Abstract
Background and objectives: Creatinine-based estimates of GFR suggest an evolving epidemic of chronic kidney disease (CKD) in U.S. adults that is inadequately explained by conventional, modifiable risk factors. Cystatin C has recently emerged as a promising measure of GFR. To enable further insights into the evolution of CKD in the U.S. population, this study aimed to examine cystatin C levels in U.S. adults.
Design, setting, participants, and measurements: Stored serum samples, measured in 2006, were used to compare cystatin C levels among adult participants in the National Health and Nutrition Examination Survey (NHANES) in two time periods, 1988–1994 (n = 6877) and 1999–2002 (n = 4563).
Results: Mean cystatin C levels (0.9 versus 0.9 mg/L, P = 0.65) and urinary albumin-creatinine ratios were similar (5.8 versus 5.9 mg/g, P = 0.19) in the 2 study eras. In contrast, standardized serum creatinine (0.8 versus 0.9 mg/dl, P < 0.0001) was higher and estimated GFR (93.2 versus 87.6 ml/min/1.73 m2, P < 0.001) was lower in 1999–2002. Similar discrepancies in population trends (when cystatin C and creatinine-based methods were used to define GFR) were present when categories of kidney function were considered, and when adjustment was made for demography and comorbid illness.
Conclusions: The disparity between temporal trends when kidney function is assessed with different measurements suggests that estimating trends in disease burden remains an open question.
Chronic kidney disease (CKD) may be important from a public health perspective; it is common, and associated with cardiovascular disease, end-stage kidney disease, death risks in community settings, and substantial health care expenditure (1–5). In the last decade, incidence of end-stage kidney disease treated with renal replacement therapy has increased substantially (5,6), as has the population-wide burden of treatable CKD risk factors, principally hypertension (7), obesity, and diabetes (8,9). However, trends in the burden of CKD are unlikely to be accurately inferred from trends in incidence of end-stage kidney disease. For example, in one notable study comparing 1976–1980 and 1988–1994, CKD prevalence did not keep pace with incidence of end-stage kidney disease treated with renal replacement therapy (10).
Precise estimates of disease trends are desirable for disease management at a public health level. In this regard, recent observations have generated concern that kidney function in representative U.S. adults may be declining without adequate explanation (11). These twin observations (increasing disease burden; inability to explain this burden with easily identifiable, modifiable risk factors) may be a cause for concern from a public health perspective.
Cystatin C is a low-molecular-weight protein with attractive properties as a measure of GFR, including near-constant production rates and free filtration by the glomerulus (12); in addition, cystatin C levels appear to be independent of sex and race, and the contribution of lean-body mass to cystatin C appears to be less than one-tenth that of true GFR in healthy adults (13).
Cystatin C levels were measured in a large, systematically sampled group of National Health and Nutrition Examination Survey (NHANES) participants from 1988–1994 and 1999–2002, enabling further insights into the evolution of CKD in the U.S. population.
Materials and Methods
Objectives
The objectives of this study were to compare representative U.S. adult participants in NHANES 1988–1994 and 1999–2002 with respect to mean cystatin C levels, the primary objective, and serum creatinine levels, GFR values estimated from serum creatinine (eGFRcreatinine) and cystatin C (eGFRcystatin C), urinary albumin-creatinine ratios (ACR), and stages of kidney function.
Study Population
NHANES surveys are cross-sectional, multistage, stratified, clustered probability samples of noninstitutionalized U.S. civilian population (14). NHANES III was performed in 2 phases (1988–1991 and 1991–1994); since 1999, NHANES has been performed continuously in 2-yr cycles. For the current study, data from the 1988–1991 and 1991–1994 cycles were combined to define an earlier study population, and data from 1999–2000 and 2001–2002 were combined to form a later population, a strategy recommended by the National Center for Health Statistics (15,16).
NHANES intentionally oversampled certain population subgroups, including the elderly, Mexican Americans, and non-Hispanic African Americans. Participants were interviewed at home, and physical examinations and blood and urine collections were performed in mobile examination centers.
