Abstract
Glial cell line-derived neurotrophic factor (GDNF) affords neuroprotection in Parkinson’s disease in accordance with its ability to bolster nigrostriatal innervation. We previously found that GDNF facilitates dopamine release in a manner dependent on adenosine A2A receptor activation. Since motor dysfunction also involves modifications of striatal glutamatergic innervation, we now tested if GDNF and its receptor system, Ret (rearranged during transfection) and GFRα1 (GDNF family receptor alpha 1) controlled the cortico-striatal glutamatergic pathway in an A2A receptor-dependent manner. GDNF (10 ng/ml) enhanced (by ≈13%) glutamate release from rat striatal nerve endings, an effect potentiated (up to ≈ 30%) by the A2A receptor agonist CGS 21680 (10 nM) and prevented by the A2A receptor antagonist, SCH 58261 (50 nM). Triple immunocytochemical studies revealed that Ret and GFRα1 were located in 50% of rat striatal glutamatergic terminals (immunopositive for vesicular glutamate transporters-1/2), where they were found to be co-located with A2A receptors. Activation of the glutamatergic system upon in vivo electrical stimulation of the rat cortico-striatal input induced striatal Ret phosphoprylation that was prevented by pre-treatment with the A2A receptor antagonist, MSX-3 (3 mg/kg). The results provide the first functional and morphological evidence that GDNF controls cortico-striatal glutamatergic pathways in a manner largely dependent on the co-activation of adenosine A2A receptors.
Keywords: cortico-striatal pathway, adenosine, A2A receptor, GDNF, glutamate, Ret/GFRα1
Introduction
Striatal circuits play a crucial role in motor control. They are triggered by cortico-striatal inputs that are tightly controlled by nigrostriatal dopaminergic innervation (Gerfen, 2004). Imbalance of motor function can result either from dysfunction of the dopaminergic nigrostriatal system, the neurochemical hallmark of Parkinson’s disease (Dauer and Przedborski, 2003), as well as from dysfunction of the cortico-striatal glutamatergic inputs, as occurs in Huntington’s disease (Cepeda et al., 2007). Dysfunction of the glutamatergic system may be a general feature of neurodegenerative diseases (Lipton and Rosenberg, 1994) and, accordingly, is also documented to be present in Parkinson’s disease (Day et al., 2006). Thus, identifying modulation systems controlling the cortico-striatal glutamatergic input may open new avenues for the generation of novel drugs to manage these neurodegenerative motor diseases.
Adenosine A2A receptor antagonists are currently a promising non-dopaminergic anti-Parkinsonian therapy, which already underwent phase III clinical trials (LeWitt et al., 2008). Interestingly, the manipulation of A2A receptors also affords beneficial effects in animal models of Huntington’s disease (reviewed in Popoli et al., 2007). This indicates that A2A receptors play a pivotal control of striatal pathways possibly by direct control of its trigger, the cortico-striatal afferents (reviewed in Schiffmann et al., 2007). Another important role of A2A receptors is their ability to tightly control the action of different growth factors (Diódenes et al., 2004; Gomes et al., 2006; Mojsilovic-Petrovic et al., 2006; Pousinha et al., 2006; Diógenes et al., 2007; Wiese et al., 2007; Fontinha et al., 2008; Fernandes et al., 2008). Of particular relevance for the present work is our previous finding that A2A receptors in the striatum play an enabling role for the facilitation of the evoked release of dopamine by glial cell line-derived neurotrophic factor, GDNF (Gomes et al., 2006). The administration of GDNF also affords beneficial effects in animal models of Parkinson’s disease (e.g. Gash et al., 1996; Tomac et al., 1995) and humans (Patel et al., 2005), in accordance with its ability to preserve and regenerate the nigrostriatal dopaminergic system (Kramer et al., 2007;, reviewed in Hurelbrink and Barker, 2004). Interestingly, the administration of GDNF also affords beneficial effects in animal models of Huntington’s disease (reviewed in Alberch et al., 2004). This indicates that GDNF acts through broader mechanism of action to preserve striatal circuits from injury, which might also involve the control of glutamatergic inputs, a hypothesis that has not yet been experimentally tested. Thus, we now tested if GDNF could modulate the activity of the cortico-striatal system and the influence of A2A receptors upon GDNF actions.
Materials and Methods
Animals
Adult (6–8 weeks-old) rats (Wistar or Sprague-Dawley) were purchased from Harlam Interfauna Iberica, SL (Barcelona, Spain) or Charles River Laboratory (Wilmington, MA). The animals were handled according to the European guidelines, the Portuguese law and the guidelines of the Institutional Care and Use Committee of the Intramural Research Program, National Institute on Drug Abuse, NIH. In the in vitro studies, the animals were deeply anesthetized (no skin pinched reaction while still breathing) with halothane before decapitation. In the in vivo experiments, animals were kept anesthetized with equithesin during all surgical procedures.
In vitro glutamate release from rat striatal synaptosomes
The release of [3H]glutamate from rat striatal nerve terminals prepared using a combined sucrose/Percoll centrifugation protocol was performed according to previous reports (see e.g. Rodrigues et al., 2005). Briefly, striatal tissue was homogenized in a medium containing 0.32 M sucrose, 1 mM EDTA, 0.1% BSA (bovine serum albumine) and 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) (pH 7.4). The homogenate was spun for 10 min 3,000 × g at 4°C and the supernatant spun again at 14,000 × g for 12 min. The pellet (P2 fraction) was resuspended in 1 ml of Percoll 45% (v/v) in Krebs-HEPES-Ringer (KHR) medium (in mM: NaCl 140, EDTA 1, KCl 5, glucose 5 and HEPES 10, pH 7.4) and spun again at 14,000 × g for 2 min. Synaptosomes were then removed from the top layer, washed once with KHR medium and resuspended in a Krebs solution (in mM: NaCl 124, KCl 3, NaH2PO4 1.25, NaHCO3 25, MgSO4 2, CaCl2 2 and glucose 10), which was gassed with a 95% O2 and 5% CO2 mixture. The nerve terminals were equilibrated at 37°C for 10 min, loaded with 0.2 μM [3H]glutamate for 5 min at 37°C, washed, layered over Whatman GF/C filters and superfused (flow rate: 0.8 ml/min) with Krebs solution for 20 min before starting collection of the superfusate. The synaptosomes were stimulated with 20 mM K+ (isomolar substitution of NaCl by KCl in the Krebs solution) at 3 and 9 min after starting sample collection (S1 and S2) triggering a release of tritium in a Ca2+-dependent manner that is mostly [3H]glutamate, gauged by HPLC (Rodrigues et al., 2005). GDNF was added 7 min before S2 onwards and their effect quantified by the modification of S2/S1 ratio versus control (i.e. absence of GDNF). When testing the action of GDNF in the presence of other drugs (CGS 21680 and/or SCH 58261), these drugs were added to the perfusion 15 min before starting sample collection and remained in the bath up to the end of the experiment; hence, they were present both in S1 and S2, and neither drugs modified the S2/S1 ratio versus control. Radioactivity was expressed in terms of disintegrations per second per milligram of protein (Bq/mg) in each chamber (see Lopes et al., 2002). Values are presented as mean ± SEM from N observations, and the significance of the means was calculated by the Student’s t test. When comparing the effect of GDNF in more than two experimental conditions, one way analysis of variance (ANOVA) followed by Bonferroni’s test was used; P values of 0.05 or less were considered to represent significant differences. Whenever a statistically significant difference between the effects of GDNF under two different experimental conditions was found, the difference was confirmed by comparing the effect of GDNF under those experimental conditions in synaptosomes from the same animal.
