Abstract
Background and objectives: The effect of mycophenolate mofetil (MMF) on T cell function has not been evaluated in patients undergoing kidney transplantation. The aim of this study was to assess the effect of 1g of MMF on T cell function, that is, intralymphocyte cytokine expression, T cell activation (CD25 and CD71), and T cell proliferation, as well as inosine monophosphate dehydrogenase (IMPDH) activity, to better understand the relationship between pharmacokinetic and pharmacodynamic markers in patients receiving the first dose of MMF before kidney transplantation.
Patients: Twenty-four patients undergoing a kidney transplantation from a living donor were enrolled in this study.
Results: Compared with baseline (before MMF intake), T cell proliferation (93%), IMPDH activity (74%), CD25 (46%), and CD71 (50%) expression significantly decreased during the first hour after MMF intake, in parallel to the rise in MPA concentration. Thereafter, all pharmacodynamic markers, except IMPDH activity, returned back to baseline level. There was a complex inverse relationship between pharmacokinetic and pharmacodynamic markers. The inhibition of T cell proliferation was highly correlated to IMPDH activity, but also to T cell activation markers.
Conclusion: The administration of MMF to patients is associated not only with a dramatic decrease in both T cell proliferation and IMPDH activity, but also with in a decrease in CD25 and CD71 expression.
To improve efficacy and to avoid the toxicity of immunosuppressive drugs, therapeutic drug monitoring (TDM) has been recommended (1). Pharmacokinetic (PK) monitoring has been used to relate immunosuppressant dose to drug exposure in vivo. However, conventional TDM of blood immunosuppressant levels may not necessarily predict the pharmacologic effects on immune cells. Pharmacodynamic monitoring (PD) is another approach in TDM and directly reflects the drug's biologic effects (2). Drug-target enzymes have been investigated as biomarkers. Calcineurin activity has been studied in transplant patients receiving a calcineurin inhibitor (3) (4). Inosine monophosphate dehydrogenase (IMPDH) activity, the target of mycophenolic acid (MPA), has been used as a pharmacodynamic marker in patients receiving mycophenolate mofetil (MMF) (5). Because transplant patients usually receive a combination of immunosuppressive drugs, more general immunological approaches for pharmacodynamic monitoring have been developed that rely on the measurement of T cell proliferation, T cell-activation markers, and T cell function via cytokine expression (6–8).
Only a few data on the pharmacokinetic-pharmacodynamic relationship are available in MPA-treated patients (9–13). PD monitoring of MMF, by measuring IMPDH activity, has been previously performed in kidney transplant patients (9). The effect of MMF on T cell function has been assessed both in vitro (8) (10) and in vivo in a rat heart-transplant model (11–13). However, data regarding the effect of MMF on lymphocyte function in patients undergoing kidney transplantation are scarce. The primary objective of this study was to assess the effect of 1 g of MMF on T cell function, that is, intralymphocyte cytokine expression, T cell activation (i.e., transferrin receptor [CD71] and IL-2 α-chain [CD25] expression), and T cell proliferation using a flow-cytometry whole-blood assay, as well as measuring IMPDH activity, in patients undergoing kidney transplantation. The second objective was to explore the relationship between pharmacokinetic and pharmacodynamic markers.
Materials and Methods
Twenty-four patients undergoing kidney transplantation from a living donor were enrolled in this study after giving their informed consent. The study was approved by the local ethics committee. The preliminary results of the first five patients have been previously reported (14). There were 17 men and 7 women, ranging in age from 23 to 61 yr. Of these, five patients were undergoing preemptive kidney transplantation. For the remaining patients, their median duration of dialysis was 22 (2–204) months. None of the patients had received any immunosuppressive drugs within the 3 mo before inclusion in the study.
Study Design
Two days before kidney transplantation (day −2), blood samples were obtained at baseline (9 a.m.) and at 1, 2, and 4 h later (H0, H1, H2, and H4). The next day at 9 a.m. (day −1), each patient received 1 g of MMF orally. Blood samples were collected just before MMF intake and at 1, 2, and 4 h later. MPA levels were obtained 1, 2, and 4 h after MMF intake. All patients were fasting overnight; only water was allowed until 1 h postdose. Dialysis sessions were performed the day before the beginning of the study (day −3), and at day −1, at the end of the study.