Measurements and Definitions
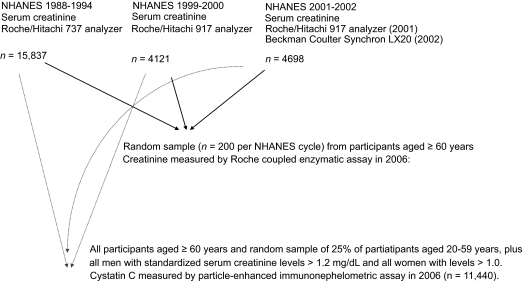
Selection factors and measurement techniques for cystatin C and serum creatinine are shown in Figure 1. Cystatin C levels were measured in stored serum samples for all participants aged ≥60 yr; for participants aged 12 to 59 yr, cystatin C levels were measured in a random sample of 25%, in all men with standardized serum creatinine levels > 1.2 mg/dl, and in all women with levels > 1.0 mg/dl. For the current study, we limited the population to participants aged ≥20 yr in whom cystatin C levels were measured. Cystatin C levels were measured by a particle-enhanced immunonephelometric assay (N Latex Cystatin C; Dade Behring, Deerfield, IL). This assay has a range of 0.23 to 7.25 mg/L, and interassay coefficients of 5.05% and 4.87% at cystatin C levels of 0.97 and 1.90 mg/L, respectively (17).
Figure 1.
Selection factors and measurement techniques for cystatin C and serum creatinine.
Serum creatinine was measured throughout by the kinetic alkaline picrate method. Between 1988 and 1994, measurements were performed at the White Sands Research Center (Coulston Foundation) laboratory (Alamogordo, NM) with a Roche/Hitachi 737 analyzer (Roche Diagnostics, Indianapolis, IN). In 1999, 2000, and 2001, measurements were performed at the Coulston Foundation laboratory with a Roche Hitachi 917 analyzer. In 2002, 2003, and 2004, measurements were performed at the Collaborative Laboratory Services laboratory (Ottumwa, IA) with a Beckman Coulter Synchron LX20 (Beckman Coulter, Fullerton, CA). For this study, serum creatinine measurements were realigned to standardized creatinine measurements obtained at the Cleveland Clinic Research Laboratory (Cleveland, OH). For creatinine standardization, 4 random samples of 200 stored surplus specimens were obtained from study participants aged ≥ 60 yr in NHANES 1988–1994, 1999–2000, 2001–2002, and 2003–2004; creatinine was measured with a Roche coupled enzymatic assay (creatininase, creatinase, sarcosine oxidase; kits no. 1775677 and 1775766) on a Roche P Module instrument (18). College of American Pathologists Creatinine Accuracy Calibration Verification/Linearity Survey LN24 samples were used to confirm that calibration of the Roche enzymatic method was traceable to methods based on liquid chromatography-isotope dilution gas chromatography mass spectrometry. Ultimately, standardized creatinine values (in mg/dl) were calculated from actual creatinine, as follows:
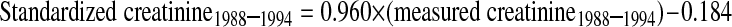 |
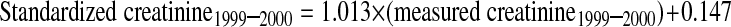 |
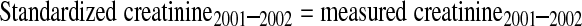 |
The abbreviated Modification of Diet in Renal Disease (MDRD) Study formula was used to estimate GFR from standardized creatinine as follows: eGFRcreatinine (ml/min/1.73 m2) = 175 × (standardized serum creatinine)−1.154 × (age)−0.203 × 0.742 (women) × 1.212 (African Americans) (19,20). The following formula was used to calculate eGFR from cystatin C levels: eGFRcystatin C (ml/min/1.73 m2) = 76.7 × cystatin C−1.19 (21). Urinary albumin and creatinine concentrations were measured at the University of Minnesota (Minneapolis, MN) from random urine spot samples by the modified kinetic Jaffe method with a Synchron AS/Astra Analyzer (Beckman Coulter). The National Kidney Foundation Kidney Disease Outcomes Quality Initiative classification system (1) was used to define categories of kidney function, except that GFR levels 30 to 59 ml/min/1.73 m2 were subdivided into two categories (22): 30 to 44 and 45 to 59 ml/min/1.73 m2, and, because of small numbers, GFR values <15 ml/min/1.73 m2 were subsumed into the < 30 category.
Current smokers were defined by affirmative answers to the questions, “Do you now smoke cigarettes?” and “Have you smoked at least 100 cigarettes in your life?” Diabetes and hypertension were defined by self-report.
