Abstract
Adenomas and nodules of the human adrenal cortex are common, whereas adrenocortical carcinomas are rare. Genes such as IGF2 have been suggested to be important in human adrenocortical tumorigenesis but their role has not been directly investigated. We describe here elements of a system in which hypotheses concerning the molecular basis for the formation of benign and malignant adrenocortical lesions can be experimentally tested. Various viral vectors have been employed in the study of adrenocortical cell biology. Because of the low proliferative rate of primary human adrenocortical (pHAC) cells, a lentiviral system is ideal for transducing these cells with genes that may alter their characteristics or cause them to acquire benign or malignant tumorigenicity. Cultures of pHAC cells were highly infectible with lentiviruses and showed a higher proliferative potential when transduced with a lentivirus encoding IGF2. For tumorigenesis studies of genetically modified adrenocortical cells, we use RAG2-/-, γc-/- mice. Using this immunodeficient mouse model, we established an orthotopic intra-adrenal cell transplantation technique for adrenocortical cells that should be be of value for future studies of the experimental conversion of human adrenocortical cells to a benign or malignant tumorigenic state.
Keywords: adrenal tumorigenesis, viral systems, immunodeficient mice, orthotopic cell transplantation
1. Introduction
Adenomas and nodules of the human adrenal cortex are common, whereas adrenocortical carcinomas are rare (Bornstein et al., 1999; Stratakis and Chrousos, 2000; Barlaskar and Hammer, 2007; Libe et al., 2007). In order to understand the pathogenesis of these adrenocortical lesions, a system is needed in which hypotheses concerning the molecular basis for the formation of nodules, adenomas and carcinomas can be experimentally tested. In this lab, we have worked toward the development of such an appropriate system. The major questions that we wish to address are (a) the experimental determination of the potential of various genes to confer on normal human adrenocortical cells the ability to form a range of tissue structures (corresponding to nodules, adenomas, and carcinomas) and (b) the potential for progression of cells from a benign to a malignant state, i.e. whether there is experimental evidence for the series nodule --> adenoma --> carcinoma in the adrenal cortex. Such progression is well established in other organs such as the colon (Vogelstein and Kinzler, 1993). Here we describe an experimental system in which we model human adrenocortical tumorigenesis by the use of primary human adrenocortical cells, viral vectors, and cell transplantation in immundodeficient mice.
In 2000, Hanahan and Weinberg proposed the concept of six classes of alterations in cell physiology, resulting from mutations or epigenetic changes, that would result in malignant growth: self-sufficiency in growth signals, insensitivity to growth inhibitory signals, resistance to apoptosis, limitless replicative potential, sustained angiogenesis, and tissue invasion and metastasis (Hanahan and Weinberg, 2000). How these principles apply to adrenocortical tumorigenesis is not yet clear; nodules and adenomas presumably have undergone fewer or different genetic changes than carcinomas. In this regard, microarray analysis to distinguish patterns of gene expression in different adrenal lesions is useful (Bornstein and Hornsby, 2005). One important gene that is more highly expressed in carcinomas is IGF2 (Giordano et al., 2003; Slater et al., 2006). Data in experimental systems show an autocrine role for IGF2 in metabolism, growth and differentiation of adrenocortical cells (Ilvesmaki et al., 1993; Weber et al., 1999). We show here the components of a complete experimental system for the study of the action of genes like IGF2 in primary human adrenocortical cells and their effects in an in vivo setting, particularly with respect to orthotopic cell transplantation in immunodeficient mice.
2. Viral vectors for delivering potentially tumorigenic factors
Viral gene delivery systems have been tremendously valuable in elucidating molecular mechanisms involved in a wide variety of diseases as well as potentially valuable as therapeutic tools. There are three viral systems that have been employed: adenovirus, retrovirus and lentivirus.