Flow Cytometry Whole-Blood Assay
The pharmacodynamic effects of MMF on lymphocyte function were assessed by calculating the percentage expression of T cell proliferation, intracellular IL-2, and TNF-α expression, as well as CD71 and CD25 expression. A validated flow-cytometry whole-blood assay was performed as described previously (8). For the measurement of intracellular cytokine expression, 96 μl of undiluted whole blood was stimulated by phorbol myristate acetate; 15 ng/ml) and ionomycin (0.75 μg/ml) for 30 min. Cytokine secretion was blocked by adding brefeldin (1 μg/ml). After 4 h of incubation at 37 °C, intracellular cytokine expression was analyzed by a three-color FACS Calibur flow cytometer (Becton Dickinson) after the blood had been labeled with monoclonal antibodies: anti-CD3-PerCP (0.5 μg/ml), anti-IL-2 PE (2 μl, 100 test/ 2 ml), and anti-TNF-α FITC (0.5 mg/ml; Becton Dickinson).
For T cell activation, surface markers (CD25 and CD71), and T cell proliferation, diluted whole blood (1:10; 200 μl of whole blood) was stimulated with concavalin A (Sigma) for 3 d. Thereafter, antigen surface markers were stained using labeled anti-CD3-PerCP (12.5 μg/ml), CD25-FITC (2 μl, 100 test/2 ml), and CD71-PE (2 μl, 100 test/2 ml) monoclonal antibodies (Becton Dickinson), and were analyzed by flow cytometry. Lymphocyte proliferation was measured by bivariate-flow cytometric analysis using directly labeled FITC monoclonal antibodies against proliferating nuclear cell-antigen (PCNA, 2.5 μl, 100 test/2 ml) and propidium iodide (1 mg/ml) labeled for DNA content. For each blood sample, one stimulated and one unstimulated sample were analyzed. In addition, each time the assay was performed, one stimulated and one unstimulated sample from a healthy volunteer served as an external control. Three thousand CD3 positive cells of each sample were analyzed with a flow cytometer.
IMPDH Activity Assay
IMPDH activity was measured as described previously (5). Mononuclear cells (MNC) were isolated from the peripheral blood by Ficoll density centrifugation. The MNCs were lysed by the addition of water and stored at −80 °C. After thawing and centrifugation, the supernatant was used for incubation, and protein determination. IMPDH activity was measured using a procedure based on chromatographic determination of produced xanthosine 5′-monophosphate during in vitro incubation of MNC lysates with inosine monophosphate and nicotinamide adenine dinucleotide. IMPDH activity has been expressed as nmol/mg prot/h.
Measurement of MPA Levels
Plasma MPA concentrations were measured using a commercially assay kit (EMIT Mycophenolic Acid Assay System, Dade Behring). The lower limit of quantification for the determination of MPA was 0.1 μg/ml. The area under the plasma concentration-time curve (AUC 0 to 4h) was calculated by the trapezoidal rule using MPA plasma-concentration data taken at 0, 1, 2, and 4 h postdosing.
Calculation of MPA Pharmacodynamics
The area under the pharmacodynamic-effect time curve (AUE 0 to 4h) was calculated using the trapezoidal rule:
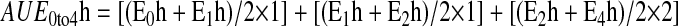 |
The relationship between plasma MPA concentrations and their effecton exvivo IMPDH activity as well on stimulated lymphocyte response were explored with sigmoid inhibitory effect model (Win Nonl inversion 5.2, Pharsight Inc., Mountain View, CA). For this analysis, all concentration and effect data from all patients from day−1 were pooled. MPA concentration associated with half-maximal inhibitory effect (IC50) on particular pharmacodynamic parameter was calculated from the formula E = (E0 + ((Emax-E0)*C)/(EC50+C), where E = effect, E0 =, effect at zero drug concentration, and Emax = effect at infinitely drug concentration. Relationship between pharmacodynamic parameters were explored with curve estimation routine in SPSS (version 15.1, SPSS Inc, Chicago, IL) comparing nonlinear regression models based on their goodness of fit. Best fits were determined graphically.
