Abstract
Forkhead O transcription factors (FOXO) playa pivotal role in the regulation of a myriad of cellular functions including cell cycle arrest, cell death, and protection from stress stimuli. Activation of cell survival pathways such as phosphoinositide-3-kinase/AKT/IKK or RAS/mitogen-activated protein kinase are known to phosphorylate FOXOs at different sites which cause FOXOs nuclear exclusion and degradation, resulting in the suppression of FOXO's transcriptional activity. Perturbation of FOXO's function leads to deregulated cell proliferation and accumulation of DNA damage, resulting in diseases such as cancer. Emerging evidence shows that active FOXO proteins are crucial for keeping cells in check; and inactivation of FOXO proteins is associated with tumorigenesis, including breast cancer, prostate cancer, glioblastoma, rhabdomyosarcoma, and leukemia. Moreover, clinically used drugs like paclitaxel, imatinib, and doxorubicin have been shown to achieve their therapeutic effects through activation of FOXO3a and FOXO3a targets. In this review, we will focus the novel functions of FOXOs revealed in recent studies and further highlight FOXOs as new therapeutic targets in a broad spectrum of cancers.
Background
Forkhead proteins, the transcription factors of winged-helix domain, are characterized by a conserved DNA-binding domain—the forkhead box among invertebrate and mammalian cells (1-3). Based on the forkhead box domain, the fork-head genes are grouped into 19 subclasses of FOX genes (4). Forkhead O transcription factors (FOXO) is one of the largest subgroups of forkhead family members. The FOXO sub-family contains four members (FOXO1, FOXO3, FOXO4, and FOXO6), which activate or repress multiple genes such as Bim and FasL involved in apoptosis (5, 6), p27kip (7) and cyclin D (8) in cell cycle regulation, GADD45a in DNA damage repair (2, 5, 6, 9), manganese superoxide dismutase in stress response (10), and glucose-6-phosphatase in metabolism (11).
FOXO3a overexpression has been shown to inhibit tumor growth in vitro and tumor size in vivo in breast cancer cells (12, 13). Furthermore, cytoplasmic location of FOXO3a seems to correlate with poor survival in patients with breast cancer (12). In addition, genetic deletion of five FOXOs alleles (FOXO1, FOXO3 and FOXO4) gives modest neoplastic phenotypes, whereas deletion of all of the FOXOs alleles generates progressive cancerous phenotypes, such as thymic lymphomas and hemangiomas. These data elucidated FOXOs as bona fide tumor suppressor genes (14). Recent studies also reveal the importance of FOXOs in preserving the self-renewal capacity of hematopoietic stem cells (15, 16), however, the details of these mechanisms are currently under work in progress.
Signals Regulating FOXO Activation and Destruction
Growth factors have been shown to down-regulate the expression and activity of FOXOs. During tumor development, the inhibition of FOXO3a's transcriptional activity promotes cell transformation, tumor progression, and angiogenesis (1, 2, 12, 17). FOXOs are regulated by a broad variety of external stimuli, such as epidermal growth factor receptors (EGFR), insulin, insulin-like growth factor, neurotrophins, nutrients, cytokines, and oxidative stress. These stimuli control FOXO protein expression, subcellular localization, or DNA binding and transcriptional activity. FOXO proteins are primarily regulated through posttranslational modifications, including phosphorylation, acetylation, ubiquitination, and possibly other modifications yet to be identified.
Many kinase pathways have been identified to activate FOXO activity, including the stress-activated c-Jun-NH2-kinase (JNK; refs. 18, 19), the mammalian orthologue of the Ste20-like protein kinase (MST1; ref. 20), and AMP-activated protein kinase (AMPK; refs. 21, 22). The phosphoinositide 3-kinase (PI3K)/AKT pathway was the first inhibitory pathway unraveled for FOXO factors, in addition to AKT, serum and glucocorticoid-induced kinase (SGK), casein kinase 1(CK1), dual-specificity tyrosine-phosphorylated-regulated kinase 1A (DYRK1A), IκB kinase β (IKKβ; ref. 12), and extracellular signal-regulated kinases 1 and 2 (ERK1/2; ref. 13; please see the following discussion) also suppressed FOXOs activity (Table 1).
