Abstract
Early blood-brain barrier (BBB) disruption, resulting from excessive neurovascular proteolysis by matrix metalloproteinases (MMPs), is closely associated with hemorrhagic transformation events in ischemic stroke. We have shown that normobaric hyperoxia (NBO) treatment reduces MMP-9 increase in the ischemic brain. The aim of this study was to determine whether NBO could attenuate MMP-9-mediated early BBB disruption following ischemic stroke. Rats were exposed to NBO (95% O2) or normoxia (30% O2) during 90-min middle cerebral artery occlusion, followed by 3-hr reperfusion. NBO-treated rats showed significant reduction in Evan’s blue extravasation in the ischemic hemisphere compared with normoxic rats. Topographically, Evan’s blue leakage was mainly seen in the subcortical regions including striatum, which was accompanied by increased gelatinolytic activity and reduced immunostaining for tight junction protein occludin. Increased gelatinolytic activities and occludin protein loss were also observed in isolated ischemic microvessels. Gel gelatin zymography identified that MMP-9 was the main enzymatic source in the cerebral microvessels. Incubation of brain slices or isolated microvessels with purified MMP-9 revealed specific degradation of occludin. Inhibition of MMP-9 by NBO or MMP-inhibitor BB1101 significantly reduced occludin protein loss in ischemic microvessels. These results suggest that NBO attenuates early BBB disruption, and inhibition of MMP-9-mediated occludin degradation is an important mechanism for this protection.
Keywords: blood-brain barrier, matrix metalloproteinases, oxygen, stroke
Introduction
Oxygen therapy for ischemic stroke has been investigated for many years. In the past several years, we and others have demonstrated that normobaric hyperoxia (NBO) treatment effectively reduces both infarct volume and neurological deficits in rodents following an ischemic stroke (Singhal et al. 2002a; Singhal et al. 2002b; Liu et al. 2004; Kim et al. 2005; Liu et al. 2006; Henninger et al. 2007; Shin et al. 2007). In human studies, NBO treatment was associated with improvements in clinical deficits and survival in selected patients with acute ischemic stroke (Singhal et al. 2005; Chiu et al. 2006). These findings have aroused enthusiasm for translating NBO therapy from experimental to clinic ischemic stroke. In addition, NBO has several distinct advantages as a stroke management: it is readily available, noninvasive, inexpensive, and can be initiated within minutes after stroke symptom onset, even by paramedics. Although some of the concerns about the potential risks associated with oxygen therapy in stroke, such as increased free radical generation (Singhal et al. 2002b; Kim et al. 2005; Liu et al. 2006) and vasoconstriction (Shin et al. 2007), have recently been addressed and were found not to be an issue, the molecular mechanism(s) for the neuroprotective effect of NBO treatment remains to be elucidated. Particularly, little is known about the impact of NBO on BBB integrity and cerebral hemorrhage, a major contributing factor to brain injury and mortality following ischemic stroke.
Matrix metalloproteinases (MMPs), especially gelatinases (MMP-2 and 9), are upregulated in cerebral ischemia and closely associated with BBB disruption (Rosenberg et al. 1998), edema formation (Pfefferkorn and Rosenberg 2003) and hemorrhagic transformation (Sumii and Lo 2002). Tight junctions are important structural components of the BBB, which span the apical region of the interendothelial clefts and restrict paracellular permeability (Wolburg and Lippoldt 2002). They are formed via complex interactions of cytoskeletal proteins and tight junction proteins, including claudins, occludin, zonula occludens and cingulin (Wolburg and Lippoldt 2002). Among these tight junction proteins, the transmembrane protein occludin is critically involved in sealing the tight junctions (Hirase et al. 1997; Lacaz-Vieira et al. 1999; Persidsky et al. 2006), and disruption of occludin alone is enough to cause functional changes of the tight junctions (Tavelin et al. 2003). Accumulating evidence indicates that hypoxia/ischemia increases BBB permeability by disrupting BBB tight junctions, for which MMPs play a pivotal role (Asahi et al. 2001; Date et al. 2006; Yang et al. 2007). Our previous results showed that NBO treatment reduced MMP-9 expression in the ischemic brain (Liu et al. 2006). Based on these observations, we hypothesized that NBO could protect BBB integrity by inhibiting MMP-mediated tight junction disruption after ischemic stroke.
In the present study, we investigated the effect of NBO on early BBB disruption using a rat model of 90-min middle cerebral artery occlusion (MCAO) with 3-hr reperfusion. Our results demonstrate that 90-min MCAO with 3-hr reperfusion induced Evan’s blue extravasation in the ischemic subcortical area in the normoxic rats, which was accompanied by increased gelatinolytic activity and occludin protein loss in the same brain region. NBO-treated rats showed significant reductions in Evan’s blue leakage, MMP-9 increase and occludin degradation in the ischemic BBB microvasculature. These findings suggest that NBO treatment attenuates early BBB disruption, and that inhibition of MMP-9-mediated degradation of the tight junction protein occludin is an important mechanism underlying the observed BBB protection.
Materials and Methods
Rat model of focal cerebral ischemia and reperfusion
The Laboratory Animal Care and Use Committee of the University of New Mexico approved all experimental protocols. Sprague – Dawley rats (Charles River Laboratories, Wilmington, MA, USA) weighing 290 to 320 g were anesthetized with isoflurane (4% for induction, 1.75% for maintenance) in N2O:O2 (70%:30%) during surgical procedures. Rats were anesthetized during the 90-min of ischemia. Body temperature was monitored continuously and maintained at 37.5°C ± 0.5°C using a heating pad.
Middle cerebral artery occlusion (MCAO) followed by reperfusion using the intraluminal model was conducted as previously described (Liu et al. 2006). Briefly, the external carotid artery (ECA), internal carotid artery (ICA) and pterygopalatine artery (PPA) of the ICA were exposed. A 4-0 silicone-coated monofilament nylon suture was inserted into the ICA via a cut on the ECA. The suture was advanced along the ICA to approximately 17 to 18 mm from the bifurcation. Reperfusion was produced by gently withdrawing the suture out of the ECA. For all MCAO animals included in this study (n = 54), successful MCAO was confirmed in vivo using a DRT4 laser Doppler flowmetry (Moor Instruments, Wilmington, NC, USA) as described in our earlier study (Liu et al. 2006).
The normoxic and NBO-treated rats received an inspiring gas mixture of 30% O2 + 70% N2 or 95% O2 + 5% CO2, respectively, during the 90-min ischemic period. In our earlier study, we found that rats breathing 95% O2 + 5% CO2 were able to maintain the ischemic penumbral tissue pO2 close to the preischemic level, and showed a relatively normal blood pH and breathing rhythm (Liu et al. 2006). Therefore, in this study, we chose 95% O2 + 5% CO2 as the NBO treatment. For the MMP inhibitor studies, BB1101 (Generous gift from A. Gearing, British Biotechnology) was delivered at a dose of 30 mg/kg body weight by intraperitoneal injection 10 minutes before MCAO started. In sham-operated animals (n = 8), the suture was inserted and advanced to the extent of 15 mm from the bifurcation, and then removed quickly, thereby avoiding ischemia. All the rats were euthanized at the end of 3-hr reperfusion, and brains removed for further analysis.
