Abstract
The acoustic startle reflex can be modulated by positive and negative emotion. There is evidence that this modulation can be influenced by voluntary attempts to regulate emotion, and that startle modulation during emotion regulation is more reflective of changes in arousal than valence. However, whether valence and arousal play similar roles in emotion regulation across different psychophysiological indices is unclear. The goal of this study was to characterize further the relative contributions of valence and arousal to changes in psychophysiological responsiveness during voluntary emotion regulation, using multiple psychophysiological measures including eyeblink startle, skin conductance, and heart rate. We studied 10 healthy adults, and found that voluntary attempts to down-regulate positive and negative emotion resulted in decreased eyeblink startle magnitude, skin conductance responses, and heart rate, relative to attempts to up-regulate emotion. These findings indicate that the volitional regulation of emotion had systematic effects on psychophysiological parameters which were similar for positive and negative emotion, suggesting that psychophysiological responsiveness during emotion regulation is more strongly influenced by the modulation of arousal than by the valence of the regulated emotion.
Introduction
Emotion regulation has been broadly defined as “the initiation of new, or the alteration of ongoing, emotional responses through the action of regulatory processes” (Ochsner & Gross, 2005, pp. 242-243). These regulatory processes may be recruited voluntarily (i.e., consciously and deliberately) or without conscious awareness to enhance, reduce, or maintain an emotion (Mauss et al., 2007). The ability to regulate emotion enables humans to maximize the experience of positive emotions while limiting the impact of negative emotions and plays an essential role in allowing humans to adapt to their surroundings, while the dysregulation of emotion has been viewed as a key component in many forms of psychopathology (Davidson, 2000; Machado & Bachevalier, 2003).
Measurement of the acoustic startle reflex is one paradigm that has been widely used to investigate emotional processing, including deliberate emotional regulation. The startle reflex is a highly conserved reflex consisting of a series of muscular contractions, and the neural circuitry underlying this reflex has been well characterized in animal models (Davis et al., 1982; Yeomans & Frankland, 1995). In humans, this reflex is measured through facial electromyography (EMG) recorded from the orbicularis oculi muscles in response to the sudden onset of an auditory stimulus (Lang et al., 1990). The modulation of the startle reflex by emotion is a widely replicated and robust finding both in animals and in humans (Koch, 1999; Lang et al., 1990) that can be demonstrated using a variety of emotionally arousing stimuli (Bradley & Lang, 2000; Vrana & Lang, 1990). In particular, it has been observed that the magnitude of the reflex is enhanced by the experience of negative emotion, and may be suppressed by positive emotion, although the magnitude of and support for the latter conclusion is less robust.
A number of studies in recent years have characterized the psychophysiological correlates of voluntary emotion regulation (Dillon & LaBar, 2005; Gross, 1998; Gross & Levenson, 1997; Jackson et al., 2000). These studies have demonstrated that conscious and deliberate attempts to regulate one's emotions can lead to a variety of physiological changes, including alterations in eyeblink startle magnitude. Jackson et al. (2000) examined the impact of voluntary up- and down-regulation of negative emotion on the startle reflex in healthy adults. The authors found that instructions to decrease emotional responses to unpleasant pictures led to decreased eyeblink startle magnitude, whereas instructions to enhance their responses led to increased startle responses.
More recent work has helped to elucidate the roles of valence and arousal in startle modulation during emotion regulation. According to the motivational priming hypothesis (Lang, 1995), the attenuation and potentiation of the startle reflex during positive and negative emotional processing, respectively, reflect differential engagement of appetitive and defensive motivational systems. One possibility is that up-regulating emotion increases motivational priming, resulting in an accentuation of these valence-specific effects, while reduced motivational priming during attempts to down-regulate emotion dampens them. It has been observed (Dillon & LaBar, 2005), however, that conscious attempts to increase or decrease emotion produce similar patterns of startle modulation for both positive and negative pictures, with increased startle responses during attempts to increase positive or negative emotion and reduced responses during attempts to down-regulate emotion, irrespective of valence. This pattern of findings suggests that startle modulation during voluntary emotion regulation may be driven more by changes in arousal than by valence.