Statistical Analyses
Data are expressed as the median with interquartile ranges or as the mean ± standard error (SEM). Because of the skewed distribution, variables were compared by nonparametric Friedmann and Wilcoxon tests. A p-value <0.05 was considered to be statistically significant.
Results
MPA Plasma Concentration After MMF Intake (Day −1)
Demographic data of the 24 patients are presented in Table 1. After 1 g of MMF, plasma MPA concentration increased to 4.9 ± 3 ng/ml at 1 h, and decreased thereafter to 2.65 ± 1.7 and 1.4 ± 1.1 ng/ml at 2 and 4 h, respectively (P < 0.0001). In all patients, but two, the maximal concentration (Cmax) was observed at 1 h. Cmax was 5.21 ± 3 ng/ml. AUC 0 to 4h was 10.4 ± 1.04 mg/L * h.
Table 1.
Patients’ characteristics at enrollment (n = 24)
| Characteristic | |
|---|---|
| Age (years) | 42.6 ± 10.5 |
| Gender | |
| male | 17 |
| female | 7 |
| Time on dialysis (months)a | 22 (2-204) |
| First transplantation | 24 |
| Type of dialysis | |
| hemodialysis | 17 |
| peritoneal dialysis | 2 |
| pre-emptive | 5 |
| Weight (kg) | 73 ± 14 |
| Body mass index (kg/m2) | 24 ± 3.9 |
| Initial nephropathy | |
| chronic glomerulonephritis | 4 |
| Ig A nephropathy | 4 |
| nephroangiosclerosis | 4 |
| membranoproliferative glomeronephritis | 3 |
| focal segmental glomerulosclerosis | 2 |
| polycystic kidney disease | 2 |
| Alport disease | 2 |
| Fabry disease | 1 |
| chronic hydronephrosis | 1 |
| systemic lupus erythematus | 1 |
| Hemoglobin level (g/dl) | 12.9 ± 1.4 |
| White blood-cell count (/mm3) | 6386 ± 2345 |
| Platelet count (/mm3) | 215,317 ± 101,219 |
Excluding patients who underwent a preemptive transplantation.
Pharmacodynamic Parameter Without MMF (Day −2)
At day −2, there was no significant change in IL-2, TNF-α, and IMPDH activity (p = ns). Conversely, there was a small increase for CD25 and for CD71 expression over the 4-h period. In addition T cell proliferation showed some minor diurnal fluctuations (Table 2).
Table 2.
Time course of pharmacodynamic parameters on day −2 and day −1
| Day −2
|
Day −1
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Median | 25th Percentile | 75th Percentile | P | Median | 25th Percentile | 75th Percentile | P | |
| IMPDH_0 h | 14.3 | 12.0 | 17.9 | 14.1 | 12.3 | 17.4 | ||
| IMPDH_1 h | 15.7 | 12.4 | 17.5 | 4.0 | 2.5 | 6.4 | ||
| IMPDH_2 h | 16.0 | 13.1 | 18.2 | 5.3 | 3.8 | 8.4 | ||
| IMPDH_4 h | 16.1 | 12.9 | 18.1 | n.s. | 8.7 | 5.4 | 13.5 | 0.0001 |
| IL-2_0 h | 35.3 | 29.6 | 48.1 | 32.9 | 24.4 | 49.3 | ||
| IL-2_1 h | 35.2 | 28.4 | 53.2 | 37.4 | 26.6 | 45.0 | ||
| IL-2_2 h | 37.9 | 28.1 | 55.5 | 36.9 | 28.6 | 43.6 | ||
| IL-2_4 h | 43.4 | 28.5 | 54.5 | n.s. | 39.2 | 28.4 | 48.1 | n.s. |
| TNF-alpha_0 h | 25.8 | 20.1 | 38.5 | 21.3 | 13.5 | 39.3 | ||
| TNF-alpha_1 h | 29.1 | 19.1 | 34.5 | 27.8 | 19.1 | 39.6 | ||
| TNF-alpha_2 h | 28.8 | 23.5 | 36.9 | 27.2 | 21.2 | 42.0 | ||
