Abstract
Background and objectives: Hemodialysis (HD)-induced myocardial stunning driven by ischemia is a recognized complication of HD, which can be ameliorated by HD techniques that improve hemodynamics. In nondialysis patients, repeated ischemia leads to chronic reduction in left ventricular (LV) function. HD may initiate and drive the same process. In this study, we examined the prevalence and associations of HD-induced repetitive myocardial injury and long-term effects on LV function and patient outcomes.
Design, setting, participants, & measurements: Seventy prevalent HD patients were assessed for evidence of subclinical myocardial injury at baseline using serial echocardiography and followed up after 12 mo. Intradialytic blood pressure, hematologic and biochemical samples, and patient demographics were also collected at both time points.
Results: Sixty-four percent of patients had significant myocardial stunning during HD. Age, ultrafiltration volumes, intradialytic hypotension, and cardiac troponin-T (cTnT) levels were independent determinants associated with its presence. Myocardial stunning was associated with increased relative mortality at 12 mo (P = 0.019). Cox regression analysis showed increased hazard of death in patients with myocardial stunning and elevated cTnT than in patients with elevated cTnT alone (P < 0.02). Patients with myocardial stunning who survived 12 mo had significantly lower LV ejection fractions at rest and on HD (P < 0.001).
Conclusions: HD-induced myocardial stunning is common, and may contribute to the development of heart failure and increased mortality in HD patients. Enhanced understanding of dialysis-induced cardiac injury may provide novel therapeutic targets to reduce currently excessive rates of cardiovascular morbidity and mortality.
Dialysis patients display hugely elevated rates of cardiac mortality (1), and this rate of cardiovascular attrition is not driven by the same risk factors, or pathophysiological processes, that are important in the general population (2). Classical complicated atherosclerotic disease appears not to be the predominant mode of death in hemodialysis (HD) patients. US registry data have identified HD as an independent risk factor for the development of de novo and recurrent heart failure with a 2-yr mortality after diagnosis of congestive heart failure as high as 51% (3).
Hemodialysis-induced myocardial stunning, as well as the potential (at least in the short term) to abrogate dialysis-induced cardiac injury with dialysis techniques that improve hemodynamic tolerability, is well described (4,5). A recent study using H215O positron emission tomography (PET) to measure myocardial blood flow (MBF) during HD in patients without angiographically significant coronary disease confirmed that HD-induced segmental left ventricular (LV) dysfunction correlates with matched reduction in MBF (6), both globally and co-localizing with segments exhibiting significant HD-induced reduction in contractile function. The details of the mechanisms involved in HD-induced myocardial stunning are currently largely unresolved. However, during HD, patients are particularly susceptible to myocardial ischemia for a number of reasons, including: high prevalence of coronary artery atheroma (7), LV hypertrophy (8), intradialytic hypotension and instability (9), and reduced coronary flow reserve (CFR) even in the absence of coronary vessel stenoses (10,11).
In the nondialysis population, repeated episodes of demand ischemia and stunning can result in chronic reduction in LV function (12,13). In HD patients, recurrent episodes of ischemia precipitated by HD may have negative consequences that lead to further myocardial injury, eventual nonviable myocardium, and irreversible reduction in LV function.
The aims of this study were to ascertain the prevalence of myocardial stunning in the HD population and understand the longer term consequences of this phenomenon on LV function, intradialytic hemodynamics, and survival. In addition, this study provided an opportunity to define the risk factors and their interactions in relation to the development of acute cardiac injury.
Materials and Methods
Subjects.
Seventy prevalent standard HD patients were recruited for a 12-mo observational cohort study from a single hospital-based hemodialysis unit of around 200 patients. Patients were excluded if they had pre-existing severe LV systolic dysfunction (NYHA IV) or inadequate echocardiographical windows (one patient excluded). All studies were conducted after the first 2-d interdialytic period, as arrhythmias and cardiac events are known to be increased after the 3-d interdialytic break.
Initial echocardiographic assessment.
Baseline assessments evaluated the presence and extent of HD-induced regional wall motion abnormalities (RWMAs). Apical two-dimensional echocardiography was performed predialysis, during HD at both 2 and 4 hrs and 30 min into the recovery period (1.5 to 3.6 MHz 3S probe, GE medical systems, Germany). Images were digitally recorded for subsequent analysis (Echo-CMS; MEDIS, The Netherlands), as described previously (14). This software has been validated using apical images and for assessment of global LV function (15,16). The technique of automated border detection has been validated for the quantitative analysis of RWMAs (17). Briefly, three consecutive heartbeats were analyzed for each time point, and endocardial borders were traced semi-automatically for each frame of the 3-beat sequence. Any anomalies were corrected for manually. Maximal displacement of the endocardial border from a center point was measured over 100 chords around the LV wall, corrected for end-diastolic LV circumference, and expressed as percentage of shortening fraction (%SF).