Table 1.
Phosphorylation and activity alteration of FOXO members are regulated by an array of protein kinases
| Kinase | FOXO1 | FOXO3 | FOXO4 | FOXO6 | E3 ligase | Effect |
|---|---|---|---|---|---|---|
| AKT/SGK | T24 | T32 | T28 | T26 | Skp2 (FOXO1) | Inhibition |
| S256 | S253 | S193 | S184 | |||
| S319 | S315 | S258 | ||||
| IKKβ | S644 | ? | Inhibition | |||
| ERK1/2 | S294, S344, S425 | MDM2 (FOXO3a) | Inhibition | |||
| CK1 | S322 | S318 | S261 | ? | Inhibition | |
| S325 | S321 | S264 | ||||
| DYRK1A | S329 | S325 | S268 | ? | Inhibition | |
| CDK2 | S249 | ? | Inhibition | |||
| AMPK | T179, S399, S413, S555, S588, S626 | Activation | ||||
| JNK | T447, T451 | Activation | ||||
| MST1 | S207 | Activation |
Three OncokinasesTargeting FOXO3a
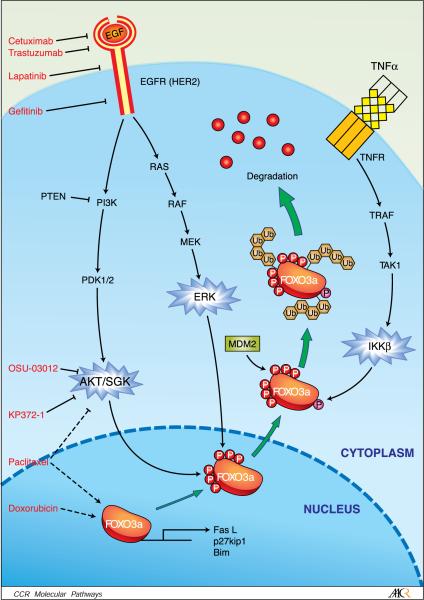
It is worth mentioning that AKT, IKK, and ERK are three commonly activated oncogenic kinases in human cancers. Interestingly, all three kinases target the same tumor suppressor gene, FOXO3a (Fig. 1). It is known that AKT, IKK, and ERK phosphorylate FOXO3a at different phosphorylation sites (Table 1) in response to growth factor and insulin stimulation from Caenorhabditis elegans to mammals. Similarly, phosphor-ylation of FOXO3a by these three oncogenic kinases results in FOXO3a translocation from the nucleus to the cytoplasm, and subsequent degradation. AKT-dependent phosphorylation of FOXO3a (Thr32, Ser253, and Ser315 for human FOXO3) enhances FOXO3a/14−3−3 interaction and promotes FOXO3a nuclear export to the cytoplasm, resulting in the repression of FOXO3a transcriptional function (2, 23). IKKh and ERK phosphorylate FOXO3a at Ser644 and Ser294, Ser344, and Ser425, respectively. All three kinase-mediated phosphorylations stimulate FOXO3a ubiquitination, resulting in its proteasomal degradation. Phosphorylation of FOXO3a by ERK may induce its conformational change and promote the physical interaction of FOXO3a with the E3 ubiquitin ligase MDM2, leading to subsequent FOXO3a ubiquitination and proteasomal degradation (13). MDM2 does not seem to serve as the E3 ligase responding to the degradation of AKT- and IKK-phosphorylated FOXO3a. However, another E3 ligase, Skp2, was reported to mediate AKT-dependant phosphorylation of FOXO1ubiquiti-nation and degradation. FOXO1 loss in which Skp2 was overexpressed has been found in a mouse lymphoma model (24). Noting this, it would be interesting to see whether Skp2 also serves as an E3 ligase for FOXO3a degradation. At this moment, it is still not yet clear which E3 ligase responds to IKKβ-mediated degradation of FOXO3a.
Fig. 1.