Measures of autonomic reactivity have been frequently adopted in studies of emotional processing. Skin conductance is widely used to index sympathetic arousal, with larger skin conductance responses (SCRs) typically observed for highly arousing stimuli. This measure generally does not differentiate reliably between positive and negative emotion (Dawson et al., 2007). Heart rate, which reflects sympathetic as well as parasympathetic activation, appears to be sensitive to changes in both arousal and valence. In particular, it has been found that viewing arousing pleasant or unpleasant pictures results in a greater parasympathetically-mediated reduction in heart rate than neutral picture viewing (e.g., Bradley et al., 2001). In addition, unpleasant pictures generally elicit more pronounced deceleration in heart rate than pleasant stimuli, reflecting heightened defensive activation (Lang, 1995). It is also clear that voluntary emotion regulatory attempts can affect autonomic reactivity. For example, attempts to decrease negative emotion through the suppression of expressive behavior (e.g., frowning) have been associated with increased sympathetic arousal and less consistently with decreased heart rate (Gross & Levenson, 1993), whereas emotion reduction through reappraisal (the cognitive reinterpretation of an event so as to change its emotional impact) generally does not increase sympathetic arousal (Gross, 1998). There is also some evidence that the up-regulation of negative emotion is associated with heightened physiological arousal (Eippert et al., 2007). However, the regulation of positive emotion (particularly its up-regulation) has received relatively little attention in psychophysiological studies to date. This is not surprising, given the prominent role of negative emotion in psychopathology and the challenges of eliciting positive emotions in laboratory settings. Further work is needed to clarify the extent to which conscious attempts to regulate positive and negative emotion result in similar patterns of changes in physiological reactivity. In addition, while changes in skin conductance and heart rate typically unfold over the course of several seconds, the startle paradigm can be used to probe relatively rapid changes in emotional state. The use of such measures in parallel may therefore provide additional insight into the time course of emotion regulation effects (e.g., to what degree the contributions of valence and arousal to emotion regulation effects change during picture processing).
The goal of the present study was to further characterize the effects of voluntary up- and down-regulation of emotion on somatic reflexes and autonomic responses. To address this aim, we collected measures of eyeblink startle, heart rate, and skin conductance in 10 healthy adults instructed to passively view or regulate their emotional responses to pleasant and unpleasant pictures. Based on previous work (e.g., Dillon and LaBar, 2005; Jackson et al., 2000), it was predicted that attempts to up-regulate positive and negative emotion would result in increased startle responses, while attempts to down-regulate positive and negative emotion would lead to decreased startle responses. Additionally, it was predicted that if arousal contributes more than valence to autonomic changes during emotion regulation, the down-regulation of both positive and negative emotion would result in reduced SCRs and increased heart rate deceleration (consistent with decreased sympathetic activation), as compared to the up-regulation of emotion. If, on the other hand, autonomic emotion regulation effects are more dependent on valence, then it was expected that attempts to up- and down-regulate emotion would elicit similar patterns of SCRs, due to the greater sensitivity of this measure to general arousal per se. It was further predicted that the up-regulation of negative emotion would evoke greater “defensive” activation, as reflected by a more pronounced deceleration in heart rate compared to down-regulating negative emotion, while the up-regulation of positive emotion would be associated with decreased defensive activation, resulting in reduced heart rate deceleration. We have outlined these specific contrasting predictions in Table 1.
Table 1.
Predicted effects of valence and arousal on the up- and down-regulation of emotion across picture categories
|
|
Picture category |
||
|---|---|---|---|
| Measure | Pleasant | Unpleasant | |
| Arousal-dependent modulation | SCRs | Increase > Decrease | Increase > Decrease |
| Startle magnitude | Increase > Decrease | Increase > Decrease | |
| Heart rate deceleration | Increase < Decrease | Increase < Decrease | |
| Valence-dependent modulation | SCRs | Increase = Decrease | Increase = Decrease |
| Startle magnitude | Increase < Decrease | Increase > Decrease | |
| Heart rate deceleration | Increase < Decrease | Increase > Decrease | |
Note. SCRs = skin conductance responses.
Method
Participants
Ten healthy right-handed adults (7 women, 3 men) with a mean age of 35.2 years (SD = 13.0) were recruited for the study. Participants were recruited from the community through advertisements and received compensation for their participation. All participants were screened for any history of neurological or psychiatric disease and provided informed consent in accordance with the Human Subjects Committee at the University of Iowa prior to their participation in this research.
