Abstract
Background and objectives: Clinically relevant kidney involvement is uncommonly described in adult patients with cystic fibrosis (CF). We sought to report on a series of patients with CF and kidney biopsy–documented renal involvement.
Design, setting, participants, & measurements: A retrospective study was undertaken in two referral centers for adult patients with CF in Paris, France. Patients who had undergone a biopsy of native kidneys between 1992 and 2008 were identified, and their medical records were reviewed.
Results: We identified 13 adult patients with CF and renal disease. Proteinuria was present in all but two cases and was associated with progressive renal impairment in four patients (median serum creatinine 85 μmol/L; range 53 to 144 μmol/L). Renal biopsy disclosed a heterogeneous spectrum of nephropathies including AA amyloidosis (n = 3), diabetic glomerulopathy (n = 3), FSGS (n = 2), minimal-change disease (n = 1), postinfectious glomerulonephritis (n = 1), IgA nephropathy related to Henoch-Schönlein purpura (n = 1), membranous nephropathy (n = 1), and chronic interstitial nephropathy (n = 1). Chronic renal failure occurred in five patients, and one patient reached ESRD.
Conclusions: Although rare, clinically significant renal disease may arise in young adult patients with CF. Given the wide spectrum of diseases that may be encountered, definite diagnosis by kidney biopsy is mandatory to optimize clinical treatment of these complex patients, particularly in the perspective of organ transplantation.
With a birth incidence ranging between 1:2500 and 1:8000 in Europe, cystic fibrosis (CF) is the most common autosomal recessive condition affecting the white population. It is caused by mutations in the CFTR gene, encoding the polyprotein cystic fibrosis transmembrane conductance regulator (CFTR), which functions as an ATP-responsive chloride channel in apical membrane of epithelial cells (1). Pathologic changes related to CFTR mutations mostly affect secretory cells, resulting in low secretion volume and increased viscosity and promoting mucosal obstruction in lung, pancreas, biliary tract, sinuses, and reproductive tract. Suppurative lung disease, respiratory failure, pancreatic insufficiency, diabetes, and biliary cirrhosis account for most of the morbidity and mortality of patients with CF (2). Advances in the care of patients with CF, mostly relying on symptomatic treatment strategies, have translated into continuous improvement of life expectancy in the past decade; it is assumed that infants who have CF and were born around 2000 may have a life expectancy as high as 50 yr (3).
CFTR is highly expressed in various segments of the nephron. It is probably involved in proximal tubule receptor–mediated endocytosis. Inactivation of CFTR leads to detection of significant, although variable, low molecular weight proteinuria in patients with CF (4). Nonetheless, there is no overt clinically relevant renal phenotype in children or teenagers with CF; however, asymptomatic nephrocalcinosis and hypercalciuria are present in up to 90 and 30% of individuals with CF, respectively (5). Not unexpected, renal stones are more common in patients with CF as compared with the control population. Urolithiasis in CF may result from enteric hyperoxaluria caused by fat malabsorption and the lack of intestinal oxalate degrading bacteria as Oxalobacter formigenes. Additional renal manifestations occasionally reported in children and teenagers include AA amyloidosis, membranoproliferative glomerulonephritis, and postinfectious glomerulonephritis (6–8). In contrast, few data on the prevalence of renal involvement and its spectrum in adulthood are available. Moreover, its impact on the management of this complex multisystemic disorder is unknown. We report a series of 13 patients who had CF and significant renal involvement and required kidney biopsy. The aim of the study was to shed light on kidney pathology and to provide data on the clinical consequences of proper identification of kidney disease in a group of adult patients with CF.
Materials and Methods
Patients and Data Collection
The study was conducted in two adult CF centers in the Paris area (Ile-de-France). Using a computerized database, we identified patients who had CF and underwent biopsy of native kidneys between January 1992 and December 2007. Patients’ medical records were reviewed, and relevant data were collected.
Hypertension was defined as BP >140/90 mmHg. Diagnosis of the nephrotic syndrome was established on proteinuria >3 g/24 h and serum albumin <30 g/L. Renal failure was defined as serum creatinine (SCr) >100 μmol/L. GFR was estimated using the Modification of Diet in Renal Disease (MDRD) simplified formula, even though this formula as well as the Cockroft-Gault formula and SCr level have not been assessed in patients who have CF and frequently exhibit gross malnutrition and low muscle mass. Severity of respiratory involvement was assessed by the forced expiratory volume in 1 s (percentage of predicted value) and the forced vital capacity (percentage of the predictive value). Diagnosis of diabetes was based on conventional World Health Organization criteria.