2.1 Adenoviruses
There is evidence that the adrenal gland is a major target for adenoviruses; a selective tropism of this virus for the adrenal cortex has been demonstrated in experimental animals (Wang et al., 2003). Previously we transduced primary bovine adrenocortical cells with recombinant adenoviruses in several studies (Wolkersdorfer et al., 2002; Alesci et al., 2002; Chen and Hornsby, 2006). Our data showed that adenoviral-mediated gene transfer can interfere with the normal function of the adrenal cortex, leading to an impairment of adrenal steroidogenesis (Wolkersdorfer et al., 2002; Alesci et al., 2002). A single intra-adrenal injection of an adenoviral vector carrying the CYP21 gene can compensate for the biochemical, endocrine, and histological alterations in 21-hydroxylase deficient mice (Tajima et al., 1999). As useful as these demonstrations are, adenoviral vectors do not fulfil the requirement of permanent genetic modification of adrenocortical cells. This leads us to consider the use of retroviruses and lentiviruses as vectors that can result in stable genetic modification of target cells.
2.2 Retroviruses
Unlike adenoviruses, retroviruses based on Moloney murine leukemia virus (MoMLV) integrate into the genome, leading to stable long-term expression. Previously, we showed that pBAC (primary bovine adrenocortical) and pHAC (primary human adrenocortical) cells co-transduced with retroviruses encoding RasG12V and SV40 T antigen can produce malignant tumors when transplanted in the subrenal capsule of immunodeficient mice. However, genetically modified pBAC cells were more aggressive in comparison to genetically modified pHAC cells (Sun et al., 2004). This may suggest that the pHAC cells may require additional factors for tumorigenicity in comparison to pBAC cells. Retroviruses remain the main means of genetic modification for investigation of experimental conversion of adrenocortical cells to a malignant state. However, retroviral systems have the limitation that they can only infect dividing cells. Adult-derived pHAC cells divide slowly in culture (Hornsby and McAllister, 1991), thus making it difficult to obtain a high percentage of infected cells using retroviruses. Therefore it is desirable to employ a method for genetic modification that provides both long-term stable expression and that would be equally applicable to both dividing and nondividing cells.
2.3 Lentiviruses
Lentiviruses are retroviruses based on HIV-1 and similar viruses that can transduce both dividing and nondividing cells (Amado and Chen, 1999; Bartosch and Cosset, 2004). Like retroviruses based on MoMLV, lentiviruses in use for genetic modification are defective and unable to replicate in target cells. We tested the efficiency of a lentivirus encoding GFP in infection of pHAC cells. The transduction efficiency was very high (Figure 1). When pHAC cells were transduced with a lentivirus encoding IGF2 they exhibited a long-term stimulation of proliferation in comparison with cells that were transduced with a control lentivirus encoding GFP (Figure 2).
Figure 1.

Primary human adrenocortical cells can be efficiently transduced with lentiviruses. Adrenocortical cells obtained from a 47 year-old male donor were cultured using conditions previously established for these cells (Hornsby and McAllister, 1991) with the exception that collagen-coated dishes were used rather than regular tissue culture plastic. A lentivirus encoding enhanced green fluorescent protein, pWPXL-GFP, was produced using conventional methods in 293T cells (Pear, 1996). One week after plating the cells in culture, unconcentrated virus was added to the cells in the presence of 20 μg/ml Polybrene for 3 hours. The efficiency of infection was monitored using fluorescence microscopy; the images above show cells photographed by fluorescence and by phase-contrast at 72 hours after infection.
Figure 2.

In vitro effects of lentivirally delivered IGF2. Adrenocortical cells obtained from a 67 year-old female donor were cultured as described in the legend to Figure 1. Cells were infected with a lentivirus encoding IGF2 (pReceiver-Lv01-IGF2 from Genecopoeia Inc.) or with a control GFP lentivirus. Following infection, cells were grown with subcultures at 10-day intervals for 30 days. After this period of time in culture, relatively few cells remained in the control culture of cells expressing GFP (a) whereas continued survival and proliferation is evident in the culture of cells expressing IGF2 (b).