Each view was divided into five segments, and %SF for the chords in each segment was averaged. Ejection fraction (EF) was calculated using the biplane disc method.
In keeping with current definitions, RWMAs were classified as segments that showed a decline in %SF >20% from baseline. Regions with evidence of persistent dysfunction in the postdialysis period were classed as stunned segments (4–6,18).
Measurement of blood pressure (BP).
BP was measured predialysis and serially every 15 min using an automated digital oscillometric device (Model UA-767, A&D Instruments, Japan). Hemodynamic instability was defined by the magnitude of reduction in systolic BP (SBP) at each time point compared with the predialysis reading.
Follow-up assessment.
An identical study session was performed 12 mo later. Demographics including hospital admissions were updated. Changes in global EF at rest and during HD were re-assessed using the biplane disc method. The percentage of shortening fraction for all myocardial regions was also recalculated for pre- and intra-HD and compared with initial values. Intradialytic BPs were recorded and biochemical markers re-assayed.
Primary objectives.
These were: (1) to assess the prevalence of HD-induced RWMAs in a standard HD population and; (2) whether HD-induced myocardial stunning would lead to a reduction in left ventricular ejection fraction (LVEF) over 12-mo.
Secondary objectives.
These included: (1) identification of variables associated with the presence of LV RWMAs; and (2) the effect of myocardial stunning on survival in HD patients.
Power calculation & statistical analysis.
To detect changes in LVEF using echocardiography between baseline and 12 mo of 3.6% (19) with a SD of 8.987 (20), the required sample size was 68 patients. This achieved a power of 90% at a significance level of 5%.
Results are presented as the mean value ± SD or the median and interquartile range (IQR) unless otherwise stated. BP data were analyzed using two-way ANOVA with Bonferroni's post test for multiple comparisons. Categorical variables between the two groups were analyzed using Fisher's exact test. Depending on Gaussian distribution, all other data were analyzed using either the paired or unpaired t test or the Mann-Whitney or Wilcoxon matched pairs tests.
Results
Baseline Study
At baseline, 64% patients (45/70) had significant RWMAs during HD, defined as a reduction in wall motion of >20% from baseline in more than two regions. There was no significant difference between the number of regions affected at 2 compared with 4 hrs (4.48 ± 1.7 versus 4.52 ± 1.9, P = 0.8). In those regions affected, there was a significant reduction in %SF at each time point compared with baseline (baseline 3.17 ± 1.28%; 2 hrs 1.75 ± 0.67%, P < 0.001; 4 hrs 1.59 ± 0.69%, P < 0.001; recovery 2.3 ± 0.94%, P < 0.01), confirming the presence of HD-induced myocardial ischemia. Although there was a significant return toward predialysis values for %SF during the recovery period from both time points during HD (P < 0.001), the persistence of RWMAs implies the development of myocardial stunning.
Patient characteristics and dialysis-related factors are shown in Table 1. Hematologic and biochemical values are summarized in Table 2. Univariate analysis showed a number of factors associated with the presence of HD-induced RWMAs. These were: advancing age (P = 0.03); higher intradialytic ultrafiltration (UF) volumes (P = 0.01); the presence of diabetes (P = 0.002); lower albumin levels (P = 0.02); and elevated cTnT concentration (P = 0.001). Patients with ischemic heart disease (IHD) were more likely to develop HD-induced RWMAs, but this was only a trend (P = 0.07). There were no other statistically significant differences with respect to underlying etiology, dialysis vintage, drug therapies, hematologic and biochemical values, or other co-morbid conditions.
Table 1.
