Abstract
Background and objectives: Relatively little is known about clinical outcomes, especially long-term outcomes, among patients who have chronic kidney disease (CKD) and experience superimposed acute renal failure (ARF; acute on chronic renal failure).
Design, setting, participants, & measurements: We tracked 39,805 members of an integrated health care delivery system in northern California who were hospitalized during 1996 through 2003 and had prehospitalization estimated GFR (eGFR) <45 ml/min per 1.73 m2. Superimposed ARF was defined as having both a peak inpatient serum creatinine greater than the last outpatient serum creatinine by ≥50% and receipt of acute dialysis.
Results: Overall, 26% of CKD patients who suffered superimposed ARF died during the index hospitalization. There was a high risk for developing ESRD within 30 d of hospital discharge that varied with preadmission renal function, being 42% among hospital survivors with baseline eGFR 30–44 ml/min per 1.73 m2 and 63% among hospital survivors with baseline eGFR 15–29 ml/min per 1.73 m2. Compared with patients who had CKD and did not experience superimposed ARF, those who did had a 30% higher long-term risk for death or ESRD.
Conclusions: In a large, community-based cohort of patients with CKD, an episode of superimposed dialysis-requiring ARF was associated with very high risk for nonrecovery of renal function. Dialysis-requiring ARF also seemed to be an independent risk factor for long-term risk for death or ESRD.
The clinical and public health importance of chronic kidney disease (CKD) is well established, with the most recent estimates indicating that the population prevalence of CKD exceeds 10% in the United States (1,2). Reduced estimated GFR (eGFR) is a strong and independent risk factor for hospitalization, cardiovascular events, and death (3). This association is most robust among people with eGFR <45 ml/min per 1.73 m2 (3).
Although CKD is a widely known risk factor for acute renal failure (ARF; also known as acute kidney injury [AKI]) (4,5), surprisingly little is known about clinical outcomes, especially long-term outcomes, among patients who have CKD and experience superimposed ARF (acute on chronic renal failure). Most published studies about ARF have focused exclusively on in-hospital outcomes with often scant, if any, follow-up after discharge. Furthermore, the few studies that did investigate long-term sequelae among survivors of ARF did not focus on patients with abnormal baseline kidney function (6–9).
We conducted this study to investigate outcomes after an episode of ARF among hospitalized patients with reduced baseline kidney function, defined as an eGFR <45 ml/min per 1.73 m2. Our end points included nonrecovery of renal function as well as death and development of ESRD that was treated with renal replacement therapy months to years after discharge.
Materials and Methods
Study Population
Kaiser Permanente of Northern California (Kaiser) is a large, integrated health care delivery system that currently insures more than 3.2 million individuals in the San Francisco Bay Area. Its population is representative of local surrounding and statewide populations with only slightly lower than population percentages at the extremes of the socioeconomic spectrum and age (10). Our database contains information on all adults (≥20 yr old) who were enrolled in Kaiser and had one or more outpatient determinations of serum creatinine between January 1, 1996, and December 31, 2003.
The study population consists of Kaiser members who were hospitalized between January 1, 1996, and December 31, 2003, and who had serum creatinine measured before hospitalization giving an eGFR <45 ml/min per 1.73 m2 by the abbreviated four-variable Modification of Diet in Renal Disease (MDRD) equation (11,12). The goal of our analysis was to compare outcomes among hospitalized patients who had eGFR <45 ml/min per 1.73 m2 and did and did not experience dialysis-requiring ARF.
Assessment of Baseline Serum Creatinine and eGFR
Measurement of serum creatinine by the Kaiser regional health plan laboratory had previously been calibrated against the MDRD core laboratory (3,13), which allowed for a more reliable estimate of GFR (14,15). Baseline kidney function was defined as the last outpatient eGFR before hospitalization, because inpatient serum creatinine measurements may not reflect usual kidney function (16,17).
In sensitivity analysis, because it is possible that the last observed outpatient serum creatinine value before hospitalization also reflects acute illness, baseline GFR was alternatively defined using the latest outpatient serum creatinine measurements >30 d before the day of admission after additionally excluding measurements that were performed in an emergency department. We also alternatively defined baseline GFR using the average of all (non–emergency department) measurements observed between 30 and 365 d before index hospitalization.