3. Immunodeficient host animal for cell transplantation studies
Another major concern for elucidation of the pathogenesis of benign and malignant lesions is the choice of an appropriate experimental model. The “gold standard” for testing the tumorigenic properties of genetically modified cells is cell transplantation in immunodeficient mice. For carcinomas, most types of immunodeficient mice can act as hosts for the growth of genetically modified human cells. There are also well-established surrogate cell culture assays for tumorigenicity, such as focus formation and anchorage-independent growth (Shin et al., 1975; Stiles et al., 1975). However, these methods are not equally applicable to experimental investigation of cells that may give rise to benign tumors as well as malignancies.
While nude and scid mice strains have been popular for testing tumorigenicity of human cells, they still retain natural killer (NK) cell activity that can affect the growth of transplanted genetically modified cells. In this lab over the last few years we have used a more profoundly immunodeficient mouse model, RAG2-/-, γc-/-, which lacks NK cell activity as well as T and B cells (Cooper et al., 2001). They serve as an excellent host for pBAC and pHAC cells that express Ras and SV40 T antigen, permitting the full expression of the malignant properties of these cells (Sun et al., 2004).
4. Sites in the body for transplantation of cells that may be tumorigenic
Cell transplantation provides a technique for the study of the physiology, cell biology, and molecular biology of adrenocortical cells in a three-dimensional vascularized tissue structure in a host animal. Cells survive, become vascularized by invasion of host endothelial cells, and secrete hormones into the circulation.
In experimental cell transplantation in immunodeficient mice, certain sites in the body and specific cell transplantation methods have been found to give superior results when investigating human cell tumorigenicity. In our studies on adrenocortical cells we have used both subrenal capsule cell transplantation and subcutaneous cell transplantation for various studies. Recently we began a study of the feasibility of orthotopic cell transplantation for adrenocortical cell studies and we report on preliminary observations on the use of this technique below.
4.1 Subcutaneous transplantation
In order to investigate the potential of cells for nodule, adenoma or carcinoma formation, a model is needed in which cells survive and function in a host animal independent of their potential to produce a malignant tumor. In conventional nude mouse tumorigenicity assays, the subcutaneous or intramuscular injection of cells that are not malignant causes them to die (Shin et al., 1975; Stiles et al., 1975). This is essentially how the assay is used; if the cells grow they are termed “tumorigenic” and if they do not grow, i.e. if they die, they are termed “nontumorigenic”. Thus, an assay in which cells are simply injected as a suspension under the skin of the mouse is unsuitable for studying cells that may not be aggressively malignant or may be normal or close to normal.
We first showed some time ago that normal pHAC and pBAC cells could survive and form a functional vascularized tissue structure when transplanted beneath the skin after being emebedded in collagen gel (Popnikolov and Hornsby, 1999). This method proved useful for malignant as well as normal cells (Sun et al., 2004). It remains a valuable technique because tumor formation in this site is readily observable by noninvasive techniques (Sun et al., 2004).
4.2 Subrenal capsule transplantation
The subrenal capsule site is an excellent site for the growth of normal and abnormal adrenocortical cells. In accordance with their genetic modifications, adrenocortical cells transplanted under the kidney capsule of immunodeficient mice can produce (i) normal functional vascularized tissue, or (ii) a benign (expanding, non-invasive) adenoma-like structure, or (iii) a malignant (invasive and metastizing) tumor. Thus, the model provides a way to investigate what genetic changes can convert normal adrenocortical cells into a benign, as well as a malignant, lesion, or produce a tissue structure with no detectable abnormalities (Thomas et al., 1997; Thomas et al., 2000; Thomas et al., 2002; Thomas et al., 2003; Huang et al., 2007). However, as excellent as this site is, it is still ectopic for adrenocortical cells, and therefore we recently turned to orthotopic cell transplantation to address this issue.
4.3 Orthotopic cell transplantation
Orthotopic transplantation models are important for a complete understanding of tumor biology (Togo et al., 1995; Singh et al., 1997; Killion et al., 1998). The properties of malignant and nonmalignant cells depend on their local microenvironment. In the environment of their normal host tissue, the behavior of cells may differ considerably from that in ectopic sites.