Brain tissue processing
Rats were sacrificed by decapitation at the end of the 3-hr reperfusion. Brains were quickly removed and chilled in ice-cold PBS for 5 minutes. Four consecutive 2-mm-thick coronary slices were sectioned from an 8-mm thick region 3 mm away from the tip of the frontal lobe, which contained the main infarction area according to our earlier studies with TTC staining (Liu et al. 2004; Liu et al. 2006). The meninges were carefully removed, and then a longitudinal cut was made 2 mm away from the midline between two hemispheres on each brain slice to exclude tissue primarily supplied by the anterior cerebral artery. Nonischemic and ischemic hemispheric tissue were then collected from each brain slice, and stored at −80°C until use.
Evaluation of BBB permeability by Evan’s blue leakage
Evan’s blue dye (2% wt/vol in PBS) was intravenously administered (3 ml/kg) via the tail vein at the start of the 3-hr reperfusion. At the end of reperfusion, the rats were transcardially perfused with 250 ml cold PBS to remove intravascular Evan’s blue dye. The brains were then removed and rapidly frozen in a −80°C freezer. 16 μm-thick cryosections were cut from the 8- mm thick brain region as mentioned above with a cryostat and mounted for confocal microscopy observation at an excitation wavelength of 542 nm and a 560-nm long-pass filter for collecting fluorescence emission (Zeiss LSM 510, Carl Zeiss Microlmaging, Thornwood, NY). BBB disruption was visualized as leakage of Evan’s blue, which appeared as red fluorescence on brain sections. Adjacent brain sections were subjected to in situ zymography analysis for MMP-2/9 activity or immunohistochemistry analysis for occludin expression, which would allow the colocalization of Evan’s blue leakage, enhanced MMP activity, and occludin degradation within the same brain region. The experimental procedures for in situ zymography and immunohistochemistry are described in detail below.
Quantitative evaluation of BBB disruption was achieved by measuring Evan’s blue content in the ischemic hemispheric tissue as reported (Singhal et al. 2002b). In brief, rat brains were quickly removed after transcardial perfusion with PBS. Nonischemic and ischemic hemispheric tissue were harvested as described above and homogenized in 50% wt/vol trichloroacetic acid (Sigma, St. Louis, MO, USA). After centrifugation, the supernatant was diluted fourfold with ethanol, and fluorescence intensity (ng/mL) was measured on a microplate fluorescence reader (FL600, Bio-Tek, Winooski, VT, USA). The total Evan’s blue content (ng) in each sample was derived from concentrations of external standards (100 – 1,000 ng/mL). The difference of dye content between ischemic and nonischemic hemispheric tissue was calculated as Evan’s blue leakage and expressed as per gram of brain tissue (ng/g).
Isolation of cerebral microvessels
Isolation of cerebral microvessels was performed as described previously (Kago et al. 2006) with modifications. The hemispheric brain tissue was minced and homogenized in 4 ml ice-cold PBS. The homogenate was filtered through a 41-μm nylon mesh (Spectrum, Irving, TX, USA), and the nylon mesh was washed three times with PBS. Microvessels retained on the mesh were then washed off and pelleted by centrifugation at 4000 g for 10 min at 4°C. The pellets were resuspended in 15% dextran T-500 and then added onto 20% dextran T-500, followed by centrifugation at 25,000g for 10 min at 4°C. The pellets were collected as the cerebral microvessels and stored at −80°C until further analysis. The purity of the microvessel preparation was verified and confirmed by light microscopy.
In situ zymography for measuring MMP-2/9 activities
The gelatinolytic activities of MMP-2/9 in isolated microvessels or cryosections from brain tissues injected with Evan’s blue were analyzed by in situ zymography using EnzCheck Collagenase Kit (Invitrogen, Carlsbad, CA, USA) following the manufacturer’sinstructions. Isolated microvessels were resuspended in PBS and allowed to attach to coverslips for 2 hrs at 37°C. Attached microvessels or brain sections were incubated for 2 hrs at 37°C in a reaction buffer containing 40 μg/ml of FITC-labeled DQ-gelatin. Gelatin-FITC is cleaved by gelatinases, yielding peptides whose fluorescence is representative of the net gelatinolytic activity. At the end of incubation, microvessels or brain sections were rinsed with PBS and then mounted on slides using Prolong Antifade Reagents (Invitrogen) for fluorescence microscopy (Olympus Optical Co. LTD). The specificity of this assay was confirmed by 1,10-phenanthroline, an MMP inhibitor provided in the kit. Reaction without DQ-gelatin did not reveal fluorescence.
Immunohistochemisty for assessing occludin protein degradation
Immunohistochemistry was performed to determine whether occludin protein degradation occurs concurrently with Evan’s blue leakage in the ischemic hemisphere. Sections from brain tissues injected with Evan’s blue were fixed with 4% paraformaldehyde in PBS. After rinsing with PBS, non-specific binding sites were blocked by preincubating tissue for 1 hr at room temperature in PBS containing 0.1% Triton X-100, 1% BSA and 5% goat serum. Sections were then incubated overnight with the primary antibody against occludin (1:500, Invitrogen) at 4° C in PBS-T (PBS plus 0.1% Tween-20) containing 0.5% BSA. Sections were washed with PBS-T and then incubated with FITC-conjugated goat anti-rabbit secondary antibody (Invitrogen) for 2 hrs at room temperature. After incubation, sections were rinsed and mounted in Prolong Antifade Reagents (Invitrogen). Slides were photographed for red fluorescence (Evan’ blue leakage) and green fluorescence (immunostaining for occludin) for each field with a fluorescent microscope (Olympus Optical Co. LTD, Japan). Sections incubated in the absence of the primary antibody were not immunoreactive.
Gelatin zymography for measuring MMP-9 in microvessels
The isolated cerebral microvessels were homogenized on ice in 20 μl TCNB buffer (in mmol/L: Tris-HCl 50, pH 7.5, CaCl2 10, NaCl 150, and 0.05% (w/v) Brij 35) with 0.1% Triton X-100 and proteinase inhibitor cocktail (Sigma). The protein concentration was determined using Bradford reagent (Bio-Rad, Hercules, CA, USA). MMP-2/9 were analyzed by gelatin zymography as previously described with modifications (Yang et al. 2007). 20 μg protein samples were separated on 10% SDS-polyacrylamide gels co-polymerized with 1 mg/ml gelatin (Sigma). Gels were washed in 2.5% Triton X-100 and then incubated for 72 hrs in a developing buffer (in mmol/L: Tris 50, pH 7.6, CaCl2 5, NaCl 0.2, and 0.02% (w/v) Brij-35) at 37°C before staining with Coomassie blue. Gels were destained until clear bands of gelatinolysis appeared. The intensities of gelatinolytic bands were analyzed using Kodak 4000 image station (Carestream Molecular Imaging, New Haven, CT, USA). A mixture of human MMP-2/9 (Chemicon, Temecula, CA, USA) was used as gelatinase standards.