PI3K/AKT, RAS/ERK, andTNFR/IKK pathways are known to phosphorylate FOXO3a at different sites (indicated by different colors; see Table 1). Phosphorylation of FOXO3a causes FOXO3a nuclear exportation, thereby suppressing FOXO's transcriptional activity. Recently, FOXO3a phosphorylation by ERK was found to lead to FOXO3a down-regulation via MDM2-mediated proteasome degradation. Inhibition of the EGFR family that governs PI3K and RAS pathways by clinically used drugs, such as cetuximab, trastuzumab, lapatinib, and gefinitib effectively up-regulates FOXO3a. Moreover, targeting AKT/SGK byOSU-03012, paclitaxel, KP372−1, and doxorubicin (see Table 2), has also been shown to achieve their therapeutic effects through activation of FOXO3a and FOXO3a targets such as FasL, Bim, and p27.
Additionally, CK1, DYRK1A, and CDK2 also phosphorylate FOXOs at various sites to inhibit FOXOs activity. It is interesting to note that AKT and ERK were shown to up-regulate CK1(25) and CDK2 (26) activity, respectively, and DYRK1A was reported to prolong ERK activity by forming and stabilizing the RAS/B-RAF/MEK1complex (27). Therefore, it would be attractive to address whether DYRK1A inactivated FOXO3a may be mediated by ERK. Phosphorylation of FOXOs by AKT, IKK, ERK, CK1, CDK2, and DYRK1A universally leads to FOXO's inhibition. However, whether CK1, DYRK1A, and CDK2 are also involved in tumorigenesis is yet to be determined. Because AKT, IKK, and ERK govern most of the signaling pathways in regulating FOXOs on top of the other kinases, inhibition of AKT, IKK, and ERK is likely to be sufficient to restore FOXO's function.
A similarly interesting story is that, in addition to FOXO3a, AKT, ERK, and IKKβ also inactivate another tumor suppressor, the tuberous sclerosis 1/2 (TSC1/2) complex. Phosphorylation of TSC2 (by AKT and ERK; refs. 28, 29) and TSC1(by IKKβ; ref. 30) results in the disruption of the TSC1/2 complex, and thereby activates the oncogenic mTOR signaling contributing to tumor progression. Similar to FOXO3a, the non – phosphorylation-mimick mutations of TSC1/2 resist inhibition of the activated kinases and block cell growth and tumorigenesis. It would be interesting to understand how these three oncogenic kinases coordinately regulate FOXO3a and TSC1/2 or if other modulations are also involved. The fact that AKT, IKKβ, and ERK can phosphorylate, down-regulate, and inactivate the tumor suppressors, FOXO3a and TSC, raises an attractive question of whether the three oncogenic kinases also target other common tumor suppressors to exert their oncogenicity. It is also appealing to ask whether other FOXO-targeting kinases, such as CK1, CDK2, and DYRK1A, can phosphorylate and regulate TSC. In all, targeting AKT, ERK, and IKKβ with inhibitors to allow the reactivation of FOXOs, TSC, and maybe more, may provide an attractive strategy for anticancer therapeutics.
Clinical-Translational Advances
FOXO factors have been shown to be deregulated in several tumor types including breast cancer, prostate cancer, glioblastoma, rhabdomyosarcoma, and leukemia (1, 31). Loss of FOXO1a through chromosomal deletion (13q14) was shown to promote androgen-independent prostate cancers (32). In addition, cytoplasmic localization or down-regulation of FOXOs via AKT, IKK, and ERK-mediated phosphorylation was observed in breast cancers (12, 13). Inactivation of FOXOs seems to be an important step in carcinogenic transformation, increasing the activity of FOXOs represents a reasonable therapeutic strategy. Although targeting oncoproteins with small molecule inhibitors is a prevailing strategy for current cancer therapy, developing small molecules that restore the function of tumor suppressors is equally important and even more challenging.
Therapeutics and Compounds Enhancing FOXO's Nuclear Localization
It was shown that the FOXO families can serve as therapeutic targets in various cancers by mediating the cytostatic and cytotoxic effects of various chemotherapeutic drugs (Table 2).
Table 2.
