Abstract
Background
Dietary studies designed to lower urinary albumin excretion rate (AER) typically reduce protein by increasing lower protein plant foods and reducing higher protein animal products.
Study Design
We evaluated AER while increasing protein intake in the Dietary Approaches to Stop Hypertension (DASH) Trial (randomized, parallel group, 8 week controlled feeding).
Setting & Participants
378 individuals without diabetes with prehypertension or stage I hypertension.
Intervention
The DASH diet, 18% energy from protein, emphasizes, among other features, lowfat dairy products; and the fruit/vegetable (FV) and Control diets, each with 15% energy from protein.
Outcome
AER
Measurements
We measured AER by immunoassay and covariates at baseline and after 8 weeks.
Results
Baseline AER had geometric mean ± standard error 4.0 ± 0.2 mg/24hr. Among 285 participants with baseline AER<7 mg/24hr, AER was unchanged by diet treatment (geometric mean 2.5 ± 0.2 mg/24hr in Control, 3.0 ± 0.2 mg/24hr in FV, 2.8 ± 0.2 mg/24hr in DASH). In contrast, among 93 participants with baseline AER ≥7 mg/24hr, end of feeding AER was lower in FV (6.6 ± 1.0 mg/24hr) than in Control (11.4 ± 1.8 mg/24hr (p=0.01) or DASH (11.7 ± 1.6 mg/24hr p = 0.005). The DASH and control diets were not different (p=0.9).
Limitations
Long term AER change not studied.
Conclusions
Reduction in AER after 8 weeks occurred only in those with high normal baseline AER in FV, in a pattern distinct from blood pressure reduction. The DASH diet did not increase AER despite 3% increase in energy from protein.
The Dietary Approaches to Stop Hypertension Trial (DASH) demonstrated a reduction in blood pressure in individuals with prehypertension or stage 1 hypertension over 8 weeks when eating a diet rich in fruits and vegetables and other plant foods, together with low-fat dairy products (the DASH diet).1 The lower blood pressure may provide unique benefits to both macrovascular disease and microvascular disease (i.e., renal function). Although primarily thought to relate to the development and progression of renal disease, urinary albumin excretion rate (AER), even at subclinical levels, may also predict an increased risk for all cause mortality and the development of cardiovascular disease.2-6 Thus, reduction of urinary albumin excretion may lower risks for both micro-and macrovascular disease.
Several controlled feeding studies have investigated dietary influences on AER, primarily under the hypothesis that decreases in protein intake would reduce AER.7-12 Most of these studies reduced protein intake by increasing plant foods at the expense of meat intake. However, some have reported that vegetable protein and animal protein have opposite associations with AER; that is, animal protein was associated with higher AER and vegetable protein with lower AER.8 We hypothesized that the fruit/vegetable diet (FV) employed in the DASH trial, which was high in nutrient- and phytochemical-rich plant foods and which lowered blood pressure, would decrease AER, even though total dietary protein did not decrease. Further we hypothesized greater AER reduction in the DASH diet, which was even higher in plant foods, but added low fat dairy foods increasing total dietary protein, and resulted in a larger blood pressure decrease than did the fruit/vegetable diet. Our overall hypothesis was that decreases in AER follow decreases in blood pressure. Thus we measured AER using frozen 24-hour urine samples obtained before and after the 8-week feeding trial with the FV or the DASH diet. Secondarily we hypothesized that reduction in AER in both the FV and DASH diets would be greater in those with high normal values of albumin excretion at baseline.
METHODS
Population
The DASH Trial was a multicenter, randomized, 3 arm, parallel design, 8 week controlled feeding study conducted between 1994 and 1996,1,13-15 Participants were nondiabetic. Entry diastolic blood pressure was 80−95 mmHg while systolic blood pressure was <160 mmHg, so that the participants aged 22−75 years (average 44 years) had untreated prehypertension or stage I hypertension. Those with reduced glomerular filtration rate were excluded.
Diets
The 3 diets that were provided to participants were prepared at 1500, 2100, 2600, and 3100 kcal. Energy intake was calibrated for weight maintenance in each participant using these base diets plus 100 kcal “unit foods”. According to chemical analysis of the diets, the Control diet contained 13.8% energy from protein, 50.5% from carbohydrate, and 35.7% from fat. The FV diet contained 15.1% energy from protein, 49.2% from carbohydrate, and 35.7% from fat. The DASH diet contained 17.9% energy from protein, 56.5% from carbohydrate, and 25.6% from fat. FV was rich in fruits, vegetables, whole grains, nuts, and reduced in refined grain, but was otherwise similar to Control. The DASH diet was even richer in vegetables, whole grains, and nuts, had reduced meat, poultry, and fish, deleted whole milk, and added low-fat dairy foods. A detailed food group comparison of the 2100 kcal diets is shown in Table 1a16 (Karanja NM, personal communication, January 5, 2006), with change in nutrient composition of the 3 diets during intervention in Table 1b. At the 2100 kcal/d level the control diet had 79 g/d of protein, the FV diet 82g/d, and the DASH diet 95 g/day of protein of which animal and plant protein respectively were 57 g and 21 g in Control, 47 g and 34 g in FV, and 61 g and 34 g in DASH.