In vitro occludin degradation by active MMP-9
In vitro occludin degradation was performed on cryosections or cerebral microvessels obtained from normal rat brains (n = 6). 16 μm-thick brain sections were cut from frozen normal rat brains as described above. Cerebral microvessels were homogenized on ice in TCNB buffer with 0.1% Triton X-100. The total homogenate was divided into several equal aliquots. Microvessel sample aliquots (10 μg protein) or cryosections were then incubated with or without 5 μg/ml human recombinant MMP-9 (R&D Systems, Minneapolis, MN, USA ) and 25 μmol/L MMP inhibitor GM6001 (Chemicon) for 2 hrs at 37°C. After incubation, MMP-9-mediated occludin degradation on cryosections was determined with immunohistochemistry as described above. Microvessel sample aliquots were subjected to western blot analysis for occludin. A decrease in the fluorescence intensity of immunostaining or in protein band intensity on western blot, following MMP-9 treatment reflected the proteolytic degradation of occludin.
Western blot analysis for occludin protein in microvessels
Cerebral microvessels were homogenized on ice in 30 μl RIPA buffer. Homogenates (10 μg of total protein) were boiled and then electrophoresed in 12% SDS-PAGE acrylamidegels, transferred onto nitrocellulose membranes (Bio-Rad), and incubated for 1 hr in TBS-T (Tris-buffered saline and 0.1% Tween20) containing 5% nonfat milk. Membranes were then incubated overnight at 4°C with primary antibody against occludin (Invitrogen) at a 1:1000 dilution, washed in TBS-T, and incubated for1 hr at room temperature with HRP-conjugated anti-rabbit antibody(1:1000; Santa Cruz Biotech). The membranes were developed with the SuperSignal West Pico HRP substrate kit (Pierce) and photographed on a Kodak 4000 image station (Carestream Molecular Imaging). To control sample loading and protein transfer, the membranes were stripped and reprobed with β-actin antibody (1:4000, Sigma).
Statistical analysis
The Data are presented as means ± SEM. Statistical analysis was carried out using student t-tests or ANOVA as indicated in the results. A value of P ≤ 0.05 was considered statistically significant.
RESULTS
Verification of successful MCAO
A total number of 54 rats subjected to MCAO showed significant reduction in regional cerebral blood flow during ischemia as monitored by laser Doppler flowmetry, with a value that dropped to 20.4 ± 3.8% of the preischemic level, which is consistent with our previous report (Liu et al. 2006). Moreover, at the end of the 3-hr reperfusion, all ischemic rats showed neurological deficits typical of MCAO, circling to the left (nonischemic side). Under the same experimental conditions, we have previously shown that NBO (95% O2) treatment increased blood PaO2 level, but did not significantly change other physiological variables including blood pH, blood PaCO2 and mean arterial blood pressure (Liu et al. 2006).
NBO attenuates early BBB disruption
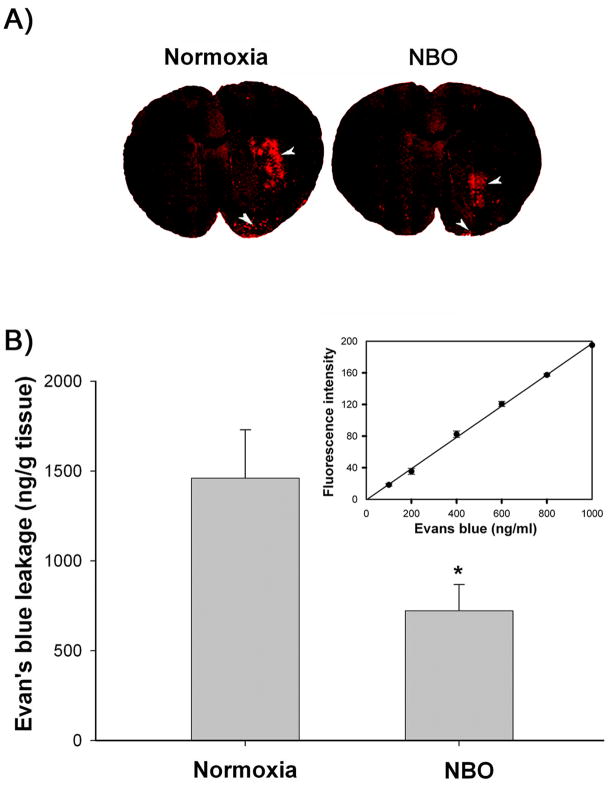
BBB disruption following 90-min ischemia and 3-hr reperfusion was assessed by measuring Evan’s blue extravasation, which on brain cryosections appeared as bright red fluorescence under a confocal microscope. As shown in Figure 1A, Evan’s blue leakage was clearly seen in the subcortical areas including the striatum of the ischemic hemisphere of the normoxic rats; there was also some leakage at the piriform cortex. In NBO-treated rats, the area of Evan’s blue leakage was markedly reduced. No dye extravasation was observed in the nonischemic hemisphere of both nomorxic and NBO-treated rats. We further quantified the extent of BBB leakage by measuring the difference of Evan’s blue contents between the ischemic and nonischemic hemispheric tissue. Analysis of Evan’s blue standards revealed a linear relationship between fluorescence intensity and dye concentration (Figure 1B, insert). Evan’s blue content in the brain tissue was calculated based on the standard curve and expressed as per gram of brain tissue (ng/g). As shown in Figure 1B, NBO-treated rats showed significantly less Evan’s blue leakage (722 ± 146) when compared with the normoxic rats (1461± 269, p<0.05, unpaired t-test). These results indicate that NBO treatment can attenuate early BBB disruption following 90-min MCAO with 3-hr reperfusion.
Figure 1.
NBO treatment significantly reduced Evan’s blue dye leakage into the ischemic brain after 90-min MCAO with 3-hr reperfusion. A) Representative confocal microscope images of brain cryosections from the normoxic and NBO-treated rats. Evan’s blue leakage (bright red fluorescence) was mainly seen in the subcortical area of the ischemic hemisphere (arrowhead), there was also some leakage at the piriform cortex (arrowhead). Experiments were repeated three times with similar results. B) Evan’s blue leakage in the brain tissue was quantified according to the external Evan’s blue standard curve which was generated by plotting the fluorescence intensity against the concentrations of Evan’s blue (Insert). Evan’s blue leakage was expressed as per gram of brain tissue (ng/g). Data are expressed as mean ± SEM (n = 7), *p < 0.05 versus the normoxic group.