Orthotopic cell transplantation has not been reported for the adrenal cortex. However, neuroblastoma cells have been transplanted into the mouse adrenal gland. These studies showed superior rates of tumor growth, vascularization, and metastasis in comparison to ectopically transplanted cells (Khanna et al., 2002; Henriksson et al., 2004; Joseph et al., 2005).
We therefore began preliminary experiments to test whether orthotopic transplantation was feasible for cells of adrenocortical origin. We established an orthotopic transplantation method using pBAC cells transduced with retroviruses encoding Ras/SV40 T antigen/hTERT/GFP (Sun et al., 2004), by injection of the cells into the adrenal glands of RAG2-/-, γc-/- mice (Figure 3). The results of these preliminary studies show that orthotopic cell transplantation by in the mouse adrenal gland is technically feasible, as SV40 T antigen-espressing cells were found within the gland and expanding beyond the gland itself.
Figure 3.
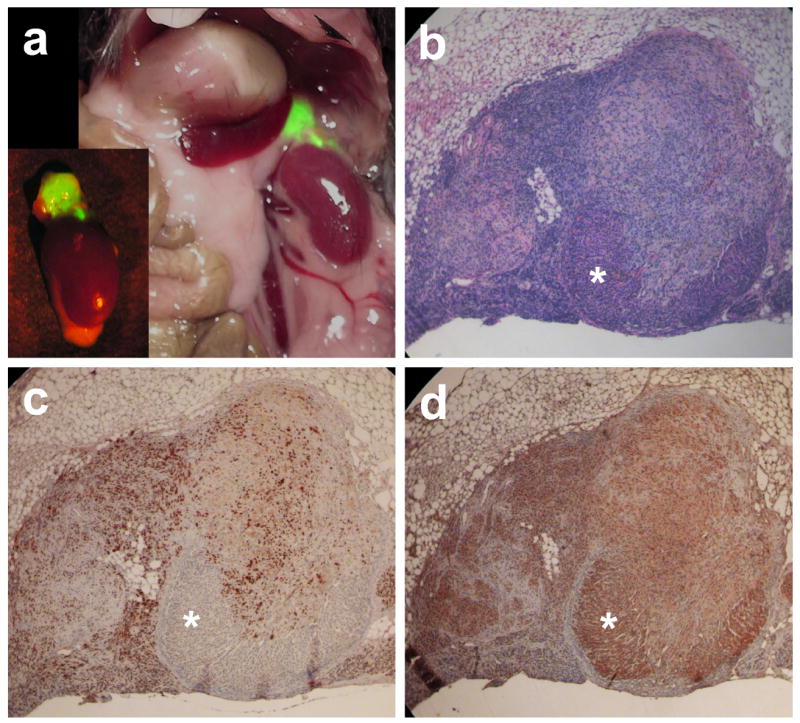
Orthotopic adrenocortical cell transplantation. Primary bovine adrenocortical (pBAC) cells were co-infected with two retroviruses (one encoding Ras, SV40 T antigen and GFP and a second encoding hTERT; Sun et al., 2004). All cells prior to transplantation were positive for expression of both SV40 T antigen and GFP. Cells (5 × 105) were embedded in a mixture of thrombin and fibrinogen (Fingert et al., 1987) and injected into the adrenal gland of female immunodeficient mice (RAG2-/-, γc-/-) using a 30 gauge needle. Nineteen days after transplantation mice were sacrificed. (a) In situ evaluation of the adrenal gland and surrounding tissues following transplantation of genetically-modified pBAC cells. Expansion of the transplanted cell population was visualized by fluorescence using a 470 nm light source. Following this photography, tissues were fixed, embedded in paraffin and sectioned for histological analysis. (b) Hematoxylin and eosin staining of tissue formed from genetically modified pBAC cells. Asterisk indicates region of remaining mouse adrenocortical tissue. (c) Demonstration of localization of genetically modified cells using immunohistochemistry with anti-SV40 T antigen (monoclonal antibody PAb416). (d) Immunohistochemistry using a polyclonal antibody against cholesterol side-chain cleavage enzyme (Calbiochem Inc.) shows remaining mouse adrenocortical cells, particularly in area indicated by asterisk.