| TNF-alpha_4 h | 29.7 | 23.6 | 36.2 | n.s. | 27.9 | 18.4 | 35.6 | 0.001 |
| CD25_0 h | 39.8 | 31.0 | 59.0 | 33.9 | 26.3 | 49.3 | ||
| CD25_1 h | 45.4 | 33.3 | 60.7 | 22.8 | 18.3 | 31.6 | ||
| CD25_2 h | 40.1 | 32.7 | 60.1 | 28.2 | 19.3 | 35.8 | ||
| CD25_4 h | 47.0 | 36.2 | 65.1 | 0.05 | 34.6 | 24.2 | 46.6 | 0.0001 |
| CD71_0 h | 26.5 | 18.7 | 33.0 | 18.7 | 14.4 | 32.5 | ||
| CD71_1 h | 30.9 | 21.4 | 36.0 | 13.2 | 10.0 | 14.1 | ||
| CD71_2 h | 28.3 | 20.2 | 37.6 | 14.0 | 11.3 | 19.4 | ||
| CD71_4 h | 37.4 | 17.9 | 43.2 | 0.05 | 19.0 | 12.6 | 28.8 | 0.0001 |
| Proliferation_0 h | 12.9 | 7.6 | 18.3 | 12.3 | 7.0 | 18.4 | ||
| Proliferation_1 h | 12.8 | 9.1 | 18.2 | 0.8 | 0.5 | 1.4 | ||
| Proliferation_2 h | 14.6 | 9.4 | 20.4 | 1.0 | 0.5 | 3.4 | ||
| Proliferation_4 h | 16.2 | 7.9 | 23.8 | 0.05 | 9.3 | 4.0 | 13.5 | 0.0001 |
IMPDH, inosine monophosphate dehydrognase.
Pharmacodynamic Parameters After MMF Intake (day −1)
Baseline values in the morning of day −1 were comparable to those values obtained the day before (Table 2). To account for the minor diurnal variation of T cell proliferation, CD25 and CD71, the values for relative inhibition on day −1, were calculated in relationship to the corresponding time point on day −2 for each patient.
During the first hour following MMF intake, T cell proliferation and IMPDH activity decreased dramatically, by 93.3% (P < 0.0001) and 73.9% (P < 0.0001), respectively. CD25 and CD71 expression compared with day −1 also significantly decreased, by 46% (P = 0.0002) and 50% (P = 0.0002), respectively. At 4 h, CD25 and CD71 levels, as well as T cell proliferation returned to almost baseline values, whereas IMPDH activity (61.9%) remained below baseline (P = 0.01; Figure 1). Intralymphocyte IL-2 expression and intralymphocyte TNF-alpha were not significantly inhibited after MMF intake.
Figure 1.
Evolution of CD25 and CD71 expressions, inosine monophosphate dehydrogenase activity, T cell proliferation, and mycophenolic acid concentrations with and without 1 g of mycophenolate mofetil intake.
Association Between Pharmacokinetic and Pharmacodynamic Markers
The relationship between MPA and CD25, CD71, proliferation as well as IMPDH activity in 24 uremic patients is shown in Figure 2. T cell proliferation and IMPDH activity were already inhibited at low MPA concentrations, with IC50 values of 1.55 and 1.60 mg/L, respectively. In contrast, only high MPA concentrations exhibited an inhibitory effect on CD25 and CD71 expression in the WinNonlin model, as evidenced by calculated high IC50 values. We could not detect any significant correlation between MPA AUC 0 to 4h and AUE 0 to 4h of any pharmacodynamic biomarker (data not shown).
Figure 2.
Mycophenolic acid concentration-dependent inhibition of CD25, CD71, T cell proliferation, and inosine monophosphate dehydrogenase activity.
