Abstract
Background
Evidence suggests that cardiovascular medications, including statins and antihypertensive medications, may delay cognitive decline in patients with Alzheimer dementia (AD). We examined the association of cardiovascular medication use and rate of functional decline in a population-based cohort of individuals with incident AD.
Methods
In the Dementia Progression Study of the Cache County Study on Memory, Health, and Aging, 216 individuals with incident AD were identified and followed longitudinally with in-home visits for a mean of 3.0 years and 2.1 follow-up visits. The Clinical Dementia Rating (CDR) was completed at each follow-up. Medication use was inventoried during in-home visits. Generalized least-squares random-effects regression was performed with CDR Sum of Boxes (CDR-Sum) as the outcome and cardiovascular medication use as the major predictors.
Results
CDR-Sum increased an average of 1.69 points annually, indicating a steady decline in functioning. After adjustment for demographic variables and the baseline presence of cardiovascular conditions, use of statins (p = 0.03) and beta-blockers (p = 0.04) was associated with a slower annual rate of increase in CDR-Sum (slower rate of functional decline) of 0.75 and 0.68 points respectively, while diuretic use was associated with a faster rate of increase in CDR-Sum (p = 0.01; 0.96 points annually). Use of calcium-channel blockers, angiotensin-converting enzyme inhibitors, digoxin, or nitrates did not affect the rate of functional decline.
Conclusions
In this population-based study of individuals with incident AD, use of statins and beta-blockers was associated with delay of functional decline. Further studies are needed to confirm these results and to determine whether treatment with these medications may help delay AD progression.
Keywords: Alzheimer disease, risk factors in epidemiology, medications, prognosis
Alzheimer disease (AD) is the leading cause of dementia in the U.S. affecting an estimated 4.5 million persons in 2004, a number that is expected to triple by the year 2030.1 AD is a major cause of disability and institutionalization in older persons, burdening caregivers both financially and emotionally. Although current FDA-approved medications for AD have well-established symptomatic effects, the effect sizes for clinical outcomes are modest and to date there is no proven therapy for primary or secondary prevention of AD.
There are many longitudinal epidemiologic studies assessing the effect of vascular conditions and treatments on AD incidence but few examining progression of AD once the diagnosis is established. Results from incidence studies suggest that cardiovascular risk factors increase the incidence of AD, including hypertension2, elevated serum cholesterol,3 diabetes,4 and atrial fibrillation.5 However, these associations are not firmly established and there are contradictory results: for example, the Framingham Health Study reported that serum cholesterol levels were not associated with incidence of AD.6 Cardiovascular medications have been similarly associated with decreased incidence of AD in pharmacoepidemiologic studies. Several case–control studies report that the use of lipid-lowering agents (LLAs), particularly statins, is associated with lower risk of incident AD.7–9 However, prospective studies have not replicated these findings.10–12 Antihypertensive use has been similarly associated with lower incidence of AD in longitudinal studies.13 These pharmacoepidemiologic observations have led logically to clinical trials of primary and secondary prevention of AD using cardiovascular medications including statins14–16 and antihypertensive medications17 with mostly negative results to date.
Identifying risk factors and potential interventions for AD progression (secondary prevention) is of obvious importance to patients, caregivers and clinicians, and for this reason it is important to examine the association of cardiovascular medication use and AD progression. It is important to use a study design that specifically addresses issues of progression because a disease-modifying factor may have differing effects at different stages of disease. For example, increased blood pressure and serum cholesterol in midlife are associated with incident AD in late life,3 while decreased blood pressure and serum cholesterol in late life18,19 are associated with an increased risk of AD in late life. Given the methodologic challenges of primary prevention trials in AD, disease modification (alteration of the rate of progression) may be a more realistic goal for the new treatments.20
The Cache County Dementia Progression Study (DPS) consists of a population-based cohort of incident dementia, and is designed to define the cognitive, functional, and neuropsychiatric trajectories of dementia patients, as well as risk factors that modify these trajectories. We sought to assess the effect of medications for treatment of vascular conditions on AD progression. The hypothesis of this article is that cardiovascular medications will alter the rate of functional decline in AD.