Follow-up duration was measured from the time of kidney biopsy until the recorded date of death or, in living patients, the date of the last follow-up visit. For genetic studies, a limited testing within CFTR had been applied routinely in most cases. including the research for the eight to 10 mutations most commonly found in European population.
In this series, indication for kidney biopsy was conservative. It was considered when urinary abnormalities or renal failure of unclear origin warranted clarification for optimal clinical management, including preparation for pulmonary transplantation.
Pathologic Analysis
Renal biopsy samples were processed for light microscopy and immunofluorescence study as described previously (9). Congo red staining was performed in all biopsies. Immunofluorescence study using anti-AA protein antibodies was used to ascertain the type of amyloidosis. All kidney biopsies were reviewed by an expert pathologist (L.-H.N.) except for one patient (patient 1), for whom kidney biopsy samples were no longer available. The Banff classification was used for the quantification of interstitial and vascular lesions.
Statistical Analysis
Owing to the small number of patients, only descriptive statistics were applied. Results are expressed as numbers (percentages) for categorical variables and as means for continuous variables.
Results
Patients’ Demographic Data
Among 510 adult patients who had CF and were attending regular follow-up in the two adult CF centers from the Paris area, 13 (2.5%) underwent a renal biopsy for significant renal disease during a 16-yr period. Presenting manifestations of CF and extrarenal involvement at the time of kidney biopsy are depicted in Table 1. In one patient (patient 5), the diagnosis of CF, suspected at the age of 22, could not be definitely ascertained before the age of 49 (Table 1).
Table 1.
Genetic and extrarenal features in 13 adult patients with CF and renal involvementa
| Patient/Gender | Age at Diagnosis of CF | Presenting Symptoms | CFTR Mutations | Extrarenal Manifestations at Kidney Biopsy
|
|||
|---|---|---|---|---|---|---|---|
| Respiratory Status (FEV1/FVC; %) | Liver | Pancreas
|
|||||
| Exocrine | Endocrine | ||||||
| 1/M | 6 mo | Digestive | F508del/F508del | 48/72 | − | + | − |
| 2/M | 12 mo | Bronchitis | F508del/F508del | 25/35 | HSM | + | − |
| 3/M | 4 mo | Bronchitis | F508del /2143delΤ | 14/36 | − | + | + |
| 4/M | 18 mo | Digestive | F508del/F508del | 23/40 | − | + | + |
| 5/F | 49 yr | Bronchitis | F508del/Y1032C | NA | − | + | − |
| 6/F | 33 yr | Bronchitis | G542X/3849+10kbC→T | NA | − | − | − |
| 7/M | 4 yr | Bronchitis | F508del/F508del | 22/41 | HM | + | + |
| 8/M | 13 yr | Bronchitis | F508del/F508del | 25/49 | − | + | + |
| 9/F | 12 yr | Bronchitis | NA | 17/28 | − | + | − |
| 10/M | Birth | Bronchitis/digestive | F508del/1717−1G→A | 25/47 | BC | + | − |
| 11/M | 9 yr | Digestive | NA | NA | BC | + | − |
| 12/M | Birth | Digestive | F508del/2789+5G→A | 35/54 | − | + | + |
| 13/F | 5 mo | Bronchitis | F508del/F508del | 33/29 | − | + | + |
BC, biliary cirrhosis; CF, cystic fibrosis; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; HM, hepatomegaly; HSM, hepatosplenomegaly; NA, not available.
Genetic Findings
Mutational analysis of CFTR yielded positive findings in 11 patients (Table 1). Six (54%) patients were F508del homozygous. No genetic data were available for two patients; however, they had a positive sweat test.
Patients Extrarenal Features at the Time of Kidney Biopsy
Pulmonary Status.
Assessment is lacking in three patients. Data regarding pulmonary status in the 10 remaining patients are summarized in Table 1. Given chronic colonization of respiratory tract by Pseudomonas aeruginosa, all patients had received multiple courses of antibiotics, including intravenous aminoglycosides.
Other Features.
