Abstract
Background and objectives: Automated peritoneal dialysis (APD) is increasingly used in comparison with continuous ambulatory peritoneal dialysis (CAPD). Although APD is expected to improve survival, convincing evidence of major advantages is lacking. The objective was to investigate whether overall mortality and technique failure of incident dialysis patients treated with APD are different from those treated with CAPD.
Design, setting, participants, & measurements: Patients on APD or CAPD at 3 mo after start of dialysis were selected from a prospective multicenter cohort study in incident dialysis patients (NECOSAD). Overall mortality was studied with an intention-to-treat design; the event was death. Technique failure was studied with an as-treated design; the event was a switch of dialysis modality. Hazard ratios (HRs) were calculated with a follow-up of 5 yr. The HRs were adjusted for gender, age, primary kidney disease, comorbidity, residual GFR, urine production and plasma albumin at 3 mo after inclusion.
Results: Eighty-seven APD and 562 CAPD patients were included. In the intention-to-treat analysis 154 CAPD and 21 APD patients died. The crude HR for overall mortality was 0.98 (95% CI: 0.62–1.54), the adjusted HR was 1.09. In the as-treated analysis 238 CAPD and 34 APD patients switched therapy, whereas 91 CAPD and 7 APD patients died. The crude HR for technique failure was 0.92 (95% CI: 0.64–1.31) and did not change after adjustment.
Conclusions: No difference was found in overall mortality and technique failure for APD compared with CAPD in incident dialysis patients.
Automated peritoneal dialysis (APD) is increasingly used in comparison with continuous ambulatory peritoneal dialysis (CAPD). Although APD has been expected to improve survival, convincing evidence of major advantages is lacking. In addition APD has potential disadvantages compared with CAPD like a possible faster decline in residual renal function (1–3), less sodium removal (3–6) and more peritoneal protein loss (6). Furthermore, APD is more expensive than CAPD. Two observational studies reported better overall and technique survival on APD (7,8). However, other studies could not demonstrate a benefit for APD in terms of survival (5,9).
Three randomized controlled trials comparing APD to CAPD have been published (10–12). The number of patients was relatively small: the largest study comprised 50 patients in the largest group (11). These were reviewed in 2006 (13,14), and no significant differences in survival were found.
Comparison of the published studies about this subject is complicated because the indications for APD differ between countries and nephrologists. At the same time, the uncertainty in the literature about the expected benefit of APD probably contributes to these differences in indications. The comparison is also complicated by the different patient selections in the studies; some included prevalent and incident APD patients (9), others included incident patients for PD (7,8) or incident dialysis patients (5,9). The definition of APD differs between the studies as well. Large prospective observational cohorts in incident dialysis patients are required to gain more insight into survival on APD compared with CAPD.
The Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD) is a large prospective, multicenter cohort study of end-stage renal disease (ESRD) patients starting on dialysis treatment. We used the NECOSAD database to investigate whether overall mortality and technique failure of incident dialysis patients treated with APD are different from those of patients treated with CAPD.
Patients and Methods
Patients
NECOSAD is a prospective multicenter cohort study in which incident adult ESRD patients were included between 1997 and 2006. Thirty-eight dialysis centers in the Netherlands participated in the study. Patients’ preference was the main reason to start APD. The patients were monitored from the start of dialysis, at 3 mo, and thereafter at 6-mo intervals until kidney transplantation or death. All invited patients gave informed consent before inclusion, and the committees of medical ethics of all participating centers approved the study.
In the present analysis, all patients treated with APD or CAPD at 3 mo after the start of dialysis (baseline) were included. This time point was chosen because patients’ switch to another therapy or deaths within this period were most probably due to their health status before the start of dialysis, rather than the dialysis modality.