Table 1.
Foods and nutrients in the DASH trial diets (Control, Fruit/Vegetable and DASH diet)
| A. Food Groups (grams/day in the 2100 kcal/day diet) | |||
|---|---|---|---|
| Control | Fruit/Vegetables | DASH | |
| Refined grain | 342 | 125 | 130 |
| Whole grain | 0 | 120 | 153 |
| Fruit | 82 | 301 | 281 |
| Fruit juice | 166 | 387 | 411 |
| Vegetables | 147 | 272 | 345 |
| Meat, poultry, fish | 192 | 172 | 139 |
| Whole milk | 66 | 57 | 0 |
| Other dairy products | 23 | 2 | 485 |
| Nuts, seeds, legumes | 0 | 27 | 36 |
| Sweets, snack foods | 152 | 28 | 22 |
| Fats, oils, salad dressings | 54 | 43 | 26 |
| B. Median nutrient content of Control diet and median differences from Control in the Fruit/Vegetable and DASH diets | |||
|---|---|---|---|
| Control | Fruit/Vegetable | DASH | |
| Protein (% kcal) | 14.0 | 1.1a | 3.8ac |
| Carbohydrate (% kcal) | 49.5 | 2.6a | 9.0ac |
| Fat (% kcal) | 36.8 | 0.1 | −9.6bd |
| Saturated fat (% kcal) | 14.3 | −1.0b | −7.1bd |
| Monounsaturated fat (% kcal) | 12.5 | 1.1a | −2.3bd |
| Polyunsaturated fat (% kcal) | 7.1 | −0.1 | 0.7ac |
| Cholesterol (mg/1000 kcal) | 117.1 | −25.8b | −48.8b |
| Dietary fiber (g/1000 kcal) | 4.9 | 8.9a | 8.7a |
| Potassium (mg/1000 kcal) | 798.1 | 1163.4a | 1212.3a |
| Calcium (mg/1000 kcal) | 207.3 | 6.4 | 357.9ac |
| Magnesium (mg/1000 kcal) | 78.8 | 105.5a | 129.4a |
| Sodium (mg/1000 kcal) | 1354.6 | −40.5 | −27.9 |
Low intake (10th percentile) in DashD or FV > high intake (90th percentile) of Control
High intake (90th percentile) in DashD or FV < low intake (10th percentile) of Control
Low intake (10th percentile) in DashD > high intake (90th percentile) of FV
High intake (90th percentile) in DashD < low intake (10th percentile) of FV
Measurement of Urine Specimens in the DASH trial
In 24-hour urine samples that had been stored at −70 °C for 6−8 years, albumin concentration was measured by a nephelometer (Dade/Behring BN100) with an enhanced sensitivity (lower detection limit 0.4 mg/L) and a coefficient of variation in 50 masked duplicate samples with values less than 40 mg/L of 9%; analytic coefficients of variation in two pools labeled at 2.5 and 25.4 mg/L were 5.7% and 6.3%, respectively. Albumin concentrations below 0.4 mg/L were undetectable and were set to this value. AER was computed as the urinary albumin concentration (mg/L) times the urine volume (L) times 24 hours/collection hours.
Because many studies do not collect timed urine samples, we also examined the urinary albumin to creatinine ratio. Creatinine measurements in urine were assessed as part of the original DASH protocol by a variation of the Jaffe procedure. Several people provided urine samples of insufficient duration, but in which albumin concentration could be assessed; thus 393 of the participants had both albumin and creatinine concentrations at baseline and 8 weeks, and several more had urine measures at baseline or 8-weeks, but not both. Following a procedure used in the Coronary Artery Risk Development in Young Adults study,17 we multiplied urinary creatinine concentration by k=0.68 in men and k=0.88 in African Americans (so 0.68*0.88 in African American men), then formed the albumin to adjusted creatinine ratio, A/kC, similar to the results of Warram et al.18 At baseline, the correlation between AER and A/kC was 0.97 (n = 406, predicted AER = −0.254 + 1.158 A/kC). The correlation for 8-week change in AER with 8-week change in A/kC was 0.74 (n=368, AER change = −0.540 + 1.078 A/kC change). Thus, the DASH trial AER values could be compared to A/kC values from other studies. For example, using the approximation that AER = 1.1 A/kC, the quartile and microalbuminuria cutpoints used in the Heart Outcomes Prevention Evaluation (HOPE) study (0.22, 0.57, 1.62, and 2 mg albumin/mmol creatinine, corresponding to 1.9, 5.0, 14.3, and 17.7 mg albumin/g creatinine) recalibrate respectively to AER values of 2.0, 5.5, 16.3, and 20.3 mg/24 hr. The relative risk for all cause mortality in the HOPE study, relative to quartile 1 of albumin excretion, was 1.08 in quartile 2, 1.46 in quartile 3, and 2.34 in quartile 4 (including microalbuminuria). When considering high normal AER, we therefore focused on 7 mg/24 hr, the 75th percentile of AER values in the DASH trial sample and the middle of the third quartile in the HOPE study. Findings concerning the influence of diet on albumin excretion were similar using either AER or A/kC (data not shown).