5. Conclusion
In summary, a suite of methods is in place that allows us to investigate the genetic modifications that may convert primary human or bovine adrenocortical cells to a fully malignant state or to various forms of benign lesions with properties of nodules or adenomas. Lentiviral transduction of pHAC cells should provide an efficient method for genetic modification of cells without excessive cell division. Orthotopic cell transplantation provides an appropriate in vivo microenvironment for the growth of genetically modified cells into a malignant or benign tissue structure. The combination of primary culture of human adrenocortical cells, lentiviral modification, and rapid orthotopic transplantation of the cells provides an excellent system for studying the genetic changes that human adrenocortical cells must acquire to exhibit various stages of malignant behavior. In the future this system could also be used to help to design therapeutic strategies for human adrenal cancer.
Acknowledgments
This work was supported by the grants from the Wilhelm Sander-Stiftung, Germany (to S.R.B.), and grants from the National Institute on Aging, Owens Medical Foundation, Shelby Rae Tengg Foundation and Glenn Foundation for Medical Research (to P.J.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alesci S, Ramsey WJ, Bornstein SR, Chrousos GP, Hornsby PJ, Benvenga S, Trimarchi F, Ehrhart-Bornstein M. Adenoviral vectors can impair adrenocortical steroidogenesis: Clinical implications for natural infections and gene therapy. Proc Natl Acad Sci USA. 2002;99:7484–7489. doi: 10.1073/pnas.062170099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amado RG, Chen IS. Lentiviral vectors--the promise of gene therapy within reach? Science. 1999;285:674–676. doi: 10.1126/science.285.5428.674. [DOI] [PubMed] [Google Scholar]
- Barlaskar FM, Hammer GD. The molecular genetics of adrenocortical carcinoma. Rev Endocr Metab Disord. 2007;8:343–348. doi: 10.1007/s11154-007-9057-x. [DOI] [PubMed] [Google Scholar]
- Bartosch B, Cosset FL. Strategies for retargeted gene delivery using vectors derived from lentiviruses. Curr Gene Ther. 2004;4:427–443. doi: 10.2174/1566523043345995. [DOI] [PubMed] [Google Scholar]
- Bornstein SR, Hornsby PJ. What can we learn from gene expression profiling for adrenal tumor management? J Clin Endocrinol Metab. 2005;90:1900–1902. doi: 10.1210/jc.2005-0065. [DOI] [PubMed] [Google Scholar]
- Bornstein SR, Stratakis CA, Chrousos GP. Adrenocortical tumors: recent advances in basic concepts and clinical management. Ann Intern Med. 1999;130:759–771. doi: 10.7326/0003-4819-130-9-199905040-00017. [DOI] [PubMed] [Google Scholar]
- Chen M, Hornsby PJ. Adenovirus-delivered DKK3/WNT4 and steroidogenesis in primary cultures of adrenocortical cells. Horm Metab Res. 2006;38:549–555. doi: 10.1055/s-2006-950500. [DOI] [PubMed] [Google Scholar]
- Cooper RN, Irintchev A, Di Santo JP, Zweyer M, Morgan JE, Partridge TA, Butler-Browne GS, Mouly V, Wernig A. A new immunodeficient mouse model for human myoblast transplantation. Hum Gene Ther. 2001;12:823–831. doi: 10.1089/104303401750148784. [DOI] [PubMed] [Google Scholar]
- Fingert HJ, Chen Z, Mizrahi N, Gajewski WH, Bamberg MP, Kradin RL. Rapid growth of human cancer cells in a mouse model with fibrin clot subrenal capsule assay. Cancer Res. 1987;47:3824–3829. [PubMed] [Google Scholar]