Synaptic localization of Ret and GFRα1
To evaluate the synaptic localization of GDNF receptors, we begun by comparing the density of these two proteins in total membranes from the whole striatum and in membranes from purified striatal nerve terminals, a strategy previously used to allocate receptors to nerve terminals (Rebola et al., 2005). Total membranes from the striatum and Percoll-purified striatal synaptosomes were prepared as in previous reports (e.g. Rebola et al., 2005). Briefly, the two striata from one rat were homogenized at 4°C in sucrose solution (0.32 M) containing 50 mM Tris ((tris(hydroxymethyl)aminomethane)-HCl), 2 mM EGTA and 1 mM dithiothreitol, pH 7.6. The resulting homogenates were centrifuged at 3,000 g for 10 min at 4°C, the supernatants collected and centrifuged at 14,000 g for 20 min at 4°C. The pellet of one of the samples was taken as the total membrane fraction and was resuspended in SDS-PAGE (sodium-dodecyl sulphate polyacrylamide gel electrophoresis) buffer (see below) for Western blot analysis. The pellet of the other sample was resuspended in 1 ml of a 45% (v/v) Percoll solution made up in a Krebs solution (composition 140 mM NaCl, 5 mM KCl, 25 mM HEPES, 1 mM EDTA, 10 mM glucose, pH 7.4). After centrifugation at 14,000 g for 2 min at 4°C, the top layer was removed (synaptosomal fraction), washed in 1 ml Krebs solution and resuspended in 10 ml of incubation buffer. This mixture was centrifuged at 14,000 g for 20 min at 4°C and the pellet corresponded to the synaptosomes. The membranes were obtained by resuspension in SDS-PAGE buffer (see below) for Western blot analysis.
Subcellular fractionation of nerve terminals
The extrasynaptic, presynaptic active zone and postsynaptic fractions from rat striatal synaptosomes were separated as in previous reports (Rodrigues et al., 2005). We have previously confirmed that this subsynaptic fractionation method allows an over 90% effective separation of active zone (SNAP (synaptosome-associated protein) 25), postsynaptic density (PSD95) and non-active zone fraction (synaptophysin) markers and can be used to access the subsynaptic distribution of receptors (see Rodrigues et al., 2005). Briefly, striata from 10 rats were homogenized at 4 °C in 15 ml of isolation solution (0.32 M sucrose, 0.1 mM CaCl2, 1 mM MgCl2, 0.1 mM phenylmethylsulfonylfluoride, PMSF). The concentration of sucrose was raised to 1.25 M by addition of 75 ml of 2 M sucrose and 30 ml of 0.1 mM CaCl2 and the suspension divided into 10 ultracentrifuge tubes. The homogenate was overlaid with 8 ml 1.0 M sucrose, 0.1 mM CaCl2 and with 5 ml of homogenization solution and centrifuged at 100,000 g for 3 h at 4 °C. Synaptosomes were collected at the 1.25/1.0 M sucrose interface, diluted 1:10 in cold 0.32 M sucrose with 0.1 mM CaCl2 and pelleted (15,000 g for 30 min at 4 °C). Pellets were resuspended in 1 ml of 0.32 M sucrose with 0.1 mM CaCl2 and a small sample taken for gel electrophoresis. Then, synaptosomal suspension was diluted 1:10 in cold 0.1 mM CaCl2 and an equal volume of 2x solubilization buffer (2% Triton X-100, 40 mM Tris, pH 6.0) was added to the suspension. The membranes were incubated for 30 min on ice with mild agitation and the insoluble material (synaptic junctions) pelleted (40,000 g for 30 min at 4 °C). The supernatant (extrasynaptic fraction) was decanted and proteins precipitated with 6 volumes of acetone at 20 °C and recovered by centrifugation (18,000 g for 30 min at 15 °C). The synaptic junctions pellet was washed in pH 6.0 solubilization buffer, resuspended in 10 ml of 1% Triton X-100 and 20 mM Tris (pH 8.0), incubated for 30 min on ice with mild agitation, centrifuged (40,000 g for 30 min at 4 °C) and the supernatant (presynaptic active zone fraction) processed as above. PMSF (1 mM) was added to the suspension in all extraction steps. The pellets from the supernatants and the final insoluble pellet (postsynaptic density fraction) were solubilized in 5% SDS, the protein concentration determined and the samples added to a 1/6 volume of 6x SDS-PAGE sample buffer for Western blot analysis.
Western blot analysis
After determining the amount of protein, each sample was diluted with 5 volumes of SDS-PAGE buffer containing 30% (v/v) glycerol, 0.6 M dithiothreitol, 10% (w/v) SDS and 375 mM Tris-HCl pH 6.8, boiled at 95 °C for 5 min. These diluted samples and the pre-stained molecular weight markers (Amersham, GE Healthcare, Lisbon, Portugal) were separated by SDS-PAGE (10% with a 4% concentrating gel) under reducing conditions and electro-transferred to polyvinylidene difluoride membranes (0.45 μm, from Amersham) in the absence or presence (for phosphorylated proteins) of 2% polyvinylpyrrolidone (PVP) and 50 mM sodium fluoride. After blocking for 2 hours at room temperature with 5% milk in Tris-buffered saline, pH 7.6 containing 0.1% Tween 20 (TBS-T), the membranes were incubated overnight at 4°C with the different antibodies, namely: rabbit anti-Ret (1:500), rabbit anti-GFRα1 (1:100), rabbit anti-phosphorylated (Ser (serine)696) Ret (1:1000), mouse anti-SNAP25 (1:20,000), mouse anti-PSD (postsynaptic density)95 (1:100,000) or mouse anti-synaptophysin (1:20,000). After four washing periods for 10 min with TBS-T containing 0.5% milk, the membranes were incubated with either alkaline phosphatase-conjugated anti-rabbit (1:20,000) or anti-mouse secondary antibody (1:10,000) or horseradish peroxidase-conjugated secondary goat anti-rabbit antibody in TBS-T containing 1% milk during 90 min at room temperature. After five 10 min-washes in TBS-T with 0.5% milk the membranes were incubated with Enhanced Chemi-Fluorescence during five minutes or with Enhanced Chemi-Luminescence (Amersham Biosciences, Piscataway, NJ) before being analysed with a VersaDoc 3000 (Biorad, Hercules, CA). The membranes were always re-probed to confirm the amount of loaded protein by measuring the immunoreactivity against β-actin. Briefly, the membranes were first incubated for 30 min with 40% methanol, then for 1 hour at room temperature with a 0.1 M glycine (pH 7.2) solution and then blocked as previously described before incubation with mouse anti-β-actin antibody (1:20,000 dilution). The membranes were then washed and incubated with the secondary antibody as described. Statistical differences were analyzed by one-way ANOVA followed by Dunnett’s multiple comparisons test.