Analysis
NHANES-recommended analytical procedures were followed and SUDAAN software (Research Triangle Institute, Research Triangle Park, NC) was used to incorporate the sampling weights implicit in this complex sample survey design (15,23). As recommended by NHANES, we used specific weights that accounted for the probability of having a cystatin C measurement, namely WTCYPEX6 for NHANES 1988–1994 and WTSCY4YR for NHANES 1999–2002. ANOVA and χ2 tests were used for unadjusted comparisons of 1988–1994 and 1999–2002 participants. Multiple logistic regression was used for multivariate comparisons of kidney function categories. An alpha level less than 0.05 was considered statistically significant.
Three sensitivity analyses were applied to comparative trends in kidney function. Serum creatinine values for 1988–1994 and 1999–2002 were compared in a reference population aged < 40 yr without diabetes or hypertension; because serum creatinine values were 0.06 mg/dl higher (P < 0.001) in 1999–2002, all creatinine-based comparisons were repeated with 0.06 subtracted from 1999–2002 levels. Second, eGFR trends were compared in subgroups defined by age, sex, and race. Finally, all analyses were repeated with eGFRcystatin defined by Equation 2 in the study by Stevens et al. (21); findings with Equation 2 were similar to those with Equation 1 and are not presented here.
Results
Participants with cystatin C measurements were very similar to their parent populations in both study eras (Table 1). In the study population of adult participants with cystatin C measurements, discriminating characteristics (P < 0.05 versus 1988–1994) of 1999–2002 participants were as follows: nonwhite race (28.3% versus 23.4%), presence of diabetes (6.7% versus 5.0%), smoking (20.5% versus 28.7%), and body mass index (28.1 versus 26.6 kg/m2).
Table 1.
Comparison of NHANES 1988–1994 and 1999–2002 participants
| Participants with Cystatin C Levels
|
All Participants
|
|||||
|---|---|---|---|---|---|---|
| 1988–1994 (n = 6877) | 1999–2002 (n = 4563) | P | 1988–1994 (n = 17,030) | 1999–2002 (n = 9471) | P | |
| Age, yrs | 44.6 (0.8) | 46.0 (0.4) | 0.12 | 44.7 (0.5) | 46.1 (0.3) | 0.03 |
| Age category, yrs (%) | <0.001 | |||||
| 20–39 | 46.2 (2.1) | 39.9 (1.3) | 46.2 (1.0) | 39.9 (1.0) | ||
| 40–59 | 31.2 (1.2) | 37.7 (1.4) | 31.2 (0.6) | 37.7 (0.8) | ||
| 60–69 | 11.4 (0.9) | 10.5 (0.6) | 11.3 (0.5) | 10.5 (0.5) | ||
| ≥ 70 | 11.3 (1.0) | 12.0 (0.5) | 11.3 (0.6) | 12.0 (0.5) | ||
| Women (%) | 52.4 (1.5) | 52.2 (1.1) | 0.94 | 52.4 (0.4) | 52.2 (0.4) | 0.82 |
| Race/ethnicity (%) | <0.001 | <0.001 | ||||
| White | 76.6 (2.0) | 71.7 (2.1) | 76.3 (1.3) | 71.5 (1.8) | ||
| African American | 10.9 (1.0) | 10.9 (1.3) | 10.9 (0.7) | 10.9 (1.2) | ||
| Hispanic | 5.1 (0.6) | 13.0 (2.2) | 5.1 (0.4) | 13.9 (2.0) | ||
| Other | 7.4 (1.2) | 4.4 (0.6) | 7.7 (0.8) | 3.7 (0.5) | ||
| Diabetes (%) | 5.0 (0.5) | 6.7 (0.4) | <0.01 | 5.5 (0.3) | 6.7 (0.3) | <0.01 |
| Hypertension (%) | 23.8 (0.9) | 26.3 (1.2) | 0.10 | 23.9 (0.6) | 25.7 (0.8) | 0.11 |
| Smoker (%) | 28.7 (1.3) | 20.5 (1.2) | <0.001 | 28.4 (0.8) | 20.5 (0.8) | <0.001 |
| Body mass index, kg/m2 | 26.6 (0.2) | 28.1 (0.2) | <0.001 | 26.5 (0.1) | 28.0 (0.1) | <0.001 |
| Body mass index category, kg/m2 (%) | <0.001 | |||||
| < 25 | 43.6 (1.5) | 36.1 (1.5) | 45.1 (0.9) | 35.2 (0.8) | ||
| 25–29 | 33.9 (1.2) | 33.9 (1.3) | 32.7 (0.6) | 34.7 (0.8) | ||
| ≥ 30 | 22.5 (1.2) | 30.0 (1.4) | 22.2 (0.7) | 30.2 (0.9) | ||
The analysis was adjusted for the sampling weights used in the study design. The chi-square test (for categorical variables) and analysis of variance (for continuous variable) were used to compare the characteristics of 1988–1994 and 1999–2002 participants. Standard errors are shown in parentheses.