Identification of Dialysis-Requiring ARF
We focused on cases of dialysis-requiring acute on chronic renal failure, which is known to be associated with high rates of morbidity and mortality. Dialysis-requiring acute on chronic renal failure episodes were identified among hospitalized patients who had outpatient measures of serum creatinine before admission. Superimposed ARF was defined as having both a peak inpatient serum creatinine greater than the last observed preadmission outpatient serum creatinine by ≥50% and receipt of dialysis during hospitalization. International Classification of Diseases, Ninth Revision procedure codes 54.98 and 39.95 and Current Procedural Terminology codes 90935, 90937, 90945, 90947, and 90999 were used to identify episodes of acute peritoneal dialysis, hemodialysis, or hemofiltration from health plan hospital discharge databases.
All analyses excluded patients who received a previous kidney transplant or were on maintenance dialysis. We cross-linked our study population with the nationally comprehensive US Renal Data System (USRDS) registry. At the time of cross-linkage, USRDS data were complete through December 31, 2003. Supplementary determination of ESRD status was performed using data from an internal Kaiser ESRD registry (18).
For comparison, we identified patients who were also hospitalized between 1996 and 2003 and who had outpatient serum creatinine measured before admission and a baseline eGFR <45 ml/min per 1.73 m2 but did not experience superimposed ARF. Only the first hospitalization for each person during the study period was considered.
Assessment of Comorbid Conditions and Reasons for Hospitalization
Relevant coexisting conditions before index hospitalization were assessed as described previously using comprehensive health plan demographic, hospitalization, ambulatory visit, laboratory, prescription medication and diagnostic code data, including age, gender, race/ethnicity, diabetes, diagnosed hypertension, known proteinuria, coronary heart disease, stroke or transient ischemic attack, peripheral artery disease, chronic heart failure, dyslipidemia, chronic lung disease, chronic liver disease, cancer, hypoalbuminemia, and diagnosed dementia (3).
As was done in previous publications, we obtained primary International Classification of Diseases, Ninth Revision codes for each admission and used this to classify hospitalizations into “cardiovascular” (390 to 459), “respiratory” (460 to 519), “gastrointestinal” (520 to 579), “infectious disease” (001 to 139), “cancer” (140 to 239), and all others (19). In addition, for the subset of patients admitted from January 1, 2000, through December 31, 2003, we calculated the Laboratory-based Acute Physiology Score (LAPS) as a measure of severity of illness (20,21).
Assessment of ESRD and Death
As mentioned, ESRD was defined as receipt of maintenance dialysis or kidney transplantation and was ascertained using information from USRDS and an internal Kaiser ESRD treatment registry (18). We ascertained development of ESRD within 30 d of discharge among patients who had CKD and whose admission was or was not complicated by acute on chronic renal failure. We chose a 30-d window given the uncertainty inherent in the start date of ESRD therapy from both USRDS and Kaiser ESRD registries. Furthermore, progression to ESRD at the time of hospital discharge or any time within this 30-d window was judged to be of similar importance clinically.
Deaths through December 31, 2003, were identified from health plan administrative databases and member proxy reporting (3), Social Security Administration files (22), and the California Automated Mortality Linkage Information System (23). Mortality tracking using the last two methods continued even for people who disenrolled from the health plan.
Statistical Analysis
We determined the proportion of patients who died during the index hospitalization and, among survivors, the proportion who experienced ESRD within 30 d after hospital discharge. Our primary end point for the long-term postdischarge analysis was death or ESRD. We examined long-term renal replacement therapy–free survival rates among patients who were alive and dialysis independent at 30 d after hospital discharge using the Kaplan-Meier product limit estimate. We determined the independent association between acute on chronic renal failure and long-term risk for death or ESRD in a Cox model that adjusted for age, gender, race/ethnicity, preadmission eGFR, and the comorbid conditions described already. To reduce confounding, we had already matched both exposed (ARF) and unexposed (non-ARF) patients for hospitalization status as part of the study design. Patients were censored on December 31, 2003. We confirmed that proportional hazards assumption was not violated by comparing estimated log (−log[survivor function]) versus time (person-years) survivor curves. In a sensitivity analyses, we further adjusted for reasons for hospitalization and LAPS.