Data Collection Procedures
At the start of dialysis treatment the following data were collected: age, sex, primary kidney disease, residual renal function (residual GFR, renal Kt/Vurea, urine production), and comorbidity. Residual renal function was expressed as the residual GFR (rGFR), which was calculated as the mean of creatinine and urea clearance adjusted for body surface area (ml/min/1.73 m2). Urea and creatinine levels were measured in plasma and urine samples. The comorbidity was scored on the basis of the number of comorbid conditions according to the comorbidity index described by Davies et al. (15). The patients were classified as having no, intermediate, or severe comorbidity. Primary kidney disease was classified according to the codes of the European Renal Association–European Dialysis and Transplant Association (16). At the start, data on social parameters were also collected. These included marital stage, the possession of children (and if so, how many), unpaid and paid work, and education level. The presence of a sleep disorder was defined as the regular use of sleep medication.
Data on biochemistry, nutritional status, and dialysis parameters were collected at the 3-mo visit and every 6 mo from the start of dialysis. The Subjective Global Assessment (SGA) scale was used to score the nutritional status. This is a standardized 7-point scale based on the clinical judgment of a dialysis nurse (17). Malnutrition was defined as an SGA-score of 5 or less.
Analysis
Numbers are presented as means and SD for continuous variables, and as numbers with valid percentages for categorical variables. For continuous variables differences were tested with t test; the χ2 was used for categorical variables. The Fisher exact test was used in case the variables did not meet the criteria for the χ2 test. A P value < 0.05 was considered significant. All statistical analyses were performed in SPSS for windows 14.0 (SPSS Inc, Chicago, IL).
Incidence rates were calculated for overall mortality and technique failure. With the Cox proportion hazards model, hazard ratios (HRs) and the associated 95% confidence intervals (CIs) were calculated for APD patients compared with CAPD patients. Patients’ survival time was calculated from baseline. Moreover, HRs were adjusted for age, gender, primary kidney disease, comorbidity, rGFR, urine production and plasma albumin at baseline. These variables are all known predictors of survival on dialysis.
Two main analyses were performed: an intention-to-treat and an as-treated analysis. The intention-to-treat analysis was used to study overall mortality. The event was death; patients were censored for all other reasons of drop-out, at withdrawal or at August 20, 2007. Maximum follow-up was 5 yr from the start of dialysis. In this analysis modality switches were ignored, and deaths were ascribed to the primary treatment modality at baseline.
To study technique failure, an as-treated design was used. The event was modality change: either to hemodialysis (HD) or to another form of peritoneal dialysis (PD); censoring was applied for death, other reasons of dropout, at withdrawal, or at August 20 2007. Thus, patients were followed from their original PD modality until their first switch to any other form of dialysis (CAPD, APD, or HD) or censoring. Patients were censored at 3 mo after the last date with available information on PD modality, when the date of withdrawal was more then 6 mo afterwards. Maximum follow-up was 5 yr from start of dialysis. Temporarily switches to HD were ignored if shorter than 3 mo.
Overall mortality was also studied with an as-treated design. Contrary to the technique survival, in this analysis, the event was death on the original treatment modality, and patients were censored at the time of modality change for all other reasons of drop-out, at withdrawal, or at August 20, 2007. If patients died within 3 mo after the switch to another dialysis modality, this switch was ignored and the death was allocated to the primary dialysis modality. The remainder of the analysis was identical to the technique failure analysis.
Stratification was used to analyze differences in overall mortality and technique failure for younger (up to 60 yr) and elderly patients. Stay on original PD modality was analyzed in the as-treated design, with technique failure and mortality as a combined end-point.
A conditional survival analysis was performed to study the long-term effect of PD modality on overall mortality and technique failure (18). First, the influence of PD modality on survival in the first 2 yr was calculated to study the short term effect. Second, we calculated the influence of PD modality at baseline on the period from 2 to 5 yr from baseline, conditionally on having survived the first 2 yr of dialysis. Thus, patients selected for the conditional analysis were those without an event in the first 2 yr. For the conditional technique failure analysis patients who stayed the first 2 yr on the same PD modality were selected.
The effect of APD compared with CAPD on survival might be different for patients who use these therapies at 1 yr after the start of dialysis. To analyze these differences, we performed an additional analysis on overall mortality. In this analysis, we included all patients treated with APD or CAPD, 1 yr after inclusion in NECOSAD. These patients were followed until death or other reasons of drop-out according to the overall mortality analysis previously described, with a maximum follow-up of 5 yr. The variables at baseline (3 mo) were used for adjustment for confounders.