Evan’s blue leakage is accompanied by occludin protein degradation and increased gelatinolytic activity
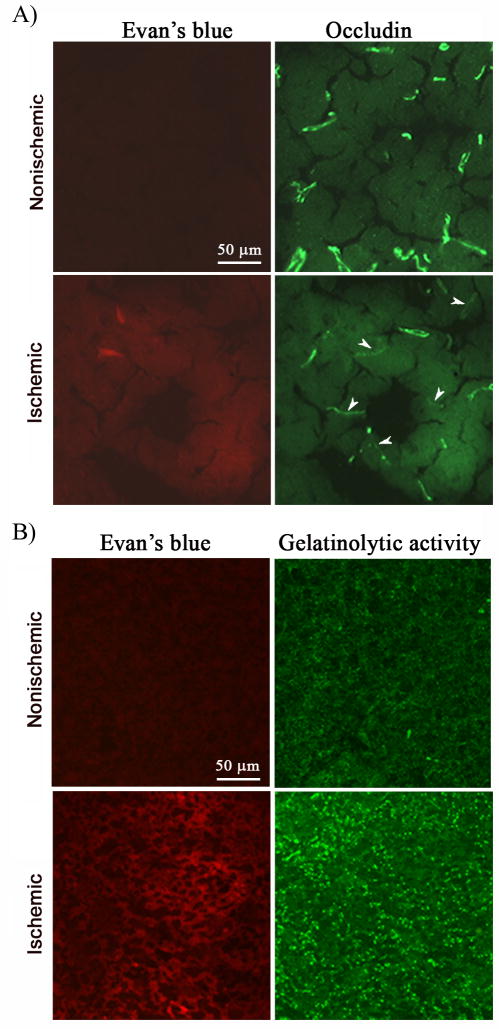
Tight junction protein occludin is considered a key molecule sealing the paracellular space between adjacent endothelial cells, and disruption of occludin alone is enough to increase tight junction permeability (Hirase et al. 1997; Lacaz-Vieira et al. 1999; Tavelin et al. 2003). To determine whether BBB disruption is associated with occludin protein degradation following ischemic stroke, we immunostained occludin protein on cryosections obtained from brain tissue injected with Evan’s blue. Microscopical photographs were taken from the corresponding subcortical areas of both hemispheres where Evan’s blue leakage was seen in the ischemic hemisphere, and the same field was photographed for both red fluorescence (Evan’ blue leakage) and green fluorescence (immunostaining for occludin). As shown in Figure 2A, immunostaining for occludin (green) was clearly seen on the microvessels of the nonischemic hemisphere, where no Evan’s leakage (red) was observed. In the ischemic hemisphere, Evan’s blue leakage was accompanied by reduced occludin staining on the microvessels, suggesting a strong association between occludin protein degradation and BBB disruption in the ischemic brain.
Figure 2.
Evan’s blue leakage was accompanied by occludin protein degradation and increased gelatinolytic activity in the ischemic brain of the normoxic rats after 90-min MCAO and 3-hr reperfusion. Occludin protein and gelatinolytic activity of MMP-2/9 were analyzed by immunohistochemistry and in situ zymography, respectively, on cryosections obtained from brain tissue injected with Evan’s blue. A) Immunostaining for occludin (green) was clearly seen on the microvessels of the nonischemic hemisphere, where no Evan’s leakage (red) was observed. In the ischemic hemisphere, Evan’s blue leakage was accompanied by reduced occludin staining on the microvessels. Arrowheads indicate cerebral microvessels with reduced occludin staining. Experiments were repeated three times with similar results. B) In situ zymography showed increased gelatinolytic activity of MMP-2/9 in the ischemic hemisphere (bright green fluorescence), where Evan’s blue leakage concurrently occurred. No Evan’s blue leakage and weak gelatinolytic activity were seen in the corresponding region of the nonischemic hemisphere. Experiments were repeated three times with similar results.
Proteolytic degradation of the BBB structural components by MMP-2/9 is an important mechanism contributing to BBB disruption in ischemic stroke (Rosenberg et al. 1998). To determine whether enhanced MMP-2/9 activity was co-localized with Evan’s blue leakage in the ischemic brain, we performed in situ zymography on cryosections obtained from brain tissue injected with Evan’s blue, in which gelatin-FITC is cleaved by MMP-2/9, yielding green fluorescent peptides. As shown in Figure 2B, increased gelatinolytic activity of MMP-2/9 was seen on the ischemic hemisphere, where Evan’s blue leakage occurred concurrently at the same location. As expected, no Evan’s blue leakage and weak gelatinolytic activity were seen in the corresponding region of the nonischemic hemisphere. These results suggest that enhanced MMP-2/9 activity may disrupt the tight junction protein occludin, leading to BBB opening in the ischemic brain. It needs to be pointed out that, in order to obtain both information on the same cryosection, the experimental procedures of in situ zymography and immunohistochemisty significantly decreased the fluorescence intensity of Evan’s blue. Obviously, immunohistochemistry which is more complicated and needs longer incubation reduced Evan’s blue fluorescence to a greater extent than in situ zymography because, although they were adjacent sections, much weaker Evan’s blue fluorescence was seen on sections subjected to immunohistochemistry analysis (Figure 2A and 2B).
Gelatinolytic activity is also increased in isolated ischemic hemispheric microvessels
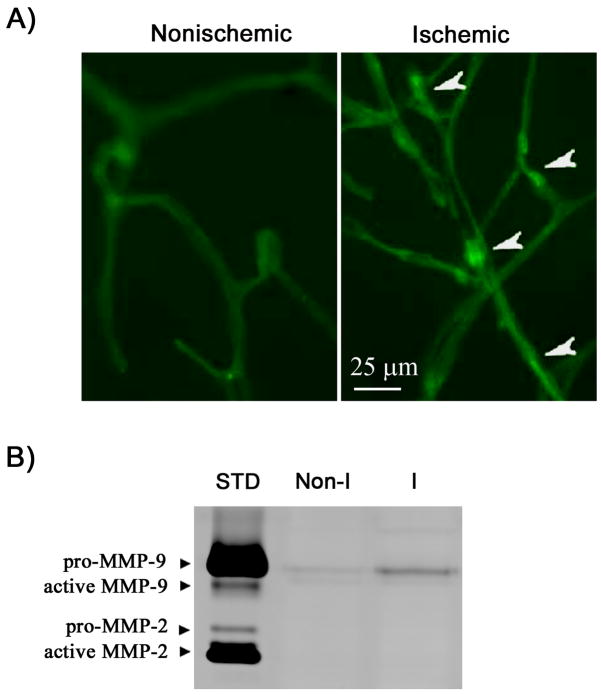
MMP-2/9 are large molecules, which restricts their proteolysis mainly to the immediate pericellular environment (Yong et al. 1998). In this context, BBB microvasculature derived MMP-2/9 would be more accessible to the barrier structural components, such as tight junction proteins, leading to their degradation. Therefore, we further examined the gelatinolytic activity of MMP-2/9 on isolated cerebral microvessels. As shown in Figure 3A, increased gelatinolytic activities (brighter or condensed fluorescence indicated with arrowheads) were seen in the microvessels isolated from the ischemic hemisphere. Nonischemic hemispheric microvessels showed much weaker staining of green fluorescence. Since in situ zymography cannot distinguish MMP-2 and MMP-9, we performed gel gelatin zymography to identify the source of gelatinolytic activity in the microvessels. As shown in Figure 3B, gelatin zymograms revealed only the pro-form of MMP-9, while no MMP-2 was detected under our experimental conditions. Moreover, consistent with the in situ zymography results, ischemia and reperfusion induced an upregulation of MMP-9 in the ischemic hemispheric microvessels (Figure 3B).