Although it seemed that the majority of ESRD cases after acute on chronic renal failure developed within 30 d of hospital discharge, we explored longer term effects of the ARF episode on risk for progression to ESRD. In this secondary analysis, death was considered a censoring event, and we adjusted for age, gender, race/ethnicity, preadmission eGFR, diabetes, diagnosed hypertension, and known proteinuria.
To attenuate confounding by differences in baseline kidney function of any observed association between ARF and subsequent risk for ESRD and death, we adjusted for baseline eGFR using finer categories (5-ml/min per 1.73 m2 increments). In a sensitivity analysis, we adjusted for baseline eGFR using cubic regression spline methods (with knots at 15 and 30 ml/min per 1.73 m2). Because the latter showed similar results, only results from the former analysis are shown.
Results
Patient Characteristics
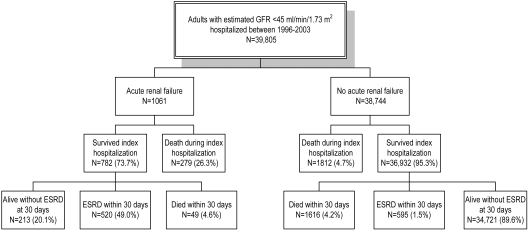
Among Kaiser members who had one or more outpatient serum creatinine levels measured from 1996 to 2003, we identified 39,805 who had CKD and were hospitalized with preadmission eGFR <45 ml/min per 1.73 m2. Among them, 1061 experienced superimposed ARF during the index hospitalization and 38,744 did not (Table 1, Figure 1). The median time lapse between last outpatient eGFR determination and index hospital admission was 17 d (interquartile range 3 to 56 d) for those who experienced superimposed ARF and 15 d (interquartile range 1 to 104 d) for those who did not.
Table 1.
Characteristics of patients who had prehospitalization eGFR <45 ml/min per 1.73 m2 and did and did not experience superimposed dialysis-requiring ARF during hospitalizationa
| Characteristic | Patients Who Had CKD and Experienced Superimposed ARF (n = 1061) | Patients Who Had CKD and Did not Experience Superimposed ARF (n = 38,744) |
|---|---|---|
| Age at hospitalization (yr; mean ± SD) | 66.6 ± 13.5 | 73.5 ± 12.9 |
| Women (n [%]) | 460 (43.4) | 22,915 (59.1) |
| Race/ethnicity | ||
| white/European | 611 (57.6) | 28,570 (73.7) |
| black/African American | 156 (14.7) | 2923 (7.5) |
| Hispanic | 104 (9.8) | 1856 (4.8) |
| Asian or Pacific Islander | 117 (11.0) | 2987 (7.7) |
| Native American | 10 (0.9) | 288 (0.7) |
| mixed or unknown | 63 (5.9) | 2120 (5.5) |
| Selected medical history (n [%]) | ||
| diabetes | 614 (57.9) | 10,517 (27.1) |
| diagnosed hypertension | 864 (81.4) | 26,993 (69.7) |
| known proteinuria | 749 (70.6) | 11,475 (29.6) |
| coronary heart disease | 532 (50.1) | 10,622 (27.4) |
| stroke or transient ischemic attack | 273 (25.7) | 6728 (17.4) |
| peripheral artery disease | 363 (34.2) | 5045 (13.0) |
| chronic heart failure | 603 (56.8) | 8414 (21.7) |
| dyslipidemia | 576 (54.3) | 13,602 (35.1) |
| chronic lung disease | 399 (37.6) | 10,059 (26.0) |
| chronic liver disease | 45 (4.2) | 816 (2.1) |
| cancer | 165 (15.6) | 6010 (15.5) |
| hypoalbuminemia | 628 (59.2) | 4990 (12.9) |
| diagnosed dementia | 31 (2.9) | 2447 (6.3) |
| Reasons for hospitalization (n [%]) | ||
| cardiovascular | 371 (35.0) | 8607 (22.2) |
| respiratory | 73 (6.9) | 2325 (6.0) |
| gastrointestinal | 40 (3.8) | 3687 (9.5) |
| infectious disease | 70 (6.6) | 965 (2.5) |
| cancer | 29 (2.7) | 2872 (7.4) |
| Laboratory test before index hospitalization | ||
| Serum creatinine (mg/dl; mean ± SD) | 3.31 ± 1.67 | 2.11 ± 1.42 |
| eGFR (ml/min per 1.73 m2; n [%]) | ||
| 30 to 44 | 294 (27.7) | 28,434 (73.4) |
| 15 to 19 | 476 (44.9) | 7763 (20.0) |
| <15 | 291 (27.4) | 2547 (6.6) |
P < 0.05 for all comparisons except for “cancer” medical history and “respiratory” reason for hospitalization. ARF, acute renal failure; eGFR, estimated GFR.