Statistical Analyses
This was an ancillary study to the DASH trial and all measurements and data analyses were performed by the authors. We tested our primary hypothesis by performing multiple linear regression of AER at week 8 on randomized diet. Because AER was highly skewed towards higher values, we used the natural logarithmic transformation, ln(AER), at week 8 as the dependent variable. Given the correlation of 0.7 between ln(AER) at baseline and 8 weeks, the estimated differences between treatment groups were approximately the same using week 8 ln(AER) as the dependent variable and adjusting for baseline ln(AER) as they would be using change in ln(AER) as the dependent variable. Adjustment for sex, race, clinic site, and recruitment cohort did not add significantly to the regression or substantially alter any regression coefficients; therefore we omitted these adjustments. Because we hypothesized that AER response to the randomized diet would vary for people with different AERs at baseline, we stratified baseline AER with cutpoints 3, 5, 7, and 10 mg/24hr. Cutpoints other than 7 were selected arbitrarily to allow examination of AER response in the DASH trial across a range of baseline AER values. In addition to this sensitivity analysis of different baseline cutpoints, we computed locally weighted smoothed lines (LOESS curves) using SAS PROC LOESS for comparison with the categorical analysis. Few DASH trial participants met the “usual” standard for microalbuminuria (30 mg/24hr).
RESULTS
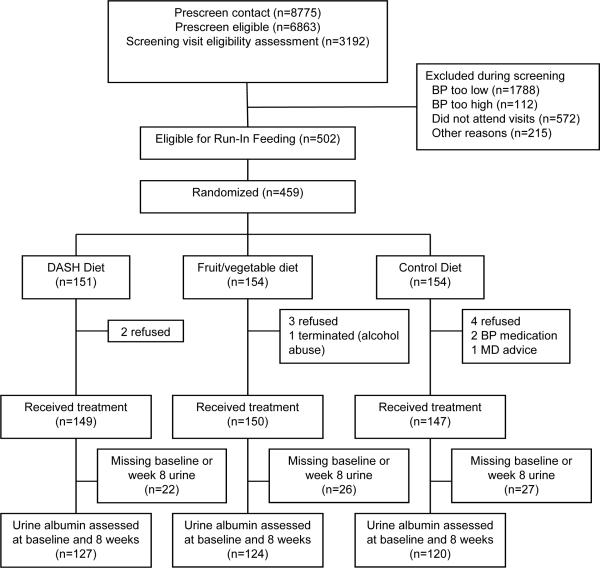
The CONSORT flow diagram of recruitment and retention in this study is provided in Figure 1. Of 459 randomized DASH participants, 378 provided 24 hour urine samples both at baseline and after 8 weeks of feeding, which formed the material for this study. No significant differences were observed in measured factors in those with and without urine samples at both timepoints. As expected given randomized assignment to diet, no statistically significant differences were observed among treatment groups for age, sex, race/ethnicity or baseline levels of blood pressure, body weight or fatness, smoking, fasting glucose, or AER (Table 2). Geometric mean ± standard error of AER at baseline was 4.0 ± 0.2 mg/24hr. Baseline AER was <3 mg/24hr in 35%, 3−4.9 mg/24hr in 26%, 5−6.9 mg/24hr in 15%, 7−9.9mg/24hr in 9%, and ≥10 mg/24hr in 15%. The tracking correlation between ln(AER) measured at baseline and at 8 weeks was 0.70. Of the 285 with AER <7 mg/24hr at baseline, 36 (13%) had an AER ≥7 mg/24hr at 8 weeks. Of the 93 who had AER ≥7 mg/24hr at baseline, 59 (63%) had AER ≥7 mg/24hr at 8 weeks.
Figure 1.
Consort diagram for study of urine albumin in the DASH study
Table 2.