Exocrine pancreatic insufficiency required enzyme replacement therapy in all but one patient. At the time of kidney biopsy, a diagnosis of diabetes, mainly relying on oral glucose tolerance test, was established in six patients (Table 2) and required treatment in four of them. Bilateral funduscopy was normal in five patients with diabetes.
Table 2.
Characteristics of diabetes in seven adult patients with CF and kidney involvementa
| Patient | Diabetes Diagnosis
|
Diabetes Treatment | Kidney biopsy
|
||||
|---|---|---|---|---|---|---|---|
| Age (yr) | Diagnostic Criteria | Precipitating Factor | HbA1c (%) | Diabetic Retinopathy | Diabetic Nephropathy | ||
| 3 | 27 | OGTT | – | OAD, insulin | 5.5 to 6.9 | No | Yes |
| 4 | 20 | FG | Cs-induced diabetes | Insulin | 5.2 | No | Yes |
| 7 | 23 | OGTT | – | Insulin | 8.0 to 11.0 | No | No |
| 8 | 33 | FG | Cs-induced diabetes | – | 6.0 | No | No |
| 11 | 32 | Kidney biopsy | – | – | 5.3 | No | Yes |
| 12 | 40 | OGTT | – | – | ΝΑ | NA | No |
| 13 | 33 | OGTT | – | OAD | 7.2 | NA | No |
Cs, corticosteroids; FG, fasting glucose; HbA1c, glycosylated hemoglobin; OAD, oral antidiabetic drug; OGTT, oral glucose tolerance test.
Renal Manifestations
Details of renal involvement are shown in Table 3. Renal manifestations were first recognized at a mean age of 31 yr (range 20 to 49 yr). Four patients had hypertension. Significant proteinuria (>0.2 g/L) was detected in 11 of 13 patients (mean 2.60 g/d; range 0.45 to 10.00). Nephrotic syndrome was present in two patients. Renal failure was noted in four patients with a SCr ranging from 105 to 144 μmol/L. Indication for kidney biopsy included persistent proteinuria (n = 11), renal failure (n = 4, including three patients on the waiting list for pulmonary transplantation), and hematuria with vasculitic purpura (n = 1).
Table 3.
Characteristics of renal involvement at kidney biopsy and last follow-up in 13 adult patients with CFa
| Patient | Age (yr)b | SCr (mmol/L)/GFR (ml/min per 1.73 m2) | Serum Albumin (g/L) | Proteinuria | Hematuria | Hypertension | Renal Pathology | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 24 | 107/78 | 40 | 0.60 g/L | − | + | Exsudative GN | – | Complete remission |
| 2 | 20 | 105/83 | 44 | 1.20 g/d | − | + | FSGS calcium oxalate crystals | ARB | NS, CRI, ARF after PT |
| 3 | 27 | 64/138 | 34 | 0.45 g/L | − | + | KW | ACEI | SCr 88 μmol/L, Pu 1.8 g/d |
| 4 | 31 | 71/119 | 27 | 1.50 g/d | − | + | KW | ACEI | SCr 156 μmol/L, NS |
| 5 | 49 | 66/88 | 28 | 9.50 g/d | − | − | AA amyloidosis | ACEI | ESRDc |
| 6 | 43 | 53/116 | 21 | 1.20 g/L | − | − | MCD, CTIN | – | Spontaneous remission, PT, SCr 150 mmol/L |
| 7 | 23 | 74/121 | 30 | 0.12 g/L | +++ | − | IgAN | ACEI | PTc |
| 8 | 33 | 69/122 | 21 | 10.00 g/d | − | − | MN | ACEI/ARB | PT |
| 9 | 23 | 140/58 | 26 | 0.15 g/L | − | − | AA amyloidosis | ACEI | PKT, SCr 60 μmol/L, Pu 0.5 g/L |
| 10 | 26 | 63/142 | 36 | >3.00 g/L | − | − | FSGS | ACEI | c |
| 11 | 32 | 97/83 | 27 | 2.20 g/L | + | − | KW | LT and PT | |
| 12 | 46 | 55/110 | 32 | 0.60 g/d | − | − | CTIN | – | PT, SCr 90 mmol/L |
| 13 | 37 | 144/51 | 23 | 1.00 g/d | − | − | AA amyloidosis | – | CRF, SCr 145 mmol/L |
ACEI, angiotensin-converting enzyme inhibitor; ARB angiotensin II receptor blocker; ARF, acute renal failure; CRI, chronic renal insufficiency; CRF, chronic renal failure; CTIN, chronic tubulointerstitial nephropathy; GN, glomerulonephritis; IgAN, IgA nephropathy; KW, Kimmelstiel-Wilson glomerulosclerosis; LT, liver transplantation; MCD, minimal-change disease; MN, membranous nephropathy; NS, nephrotic syndrome; PKT, pulmonary and kidney transplantation; PT, pulmonary transplantation; Pu, proteinuria; SCr, serum creatinine.