Figure 1.
Outcomes among 39,805 hospitalized patients with chronic kidney disease (CKD) and preadmission estimated GFR (eGFR) <45 ml/min per 1.73 m2 (acute renal failure [ARF] is defined ≥50% increase in serum creatinine and receipt of inpatient acute dialysis).
Validation of Exposure and Outcome Ascertainment
Overall, patients who had CKD and experienced ARF were more likely to have been hospitalized in the intensive care unit, received mechanical ventilation, experienced sepsis or hyperbilirubinemia, and undergone cardiac catheterization or surgery compared with patients who had CKD and did not experience ARF (24). Among the 1061 acute on chronic cases, a review of medical records among a random sample of 100 by a board-certified nephrologist (J.D.O.) confirmed that all had superimposed ARF and 99 received acute dialysis. In one instance, acute dialysis was initially planned but not performed, and the patient died. There were no observed cases of elective hospitalization for initiation of maintenance dialysis. This was consistent with the typical clinical practice of Kaiser nephrologists, whose general policy was to avoid hospitalizing patients for elective initiation of maintenance dialysis. The causes of ARF for these random 100 cases are shown in Table 2 and are comparable to those reported in previous studies (25) (causes were classified on the basis of a system used in the published literature [25]).
Table 2.
Cause of ARF by random chart review (N = 100)a
| Cause | n |
|---|---|
| Decreased renal perfusion (including volume contraction, congestive heart failure, hypotension, and cardiac arrest) | 76 |
| Medication related | 0 |
| Radiocontrast media | 6 |
| Postoperative | 6 |
| Sepsis | 21 |
| Other | 6 |
Total exceeds 100 because some cases had more than one contributing cause.
Among the 100 randomly selected individuals, we classified 46 patients as developing ESRD within 30 d of discharge. Additional chart review demonstrated that 44 of the 46 patients had confirmed ESRD treated with maintenance dialysis (positive predictive value 96%). Finally, of the 44 patients with confirmed ESRD, none recovered kidney function within 3 mo of initiation of dialysis by chart review.
Short-Term Patient and Kidney Survival
Among the 1061 patients who had CKD and experienced superimposed dialysis-requiring ARF, 279 (26%) died before hospital discharge. Of the 782 survivors, 520 developed ESRD within 30 d of hospital discharge, which translates into a rate of 49.0%—or 66.5% among survivors (Figure 1). By comparison, only 1.5% of patients who had CKD and did not experience ARF developed ESRD within 30 d of hospital discharge (Figure 1).
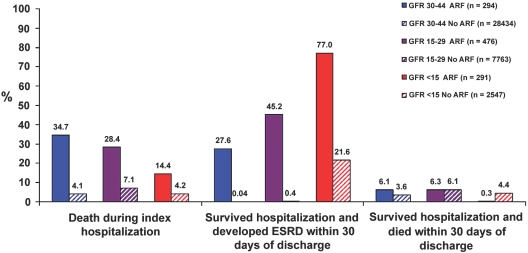
The risk for ESRD varied by level of baseline eGFR (Figure 2). For example, among those with baseline eGFR 30 to 44 ml/min per 1.73 m2, it was 27.6% (81 of 294 admitted patients with CKD), representing 42.2% among the 192 survivors of hospitalization. Among those with baseline eGFR 15 to 29 ml/min per 1.73 m2, it was 45.2% (215 of 476 admitted patients with CKD), representing 63.0% among the 341 survivors of hospitalization.