Baseline characteristics according to randomized treatment assignment in DASH trial participants with 24 hour urinary albumin measured both at baseline and end of intervention 8 weeks later
| Control n = 127 | Fruits and Vegetables n = 124 | DASH Diet n = 127 | |||||
|---|---|---|---|---|---|---|---|
| Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | p** | |
| Age (yr) | 44.9 | 11.1 | 45.7 | 10.6 | 44.2 | 10.2 | 0.5 |
| Men (%) | 55% | 51% | 50% | 0.7 | |||
| Race/ethnicity | |||||||
| White (%) | 38% | 38% | 35% | 0.8 | |||
| Africian American (%) | 57% | 57% | 60% | 0.9 | |||
| Other (%) | 5% | 5% | 6% | 0.9 | |||
| Systolic blood pressure (mmHg) | 131.1 | 12.6 | 131.7 | 12.0 | 130.9 | 11.4 | 0.9 |
| Diastolic blood pressure (mmHg) | 85.4 | 6.3 | 84.2 | 6.7 | 84.1 | 6.2 | 0.3 |
| Hypertensive (%) | 32% | 32% | 25% | 0.4 | |||
| Weight (kg) | 81.7 | 14.9 | 82.5 | 13.5 | 83.6 | 14.9 | 0.6 |
| Body mass index (kg/m2) | 27.9 | 3.8 | 28.3 | 3.9 | 28.5 | 4.0 | 0.4 |
| Current cigarette smoker (%) | 8% | 12% | 9% | 0.5 | |||
| Fasting plasma glucose (mg/dL)* | 88.0 | 8.4 | 89.0 | 7.8 | 90.2 | 9.8 | 0.4 |
| Albumin excretion rate (mg/24 hr) | 4.1 | 3.6 | 3.9 | 4.7 | 4.0 | 5.2 | 1.00 |
| Albumin excretion rate ≥ 7 mg/24 hr (%) | 21% | 28% | 24% | 0.4 | |||
| Geometric mean albumin excretion rate (mg/24 hr) among those with albumin excretion rate ≥ 7 mg/24 hr | 17.9 | 16.1 | 13.3 | 0.2 | |||
| Microalbuminuria (albumin excretion rate ≥ 25 mg/24 hr (%) | 4% | 2% | 2% | 0.7 | |||
Number of participants with plasma glucose measured: Control, 71; Fruits and vegetables, 71; Dash Diet 72
p from F-test for any difference among treatment groups
Effect of Diet on AER at 8 weeks
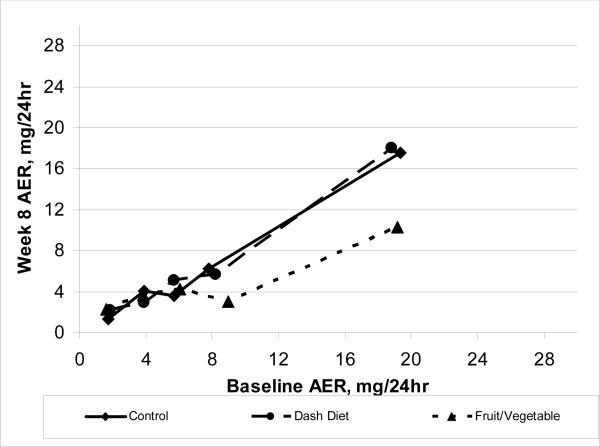
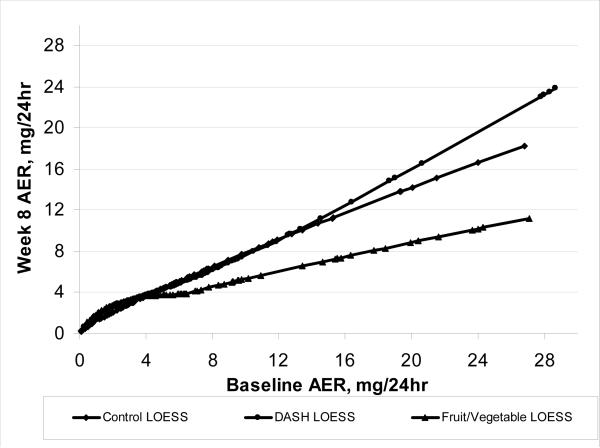
There were no overall differences in week 8 AER across treatment groups whether unadjusted (n = 415 for this analysis only, p = 0.6, 2 df) or adjusted for baseline AER (n = 378, p = 0.6, 2 df). However, an interaction was detected (p = 0.003, 8 df) in which week 8 AER was affected by treatment in the 25% of participants with baseline AER ≥7 mg/24 hr, but was unaffected in the 75% of participants with lower AER (Figure 2A), a conclusion supported by LOESS curves also plotted in Figure 2B. AER was similar for all 3 treatments for participants with baseline AER values <7 mg/24 hr (Table 3, Figure 2). In contrast, among participants with baseline AER ≥7 mg/24 hr, week 8 AER was lower in participants eating FV than in those eating either the DASH or Control diet. The week 8 AER was similar between DASH and Control diets (Figure 2).
Figure 2A.