Results
At baseline, 562 patients who were treated with CAPD and 87 patients who were treated with APD were included. The physical characteristics of these patients are presented in Table 1; their social characteristics are presented in Table 2. No major differences were present at baseline between the CAPD and the APD patients. The adjusted analyses could be performed in 617 of the 649 included patients.
Table 1.
Baseline characteristics
| Variable | CAPD (n = 562) | APD (n = 87) | P |
|---|---|---|---|
| Gender (male) | 370 (65.8%) | 68 (78.2%) | 0.02 |
| Age (yrs) | 53.4 (14.5) | 51.5 (16.3) | 0.32 |
| Systolic blood pressure (mmHg) | 140.4 (21.1) | 138.7 (21.2) | 0.49 |
| Diastolic blood pressure (mmHg) | 84.5 (12.1) | 83.6 (11.5) | 0.49 |
| Residual GFR (ml/min/1.73 m2) | 4.4 (3.4) | 4.3 (3.0) | 0.79 |
| Urine production (l/24 h) | 1.2 (0.8) | 1.0 (0.7) | 0.01 |
| Primary kidney disease | |||
| diabetes mellitus | 85 (15.2%) | 15 (17.4%) | 0.14 |
| glomerulonephritis | 110 (19.6%) | 13 (15.1%) | |
| renal vascular disease | 73 (13.0%) | 5 (5.8%) | |
| other | 293 (52.2%) | 53 (61.6%) | |
| SGA score | |||
| ≤5 | 89 (17.6%) | 11 (19.3%) | 0.66 |
| 6 | 182 (35.9%) | 17 (29.8%) | |
| 7 | 236 (46.5%) | 29 (50.9%) | |
| Davies score | |||
| no comorbidity | 324 (57.7%) | 61 (70.1%) | 0.09 |
| intermediate comorbidity | 199 (35.4%) | 22 (25.3%) | |
| high comorbidity | 39 (6.9%) | 4 (4.6%) | |
| Hemoglobin (g/dl) | 11.8 (1.6) | 11.8 (1.4) | 0.84 |
| Albumin (g/dl) | 3.6 (0.5) | 3.7 (0.5) | 0.11 |
Age, residual GFR (glomerular filtration rate), urine production, blood pressure, albumin and hemoglobin, are expressed as mean values (standard deviation). Gender, primary kidney disease and SGA (Subjective Global Assessment scale) are expressed as numbers (valid percentages). Davies scores are expressed as numbers (percentages). GFR, glomerular filtration rate; SGA score, Standard Global Assessment score, higher scores indicate better nutritional status (7, well nourished; 6, nourished; ≤5 malnourished).
Table 2.
Social characteristics at baseline
| Variable | CAPD (n = 562) | APD (n = 87) | P |
|---|---|---|---|
| Married/living with partner | 417 (77.4%) | 56 (71.8%) | 0.28 |
| Marital state | |||
| married | 417 (77.4%) | 56 (71.8%) | 0.54 |
| divorced | 42 (7.8%) | 7 (9.0%) | |
| widow | 25 (4.6%) | 3 (3.4%) | |
| never married | 55 (10.2%) | 12 (15.4%) | |
| Day job (not paid) | |||
| Retired | 154 (38.6%) | 25 (46.3%) | 0.09 |
| Student | 12 (3.0%) | 5 (9.3%) | |
| Unemployed | 23 (5.8%) | 2 (3.7 5) | |
| Medically incapable of work because of kidney disease | 97 (17.3%) | 13 (24.1%) | |
| Medically incapable of work because of other reason | 51 (12.8%) | 6 (11.1%) | |
| Household and / or children | 62 (15.5%) | 3 (5.6%) | |
| Day job (paid) | 176 (32.8%) | 29 (33.3%) | 0.41 |
| Education | |||
| low | 259 (48.1%) | 28 (36.4%) | 0.05 |
| high | 280 (51.9%) | 49 (63.6%) | |
| Children | 404 (74.7%) | 53 (68.8%) | 0.27 |
| Number of children | 1.9 (1.7) | 1.5 (1.2) | 0.05 |
| Sleep problem | 67 (13%) | 5 (7%) | 0.17 |
Hours of paid work and number of children are expressed as mean values (standard deviation). Married / living with partner, day job (not paid), day job (paid), education, children and sleep problems are expressed as numbers (valid percentages). Low education level defined as primary school, lower vocational training; high education level defined as lower general secondary education, pre-university education, high vocational training, or university.