Patient characteristics and dialysis-related factors in all patients and in groups based on the presence (with) or absence (without) of dialysis-induced regional wall motion abnormalities (RWMAs)
| Characteristics | All Patients (n = 70) | Patients with RWMAs (n = 45) | Patients without RWMAs (n = 25) | P value |
|---|---|---|---|---|
| Age (mean, years) | 63.1 ± 13.7 | 65.6 ± 1.9 | 59.4 ± 3 | 0.03 |
| Male: Female | 47: 23 | 28: 17 | 19: 6 | 0.29 |
| Dialysis vintage (mean, months) | 45.2 ± 32.3 | 43.5 ± 31 | 48.1 ± 34.8 | 0.57 |
| Intradialytic UF volume (mean, L) | 1.95 ± 0.83 | 2.13 ± 0.91 | 1.59 ± 0.75 | 0.01 |
| Kt/V urea | 1.3 ± 0.2 | 1.3 ± 0.2 | 1.3 ± 0.3 | 0.88 |
| Smoker | 11 (16%) | 7 (16%) | 4 (16%) | 1 |
| Ethnicity (n) | – | – | – | – |
| Caucasian | 65 (93%) | 43 (96%) | 22 (88%) | 0.34 |
| Afro-Caribbean | 1 (1%) | 1 (2%) | 0 (0%) | 1 |
| Asian | 4 (5%) | 1 (2%) | 3 (12%) | 0.13 |
| Etiologies (n) | – | – | – | – |
| Diabetic nephropathy | 22 (31%) | 18 (40%) | 4 (16%) | 0.06 |
| Glomerular disease | 12 (17%) | 8 (18%) | 4 (16%) | 1 |
| APKD | 7 (10%) | 5 (11%) | 2 (8%) | 1 |
| Urological | 6 (9%) | 3 (7%) | 3 (12%) | 0.66 |
| Unknown | 9 (13%) | 5 (11%) | 4 (16%) | 0.71 |
| Other | 14 (29%) | 6 (13%) | 8 (32%) | 0.12 |
| Co-morbidities (n) | – | – | – | – |
| Ischaemic heart disease | 24 (34%) | 19 (42%) | 5 (20%) | 0.071 |
| Diabetes mellitus | 28 (40%) | 24 (53%) | 4 (16%) | 0.002 |
| Hypertension | 42 (60%) | 27 (60%) | 15 (60%) | 1 |
| Hyperlipidaemia | 31 (44%) | 21 (47%) | 10 (40%) | 0.62 |
| Left ventricular hypertrophy | 42 (60%) | 27 (60%) | 15 (60%) | 1 |
APKD, adult polycystic kidney disease; UF, ultrafiltration.
Table 2.
Baseline haematological and biochemical data stratified based on the presence (with) or absence (without) of dialysis-induced regional wall motion abnormalities (RWMAs) taken before and after HD
| Parameter | Predialysis blood testing
|
Postdialysis blood testing
|
||||
|---|---|---|---|---|---|---|
| Patients with RWMAs | Patients without RWMAs | P value | Patients with RWMAs | Patients without RWMAs | P value | |
| Haemoglobin (g/dl) | 11 ± 1.3 | 11.5 ± 1 | 0.15 | 11.4 ± 1.4 | 12 ± 1.5 | 0.14 |
| Haematocrit (%) | 36 ± 4 | 36 ± 2 | 0.35 | 36 ± 4 | 37 ± 4 | 0.44 |
| Na+ (mEq/L) | 138 ± 3 | 139 ± 4 | 0.18 | 138 ± 2 | 139 ± 3 | 0.1 |
| K+ (mEq/L) | 4.8 ± 0.8 | 4.7 ± 0.9 | 0.89 | 3.0 ± 0.4 | 3.0 ± 0.5 | 0.69 |
| Urea nitrogen (mg/dl) | 59.1 ± 15.1 | 55.5 ± 14.0 | 0.33 | 17.4 ± 6.2 | 17.4 ± 7.0 | 0.81 |
| Creatinine (mgl/dl) | 8.8 ± 2.5 | 9.8 ± 2.9 | 0.13 | 3.4 ± 1.2 | 3.9 ± 1.7 | 0.12 |
| Phosphorus (mg/dl) | 5.3 ± 1.2 | 5.0 ± 1.9 | 0.42 | 2.5 ± 0.6 | 2.5 ± 0.6 | 0.61 |
| Bicarbonate (mEq/L) | 22.7 ± 3.0 | 22.8 ± 3.2 | 0.87 | 27.8 ± 2.8 | 28.4 ± 2.5 | 0.44 |
| Calcium (mg/dl) | 9.68 ± 0.6 | 9.6 ± 0.6 | 0.58 | 9.4 ± 0.4 | 9.6 ± 0.44 | 0.84 |
| Albumin (g/dl) | 3.5 ± 0.4 | 3.7 ± 0.3 | 0.02 | 3.7 ± 0.5 | 3.9 ± 0.6 | 0.04 |
| hsCRP (mg/L) | 1.2 ± 1.0 | 0.8 ± 0.7 | 0.13 | – | – | – |
| cTnT (ng/ml) | 0.098 ± 0.08 | 0.036 ± 0.04 | 0.001 | – | – | – |
Postdialysis cardiac troponin-T (cTnT) levels were not taken due to an insufficient time lapse needed to detect a significant change. hsCRP, high sensitivity C-reactive protein.