Clinical anticancer treatments target FOXO3a through three oncogenic kinases (AKT, IKK, and ERK) and various signaling pathways
| Cancer type | Drug or therapy | Protein targeted | AKT, IKK, and ERK affected | Signal pathway affected |
|---|---|---|---|---|
| Breast cancer | Paclitaxel | FOXO3a | AKT | Effected tubulin, decrease in AKT and increase in JNK activity, leading to FOXO3a activation and induction of Bim (33, 34) |
| Acute myeloid leukemia | KP372−1 | FOXO3a | AKT | Inhibition of AKT activity, activating FOXO3a and inducing apoptosis (35) |
| Chronic myeloid leukemia | Imatinib (STI571 or Glivec) | FOXO3a | AKT and ERK | BCR-ABL inhibition, leading to FOXO3a dephosphorylation and Bim-dependent apoptosis; down-regulation of ID1 and erythroid differentiation (39, 40) |
| Osteosarcoma, breast cancer | Ionizing radiation (IR or UV) | FOXO3a | ? | Induction of FOXO3a in p53-null cells and enhanced ATM activity (9, 44) |
| Breast, prostate, kidney, ovarian, non–small cell lung cancer | Trastuzumab, cetuximab, lapatinib, gefitinib | FOXO3a | AKT and ERK | EGFR blockade; induction of FOXO3a and expression of proapoptotic FOXO3a target BNIP3L1 (42) |
| Leukemia | Doxorubicin | FOXO3a | ? | Activation of FOXO3a and MDR1 (38) |
| Melanoma | Adenovirus-mediated transfer of constitutively active FOXO3A | FOXO3a | ? | Activation of FOXO3a and induction of apoptosis (46) |
| Breast cancer | OSU-03012/tamoxifen | FOXO3a | AKT | Inhibition of PDK-1/AKT activity, activating FOXO3a and sensitizing ER-negative breast cancer cells to tamoxifen (41) |
For example, the chemotherapeutic drugs paclitaxel (33, 34) and KP372−1(a multiple kinase inhibitor; ref. 35), which are currently used in the treatment of breast carcinoma and acute myeloid leukemia, can activate FOXO3a by reducing AKT activity. Paclitaxel also activates c-Jun-NH2-kinase, which phosphorylates several sites within the DNA-binding domain of FOXO3a (34). These phosphorylation residues reduce the interaction between FOXO3a with 14−3−3 protein, leading to impaired nuclear export of FOXO3a mediated by 14−3−3, and thereby increasing FOXO3a activity (23, 34).
Some attempts were made to promote FOXO's function by regulating its subcellular localization. For example, screening of marine natural product extracts identified a bromotyrosine derivative, psammaplysene A, that can cause the relocalization of FOXO1to the nucleus in PTEN-deficient cells (36). In addition, CRM1inhibitors have also been discovered to enhance FOXO1activity by blocking FOXO1nuclear export (37).
Therapeutics Activating FOXOs and Downstream Genes
Several findings have concluded the clinical importance of activating FOXO3a and its downstream genes in various anticancer therapeutics (31). TP53, a well-known tumor suppressor gene, is mutated in 50% of human cancers. These p53 mutations play an essential role in tumorigenesis and drug resistance. Different from p53, FOXO3a mutation has not yet been found in human cancer, which makes therapeutics activating FOXO3a more appealing than others. And indeed, activation of FOXO3a in p53-mutated cancer cells is sufficient for tumor suppression (12, 13). For example, doxorubicin has shown to activate FOXO3a to induce the expression of the multidrug resistance gene ABCB1 (MDR1) in K562 doxorubicin-sensitive leukemic cells (38). In chronic myeloid leukemia, inhibition of BCR-ABL by imatinib activates FOXO3a and induces Bim-dependent apoptosis (39). Imatinib also induces erythroid differentiation through repression of the ID1 gene transcription by FOXO3a (40).