Albumin excretion rate (AER) after feeding (week 8) in the DASH Trial according to intervention group and baseline AER level. Categorical analysis: <3 (n=132), 3−4.9 (n=98), 5−6.9 (n=55), 7−9.9 (n=35), and ≥10 (n=58) mg/24hr. Each point is plotted over its treatment-specific baseline mean AER. p-value for interaction = 0.007 with 8 and 363 degrees of freedom. Pairwise comparisons of the Fruit/Vegetable diet vs. the Control (p=0.08) or Dash diet p=0.05 for baseline AER 7−9.9 mg/24hr; and p=0.04 for both comparisons within AER ≥10 mg/24hr. Control less than both Dash Diet and Fruit/Vegetable diet for baseline AER <3 mg/24hr, each p < 0.02.
Figure 2B.
Albumin excretion rate (AER) after feeding (week 8) in the DASH Trial according to intervention group and baseline AER level. Smoothed line analysis: LOESS curves (fitted using SAS PROC LOESS) show AER after feeding (week 8) as a function of baseline AER level by treatment. Baseline AER values above 30 were not plotted (DASH diet n = 3, Control diet n = 5, Fruit/Vegetable diet n = 3). The LOESS curves follow the general pattern of the means in the baseline AER categories.
Table 3.
Geometric means of albumin excretion rate (AER) after 8 weeks of intervention by treatment diet within baseline AER categories
| Baseline AER < 7 mg/24 hr | Baseline AER ≥ 7 mg/24 hr | ||||||
|---|---|---|---|---|---|---|---|
| Control | Fruit/Vegetable | Dash Diet | Control | Fruit/Vegetable | DASH diet | p Fruit/Vegetable – Control | p Fruit/Vegetable – DASH diet |
| 2.5 | 3.0 | 2.8 | 11.4 | 6.6 | 11.7 | 0.007 | 0.003 |
Notes:
For baseline AER < 7 mg/24 hr, all pairwise comparisons, p > 0.05
For baseline AER ≥ 7 mg/24 hr, all pairwise comparisons of DASH and Control diets, p > 0.05.
P for interaction = 0.003 (2df) testing any difference in outcome pattern between baseline AER <7 mg/24 hr and baseline AER ≥ 7 mg/24 hr.
Mediation by blood pressure or sodium intake changes during intervention
Mean systolic/diastolic blood pressure changes during intervention in the subgroup with AER ≥ 7 mg/24hr were −0.7/0.1 mmHg in Control, −3.6/−1.4 mmHg in FV, and −4.9/−2.1 in the DASH diet, similar to findings in the people with AER < 7 mg/24 hr and in the overall group of 378 people analyzed in this paper and in all 459 DASH participants. The intervention related AER changes were essentially unaltered by simultaneous adjustment for concurrent changes in systolic and diastolic blood pressure. Similarly, 24 hour urinary sodium and potassium at baseline did not differ significantly among treatments. Concordant with the small change in sodium intake during intervention (Table 1), change in urinary sodium during the study was small, increasing 5% in Control, decreasing 2% in the DASH diet, and decreasing 7% in FV. Adjustment for change in urinary sodium did not alter the findings.
DISCUSSION
Among the men and women in the DASH trial, all of whom had prehypertension or elevated blood pressure, eating the FV diet led to a reduction in week 8 AER in those with AER ≥7 mg/24hr at baseline, a range found to be associated with increased long term risk of all cause death and heart disease.2-6 Thus part of our hypothesis was sustained. That is, AER was reduced by FV in those with higher baseline AER), although the AER changes were independent of blood pressure changes. However, FV did not affect AER if it was initially <7 mg/24hr. The other part of our hypothesis was not sustained. That is, the DASH diet did not affect AER at all even though it lowered blood pressure the most. However, the 3.8% higher protein intake in the DASH diet compared with the Control diet might have been expected to raise AER.19 It is therefore of interest that the DASH diet did not increase AER, even in those in the higher AER range.
The HOPE Trial,2 measuring albumin/creatinine ratio (A/C), demonstrated a gradation of risk for adverse cardiovascular disease outcomes among people with diabetes or a history of myocardial infarction at baseline, even into the higher segment of their reference range for A/C. We utilized the results of the HOPE Trial2 to justify exploring the association of FV and the DASH diet to albuminuria in the high normal range of AER. Others have also investigated high normal AER values, for example, a Brazilian study20 that identified excess risk of future elevated AER and death associated with AER ≥5 mg/24hr in people with type 2 diabetes followed for 8 years. AER, which is a potential marker of endothelial dysfunction and a predictor of macrovascular adverse events, may have significant impact above a threshold (7 mg/24hr) that is well below that usually defining microalbuminuria (25 mg/24hr). We acknowledge that the cutpoint 7 mg/24hr is somewhat arbitrary. For example, any cutpoint from 5−10 mg/24 hr would fit our data fairly well and also be concordant with the AER value at which increase in long term risk was seen in the HOPE trial2. Therefore, the reduction in AER observed in this study with FV would potentially be important to reducing risk if it were sustained in the long run. Dullaart et al.21 showed that low AER can be maintained in diabetics over 2 years when eating a diet in which intake of animal protein was reduced from 10% to 5% of energy, while intake of plant protein remained constant at 5% of energy. We assume that an underlying defect such as glomerular or endothelial dysfunction that leads to increased albumin excretion in excess of 7 mg/24hr may reflect generalized arterial disease. We do not know whether the short term AER reduction we observed in this study corrected the hypothesized underlying defect in the vascular endothelium.