Figure 2.
Outcomes during and immediately after hospitalization for patients who had CKD and did or did not experience an episode of superimposed ARF, stratified by level of preadmission eGFR (all P < 0.01 for trend across GFR categories).
In sensitivity analysis, when baseline renal function was defined using latest outpatient serum creatinine >30 d before admission, the risk for ESRD was 31.0% for patients with eGFR 30 to 44 ml/min per 1.73 m2 and 54.1% for patients with eGFR 15 to 29 ml/min per 1.73 m2. When baseline renal function was defined using average of creatinine measurements between 30 and 365 d before hospitalization, risk for ESRD was 39.7% for patients with eGFR 30 to 44 ml/min per 1.73 m2 and 54.4% for patients with eGFR 15 to 29 ml/min per 1.73 m2.
Long-Term Patient and Kidney Survival
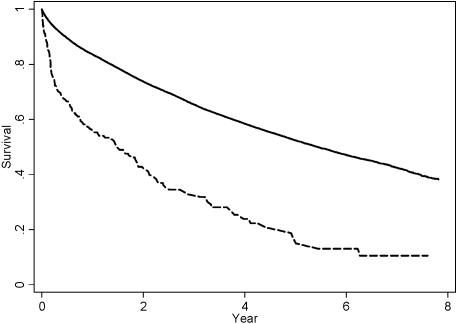
The 213 patients who developed acute on chronic renal failure and did not experience ESRD or death within 30 d of hospital discharge were compared with the 34,721 corresponding individuals who had ARF and survived and were dialysis-independent 30 d after hospital discharge (Figure 1). Among these 213 patients with acute on chronic renal failure, 12.7% developed ESRD and 19.7% died within 6 mo after hospital discharge. In comparison, among patients who had CKD and did not experience acute on chronic renal failure, the corresponding figures were 1.7 and 7.4%. Figure 3 shows the survival curves for the combined outcome of death or ESRD (ESRD-free survival) for patients who had CKD and did and did not experience superimposed ARF.
Figure 3.
Long-term ESRD-free survival (absence of death or ESRD) among patients who had CKD and who did not develop ESRD or death within 30 d of discharge from index hospitalization. Dashed line represents those who experienced superimposed ARF (n = 213); solid line represents those who did not (n = 34,721).
After adjustment for age, gender, race/ethnicity, preadmission eGFR, diabetes, diagnosed hypertension, known proteinuria, coronary heart disease, stroke or transient ischemic attack, peripheral artery disease, chronic heart failure, dyslipidemia, chronic lung disease, chronic liver disease, cancer, hypoalbuminemia, and diagnosed dementia, an episode of acute on chronic renal failure was associated with a 30% increase in long-term risk for death or ESRD (adjusted hazard ratio [HR]1.30; 95% confidence interval [CI] 1.04 to 1.64). Further adjustment for reasons for hospitalization did not materially alter the results (adjusted HR 1.36; 95% CI 1.08 to 1.72); neither did controlling for severity of illness using LAPS (adjusted HR 1.37; 95% CI 1.01 to 1.86).
In a secondary analysis that focused only on ESRD as outcome, after controlling for age, gender, race/ethnicity, preadmission eGFR, diabetes, diagnosed hypertension, and known proteinuria, acute on chronic renal failure was associated with a 47% increase in risk for ESRD, although this did not reach conventional threshold of statistical significance (adjusted HR 1.47; 95% CI 0.95 to 2.28). Similar results were seen in sensitivity analysis using alternative definitions of baseline renal function as describe previously.
Discussion
Our study provides novel data regarding the consequences of an episode of acute on chronic renal failure. We noted that a very large fraction of survivors of acute on chronic renal failure—more than one half—went on to require long-term dialysis within 30 d of discharge. Furthermore, acute on chronic renal failure was an independent risk factor for death or ESRD months to years after the index hospitalization. These findings highlight the importance of superimposed ARF episodes among patients with preexisting CKD.