Age at the time of kidney biopsy.
Death.
Pathology
Details of renal pathology from kidney biopsy results are shown in Table 4. Renal lesions were notably heterogeneous among the patients and combined two different lesions in two patients (patients 2 and 6).
Table 4.
Pathologic findings on kidney specimens in 13 adult patients with CFa
| Patient | Light Microscopy
|
Immunofluorescence | Diagnosis | ||
|---|---|---|---|---|---|
| Glomeruli (Sclerotic Glomeruli) | Tubules/Interstitium | Vessels | |||
| 1 | Endocapillary proliferation, mesangial neutrophils infiltration | – | – | Glomerular C3 deposits, humps | Postinfectious GN |
| 2 | FSGS | ci2/ct2 calcium oxalate crystals | ah0/cv0 | Negative | FSGS |
| 3 | KW (6/17) | ci2/ct2 | ah2/cv1 | Linear IgA, IgG, and albumin deposits | Diabetic glomerulopathy |
| 4 | KW (1/17) | ci1/ct1 | ah3/cv1 | Linear IgA, IgG, and albumin deposits | Diabetic glomerulopathy |
| 5 | Mesangial and subepithelial amyloid deposits (7/20) | ci2/ct2 amyloid deposits | ah2/cv2 amyloid deposits | AA amyloidosis | Diffuse amyloidosis AA subtype |
| 6 | Normal (1/3) | ci1/ct1 | ah0/cv0 | Negative | MCD/CTIN |
| 7 | Diffuse mesangial proliferation (0/18) | ci1/ct0 | ah0/cv1 | Mesangial IgA deposits | IgAN |
| 8 | Normal (0/16) | i0/t0 | ah0/cv1 | Subepithelial IgG deposits | MN |
| 9 | Mesangial amyloid deposits (2/14) | i0/t0 intratubular vacuoles | Arteriolar amyloidosis | AA amyloidosis | Diffuse amyloidosis AA subtype |
| 10 | FSGS (1/14) | ci1/ct0 | ah1/cv0 | Negative | FSGS |
| 11 | KW (0/10) | ci1/ct1 | ah0/cv1 | Linear IgA, IgG, and albumin deposits | Diabetic glomerulopathy |
| 12 | Normal (2/21) | ci2/ct2 | ah0/cv0 cellular infiltrate, intratubular pigments | Negative | CTIN |
| 13 | Mesangial and subepithelial amyloidosis (4/23) | ci2/ct2 amyloidosis | Amyloidosis ah2/cv1 | AA amyloidosis | Diffuse amyloidosis AA subtype |
ah, arteriolar hyaline thickening; ci, interstitial fibrosis; ct, tubular atrophy; cv, vacular fibrous intimal thickening.
Glomerular Lesions
Twelve patients had glomerular involvement. The most frequent pattern was Kimmelstiel-Wilson (KW) nodular glomerulosclerosis, noted in three of 13 patients. Typical KW lesions contrasted with the absence of diabetic retinopathy in patients 3 and 4; more striking, diabetic glomerulopathy was diagnosed in patient 11 in the absence of previously known diabetes (Figure 1). Glycosylated hemoglobin was 5.3% at the time of kidney biopsy, and meticulous review of medical records failed to disclose abnormal fasting glucose before kidney biopsy. In contrast, four patients with diabetes (patients 7, 8, 12, and 13) had nondiabetic nephropathy, including three with glomerular lesions.