Spearman univariate correlation revealed significant correlations between a number of these same variables and the severity of myocardial stunning measured by magnitude of reduction in %SF, including: reduction in SBP (r2 0.4, P = 0.001); higher UF volumes (r2 0.28, P = 0.02); higher plasma cTnT concentration (r2 0.29, P = 0.02), and increasing age (r2 0.29, P = 0.014).
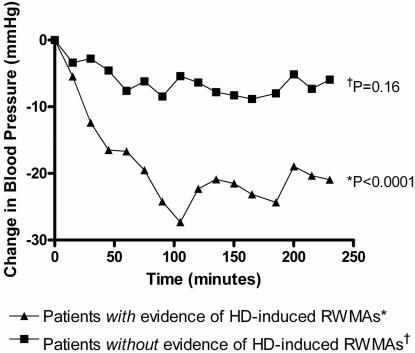
At baseline there was a significant reduction in SBP during HD in patients with HD-induced RWMAs (P < 0.0001); however, patients without such RWMAs did not have a significant reduction in their SBP (P = 0.16), Figure 1.
Figure 1.
Reduction in systolic BP (SBP) during hemodialysis (HD) in patients with and without HD-induced regional wall motion abnormalities (RWMAs). At baseline there was a significant reduction in SBP during HD in patients with HD-induced RWMAs (P < 0.0001); however, patients without such RWMAs did not have a significant reduction in their SBP (P = 0.16).
Stepwise multivariate analysis of factors contributing to the presence of RWMAs revealed that the following were all independent variables associated with HD-induced RWMAs: maximum reduction in SBP per mmHg (OR 1.1, CI 1.02 to 1.1, P = 0.002); UF volume per liter (OR 5.1, CI 1.33 to 19.7, P = 0.007); age per year (OR 1.26, CI 1.04 to 1.54, P = 0.004); and cTnT concentrations per μg/L (OR 1.07, 1.01 to 1.13, P = 0.018) (Nagelkerke R2 = 0.6 of the model overall). The nonlinear increasing effects of UF volume and BP on risk of developing HD-induced cardiac injury are illustrated in Table 3. All other factors, including diabetes and IHD, did not enter the equation.
Table 3.
The effect of increasing ultrafiltration (UF) volume and worsening intradialytic haemodynamics on the development of HD-induced RWMAs
| Factor associated with presence of myocardial stunning | Odds Ratio | P value |
|---|---|---|
| UF volume during HD of 1L | 5.1 | 0.007 |
| UF volume during HD of 1.5L | 11.6 | |
| UF volume during HD of 2L | 26.2 | |
| Maximum SBP reduction during HD of 10 mmHg | 1.8 | 0.002 |
| Maximum SBP reduction during HD of 20 mmHg | 3.3 | |
| Maximum SBP reduction during HD of 30 mmHg | 6.0 |
Follow-up at One Year
After 12 mo, 51 patients entered follow-up. Nineteen patients were censored from final analysis (died [n = 9]), transplanted [n = 6], changed dialysis modality [n = 3], and withdrawal of consent [n = 1]).
Of these, 74% (38/51) had significant RWMAs during HD. Of the 31 follow-up patients with evidence of myocardial stunning at baseline, 94% (29/31) continued to have evidence of significant RWMAs during HD, and 45% (14/31) had an increase in the number of affected regions. Two patients had a reduction in the number of RWMAs below significance. Of the 20 follow-up patients without evidence of RWMAs at baseline, 45% (9/20) showed development of significant RWMAs; however, 55% (11/20) continued to be unaffected.
At baseline, LVEF at rest (LVEFrest) was not significantly different between patients who did and did not develop HD-induced RWMAs (62.1 ± 11.4% versus 60.1 ± 7.7%, P = 0.4). After 12 mo, LVEFrest had significantly deteriorated in patients with RWMAs (62.1 ± 11.4% versus 54.7 ± 10.1%, P < 0.0008) but remained unchanged in those patients without (60.1 ± 7.7% versus 55.8 ± 5.5%, P = 0.09). Baseline LVEF at peak dialytic stress (LVEFHD) was significantly lower in patients who had RWMAs compared with those who did not (57.3 ± 10.1% versus 67 ± 6.8%, P < 0.0001). Although there was a reduction in LVEFHD after 12 mo in patients both with and without HD-induced RWMAs (57.3 ± 10.1% versus 50.9 ± 8.9, P < 0.001 and 67 ± 6.8% versus 61.3 ± 9.3, P = 0.007 respectively), the difference in LVEFHD between the two groups remained significant (61.3 ± 9.3 versus 50.9 ± 8.9, P < 0.0001).