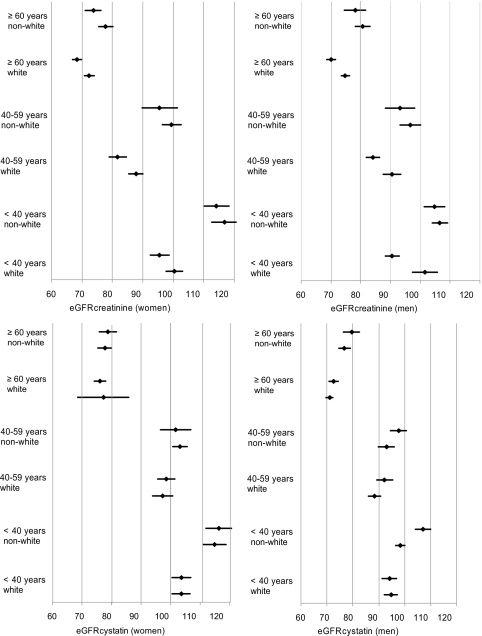
Mean cystatin C levels (0.9 versus 0.9 mg/L), eGFRcystatin C (94.6 versus 93.1 versus ml/min/1.73 m2), and urinary ACR (5.9 versus 5.8 mg/g) were similar in the two study eras (Table 2). In contrast, standardized serum creatinine (0.9 versus 0.8 mg/dl) was higher and eGFRcreatinine was lower (87.6 versus 93.2 ml/min/1.73 m2) in 1999–2002. Figure 2 illustrates eGFRcystatin C and eGFRcreatinine values by study era in subgroups defined by age, sex, and race. The qualitative pattern described above, with similar eGFRcystatin C in both time frames and lower eGFRcreatinine in 1999–2002, was maintained in the subgroups.
Table 2.
Comparison of cystatin C, creatinine, eGFR, and CKD stage, NHANES 1988–1994 and 1999–2004 (n = 11,440)
| Variables | 1988–1994 | 1999–2004 | Pa |
|---|---|---|---|
| Cystatin C, mg/L | 0.9 (< 0.1) | 0.9 (< 0.1) | 0.65 |
| eGFRcystatin C, ml/min/1.73 m2 | 93.1 (0.8) | 94.6 (0.9) | 0.17 |
| eGFRcystatin C (%) | |||
| ≥ 90 | 56.2 (1.7) | 58.7 (1.8) | 0.28 |
| 60–89 | 37.4 (1.4) | 34.3 (1.5) | 0.14 |
| 45–59 | 4.3 (0.4) | 4.8 (0.5) | 0.48 |
| 30–44 | 1.7 (0.2) | 1.5 (0.2) | 0.67 |
| < 30 | 0.4 (0.1) | 0.6 (0.1) | 0.12 |
| CKD stage, eGFRcystatin C, ACR (%) | |||
| normal | 84.9 (1.1) | 85.1 (0.9) | 0.87 |
| stage 1 | 3.9 (0.5) | 4.0 (0.5) | 0.88 |
| stage 2 | 4.7 (0.4) | 3.9 (0.4) | 0.11 |
| stage 3a | 4.3 (0.4) | 4.8 (0.5) | 0.43 |
| stage 3b | 1.7 (0.2) | 1.6 (0.2) | 0.72 |
| stage 4/5 | 0.4 (0.1) | 0.6 (0.1) | 0.11 |
| Standard creatinine in 1988–1994 and 1999–2004 | |||
| Creatinine, mg/dl | 0.8 (< 0.1) | 0.9 (< 0.1) | < 0.001 |
| eGFRcreatinine, ml/min/1.73 m2 | 93.2 (0.7) | 87.6 (0.7) | < 0.001 |
| eGFRcreatinine (%) | |||
| ≥ 90 | 53.2 (1.9) | 41.9 (1.7) | < 0.001 |
| 60–89 | 41.1 (1.8) | 49.7 (1.5) | < 0.001 |
| 45–59 | 4.1 (0.3) | 6.1 (0.4) | < 0.01 |
| 30–44 | 1.2 (0.1) | 1.8 (0.2) | 0.01 |
| < 30 | 0.3 (< 0.1) | 0.5 (0.1) | < 0.01 |
| CKD stage, eGFRcreatinine, ACR (%) | |||
| normal | 85.2 (1.0) | 83.6 (0.8) | 0.18 |
| stage 1 | 4.3 (0.6) | 3.9 (0.5) | 0.67 |
| stage 2 | 4.9 (0.4) | 3.9 (0.4) | 0.10 |
| stage 3a | 4.1 (0.3) | 6.2 (0.4) | < 0.01 |
| stage 3b | 1.2 (0.1) | 1.8 (0.2) | 0.01 |
| Standard creatinine in 1988–1994, minus 0.06 in 1999–2004b | |||