Immunocytochemical analysis of striatal nerve terminals
The double or triple labelling immunocytochemical analysis to quantify the localization of Ret and GFRα1 receptors in striatal nerve terminals or their co-localization with A2A receptor was performed essentially as in previous reports (e.g. Rodrigues et al., 2005). Briefly, striatal synaptosomes were obtained through a discontinuous Percoll gradient. This procedure for preparation of the synaptosomes is crucial to reduce the amount of postsynaptic density material. In fact, immunocytochemical analysis of the synaptosomes obtained with this discontinuous Percoll gradient showed that less than 1% of the synaptophysin-positive elements were labelled by an anti-PSD95 antibody (Rebola et al., 2005). Briefly, striatal tissue was homogenized in a medium containing 0.25 M sucrose and 5 mM TES (pH 7.4). The homogenate was spun for 3 min 2,000 g at 4°C and the supernatant spun again at 9,500 g for 13 min. Then, the pellets were re-suspended in 8 mL of 0.25 M sucrose and 5 mM TES (pH 7.4) and 2 mL were placed onto 3 mL of Percoll discontinuous gradients containing 0.32 M sucrose, 1 mM EDTA, 0.25 mM dithiothreitol and 3, 10, or 23% Percoll, pH 7.4. The gradients were centrifuged at 25,000 g for 11 min at 4°C. The synaptosomes were collected between the 10 and 23% Percoll bands, they were washed in 15 mL of HEPES buffered medium (140 mM NaCl, 5 mM KCl, 5 mM NaHCO3, 1.2 mM NaH2PO4, 1 mM MgCl2, 10 mM glucose, and 10 mM HEPES, pH 7.4) and recovered by centrifugation at 22,000 g for 11 min at 4°C. These striatal synaptosomes were placed onto coverslips previously coated with poly-L-lysine, fixed with 4% paraformaldehyde for 15 min and washed twice with PBS medium (140 mM NaCl, 3 mM KCl, 20 mM NaH2PO4, 15 mM KH2PO4, pH 7.4). The synaptosomes were permeabilized in PBS with 0.2% Triton X-100 for 10 min and then blocked for 1 h in PBS with 3% BSA and 5% normal rat serum. The synaptosomes were then washed twice with PBS and incubated for 1 h at room temperature with different mixtures of primary antibodies, namely: rabbit anti-Ret (1:100) or rabbit anti-GFRα1 (1:100) and mouse anti-synaptophysin (1:200) antibodies; anti-Ret or anti-GFRα1 and either guinea-pig anti-vesicular glutamate transporter type 1 (vGluT1, 1:1000), anti-vGluT2 (1:1000), mouse anti-tyrosine hydroxylase (TH, 1:500), guinea-pig anti-vesicular GABA transporter (vGAT, 1:1000) or guinea-pig anti-vesicular acetylcholine transporter (vAChT, 1:500) antibodies; anti-Ret or anti-GFRα1 together with goat anti-A2A receptor antibody (1:200) and either anti-vGluT1 and 2 or anti-TH antibodies; anti-A2A receptor antibody and either anti-TH or rat anti-dopamine transporter (DAT, 1:500) antibody. For the double immunocytochemical labelling, the synaptosomes were then washed three times with PBS with 3% BSA and incubated for 1 h at room temperature with AlexaFluor-598 (red)-labelled donkey anti-guinea pig or anti-rat or anti-goat or anti-mouse or anti-rat IgG antibodies (1:200 for each), carefully washed with PBS and then incubated for 1 hour at room temperature with AlexaFluor-488 (green)-labelled donkey anti-rabbit or anti-goat IgG antibodies (1:200 for each). For the triple immunocytochemical labelling, the synaptosomes were first incubated with AlexaFluor-488-labelled donkey anti-goat antibody (1:200) and then with a mixture of AlexaFluor-598-labelled donkey anti-mouse or anti-rat or anti-guinea-pig and AlxaFluor-350 (blue)-labelled goat anti-rabbit antibodies (1:200 for each), to avoid recognition of the goat anti-rabbit by the donkey anti-goat antibody (see 23). We confirmed that none of the secondary antibodies produced any signal in preparations to which the addition of the corresponding primary antibody was omitted. Most importantly, we confirmed that the individual signals in double-labelled fields are not enhanced over the signals under single-labelling conditions. After washing and mounting on slides with Prolong Antifade, the preparations were visualized in a Zeiss Axiovert 200 inverted fluorescence microscope equipped with a cooled CCD camera and analyzed with MetaFluor 4.0 software. Each coverslip (three to four per experiment) was analyzed by counting three different fields and in each field a minimum of 50 individualized elements. The values are presented as mean ± S.E.M. of N experiments (i.e. in preparation obtained from different rats).
In vivo electrical cortical stimulation
The animals were implanted unilaterally under Equithesin (NIDA Pharmacy, Baltimore, MD) anesthesia with bipolar stainless steel electrodes, 0.15 mm in diameter, (Plastics One, Roanoke, VA) into the orofacial area of the lateral agranular motor cortex (3 mm anterior, 3 mm lateral and4.2 mm below to bregma). The electrodes and a head holder (connected to a swivel during stimulation) were fixed on the skull with stainless steel screws and dental acrylic resin. Five days after surgery, rats were placed in individual bowl chambers and the implanted electrodes were attached to an electrical stimulator(Grass S88K; Grass Instruments Co., W. Warwick, RI). Ten min before cortical stimulation the animals were given an i.p. (intra-peritoneal) administration of either saline, the A2A receptor antagonist, MSX-3 (3 mg/kg) or the A1 receptor antagonist, CPT (4.8 mg/kg). The doses of the adenosine antagonists were previously shown to provide motor activation by selectively antagonizing A1 receptors (CPT) or A2A receptors (MSX-3) (Karcz-Kubicha et al., 2003). In these in vivo experiments the A2A receptor antagonist, MSX-3, was used instead of SCH 58261, which was used in vitro. This was because we had previously characterized SCH 58261 (but not MSX-3) in the same in vitro conditions and MSX-3 (but not SCH 58261) in the same in vivo conditions as those used in the present work. Both compounds have demonstrated their selectivity for A2A receptors at the concentration (SCH 58261, 50 nM) and dose (MSX-3, 3mg/kg i.p.) now used, as shown by us before (Ciruela et al., 2006; Karcz-Kubicha et al., 2003; Quiroz et al., 2006). After 10 min of habituation, biphasic current pulse trains(pulse 0.1 msec; 150–200 μA, 100 Hz, 160 msec trains repeating once per second) were delivered using two-coupled constant current units (Grass PSIU6; Grass Instruments Co.). The intensity was 150 μA for most cases or it was increased up to 200 μA, until small jaw movements were observed. The cases that failed to show visible somatic movements (less than 10%) were excluded from additional analysis. In no case did animals display evidence of seizure activity from the electrical stimulation. Stimulation was applied for 20 min and the animals were killed immediately after the stimulation offset. The brains were rapidly extracted, frozen in dry ice-cold isopentane, and stored at −80°C. Subsequently, unilateral tissue punches of the lateral striatum (16 gauge) at the AP (anterioposterior) level of bregma 0.0 were obtained from 1 mm-thick coronal sections cut in the cryostat at −20°C. We have previously shown that this is the striatal area with maximal ERK (extracellular signal-regulated kinases) 1/2 phosphorylation following cortical stimulation in the orofacial area (Quiroz et al., 2006). The rostral side of the coronal sections was localized approximately at bregma 0.5 mm and the caudal side at bregma −0.5 mm. The tissue punches were sonicated for 10–15 s in homogenization buffer (200 μL 1% SDS in deionized ultrapure sterile water).
Materials
2-[p-(2 carboxyethyl)phenethylamino]-5′-N-ethylcarboxamido adenosine (CGS 21680), 8-cyclopentyl-1,3-dimethyl xanthine (CPT ) and 3, 7-dihydro-8-[(1E)-2-(3-ethoxyphenyl)ethenyl]-7 methyl-3-[3-(phosphooxy)propyl-1-(2 propynil)-1H-purine-2,6-dione (MSX-3) and were from Sigma (St. Louis, MO), 7-(2-phenylethyl)-5-amino-2-(2-furyl)-pyrazolo-[4,3-e]-1,2,4 triazolol[1,5c]pyrimidine (SCH 58261) was a generous from S. Weiss (Vernalis, UK), glial cell line-derived neurotrophic factor (GDNF) was from Promega (Madison, WI) and [3H]glutamate (specific activity 50 Ci/mmol) was from Amersham (GE Heathcare, Lisbon, Portugal). CGS 21680 and SCH 58261 were made up in 5 and 10 mM stock solutions, respectively, in DMSO. GDNF stock solution was prepared in distilled water, at a final concentration of 0.1 mg/ml. CGS 21680 stock solutions were kept frozen at −20°C until use, while SCH 58261 aliquots were kept at 4°C until use. MSX-3 and CPT were dissolved in sterile saline (with a few drops of 0.1 N NaOH for MSX-3; final pH 7.4).