Figure 3.
MMP-9 induction in the ischemic cerebral microvessels isolated from the normoxic rats after 90-min MCAO with 3-hr reperfusion. A) In situ zymography showing gelatinolytic activity of MMP-2/9 in isolated nonischemic and ischemic hemispheric microvessels. Increased gelatinolytic activities in the ischemic microvessels are shown by brighter or condensed fluorescence (arrowhead). B) Representative gelatin zymogram showing the expression of MMP-2/9 in nonischemic (Non-I) and ischemic (I) microvessels. 92 kDa proform of MMP-9 was increased in the ischemic hemispheric microvessels, while no MMP-2 was observed. STD is a mixture of standard MMP-2/9.
In vitro degradation of occludin protein by MMP-9
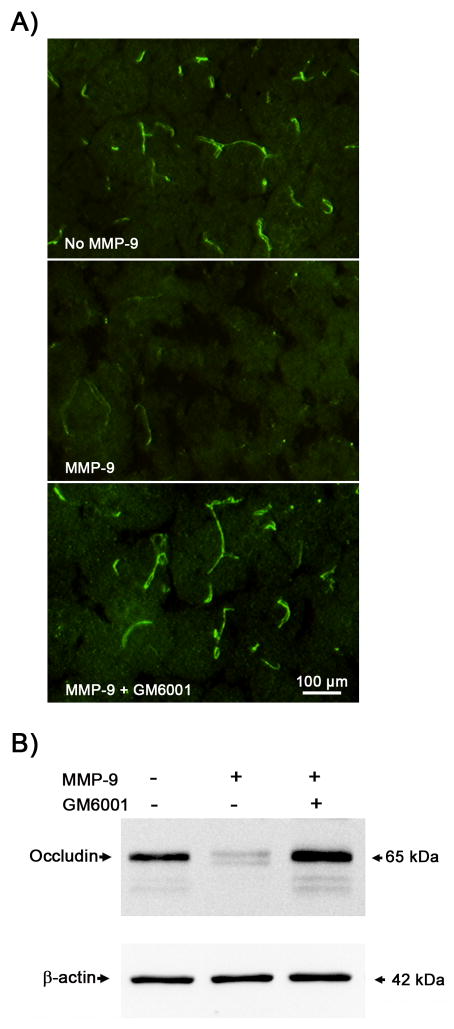
Our above results showed that enhanced gelatinolytic activity co-existed with occludin protein loss at the same brain tissue location where the BBB was disrupted (Figure 2A and 2B). This result implies a strong association between MMP increase and occludin protein degradation in the ischemic brain. To further establish the causal relationship between them, we performed in vitro occludin degradation by incubating brain cryosections or microvessel extracts with or without purified MMP-9 (5 μg/ml) and MMP inhibitor GM6001 (25 μmol/L) at 37°C for 2 hours. After incubation, MMP-mediated occludin degradation in brain sections and microvessel extracts was determined by immunohistochemistry and western blot, respectively. As expected, the immunostaining of occludin almost completely disappeared after incubation with MMP-9, which was fully reversed by GM6001 (Figure 4A). MMP-dependent occludin degradation was also observed in microvessel extracts incubated with MMP-9. Figure 4B showed that the protein band of occludin almost disappeared after incubation with MMP-9 and GM6001 completely blocked this degradation. As expected, MMP-9 had no effect on β-actin. These results indicate that tight junction protein occludin is a substrate of MMP-9, and MMP-9-mediated occludin degradation may represent an important mechanism for BBB disruption in ischemic stroke.
Figure 4. In vitro.
degradation of occludin protein by MMP-9. Brain cryosections or cerebral microvessel extracts obtained from normal rat brains were incubated with or without purified MMP-9 (5 μg/ml) and MMP inhibitor GM6001 (25 μmol/L) at 37°C for 2 hrs. After incubation, MMP-mediated occludin degradation in brain sections and microvessel extracts was determined by immunohistochemistry and western blot, respectively. A) The immunostaining of occludin (green) in the brain tissue almost completely disappeared after incubation with MMP-9, which was completely reversed by GM6001. Experiments were repeated three times with similar results. B) Representative western blots showed similar MMP-dependent occludin degradation in cerebral microvessel extracts. Aliquots (10 μg) of cerebral microvessel lysates were treated as indicated before they were subjected to western blot analysis. Experiments were repeated three times with similar results.
NBO inhibits MMP-9-mediated occludin degradation in the ischemic hemispheric microvessels
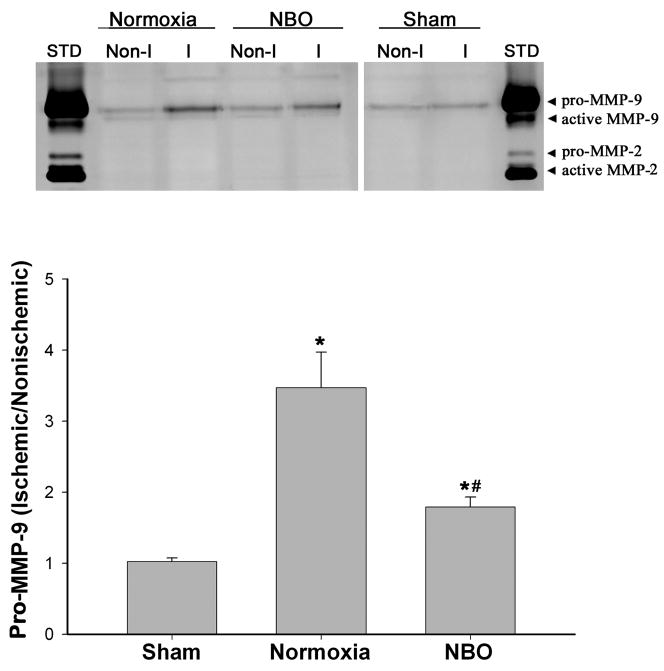
Our results so far have shown that (a) NBO treatment reduced early BBB disruption, and (b) MMP-9-mediated occludin degradation may be the underlying mechanism for BBB disruption after 90-min MCAO and 3-hr reperfusion. Previous studies by us and others have demonstrated that NBO inhibited MMP-9 increase in the ischemic brain tissue (Kim et al. 2005; Liu et al. 2006). We therefore speculated that NBO may protect the BBB through inhibiting MMP-9-mediated occludin degradation in the BBB microvasculature. To test this possibility, we first examined the effect of NBO on MMP-9 expression in the ischemic hemispheric microvessels. Gel gelatin zymography was performed to quantitatively analyze MMP-9 expression in isolated hemispheric microvessels from normoxic, NBO-treated and sham-operated rats. Representative zymograms revealed that MMP-9 expression was drastically increased in the ischemic hemispheric microvessels from normoxic rats as compared to sham-operated rats, while NBO-treated rats showed a significantly reduced MMP-9 expression compared with the normoxic group (Figure 5, upper panel). As expected, there was no significant difference in MMP-9 levels in the nonischemic hemispheric microvessels among three animal groups. The band intensity of MMP-9 was quantitated, and MMP-9 increase was expressed as a hemispheric MMP-9 ratio (ischemic/nonischemic). Compared with sham-operated rats, 90-min MCAO and 3-hr reperfusion significantly increased the hemispheric MMP-9 ratio in the normoxic and NBO-treated rats (*P < 0.05 by AVONA). More importantly, NBO-treated rats showed a significantly smaller MMP-9 ratio in comparison to the normoxic rats (#P<0.05 by AVONA, Figure 5 bottom panel). These results indicate that NBO treatment indeed inhibits MMP-9 induction in the ischemic BBB microvasculature.