| Creatinine, mg/dl | 0.8 (< 0.1) | 0.8 (< 0.1) | 0.80 |
| eGFRcreatinine, ml/min/1.73 m2 | 93.2 (0.7) | 95.9 (0.8) | 0.02 |
| eGFRcreatinine (%) | |||
| ≥ 90 | 53.2 (1.9) | 55.1 (1.5) | 0.48 |
| 60–89 | 41.1 (1.8) | 38.9 (1.4) | 0.37 |
| 45–59 | 4.1 (0.3) | 3.9 (0.3) | 0.69 |
| 30–44 | 1.2 (0.1) | 1.5 (0.2) | 0.19 |
| < 30 | 0.3 (< 0.1) | 0.5 (0.1) | 0.02 |
| CKD stage, eGFRcreatinine, ACR (%) | |||
| normal | 85.2 (1.0) | 85.8 (0.8) | 0.63 |
| stage 1 | 4.3 (0.6) | 5.1 (0.6) | 0.29 |
| stage 2 | 4.9 (0.4) | 3.1 (0.4) | < 0.01 |
| stage 3a | 4.1 (0.3) | 4.0 (0.3) | 0.77 |
| stage 3b | 1.2 (0.1) | 1.5 (0.2) | 0.16 |
| ACR, mg/g | 5.8 (3.6–11.2) | 5.9 (3.9–11.0) | 0.19 |
| ACR (%) | |||
| < 30 | 89.0 (0.9) | 89.7 (0.7) | 0.45 |
| 30–299 | 8.1 (0.8) | 8.6 (0.7) | 0.65 |
| ≥ 300 | 3.0 (0.4) | 1.7 (0.2) | 0.01 |
ACR, urinary albumin-creatinine ratio; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate. CKD stages: stage 1, eGFR ≥ 90 ml/min/1.73 m2, ACR ≥ 30; stage 2, eGFR 60–89 ml/min/1.73 m2, ACR ≥ 30; stage 3a, eGFR 45–59 ml/min/1.73 m2; stage 3b, eGFR 30–44 ml/min/1.73 m2; stage 4 eGFR 15–30; stage 5 eGFR < 15 ml/min/1.73 m2.
Comparing 1988–1994 and 1999–2004. The analysis was adjusted for the sampling weights used in the study design. The chi-square test (for categorical variables) and analysis of variance (for continuous variables) were used to compare the characteristics of 1988–1994 and 1999–2002 participants. Standard errors are shown in parentheses except for ACR, where median and interquartile range are shown. For kidney function outcomes, participants in each kidney function category were compared with all other participants. For example, for eGFRcystatin C, 45–59 ml/min/1.73 m2, participants with this characteristic were compared with participants with eGFRcystatin C ≥ 60 or <45 ml/min/1.73 m2.
When reference populations (ages 20 to 39 yr without diabetes or hypertension) were compared, serum creatinine values were 0.06 mg higher in the 1999–2002 than the 1988–1994 population.
Figure 2.
Mean eGFRcystatin and eGFRcreatinine levels within subgroups defined by sex, age, and race. The lower and upper members of each two-member cluster represent 1988–1994 and 1999–2002, respectively. Error bars represent 95% confidence intervals. eGFR, estimated GFR.