METHODS
Participant Screening
The DPS enrolls participants with incident dementia screened from the population of Cache County, Utah via procedures of the Cache County Study on Memory, Health, and Aging (CCSMHA). The protocols have been reported in detail elsewhere.21 Briefly, of the 5677 permanent residents of Cache County Utah aged 65 or older on January 1, 1995, 5092 (90%) enrolled in the study and underwent a multistage screening and assessment. Individuals with prevalent dementia were identified at the initial study wave (1995–1996) and those developing incident dementia were identified at two follow-up waves (1998–1999 and 2002–2003). At each wave, participants were screened for dementia using the revised Modified Mini-Mental State Examination for epidemiologic studies (3MS).22. Individuals whose 3MS scores (adjusted for education and sensory deficits) fell below 87 out of a possible 100 were studied further using the Dementia Questionnaire.23 Participants with suspected dementia or its prodrome underwent baseline clinical assessment, including an interview to ascertain medical, cognitive, and demographic history, a brief medical and neurological examination, and a neuropsychological test battery. Following case review by a study geropsychiatrist and neuropsychologist with the clinical assessment team, individuals with suspected dementia were referred for an MRI scan, laboratory studies, and geropsychiatry evaluation. All available information were reviewed by a panel of experts who assigned final dementia diagnoses (see below). Participants with a diagnosis of incident dementia (N = 432) over the two waves of follow-up in CCSMHA had the option of continuing in the DPS. Of these, 250 participants (58%) had probable or possible AD without concomitant vascular dementia. DPS participants were followed with in-home assessments, similar to that of the CCSMHA, by an interdisciplinary specialty geropsychiatry team. The baseline assessment for the DPS is considered to be the visit that established the diagnosis of dementia in CCSMHA. Two hundred sixteen (86%) of the 250 participants had sufficient baseline data available for analysis, and of these 135 (63%) of the 250 AD participants had at least 1 additional follow-up and, therefore, comprise our longitudinal sample. It should be noted that DPS is an ongoing study and the major reason for small numbers of participants at visits 3–5 is because the study is not yet complete, rather than loss to follow-up.
Assessment of Dementia and Dementia Severity
Dementia diagnoses were assigned by a panel of experienced clinicians in geropsychiatry, neurology, and neuropsychology after thorough review of all available information including results of the clinical assessment, geropsychiatry exam, and neuroimaging and laboratory studies. The assessment included interview of the participant as well as a proxy informant; the proxy informant was generally a family member with the most current knowledge of the participant’s condition. Dementia was diagnosed according to DSM–III–R criteria24 and AD diagnoses were made according to National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer Disease and Related Disorders Association (NINCDS/ADRDA) criteria.25 Participants were included if they were diagnosed with possible or probable AD and were excluded if they had other primary or secondary diagnoses including vascular dementia.
The severity of dementia was measured with the Clinical Dementia Rating Scale (CDR).26 The CDR uses a 5-point anchored ordinal scale to characterize six domains of cognitive and functional performance: memory, orientation, judgment, community, hobbies, and personal care. The CDR is assessed with a semistructured interview and has been demonstrated to have excellent reliability and validity. Scores are reported here both as a composite CDR score (CDR-composite) and Sum of Boxes (CDR-Sum), which is the sum of individual ratings in each of the six domains, with a range of 0 (no impairment) to 30 (maximum impairment in all domains). The primary outcome was CDR-Sum, which was chosen instead of CDR because of its greater range and sensitivity to change in MCI and AD as demonstrated in epidemiological27 and functional MRI studies.28 The Mini-Mental State Exam (MMSE)29 is a 30-item brief cognitive exam assessing domains of orientation, immediate and delayed recall, attention, calculation, language, and praxis. MMSE was examined as a secondary outcome.
The General Medical Health Rating Scale (GMHR) was administered at baseline. The GMHR is a measure validated to assess overall medical acuity in dementia.30
Assessment of Vascular Conditions and Medications
History of vascular risk factors was obtained at each examination via a combination of proxy- and self-report for all visits that preceded the onset of dementia through interview data from the CCSMHA. Updated information at the clinical assessment (which is the visit when the diagnosis of dementia was established) was obtained from a proxy informant. Information about cerebrovascular disease was obtained through questions such as “Has a doctor or nurse told you that you had a stroke?” (directed to the participant) or “has a doctor told your loved one that he or she has had a stroke?” (directed to the proxy informant). Cardiovascular conditions were ascertained through direct questions for heart attack, or chronic conditions such as diabetes, and hypertension. Vascular risk factors included self-reported history of diabetes, history of cardiovascular disease (defined as MI, Calif. BG, or angina), current diagnosis of atrial fibrillation, and measured systolic blood pressure.
At each interview, a detailed inventory of all over-the-counter and prescription medications in current use was completed. These inventories began with a visual inspection of all available medication vials; this inspection collected the vast majority of the medication data. This was followed by probe questions of proxy informants to identify other medications that may not have been disclosed. As a memory aid, the most common medications were shown to participants on large print “drug cards.” For participants residing in institutions, this information was obtained from institutional (i.e., nursing home) records. Proxy informants were asked further questions about the form, dosage, indication, start date, and duration of medication use. Cardiovascular medications were classified as angiotensin converting enzyme (ACE) inhibitors, β-blocking antia-drenergics (β-blockers), calcium ion channel blockers, diuretics, LLAs (further categorized into statins versus other agents), nitrates, platelet inhibitors, or digoxin.