Figure 5.
NBO treatment significantly inhibited MMP-9 induction in the ischemic cerebral microvessels after 90-min MCAO with 3-hr reperfusion. Cerebral microvessels were homogenized in TCNB buffer and protein extracts (20 μg) were subjected to gel gelatin zymography analysis. Upper panel: representative gelatin zymograms showing the expression of 92 kDa proform of MMP-9 in nonischemic (Non-I) and ischemic (I) microvessels isolated from sham-operated, normoxic and NBO-treated rats. STD is a mixture of standard MMP-2/9. Lower panel: MMP-9 band intensity was quantified, and the relative quantity of MMP-9 was expressed as MMP-9 ratio (ischemic/nonischemic hemispheric microvessels). *p < 0.05 versus sham group; #p < 0.05 versus normoxic group. Data are expressed as mean ± SEM, n = 4 in the sham group, n = 6 in each of the normoxic and NBO groups.
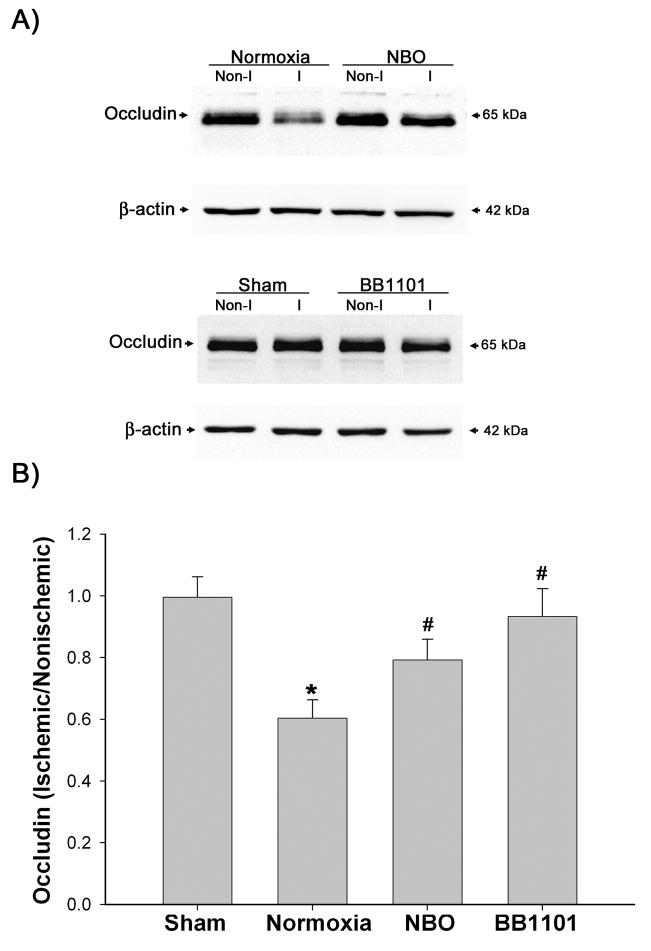
Next, we examined the effect of NBO treatment on occludin degradation in the ischemic hemispheric microvessels. Occludin protein was analyzed by Western blot. Representative blots are shown in Figure 6A. The band intensity of occludin protein was quantitated, and the different occludin protein levels between ischemic and nonischemic hemispheric microvessels were expressed as hemispheric ratios (ischemic/nonischemic) after normalization to β-actin. Quantitative data for occludin protein expression are shown in Figure 6B. As expected, sham-operated rats showed similar protein level of occludin in both hemispheric microvessels, with a hemispheric ratio close to 1.0. In the normoxic rats, occludin protein levels were significantly reduced in the ischemic cerebral hemispheric microvessels (*P < 0.05 vs sham group, AVONA), which was consistent with our immunohistochemisry results shown in Figure 2A. In agreement with its inhibition on MMP-9 induction, NBO treatment prevented occludin degradation in the ischemic hemispheric microvessels and partially restored the hemispheric occludin ratio from 0.56 to 0.79 (#P < 0.05 vs normoxic group, AVONA). To further support a role of MMP-9 in mediating occludin degradation in the microvessels, we pretreated rats with BB1101, a broad spectrum MMP inhibitor, which was shown to inhibit BBB disruption in our recent experimental stroke study (Yang et al. 2007). Indeed, BB1101 reversed the hemispheric occludin ratio back to 0.93 (#P < 0.05 vs normoxic group, AVONA). No significant difference was observed in occludin protein levels in the nonischemic hemispheric microvessels between animal groups. These results suggest that NBO may reduce occludin degradation in the ischemic cerebral microvessels through inhibiting MMP-9.
Figure 6.
The effect of NBO and MMP inhibitor BB1101 on the degradation of occludin protein in ischemic hemispheric microvessels after 90-min MCAO with 3-hr reperfusion. Cerebral microvessel lysates (10 μg) were analyzed for occludin protein by western blot. As a loading control, the blots were stripped and reprobed withβ-actin antibody. A) Representative blots of occludin and corresponding β-actin. Non-I and I represent nonischemic and ischemic hemispheric microvessels, respectively. B) The relative quantity of protein was calculated after normalization to β-actin and expressed as an occludin ratio (ischemic/nonischemic hemispheric microvessels). The occludin ratio was significantly reduced in normoxic rats (*p < 0.05 versus sham group). NBO treatment or BB1101 significantly reversed the reduction of occludin protein in ischemic hemispheric microvessels (#p < 0.05 versus normoxic group). Data are expressed as mean ± SEM, n = 4 in the sham group, n = 7 in each of the normoxic, NBO-, and BB1101-treated groups.
Discussion
Our recent study showed that NBO treatment maintained ischemic penumbral oxygenation status close to the pre-ischemic level, resulting in a significant reduction in infarction volume and improved neurological function in a rat model of transient focal cerebral ischemia (Liu et al. 2006). In the present study, we investigated the possible underlying mechanism for the neuroprotective effect of NBO with a focus on BBB damage. We found that 90-min MCAO with 3-hr reperfusion induced Evan’s blue dye extravasation in the ischemic subcortical area, which was accompanied by increased gelatinolytic activity and occludin protein loss at the same brain tissue location. Incubation of brain tissue or cerebral microvessels with MMP-9 revealed MMP-dependent occludin degradation. NBO treatment significantly reduced Evan’s blue leakage, MMP-9 increase and occludin degradation in ischemic cerebral microvessels. These findings suggest that NBO treatment attenuated early BBB disruption by inhibiting MMP-9-mediated degradation of the tight junction protein occludin in the ischemic BBB microvasculature.