Due to the potent antitumor activity of FOXO3a, drugs that activate FOXO3a can be used in combination with other therapeutic agents to sensitize tumor cells. A novel phosphoinositide-dependent protein kinase-1/AKT inhibitor, OSU-03012, was recently reported to sensitize ER-negative breast cancer cells to tamoxifen through desuppressing AKT downstream effectors, including FOXO3a and p27 (41). It was also shown that EGFR family blockade by the blocking antibodies (such as trastuzumab or cetuximab) could inhibit the PI3K pathway and induce FOXO3a activity, which leads to transcriptional activation of the proapoptotic BNIP3L gene (42). Blocking EGFR/HER2 was used as single agent and in combination with other agents in clinical trials against breast, prostate, kidney, ovarian, and lung cancers (31). It would also be intriguing to see if the re-introduction of active FOXO3a could sensitize resistant cancer cells to small molecules targeting the EGFR family (such as lapatinib and gefitinib; ref. 31).
Activation of FOXO3s can also be effective in overcoming tumor resistance against radiotherapy (31). It was reported that FOXO3a activation efficiently induced Bim and apoptosis in p53-null osteosarcoma cell lines exposed to ionizing radiation (9). Recently, FOXO3a was shown to enhance ataxia telangiectesia mutated (ATM) activity through the binding of the carboxyl terminal domain of FOXO3a to the FAT (FRAP, ATM, and TRRAP; ref. 43) domain of ATM, thereby contributing to the activation of ATM (44). These data suggest that FOXO3a may be an important factor for radiation-inducing apoptosis. A combination therapy of radiation with chemo-therapy targeting FOXO3a may be able to sensitize resistant tumor cells to radiotherapy. Especially with our recent findings of FOXO3a regulation by the RAS-MEK-ERK pathway, AZD6244, a MEK1/2 inhibitor that is currently used in phase II clinical trials in various cancers (45), might serve as a potential candidate to synergize with other anticancer therapeutics through activating FOXO3a.
It was reported that adenovirus-mediated transfer of constitutively active FOXO3a induces apoptosis in melanoma cells (46). Further investigation needs to be done in vivo with various cancer types (31). Gene therapy using cancer-specific expression vector has shown both effectiveness and specificity in killing human tumors (47-50). Therefore, using a cancer-specific gene therapy to deliver functional FOXO3a may also provide a potential anticancer therapeutic strategy.
However, it is worth noting that targeting FOXO proteins may be complicated by potential feedback mechanisms. Most current evidence supports the notion that activation of FOXO3a is an important player in mediating cell apoptosis in a broad spectrum of tumors (31). Alternatively, FOXO1 has recently been shown to increase protein kinase B phosphorylation by repressing expression of TRB3, a pseudokinase inhibiting protein kinase B phosphorylation (31, 51). The FOXO homologue, DAF16, has been shown to induce the expression of the insulin receptor and increase PI3K activity in C. elegans (52). Thus, FOXO transcription factors may engage in a feedback regulation towards the upstream PI3K/AKT pathway. Therefore, while exploring FOXOs as a therapeutic agent, one may need to consider prevention of FOXO's feedback loop. Our knowledge of FOXO is continually advancing and recent studies reveal great rationales and opportunities to adopt FOXO family members as therapeutic targets in cancers. AKT, IKKh, and ERK phosphorylate and down-regulate the tumor suppressors FOXO3a and TSC. Restoring FOXO's and TSC's activity from the regulation of these kinases can be effective for cancer treatment. In recent years, the development and success of multitargeted agents has shed light on treating cancers by inhibiting more than one receptor tyrosine kinase at once. For example, lapatinib, a small molecule inhibitor, has shown effectiveness in targeting two EGFR family members, EGFR and HER2, which used to be therapeutically aimed by single targeting reagents, such as gefitinib (a receptor tyrosine kinase inhibitor) and trastuzumab (a humanized monoclonal antibody), respectively (53, 54). Herein, based on the previous findings in our model, developing therapeutic agents to multitarget AKT, IKKβ, and ERK will be expected to boost antitumor activity through the maximal activation of FOXOs and TSC, and also achieve maximal therapeutic efficacy with the least resistance to cancer treatment.
Acknowledgments
We thank Drs. Chun-Ju Chang, Jeng C. Cheng, Stephanie A. Miller, and Adam M. LaBaff for editing this manuscript. We also thank Jung-Mao Hsu for the diagram.