Association Between Pharmacodynamic Markers
Figure 3 depicts the relationship between IMPDH activity and T cell proliferation in patients receiving 1g MMF. As expected, in general high IMPDH activity level were associated with higher proliferative activity, however this relationship was not very strong because a high variability of stimulation dependent proliferation is observed with high IMPDH activity. Interestingly, low IMPDH values, mostly seen during a time period of profound inhibition of the enzyme, were clearly associated with a very low proliferative response in the majority of samples. Because of the timely dissociation of IMPDH activity, which remained suppressed for at least 4 h, and cell proliferation, which already returned to baseline after 4 h, we separately analyzed the pharmacodynamic relationship between IMPDH activity and cell proliferation in the first 2 h after drug intake. Figure 3b clearly demonstrates that during the first 2 h after MMF intake low IMPDH activity always was associated with a diminished proliferative response, suggesting that lymphocyte proliferation depends on a certain threshold of IMPDH activity. An IMPDH value of 8.65nmol/h was estimated to result in 50% inhibition of cell proliferation using activity levels instead of concentration data in the sigmoid Emax PK-PD-model. We found only weak or no associations with a coefficient of determination <0.1 between IMPDH activity and the other PD parameter (CD71: r = 0.30, CD25: r = 0.23; IL-2: r = 0.01; TNF-α: r = −0.08).
Figure 3.
Inosine monophosphate dehydrogenase activity-dependent inhibition of T cell proliferation during the 4 h (A) and 2 h (B) after 1 g mycophenolate mofetil intake.
In addition, we analyzed cellular activation markers in relationship to cell proliferation. High expression of CD71 and CD25 was associated with high proliferative activity (Fig. 4), whereas intracellular IL-2 and TNF-α expression were not associated with cell proliferation (not shown).
Figure 4.
Correlation between T cell proliferation and both CD25 and CD71 expression.
Discussion
This is an extensive evaluation on the pharmacodynamic effects of MPA using two different technologies (whole blood, FACS technology versus isolated cells target enzymes) after the first dose of MMF in a treatment-naïve population just before kidney transplantation. Our results suggest that despite rather low MPA levels in these uremic population after the first dose of MMF, (1) MPA not only inhibits IMPDH activity and T cell proliferation, but also inhibits transiently T cell activation, mainly CD25; (2) there was MPA concentration-dependent inhibition of pharmacodynamic markers; (3) the inhibition of T cell proliferation depends on the inhibition of IMPDH activity in patients receiving MMF; and (4) T cell activation and T cell proliferation were associated in these patients. Finally, similar to a previous report by Queneumer et al., who found in vitro that T cell proliferation was inhibited by the presence of MPA without a decrease of IL-2 level (15), we also did not observe a decrease in intralymphocyte IL-2 expression. The fact that MPA does not influence intracellular IL-2 production, provides some evidence that MPA has unspecific effects on all T cell activation processes
Previous experiments had shown in vitro (8,10) and in vivo in nonhuman animal models that MMF (12,13) as well as its active metabolite (10), that is, MPA, has a high potency to inhibit T cell proliferation and lymphocyte surface-antigen expression. MPA dose-dependent inhibition of CD25 has been demonstrated (10–12). In a rat heart-transplant model, both MPA (11) and MMF (12,13) therapies suppressed T cell proliferation and T cell-activation markers (CD25 and CD134). These effects correlated with MPA levels and the severity of histologic scores of rejection (11–13). In kidney-transplant patients, Sankatssing et al. reported that MMF inhibits T cell proliferation without lowering intracellular dGTP (16). The present study, performed in uremic patients, further confirms and extends the observation that MPA not only inhibits IMPDH activity, but also influences T cell proliferation and activation. In the absence of any immunosuppressive drug, no significant difference in T cell activation and T cell proliferation was observed between uremic dialysis patients and healthy volunteers (17). In the present study, 1 h after MMF intake in uremic patients, T cell proliferation and IMPDH activity decreased dramatically, by 93.3% and 73.9%, respectively. At this time point, CD25 and CD71 expression also significantly decreased, by 46% and 50%, respectively. According to a previous report by Gummert et al., the maximum efficacy of MPA (10,11) was lower for T cell-activation markers compared with T cell proliferation. In the present study, we similarly observed differences between the effective MPA concentrations. The IC50 value for both cell proliferation and IMPDH activity was much lower