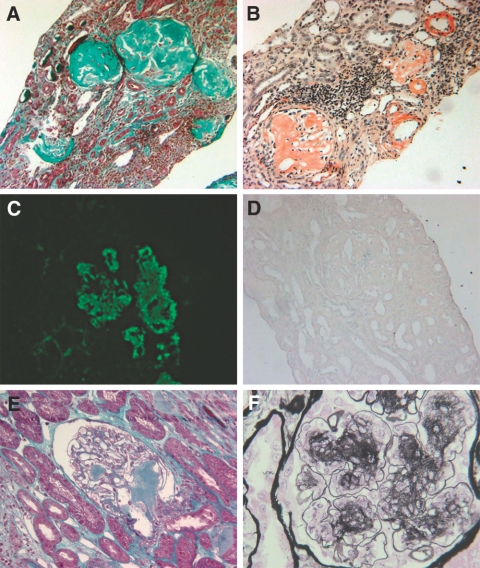
Figure 1.
(A through D) Patient 5. (A) Light microscopy (Masson's trichrome). Massive amyloidosis with destruction of glomeruli. (B) Light microscopy (Congo red staining). Deposits are stained by Congo red. (C) Immunofluorescence study (anti-serum amyloid A antibody staining). Massive glomerular (*) as well as interstitial amyloid deposits are revealed. (D) Light microscopy (Wright technique). Congo red staining disappears after treatment by permanganate potassium. (E) Patient 12. Light microscopy (Masson's trichrome). Relatively mild glomerular amyloid deposits (#). (F) Patient 11. (F) Light microscopy (Jones staining). Nodular accumulation of mesangial matrix (&) and presence of aneurysmal dilations of the glomerular capillaries (¶). Magnifications: ×100 in A through E; ×250 in F.
After comprehensive evaluation of clinical and histologic findings, the final diagnosis included diabetic glomerulopathy (n = 3); AA amyloidosis (n = 3) with diffuse amyloidosis deposits in the mesangium, interstitium, and vessels walls (Figure 1); focal and segmental glomerular lesions (n = 2); minimal-change disease (n = 1); acute endocapillary glomerulonephritis (n = 1); IgA nephropathy related to Henoch-Schönlein purpura (n = 1); and membranous nephropathy (n = 1).
Tubulointerstitial and Vascular Lesions
One female patients (patient 12; Figure 1) exhibited chronic tubulointerstitial nephropathy in the absence of primary glomerulopathy. Among the 12 remaining patients, significant interstitial fibrosis were present in seven (Table 4). Vascular lesions were mild or absent in all patients.
Outcome
Mean follow-up after kidney biopsy was 5 yr (range 1 to 16 yr). Seven patients underwent solid organ transplantation during the course of nephropathy: Five underwent lung transplantation alone (patients 2, 6, 7, 8, and 12), one had a combined lung and liver transplantation (patient 11), and one had a combined renal and lung transplant (patient 9).
Three patients died during follow-up. The cause of death was severe sepsis on dialysis (patient 5), chronic lung rejection (patient 7), and progressive respiratory failure (patient 10).
Renal complete remission occurred in patient 1 with postinfectious glomerulonephritis. Progressive renal failure occurred in six patients (patients 2, 4, 5, 6, 9, 10, 11, 12, and 13), after lung transplantation in two patients (patients 2 and 6). One patient (patient 5) reached ESRD. One patient underwent preemptive kidney transplantation (patient 9).
Impact of Accurate Kidney Assessment on Clinical Management
In the context of multisystem disease shared by patients with CF, definite identification of the renal pathologic process was helpful for the proper treatment of most patients. For instance, ruling out diabetic glomerulopathy in four of seven patients with diabetes (Table 2) proved useful. In contrast, detection of a significant diabetic microangiopathy in patient 11 led to the diagnosis of diabetes and to adequate diet and proper medication. Renal pathology also directly contributed to transplantation policy among the patients: In patient 9, severe tubulointerstitial lesions associated with AA amyloidosis prompted combining lung and kidney transplantation; similarly, patient 13 is currently on the waiting list for lung and kidney transplantation. In patient 12, significant interstitial nephritis before lung transplantation resulted in withdrawal of calcineurin inhibitors from the immunosuppressive regimen after engraftment.