After 12 mo, both groups of patients (with and without evidence of HD-induced RWMAs) exhibited a significant deterioration in SBP during HD. Mean reduction was greater in patients with RWMAs at both time points (-18.6 ± 6.9 mmHg to −23.3 ± 7.2 mmHg, P < 0.01 and −7 ± 3.6 mmHg to −14 ± 4.8 mmHg, P < 0.001, respectively). However, the separation remained between patients developing RWMAs versus those who did not (−23.3 ± 7.2 mmHg versus −14 ± 4.8 mmHg, P < 0.001).
Presence of HD-induced Myocardial Stunning and Patient Survival
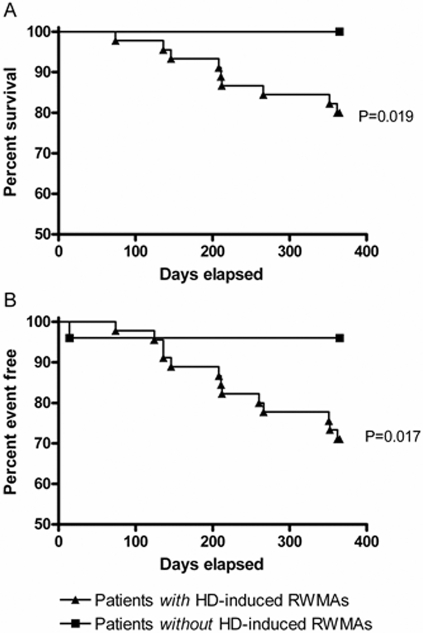
The presence of HD-induced RWMAs was associated with increased relative mortality at 12 mo (P = 0.019). Survival to a composite end point of mortality and first cardiovascular event also demonstrated almost complete separation between patients with and without HD- induced myocardial injury, with only one patient in the unaffected group, compared with 13 in the myocardial stunning group (P = 0.017, hazards ratio of 8, 95% CI 1.264 to 10.99, Figure 2). Death resulted overwhelmingly from cardiovascular causes.
Figure 2.
The association of hemodialysis-induced RWMAs with mortality and outcome. (A) The development of HD-induced RWMAs was associated with increased relative mortality at 12 mo (P = 0.019) and (B) reduced survival to a composite end point of mortality and time to first cardiovascular event (P = 0.017).
Higher baseline plasma cTnT concentrations were associated with mortality after 1 yr (0.067 ± 0.076 μmol/L versus 0.136 ± 0.059 μmol/L, P < 0.001). Cox regression analysis demonstrated an increased hazard of death in patients with RWMAs and an elevated cTnT than in patients with elevated cTnT alone (P = 0.02).
Discussion
This study demonstrates for the first time that dialysis-induced cardiac injury is common and associated with reductions in myocardial contractile function and patient survival. Although the exact influence of a number of biologic variables within this model remains unknown, we have identified specific elements of the dialysis process that may drive heart failure and other adverse cardiovascular outcomes in HD patients.
Diabetes was associated with the presence of HD-induced RWMAs. IHD was of only borderline importance, but it is possible that with a larger sample size this association would have reached statistical significance. In the HD population there is increasing evidence that decline in LVEF is not due to traditional cardiac risk factors (2). Indeed, HD patients with diabetes and normal epicardial coronary anatomy have significantly reduced CFR (11) predisposing to the development of demand ischemia, but at present, the exact nature of the relationship between diabetes, coronary artery disease, and HD-induced myocardial stunning remains unclear.
Lower serum albumin levels were associated with dialysis-induced RWMAs (on a univariate basis only). Considerable evidence exists linking malnutrition and inflammation with atherosclerosis in end-stage renal patients (21). Other inflammatory markers (e.g., IL-6) may provide additional information about the association between HD-induced myocardial stunning, malnutrition, and inflammation but was outside the scope of this study.
After 12 mo, over 90% of patients affected at baseline continued to have RWMAs precipitated by HD, and nearly half had progressed with greater numbers of regions involved. In addition, nearly half of patients unaffected at baseline had subsequently developed RWMAs on HD. Two patients with an apparent improvement did in fact merely transit to globally reduced %SF in all segments. Myocardial stunning is just the beginning of a disease continuum involving myocardial hibernation and fibrosis leading to fixed segmental LV systolic dysfunction (22). As such, these patients with fewer regions displaying a reduction in %SF during HD may represent progression of disease from myocardial stunning to fixed systolic dysfunction.