NBO has been advocated as a potential treatment for ischemic stroke (Henninger and Fisher 2006) based on the following facts: (1) recent experimental and clinical pilot studies support a beneficial effect of NBO in focal cerebral ischemia (Miyamoto and Auer 2000;Singhal et al. 2002a; Singhal et al. 2005; Chiu et al. 2006; Liu et al. 2006; Henninger et al. 2007; Shin et al. 2007); (2) NBO is simple to administer, is noninvasive, and can be started promptly after stroke symptom onset, which enables NBO as a potential stroke treatment because brain cells start dying quickly after the blood flow to the brain stops. Since early BBB disruption is closely associated with hemorrhagic transformation and poor clinical outcome following acute thrombolysis in ischemic stroke (Latour et al. 2004), we focused our current study on investigating the effect of NBO on ischemia-induced BBB damage as a possible neuroprotective mechanism of NBO treatment. Our results clearly demonstrated that 90-min MCAO with 3 hr-reperfusion induced Evan’s blue leakage in the subcortical area of the normoxic rats, and NBO treatment significantly attenuated this BBB disruption.
We next investigated the mechanisms at work in NBO-induced BBB protection. Tight junctions are important structural components of the BBB, which span the apical region of the interendothelial clefts and restrict paracellular permeability (Wolburg and Lippoldt 2002). Experimental evidence indicates that MMP-mediated tight junction disruption is an important mechanism leading to an excessive increase in BBB permeability following cerebral hypoxia or ischemia (Asahi et al. 2001; Date et al. 2006; Yang et al. 2007). The tight junction protein occludin is an important protein to seal the tight junctions (Hirase et al. 1997; Lacaz-Vieira et al. 1999; Tavelin et al. 2003). Several studies suggest that occludin is a substrate for MMP-2/9 (Hawkins et al. 2007; Yang et al. 2007), however, MMP-independent degradation of occludin has also been reported (Reijerkerk et al. 2006). From the in vitro occludin degradation experiments, we clearly showed that occludin is a substrate of MMP-9. More importantly, we co-localized Evan’s blue leakage, occludin degradation and the increase of gelatinolytic activity at the same tissue location of the ischemic hemisphere, suggesting a causal link between MMP-mediated occludin degradation and BBB disruption in the ischemic brain. MMP-2/9 are large molecules, which restricts their proteolysis mainly to the immediate pericellular environment (Yong et al. 1998), therefore, BBB microvasculature derived MMP-2/9 may represent an important enzymatic source which can directly interact with the BBB structural components, leading to their degradation. In this context, we further examined the change of MMP-2/9 expression in isolated cerebral microvessels. Our in situ and gel zymography analyses showed increased gelatinolytic activity in the ischemic hemisphere microvessels, and that MMP-9, but not MMP-2, was identified as the main enzymatic source. These results suggest that MMP-9-induced occludin degradation may represent an important mechanism for BBB disruption in focal cerebral ischemia. It is worth pointing out that although MMP-9 is the major gelatinase produced in the BBB microvasculature, astrocyte-derived MMP-2/9 would also presumably have access to the neurovascular barrier structural components because the plasma membranes of astrocyte end-feet are contiguous with the basal lamina of the BBB (Hawkins and Davis 2005). However, this mechanism is likely at work at late reperfusion time points because our previous study showed that disruption of tight junction proteins seen early in endothelial cells appeared in the surrounding astrocytes at the end of 24 hrs reperfusion(Yang et al. 2007). Besides occludin, claudins are a family of four-membrane spanning proteins sealing the paracellular space between adjacent endothelial cells (Fanning et al. 1999). Our earlier study demonstrated that increased MMP-2/9 in the ischemic brain tissue mediated the translocation of claudin-5 from endothelial cells to surrounding astrocytes, rather than causing its degradation (Yang et al. 2007). Similarly, we found that incubation of microvessel extracts with active MMP-9 did not show any degradation of claudin-5 protein (data not shown), further indicating claudin-5 is not a substrate of MMP-9. One possible explanation for the observed MMP-mediated claudin-5 reorganization could be that MMPs may interrupt the interaction between tight junction proteins by degrading some types of these proteins, such as occludin as shown in this study and zonula occludens (Asahi et al. 2001), thus facilitating the translocation of claudin-5 from endothelial cells to surrounding astrocytes. This possibility requires further investigation in future studies. To determine whether NBO protects the BBB through inhibiting MMP-9-mediated occludin degradation, we examined the effects of NBO on MMP-9 and occludin protein expression in the cerebral microvessels. Indeed, we found that NBO treatment significantly reduced MMP-9 induction as well as occludin protein degradation in the ischemic hemispheric microvessels. As discussed above, our in vitro experiments demonstrate that occludin is a substrate for MMP-9. To further support a role of MMP-9 in occludin degradation in vivo, we pretreated ischemic rats with a broad spectrum MMP inhibitor BB1101 and examined its effect on occludin degradation in the ischemic cerebral microvessels. Consistent with its protective effect on BBB integrity observed in recent experimental stroke studies (Yang et al. 2007; Sood et al. 2008), we found that BB1101 significantly inhibited occludin degradation in the ischemic microvessels. These results suggest that NBO may maintain tight junction integrity by inhibiting MMP-9-mediated occludin degradation in the BBB microvasculature.
In summary, the present study suggests that MMP-, particularly MMP-9, mediated occludin degradation may represent an important mechanism for early BBB disruption in transient focal cerebral ischemia. More importantly, our results demonstrate that NBO treatment can reduce the MMP-9 increase and occludin degradation in ischemic BBB microvasculature, leading to a significant reduction in early BBB disruption. Because delayed reperfusion-associated brain edema and hemorrhage occur as a result of the BBB disruption, and MMP-9 is considered to be the critical mediator in this process, our results provide important mechanistic evidence in support of using NBO as a useful neuroprotective treatment for ischemic stroke, and potentially as an effective combination therapy to extend the therapeutic time window for additional intervention (e.g., thrombolysis).
Acknowledgments
This work was supported in part by grants from National Institutes of Health (P20 RR15636 and R01 AG031725), and American Heart Association (0555669Z and 0765461Z).