compared with those values for CD25 and CD71, suggesting that high MPA concentrations may elicit additional inhibitory effects on T cell activation. The rapid decrease of CD25 and CD71 expression within 1 h suggests that this might be an as-yet-unknown direct effect of MPA, however we can not exclude that the observed effects on T cell activation are also a downstream consequence of IMPDH-inhibition and subsequent nucleotide depletion. It was shown in vitro that reduced levels of purine are responsible for a decrease in the concentration of sugars on the cell surface (18). Therefore, because CD25 is glycosylated, MPA may inhibit glycosylation in vivo (19). In addition, low levels of purine may limit the synthesis of CD25-mRNA, thus inhibiting its expression (20). Finally, MPA was shown to suppress guanine nucleotide proteins (G-proteins), which are required for the activation of some T cell surface antigens (21). Similarly, it has been previously demonstrated that brequinar, an inhibitor of de novo pyrimidine synthesis, suppresses not only T cell proliferation, but also IL-2 receptor expression (22). The inhibition of mRNA synthesis because of the decrease in ATP, induced by either MMF or brequinar, may explain the suppression of CD25 expression (23,24). The fact that we only observed a rather weak inhibition of CD25 expression only at high MPA concentrations provides some circumstantial evidence for the utility of an additional anti-CD25 monoclonal antibody to sufficiently suppress acute rejection
In dialysis patients, at 1 h after the first dose of 1 g of MMF, a maximal inhibition of IMPDH activity ranging from 76% to 100% was observed (25). Similarly, in stable renal allograft recipients, maximal inhibition of IMPDH activity (87% ± 8%) was observed at 1 h after MMF intake (26). In both populations, IMPDH activity inhibition persisted for 4 h, returning to baseline after 11 h and before the next dose (25–27). Langman et al. also found that significant inhibition of IMPDH activity persisted for at least 8 h despite low MPA levels (28). More recently, Vethe et al. demonstrated that in kidney allograft recipients, the maximal inhibition of IMPDH activity occurs 1 h after MMF intake (29). In this latter study, IMPDH activity had returned to predose values approximately 6 h postdose. Here in this MPA-naïve pretransplant population, we again confirm previous observations of an inhibitory Emax model relationship between MPA and IMPDH activity. MPA concentrations above the IC50 value of 1.6 mg/L caused a > 50% enzyme inhibition; further increasing MPA concentrations had only minor additional effects on the degree of inhibition.
Similar to the rat heat-transplant model (11–13), we observed a correlation between MPA concentrations and T cell proliferation and T cell activation. Klupp et al. found that, in rat heart transplant recipients, histologic graft-rejection scores correlated with T cell proliferation and CD71 expression (r2 = 0.85 and 0.81, respectively) more highly than with MPA plasma levels (r2 = 0.45; 13). Variations in correlation between PD effects and PK concentration seem to confirm that immunosuppressive drugs produce a variety of responses in patients even when drug level and the drug dose are similar.
In the present study, there was a good correlation between the inhibition of IMPDH activity and T cell proliferation. Under profound inhibition of IMPDH activity, we observed a strong inhibition of cellular proliferation as well. Our data are highly suggestive that a threshold IMPDH activity is required for cellular proliferation, especially in the first hours after MPA intake. With less inhibition of IMPDH enzyme activity over time, it seems conceivable that nucleotide synthesis was partially restored. Although an escape phenomenon and methodological variability could explain some of the results, we observed only minor cellular proliferation under low IMPDH activity in all patients tested during the first hours following MMF intake. Therefore, our study provides some new insights in the relationship between IMPDH activity and cell proliferation in kidney transplant recipients. Herein, we did not assess T cell proliferation after a prolonged exposure to MMF. In addition, the pronounced anti-proliferative effect observed after MMF monotherapy may be different in case of concomitant immunosuppressive drug intake. Further studies are required to better understand the immunosuppressive effect of MMF.
In conclusion, our data show that the administration of 1 g of MMF to uremic patients is associated not only with a dramatic decrease in both T cell proliferation and IMPDH activity, but also with T cell activation. Maximal inhibition of IMPDH and proliferation occur at drug concentrations, which are significantly lower than those targeted in clinical practice. The inhibition of IMPDH activity under a certain threshold seems to be required for the inhibition of T cell proliferation.
Disclosures
None.