Mortality Analyses
In the intention-to-treat analysis, 154 CAPD (27%) and 21 APD (24%) patients died. Myocardial ischemia and infarction were the most frequent causes of death in the group with a known cause. The causes of death are listed in Table 3.
Table 3.
Causes of death in the overall mortality analysis
| Cause | CAPD | APD |
|---|---|---|
| Myocardial ischemia and infarction | 26 (17%) | 7 (33%) |
| Heart failure | 13 (8%) | 0 (0%) |
| Cerebrovascular accident | 10 (6%) | 1 (5%) |
| Infection | 22 (14%) | 3 (14%) |
| Discontinuation treatment | 22 (14%) | 3 (14%) |
| Malignancies | 14 (9%) | 0 (0%) |
| Unknown / unavailable | 48 (31%) | 7 (33%) |
| Total | 154 | 21 |
Causes of death in the patients studied for overall mortality with an intention-to-treat design.
Ninety CAPD patients (16%) completed the follow-up of 5 yr compared with 12 APD patients (14%). Reasons for censoring in the CAPD group were transplantation for 204 patients (36%) and recovery of kidney function in three patients (1%). In the APD group, 29 patients (33%) were censored because of transplantation, and two (2%) because of recovery.
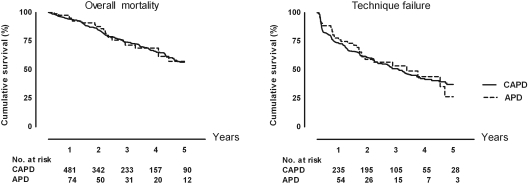
The incidence rate of death was 110 per 1000 person-years on CAPD, and 106 per 1000 person-years on APD. Figure 1 shows the Kaplan-Meier curves for overall mortality in CAPD and APD patients. The crude HR of APD compared with CAPD for overall mortality was 0.98 (95% CI: 0.62 to 1.54). After adjustment for the previously mentioned variables, the HR was 1.09 (95% CI: 0.65 to 1.83). Adjustment for social factors or potential dialysis center effects in addition to the other confounders did not change the HRs.
Figure 1.
Left: Kaplan-Meier curve of overall mortality on automated peritoneal dialysis compared with continuous ambulatory peritoneal dialysis. Right: Kaplan-Meier curve of pure technique failure on automated peritoneal dialysis compared with continuous ambulatory peritoneal dialysis. The numbers under the graphs show the number of patients at risk.
After stratification for age, the group with younger patients consisted of 370 CAPD and 57 APD patients. In the group aged > 60 yr, 57 patients started on CAPD and 30 on APD. The HRs were similar for both groups of age and did not change after adjustment. These results are summarized in Table 4.
Table 4.