The antibodies used were as follows: goat anti-adenosine A2A receptor, rabbit anti-GFRα1 and rabbit anti-Ret antibodies (Santa Cruz Biotechnology, Santa Cruz CA), rabbit anti-phosphorylated Ret (at serine 696) antibody (Novus Biologicals, Litlleton CO), guinea-pig anti-vesicular glutamate transporters type 1 and type 2 (vGluT1,2), guinea-pig anti-vesicular acetylcholine transporter (vAChT), mouse anti-tyrosine hydroxilase (TH), rat anti-dopamine transporter (DAT) and mouse anti-PSD95 antibodies (Chemicon Europe, Southampton, UK), guinea-pig anti-vesicular GABA transporter (vGAT) antibody (Calbiochem, Darmstadt, Germany), mouse anti-SNAP25, mouse anti-synaptophysin and mouse anti-β-actin (Sigma-Aldrich Ibérica, Sintra, Portugal), alkaline phosphatase-labelled goat anti-rabbit or anti-mouse antibodies (Amersham, GE Healthcare, Lisbon, Portugal), AlexaFluor-598-labelled donkey anti-guinea pig, anti-rat, anti-goat and anti-mouse, AlexaFluor-488-labelled donkey anti-rabbit or anti-goat and AlexaFluor-350-labelled goat anti-rabbit antibodies (Molecular Probes, Alfagene, Lisbon, Portugal).
Results
GDNF facilitates glutamate release in an adenosine A2A receptor-dependent manner
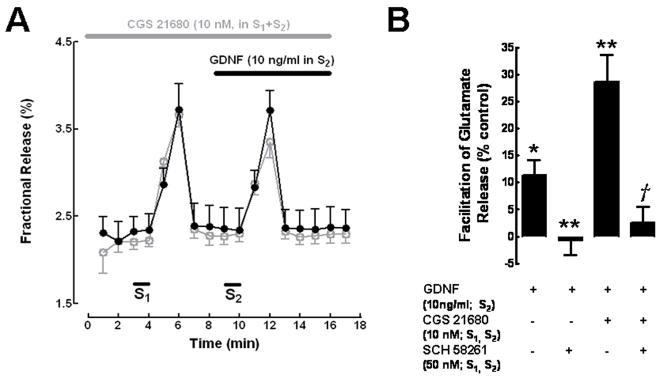
In striatal synaptosomes incubated with [3H]glutamate, the initial (first three collected samples) basal release of tritium was 2.72±0.05% of the total tritium incorporated in the synaptosomes (fractional release) (N=6). The first stimulation period (S1) caused a near 1.5 fold increase in the amount of tritium release; the second stimulation period (S2) caused a slightly smaller increase in tritium release than the first one, so the S2/S1 ratio obtained was 0.91±0.05 (N=6). To evaluate the action of GDNF upon glutamate release from synaptosomes, we used a concentration of GDNF (10 ng/ml) known to facilitate dopamine release from the same preparation (Gomes et al., 2006) as well as to increase dopamine levels and number of dopaminergic neurons in culture (Schatz et al., 1999). When GDNF (10 ng/ml) was present during S2, the S2/S1 ratio was increased to 1.1±0.08 (P<0.05 as compared with absence of GDNF in the same synaptosomal batch), corresponding to a 13.1±2.8% (N=5, P<0.05) enhancement of 3H-glutamate release (Fig. 1B).
Fig. 1.
GDNF enhancement of K+-evoked [3H]glutamate release from striatal synaptosomes is dependent on the activation of adenosine A2A receptors. (A) Averaged time course of [3H] glutamate release experiments. Striatal synaptosomes were labelled with [3H]glutamate as described in Methods. Release was evoked twice (S1 and S2) by chemical stimulation (20 mM KCl, 30 sec), as indicated by the bars above the abscissa. CGS 21680 was added 15 min before S1 onwards, and was present throughout all the experiment in both control and test conditions (indicated by the upper grey horizontal bar). GDNF was added to the test chambers 2 min before S2, as indicated by the upper black horizontal bar (filled circles in black), whereas it was not added to the parallel control chambers (open circles in grey). Each point represents the mean±SEM of the results obtained in 3 experiments performed in duplicate. (B) Blockade of the effect of GDNF (added before S2) by the A2A receptor antagonist, SCH 58268 (added before S1), and its potentiation by the A2A receptor agonist, CGS 21680 (added before S1). The effect of GDNF on the evoked release of glutamate was estimated by changes in the amount of [3H]glutamate release in S2 as referred to S1 (S2/S1 ratio). 0% represents the S2/S1 ratio in the absence of GDNF. Results are mean±SEM from 3–5 experiments performed in duplicate. *P<0.05 as compared with 0% (Students’ t test); **P<0.05 as compared with the effect of GDNF in the absence of other drugs (left column) (one-way ANOVA analysis followed by Bonferroni’s test); †P<0.05 as compared with the effect of GDNF in the presence of CGS 21680 (third column from left) (one-way ANOVA analysis followed by Bonferroni’s test). The amount of tritium released during S1 under the different conditions was not significantly different (P>0.05, one-way ANOVA analysis followed by Bonferroni’s test); the presence of CGS 21680 or SCH 58268 also did not significantly affect the S2/S1 ratio (P>0.05, one-way ANOVA analysis followed by Bonferroni’s test).
When studying the ability of GDNF to facilitate dopamine release (Gomes et al., 2006), we found that this effect was tightly controlled by the activation of adenosine A2A receptors. To evaluate whether activation of A2A receptors could influence the action of GDNF on glutamate release, experiments were designed where the selective A2A receptor antagonist, SCH58261 (50 nM) (Zocchi et al., 1996) or the selective agonist CGS 21680 (10 nM) (Jarvis et al., 1989), were present during S1 and S2 (see Methods). As illustrated in Fig. 1B, the GDNF-induced enhancement of dopamine release was abolished (P<0.05, n=3) when the A2A receptor antagonist was present. In two of these experiments the effect of GDNF in the absence of SCH58261 was alto tested in the same synaptosomal batch and the usual excitatory effect of GDNF (10 ng/ml) was observed. The abolishment of the effect of GDNF by the A2A receptor antagonist suggests that the effect of GDNF in striatal glutamatergic nerve endings requires tonic activation of adenosine A2A receptors. In the presence of CGS 21680, GDNF caused a marked increase in the S2/S1 ratio (Fig. 1A), corresponding to a facilitatory effect of 29±6.9% (N=3). This effect of GDNF was significantly (P<0.05) greater than that observed in the absence of CGS 21680 (Fig. 1B); the potentiation of the GDNF effect by CGS 21680 should be attributed to A2A receptor activation, since it was prevented by the selective (Zocchi et al., 1996) A2A receptor antagonist SCH 58261 (50 nM; Fig. 1B). The presence of CGS 21680 (10 nM) or SCH 58261 (50 nM) during S1 and S2 did not significantly modify the S2/S1 ratio (P>0.05). The amount of tritium released during S1 in the absence of any drug or in the presence of CGS 21680 (10 nM) or SCH 58261 (50 nM) was also not significantly different (P>0.05).
GDNF receptor proteins are located in striatal nerve terminals
The GDNF receptor is composed of a complex of different proteins, one that binds GDNF, GFRα1, and another, Ret, that once activated by the GDNF-GFRα1 complex, initiates a cascade of phosphorylation events, the first step being its auto-phosphorylation (Sariola and Saarma, 2003). As illustrated in Fig. 2, both Ret (Fig. 2A,B) and GFRα1 (Fig. 2A,C) immunoreactivity were present in synaptosomal membranes with a density similar to that found in total membranes from the whole striatum.
Fig. 2.