Data Analysis
Demographic variables were compared between participants who used a specific class of medication versus those who did not, and Student’s t-test (for continuous variables) or chi-squared analyses (for categorical variable) were performed to assess whether the variables differed significantly between users and nonusers, using p < 0.05 as the test for statistical significance. The primary statistical analysis of outcome (change in CDR-Sum) was performed using generalized-least squares linear mixed models, which allows for assessment of the effect of multiple covariates in a longitudinal analysis controlling for within-subject variation. The outcome was CDR-Sum. An unstructured correlation structure was used. Covariates were chosen according to 1) statistical significance in univariate generalized linear models, using p < 0.10 as cutoff for significance; 2) covariates reported to be associated with AD incidence or progression from the literature; 3) medications were included as covariates only if >10 participants took the medication at baseline (platelet inhibitors and LLAs other than statins were excluded for this reason). For each medication variable, we tested for baseline correlation with CDR-Sum and interaction with time. Time was treated as a continuous variable. Covariates included age, gender, education, and baseline MMSE. All analyses were conducted using Stata Version 8.2 (StataCorp, College Station, Tex.).
RESULTS
Of the 216 participants with incident AD and who had baseline CDR and MMSE scores, 135 (62.5%) had at least 1 additional follow-up. The flow chart for recruitment and attrition have been previously published.31 The attrition between baseline and follow-up was attributable to death in 59 participants, refusal in 11 participants, and not yet reached time for follow-up in 11 participants. The mean duration from baseline to follow-up visit was 1.6 years (SD 6.5), and subsequent visits were biennial (every 6 months). Compared with participants who had no follow-up visits, participants with ≥1 follow-up visit were younger (mean age 84.2 years [SD 6.5] versus 87.3 years [SD 6.2], Student’s t-test p < 0.01 [df = 214]), had longer duration of dementia (mean 2.1 year [SD 1.3] versus 1.7 years [SD 1.2], Student’s t-test p < 0.05 [df = 214]), and had a higher baseline MMSE score (mean 22.3 [SD 4.3] versus 20.4 [4.8], Student’s t-test p < 0.01 [df = 214]), but did not differ in race, sex, CDR composite or CDR-Sum scores, or prevalence of vascular conditions. These participants were followed for a mean of 3.0 years (range: 0.8–9.5) and 2.1 follow-up visits (range: 1–5). Mean CDR-composite score at baseline was 1.1 (SD 0.54) indicating mild severity of dementia.
Table 1 presents baseline covariates and outcome measures stratified by medication use. The participants were on average very elderly (mean 85.4 years, SD 6.5), mildly demented (CDR mean 1.1, SD 0.53), had 13.2 years education (SD 2.8), and were 100% white reflecting the ethnic make-up of Cache County. Their mean duration of dementia was 1.9 years (SD 1.3). Rates of baseline cardiovascular medication use ranged from 12–16%. Overall, the differences between users and nonusers of medication were not remarkable. Participants who used any class of medications had higher GMHR than participants who did not use medications (Student’s t-test, p < 0.01 for all medication classes). Participants who used statins were younger than non-users, but there were no other differences in age, sex, or MMSE between medication users and non-users. Participants with all vascular conditions assessed were more likely to use statins; participants with angina more likely to use beta-blockers, and participants with MI and diabetes more likely to use ACE inhibitors.
TABLE 1.
Cardiovascular Medication Use and Prevalence of Vascular Conditions in Dementia Progression Study
| Number of Participants (%) |
Age (years) |
% Female |
Education (years) |
Baseline MMSE |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Medication Use | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No |
| Statins | 31 (15%) | 183 (85%) | 83.0 (6.2) | 85.7 (6.5) | 58 | 71 | 13.9 (2.6) | 13.2 (2.8) | 22.6 (3.7) | 21.4 (4.7) |
| Beta-Blockers | 33 (15%) | 181 (85%) | 86.3 (6.6) | 85.2 (6.6) | 79 | 67 | 12.9 (2.2) | 13.3 (2.9) | 22.4 (3.5) | 21.5 (4.7) |
| ACE Inhibitors | 32 (15%) | 182 (85%) | 84.4 (5.9) | 85.5 (6.6) | 59 | 71 | 13.3 (3.7) | 13.3 (2.6) | 21.9 (5.0) | 21.6 (4.5) |
| Calcium Channel Blockers | 36 (16%) | 179 (84%) | 85.8 (5.7) | 85.3 (6.7) | 71 | 69 | 13.5 (3.1) | 13.2 (2.8) | 21.8 (5.1) | 21.6 (4.5) |
| Diuretics | 42 (20%) | 172 (80%) | 86.6 (6.3) | 85.0 (6.6) | 81 | 66 | 13.1 (2.2) | 13.3 (3.0) | 22.3 (4.2) | 21.4 (4.6) |
|
Angina (%) |
MI (%) |
Stroke (%) |
Diabetes (%) |
|||||||
| Medication Use | Yes | No | Yes | No | Yes | No | Yes | No | ||
|
| ||||||||||
| Statins | 32.3 | 9.3 | 38.7 | 13.7 | 19.4 | 4.4 | 35.5 | 14.2 | ||
| Beta-Blockers | 27.3 | 9.9 | 24.2 | 16.0 | 6.0 | 6.6 | 21.2 | 16.6 | ||
| ACE Inhibitors | 9.4 | 13.2 | 34.4 | 14.3 | 9.4 | 6.0 | 37.5 | 13.7 | ||
| Calcium Channel Blockers | 17.1 | 11.7 | 25.7 | 15.6 | 11.4 | 5.5 | 22.9 | 16.2 | ||
| Diuretics | 21.4 | 10.5 | 19.0 | 16.9 | 9.5 | 5.8 | 23.8 | 15.7 | ||
Notes: N = 216 participants assessed at baseline. Statistic used was Student’s t (df = 212) for continuous variables and Pearson’s χ2(1) for categorical variables. Continuous variables are expressed as mean (SD) or N (%). Variables that differed significantly (p < .05) by medication use are in bold face.