The strengths of this study include the large and diverse patient sample, careful definition of prehospitalization CKD severity using outpatient determinations of serum creatinine (in contrast to “baseline” kidney function determined during the hospitalization, which may not truly reflect usual renal function) (19,25–27), calibration of serum creatinine with the MDRD laboratory, and adjustment for multiple potential confounders. We also performed a chart validation study of our approach to identify acute on chronic renal failure, which strengthened our confidence in the observed results.
The high rates of kidney function nonrecovery among patients who have CKD and experience superimposed ARF contrast sharply with observations made among patients without CKD (6–8). For example, Schiffl (9,28) followed 425 patients who had documented baseline serum creatinine ≤1.3 mg/dl and eGFR ≥90 ml/min per 1.73 m2 and experienced an episode of dialysis-requiring acute tubular necrosis. None of those who survived hospitalization remained dialysis dependent, and, in the subsequent year, only one patient progressed to ESRD. Although other case series of ARF outcome among patients without preexisting CKD reported higher rates of ESRD (e.g., 17% of survivors within 90 d of presentation [29]), the proportions are still much lower than what we observed in our cohort, which focused on patients with preexisting CKD.
The inpatient mortality risk in our cohort is lower that that observed in the Schiffl study (47%) (9) and others. This is consistent with the existing literature that de novo ARF is associated with higher short-term mortality than acute on chronic renal failure (30), perhaps because in the latter, a lesser degree of nephrotoxic and systemic insult is required for the patient to experience superimposed ARF that requires dialysis (31).
Previous studies of long-term outcomes after ARF did include patients with preexisting CKD; however, virtually all previous studies were limited by lack of a control (non-ARF) population (i.e., they were designed as case series and not as cohort studies) (32–36). They also often had limited generalizability as a result of the nature of the patients studied (e.g., only patients who were treated with continuous renal replacement therapies [32], only those who were in the intensive care unit [34,35]). Several studies did not gather data on long-term kidney function outcomes (37). Two articles that were based on elderly Medicare patients who were hospitalized for myocardial infarction reported that acute changes in serum creatinine during hospitalization were independently associated with higher future risk for ESRD and death (16,17). Neither of these studies addressed whether the ARF (or AKI) occurred against a background of normal or abnormal renal function. Residual confounding may explain the reported associations between very minor changes in serum creatinine (e.g., as small as 0.1 mg/dl) and adverse outcomes in the distant future (16). Our findings are consistent with a recently published study of Medicare beneficiaries (aged ≥67 yr) that showed that the risk for ESRD 2 yr after live hospital discharge was much higher among patients who had CKD and experienced superimposed ARF/AKI than patients who had CKD and did not (79.45 versus 19.88 cases per 1000 patients). CKD and AKI status were determined in that study using administrative diagnostic codes, and AKI cases that did or did not require dialysis both were combined in the analysis (38).