GDNF receptor protein (cRet and GFRα1) are located in synapses, namely in the presynaptic active zone of striatal nerve terminals. (A) Representative Western blots showing that both cRet and GFRα1 immunoreactivity is located in membranes from purified nerve terminals with a density similar to that found in total striatal membranes. This is quantified in panels (B) and (C), which are mean±SEM of 4 experiments. (D) Representative Western blots showing that cRet and GFRα1 immunoreactivity is mainly located in the presynaptic active zone and in the postsynaptic density, as evaluated upon subsynaptic fractionation of purified striatal nerve terminals into the presynaptic active zone (enriched in SNAP25), postsynaptic density (enriched in PSD95) and extra-synaptic fraction (enriched in synaptophysin); the last lane (SF) shows immunoreactivity in the synaptosomal fraction before subsynaptic fractionation. This is quantified in panel (E) and (F), which displays the mean±SEM of 5 experiments. (G) Representative double labelling immunocytochemical co-localization of the cRet and GFRα1 immunoreactivity with synaptophysin in striatal nerve terminals. As shown in (H), more than half of striatal nerve terminals (synaptophysin-immunopositive) were endowed with cRet and GFRα1 immunoreactivity (mean±SEM of 3–4 experiments).
Presynaptic modulation systems can either directly control the release of neurotransmitters or indirectly modify it through the control of the metabolism and/or viability of synaptic contacts. The former are expected to be located in the active zone (i.e. where neurotransmitter release takes place), where the later are not required to display such a confined localization. To gauge the possible relevance of the GDNF receptor system as a modulator of neurotransmitter release, we next investigated the sub-synaptic localization of Ret and GFRα1, in particular their localization in the active zone. As illustrated in Fig. 2D, both proteins were present in the active zone. Thus, when quantifying the relative density of Ret and GFRα1 immunoreactivity in the 3 sub-synaptic fractions (presynaptic active zone, postsynaptic density and extra-synaptic fractions) in 3 different assays from different groups of rats, it was found that Ret and GFRα1 immunoreactivity is mainly located in the presynaptic active zone fraction (34±5.5% and 35±7.3% of total immunoreactivity, N=5) and in the postsynaptic density (50±3.0% and 52±8.9% of total immunoreactivity, N=5) and has a significantly (P<0.05) lower relative abundance in the extrasynaptic fraction of nerve terminals (17±3.4% and 12±3.1% of total immunoreactivity, N=5) (Fig. 2E,F).
We then wanted to estimate if the proteins constituting the GDNF receptor were confined to a reduced number of nerve terminals or if most nerve terminals in the striatum were equipped with this modulation system. For that purpose, we carried out a double immunocytochemical labelling of Ret or GFRα1 with a marker of nerve terminals, synaptophysin, a protein located in synaptic vesicles. As illustrated in Fig. 2G, there was a co-localization of synaptophysin with either Ret or GFRα1. Thus, 56±2.3% (N=3) of the general population of striatal nerve terminals, identified as synaptophysin immunopositive elements were also endowed with Ret receptor component and 61±1.2% (N=4) of the referred elements are equipped with GFRα1 component of the receptor (Fig. 2H). Therefore, a high proportion of the nerve endings at the striatum possess GDNF receptor complex.
Mapping GDNF receptor components in different types of striatal nerve terminals
The striatum is a complex network of neuronal circuits, which are triggered by glutamatergic afferents, are mainly GABAergic in nature and are modulated by a main external dopaminergic nigrostriatal input and by cholinergic interneurons (Gerfen, 2004). To understand the role of the GDNF presynaptic modulation system, it is important to determine which type(s) of nerve terminals are equipped with the GDNF receptor system. To tackle this question, we carried out double immunocytochemical studies, looking at the co-localization of Ret or of GFRα1 immunoreactivity with markers of the different types of nerve terminals. As shown in Fig. 3A, only few GABAergic terminals (immunopositive for vesicular GABA transporters) were endowed with Ret (18±2.5%, Fig. 3B) or GFRα1 (21±1.2%, Fig. 3C) immunoreactivity (N=4). The percentage of cholinergic terminals (immunopositive for vesicular acetylcholine transporters) equipped with at least one of the GDNF receptor components is even lower (Ret: 4.7±2.7%; GFRα1: 5.3±3.1%, N=4, Fig. 3B,C). In contrast, numerous dopaminergic terminals (immunopositive for tyrosine hydroxylase) were immunopositive for GDNF receptor proteins (Ret: 61±1.6%; GFRα1: 56±0.3%, N=3–4, Fig. 3B,C). Likewise, a large percentage of striatal glutamatergic terminals (immunopositive for vesicular glutamate transporters 1 and 2) were immunopositive for GDNF receptor components (Ret: 45±2.4%; GFRα1:53±0.9%, N=3–4, Fig. 3B,C). These results show that the presynaptic GDNF receptor proteins are mainly located in dopaminergic and glutamatergic terminals, and are consistent with the ability of GDNF to enhance the release of dopamine (Gomes et al., 2006) and glutamate (present work) from striatal nerve endings.
Fig. 3.
GDNF receptor protein (cRet and GFRα1) are mainly located in dopaminergic and glutamatergic, rather than GABAergic and cholinergic, nerve terminals in the rat striatum. (A) Representative double labelling immunocytochemical co-localization of cRet (3 panels in the left of each row) or GFRα1 (3 panels in the right of each row) and different markers of different types of nerve terminals, namely tyrosine hydroxylase (TH, marker of dopaminergic neurons, first row), vesicular glutamate transporters types 1 and 2 (vGluT1/2, markers of glutamatergic terminals, second row), vesicular GABA transporter (vGAT, marker of GABAergic terminals, third row) and vesicular acetylcholine transporter (vAChT, marker of cholinergic terminals, last row). (B) and (C) display the average number of each type of terminals endowed with either cRET (B) or GFRα1 (C), showing that these proteins constituting the GDNF receptor are more abundantly located in dopaminergic and glutamatergic terminals of the rat striatum. The results are mean±SEM of 3–4 experiments.
GDNF and adenosine A2A receptor are co-located in striatal glutamatergic and dopaminergic nerve terminals
This tight control by A2A receptors of the effects of GDNF in both glutamatergic and dopaminergic terminals should require that both receptor system should be located in a subset of both glutamatergic and dopaminergic terminals, which we attempted to demonstrate using triple immunocytochemical analysis of the GDNF receptor proteins (Ret and GFRα1), A2A receptors and markers of either glutamatergic (vGluT1/2) or dopaminergic (TH) terminals.
As illustrated in Fig. 4A,B, 13±0.9% (N=3) of the vGluT1/2-positive striatal nerve terminals are simultaneously equipped with both A2A and Ret receptor and 9.4±1.6% (N=3) of the vGluT1/2 positive nerve terminals are equipped with both A2A and GFRα1 receptor. Likewise, we also found that 12±1.0% (N=3) of the TH-positive nerve terminals are equipped with both A2A and Ret receptor and that 11±0.4% (N=3) of the TH-positive nerve terminals are equipped with both A2A and GFRα1 receptor (Fig. 4C,D).
Fig. 4.
GDNF receptor protein (cRet and GFRα1) are co-located with adenosine A2A receptors in both glutamatergic and dopaminergic nerve terminals of the rat striatum. (A) Representative triple labelling immunocytochemical co-localization of adenosine A2A receptors and either cRet (upper row) or GFRα1 (lower row) in glutamatergic nerve terminals (immunopositive for vesicular glutamate transporters types 1 and 2, vGluT1/2), which is quantified in panel (B) (mean±SEM of 3 experiments). (C) Representative triple labelling immunocytochemical co-localization of adenosine A2A receptors and either cRet (upper row) or GFRα1 (lower row) in dopaminergic nerve terminals (immunopositive for tyrosine hydroxylase, TH), which is quantified in panel (D) (mean±SEM of 3 experiments). (E) Representative double labelling immunocytochemical co-localization of adenosine A2A receptors in dopaminergic terminals, labelled either with tyrosine hydroxylase (TH, upper row) or dopamine transporters (DAT, lower row) and either cRet (upper row). (F) displays the average number of each type of TH-or DAT-immunopositive terminals endowed with A2A receptors (mean±SEM of 5–6 experiments).