Table 2 presents the results of generalized-least squares linear mixed models for CDR-Sum. In univariate analyses, baseline use of statins (β = −1.10, z = −3.20, p = 0.001) and beta-blockers (β = −0.92, z = −2.73, p = 0.006) use were associated with significantly slower increase of CDR-Sum (slower rate of decline). In other words, participants taking statins experienced 1.1 points slower annual decline in CDR-Sum than participants not taking statins, while participants taking beta-blockers experienced 0.92 points slower annual decline than those not taking beta-blockers. Although diuretic use was not associated with CDR-Sum trajectory in univariate analyses, it was included as a covariate in the multivariate analysis due to a significant interaction with beta-blocker use. Use of ACE inhibitors, calcium channel blockers, nitrates, or digoxin did not alter CDR-Sum trajectory and thus were not included in multivariate analyses.
TABLE 2.
CDR Sum of Boxes vs. Medication Use
| Univariate Models |
Multivariate Model |
|||
|---|---|---|---|---|
| Variable | Coeff (95% CI) | Coeff * Time (95% CI) | Coeff (95% CI) | Coeff * Time (95% CI) |
| Statins | −.72 (−2.3, .85) | −1.10 (−1.78, −.42) | −.21 (−1.5, 1.0) | −.75 (−1.4, −.072) |
| Beta-blockers | −.22 (−1.75, 1.32) | −.92 (−1.57, −.25) | −.22 (−1.5, 1.0) | −.68 (−1.33, −.032) |
| Diuretics | .14 (−1.30, 1.58) | .53 (−.28, 1.34) | .00 (−1.22, 1.23) | .96 (.21, 1.71) |
| Time | 1.49 (1.31, 1.66) | N/A | 1.69 (1.51, 1.86) | N/A |
Notes: Generalized linear mixed regression models of CDR Sum of Boxes. Medication use is represented by two coefficients: 1) “coeff” = baseline (cross-sectional) effect of medication use 2) “coeff*time” = interaction of baseline medication use with time. The former variable controls for baseline differences in medication use, the latter accounts for predictive value of baseline use with longitudinal changes in CDR Sum of Boxes. The multivariate model includes the three medication use variables, controlling for the following covariates at baseline: age, gender, education, MMSE, systolic blood pressure, the presence of any apoE4 allele, MMSE, and GMHR. (N = 216 subjects. Variables whose coefficients contributed significantly to the model at p < .05 using a z-score statistic are indicated in boldface italic).
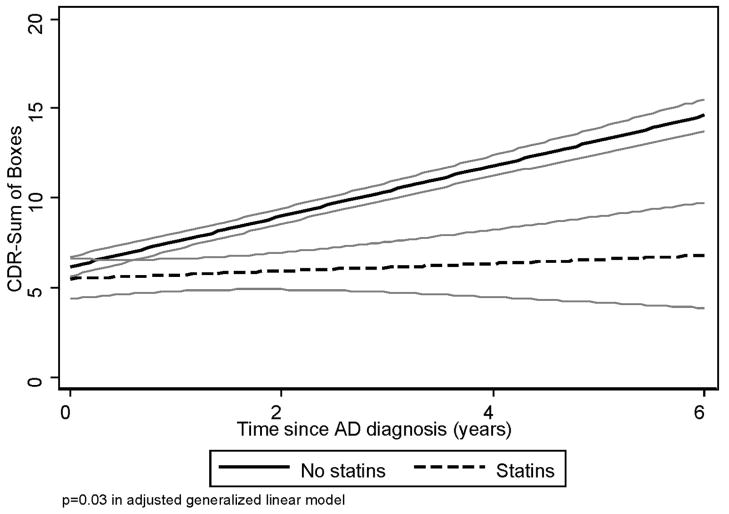
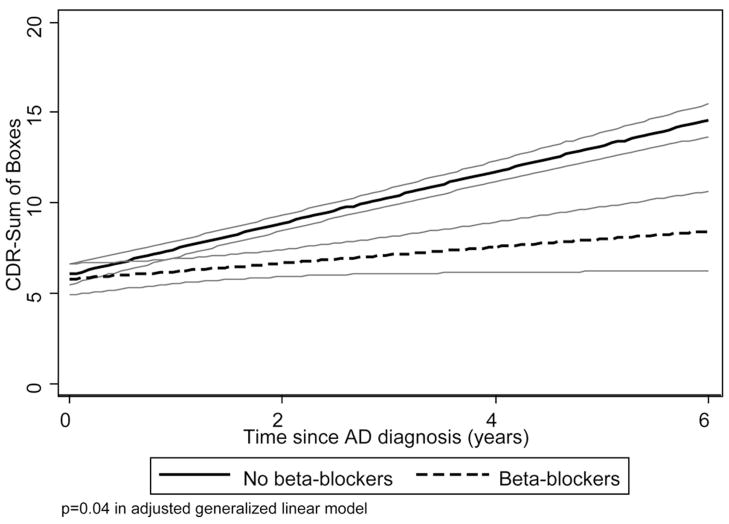
Multivariate analyses are presented in Table 2. CDR-Sum increased by 1.69 points annually. None of the three medication classes included in the model was associated with baseline CDR-Sum level. Use of statins was associated with a reduction in the rate of increase in CDR-Sum of 0.75 points annually (β = −0.75, z = −2.17, p = 0.03), reflecting slower functional decline. Similarly, use of beta-blockers was associated with a reduction in the rate of increase in CDR-Sum of 0.68 points annually (β = −.68, z = −2.06, p = 0.04). Use of diuretics was associated with more rapid increase in CDR-Sum of 0.96 points annually (β = 0.96, z = 2.56, p = 0.01), reflecting faster functional decline. The interaction between beta-blocker and systolic blood pressure did not significantly affect baseline CDR-Sum nor its trajectory (data not shown). Our sample size was too small to examine specific types of diuretic classes. The presence of vascular conditions (angina, history of myocardial infarction, history of stroke, diagnosis of diabetes) did not significantly alter the coefficients when added to the multivariate model, nor did dementia duration (data not shown). Best-Fit models for use of statins, and beta-blockers are illustrated in Figs 1, 2. Diuretics were not graphed due to lack of significance in univariate analyses.