- Giordano TJ, Thomas DG, Kuick R, Lizyness M, Misek DE, Smith AL, Sanders D, Aljundi RT, Gauger PG, Thompson NW, Taylor JM, Hanash SM. Distinct transcriptional profiles of adrenocortical tumors uncovered by DNA microarray analysis. Am J Pathol. 2003;162:521–531. doi: 10.1016/S0002-9440(10)63846-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Henriksson KC, Almgren MA, Thurlow R, Varki NM, Chang CL. A fluorescent orthotopic mouse model for reliable measurement and genetic modulation of human neuroblastoma metastasis. Clin Exp Metastasis. 2004;21:563–570. doi: 10.1007/s10585-004-4091-5. [DOI] [PubMed] [Google Scholar]
- Hornsby PJ, McAllister JM. Culturing steroidogenic cells. In: Waterman MR, Johnson EF, editors. Methods in Enzymology. Vol. 206. Academic; San Diego: 1991. pp. 371–380. [DOI] [PubMed] [Google Scholar]
- Huang Q, Chen M, Liang S, Acha V, Liu D, Yuan F, Hawks CL, Hornsby PJ. Improving cell therapy -- experiments using a cell transplantation model in immunodeficient mice. Mech Age Dev. 2007;128:25–30. doi: 10.1016/j.mad.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilvesmaki V, Blum WF, Voutilainen R. Insulin-like growth factor-II in human fetal adrenals: Regulation by ACTH, protein kinase C and growth factors. J Endocrinol. 1993;137:533–542. doi: 10.1677/joe.0.1370533. [DOI] [PubMed] [Google Scholar]
- Joseph JM, Gross N, Lassau N, Rouffiac V, Opolon P, Laudani L, Auderset K, Geay JF, Muhlethaler-Mottet A, Vassal G. In vivo echographic evidence of tumoral vascularization and microenvironment interactions in metastatic orthotopic human neuroblastoma xenografts. Int J Cancer. 2005;113:881–890. doi: 10.1002/ijc.20681. [DOI] [PubMed] [Google Scholar]
- Khanna C, Jaboin JJ, Drakos E, Tsokos M, Thiele CJ. Biologically relevant orthotopic neuroblastoma xenograft models: primary adrenal tumor growth and spontaneous distant metastasis. In Vivo. 2002;16:77–85. [PubMed] [Google Scholar]
- Killion JJ, Radinsky R, Fidler IJ. Orthotopic models are necessary to predict therapy of transplantable tumors in mice. Cancer Metastasis Rev. 1998;17:279–284. doi: 10.1023/a:1006140513233. [DOI] [PubMed] [Google Scholar]
- Libè R, Fratticci A, Bertherat J. Adrenocortical cancer: pathophysiology and clinical management. Endocr Relat Cancer. 2007;14:13–48. doi: 10.1677/erc.1.01130. [DOI] [PubMed] [Google Scholar]
- Pear W. Transient transfection methods for preparation of high-titer retroviral supernatants. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 1996. pp. 9.11.1–9.11.18. [DOI] [PubMed] [Google Scholar]
- Popnikolov NK, Hornsby PJ. Subcutaneous transplantation of bovine and human adrenocortical cells in collagen gel in scid mice. Cell Transplantation. 1999;8:617–625. doi: 10.1177/096368979900800608. [DOI] [PubMed] [Google Scholar]
- Shin SI, Freedman VH, Risser R, Pollack R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc Natl Acad Sci USA. 1975;72:4435–9. doi: 10.1073/pnas.72.11.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RK, Tsan R, Radinsky R. Influence of the host microenvironment on the clonal selection of human colon carcinoma cells during primary tumor growth and metastasis. Clin Exp Metastasis. 1997;15:140–50. doi: 10.1023/a:1018400826845. [DOI] [PubMed] [Google Scholar]
- Slater EP, Diehl SM, Langer P, Samans B, Ramaswamy A, Zielke A, Bartsch DK. Analysis by cDNA microarrays of gene expression patterns of human adrenocortical tumors. Eur J Endocrinol. 2006;154:587–98. doi: 10.1530/eje.1.02116. [DOI] [PubMed] [Google Scholar]