The results of our study should be interpreted in the context of the study design. We a priori designed our study to focus only on patients with eGFR <45 ml/min per 1.73 m2, because we wanted to be confident that our study population consisted of true CKD cases, recognizing that the MDRD equation, even with calibration of the serum creatinine measurement, may misclassify certain patients who have low eGFR in the 45- to 60-ml/min per 1.73 m2 range and may actually have near-normal kidney function (39). Another consideration is that the association between reduced eGFR and adverse outcomes is more robust among those with eGFR <45 ml/min per 1.73 m2 (3). We may have failed to identify individuals who developed ESRD defined as low GFR not compatible with life but who were not initiated on dialysis (e.g., because of advanced age, significant comorbidity, or personal preferences) and hence underestimated the true magnitude of risk associated with ARF. We were not able to define precisely the cause of ARF; however, we believe that it would be unlikely for reversible prerenal or postrenal causes of ARF to lead to initiation of acute dialysis, and most cases of severe hospital-acquired intrinsic ARF are due to acute tubular necrosis (40). We considered all forms of acute renal replacement therapy as “dialysis,” and we did not have information regarding details of treatment such as dialysis dosage. Among patients with advanced CKD, it may be difficult to distinguish between the final stages of progression to ESRD and potentially reversible superimposed ARF, because relatively small changes in GFR will result in large changes in serum creatinine, so observations among those with preadmission eGFR <15 ml/min per 1.73 m2 should be interpreted with caution; however, results of our medical records review validation study provided strong evidence that we did not erroneously include among cases patients who were electively admitted for initiation of long-term dialysis. Because most the cases of ESRD among this cohort occurred within 30 d of hospital discharge (including patients who never recovered sufficient kidney function to come off acute dialysis), power was limited in ascertaining the association between acute on chronic renal failure and long-term risk for ESRD, which is reflected in the larger CI in our secondary analysis. In our regression analyses, potential confounders such as diabetes were considered only as binary variables, so there may be residual confounding by severity of diabetes and other comorbidities. Extensive efforts were undertaken to adjust for potential confounding, but residual confounding is possible in any observational study. However, we emphasize that our most notable finding—that more than half of those who survived acute on chronic renal failure developed ESRD within 30 d of hospital discharge—is not dependent on completeness or correctness of any regression model. Although our study was conducted among an ethnically and socioeconomically diverse cohort, the results may not be completely generalizable to the uninsured or to people in other health care settings.
Conclusions
Among a large cohort of hospitalized individuals with CKD (eGFR <45 ml/min per 1.73 m2), we found that an episode of superimposed ARF was associated with clinically significant increases in the risks for short- and long-term sequelae, including death and ESRD. The risk of ARF and its subsequent complications should assume a secure place alongside the risks of cardiovascular disease, malnutrition, anemia, disorders of bone and mineral metabolism, and other important complications of CKD when considering its public health implications and priority areas for research in prevention and therapeutics.
Disclosures
None.
Acknowledgments
This work was supported by a grant (R01 DK67126) from the National Institute of Diabetes and Digestive and Kidney Diseases. The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
We thank Niela Pomernacki, RD, for expert technical assistance on the study.
Published online ahead of print. Publication date available at www.cjasn.org.
Some of the data reported here were supplied by the US Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G: National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med 139: 137–147, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Hou SH, Bushinsky DA, Wish JB, Cohen JJ, Harrington JT: Hospital-acquired renal insufficiency: A prospective study. Am J Med 74: 243–248, 1983 [DOI] [PubMed] [Google Scholar]
- 5.Leblanc M, Kellum JA, Gibney RT, Lieberthal W, Tumlin J, Mehta R: Risk factors for acute renal failure: Inherent and modifiable risks. Curr Opin Crit Care 11: 533–536, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Lowe KG: The late prognosis in acute tubular necrosis: An interim follow-up report on 14 patients. Lancet 1: 1086–1088, 1952 [DOI] [PubMed] [Google Scholar]
- 7.Finkenstaedt JT, Merrill JP: Renal function after recovery from acute renal failure. N Engl J Med 254: 1023–1026, 1956 [DOI] [PubMed] [Google Scholar]
- 8.Briggs JD, Kennedy AC, Young LN, Luke RG, Gray M: Renal function after acute tubular necrosis. BMJ 3: 513–516, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiffl H: Renal recovery from acute tubular necrosis requiring renal replacement therapy: A prospective study in critically ill patients. Nephrol Dial Transplant 21: 1248–1252, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Krieger N: Overcoming the absence of socioeconomic data in medical records: Validation and application of a census-based methodology. Am J Public Health 82: 703–710, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Greene T, Kusek JW, Beck GJ: A simplified equation to predict glomerular filtration rate from serum creatinine [Abstract]. J Am Soc Nephrol 11: 155A, 2000 [Google Scholar]