FIGURE 1. CDR Sum of Boxes vs. Statin Use.
Notes: A best-fit linear regression model for CDR Sum of Boxes vs. time were used to model the progression of CDR Sum of Boxes over time, in 216 subjects with pure AD in DPS, stratified by baseline use of statins. 95% confidence limits for the model are presented in gray outline.
FIGURE 2. CDR Sum of Boxes vs. Beta-Blocker Use.
Notes: A best-fit linear regression model for CDR Sum of Boxes vs. time were used to model the progression of CDR Sum of Boxes over time, in 216 subjects with pure AD in DPS, stratified by baseline use of beta-blockers. 95% confidence limits for the model are presented in gray outline.
Results using MMSE as an outcome were significant only for protective effect of statins: MMSE declined by a mean of 4.6 points annually, a decline that was reduced by 1.5 points annually in participants using statins (data not shown).
CONCLUSION
In a population-based sample of 216 participants with incident AD followed in a prospective longitudinal manner for a mean of 3.14 years, the use of statins and beta-blockers at baseline was associated with a slower rate of functional decline as measured by the CDR-Sum. CDR-Sum increased 1.69 points annually overall indicating that the typical course of AD in this cohort was progressive functional decline. This rate of decline was slower than the 2.9 point annual decline reported by Bhargava et al.32 and the 2.17 point annual decline reported by Pavlik et al.,27 both for referral clinic cohorts. These differences are not surprising: the DPS cohort was older and derived from population-based sampling, which are both factors associated with a more benign course of disease. Our results with MMSE as an alternative outcome were significant only for protective effect of statins, which is expected given that MMSE is a less sensitive measure of change than CDR-Sum.
Statins appeared protective, slowing the rate of increase in CDR-Sum by approximately 0.75 points annually (44% decrease in rate of functional decline). To our knowledge this is the first cohort study to report a protective effect of statins in established AD and extends the results reported from longitudinal studies of AD incidence and clinical trials. There are several potential protective mechanisms of statin treatment. Statins may alter the cholesterol content of lipid rafts in the neuronal membrane which can then alter the activity of β-secretase, shifting the metabolism of amyloid precursor protein from β- to α-cleavage.33–35 Excess dietary cholesterol has been shown to lead to amyloid-β accumulation in mouse models,36 and statin treatment could mediate this effect. Statin treatment improved learning, memory, and hippocampal long-term potentiation in transgenic mice overexpressing human familial autosomal dominant genes for AD.37 In addition, statins have numerous biologic effects other than alteration of cholesterol metabolism, including anti-inflammatory and antioxidant effects.38 One trial of atorvastatin has reported positive effects on cognition and mood in mild to moderate AD,16 and a community-based observational study reported that premortem statin use was associated with less evidence of AD-like pathologic changes at autopsy.39 The Alzheimer Disease Cooperative Study trial of simvastatin for secondary prevention in AD should directly address the efficacy of statins for delaying progression of AD.