Higher UF volumes, intradialytic hemodynamic instability and raised biochemical markers of myocardial damage (cTnT) were all associated with the presence and severity of HD-induced RWMAs. Logistic regression analysis confirmed these factors were independent determinants of myocardial stunning and that the risk associated with higher UF volumes and greater drops in SBP increased disproportionately with each additional unit of measure. It is certainly not unusual in clinical practice for patients to have UF volumes set at two liters or more and to experience a drop in SBP of more than 20 mmHg. Previous studies into HD-induced RWMAs have demonstrated that UF volume during dialysis is not the only factor that determines their development (4,5). Similarly in this study, there were no significant differences in UF volume between the studies at baseline and at 12 mo.
Hemodialysis-induced myocardial stunning was associated with increased mortality and time to first cardiovascular event. Intradialytic hypotension (IDH) is an independent risk factor for mortality in HD patients (23). Although there is evidence that both larger interdialytic weight gain (24) and higher UF rates (25) are associated with worse survival, there are currently no available data illustrating the potential differential effects of volume and rate. Although some patients with high UF requirements did not develop RWMAs, overall there was a significant association between UF volumes and the presence of RWMAs. This finding is crucially important and represents a potential therapeutic target. More frequent daily or nocturnal HD therapies can improve cardiovascular outcome measures and quality of life (26,27). Such treatments have been shown to improve both intradialytic hemodynamics (by reducing IDH) (28) and plasma concentrations of biochemical markers of myocardial injury (29). We have already demonstrated that dialysis-based strategies such as cooled dialysate can improve intradialytic hemodynamics and reduce myocardial stunning (5). It could rightly be argued that a decline in SBP is a result rather than a cause of myocardial stunning; either way, targeting treatments that minimize UF rates and improve IDH should prevent the acute development of RWMAs and may improve long-term cardiovascular outcomes.
At baseline, both groups of patients had similar LVEFrest. During HD, patients with significant RWMAs had a significant drop in their LVEFHD. After 12 mo, LVEFrest had fallen in patients with HD-induced RWMAs, and their ability to respond to the challenge of HD was further compromised. Patients without significant RWMAs at baseline maintained their LVEFrest, but LVEFHD was significantly reduced. This is entirely consistent with the pattern of injury associated with ischemic cardiomyopathy in the nondialysis population. Repetitive ischemic injury initially causes a loss of contractile reserve that may lead to myocardial fibrosis and resting dysfunction (30). In HD patients, repetitive HD-induced RWMAs led to a reduction in contractile reserve and then resting LV systolic dysfunction. This process of deterioration has already begun in patients unaffected by HD-induced myocardial injury at baseline, as almost half now have evidence of HD-induced RWMAs and reduced contractile reserve. This also has major potential clinical implications. In the nondialysis population, timely revascularization can lead to an improvement in LVEF, heart failure and prognosis (31). In addition to modifications to the HD procedure, treatment of underlying coronary artery disease may slow progression of heart failure and improve outcome.
It is well recognized that cTnT levels are often elevated in dialysis patients and that elevated levels predict mortality (32). In addition to the association with presence and severity of HD-induced RWMAs, elevated plasma cTnT levels were also associated with an increased hazard of death. A recent study of cardiac troponins and outcome in acute heart failure revealed associations with lower SBP, lower LVEF, and higher mortality (33). CTnT may therefore provide a useful tool to identify vulnerable patients who might benefit from modified dialysis therapies.
This study has limitations, the principle being its observational nature. Despite being repeatable and quantitative, echocardiography was the only technique used to quantify functional decline. Other techniques may have provided additional information but are often impractical and difficult to perform during dialysis. In any case, a load independent way of assessing regional myocardial function is needed to fully elucidate the complex mechanisms behind HD- induced RWMAs. The mortality observations are derived from a small number of patient deaths, and the study is clearly underpowered in this respect. Consequently, further analysis looking at the independent contribution of other variables on mortality and composite outcome was not practicable, and no further conclusions are derived from these results. However, statistical significance was achieved and the clear separation for cardiovascular events and mortality between the two groups is compelling.
Conclusions
Conventional HD exerts a significant acute cardiovascular stress, the exact consequences of which are poorly understood. This study supports the contention that subclinical myocardial ischemia is commonly precipitated by dialysis. Such episodes of ischemia are associated with longer term loss of systolic cardiac function, increased cardiac events, and reduced patient survival. This may be an important process in a spectrum of mechanisms contributing to poor cardiovascular outcome in HD patients.