Relative to the Control diet in the DASH study, FV is rich in fruits and vegetables, but is otherwise similar to a typical American diet. The DASH diet also emphasizes fruits and vegetables, but in addition emphasizes low fat dairy and whole grains and nuts, while reducing fats, refined grains and sweets, as well as small reductions in meat, fish and poultry (Table 1). From a nutrient perspective, the FV diet, compared to the Control diet added 13 g/day plant protein and many other plant constituents. However, total dietary protein was similar to the control diet owing to a 10g/day reduction in animal protein. The DASH diet was somewhat richer than FV in the plant constituents of FV, and had more dairy and total protein. The DASH diet compared to the Control diet had more total protein, but similar amounts of animal protein, substituting dairy for meat protein, and more plant protein.
Only 3 other clinical trials were identified that compared diet and albumin excretion rate in nondiabetic persons. In two 3 week cross-over studies in 6 men7 and 10 men,8 reducing animal protein intake by increasing plant foods led to 50% reductions of AER within its normal range. The third study, a parallel design, 6-month weight loss experiment by Skov et al.,22 showed similar AER decreases in both the low and high protein intake diet groups. Other weight loss studies have shown reduced AER after weight loss,23,24 which might therefore have masked other dietary effects in the Skov et al. study.22 In studies of patients with type 2 diabetes, usually with microalbuminuria, low protein diets were usually formed by increasing plant food, often with properties such as increasing fiber and other phytonutrients, and led to statistically significantly reduced AER in several studies;9-12,19,25 or reduced estimates of AER that did not achieve statistical significance in two others.26,27
In contrast, in a 4 week cross-over design Gross et al.28 studied usual red meat vs. a low protein diet that, like the DASH diet, replaced animal protein in the usual diet with vegetable and milk protein. In these patients with type 2 diabetes, whether in the 15 with normoalbuminuria or the 13 with microalbuminuria, he found no difference in AER; however a third diet that replaced red meat with chicken reduced AER compared to usual and low protein diets in the 13 patients with microalbuminuria. Texeira et al.29 performed a cross-over study in diabetic men in which one of the 8 week long diets included 23.4% of energy from soy protein and the other 22.7% of energy from the milk protein casein, both of which are high protein content in a human diet. AER was reduced eating the soy protein diet containing a large amount of isoflavones compared to the casein diet. Urinary albumin excretion was negatively correlated with plasma total isoflavones (r = −0.44), daidzein (r = −0.33), and O-desmethylangolesin (r = −0.39).
Our finding is novel among short term trials that reduced AER, in that the total protein content of the FV diet was 1% of energy intake higher than in the Control diet; and the DASH diet had similar plant protein and other plant constituent contents to FV, but much higher dairy protein. With these views of the diets in mind, our results are concordant with alternative interpretations. The first is that only animal protein enhances albumin leakage, while plant protein protects, as suggested by Teixeira et al.29 The second is the idea that the combinations of phytochemicals provided by the FV diet, such as isoflavones29 or any of thousands of known and unknown compounds with antioxidant properties30 that in combination could alleviate inflammation and endothelial dysfunction,31,32 reduced AER only in those with high normal AER. If these phytochemicals reduce albuminuria, some characteristics of the DASH diet may have cancelled the potential effect of animal protein; that is, the response of the AER in the DASH diet could be seen as the sum of the AER reduction from a common component also in the FV diet and an AER increase from another part of the DASH diet (e.g., increases in animal/dairy protein intake).
We conclude that FV lowered the slightly elevated AER over 8 weeks. Within the short term scope of the DASH trial, our findings disassociate the outcomes of reduced blood pressure vs. reduced AER, contrary to our primary hypotheses and what might be expected from other observational studies and clinical trials. Yet despite substantially greater protein content in the DASH than the Control diet, the DASH diet did not increase albuminuria compared to control. Our results support the utilization of a diet high in fruits, vegetables and unrefined grains to lower the potential risks of both microvascular and macrovascular disease.
ACKNOWLEDGEMENTS
We thank Gayle Meltesen at the DASH Coordinating Center at the Kaiser Permanente Center for Health Research, Portland, OR for providing data processing services.