Beta-blockers had a similarly protective effect, reducing the increase in CDR-Sum by 0.68 units annually (40% decrease in rate of functional decline). To our knowledge this is a novel finding in the published literature on AD progression. This finding did not appear to be solely due to adequate control of systolic blood pressure since the interaction between beta-blocker use and systolic blood pressure did not significantly affect CDR-Sum. There are published clinical trials reporting the effect of beta-blockers on dementia incidence, but none reporting effect on dementia progression. The Systolic Hypertension in the Elderly Program used atenolol as second-step treatment, and reported no effect on dementia incidence.40 The Honolulu, Hawaii, Asia Aging Study found that a longer duration of hypertensive treatment was associated with reduced incidence of dementia,41 but did not distinguish between different antihypertensive classes. There are several possible mechanisms for neuroprotection in AD by beta-blockers. The beta-blocker carvedilol reduced infarct size and neurologic deficits in a rat model of transient focal stroke, and this neuroprotection was associated with decreased apoptosis and in brain levels of the proinflammatory cytokines TNF-α and IL-1-β.42 Since there is increasing evidence that neurotoxicity in AD is mediated through release of proinflammatory cytokines by activated microglia,43 it is possible that beta-blockers are acting as anti-inflammatory agents in the AD brain. Another possible mechanism is that beta-blockers improve cardiac output in subclinical congestive heart failure and/or coronary artery disease, thus improving brain perfusion and conceivably improving cognitive function.
Diuretic use was associated with more rapid functional decline by 1.0 CDR-Sum units annually (59% increase). This is opposite in effect to reported data from the CCSMHA that diuretics are associated with lower incidence of AD.13 This difference in effect may be attributable to the stage-specific effect of risk factors, to a greater burden of medical illness, or to higher-order interactions between medication use and illness that cannot be addressed by a sample of this size.
The major limitation of the study is that the limited number of participants and follow-ups results in in-sufficient power to examine the interaction of medication treatments with vascular risk factors (and other potential interactions) over time. When the follow-up visits are completed, even with the high rate of attrition due to death in this very elderly cohort, this dataset should be adequately powered to address these crucial issues. Other limitations of the study include: 1) limited duration of follow-up, which limits the power of the study to detect change; 2) lack of laboratory studies to assess changes in lipid levels associated with LLA use, for example; 3) no objective assessment of medication adherence; 4) since participants were enrolled in DPS with incident AD, most of the subjects had mild dementia severity limiting the generalizability to more advanced disease stages.
Strengths of the study include 1) population-based sample which is more likely to reflect the natural variability and course of AD than clinical populations which include referral bias; 2) in most cases, participants have been followed prior to onset of dementia in the Cache County Study on Memory, Health, and Aging and thus incident dementia cases are identified close to onset of dementia as evidenced by the short duration of dementia (<2 years) at baseline; 3) systematic diagnosis of AD by a geriatric multispecialty team in a consensus conference; 4) data analysis using prospective design; 5) systematic assessment of medication use combining visual inspection of medicine cabinet with interview of proxy informants.
These results add to the data suggesting the protective effect of statins on AD progression and suggest an additional protective effect of beta-blockers, while casting doubt on the previously reported protective effect of diuretics. If replicated in epidemiologic and clinical studies, these findings could have substantial impact on our ability to slow the progressive functional decline of AD and thus diminish the tremendous burden of disability on AD patients and caregivers.
Footnotes
These results have been presented previously in part at the American College of Neuropsychopharmacology 45th Annual Meeting, Hollywood, Florida, December 3–7, 2006.