Abbreviations
- MMP
Matrix metalloproteinase
- NBO
normobaric hyperoxia
- BBB
blood brain barrier
- MCAO
middle cerebral artery occlusion
- TTC
2,3,5-triphenyltetrazolium chloride
References
- Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu EH, Liu CS, Tan TY, Chang KC. Venturi mask adjuvant oxygen therapy in severe acute ischemic stroke. Arch Neurol. 2006;63:741–744. doi: 10.1001/archneur.63.5.741. [DOI] [PubMed] [Google Scholar]
- Date I, Takagi N, Takagi K, Tanonaka K, Funakoshi H, Matsumoto K, Nakamura T, Takeo S. Hepatocyte growth factor attenuates cerebral ischemia-induced increase in permeability of the blood-brain barrier and decreases in expression of tight junctional proteins in cerebral vessels. Neurosci Lett. 2006;407:141–145. doi: 10.1016/j.neulet.2006.08.050. [DOI] [PubMed] [Google Scholar]
- Fanning AS, Mitic LL, Anderson JM. Transmembrane proteins in the tight junction barrier. J Am Soc Nephrol. 1999;10:1337–1345. doi: 10.1681/ASN.V1061337. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Lundeen TF, Norwood KM, Brooks HL, Egleton RD. Increased blood-brain barrier permeability and altered tight junctions in experimental diabetes in the rat: contribution of hyperglycaemia and matrix metalloproteinases. Diabetologia. 2007;50:202–211. doi: 10.1007/s00125-006-0485-z. [DOI] [PubMed] [Google Scholar]
- Henninger N, Fisher M. Normobaric hyperoxia - a promising approach to expand the time window for acute stroke treatment. Cerebrovasc Dis. 2006;21:134–136. doi: 10.1159/000090446. [DOI] [PubMed] [Google Scholar]
- Henninger N, Bouley J, Nelligan JM, Sicard KM, Fisher M. Normobaric hyperoxia delays perfusion/diffusion mismatch evolution, reduces infarct volume, and differentially affects neuronal cell death pathways after suture middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 2007;27:1632–42. doi: 10.1038/sj.jcbfm.9600463. [DOI] [PubMed] [Google Scholar]
- Hirase T, Staddon JM, Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Fujimoto K, Tsukita S, Rubin LL. Occludin as a possible determinant of tight junction permeability in endothelial cells. J Cell Sci. 1997;110 ( Pt 14):1603–1613. doi: 10.1242/jcs.110.14.1603. [DOI] [PubMed] [Google Scholar]
- Kago T, Takagi N, Date I, Takenaga Y, Takagi K, Takeo S. Cerebral ischemia enhances tyrosine phosphorylation of occludin in brain capillaries. Biochem Biophys Res Commun. 2006;339:1197–1203. doi: 10.1016/j.bbrc.2005.11.133. [DOI] [PubMed] [Google Scholar]
- Kim HY, Singhal AB, Lo EH. Normobaric hyperoxia extends the reperfusion window in focal cerebral ischemia. Ann Neurol. 2005;57:571–575. doi: 10.1002/ana.20430. [DOI] [PubMed] [Google Scholar]
- Lacaz-Vieira F, Jaeger MM, Farshori P, Kachar B. Small synthetic peptides homologous to segments of the first external loop of occludin impair tight junction resealing. J Membr Biol. 1999;168:289–297. doi: 10.1007/s002329900518. [DOI] [PubMed] [Google Scholar]
- Latour LL, Kang DW, Ezzeddine MA, Chalela JA, Warach S. Early blood-brain barrier disruption in human focal brain ischemia. Ann Neurol. 2004;56:468–477. doi: 10.1002/ana.20199. [DOI] [PubMed] [Google Scholar]
- Liu S, Shi H, Liu W, Furuichi T, Timmins GS, Liu KJ. Interstitial pO2 in ischemic penumbra and core are differentially affected following transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2004;24:343–349. doi: 10.1097/01.WCB.0000110047.43905.01. [DOI] [PubMed] [Google Scholar]
- Liu S, Liu W, Ding W, Miyake M, Rosenberg GA, Liu KJ. Electron paramagnetic resonance-guided normobaric hyperoxia treatment protects the brain by maintaining penumbral oxygenation in a rat model of transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:1274–1284. doi: 10.1038/sj.jcbfm.9600277. [DOI] [PubMed] [Google Scholar]
- Miyamoto O, Auer RN. Hypoxia, hyperoxia, ischemia, and brain necrosis. Neurology. 2000;54:362–371. doi: 10.1212/wnl.54.2.362. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1:223–236. doi: 10.1007/s11481-006-9025-3. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn T, Rosenberg GA. Closure of the blood-brain barrier by matrix metalloproteinase inhibition reduces rtPA-mediated mortality in cerebral ischemia with delayed reperfusion. Stroke. 2003;34:2025–2030. doi: 10.1161/01.STR.0000083051.93319.28. [DOI] [PubMed] [Google Scholar]
- Reijerkerk A, Kooij G, van der Pol SM, Khazen S, Dijkstra CD, de Vries HE. Diapedesis of monocytes is associated with MMP-mediated occludin disappearance in brain endothelial cells. Faseb J. 2006;20:2550–2552. doi: 10.1096/fj.06-6099fje. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Estrada EY, Dencoff JE. Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke. 1998;29:2189–2195. doi: 10.1161/01.str.29.10.2189. [DOI] [PubMed] [Google Scholar]
- Shin HK, Dunn AK, Jones PB, Boas DA, Lo EH, Moskowitz MA, Ayata C. Normobaric hyperoxia improves cerebral blood flow and oxygenation, and inhibits peri-infarct depolarizations in experimental focal ischaemia. Brain. 2007;130:1631–1642. doi: 10.1093/brain/awm071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal AB, Dijkhuizen RM, Rosen BR, Lo EH. Normobaric hyperoxia reduces MRI diffusion abnormalities and infarct size in experimental stroke. Neurology. 2002a;58:945–952. doi: 10.1212/wnl.58.6.945. [DOI] [PubMed] [Google Scholar]
- Singhal AB, Wang X, Sumii T, Mori T, Lo EH. Effects of normobaric hyperoxia in a rat model of focal cerebral ischemia-reperfusion. J Cereb Blood Flow Metab. 2002b;22:861–868. doi: 10.1097/00004647-200207000-00011. [DOI] [PubMed] [Google Scholar]
- Singhal AB, Benner T, Roccatagliata L, Koroshetz WJ, Schaefer PW, Lo EH, Buonanno FS, Gonzalez RG, Sorensen AG. A pilot study of normobaric oxygen therapy in acute ischemic stroke. Stroke. 2005;36:797–802. doi: 10.1161/01.STR.0000158914.66827.2e. [DOI] [PubMed] [Google Scholar]
- Sood RR, Taheri S, Candelario-Jalil E, Estrada EY, Rosenberg GA. Early beneficial effect of matrix metalloproteinase inhibition on blood-brain barrier permeability as measured by magnetic resonance imaging countered by impaired long-term recovery after stroke in rat brain. J Cereb Blood Flow Metab. 2008;28:431–438. doi: 10.1038/sj.jcbfm.9600534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumii T, Lo EH. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke. 2002;33:831–836. doi: 10.1161/hs0302.104542. [DOI] [PubMed] [Google Scholar]
- Tavelin S, Hashimoto K, Malkinson J, Lazorova L, Toth I, Artursson P. A new principle for tight junction modulation based on occludin peptides. Mol Pharmacol. 2003;64:1530–1540. doi: 10.1124/mol.64.6.1530. [DOI] [PubMed] [Google Scholar]
- Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002;38:323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- Yong VW, Krekoski CA, Forsyth PA, Bell R, Edwards DR. Matrix metalloproteinases and diseases of the CNS. Trends Neurosci. 1998;21:75–80. doi: 10.1016/s0166-2236(97)01169-7. [DOI] [PubMed] [Google Scholar]