Materials and Design
The stimuli used for this task included 112 color pictures selected from the International Affective Picture System (IAPS; Lang et al., 2005). Stimuli were presented on a PC computer screen 0.5 m in front of the participant using Presentation software. Sixteen of the pictures were neutral, 48 were pleasant, and 48 were unpleasant based on normative ratings. Pleasant and unpleasant pictures were matched as closely as possible on rated arousal. Each picture was presented for 8 s, with an interstimulus interval of 14 s. The acoustic startle probe was a 50-ms burst of white noise with an instantaneous rise time and a magnitude of 95 dB, presented binaurally through headphones. Startle probes were delivered during 75% of picture presentations and were evenly distributed across picture valence categories. To characterize changes in startle modulation that may occur over the course of picture viewing (e.g., Sutton et al., 1997), probes were delivered either at 4 or 7 s following picture onset. Picture trials were organized in two pseudorandomized orders that were counterbalanced for order of presentation, instruction type, and startle probe time. No more than three trials of the same emotion category, regulation instruction, or probe time were presented consecutively.
EMG activity from the orbicularis oculi was collected using two In Vivo Metrics (Healdsburg, CA) recording electrodes placed directly below the left eye using the placement recommended by Fridlund & Cacioppo (1986). Electrode impedances were less than 10,000 ohms. Raw signals were recorded using Biopac (Biopac Systems, Santa Barbara, CA) EMG150 amplifiers passing 30-500 Hz, with a gain multiplication of 5000, and a Biopac MP150 interface sampled all EMG data at a rate of 1000 Hz. The MP150 recorded the EMG signal, which was then half-wave rectified and integrated with a 10-ms time constant. Heart rate was measured using two electrocardiograph electrodes, with one placed on the right side of the neck and the other on the left side of the torso 2 cm below the rib cage. Skin conductance was measured using two Ag-AgCl electrodes placed on the thenar and hypothenar surfaces of the left palm. Autonomic signals were recorded at 500 Hz using a Biopac MP150 system including amplifiers for ECG and SCR collection.
Procedure
After providing informed consent to participate in the study, electrodes were placed on each participant. Prior to the first 48 picture trials (16 pleasant, 16 neutral, 16 unpleasant), participants were instructed to pay attention to each picture for the full time that it appeared on the screen and to ignore the noises heard over the headphones (passive viewing condition). For the remaining 64 picture trials (32 pleasant, 32 unpleasant), participants were instructed to either increase or decrease the emotional response elicited by each picture. Participants were told to use whatever regulation strategies they felt were most effective and that they should pay attention to each picture and continue increasing or decreasing their emotional response for the full duration of the trial. Before the experimental stimuli were presented, participants were provided with several practice stimuli and startle probes. Additional instructions were provided to participants who did not appear to understand the task or were unable to describe specific regulation strategies after completing the practice trials. The regulation instruction was indicated during the task by a one word instruction (“Increase” or “Decrease”) presented onscreen for 2 s immediately prior to each picture. A number of breaks were included throughout the task in order to minimize fatigue. At the end of the experiment, all electrodes were removed, and participants were asked to rate on a 5-point scale how difficult it was to regulate their emotions for each instruction type (1 = not difficult at all, 5 = extremely difficult) and to describe the specific strategies they used to increase and decrease their emotional responses. They were then asked to view the same set of pictures used in the startle task and provide ratings of valence and arousal for each picture using the Self-Assessment Manikin (SAM; Bradley & Lang, 1994). The SAM is a 9-point rating scale for both valence (1 = highly unpleasant, 9 = highly pleasant) and arousal (1 = low arousal, 9 = high arousal). After the ratings were completed, the participants were debriefed.
Data Reduction and Analysis
EMG responses to startle probes were reduced to eyeblink reflex magnitudes using the following procedure. Peak detection was performed on the integrated EMG response to each probe (between 0 and 150 ms post-noise burst). Eyeblink reflex magnitudes were calculated by subtracting the amount of integrated EMG activity at reflex onset from the peak amplitude (maximum activity between 0 and 150 ms after probe onset). Each individual startle response was viewed by an experimenter. EMG blink magnitudes are expressed in the standardized z-score metric (i.e., M = 0, SD = 1) using the overall mean and standard deviation from each participant across all startle responses. Skin conductance response magnitude was scored as the greatest change above 0.02 microSiemens occurring in a 1 – 4 s time window following picture onset. A log transformation (log [SCR + 1]) was then performed to normalize the SCR data. Heart rate response magnitude was calculated in 500 ms bins in a 0 – 8 s time window following stimulus onset relative to a 1 s baseline period immediately prior to stimulus onset.