References
- 1.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 2.Skoog I, Lernfelt B, Landahl S, Palmertz B, Andreasson LA, Nilsson L, Persson G, Oden A, Svanborg A. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347:1141–1145. doi: 10.1016/s0140-6736(96)90608-x. [DOI] [PubMed] [Google Scholar]
- 3.Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A. Midlife vascular risk factors and Alzheimer’s disease in later life: Longitudinal, population based study. BMJ. 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: the rotterdam study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 5.Forti P, Maioli F, Pisacane N, Rietti E, Montesi F, Ravaglia G. Atrial fibrillation and risk of dementia in non-demented elderly subjects with and without mild cognitive impairment. Neurol Res. 2006;28:625–629. doi: 10.1179/016164106X130461. [DOI] [PubMed] [Google Scholar]
- 6.Tan ZS, Seshadri S, Beiser A, Wilson PW, Kiel DP, Tocco M, D’Agostino RB, Wolf PA. Plasma total cholesterol level as a risk factor for Alzheimer disease: the framingham study. Arch Intern Med. 2003;163:1053–1057. doi: 10.1001/archinte.163.9.1053. [DOI] [PubMed] [Google Scholar]
- 7.Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet. 2000;356:1627–1631. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- 8.Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol. 2000;57:1439–1443. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- 9.Green RC, McNagny SE, Jayakumar P, Cupples LA, Benke K, Farrer LA. Statin use and the risk of Alzheimer’s disease: the MIRAGE study. Alzheimer’s Dementia. 2006;3:96–103. doi: 10.1016/j.jalz.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Rea TD, Breitner JC, Psaty BM, Fitzpatrick AL, Lopez OL, Newman AB, Hazzard WR, Zandi PP, Burke GL, Lyketsos CG, Bernick C, Kuller LH. Statin use and the risk of incident dementia: the cardiovascular health study. Arch Neurol. 2005;62:1047–1051. doi: 10.1001/archneur.62.7.1047. [DOI] [PubMed] [Google Scholar]
- 11.Zandi PP, Sparks DL, Khachaturian AS, Tschanz J, Norton M, Steinberg M, Welsh-Bohmer KA, Breitner JC Cache County Study investigators. Do statins reduce risk of incident dementia and Alzheimer disease? the cache county study. Arch Gen Psychiatry. 2005;62:217–224. doi: 10.1001/archpsyc.62.2.217. [DOI] [PubMed] [Google Scholar]
- 12.Li G, Higdon R, Kukull WA, Peskind E, Van Valen Moore K, Tsuang D, van Belle G, McCormick W, Bowen JD, Teri L, Schellenberg GD, Larson EB. Statin therapy and risk of dementia in the elderly: a community-based prospective cohort study. Neurology. 2004;63:1624–1628. doi: 10.1212/01.wnl.0000142963.90204.58. [DOI] [PubMed] [Google Scholar]
- 13.Khachaturian AS, Zandi PP, Lyketsos CG, Hayden KM, Skoog I, Norton MC, Tschanz JT, Mayer LS, Welsh-Bohmer KA, Breitner JC. Antihypertensive medication use and incident Alzheimer disease: the cache county study. Arch Neurol. 2006;63:686–692. doi: 10.1001/archneur.63.5.noc60013. [DOI] [PubMed] [Google Scholar]
- 14.Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Meinders AE, Norrie J, Packard CJ, Perry IJ, Stott DJ, Sweeney BJ, Twomey C, Westendorp RG PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk: pravastatin in elderly individuals at risk of vascular disease (PROSPER): A randomised controlled trial. Lancet. 2002;360:1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 15.Simons M, Schwarzler F, Lutjohann D, von Bergmann K, Beyreuther K, Dichgans J, Wormstall H, Hartmann T, Schulz JB. Treatment with simvastatin in normocholesterolemic patients with Alzheimer’s disease: a 26-week randomized, placebo-controlled, double-blind trial. Ann Neurol. 2002;52:346–350. doi: 10.1002/ana.10292. [DOI] [PubMed] [Google Scholar]
- 16.Sparks DL, Sabbagh MN, Connor DJ, Lopez J, Launer LJ, Browne P, Wasser D, Johnson-Traver S, Lochhead J, Ziolwolski C. Atorvastatin for the treatment of mild to moderate Alzheimer disease: preliminary results. Arch Neurol. 2005;62:753–757. doi: 10.1001/archneur.62.5.753. [DOI] [PubMed] [Google Scholar]
- 17.Skoog I, Lithell H, Hansson L, Elmfeldt D, Hofman A, Olofsson B, Trenkwalder P, Zanchetti AS C.OPE Study Group. Effect of baseline cognitive function and antihypertensive treatment on cognitive and cardiovascular outcomes: study on Cognition and prognosis in the elderly (SCOPE) Am J Hypertens. 2005;18:1052–1059. doi: 10.1016/j.amjhyper.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 19.Mielke MM, Zandi pp, Sjogren M, Gustafson D, Ostling S, Steen B, Skoog I. High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology. 2005;64:1689–1695. doi: 10.1212/01.WNL.0000161870.78572.A5. [DOI] [PubMed] [Google Scholar]
- 20.Green RC, DeKosky ST. Primary prevention trials in Alzheimer disease. Neurology. 2006;67:S2–S5. doi: 10.1212/wnl.67.9_suppl_3.s2. [DOI] [PubMed] [Google Scholar]
- 21.Breitner JC, Wyse BW, Anthony JC, Welsh-Bohmer KA, Steffens DC, Norton MC, Tschanz JT, Plassman BL, Meyer MR, Skoog I, Khachaturian A. APOE-epsilon4 count predicts age when prevalence of AD increases, then declines: the cache county study. Neurology. 1999;53:321–331. doi: 10.1212/wnl.53.2.321. [DOI] [PubMed] [Google Scholar]