Hazard ratios
| Group | Overall mortality | Technique failure |
|---|---|---|
| Complete group | ||
| crude | 0.98 (0.62 to 1.54) | 0.92 (0.64 to 1.31) |
| adjusted | 1.09 (0.65 to 1.83) | 0.91 (0.63 to 1.32) |
| Age ≤60 yr | ||
| crude | 1.08 (0.53 to 2.18) | 0.69 (0.43 to 1.10) |
| adjusted | 1.34 (0.60 to 2.98) | 0.69 (0.43 to 1.11) |
| Age >60 yr | ||
| crude | 1.04 (0.57 to 1.89) | 1.77 (0.99 to 3.14) |
| adjusted | 0.95 (0.46 to 1.97) | 1.63 (0.87 to 3.05) |
| Analysis 0 to 2 yr | ||
| crude | 0.80 (0.41 to 1.53) | 0.91 (0.61 to 1.35) |
| adjusted | 0.98 (0.44 to 2.17) | 0.93 (0.62 to 1.39) |
| Conditional analysis 2 to 5 yr | ||
| crude | 1.23 (0.65 to 2.32) | 0.96 (0.41 to 2.25) |
| adjusted | 1.22 (0.61 to 2.41) | 1.00 (0.38 to 2.65) |
Hazard ratios and the associated 95% confidence intervals for overall mortality and technique failure on automated peritoneal dialysis compared with continuous ambulatory peritoneal dialysis. Adjustment was performed for age, gender, primary kidney disease, comorbidity, residual glomerular filtration rate, urine production, and plasma albumin at baseline.
When we analyzed overall mortality with the as-treated design as described in Patients and Methods, the incidence rate was 106 per 1000 person-years on CAPD and 54 per 1000 person-years on APD. The crude HR was 0.51 (95% CI: 0.24 to 1.11); after adjustment the HR was 0.75 (95% CI: 0.30 to 1.89).
Technique Failure
In the as-treated analysis, 238 CAPD patients (42%) switched therapy, compared with 34 (39%) in the APD group. Of these, 103 CAPD (18%) and 22 APD (25%) patients switched to HD, whereas 135 CAPD (24%) patients switched to APD and 12 APD patients (14%) made the switch to CAPD. During follow-up, 91 CAPD (16%) patients died, compared with seven APD patients (8%). The follow-up was completed by 28 CAPD patients (5%) and 3 APD patients (3%). One hundred four CAPD patients (18%) and 21 APD patients (24%) were censored for transplantation. Three CAPD patients (1%) were censored because of recovery of renal function. No patients recovered in the APD group.
The incidence rate of technique failure was 279 per 1000 person-years on CAPD and 263 per 1000 person-years on APD. Figure 1 shows the Kaplan-Meier curves for technique failure in CAPD and APD patients. The crude HR of APD compared with CAPD for technique failure was 0.92 (95% CI: 0.64 to 1.31). After adjustment, the HR was 0.91 (95% CI: 0.63 to 1.32). Adjustment for social factors or potential dialysis center effects on top of the other confounders did not change the HRs.
Stratification for age provided a crude HR of 0.69 (95% CI: 0.43 to 1.10) for technique failure in patients up to 60 yr of age. After adjustment the HR did not change. The crude HR for technique failure in the elderly group was 1.77 (95% CI: 0.99 to 3.14). After adjustment the HR was 1.63 (95% CI: 0.87 to 3.05). The HRs are summarized in Table 4.
Stay on Original PD Modality
To assess whether the time on original PD was different between APD and CAPD, we combined overall mortality and technique survival in the as-treated design. The events were death or switch to another form of dialysis and death; drop-out for all other reasons was censored. For duration on original PD modality, the crude HR was 0.81 (95% CI: 0.58 to 1.12). The adjusted HR was 0.86 (95% CI: 0.61 to 1.22).
Conditional Analysis
In this analysis the first 2 yr were compared with the last 3 yr of follow-up. During the first 2 yr, 84 CAPD (15%) and 10 APD (11%) patients died in the intention-to-treat analysis. The crude HR for overall mortality was 0.80 (CI 95%: 0.41 to1.53) for the first 2 yr. After adjustment the HR was 0.98 (95% CI: 0.44 to 2.17). To calculate the HR for the long-term effect, 332 patients who initially started on CAPD and 44 patients who started on APD were included. Seventy CAPD patients and 11 APD patients died during the last 3 yr. The crude HR of mortality for this period was 1.23 (95% CI: 0.65 to 2.32); after adjustment, the HR was 1.22 (95% CI: 0.61 to 2.41).