A preliminary version of this work was presented in March, 2004 as a poster at the American Heart Association Council on Epidemiology and Prevention. Circulation 109(7): 21−22, 2004 Support: This study was supported by a grant from the National Institutes of Health, R01 HL071121.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: None.
REFERENCES
- 1.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 2.Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 3.Hillege HL, Fidler V, Diercks GF, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106:1777–1782. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 4.Arnlov J, Evans JC, Meigs JB, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112:969–975. doi: 10.1161/CIRCULATIONAHA.105.538132. [DOI] [PubMed] [Google Scholar]
- 5.de Zeeuw D, Remuzzi G, Parving HH, et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation. 2004;110:921–927. doi: 10.1161/01.CIR.0000139860.33974.28. [DOI] [PubMed] [Google Scholar]
- 6.Jager A, Kostense PJ, Ruhe HG, et al. Microalbuminuria and peripheral arterial disease are independent predictors of cardiovascular and all-cause mortality, especially among hypertensive subjects: five-year follow-up of the Hoorn Study. Arterioscler Thromb Vasc Biol. 1999;19:617–624. doi: 10.1161/01.atv.19.3.617. [DOI] [PubMed] [Google Scholar]
- 7.Viberti G, Bognetti E, Wiseman MJ, Dodds R, Gross JL, Keen H. Effect of protein-restricted diet on renal response to a meat meal in humans. Am J Physiol. 1987;253:F388–F393. doi: 10.1152/ajprenal.1987.253.3.F388. [DOI] [PubMed] [Google Scholar]
- 8.Kontessis P, Jones S, Dodds R, et al. Renal, metabolic and hormonal responses to ingestion of animal and vegetable proteins. Kidney Int. 1990;38:136–144. doi: 10.1038/ki.1990.178. [DOI] [PubMed] [Google Scholar]
- 9.Pecis M, de Azevedo MJ, Gross JL. Chicken and fish diet reduces glomerular hyperfiltration in IDDM patients. Diabetes Care. 1994;17:665–672. doi: 10.2337/diacare.17.7.665. [DOI] [PubMed] [Google Scholar]
- 10.Hansen HP, Christensen PK, Tauber-Lassen E, Klausen A, Jensen BR, Parving HH. Low-protein diet and kidney function in insulin-dependent diabetic patients with diabetic nephropathy. Kidney Int. 1999;55:621–628. doi: 10.1046/j.1523-1755.1999.00274.x. [DOI] [PubMed] [Google Scholar]
- 11.Raal FJ, Kalk WJ, Lawson M, et al. Effect of moderate dietary protein restriction on the progression of overt diabetic nephropathy: a 6-mo prospective study. Am J Clin Nutr. 1994;60:579–585. doi: 10.1093/ajcn/60.4.579. [DOI] [PubMed] [Google Scholar]
- 12.Jibani MM, Bloodworth LL, Foden E, Griffiths KD, Galpin OP. Predominantly vegetarian diet in patients with incipient and early clinical diabetic nephropathy: effects on albumin excretion rate and nutritional status. Diabet Med. 1991;8:949–953. doi: 10.1111/j.1464-5491.1991.tb01535.x. [DOI] [PubMed] [Google Scholar]
- 13.Appel LJ, Vollmer WM, Obarzanek E, et al. Recruitment and baseline characteristics of participants in the Dietary Approaches to Stop Hypertension trial. DASH Collaborative Research Group. J Am Diet Assoc. 1999;99:S69–S75. doi: 10.1016/s0002-8223(99)00419-8. [DOI] [PubMed] [Google Scholar]
- 14.Vogt TM, Appel LJ, Obarzanek E, et al. Dietary Approaches to Stop Hypertension: rationale, design, and methods. DASH Collaborative Research Group. J Am Diet Assoc. 1999;99:S12–S18. doi: 10.1016/s0002-8223(99)00411-3. [DOI] [PubMed] [Google Scholar]
- 15.Sacks FM, Appel LJ, Moore TJ, Obarzanek E, et al. A dietary approach to prevent hypertension: a review of the Dietary Approaches to Stop Hypertension (DASH) Study. Clin Cardiol. 1999;22:III6–III10. doi: 10.1002/clc.4960221503. [DOI] [PubMed] [Google Scholar]
- 16.Karanja NM, Obarzanek E, McCullough ML, et al. Descriptive characteristics of the dietary patterns used in the Dietary Approaches to Stop Hypertension trial. J Amer Diet Assoc. 1999;99:s19–s27. doi: 10.1016/s0002-8223(99)00412-5. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs DR, Jr, Murtaugh MA, Steffes M, Yu X, Roseman J, Goetz FC. Gender- and race-specific determination of albumin excretion rate using albumin-to-creatinine ratio in single, untimed urine specimens: the Coronary Artery Risk Development in Young Adults Study. Am J Epidemiol. 2002;155:1114–1119. doi: 10.1093/aje/155.12.1114. [DOI] [PubMed] [Google Scholar]