Acknowledgments
We thank the Francophone Society of Transplantation for its financial support (N.K.). T.B. and J.N. received grants from Novartis and Hoffmann-LaRoche, respectively. The study was supported by a research grant from Roche, Germany. Preliminary data have been presented in 2003 as a poster at the 38th Renal Week of the American Society of Nephrology.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Klupp J, Holt DW, van Gelder T: How pharmacokinetic and pharmacodynamic drug monitoring can improve outcome in solid organ transplant recipients. Transpl Immunol 9: 211–214, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Dambrin C, Klupp J, Morris RE: Pharmacodynamics of immunosuppressive drugs. Curr Opin Immunol 12: 557–562, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Halloran PF, Helms LM, Kung L, Noujaim J: The temporal profile of calcineurin inhibition by cyclosporine in vivo. Transplantation 68: 1356–1361, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Grinyo JM, Cruzado JM, Millan O, Caldes A, Sabate I, Gil-Vernet S, Seron D, Brunet M, Campistol JM, Torras J, Martorell J: Low-dose cyclosporine with mycophenolate mofetil induces similar calcineurin activity and cytokine inhibition as does standard-dose cyclosporine in stable renal allografts. Transplantation 78: 1400–1403, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Glander P, Braun KP, Hambach P, Bauer S, Mai I, Roots I, Waiser J, Fritsche L, Neumayer HH, Budde K: Non-radioactive determination of inosine 5′-monophosphate dehydrogenase (IMPDH) in peripheral mononuclear cells. Clin Biochem 34: 543–549, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Barten MJ, Gummert JF, van Gelder T, Shorthouse R, Morris RE: Flow cytometric quantitation of calcium-dependent and -independent mitogen-stimulation of T cell functions in whole blood: Inhibition by immunosuppressive drugs in vitro. J Immunol Methods 253: 95–112, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Barten MJ, van Gelder T, Gummert JF, Shorthouse R, Morris RE: Novel assays of multiple lymphocyte functions in whole blood measure: New mechanisms of action of mycophenolate mofetil in vivo. Transpl Immunol 10: 1–14, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Bohler T, Nolting J, Kamar N, Gurragchaa P, Reisener K, Glander P, Neumayer HH, Budde K, Klupp J: Validation of immunological biomarkers for the pharmacodynamic monitoring of immunosuppressive drugs in humans. Ther Drug Monit 29: 77–86, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Glander P, Hambach P, Braun KP, Fritsche L, Giessing M, Mai I, Einecke G, Waiser J, Neumayer HH, Budde K: Pre-transplant inosine monophosphate dehydrogenase activity is associated with clinical outcome after renal transplantation. Am J Transplant 4: 2045–2051, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Gummert JF, Barten MJ, Sherwood SW, van Gelder T, Morris RE: Pharmacodynamics of immunosuppression by mycophenolic acid: Inhibition of both lymphocyte proliferation and activation correlates with pharmacokinetics. J Pharmacol Exp Ther 291: 1100–1112, 1999 [PubMed] [Google Scholar]
- 11.Gummert JF, Barten MJ, van Gelder T, Billingham ME, Morris RE: Pharmacodynamics of mycophenolic acid in heart allograft recipients: Correlation of lymphocyte proliferation and activation with pharmacokinetics and graft histology. Transplantation 70: 1038–1049, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Barten MJ, van Gelder T, Gummert JF, Boeke K, Shorthouse R, Billingham ME, Morris RE: Pharmacodynamics of mycophenolate mofetil after heart transplantation: New mechanisms of action and correlations with histologic severity of graft rejection. Am J Transplant 2: 719–732, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Klupp J, van Gelder T, Dambrin C, Regieli JJ, Boeke K, Billingham ME, Morris RE: Sustained suppression of peripheral blood immune functions by treatment with mycophenolate mofetil correlates with reduced severity of cardiac allograft rejection. J Heart Lung Transplant 23: 334–351, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Kamar N, Glander P, Nolting J, Bohler T, Hambach P, Liefeldt L, Neumayer HH, Klupp