As defined by eGFRcystatin C and ACR, distributions of CKD stages were similar in 1988–1994 and 1999–2002. As defined by eGFRcreatinine and ACR, stage 3a (6.2% versus 4.1%), stage 3b (1.8% versus 1.2%), and eGFR <30 ml/min/1.73 m2 (0.5% versus 0.3%) were all more prevalent in the 1999–2002 population (Table 2). The prevalence of CKD was higher on the basis of cystatin C-based estimates in both earlier (11.1% versus 10.5%) and later (12.4% versus 10.9%) time periods. Creatinine values were 0.06 mg/dl higher in 1999–2002 among participants aged 20 to 39 yr without diabetes or hypertension. When 0.06 mg/dl was subtracted from all 1999–2002 creatinine values, the proportion of participants with CKD stage 2 fell between study periods (4.9% versus 3.1%) and the proportion with stages 4/5 rose (0.3% versus 0.5%).
Table 3 shows adjusted prevalence ratios of CKD stages in 1999–2002, using 1988–1994 as reference group. With staging based on eGFRcystatin C and ACR, statistically significant changes were observed only for stage 2 (adjusted prevalence ratio, 0.70). With creatinine-based methods, stage 2 was less prevalent (ratio, 0.73) and stages 3a (ratio, 1.50), 3b (ratio, 1.26) and 4/5 (ratio, 1.90) were more prevalent. When 0.06 mg/dl was subtracted from serum creatinine values in 1999–2002, stage 2 declined (ratio, 0.57) and stages 4/5 became more prevalent (ratio, 1.77).
Table 3.
Cystatin C, creatinine, eGFR, and CKD Stage, 1999–2002 vs. 1988–1994, multivariate analysisa
| Variables | Adjusted Prevalence Ratios for 1999–2002 (vs. 1998–1994) | P |
|---|---|---|
| eGFRcystatin C (ml/min/1.73 m2) | Ratio (95% CI) | |
| ≥ 90 | 1.31 (1.02–1.67) | 0.03 |
| 60–89 | 0.81 (0.67–1.00) | 0.05 |
| 45–59 | 0.95 (0.74–1.22) | 0.69 |
| 30–44 | 0.77 (0.53–1.13) | 0.18 |
| < 30 | 1.10 (0.70–1.74) | 0.66 |
| CKD stage, eGFRcystatin C, ACR | ||
| normal | 1.16 (0.94–1.41) | 0.16 |
| stage 1 | 1.09 (0.73–1.64) | 0.66 |
| stage 2 | 0.70 (0.53–0.93) | 0.02 |
| stage 3a | 0.96 (0.75–1.23) | 0.76 |
| stage 3b | 0.78 (0.54–1.14) | 0.20 |
| stage 4/5 | 1.12 (0.71–1.76) | 0.62 |
| Standard creatinine in 1988–1994 and 1999–2004 | ||
| eGFRcreatinine(ml/min/1.73 m2) | ||
| ≥ 90 | 0.59 (0.45–0.76) | <0.001 |
| 60–89 | 1.44 (1.18–1.76) | <0.001 |
| 45–59 | 1.49 (1.20–1.84) | <0.001 |
| 30–44 | 1.24 (0.99–1.56) | 0.06 |
| < 30 | 1.88 (1.22–2.90) | <0.01 |
| CKD stage, eGFRcreatinine, ACR | ||
| normal | 0.95 (0.81–1.13) | 0.57 |
| stage 1 | 0.93 (0.64–1.34) | 0.69 |
| stage 2 | 0.73 (0.54–0.99) | 0.04 |
| stage 3a | 1.50 (1.21–1.87) | <0.001 |
| stage 3b | 1.26 (1.00–1.58) | 0.05 |
| stage 4/5 | 1.90 (1.23–2.94) | <0.01 |
| Standard creatinine in 1988–1994, minus 0.06 in 1999–2004b | ||
| eGFRcreatinine(ml/min/1.73 m2) | ||
| ≥ 90 | 1.21 (0.95–1.55) | 0.12 |
| 60–89 | 0.87 (0.70–1.07) | 0.18 |
| 45–59 | 0.86 (0.69–1.08) | 0.19 |
| 30–44 | 1.03 (0.81–1.30) | 0.82 |
| < 30 | 1.76 (1.11–2.77) | 0.02 |
| CKD stage, eGFRcreatinine, ACR | ||
| Normal | 1.16 (0.97–1.38) | 0.10 |
| stage 1 | 1.22 (0.86–1.73) | 0.27 |
| stage 2 | 0.57 (0.42–0.79) | <0.001 |
| stage 3a | 0.87 (0.70–1.09) | 0.23 |
| stage 3b | 1.04 (0.82–1.31) | 0.74 |
| stage 4/5 | 1.77 (1.12–2.81) | 0.02 |
| ACR, mg/g | ||
| < 30 | 1.17 (0.95–1.44) | 0.14 |
| 30–299 | 1.04 (0.80–1.35) | 0.77 |
| ≥ 300 | 0.45 (0.31–0.67) | <0.001 |
ACR, urinary albumin-creatinine ratio; CKD, chronic kidney disease; CI, confidence interval; eGFR, estimated glomerular filtration rate. CKD stages: stage 1, eGFR ≥ 90 ml/min/1.73 m2, ACR ≥ 30; stage 2, eGFR 60–89 ml/min/1.73 m2, ACR ≥ 30; stage 3a, eGFR 45–59 ml/min/1.73 m2; stage 3b, eGFR 30–44 ml/min/1.73 m2; stage 4 eGFR 15–30 ml/min/1.73 m2; stage 5 eGFR < 15 ml/min/1.73 m2.