Grant support: NIH grant P01 CA 099031, M. D. Anderson Cancer Center Specialized Programs of Research Excellence in breast cancer CA116199, and was partially supported by the National Breast Cancer Foundation, Inc., the Patel Memorial Breast Cancer Research Foundation, the Breast Cancer Research Foundation grant, the Marcus Foundation, and the Kadoorie Charitable Foundation.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Arden KC. Multiple roles of FOXO transcription factors in mammalian cells point to multiple roles in cancer. Exp Gerontol. 2006;41:709–17. doi: 10.1016/j.exger.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 2.Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–25. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 3.Tsai KL, Sun YJ, Huang CY, Yang JY, Hung MC, Hsiao CD. Crystal structure of the human FOXO3a-DBD/DNA complex suggests the effects of post-translational modification. Nucleic Acids Res. 2007;35:6984–94. doi: 10.1093/nar/gkm703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14:142–6. [PubMed] [Google Scholar]
- 5.Finnberg N, El-Deiry WS. Activating FOXO3a, NF-κB and p53 by targeting IKKs: an effective multi-faceted targeting of the tumor-cell phenotype? Cancer Biol Ther. 2004;3:614–6. doi: 10.4161/cbt.3.7.1057. [DOI] [PubMed] [Google Scholar]
- 6.Tran H, Brunet A, Griffith EC, Greenberg ME. The many forks in FOXO's road. Sci STKE. 2003;2003:RE5. doi: 10.1126/stke.2003.172.re5. [DOI] [PubMed] [Google Scholar]
- 7.Dijkers PF, Medema RH, Pals C, et al. Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27(KIP1). Mol Cell Biol. 2000;20:9138–48. doi: 10.1128/mcb.20.24.9138-9148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt M, Fernandez de Mattos S, van der Horst A, et al. Cell cycle inhibition by FoxO forkhead transcription factors involves downregulation of cyclin D. Mol Cell Biol. 2002;22:7842–52. doi: 10.1128/MCB.22.22.7842-7852.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang JY, Xia W, Hu MC. Ionizing radiation activates expression of FOXO3a, Fas ligand, and Bim, and induces cell apoptosis. Int J Oncol. 2006;29:643–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Kops GJ, Dansen TB, Polderman PE, et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–21. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 11.Onuma H, Vander Kooi BT, Boustead JN, Oeser JK, O'Brien RM. Correlation between FOXO1a (FKHR) and FOXO3a (FKHRL1) binding and the inhibition of basal glucose-6-phosphatase catalytic subunit gene transcription by insulin. Mol Endocrinol. 2006;20:2831–47. doi: 10.1210/me.2006-0085. [DOI] [PubMed] [Google Scholar]
- 12.Hu MC, Lee DF, Xia W, et al. IκB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–37. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- 13.Yang JY, Zong CS, Xia W, et al. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol. 2008;10:138–48. doi: 10.1038/ncb1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paik JH, Kollipara R, Chu G, et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–23. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyamoto K, Araki KY, Naka K, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–12. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Tothova Z, Kollipara R, Huntly BJ, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–39. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Potente M, Urbich C, Sasaki K, et al. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J Clin Invest. 2005;115:2382–92. doi: 10.1172/JCI23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Essers MA, deVries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC. Functional interaction between β-catenin and FOXO in oxidative stress signaling. Science. 2005;308:1181–4. doi: 10.1126/science.1109083. [DOI] [PubMed] [Google Scholar]