- 18.Warram JH, Gearin G, Laffel L, Krolewski AS. Effect of duration of type I diabetes on the prevalence of stages of diabetic nephropathy defined by urinary albumin/creatinine ratio. J Am Soc Nephrol. 1996;7:930–937. doi: 10.1681/ASN.V76930. [DOI] [PubMed] [Google Scholar]
- 19.Mandayam S, Mitch WE. Dietary protein restriction benefits patients with chronic kidney disease. Nephrology. 2006;11:53–57. doi: 10.1111/j.1440-1797.2006.00528.x. [DOI] [PubMed] [Google Scholar]
- 20.Murassi M, Campagnolo N, Beck MO, Gross JL, Silveiro SP. High-normal levels of albuminuria predict the development of micro- and macroalbuminuria and increased mortality in Brazilian Type 2 diabetic patients: an 8-year follow-up study. Diabet Med. 2007;24:1136–1142. doi: 10.1111/j.1464-5491.2007.02209.x. [DOI] [PubMed] [Google Scholar]
- 21.Dullaart RP, Beusekamp BJ, Meijer S, van Doormaal JJ, Sluiter WJ. Long-term effects of protein-restricted diet on albuminuria and renal function in IDDM patients without clinical nephropathy and hypertension. Diabetes Care. 1993;16:483–492. doi: 10.2337/diacare.16.2.483. [DOI] [PubMed] [Google Scholar]
- 22.Skov AR, Toubro S, Bulow J, Krabbe K, Parving HH, Astrup A. Changes in renal function during weight loss induced by high vs low-protein low-fat diets in overweight subjects. Int J Obes Relat Metab Disord. 1999;23:1170–1177. doi: 10.1038/sj.ijo.0801048. [DOI] [PubMed] [Google Scholar]
- 23.Chagnac A, Weinstein T, Herman M, Hirsh J, Gafter U, Ori Y. The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol. 2003;14:1480–1486. doi: 10.1097/01.asn.0000068462.38661.89. [DOI] [PubMed] [Google Scholar]
- 24.Ohashi H, Oda H, Ohno M, Watanabe S. Weight reduction improves high blood pressure and microalbuminuria in hypertensive patients with obesity. Nippon Jinzo Gakkai Shi. 2001;43:333–339. Japanese. [PubMed] [Google Scholar]
- 25.Chang FT, Shin SJ, Hu SH, Tsai JH. Reduced urinary protein after dietary protein restriction instruction in proteinuric diabetic patients. J Formos Med Assoc. 1993;92:1060–1065. [PubMed] [Google Scholar]
- 26.Wheeler ML, Fineberg SE, Fineberg NS, Gibson RG, Hackward LL. Animal versus plant protein meals in individuals with type 2 diabetes and microalbuminuria: effects on renal, glycemic, and lipid parameters. Diabetes Care. 2002;25(8):1277–1282. doi: 10.2337/diacare.25.8.1277. [DOI] [PubMed] [Google Scholar]
- 27.Pomerleau J, Verdy M, Garrel DR, Nadeau MH. Effect of protein intake on glycaemic control and renal function in type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1993;36:829–834. doi: 10.1007/BF00400358. [DOI] [PubMed] [Google Scholar]
- 28.Gross JL, Zelmanovitz T, Moulin CC, et al. Effect of a chicken-based diet on renal function and lipid profile in patients with type 2 diabetes: a randomized crossover trial. Diabetes Care. 2002;25:645–651. doi: 10.2337/diacare.25.4.645. [DOI] [PubMed] [Google Scholar]
- 29.Teixeira SR, Tappenden KA, Carson L, et al. Isolated soy protein consumption reduces urinary albumin excretion and improves the serum lipid profile in men with type 2 diabetes mellitus and nephropathy. J Nutr. 2004;134:1874–1880. doi: 10.1093/jn/134.8.1874. [DOI] [PubMed] [Google Scholar]
- 30.Halvorsen BL, Carlsen MH, Phillips KM, et al. Content of redox-active compounds (ie, antioxidants) in foods consumed in the United States. Am J Clin Nutr. 2006;84:95–135. doi: 10.1093/ajcn/84.1.95. [DOI] [PubMed] [Google Scholar]
- 31.Nettleton JA, Steffen LM, Mayer-Davis EJ, et al. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2006;83:1369–1379. doi: 10.1093/ajcn/83.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nettleton JA, Steffen LM, Palmas W, Burke GL, Jacobs DR., Jr Associations between microalbuminuria and animal foods, plant foods, and dietary patterns in the Multiethnic Study of Atherosclerosis. Am J Clin Nutr. 2008;87:1825–1836. doi: 10.1093/ajcn/87.6.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]