In the as-treated analysis for technique failure, 193 (34%) CAPD patients and 28 (32%) APD patients switched therapy during the first 2 yr. The crude HR for technique failure was 0.91 (95% CI: 0.61 to 1.35) during this period, after adjustment the HR was 0.93 (95% CI: 0.62 to 1.39). In the conditional analysis for the long-term effect, 185 CAPD and 24 APD patients were included. Forty-five patients switched therapy in the CAPD group compared with 6 patients in the APD group during the last 3 yr. For this period the crude HR for technique failure was 0.96 (95% CI: 0.41 to 2.25), the adjusted HR was 1.00 (95% CI: 0.38 to 2.65).
Comparison at 1 Yr After Start of Dialysis
One year after inclusion in NECOSAD, 375 patients used CAPD as renal replacement therapy compared with 138 APD patients. In this additional analysis, the APD group contained more men than the CAPD group (74% versus 65%). Furthermore, the patients on APD group had a higher plasma albumin compared with the CAPD patients; median 38.0 (range, 24.0 to 49.0) versus 36.0 g/L (range, 24.0 to 49.0). The other physical parameters were not different.
During follow-up, 108 (29%) CAPD patients and 28 (20%) APD patients died. The crude HR for overall survival on APD compared with CAPD was 0.73 (95% CI: 0.48 to 1.10). The adjusted HR was 0.82 (95% CI: 0.51 to 1.32).
Discussion
This study showed no difference in overall mortality and technique failure for patients treated with APD compared with those treated with CAPD. Moreover, no short-term or long-term effect of PD modality on overall mortality or technique failure was found. Neither was there an effect of PD modality on overall mortality or technique failure in patients who were on one of these treatments 1 yr after the start of dialysis.
Stratification for age did not change the results significantly, although the point estimates for technique failure in the younger groups were lower than those in the older group. This indicates that younger patients remain on APD longer, whereas older patients remain on CAPD longer. Furthermore, we observed that the majority of patients who failed on APD switched to HD, whereas patients who failed on CAPD switched to APD. An explanation for the low point estimate in the younger group could be that these patient and their physicians were more motivated to keep them on APD to avoid HD. In older patients, APD can be less favorable because of a higher prevalence of comorbidities, which can make it difficult to handle the machine. Therefore, it can be hypothesized that these patients and their physicians try to keep CAPD as their dialysis modality.
Several potential sources of bias and confounding exist for this observational study. The comparison of two therapies in an observational design makes confounding by indication the most important obstacle. However, at baseline, no major differences in medical or social characteristics were present between the CAPD and APD patients. Only urine production was significantly different when tested, although we do not consider a mean difference of 200 ml/24 h clinically important. Because several small differences together might introduce a difference between the groups, we corrected for many possible confounders. Because no major differences were observed after this adjustment, confounding by indication does not seem to be a significant problem. However, residual confounding might still be present, as a result of remaining confounding variables related to the indication for APD or CAPD that were unaccounted for. Because incident patients were included on their original therapy, failure on CAPD could not have been an indication to start APD. Thus, the main indication for APD must have been the preference of the patient or her or his physician, which is difficult to measure, and thus to correct for.
A limitation of this study might be the relatively low number of APD patients (13%) in the major analyses. This was mostly due to the inclusion of only incident PD patients. We added an additional analysis 1 yr after the start of dialysis, to increase the number of APD patients. Despite this addition, no difference in survival between the two modalities was found. Another reason for the low number of APD patients was the inclusion period of NECOSAD, which started in 1997 and closed in 2006. In these years fewer patients started on APD than nowadays.
The results of our study are in line with those of the three small randomized controlled trials (10–12) and two observational studies on this subject (5,9). The main problem with the randomized trials is the size of the cohorts, with a maximum of 50 patients in the largest one (11). In the study from Rodriquez-Carmona et al. (5), more patients were included (53 CAPD and 51 APD patients), although this was still a limited number. This study showed similar results as ours, but the possibility of survival bias cannot be excluded in that study, since inclusion was restricted to PD patients who remained on the same modality for the first 2 yr (5). In contrast to our study, the main analysis in the large cohort of the ANZDATA registry compared patients with at least one episode on APD with patients treated with CAPD only (9). Therefore in this study an effect of previous CAPD therapy cannot be excluded. For this reason we defined our groups at start of dialysis, ruling out any influence of previous dialysis modalities. Furthermore, since APD patients tend to switch to HD, while CAPD patients tend to switch to APD, in our technique failure analysis a switch to any other form of dialysis (including the other PD modality) was considered an event. In the ANZDATA registry switches to another PD modality were not considered as technique failure. Despite the differences in design, our results are in line with those of the ANZDATA study.