Further consideration of interventions reducing the acute impact of dialysis on the cardiovascular system should be considered an urgent priority, allowing selection of optimal therapies for future large-scale RCT study of “hard” end points. This is of particular importance given the almost universal lack of effectiveness, in HD patients, of current cardiovascular interventions developed within the nondialysis-based population.
Disclosures
None.
Acknowledgments
This study was jointly sponsored by the University of Nottingham and Derby Hospitals NHS Foundation Trust. This study was also funded by a grant from Kidney Research (UK), Kings Chambers, Priestgate, Peterborough, PE1 1FG, UK (Grant reference number RP4/2/05).
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Foley RN, Parfrey PS, Sarnak MJ: Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol 9(12 Suppl): S16–S23, 1998 [PubMed] [Google Scholar]
- 2.Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD: Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int 63: 793–808, 2003 [DOI] [PubMed] [Google Scholar]
- 3.US RDS. Annual data report: Atlas of chronic kidney disease and end-stage renal disease in the US. National Institutes of Health, Diabetes and Digestive and Kidney Diseases. Bethesda, MD. 2007
- 4.Selby NM, Lambie SH, Camici PG, Baker CS, McIntyre CW: Occurrence of regional left ventricular dysfunction in patients undergoing standard and biofeedback dialysis. Am J Kidney Dis 47: 830–841, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Selby NM, Burton JO, Chesterton LJ, McIntyre CW: Dialysis induced regional left ventricular dysfunction is ameliorated by cooling the dialysate. Clin J Am Soc Nephrol 1: 1216–1225, 2006 [DOI] [PubMed] [Google Scholar]
- 6.McIntyre CW, Burton JO, Selby NM, Leccisotti L, Korsheed S, Baker CS, Camici PG: Haemodialysis induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol 3: 19–26, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung AK, Sarnak MJ, Yan G, Berkoben M, Heyka R, Kaufman A, Lewis J, Rocco M, Toto R, Windus D, Ornt D, Levey AS: Cardiac diseases in maintenance hemodialysis patients: Results of the HEMO Study. Kidney Int 65: 2380–2389, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Silberberg JS, Barre PE, Prichard SS, Sniderman AD: Impact of left ventricular hypertrophy on survival in end-stage renal disease. Kidney Int 36: 286–290, 1989 [DOI] [PubMed] [Google Scholar]
- 9.Bos WJ, Bruin S, van Olden RW, Keur I, Wesseling KH, Westerhof N, Krediet RT, Arisz LA: Cardiac and hemodynamic effects of hemodialysis and ultrafiltration. Am J Kidney Dis 35: 819–826, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Tok D, Gullu H, Erdogan D, Topcu S, Ciftci O, Yildirim I, Muderrisoglu H: Impaired coronary flow reserve in hemodialysis patients: A transthoracic Doppler echocardiographic study. Nephron Clin Pract 101: c200–c206, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Ragosta M, Samady H, Isaacs RB, Gimple LW, Sarembock IJ, Powers ER: Coronary flow reserve abnormalities in patients with diabetes mellitus who have end-stage renal disease and normal epicardial coronary arteries. Am Heart J 147: 1017–1023, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Homans DC, Laxson DD, Sublett E, Lindstrom P, Bache RJ: Cumulative deterioration of myocardial function after repeated episodes of exercise-induced ischemia. Am J Physiol 256(5 Pt 2): H1462–H1471 [DOI] [PubMed]
- 13.Bolli R: Myocardial ‘stunning’ in man. Circulation 86: 1671–1691, 1992 [DOI] [PubMed] [Google Scholar]
- 14.Bosch JG, Savalle LH, van Burken G, Reiber JH: Evaluation of a semiautomatic contour detection approach in sequences of short-axis two-dimensional echocardiographic images. J Am Soc Echocardiogr 8: 810–821, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Bosch JG, Mitchell SC, Lelieveldt BP, Nijland F, Kamp O, Sonka M, Reiber JH: Automatic segmentation of echocardiographic sequences by active appearance motion models. IEEE Trans Med Imaging 21: 1374–1383, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Sugioka K, Hozumi T, Yagi T, Yamamuro A, Akasaka T, Takeuchi K, Homma S, Yoshida K, Yoshikawa J: Automated quantification of left ventricular function by the automated contour tracking method. Echocardiography 20: 313–318, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Tsai LM, Chen TP, Chen TS, Chen JH: Application of automatic boundary detection for computerized quantitative analysis of left ventricular