The analysis was adjusted for the sampling weights used in the study design. Logistic regression was used was used to calculate prevalence ratios. For kidney function outcomes, participants in each kidney function category were compared with all other participants. For example, for eGFRcystatin C45–59 ml/min/1.73 m2, participants with this characteristic were compared with participants with eGFRcystatin C ≥ 60 or < 45 ml/min/1.73 m2. Adjustment was made for age, sex, race, body mass index, and self-reported diabetes, hypertension, and smoking.
When reference populations (aged 20 to 39 yr without diabetes or hypertension) were compared, serum creatinine values were 0.06 mg higher in the 1999–2002 than the 1988–1994 population.
Discussion
We found substantial declines in eGFR values on the basis of standardized serum creatinine levels. In contrast, serum cystatin C levels (the primary outcome for this study) and ACRs showed no such trends.
Ideally, to compare a given biologic analyte in different eras, the following might be desirable: measurement in all participants and a single, accurate assay throughout. In this study, while all study participants had serum creatinine measurements, three analyzers were used for the original measurements. Standardization to a single reference standard in 2006 included approximately 1% of NHANES 1988–1994 participants and was restricted to those aged 60 yr or older (18). For cystatin C, a systematic sampling design was used; more than 40% of participants were sampled using a single assay. Although issues related to differential sample drift for stored creatinine and cystatin C are theoretically possible, this seems an unlikely explanation for the findings we observed for cystatin C and creatinine-based GFR estimates. It is tempting to speculate that differences in sampling strategies or residual differences in serum creatinine calibration between study eras may be responsible. This being said, it is also possible that findings based on serum creatinine may be correct. In this regard, the findings for eGFR < 30 ml/min/1.73 m2 were notable: creatinine-based methods showed 88% and 76% rises in adjusted prevalence ratios, whereas cystatin C-based methods showed no such trend. In addition, differential evolution of nonrenal and renal cystatin C determinants could explain static cystatin C values in the face of a real fall in GFR values.
Some of the population findings based on serum creatinine in this study are similar to those reported by Coresh et al. (11), who found that prevalence of creatinine-based estimated GFR levels < 60 ml/min/1.73 m2 rose by a factor of 1.4, from 5.63% to 8.04%, over an interval of approximately a decade (NHANES 1999–2004 versus NHANES 1988–1994). Of note, the higher prevalence of GFR values < 60 ml/min/1.73 m2 among later participants was inadequately explained by changes in age distribution or by changes in burdens of diabetes, obesity, or hypertension. Other noticeable features of the more recent report were mean serum creatinine values 0.04 mg/dl higher in 1999–2004 study participants aged 20 to 39 yr without hypertension or diabetes (11). These findings differed from those of a previous report based on nonstandardized serum creatinine values in NHANES 1988–1994 and 1999–2000, which showed stable CKD prevalence estimates (24).