Since the localization of A2A receptors in dopaminergic terminals is frequently questioned (e.g. Svenningsson et al., 1999), we carried out a confirmatory double immunocytochemical studies using striatal synaptosomes to access the presence of A2A receptor in striatal dopaminergic nerve terminals [labelled with tyrosine hydroxylase (TH) or dopamine transporter (DAT) immunoreactivity]. We found that 21±4.3% (N=6) of the TH-immunopositive striatal nerve terminals were endowed with A2A receptor immunoreactivy and 22±1.1% (N=5) of the DAT-immunopositive striatal nerve terminals were endowed with A2A receptor immunoreactivity (Fig. 4E,F). This constitutes the first direct demonstration for the presence of A2A receptors in dopaminergic terminals in the striatum, reinforcing previous functional data showing that A2A receptors enable GDNF actions in striatal dopaminergic nerve endings (Gomes et al., 2006).
Cortical stimulation induces GDNF receptor phosphorylation in the striatum, an action that also requires A2A receptor tonic activation
It was previously reported (Quiroz et al., 2006) that stimulation in the orofacial area of the lateral agranular motor cortex induces phosphorylation of ERK1/2 and GluR1 (glutamate receptor 1) at Ser845 in the lateral caudate-putamen at the AP level of bregma 0.0±1.0 mm and, mostly relevant for the present work, this phosphorylation was dependent on A2A receptor activation. Western blot assays were therefore performed from punches of the lateral striatum after cortical electrical stimulation in vivo (demonstrated by the selective elicitation of jaw movements) to evaluate the possible induction of Ret phosphorylation. The dependency on A2A receptor activation was evaluated in a parallel group of animals where cortical stimulation was performed after administration of a selective A2A receptor antagonist, MSX-3 (3 mg/kg, i.p.).
Cortical stimulation in the orofacial area of the lateral agranular motor cortex induced an increase (near 40%) in Ret phosphorylation in the ipsilateral striatum compared to electrode-implanted non-stimulated controls (Fig. 5A, 1st and 2nd columns). In animals under MSX-3, cortical stimulation failed to induce Ret phosphorylation (Fig. 5A, 3rd and 4th columns). In contrast, previous systemic administration of the A1 receptor antagonist CPT (4.8 mg/kg, i.p.) did not counteract the influence of cortical stimulation upon Ret phosphorylation (Fig. 5A, 5th and 6th columns). As shown in Fig. 5B, total Ret levels were not significantly affected by cortical stimulation in any group of animals.
Fig. 5.
Cortico-striatal stimulation triggers the activation of GDNF receptors in an adenosine A2A receptor-dependent manner. (A) Striatal Ret phosphorylation induced by cortical electrical stimulation. The A2A receptor antagonist MSX-3, but not the A1 receptor antagonist CPT, counteracts Ret phosphorylation induced by cortical electrical stimulation (Ret molecular weight: 170 kDa). Results are shown in means ± SEM (N=6–8/group) of representative Western blots. *P<0.05 compared with the vehicle (Veh)-treated group (ANOVA with Dunnett’s post-hoc test); (B) Striatal total Ret after cortical electrical stimulation. No significant differences in the values of total Ret between the different sham and stimulated groups, with or without the A2A receptor antagonist MSX-3 or the A1 receptor antagonist CPT, were observed. (Ret molecular weight: 170 kDa). Results are shown in means ± SEM (N=5–7/group) of representative Western blots. *P<0.05 compared with the vehicle (Veh)-treated group (ANOVA with Dunnett’s post-hoc test).
Discussion
The main findings of the present work are: (a) GDNF-induced enhancement of glutamate release requires co-activation of A2A receptors; (b) GDNF receptor proteins are located in striatal nerve terminals, mainly glutamatergic and dopaminergic, and are co-located with adenosine A2A receptors; (c) in vivo stimulation of the cortical (glutamatergic) afferents triggers phosphorylation of the GDNF receptor protein, Ret, in the striatum, an effect that requires the tonic activation of A2A receptors.
These observations allow ascribing a novel role for GDNF in the striatum, acting on a short time scale as a presynaptic neuromodulator, apart from acting in a larger time scale as a trophic factor. In fact, we now report that GDNF directly controls the release of glutamate, in accordance with the localization of the GDNF receptor proteins in glutamatergic terminals. These GDNF receptor proteins are also located in dopaminergic terminals, supporting our previous report showing that GDNF also enhances the release of dopamine (Gomes et al., 2006). This ability of GDNF to modulate neurotransmitter release seems restricted to the control of glutamate and dopamine since only few GABAergic and cholinergic terminals were endowed with Ret/GFRα1 immunoreactivity. The observation that GDNF controls the two key transmitter systems involved in defining the firing pattern of striatal circuits has profound implications for our conception of the role of GDNF in striatal circuitry. This simultaneous ability of GDNF to presynaptically control the release of glutamate (the trigger of striatal circuits) as well as the release of dopamine (the main modulator defining the ON state of the targeted medium spiny neurons) predicts a powerful effect of GDNF on striatal plasticity, which is considered a key event both in physiological function of striatal circuits as well as major adapting and contributing factor in neurodegenerative diseases affecting striatal circuits (Calabresi et al., 2007).
The present observation that GDNF directly controls cortico-striatal synapses is also of interest for our current understanding of the mechanisms underlying the beneficial effects afforded by GDNF in neurodegenerative diseases affecting striatal circuits. In fact, it is usually conceived that the beneficial effects of GDNF in Parkinson’s disease result from its protective and/or restorative effects acting as a growth factor on nigrostriatal dopaminergic neurons (reviewed in Hurelbrink and Barker, 2004). Interestingly, GDNF is most effective in animal models of Parkinson’s disease when applied in the striatal parenchyma rather than in the nigra (reviewed in Hurelbrink and Barker, 2004), which is at odds with its expected direct effect on dopaminergic neurons. Furthermore, GDNF also affords beneficial effects in animal models of Huntington’s disease (reviewed in Alberch et al., 2004), where the control of the viability of dopaminergic neurons is not considered a prominent etiological factor. In contrast, the parallel beneficial role of GDNF in different neurodegenerative diseases affecting striatal circuits can be adequately explained by considering the direct effects of GDNF within the striatum and, according to the present data, in glutamatergic and dopaminergic terminals. In fact, there is increasing evidence that neurodegeneration begins with a synaptic dysfunction, which later evolves into an overt damage of neurons through a dying back mechanism (Coleman and Perry, 2002; Wishart et al., 2006). This has been documented in either Parkinson’s (reviewed in Dauer and Przedborski, 2003) or Huntington’s disease (Li et al., 2001; 2003). Thus, any synaptic modulation system able either to normalise, restore or prevent dysfunction of deregulated striatal synapses may potentially afford a protective effect in each of these neurodegenerative conditions.
Another major conclusion from this study is the re-enforcement of the importance of adenosine A2A receptors in the control of the effects operated by GDNF. In fact, we now observed that GDNF causes a minor effect on the release of glutamate, which was enabled upon activation of A2A receptors. Likewise, we previously reported that A2A receptor activation by endogenous adenosine is a pre-requisite for the ability of GDNF to facilitate striatal dopamine release, since the effect was fully lost upon A2A receptor blockade (Gomes et al., 2006). Furthermore, we now obtained direct morphological evidence to support this tight interaction between A2A and GDNF receptors, showing for the first time the co-location of GDNF and A2A receptors at both dopaminergic and glutamatergic terminals in the striatum. We also extended these in vitro approaches with in vivo studies further supporting the pivotal role of A2A receptors to enable the functioning of the modulation system operated by GDNF. Thus, we observed that the cortical stimulation of the orofacial area of the lateral agranular motor cortex increased Ret phosphorylation in the ipsilateral striatum compared to electrode-implanted non-stimulated controls, an effect counteracted by the selective A2A receptor antagonist MSX-3, but not by the A1 receptor antagonist CPT. These results are consistent with the hypothesis that stimulation of cortical afferents promotes activation of the effector of the GDNF receptor in the striatum, a process that requires co-activation of A2A receptors.