J, Budde K: Effect of mycophenolate mofetil monotherapy on T-cell functions and inosine monophosphate dehydrogenase activity in patients undergoing a kidney transplantation. Transplant Proc 38: 2292–2294, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Quemeneur L, Flacher M, Gerland LM, Ffrench M, Revillard JP, Bonnefoy-Berard N: Mycophenolic acid inhibits IL-2-dependent T cell proliferation, but not IL-2-dependent survival and sensitization to apoptosis. J Immunol 169: 2747–2755, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Sankatsing SU, Prins JM, Yong SL, Roelofsen J, van Kuilenburg AB, Kewn S, Back DJ, Bemelman FJ, ten Berge IJ: Mycophenolate mofetil inhibits T-cell proliferation in kidney transplant recipients without lowering intracellular dGTP and GTP. Transpl Int 21: 1066–1071, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Bohler T, Canivet C, Le Nguyen PN, Galvani S, Thomsen, Durand D, Salvayre R, Nègre-Salvayre A, Rostaing L, Kamar, N: Cytokines correlate with age in healthy volunteers, dialysis patients and kidney -transplant patients. Cytokines 45: 169–173, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Hayes GR, Williams A, Costello CE, Enns CA, Lucas JJ: The critical glycosylation site of human transferrin receptor contains a high-mannose oligosaccharide. Glycobiology 5: 227–232, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Laurent AF, Dumont S, Poindron P, Muller CD: Mycophenolic acid suppresses protein N-linked glycosylation in human monocytes and their adhesion to endothelial cells and to some substrates. Exp Hematol 24: 59–67, 1996 [PubMed] [Google Scholar]
- 20.Catapano CV, Dayton JS, Mitchell BS, Fernandes DJ: GTP depletion induced by IMP dehydrogenase inhibitors blocks RNA-primed DNA synthesis. Mol Pharmacol 47: 948–955, 1995 [PubMed] [Google Scholar]
- 21.Mustelin T: GTP dependence of the transduction of mitogenic signals through the T3 complex in T lymphocytes indicates the involvement of a G-protein. FEBS Lett 213: 199–203, 1987 [DOI] [PubMed] [Google Scholar]
- 22.Forrest TL, Ware RE, Howard T, Jaffee BD, Denning SM: Novel mechanisms of brequinar sodium immunosuppression on T cell activation. Transplantation 58: 920–926, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Fairbanks LD, Bofill M, Ruckemann K, Simmonds HA: Importance of ribonucleotide availability to proliferating T-lymphocytes from healthy humans. Disproportionate expansion of pyrimidine pools and contrasting effects of de novo synthesis inhibitors. J Biol Chem 270: 29682–29689, 1995 [PubMed] [Google Scholar]
- 24.Qiu Y, Fairbanks LD, Ruckermann K, Hawrlowicz CM, Richards DF, Kirschbaum B, Simmonds HA: Mycophenolic acid-induced GTP depletion also affects ATP and pyrimidine synthesis in mitogen-stimulated primary human T-lymphocytes. Transplantation 69: 890–897, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Glander P, Hambach P, Braun KP, Fritsche L, Waiser J, Mai I, Neumayer HH, Budde K: Effect of mycophenolate mofetil on IMP dehydrogenase after the first dose and after long-term treatment in renal transplant recipients. Int J Clin Pharmacol Ther 41: 470–476, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Budde K, Braun KP, Glander P, Bohler T, Hambach P, Fritsche L, Waiser J, Mai I, Neumayer HH: Pharmacodynamic monitoring of mycophenolate mofetil in stable renal allograft recipients. Transplant Proc 34: 1748–1750, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Budde K, Glander P, Braun KP, Bohler T, Waiser J, Fritsche L, Mai I, Neumayer H: Pharmacodynamic monitoring of mycophenolate mofetil in renal allograft recipients. Transplant Proc 33: 3313–3315, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Langman LJ, LeGatt DF, Halloran PF, Yatscoff RW: Pharmacodynamic assessment of mycophenolic acid-induced immunosuppression in renal transplant recipients. Transplantation 62: 666–672, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Vethe NT, Mandla R, Line PD, Midtvedt K, Hartmann A, Bergan S: Inosine monophosphate dehydrogenase activity in renal allograft recipients during mycophenolate treatment. Scand J Clin Lab Invest 66: 31–44, 2006 [DOI] [PubMed] [Google Scholar]