Discussion
Clinically relevant renal involvement is considered uncommon in patients with CF. This may be partly explained by (1) the lack of clear renal phenotype directly related to the mutated CFTR protein; (2) the low prevalence of symptomatic urolithiasis, as compared with the high prevalence of hypercalciuria; (3) the poor reliability of SCr and inadequacy of Cockcroft and MDRD formulas to provide accurate estimate of GFR and, hence, the prevalence of chronic renal failure; (4) the excellent tolerance of repeated high-dosage courses of nephrotoxic drugs, including aminoglycosides (however, aminoglycoside-induced acute renal failure may be an underrecognized cause of morbidity in patients with CF [10]); and (5) reluctance of CF-specialized teams to perform renal invasive tests (GFR assessment by reference methods and/or kidney biopsies) in patients with CF.
Conversely, better knowledge of renal involvement in patients with CF is mandatory for two reasons. Continuous improvement in the treatment of these patients translates into significant increase in life expectancy (from approximately 20 yr in 1960 to an expected 50 yr for patients born in the early 2000s) but an increased risk for developing renal disease related to cumulative drug toxicity or to complications of chronic infections (AA amyloidosis) or organ failure (CF-related diabetes). Moreover, increasing acceptance of patients with CF for nonkidney transplantation requires accurate assessment of the kidney function for proper pre- and posttransplantation management.
In a large cohort of 510 adult patients with CF, 2.5% underwent kidney biopsy. Although low, this incidence rate should not be misinterpreted: All biopsies were performed on a conservative basis and most of them concentrated in the most recent period, thus suggesting an increasing requirement. The issue of whether renal disease is more likely in patients with CF compared with the general population cannot be addressed on the basis of the data of our study. Indeed, patients who had CF and were followed in the two referral centers that participated in the study were not systematically screened for renal disease. Only a prospective study may help to address this issue.
The main study regarding nephropathy in patients with CF was conducted by Abramowsky and Swinehart (11) in autopsies from 34 pediatric and adult patients with CF (age range 4 mo to 35 yr). The histologic changes included glomerulomegaly, a mesangiopathic lesion, and tubulointerstitial disease frequently associated with acute and chronic tubular injury. Diabetic lesions were not seen. No correlation was found between the severity and type of renal histologic lesion and patient age or duration of CF.
In this report, we included 13 adult patients (range 16 to 49 yr) who had developed a nephropathy that led to a kidney biopsy. In the setting of a multisystemic disease such as CF (in this study, diabetes was present in seven patients and liver cirrhosis in one patient), kidney biopsy seems mandatory to ascertain the type of nephropathy. This is corroborated by the high heterogeneity of renal lesions noted in our patients: Eight distinct types of nephropathies in 13 biopsies, with glomerular diseases being predominant.
Some types of nephropathies, such as postinfectious glomerulonephritis, or AA amyloidosis were expected in patients with CF. For instance, in a retrospective review of 33 autopsies of patients with CF, 33% had amyloidosis, principally in the spleen, liver, and kidney (12). The prognosis of patients with CF and AA amyloidosis is generally poor, with nearly all patients dying within 1 yr of clinical presentation; this was the case of our patient 5. However, two other patients with AA amyloidosis (patients 9 and 13) presented with a milder evolution of AA amyloidosis. Aggressive antibiotic treatment strategies and other genetic factors (marenostrin and TNF receptor) might modulate the risk and the severity of AA amyloidosis in patients with CF.
Oxalate calcium deposits were noted in only one of our 13 patients, although exocrine pancreatic dysfunction, a major risk factor for enteric hyperoxaluria, was present in all but one patient. Our data contrast with previous reports underlying an increased prevalence of calcium oxalate lithiasis (5% in patients with CF compared with 1 to 2% in the overall population) and medullary nephrocalcinosis (>90%) in autopsy studies (5,13).
Typical features of diabetic nephropathy were found in three of our 13 patients. Diabetes is present in up to 20% of patients with CF at the age of 20 yr and in 50% of the surviving patients at the age of 50 yr (14); however, the precise prevalence of diabetic microangiopathy, including nephropathy, in patients with CF remains ill-defined. In two recent studies, the incidence of microalbuminuria and retinopathy among patients with CF-related diabetes ranged from 14 to 21% and 10 to 16%, respectively (15,16).
