PubMed] [Google Scholar]
- 12.Nickenig G, Harrison DG. The AT(1)-type angiotensin receptor in oxidative stress and atherogenesis. Part I. Oxidative stress and atherogenesis. Circulation. 2002;105(3):393–396. doi: 10.1161/hc0302.102618. [DOI] [PubMed] [Google Scholar]
- 13.Nickenig G. Central role of the AT(1)-receptor in atherosclerosis. J Hum Hypertens. 2002;16(3):S26–S33. doi: 10.1038/sj.jhh.1001436. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki Y, Ruiz-Ortega M, Lorenzo O, et al. Inflammation and angiotensin II. Int J Biochem Cell Biol. 2003;35(6):881–900. doi: 10.1016/s1357-2725(02)00271-6. [DOI] [PubMed] [Google Scholar]
- 15.Dahlof B, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 16.Demers C, et al. Impact of candesartan on nonfatal myocardial infarction and cardiovascular death in patients with heart failure. JAMA. 2005;294(14):1794–1798. doi: 10.1001/jama.294.14.1794. [DOI] [PubMed] [Google Scholar]
- 17.Mehta JL. Modulation of arterial thrombosis by angiotensin-converting enzyme inhibition and angiotensin II type 1-receptor blockade. Am J Cardiol. 1998;82(10A):53S–56S. doi: 10.1016/s0002-9149(98)00678-x. [DOI] [PubMed] [Google Scholar]
- 18.Heeneman S, Sluimer JC, Daemen MJAP. Angiotensin converting enzyme and vascular remodeling. Circ Res. 2007;101:441–454. doi: 10.1161/CIRCRESAHA.107.148338. [DOI] [PubMed] [Google Scholar]
- 19.Tsutamoto T, Wada A, Maeda K, et al. Angiotensin II type 1 receptor antagonist decreases plasma levels of tumor necrosis factor alpha, interleukin-6 and soluble adhesion molecules in patients with chronic heart failure. J Am Coll Cardiol. 2000;35(3):714–721. doi: 10.1016/s0735-1097(99)00594-x. [DOI] [PubMed] [Google Scholar]
- 20.Donaire JA, Ruilope LM. Angiotensin receptor blockade in diabetic renal disease-focus on candesartan. Diabetes Res Clin Pract. 2007;76(1):S22–S30. doi: 10.1016/j.diabres.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Ghiadoni L, Virdis A, Magagna A, Taddei S, Salvetti A. Effect of the angiotensin II type 1 receptor blocker candesartan on endothelial function in patients with essential hypertension. Hypertension. 2000;35(1 Pt 2):501–506. doi: 10.1161/01.hyp.35.1.501. [DOI] [PubMed] [Google Scholar]
- 22.Koh KK, Ahn JY, Han SH, et al. Pleiotropic effects of angiotensin II receptor blocker in hypertensive patients. J Am Coll Cardiol. 2003;42(5):905–910. doi: 10.1016/s0735-1097(03)00846-5. [DOI] [PubMed] [Google Scholar]
- 23.Igarashi M, Hirata A, Yamaguchi H, et al. Candesartan inhibits carotid intimal thickening and ameliorates insulin resistance in balloon-injured diabetic rats. Hypertension. 2001;38(6):1255–1259. doi: 10.1161/hy1101.095537. [DOI] [PubMed] [Google Scholar]
- 24.Koh KK, Quon MJ, Han SH, et al. Vascular and metabolic effects of candesartan: insights from therapeutic interventions. J Hypertens Suppl. 2006;24(1):S31–S38. doi: 10.1097/01.hjh.0000220404.38622.6a. [DOI] [PubMed] [Google Scholar]
- 25.Dohi Y, Ohashi M, Sugiyama M, et al. Candesartan reduces oxidative stress and inflammation in patients with essential hypertension. Hypertens Res. 2003;26(9):691–697. doi: 10.1291/hypres.26.691. [DOI] [PubMed] [Google Scholar]
- 26.Koh KK, Quon MJ, Han SH, et al. Anti-inflammatory and metabolic effects of candesartan in hypertensive patients. Int J Cardiol. 2006;108(1):96–100. doi: 10.1016/j.ijcard.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 27.Devaraj S, Glaser N, Griffen S, et al. Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes. 2006;55(3):774–779. doi: 10.2337/diabetes.55.03.06.db05-1417. [DOI] [PubMed] [Google Scholar]