- 19.Wang MC, Bohmann D, Jasper H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell. 2005;121:115–25. doi: 10.1016/j.cell.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 20.Lehtinen MK, Yuan Z, Boag PR, et al. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125:987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 21.Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27:2276–88. doi: 10.1038/onc.2008.21. [DOI] [PubMed] [Google Scholar]
- 22.Greer EL, Oskoui PR, Banko MR, et al. The energy sensorAMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem. 2007;282:30107–19. doi: 10.1074/jbc.M705325200. [DOI] [PubMed] [Google Scholar]
- 23.Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 24.Huang H, Regan KM, Wang F, et al. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc Natl Acad Sci U S A. 2005;102:1649–54. doi: 10.1073/pnas.0406789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giamas G, Hirner H, Shoshiashvili L, et al. Phosphorylation of CK1δ: identification of Ser370 as the major phosphorylation site targeted by PKA in vitro and in vivo. Biochem J. 2007;406:389–98. doi: 10.1042/BJ20070091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lents NH, Keenan SM, Bellone C, Baldassare JJ. Stimulation of the Raf/MEK/ERK cascade is necessary and sufficient for activation and Thr-160 phosphorylation of a nuclear-targeted CDK2. J Biol Chem. 2002;277:47469–75. doi: 10.1074/jbc.M207425200. [DOI] [PubMed] [Google Scholar]
- 27.Kelly PA, Rahmani Z. DYRK1A enhances the mitogen-activated protein kinase cascade in PC12 cells by forming a complex with Ras, B-Raf, and MEK1. Mol Biol Cell. 2005;16:3562–73. doi: 10.1091/mbc.E04-12-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–57. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 29.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–93. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 30.Lee DF, Kuo HP, Chen CT, et al. IKKβ suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440–55. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 31.Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–59. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 32.Dong XY, Chen C, Sun X, et al. FOXO1A is a candidate for the 13q14 tumor suppressor gene inhibiting androgen receptor signaling in prostate cancer. Cancer Res. 2006;66:6998–7006. doi: 10.1158/0008-5472.CAN-06-0411. [DOI] [PubMed] [Google Scholar]
- 33.Sunters A, Fernandez de Mattos S, Stahl M, et al. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J Biol Chem. 2003;278:49795–805. doi: 10.1074/jbc.M309523200. [DOI] [PubMed] [Google Scholar]
- 34.Sunters A, Madureira PA, Pomeranz KM, et al. Paclitaxel-induced nuclear translocation of FOXO3a in breast cancer cells is mediated by c-Jun NH2-terminal kinase and Akt. Cancer Res. 2006;66:212–20. doi: 10.1158/0008-5472.CAN-05-1997. [DOI] [PubMed] [Google Scholar]
- 35.Zeng Z, Samudio IJ, Zhang W, et al. Simultaneous inhibition of PDK1/AKT and Fms-like tyrosine kinase 3 signaling by a small-molecule KP372−1 induces mitochondrial dysfunction and apoptosis in acute myelogenous leukemia. Cancer Res. 2006;66:3737–46. doi: 10.1158/0008-5472.CAN-05-1278. [DOI] [PubMed] [Google Scholar]
- 36.Schroeder FC, Kau TR, Silver PA, Clardy J. The psammaplysenes, specific inhibitors of FOXO1a nuclear export. J Nat Prod. 2005;68:574–6. doi: 10.1021/np049624z. [DOI] [PubMed] [Google Scholar]
- 37.Kau TR, Schroeder F, Ramaswamy S, et al. A chemical genetic screen identifies inhibitors of regulated nuclear export of a Forkhead transcription factor in PTEN-deficient tumor cells. Cancer Cell. 2003;4:463–76. doi: 10.1016/s1535-6108(03)00303-9. [DOI] [PubMed] [Google Scholar]
- 38.Hui RC, Francis RE, Guest SK, et al. Doxorubicin activates FOXO3a to induce the expression of multidrug resistance gene ABCB1 (MDR1) in K562 leukemic cells. Mol CancerTher. 2008;7:670–8. doi: 10.1158/1535-7163.MCT-07-0397. [DOI] [PubMed] [Google Scholar]