Our study suggests that patients with diabetes and with CF may have an atypical nephropathy presentation compared with the population with diabetes and without CF: One patient (patient 11) had typical features of KW in renal biopsy in the absence of previously known diabetes. In two other patients (patients 3 and 4), KW renal features contrasted with a well-controlled diabetes and the absence of diabetic nephropathy. These data, as well as the fact that patients with diabetes may present with nondiabetic nephropathy (patients 7, 8, 12, and 13), plead once again for large indications for renal biopsies in patients with CF, including those with diabetes. Characteristic KW nodular glomerulosclerosis has been rarely reported in patients with CF in the absence of abnormal glucose metabolism and may be related to the inflammatory cytokine profile present in patients with CF (17). This peculiar pathologic feature also was recently associated with hypertension, smoking, and metabolic syndrome (18).
Other types of glomerulopathy seen in our patients may be merely coincidental; however, IgA nephropathy was previously reported in patients with CF (19). Moreover, glomerular IgA deposits have been reported in the setting of Staphylococcus aureus infections (20), an increasingly recognized complication in patients with CF (21).
Interstitial chronic lesions seen in most of our patients may result from a prolonged exposure to nephrotoxic drugs, mainly antibiotics used at high dosages in case of pulmonary infections. For instance, repeated aminoglycoside use in pediatric patients with CF is associated with long-term renal damage. Careful monitoring of trough and peak serum drug levels are needed to ensure therapeutic adequacy and avoid nephrotoxicity (22).
Another input of kidney biopsy in patients with CF is the evaluation of the extent of renal fibrosis, particularly in view of lung transplantation. Pulmonary transplantation is a treatment option for end-stage CF, but it will have a deleterious impact on renal function. It was shown that GFR decreased by 50% at 1 mo after lung transplantation in patients with CF and might continue to deteriorate at a rate of 10 ml/min per yr (23). In a study by Lefaucheur et al. (24), accelerated renal function loss occurred in 32.5% of patients with CF after pulmonary transplantation and was correlated with the risk for ESRD. The histologic lesions differed according to the time of onset of renal deterioration: Early-onset episodes were associated with oxalate nephropathy and/or a pigmented tubulopathy, whereas late-onset episodes were due to arteriosclerosis and arteriolosclerosis. More than 90% of the reviewed biopsies disclosed typical features of calcineurin inhibitor nephrotoxicity.
As already stated, the estimation of GFR using creatinine is not a reliable estimation of renal function. This is illustrated by patients 3 and 12, in whom “normal-range” SCr level contrasted with extensive renal sclerotic lesions. Thus, the evaluation of renal sclerotic lesions proves to be of great help for clinicians who care for patients who have CF and awaiting lung transplantation. The presence of extensive renal sclerotic lesions urges clinicians to avoid nephrotoxic immunosuppressive agents (as in patient 13) and may lead to discussion of combined renal and lung transplantation (as in patients 9 and 13).
In all, the spectrum of renal diseases in adult patients with CF proves to be very heterogeneous, AA amyloidosis and diabetic nephropathy being the main types of nephropathies. Thus, indications for kidney biopsy should be large in patients with CF to determine the type of nephropathy and help clinicians in the treatment of these patients, particularly in the perspective of organ transplantation.
Disclosures
None.
Published online ahead of print. Publication date available at www.cjasn.org.
F.F.'s current affiliation is Molecular Genetics and Rheumatology Section, Faculty of Medicine, Imperial College, Hammersmith Campus, London, United Kingdom.