- Stiles CD, Desmond W, Jr, Sato G, Saier MH., Jr Failure of human cells transformed by simian virus 40 to form tumors in athymic nude mice. Proc Natl Acad Sci USA. 1975;72:4971–4975. doi: 10.1073/pnas.72.12.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratakis CA, Chrousos GP. Adrenal cancer. Endocrinol Metab Clin North Am. 2000;29:15–25. doi: 10.1016/s0889-8529(05)70113-4. [DOI] [PubMed] [Google Scholar]
- Sun B, Huang Q, Liu S, Chen M, Hawks CL, Wang L, Zhang C, Hornsby PJ. Progressive loss of malignant behavior in telomerase-negative tumorigenic adrenocortical cells and restoration of tumorigenicity by human telomerase reverse transcriptase. Cancer Res. 2004;64:6144–6151. doi: 10.1158/0008-5472.CAN-04-1376. [DOI] [PubMed] [Google Scholar]
- Tajima T, Okada T, Ma XM, Ramsey W, Bornstein S, Aguilera G. Restoration of adrenal steroidogenesis by adenovirus-mediated transfer of human cytochrome P450 21-hydroxylase into the adrenal gland of 21-hydroxylase-deficient mice. Gene Ther. 1999;6:1898–903. doi: 10.1038/sj.gt.3301018. [DOI] [PubMed] [Google Scholar]
- Thomas M, Northrup SR, Hornsby PJ. Adrenocortical tissue formed by transplantation of normal clones of bovine adrenocortical cells in scid mice replaces the essential functions of the animals’ adrenal glands. Nature Med. 1997;3:978–983. doi: 10.1038/nm0997-978. [DOI] [PubMed] [Google Scholar]
- Thomas M, Yang L, Hornsby PJ. Formation of functional tissue from transplanted adrenocortical cells expressing telomerase reverse transcriptase. Nature Biotechnol. 2000;18:39–42. doi: 10.1038/71894. [DOI] [PubMed] [Google Scholar]
- Thomas M, Wang X, Hornsby PJ. Human adrenocortical cell xenotransplantation: Model of cotransplantation of human adrenocortical cells and 3T3 cells in scid mice to form vascularized functional tissue and prevent adrenal insufficiency. Xenotransplantation. 2002;9:58–67. doi: 10.1046/j.0908-665x.2001.00138.x. [DOI] [PubMed] [Google Scholar]
- Thomas M, Hawks CL, Hornsby PJ. Adrenocortical cell transplantation in scid mice: the role of the host animals’ adrenal glands. J Steroid Biochem Mol Biol. 2003;85:285–290. doi: 10.1016/s0960-0760(03)00212-7. [DOI] [PubMed] [Google Scholar]
- Togo S, Shimada H, Kubota T, Moossa AR, Hoffman RM. Host organ specifically determines cancer progression. Cancer Res. 1995;55:681–684. [PubMed] [Google Scholar]
- Vogelstein B, Kinzler KW. The multistep nature of cancer. Trends Genet. 1993;9:138–141. doi: 10.1016/0168-9525(93)90209-z. [DOI] [PubMed] [Google Scholar]
- Wang Y, Groot-Wassink T, Lemoine NR, Vassaux G. Cellular characterization of the tropism of recombinant adenovirus for the adrenal glands. Eur J Clin Invest. 2003;33:794–798. doi: 10.1046/j.1365-2362.2003.01216.x. [DOI] [PubMed] [Google Scholar]
- Weber MM, Fottner C, Schmidt P, Brodowski KM, Gittner K, Lahm H, Engelhardt D, Wolf E. Postnatal overexpression of insulin-like growth factor II in transgenic mice is associated with adrenocortical hyperplasia and enhanced steroidogenesis. Endocrinology. 1999;140:1537–43. doi: 10.1210/endo.140.4.6660. [DOI] [PubMed] [Google Scholar]
- Wolkersdorfer GW, Bornstein SR, Higginbotham JN, Hiroi N, Vaquero JJ, Green MV, Blaese RM, Aguilera G, Chrousos GP, Ramsey WJ. A novel approach using transcomplementing adenoviral vectors for gene therapy of adrenocortical cancer. Horm Metab Res. 2002;34:279–287. doi: 10.1055/s-2002-33255. [DOI] [PubMed] [Google Scholar]