- 39.Essafi A, Fernandez de Mattos S, Hassen YA, et al. Direct transcriptional regulation of Bim by FoxO3a mediates STI571-induced apoptosis in Bcr-Abl-expressing cells. Oncogene. 2005;24:2317–29. doi: 10.1038/sj.onc.1208421. [DOI] [PubMed] [Google Scholar]
- 40.Birkenkamp KU, Essafi A, van der Vos KE, et al. FOXO3a induces differentiation of Bcr-Abl-transformed cells through transcriptional down-regulation of Id1. J Biol Chem. 2007;282:2211–20. doi: 10.1074/jbc.M606669200. [DOI] [PubMed] [Google Scholar]
- 41.Weng SC, Kashida Y, Kulp SK, et al. Sensitizing estrogen receptor-negative breast cancer cells to tamoxifen with OSU-03012, a novel celecoxib-derived phosphoinositide-dependent protein kinase-1/Akt signaling inhibitor. Mol CancerTher. 2008;7:800–8. doi: 10.1158/1535-7163.MCT-07-0434. [DOI] [PubMed] [Google Scholar]
- 42.Real PJ, Benito A, Cuevas J, et al. Blockade of epidermal growth factor receptors chemosensitizes breast cancer cells through up-regulation of Bnip3L. Cancer Res. 2005;65:8151–7. doi: 10.1158/0008-5472.CAN-05-1134. [DOI] [PubMed] [Google Scholar]
- 43.Bosotti R, Isacchi A, Sonnhammer EL. FAT: a novel domain in PIK-related kinases. Trends Biochem Sci. 2000;25:225–7. doi: 10.1016/s0968-0004(00)01563-2. [DOI] [PubMed] [Google Scholar]
- 44.Tsai WB, Chung YM, Takahashi Y, Xu Z, Hu MC. Functional interaction between FOXO3a and ATM regulates DNA damage response. Nat Cell Biol. 2008;10:460–7. doi: 10.1038/ncb1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davies BR, Logie A, McKay JS, et al. AZD6244 (ARRY-142886), a potent inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2 kinases : mechanism of action in vivo, pharmacokinetic/pharmacodynamic relationship, and potential for combination in preclinical models. Mol CancerTher. 2007;6:2209–19. doi: 10.1158/1535-7163.MCT-07-0231. [DOI] [PubMed] [Google Scholar]
- 46.Gomez-Gutierrez JG, Souza V, Hao HY, et al. Adenovirus-mediated gene transfer of FKHRL1 triple mutant efficiently induces apoptosis in melanoma cells. Cancer Biol Ther. 2006;5:875–83. doi: 10.4161/cbt.5.7.2911. [DOI] [PubMed] [Google Scholar]
- 47.Xie X, Xia W, Li Z, et al. Targeted expression of BikDD eradicates pancreatic tumors in noninvasive imaging models. Cancer Cell. 2007;12:52–65. doi: 10.1016/j.ccr.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 48.Lee CM, Lo HW, Shao RP, et al. Selective activation of ceruloplasmin promoter in ovarian tumors: potential use for gene therapy. Cancer Res. 2004;64:1788–93. doi: 10.1158/0008-5472.can-03-2551. [DOI] [PubMed] [Google Scholar]
- 49.Day CP, Rau KM, Qiu L, et al. Mutant Bik expression mediated by the enhanced minimal topoisomerase IIα promoter selectively suppressed breast tumors in an animal model. Cancer Gene Ther. 2006;13:706–19. doi: 10.1038/sj.cgt.7700945. [DOI] [PubMed] [Google Scholar]
- 50.Chen JS, Liu JC, Shen L, et al. Cancer-specific activation of the survivin promoter and its potential use in gene therapy. Cancer GeneTher. 2004;11:740–7. doi: 10.1038/sj.cgt.7700752. [DOI] [PubMed] [Google Scholar]
- 51.Matsumoto M, Han S, Kitamura T, Accili D. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J Clin Invest. 2006;116:2464–72. doi: 10.1172/JCI27047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Puig O, Tjian R. Transcriptional feedback control of insulin receptor by dFOXO/FOXO1. Genes Dev. 2005;19:2435–46. doi: 10.1101/gad.1340505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wheatley-Price P, Shepherd FA. Epidermal growth factor receptor inhibitors in the treatment of lung cancer: reality and hopes. Curr Opin Oncol. 2008;20:162–75. doi: 10.1097/CCO.0b013e3282f335a3. [DOI] [PubMed] [Google Scholar]
- 54.Harari PM, Allen GW, Bonner JA. Biology of interactions: antiepidermal growth factor receptor agents. J Clin Oncol. 2007;25:4057–65. doi: 10.1200/JCO.2007.11.8984. [DOI] [PubMed] [Google Scholar]