Our results are in contrast with two large observational studies, which showed a survival benefit for APD (7,8). Both studies included incident PD patients; therefore, they could theoretically have been treated with HD before PD. The Mexican study had the longest follow-up (3 yr), but it was a retrospective single-center study in patients only treated with solutions and machines of one company (8). The registry study from the United States had a short follow-up of one year (7). In both studies adjustment for possible confounders was hampered by the quality of limited data at baseline.
Our results imply that APD should not be prescribed to improve survival of incident PD patients, neither the intention-to-treat design, nor the as-treated analyses showed that survival might be better on APD compared with CAPD. Stratification for age, the conditional analysis and the analysis after one year all showed no effect on overall mortality and technique failure. Although not investigated in the present study a switch from CAPD to APD may extend the time on PD.
In conclusion, we found no difference in overall mortality and technique failure for APD compared with CAPD in incident dialysis patients. Therefore, the choice to start either one of these therapies should be based on other factors than survival, such as quality of life, patients’ preference, or available resources. Thus, countries in which prescription of APD is limited because of limited resources do not provide an inferior therapy for their patients.
Disclosures
This work was supported by grants from Baxter Healthcare, the Dutch Kidney Foundation and the Dutch National Health Insurance Board. The funding sources were involved in neither the collection, interpretation, and analysis of the data, nor the decision for the writing and submission of this report for publication.
Acknowledgments
The nursing staff of the participating hospitals dialysis centers, the trial nurses, and the staff of the NECOSAD trial office are acknowledged for their assistance in the collection and management of data for this study.
The members of Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD) Study Group include A. J. Apperloo, J. A. Bijlsma, M. Boekhout, W. H. Boer, P. J. M. van der Boog, H. R. Büller, M. van Buren, F. Th. de Charro, C. J. Doorenbos, M. A. van den Dorpel, A. van Es, W. J. Fagel, G.W. Feith, C. W. H. de Fijter, L. A. M. Frenken, W. Grave, J. A. C. A. van Geelen, P. G. G. Gerlag, J. P. M. C. Gorgels, R. M. Huisman, K. J. Jager, K. Jie, W. A. H. Koning-Mulder, M. I. Koolen, T. K. Kremer Hovinga, A. T. J. Lavrijssen, A. J. Luik, J. van der Meulen, K. J. Parlevliet, M. H. M. Raasveld, F. M. van der Sande, M. J. M. Schonck, M. M. J. Schuurmans, C. E. H. Siegert, C. A. Stegeman, P. Stevens, J. G. P. Thijssen, R. M. Valentijn, G. H. Vastenburg, C. A. Verburgh, H. H. Vincent, and P. F. Vos.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Hiroshige K, Yuu K, Soejima M, Takasugi M, Kuroiwa A: Rapid decline of residual renal function in patients on automated peritoneal dialysis. Perit Dial Int 16: 307–315, 1996 [PubMed] [Google Scholar]
- 2.Hufnagel G, Michel C, Queffeulou G, Skhiri H, Damieri H, Mignon F: The influence of automated peritoneal dialysis on the decrease in residual renal function. Nephrol Dial Transplant 14: 1224–1228, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Carmona A, Fontan MP: Sodium removal in patients undergoing CAPD and automated peritoneal dialysis. Perit Dial Int 22: 705–713, 2002 [PubMed] [Google Scholar]