This functional interplay between A2A receptors and GDNF may have important physio-pathological consequences. The ability of the GNDF system to facilitate the release of glutamate and to become activated upon recruitment of the cortico-striatal pathway suggests that GDNF might be part of a positive feedback loop potentiating corticostriatal transmission. This loop is under tight control by adenosine A2A receptor, so that GDNF facilitates the release of transmitters, which in turn may result in enhanced synaptic activity and further facilitates activation of the GDNF receptor complex. Presynaptic A2A receptors localized in glutamatergic nerve terminals have been recently demonstrated to exert an important modulatory role of striatal glutamate release (Ciruela et al., 2006). The main source of adenosine that activates these presynaptic A2A receptors seems to be ATP which is co-released with glutamate (Ferré et al., 2005). A2A receptors localized in glutamatergic nerve terminals form heteromers with A1 receptors and function as a concentration-dependent switch, by which low concentrations of adenosine (by acting at A1 receptors) inhibit glutamate release and high concentrations of adenosine (by acting at A2A receptors, which shuts down A1 receptor signalling), facilitate glutamate release (Ferré et al., 2007). The present results indicate that GDNF provides an additional key component for the operation of the A1-A2A receptor heteromer. Whereas it is known that A2A receptors control synaptic plasticity in the basal ganglia (D’Alcantara et al., 2001), it remains to be defined if GDNF participates in synaptic plasticity changes at cortico-striatal synapses in an adenosine A2A receptor dependent manner. Likewise, the functional interaction between A2A receptors and GDNF to control the demise of neurodegenerative processes affecting striatal circuits should also be considered. In fact, both GDNF as well as antagonists of adenosine A2A receptors are currently pursued as promising anti-Parkinsonian novel therapies (Barker, 2006). Our present findings that the tonic activation of A2A receptors determines the activation and function of the GDNF modulation system, clearly point towards the need of further studies on the consequences of long-term therapy with A2A receptor blockers in neurodegenerative diseases where GDNF may have a beneficial role.
In summary, we now provide the first direct morphological evidence indicating that GDNF receptor proteins are located in striatal glutamatergic and dopaminergic nerve terminals and that these receptors are co-located with A2A receptors in a subset of these terminals. This supports the ability of GDNF to facilitate glutamate and dopamine release in a manner dependent on the activation of A2A receptors in both dopaminergic (Gomes et al., 2006) and glutamatergic nerve terminals (present results). Phosphorylation of Ret, the first step of the transducing signalling triggered by GDNF after binding to its receptor, also requires co-activation of A2A receptors, further suggesting that GDNF actions in the striatum are seriously impaired when A2A receptors are not free to be activated by its endogenous ligand. Altogether, these findings prompt a novel view on the role of GDNF in striatal circuits, where it fulfils a synaptic short term neuromodulation role apart from its long term role as a neurotrophic factor. Furthermore, the synaptic localization of the GDNF modulation system controlling glutamatergic synapses in a manner tightly controlled by A2A receptors opens a novel working hypothesis to understand the control of synaptic plasticity and neurodegeneration by these two receptor systems.
Acknowledgments
This work was supported by Fundacao para a Ciencia e para a Tecnologia, Portugal (work at Lisboa and Coimbra) and by the Intramural Research funds of the National Institute on Drug Abuse, NIH, USA and Faculdade de Medicina, Universidade de Lisboa, Portugal.
Abbreviations
- AP
anteroposterior
- BSA
bovine serum albumine
- CGS 21680
2-[p-(2 carboxyethyl)phenethylamino]-5′-N-ethylcarboxamido adenosine
- CPT
8-cyclopentyl-1,3-dimethylxanthine
- DAT
dopamine transporter
- ERK
extracellular signal-regulated kinases
- GDNF
Glial cell line-derived neurotrophic factor
- GFRα1
GDNF family receptor alpha 1
- GluR1
glutamate receptor subtype 1
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid
- i.p
intra-peritoneal
- KHR
Krebs-HEPES-Ringer
- MSX-3
3,7-dihydro-8-[(1E)-2-(3-ethoxyphenyl)ethenyl]-7 methyl-3-[3-(phosphooxy)propyl-1-(2 propynil)-1H-purine-2,6-dione
- PBS
phosphate buffer saline
- PMSF
phenylmethylsulfonylfluoride
- PVP
polyvinylpyrrolidone
- PSD
post-synaptic density
- Ret
rearranged during transfection
- SCH 5 8 2 6 1
7-(2-phenylethyl)-5-amino-2-(2-furyl)-pyrazolo-[4,3-e]-1,2,4 triazolol[1,5c]pyrimidine
- Ser
serine
- SDS-PAGE
sodium-dodecyl sulphate polyacrylamide gel electrophoresis
- SNAP
synaptosome-associated protein
- TBS
Tris-buffered saline
- Tris
tris(hydroxymethyl)aminomethane
- TH
tyrosine hydroxylase
- vAChT
vesicular acethylcholine transporter
- vGAT
vesicular GABA transporter
- vGlut1
vesicular glutamate transporter subtype 1
- vGlut2
vesicular glutamate transporter subtype 2
References
- Alberch J, Pérez-Navarro E, Canals JM. Neurotrophic factors in Huntington’s disease. Prog Brain Res. 2004;146:195–229. doi: 10.1016/s0079-6123(03)46014-7. [DOI] [PubMed] [Google Scholar]
- Barker RA. Continuing trials of GDNF in Parkinson’s disease. Lancet Neurol. 2006;5:285–286. doi: 10.1016/S1474-4422(06)70386-6. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30:211–219. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Wu N, André VM, Cummings DM, Levine MS. The corticostriatal pathway in Huntington’s disease. Prog Neurobiol. 2007;81:253–271. doi: 10.1016/j.pneurobio.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruela F, Casadó V, Rodrigues RJ, Lujan R, Burgueño J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, Cortés A, Canela EI, Lopez-Gimenez JF, Milligan G, Lluis C, Cunha RA, Ferré S, Franco R. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J Neurosci. 2006;26:2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MP, Perry VH. Axon pathology in neurological disease: a neglected therapeutic target. Trends Neurosci. 2002;25:532–537. doi: 10.1016/s0166-2236(02)02255-5. [DOI] [PubMed] [Google Scholar]
- D’Alcantara P, Ledent C, Swillens S, Schiffmann SN. Inactivation of adenosine A2A receptors impairs long term potentiation in the accumbens nucleus without altering basal synaptic transmission. Neuroscience. 2001;107:455–464. doi: 10.1016/s0306-4522(01)00372-4. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun Z, Sampson AR, Mugnaini E, Deutch AY, Sesack SR, Arbuthnott GW, Surmeier DJ. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nature Neurosci. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- Diógenes MJ, Fernandes CC, Sebastião AM, Ribeiro JA. Activation of adenosine A2A receptor facilitates brain-derived neurotrophic factor modulation of synaptic transmission in hippocampal slices. J Neurosci. 2004;24:2905–2913. doi: 10.1523/JNEUROSCI.4454-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diógenes MJ, Assaife-Lopes N, Pinto-Duarte A, Ribeiro JA, Sebastião AM. Influence of age on BDNF modulation of hippocampal synaptic transmission: interplay with adenosine A2A receptors. Hippocampus. 2007;17(7):577–85. doi: 10.1002/hipo.20294. [DOI] [PubMed] [Google Scholar]