The limitations of this study deserve attention. Gold standard measurements of kidney function, such as insulin or radioisotope clearance, were not performed. The study was cross-sectional; hence, identification of participants with progressive loss of kidney function was not feasible. When conditions are defined by numerical variables that vary intrinsically within individuals, such as GFR or urinary protein excretion, cross-sectional studies can provide reasonable estimates of disease prevalence; all things being equal, it would be expected that individuals showing atypically high values of a given variable would be balanced by individuals with atypically low levels at the time of measurement. In contrast, cross-sectional designs are unsuitable for identifying individual cases, as demonstrating persistently abnormal values requires repeated measurements. In addition, the presence of diabetes and hypertension was based on self-report; possibly, differential ascertainment of these parameters may have occurred in 1988–1994 and 1999–2004.
Despite its limitations, we believe this study provides useful information. The population prevalence of CKD is clearly challenging in scope, and finding an increasing disease burden without apparent explanation could be seen as a major public health concern, as it implies an ongoing epidemic without logical targets for intervention. The disparity between temporal trends when kidney function is addressed with different measurements suggests that estimating trends in disease burden remains an open question.
Disclosures
None.
Acknowledgments
This study was performed as a deliverable under National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health Contract No. HHSN267200715002C. The authors have no competing financial interests with regard to this manuscript. The authors thank United States Renal Data System colleagues Beth Forrest for regulatory assistance, Shane Nygaard for manuscript preparation, and Nan Booth for manuscript editing.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Kidney Disease Outcome Quality Initiative. Am J Kidney Dis 39[suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 2.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, de Zeeuw D, Hostetter TH, Lameire N, Eknoyan G: Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 67: 2089–2100, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Foley RN, Murray AM, Li S, Herzog CA, McBean AM, Eggers PW, Collins AJ: Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 16: 489–495, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW: Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 5.United States Renal Data System: U.S.RDS 2008 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2008
- 6.United States Renal Data System: U.S.RDS 2007 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2008. Available from: http://www.usrds.org/2007/view/02_incid_prev.asp Accessed October 3, 1008
- 7.Hajjar I, Kotchen TA: Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA 290: 199–206, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS: Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 289: 76–79, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP: The continuing epidemics of obesity and diabetes in the United States. JAMA 286: 1195–1200, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Hsu CY, Vittinghoff E, Lin F, Shlipak MG: The incidence of end-stage renal disease is increasing faster than the prevalence of chronic renal insufficiency. Ann Intern Med 141: 95–101, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Grubb A, Simonsen O, Sturfelt G, Truedsson L, Thysell H: Serum concentration of cystatin C, factor D and beta 2-microglobulin as a measure of glomerular filtration rate. Acta Med Scand 218: 499–503, 1985 [DOI] [PubMed] [Google Scholar]
- 13.Macdonald J, Marcora S, Jibani M, Roberts G, Kumwenda M, Glover R, Barron J, Lemmey A: GFR estimation using cystatin C is not independent of body composition. Am J Kidney Dis 48: 712–719, 2006 [DOI] [PubMed] [Google Scholar]
- 14.National Center for Health Statistics and Centers for Disease Control and Prevention: Analytic and reporting guidelines: The third National Health and Nutrition Examination Survey, NHANES III (1988–94). Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/nh3guide.pdf
- 15.National Health and Nutrition Examination Survey (NHANES). Analytic and Reporting Guidelines. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. Available from: http://www.cdc.gov/nchs/nhanes.htm
- 16.National Health and Nutrition Examination Survey (NHANES). NHANES Analytic Guidelines, June 2004 Version. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_general_guidelines_june_04.pdf
- 17.National Health and Nutrition Examination Survey (NHANES). Documentation, Codebook, and Frequencies: Surplus Specimen Laboratory Component: Cystatin C (Surplus Sera). Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_01_02/sscyst_b.pdf
- 18.Selvin E, Manzi J, Stevens LA, Van LF, Lacher DA, Levey AS, Coresh J: Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis 50: 918–926, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F: Using standardized serum creatinine values in the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van LF, Bruce RD, III, Zhang YL, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51: 395–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 23.National Health and Nutrition Examination Survey (NHANES). Analytic and Reporting Guidelines. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/nhanes_analytic_guidelines_dec_2005.pdf
- 24.Coresh J, Byrd-Holt D, Astor BC, Briggs JP, Eggers PW, Lacher DA, Hostetter TH: Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol 16: 180–188, 2005 [DOI] [PubMed] [Google Scholar]