regional wall motion by two-dimensional echocardiography. J Ultrasound Med 16: 177–182, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Burton JO, Korsheed S, Grundy BJ, McIntyre CW: Hemodialysis induced left ventricular dysfunction is associated with an increase in ventricular arrhythmias. Ren Fail 30: 701–709, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Rame JE, Ramilo M, Spencer N, Blewett C, Mehta SK, Dries DL, Drazner MH: Development of a depressed left ventricular ejection fraction in patients with left ventricular hypertrophy and a normal ejection fraction. Am J Cardiol 93: 234–237, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Zoccali C, Benedetto FA, Mallamaci F, Tripepi G, Giacone G, Cataliotti A, Seminara G, Stancanelli B, Malatino LS: Prognostic value of echocardiographic indicators of left ventricular systolic function in asymptomatic dialysis patients. J Am Soc Nephrol 15: 1029–1037, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Pecoits-Filho R, Lindholm B, Stenvinkel P: The malnutrition, inflammation, and atherosclerosis (MIA) syndrome – The heart of the matter. Nephrol Dial Transplant 17 Suppl 11: 28–31, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Canty JM, Jr., Fallavollita JA: Chronic hibernation and chronic stunning: A continuum. J Nucl Cardiol 7: 509–527, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Shoji T, Tsubakihara Y, Fujii M, Imai E: Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int 66: 1212–1220, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Kalantar-Zadeh K, Bunnapradist S, Regidor DL, Streja E, Pillon L, McAllister CJ, Kopple JD: Greater interdialytic fluid retention is associated with poor survival in maintenance hemodialysis patients [Abstract]. J Am Soc Nephrol 18: 724A, 2007 [Google Scholar]
- 25.Movilli E, Gaggia P, Zubani R, Camerini C, Vizzardi V, Parrinello G, Savoldi S, Fischer MS, Londrino F, Cancarini G: Association between high ultrafiltration rates and mortality in uraemic patients on regular haemodialysis. A 5-year prospective observational multicentre study. Nephrol Dial Transplant 22: 3547–3552, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Culleton BF, Walsh M, Klarenbach SW, Mortis G, Scott-Douglas N, Quinn RR, Tonelli M, Donnelly S, Friedrich MG, Kumar A, Mahallati H, Hemmelgarn BR, Manns BJ: Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: A randomized controlled trial. JAMA 298: 1291–1299, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Chan C, Floras JS, Miller JA, Pierratos A: Improvement in ejection fraction by nocturnal haemodialysis in end-stage renal failure patients with coexisting heart failure. Nephrol Dial Transplant 17: 1518–1521, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Okada K, Abe M, Hagi C, Maruyama T, Maruyama N, Ito K, Higuchi T, Matsumoto K, Takahashi S: Prolonged protective effect of short daily hemodialysis against dialysis-induced hypotension. Kidney Blood Press Res 28: 68–76, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Odar-Cederlof I, Bjellerup P, Williams A, Blagg CR, Twardowski Z, Ting G, Kjellstrand CM: Daily dialyses decrease plasma levels of brain natriuretic peptide (BNP), a biomarker of left ventricular dysfunction. Hemodial Int 10: 394–398, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Schinkel AF, Bax JJ, van Domburg R, Elhendy A, Valkema R, Vourvouri EC, Sozzi FB, Roelandt JR, Poldermans D: Dobutamine-induced contractile reserve in stunned, hibernating, and scarred myocardium in patients with ischemic cardiomyopathy. J Nucl Med 44: 127–133, 2003 [PubMed] [Google Scholar]
- 31.Bax JJ, Poldermans D, Elhendy A, Cornel JH, Boersma E, Rambaldi R, Roelandt JR, Fioretti PM: Improvement of left ventricular ejection fraction, heart failure symptoms and prognosis after revascularization in patients with chronic coronary artery disease and viable myocardium detected by dobutamine stress echocardiography. J Am Coll Cardiol 34: 163–169, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Khan NA, Hemmelgarn BR, Tonelli M, Thompson CR, Levin A: Prognostic value of troponin T and I among asymptomatic patients with end-stage renal disease: A meta-analysis. Circulation 112: 3088–3096, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Peacock W 4th, De Marco T, Fonarow GC, Diercks D, Wynne J, Apple FS, Wu AH; ADHERE Investigators: Cardiac troponin and outcome in acute heart failure. N Engl J Med 358: 2117–2126, 2008 [DOI] [PubMed] [Google Scholar]