- Fernandes CC, Pinto-Duarte A, Sebastião AM, Ribeiro JA. Postsynaptic action of brain-derived neurotrophic factor attenuates alpha7 nicotinic acetylcholine receptor-mediated responses in hippocampal interneurons. J Neurosci. 2008;28(21):5611–5618. doi: 10.1523/JNEUROSCI.5378-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Borycz J, Goldberg SR, Hope BT, Morales M, Lluis C, Franco R, Ciruela F, Cunha RA. Role of adenosine in the control of homosynaptic plasticity in striatal excitatory synapses. J Integr Neurosci. 2005;4:445–464. doi: 10.1142/s0219635205000987. [DOI] [PubMed] [Google Scholar]
- Ferré S, Ciruela F, Woods AS, Lluis C, Franco R. Functional relevance of neurotransmitter receptor heteromers in the central nervous system. Trends Neurosci. 2007;30:440–446. doi: 10.1016/j.tins.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Fontinha BM, Diógenes MJ, Ribeiro JA, Sebastião AM. Enhancement of long-term potentiation by brain-derived neurotrophic factor requires adenosine A2A receptor activation by endogenous adenosine. Neuropharmacology. 2008;54 (6):924–933. doi: 10.1016/j.neuropharm.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Gash DM, Zhang Z, Ovadia A, Cass WA, Yi A, Simmerman L, Russell D, Martin D. Functional recovery in parkinsonian monkeys treated with GDNF. Nature. 1996;380:252–255. doi: 10.1038/380252a0. [DOI] [PubMed] [Google Scholar]
- Gerfen GR. Basal ganglia. In: Paxinos G, editor. The Rat Nervous System. Elsevier Academic Press; Amsterdam: 2004. [Google Scholar]
- Gomes CA, Vaz SH, Ribeiro JA, Sebastião AM. Glial cell line-derived neurotrophic factor (GDNF) enhances dopamine release from striatal nerve endings in an adenosine A2A receptor-dependent manner. Brain Res. 2006;1113:129–136. doi: 10.1016/j.brainres.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Hurelbrink CB, Barker RA. The potential of GDNF as a treatment for Parkinson’s disease. Exp Neurol. 2004;185:1–6. doi: 10.1016/j.expneurol.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Jarvis MF, Schulz R, Hutchison AJ, Do UH, Sills MA, Williams M. [3H]CGS 21680, a selective A2 adenosine receptor agonist directly labels A2 receptor in rat brain. J Pharmacol Exp Ther. 1989;251:888–893. [PubMed] [Google Scholar]
- Karcz-Kubicha M, Quarta D, Hope BT, Antoniou K, Muller CE, Morales M, Schindler CW, Goldberg SR, Ferré S. Enabling role of adenosine A1 receptors in adenosine A2A receptor-mediated expression of c-fos. Eur J Neurosci. 2003;18:296–302. doi: 10.1046/j.1460-9568.2003.02747.x. [DOI] [PubMed] [Google Scholar]
- Kramer ER, Aron L, Ramakers GM, Seitz S, Zhuang X, Beyer K, Smidt MP, Klein R. Absence of Ret signaling in mice causes progressive and late degeneration of the nigrostriatal system. PLoS Biol. 2007;5:e39. doi: 10.1371/journal.pbio.0050039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeWitt PA, Guttman M, Tetrud JW, Tuite PJ, Mori A, Chaikin P, Sussman NM 6002-US-005 Study Group. Adenosine A2A receptor antagonist istradefylline (KW-6002) reduces “off” time in Parkinson’s disease: a double-blind, randomized, multicenter clinical trial (6002-US-005) Ann Neurol. 2008;63(3):295–302. doi: 10.1002/ana.21315. [DOI] [PubMed] [Google Scholar]
- Li H, Li SH, Yu ZX, Shelbourne P, Li XJ. Huntingtin aggregate-associated axonal degeneration is an early pathological event in Huntington’s disease mice. J Neurosci. 2001;21:8473–8481. doi: 10.1523/JNEUROSCI.21-21-08473.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Plomann M, Brundin P. Huntington’s disease: a synaptopathy? Trends Mol Med. 2003;9:414–420. doi: 10.1016/j.molmed.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- Lopes LV, Cunha RA, Kull B, Fredholm BB, Ribeiro JA. Adenosine A(2A) receptor facilitation of hippocampal synaptic transmission is dependent on tonic A(1) receptor inhibition. Neuroscience. 2002;112(2):319–29. doi: 10.1016/s0306-4522(02)00080-5. [DOI] [PubMed] [Google Scholar]
- Mojsilovic-Petrovic J, Jeong GB, Crocker A, Arneja A, David S, Russell DS, Kalb RG. Protecting motor neurons from toxic insult by antagonism of adenosine A2a and Trk receptors. J Neurosci. 2006;26:9250–9263. doi: 10.1523/JNEUROSCI.1856-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NK, Bunnage M, Plaha P, Svendsen CN, Heywood P, Gill SS. Intraputamenal infusion of glial cell line-derived neurotrophic factor in PD: a two-year outcome study. Ann Neurol. 2005;57:298–302. doi: 10.1002/ana.20374. [DOI] [PubMed] [Google Scholar]
- Popoli P, Blum D, Martire A, Ledent C, Ceruti S, Abbracchio MP. Functions, dysfunctions and possible therapeutic relevance of adenosine A2A receptors in Huntington’s disease. Prog Neurobiol. 2007;81:331–348. doi: 10.1016/j.pneurobio.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Pousinha PA, Diógenes MJ, Ribeiro JA, Sebastião AM. Triggering of BDNF facilitatory action on neuromuscular transmission by adenosine A2A receptors. Neurosci Lett. 2006;404 (1–2):143–7. doi: 10.1016/j.neulet.2006.05.036. [DOI] [PubMed] [Google Scholar]
- Quiroz C, Gomes C, Pak AC, Ribeiro JA, Goldberg SR, Hope BT, Ferré S. Blockade of adenosine A2A receptors prevents protein phosphorylation in the striatum induced by cortical stimulation. J Neurosci. 2006;26:10808–10812. doi: 10.1523/JNEUROSCI.1661-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebola N, Canas PM, Oliveira CR, Cunha RA. Different synaptic and subsynaptic localization of adenosine A2A receptors in the hippocampus and striatum of rat. Neuroscience. 2005;132:893–903. doi: 10.1016/j.neuroscience.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Rodrigues RJ, Alfaro TM, Rebola N, Oliveira CR, Cunha RA. Co-localization and functional interaction between adenosine A2A and metabotropic group 5 receptors in glutamatergic nerve terminals of the rat striatum. J Neurochem. 2005;92:433–441. doi: 10.1111/j.1471-4159.2004.02887.x. [DOI] [PubMed] [Google Scholar]
- Sariola H, Saarma M. Novel functions and signalling pathways for GDNF. J Cell Sci. 2003;116:3855–3862. doi: 10.1242/jcs.00786. [DOI] [PubMed] [Google Scholar]
- Schatz DS, Kaufmann WA, Saria A, Humpel C. Dopamine neurons in a simple GDNF-treated meso-striatal organotypic co-culture model. Exp Brain Res. 1999;127:270–278. doi: 10.1007/s002210050796. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Fisone G, Moresco R, Cunha RA, Ferre S. Adenosine A2A receptors and basal ganglia physiology. Progr Neurobiol. 2007;83:277–292. doi: 10.1016/j.pneurobio.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999;59:355–396. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Tomac A, Lindqvist E, Lin LF, Ogren SO, Young D, Hoffer BJ, Olson L. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995;373:335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- Wiese S, Jablonka S, Holtmann B, Orel N, Rajagopal R, Chao MV, Sendtner M. Adenosine receptor A2A-R contributes to motoneuron survival by transactivating the tyrosine kinase receptor TrkB. Proc Natl Acad Sci U S A. 2007;2007;104(43):17210–5. doi: 10.1073/pnas.0705267104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart TM, Parson SH, Gillingwater TH. Synaptic vulnerability in neurodegenerative disease. J Neuropathol Exp Neurol. 2006;65:733–739. doi: 10.1097/01.jnen.0000228202.35163.c4. [DOI] [PubMed] [Google Scholar]
- Zocchi C, Ongini E, Conti A, Monopoli A, Negretti A, Baraldi PG, Dionisotti S. The non-xanthine heterocyclic compound SCH 58261 is a new potent and selective A2A adenosine eceptor antagonist. J Pharmacol Exp Ther. 1996;276:398–404. [PubMed] [Google Scholar]