- 4.Ortega O, Gallar P, Carreno A, Gutierrez M, Rodriguez I, Oliet A, Vigil A, Gimenez E: Peritoneal sodium mass removal in continuous ambulatory peritoneal dialysis and automated peritoneal dialysis: Influence on blood pressure control. Am J Nephrol 21: 189–193, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Carmona A, Perez-Fontan M, Garca-Naveiro R, Villaverde P, Peteiro J: Compared time profiles of ultrafiltration, sodium removal, and renal function in incident CAPD and automated peritoneal dialysis patients. Am J Kidney Dis 44: 132–145, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Westra WM, Kopple JD, Krediet RT, Appell M, Mehrotra R: Dietary protein requirements and dialysate protein losses in chronic peritoneal dialysis patients. Perit Dial Int 27: 192–195, 2007 [PubMed] [Google Scholar]
- 7.Guo A, Mujais S: Patient and technique survival on peritoneal dialysis in the United States: Evaluation in large incident cohorts. Kidney Int 64: S3–12, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Sanchez AR, Madonia C, Rascon-Pacheco RA: Improved patient/technique survival and peritonitis rates in patients treated with automated peritoneal dialysis when compared to continuous ambulatory peritoneal dialysis in a Mexican PD center. Kidney Int 73: S76–S80, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Badve SV, Hawley CM, McDonald SP, Mudge DW, Rosman JB, Brown FG, Johnson DW: Automated and continuous ambulatory peritoneal dialysis have similar outcomes. Kidney Int 73: 480–488, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Bro S, Bjorner JB, Tofte-Jensen P, Klem S, Almtoft B, Danielsen H, Meincke M, Friedberg M, Feldt-Rasmussen B: A prospective, randomized multicenter study comparing APD and CAPD treatment. Perit Dial Int 19: 526–533, 1999 [PubMed] [Google Scholar]
- 11.de Fijter CW, Oe LP, Nauta JJ, van der MJ, Verbrugh HA, Verhoef J, Donker AJ: Clinical efficacy and morbidity associated with continuous cyclic compared with continuous ambulatory peritoneal dialysis. Ann Intern Med 120: 264–271, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Iles-Smith H, Curwell J, Gokal R: Comparative evaluation of CAPD and PD-plus effectiveness. EDTNA ERCA J 25: 27–29, 1999 [PubMed] [Google Scholar]
- 13.Rabindranath KS, Adams J, Ali TZ, Daly C, Vale L, Macleod AM: Automated vs continuous ambulatory peritoneal dialysis: A systematic review of randomized controlled trials. Nephrol Dial Transplant 22: 2991–2998, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Rabindranath KS, Adams J, Ali TZ, Macleod AM, Vale L, Cody J, Wallace SA, Daly C: Continuous ambulatory peritoneal dialysis versus automated peritoneal dialysis for end-stage renal disease. Cochrane Database Syst Rev CD006515, 2007 [DOI] [PMC free article] [PubMed]
- 15.Davies SJ, Russell L, Bryan J, Phillips L, Russell GI: Comorbidity, urea kinetics, and appetite in continuous ambulatory peritoneal dialysis patients: Their interrelationship and prediction of survival. Am J Kidney Dis 26: 353–361, 1995 [DOI] [PubMed] [Google Scholar]
- 16.van Dijk PC, Jager KJ, de CF, Collart F, Cornet R, Dekker FW, Gronhagen-Riska C, Kramar R, Leivestad T, Simpson K, Briggs JD: Renal replacement therapy in Europe: The results of a collaborative effort by the ERA-EDTA registry and six national or regional registries. Nephrol Dial Transplant 16: 1120–1129, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Visser R, Dekker FW, Boeschoten EW, Stevens P, Krediet RT: Reliability of the 7-point subjective global assessment scale in assessing nutritional status of dialysis patients. Adv Perit Dial 15: 222–225, 1999 [PubMed] [Google Scholar]
- 18.Dekker FW, de MR, van Dijk PC, Zoccali C, Jager KJ: Survival analysis: Time-dependent effects and time-varying risk factors. Kidney Int 74: 994–997, 2008 [DOI] [PubMed] [Google Scholar]