- 22.Tschanz JT, Welsh-Bohmer KA, Plassman BL, Norton MC, Wyse BW, Breitner JC Cache County Study Group. An adaptation of the modified mini-mental state examination: analysis of demographic influences and normative data: the cache county study. Neuropsychiatry Neuropsychol Behav Neurol. 2002;15:28–38. [PubMed] [Google Scholar]
- 23.Silverman JM, Breitner JC, Mohs RC, Davis KL. Reliability of the family history method in genetic studies of Alzheimer’s disease and related dementias. Am J Psychiatry. 1986;143:1279–1282. doi: 10.1176/ajp.143.10.1279. [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Revised 3rd. Washington, D.C: American Psychiatric Association; 1987. [Google Scholar]
- 25.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 26.Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 27.Pavlik VN, Doody RS, Massman PJ, Chan W. Influence of premorbid IQ and education on progression of Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;22:367–377. doi: 10.1159/000095640. [DOI] [PubMed] [Google Scholar]
- 28.Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, Dale AM, Stern CE, Blacker D, Albert MS, Sperling RA. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 30.Lyketsos CG, Galik E, Steele C, Steinberg M, Rosenblatt A, Warren A, Sheppard JM, Baker A, Brandt J. The general medical health rating: a bedside global rating of medical comorbidity in patients with dementia. J Am Geriatr Soc. 1999;47:487–491. doi: 10.1111/j.1532-5415.1999.tb07245.x. [DOI] [PubMed] [Google Scholar]
- 31.Mielke MM, Rosenberg PB, Tschanz JT, Cook L, Corcoran C, Norton MC, Green RC, Welsh-Bohmer KA, Breitner JC, Munger RG, Lyketsos CG. Vascular factors predict rate of progression in Alzheimer’s disease. Neurology. 2007;69:1850–1858. doi: 10.1212/01.wnl.0000279520.59792.fe. [DOI] [PubMed] [Google Scholar]
- 32.Bhargava D, Weiner MF, Hynan LS, Diaz-Arrastia R, Lipton AM. Vascular disease and risk factors, rate of progression, and survival in Alzheimer’s disease. J Geriatr Psychiatry Neurol. 2006;19:78–82. doi: 10.1177/0891988706286505. [DOI] [PubMed] [Google Scholar]
- 33.Sjogren M, Mielke M, Gustafson D, Zandi P, Skoog I. Cholesterol and Alzheimer’s disease–is there a relation? Mech Ageing Dev. 2006;127:138–147. doi: 10.1016/j.mad.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 34.Racchi M, Baetta R, Salvietti N, Ianna P, Franceschini G, Paoletti R, Fumagalli R, Govoni S, Trabucchi M, Soma M. Secretory processing of amyloid precursor protein is inhibited by increase in cellular cholesterol content. Biochem J. 1997;322(Pt 3):893–898. doi: 10.1042/bj3220893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simons M, Keller P, De Strooper B, Beyreuther K, Dotti CG, Simons K. Cholesterol depletion inhibits the generation of beta-amyloid in hippocampal neurons. Proc Natl Acad Sci USA. 1998;95:6460–6464. doi: 10.1073/pnas.95.11.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Refolo LM, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS, Sambamurti K, Duff K, Pappolla MA. Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- 37.Li L, Cao D, Kim H, Lester R, Fukuchi K. Simvastatin enhances learning and memory independent of amyloid load in mice. Ann Neurol. 2006;60:729–739. doi: 10.1002/ana.21053. [DOI] [PubMed] [Google Scholar]
- 38.Wolozin B, Manger J, Bryant R, Cordy J, Green RC, McKee A. Re-assessing the relationship between cholesterol, statins and Alzheimer’s disease. Acta Neurol Scand Suppl. 2006;185:63–70. doi: 10.1111/j.1600-0404.2006.00687.x. [DOI] [PubMed] [Google Scholar]
- 39.Li G, Larson EB, Sonnen JA, Shofer JB, Petrie EC, Schantz A, Peskind ER, Raskind MA, Breitner JC, Montine TJ. Statin therapy is associated with reduced neuropathologic changes of Alzheimer disease. Neurology. 2007;69:878–885. doi: 10.1212/01.wnl.0000277657.95487.1c. [DOI] [PubMed] [Google Scholar]
- 40.Di Bari M, Pahor M, Franse LV, Shorr RI, Wan JY, Ferrucci L, Somes GW, Applegate WB. Dementia and disability outcomes in large hypertension trials: lessons learned from the systolic hypertension in the elderly program (SHEP) trial. Am J Epidemiol. 2001;153:72–78. doi: 10.1093/aje/153.1.72. [DOI] [PubMed] [Google Scholar]
- 41.Peila R, White LR, Masaki K, Petrovitch H, Launer LJ. Reducing the risk of dementia: efficacy of long-term treatment of hypertension. Stroke. 2006;37:1165–1170. doi: 10.1161/01.STR.0000217653.01615.93. [DOI] [PubMed] [Google Scholar]
- 42.Savitz SI, Erhardt JA, Anthony JV, Gupta G, Li X, Barone FC, Rosenbaum DM. The novel beta-blocker, carvedilol, provides neuroprotection in transient focal stroke. J Cereb Blood Flow Metab. 2000;20:1197–1204. doi: 10.1097/00004647-200008000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Rosenberg PB. Clinical aspects of inflammation in Alzheimer’s disease. Int Rev Psychiatry. 2005;17:503–514. doi: 10.1080/02646830500382037. [DOI] [PubMed] [Google Scholar]