References
- 1.Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buchwald M, Tsui LC: Identification of the cystic fibrosis gene: Genetic analysis. Science 245: 1073–1080, 1989 [DOI] [PubMed] [Google Scholar]
- 2.Ratjen F, Döring G: Cystic fibrosis. Lancet 361: 681–689, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Dodge JA, Lewis PA, Stanton M, Wilsher J: Cystic fibrosis mortality and survival in the UK: 1947–2003. Eur Respir J 29: 522–526, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Jouret F, Bernard A, Hermans C, Dom G, Terryn S, Leal T, Lebecque P, Cassiman JJ, Scholte BJ, de Jonge HR, Courtoy PJ, Devuyst O: Cystic fibrosis is associated with a defect in apical receptor–mediated endocytosis in mouse and human kidney. J Am Soc Nephrol 18: 707–718, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Katz SM, Krueger LJ, Falkner B: Microscopic nephrocalcinosis in cystic fibrosis. N Engl J Med 319: 263–266, 1988 [DOI] [PubMed] [Google Scholar]
- 6.Mc Laughlin AM, Crotty TB, Egan JJ, Watson AJ, Gallagher CG: Amyloidosis in cystic fibrosis: A case series. J Cyst Fibros 5: 59–61, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Melzi ML, Costantini D, Giani M, Appiani AC, Giunta AM: Severe nephropathy in three adolescents with cystic fibrosis. Arch Intern Med 66: 1444–1447, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soriano E, Fischman D, Cheriyath P: Membranoproliferative glomerulonephritis in patients with cystic fibrosis: Coincidence or comorbidity? A case series. South Med J 101: 641–645, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Fakhouri F, Darré S, Droz D, Lemaire M, Nabarra B, Machet MC, Chauveau D, Lesavre P, Grünfeld JP, Noël LH, Knebelmann B: Mesangial IgG glomerulonephritis: A distinct type of primary glomerulonephritis. J Am Soc Nephrol 13: 379–387, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Mukherjee R, Whitehouse J, Honeybourne D: Renal impairment in cystic fibrosis. Thorax 63: 473, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Abramowsky CR, Swinehart GL: The nephropathy of cystic fibrosis: A human model of chronic nephrotoxicity. Hum Pathol 13: 934–939, 1982 [DOI] [PubMed] [Google Scholar]
- 12.McGlennen RC, Burke BA, Dehner LP: Systemic amyloidosis complicating cystic fibrosis: A retrospective pathologic study. Arch Pathol Lab Med 110: 879–884, 1986 [PubMed] [Google Scholar]
- 13.Sidhu H, Hoppe B, Hesse A, Tenbrock K, Bromme S, Rietschel E, Peck AB: Absence of Oxalobacter formigenes in cystic fibrosis patients: A risk factor for hyperoxaluria. Lancet 352: 1026–1029, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Lanng S, Hansen A, Thorsteinsson B, Nerup J, Koch C: Glucose tolerance in patients with cystic fibrosis: Five year prospective study. BMJ 311: 655–659, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Berg JM, Morton AM, Kok SW, Pijl H, Conway SP, Heijerman HG: Microvascular complications in patients with cystic fibrosis-related diabetes (CFRD). J Cyst Fibros 7: 515–519, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Schwarzenberg SJ, Thomas W, Olsen TW, Grover T, Walk D, Milla C, Moran A: Microvascular complications in cystic fibrosis-related diabetes. Diabetes Care 30: 1056–1061, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Westall GP, Binder J, Kotsimbos T, Topliss D, Thomson N, Dowling J, Wilson JW: Nodular glomerulosclerosis in cystic fibrosis mimics diabetic nephropathy. Nephron Clin Pract 96: c70–c75, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Li W, Verani RR: Idiopathic nodular glomerulosclerosis: A clinicopathologic study of 15 cases. Hum Pathol 39: 1771–1776, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Bhatt N, Bhatt N: IgA nephropathy in cystic fibrosis. Clin Nephrol 67: 403–404, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Satoskar AA, Nadasdy G, Plaza JA, Sedmak D, Shidham G, Hebert L, Nadasdy T: Staphylococcus infection-associated glomerulonephritis mimicking IgA nephropathy. Clin J Am Soc Nephrol 1: 1179–1186, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Dasenbrook EC, Merlo CA, Diener-West M, Lechtzin N, Boyle MP: Persistent methicillin-resistant Staphylococcus aureus and rate of FEV1 decline in cystic fibrosis. Am J Respir Crit Care Med 178: 814–821, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Sermet-Gaudelus I, Ferroni A, Gaillard JL, Silly C, Chretiennot C, Lenoir G, Berche P: Antibiotic therapy in cystic fibrosis: I. Pharmacologic specifics of antibiotics. Arch Pediatr 7: 519–528, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Broekroelofs J, Navis GJ, Stegeman CA, van der Bij W, de Boer WJ, de Zeeuw D, de Jong PE: Long-term renal outcome after lung transplantation is predicted by the 1-month postoperative renal function loss. Transplantation 69: 1624–1628, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Lefaucheur C, Nochy D, Amrein C, Chevalier P, Guillemain R, Cherif M, Jacquot C, Glotz D, Hill GS: Renal histopathological lesions after lung transplantation in patients with cystic fibrosis. Am J Transplant 8: 1901–1910, 2008 [DOI] [PubMed] [Google Scholar]