- 13.Landis JR, Gaughan C, Joffe M, CRIC Study Investigators: Inter-laboratory serum creatinine (sCr) calibration study [Abstract]. J Am Soc Nephrol 14: 294A, 2003 [Google Scholar]
- 14.Hsu CY, Chertow GM, Curhan GC: Methodological issues in studying the epidemiology of mild to moderate chronic renal insufficiency. Kidney Int 61: 1567–1576, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Coresh J, Astor BC, McQuillan G, Kusek J, Greene T, Van Lente F, Levey AS: Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis 39: 920–929, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Newsome BB, McClellan W, Coffey CS, Allison JJ, Kiefe CI, Warnock DG: Survival advantage of black patients with kidney disease after myocardial infarction. Clin J Am Soc Nephrol 1: 993–999, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Parikh CR, Coca SG, Wang Y, Masoudi FA, Krumholz HM: Long-term prognosis of acute kidney injury after acute myocardial infarction. Arch Intern Med 168: 987–995, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV: Ethnic disparities in diabetic complications in an insured population. JAMA 287: 2519–2527, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Escobar GJ, Greene JD, Scheirer P, Gardner MN, Draper D, Kipnis P: Risk-adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care 46: 232–239, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Hayward RA: Access to clinically-detailed patient information: A fundamental element for improving the efficiency and quality of healthcare. Med Care 46: 229–231, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Cowper DC, Kubal JD, Maynard C, Hynes DM: A primer and comparative review of major US mortality databases. Ann Epidemiol 12: 462–468, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Arellano MG, Petersen GR, Petitti DB, Smith RE: The California Automated Mortality Linkage System (CAMLIS). Am J Public Health 74: 1324–1330, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu CY, Ordonez JD, Chertow GM, Fan D, McCulloch CE, Go AS: The risk of acute renal failure in patients with chronic kidney disease. Kidney Int 74: 101–107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nash K, Hafeez A, Hou S: Hospital-acquired renal insufficiency. Am J Kidney Dis 39: 930–936, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Chertow GM, Christiansen CL, Cleary PD, Munro C, Lazarus JM: Prognostic stratification in critically ill patients with acute renal failure requiring dialysis. Arch Intern Med 155: 1505–1511, 1995 [PubMed] [Google Scholar]
- 27.Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP: A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol 16: 162–168, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Schiffl H, Fischer R: Five-year outcomes of severe acute kidney injury requiring renal replacement therapy. Nephrol Dial Transplant 23: 2235–2241, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Bhandari S, Turney JH: Survivors of acute renal failure who do not recover renal function. QJM 89: 415–421, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Mehta RL, Pascual MT, Soroko S, Savage BR, Himmelfarb J, Ikizler TA, Paganini EP, Chertow GM: Spectrum of acute renal failure in the intensive care unit: The PICARD experience. Kidney Int 66: 1613–1621, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Waikar SS, Liu KD, Chertow GM: Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol 3: 844–861, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Morgera S, Kraft AK, Siebert G, Luft FC, Neumayer HH: Long-term outcomes in acute renal failure patients treated with continuous renal replacement therapies. Am J Kidney Dis 40: 275–279, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Ahlstrom A, Tallgren M, Peltonen S, Rasanen P, Pettila V: Survival and quality of life of patients requiring acute renal replacement therapy. Intensive Care Med 31: 1222–1228, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Bagshaw SM, Laupland KB, Doig CJ, Mortis G, Fick GH, Mucenski M, Godinez-Luna T, Svenson LW, Rosenal T: Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: A population-based study. Crit Care 9: R700–R709, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lins RL, Elseviers MM, Daelemans R: Severity scoring and mortality 1 year after acute renal failure. Nephrol Dial Transplant 21: 1066–1068, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Liano F, Felipe C, Tenorio MT, Rivera M, Abraira V, Saez-de-Urturi JM, Ocana J, Fuentes C, Severiano S: Long-term outcome of acute tubular necrosis: A contribution to its natural history. Kidney Int 71: 679–686, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Korkeila M, Ruokonen E, Takala J: Costs of care, long-term prognosis and quality of life in patients requiring renal replacement therapy during intensive care. Intensive Care Med 26: 1824–1831, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ: Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20: 223–228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG: Using serum creatinine to estimate glomerular filtration rate: Accuracy in good health and in chronic kidney disease. Ann Intern Med 141: 929–937, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Lameire N, Van Biesen W, Vanholder R: Acute renal failure. Lancet 365: 417–430, 2005 [DOI] [PubMed] [Google Scholar]