- 28.Dasu MR, Devaraj S, Du Clos TW, Jialal I. The biological effects of CRP are not attributable to endotoxin contamination: evidence from TLR4 knockdown human aortic endothelial cells. J Lipid Res. 2007;48(3):509–512. doi: 10.1194/jlr.C600020-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Devaraj S, Venugopal SK, Singh U, Jialal I. Hyperglycemia induces monocytic release of interleukin-6 via induction of protein kinase C-α and -β. Diabetes. 2005;54:85–91. doi: 10.2337/diabetes.54.1.85. [DOI] [PubMed] [Google Scholar]
- 30.Dasu MR, Devaraj S, Jialal I. High glucose induces IL-1beta expression in human monocytes: mechanistic insights. Am J Physiol Endocrinol Metab. 2007;293(1):E337–E346. doi: 10.1152/ajpendo.00718.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuguchi T, Musikacharoen T, Ogawa T, Yoshikai Y. Gene expressions of Toll-like receptor 2, but not Toll-like receptor 4, is induced by LPS and inflammatory cytokines in mouse macrophages. J Immunol. 2000;165:5767–5772. doi: 10.4049/jimmunol.165.10.5767. [DOI] [PubMed] [Google Scholar]
- 32.Medvedev AE, Kopydlowski KM, Vogel SN. Inhibition of lipopolysaccharide-induced signal transduction in endotoxin-tolerized mouse macrophages: dysregulation of cytokine, chemokine, and Tolllike receptor 2 and 4 gene expression. J Immunol. 2000;164:5564–5574. doi: 10.4049/jimmunol.164.11.5564. [DOI] [PubMed] [Google Scholar]
- 33.Niessner A, Steiner S, Speidl WS, et al. Simvastatin suppresses endotoxin-induced upregulation of toll-like receptors 4 and 2 in vivo. Atherosclerosis. 2006;189(2):408–413. doi: 10.1016/j.atherosclerosis.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 34.Methe H, Kim JO, Kofler S, Nabauer M, Weis M. Statins decrease Toll-like receptor 4 expression and downstream signaling in human CD14+ monocytes. Arterioscler Thromb Vasc Biol. 2005;25(7):1439–1445. doi: 10.1161/01.ATV.0000168410.44722.86. [DOI] [PubMed] [Google Scholar]
- 35.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 36.Uematsu S, Akira S. Toll-like receptors and innate immunity. J Mol Med. 2006;84(9):712–725. doi: 10.1007/s00109-006-0084-y. [DOI] [PubMed] [Google Scholar]
- 37.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13(5):8156–8825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 38.Kawai T, Adachi O, Ogawa T, Takeda S, Akira S. Unresponsiveness of MyD88 deficient mice to endotoxin. Immunity. 1999;11(1):115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 39.Stoll LL, Denning GM, Weintraub NL. Endotoxin, TLR4 signaling and vascular inflammation: potential therapeutic targets in cardiovascular disease. Curr Pharm Des. 2006;12(32):4229–4245. doi: 10.2174/138161206778743501. [DOI] [PubMed] [Google Scholar]
- 40.Mullick AE, Tobias PS, Curtiss LK. Toll-like receptors and atherosclerosis: key contributors in disease and health? Immunol Res. 2006;34(3):193–209. doi: 10.1385/IR:34:3:193. [DOI] [PubMed] [Google Scholar]
- 41.Michelsen KS, Wong MH, Shah PK, et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA. 2004;101(29):10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu X, Ukai T, Yumoto H, et al. Toll-like receptor 2 plays a critical role in the progression of atherosclerosis that is independent of dietary lipids. Atherosclerosis. 2007 April 25; doi: 10.1016/j.atherosclerosis.2007.03.025. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Creely SJ, McTernan PG, Kusminski CM, et al. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292(3):E740–E747. doi: 10.1152/ajpendo.00302.2006. [DOI] [PubMed] [Google Scholar]
- 44.Fazio S, Linton MF. The inflamed plaque: cytokine production and cellular cholesterol balance in the vessel wall. Am J Cardiol. 2001;88(2A):12E–15E. doi: 10.1016/s0002-9149(01)01717-9. [DOI] [PubMed] [Google Scholar]