The general approach for the analysis of psychophysiological data during passive picture viewing was a repeated measures analysis of variance (ANOVA) with picture category (pleasant, neutral, unpleasant) as a within-subjects factor. Effects of regulation instruction were examined using a 2 × 2 repeated measures ANOVA, with instruction type (increase, decrease) as within-subjects factors. In addition, analyses of eyeblink startle data included probe time (4 s, 7 s) as a within-subjects factor. The relationships between ratings of difficulty and psychophysiological variables were examined across instruction types and picture categories using Pearson's correlation coefficient. Planned polynomial contrasts were conducted to examine the pattern of responses across picture categories. Post-hoc pairwise comparisons were performed using the Bonferroni procedure. As recommended by Maxwell & Delaney (1990), all analyses on within-subjects variables used the mixed-model univariate ANOVA, as opposed to a multivariate approach, due to the relatively small sample size. The Greenhouse-Geisser epsilon correction procedure (Geisser & Greenhouse, 1959) was used in order to control for the inflated Type I error rate associated with the mixed-model univariate ANOVA when the sphericity assumption is not met (Vasey & Thayer, 1987). A measure of effect size (partial eta-squared) is included for each ANOVA.
Results
Stimulus Ratings
Table 2 presents means and standard deviations for ratings of affective valence and arousal. The ratings were analyzed with a one-way repeated measures ANOVA, with picture category (pleasant, neutral, unpleasant) as a within-subjects variable. Valence and arousal ratings of the pictures generally conformed to expectations based on the a priori classifications derived from normative data. A main effect of picture category for valence ratings (F(2,18) = 101.1, p < 0.001, partial eta-squared = 0.92) indicated that participants rated the pictures in the predicted direction, with higher ratings for pleasant compared to neutral (p < 0.001), neutral compared to unpleasant (p < 0.001), and pleasant compared to unpleasant pictures, (p < 0.001). There was similarly a main effect of picture category for the ratings of arousal (F(2,18) = 56.7, p < 0.001, partial eta-squared = 0.86), with unpleasant (p < 0.001) and pleasant (p < 0.001) pictures rated as more arousing than neutral pictures, and unpleasant pictures rated as more arousing than pleasant pictures (p < 0.001).
Table 2.
Picture ratings for valence and arousal, as a function of picture category
| Picture category |
|||
|---|---|---|---|
| Neutral | Pleasant | Unpleasant | |
| Valence ratings | 5.1 ± 0.2 | 6.4 ± 0.6 | 2.4 ± 0.7 |
| Arousal ratings | 1.5 ± 1.3 | 4.0 ± 1.7 | 5.9 ± 1.8 |
Note. Ratings are given on a 9-point scale, with higher numbers indicating greater pleasantness or arousal. Means and standard deviations are reported.
Psychophysiological Effects During Picture Viewing
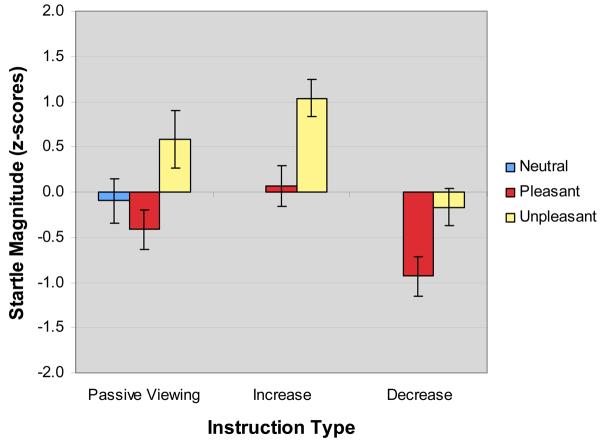
The standardized means and standard errors of the mean for startle magnitude across picture categories and instruction types are presented in Figure 1. A 3 Picture Category (pleasant, neutral, unpleasant) × 2 Probe Time (4 s, 7 s) analysis of startle response data revealed a main effect of picture category, F(2,18) = 6.0, p < 0.05, partial eta-squared = 0.40. Follow-up analyses revealed that startle responses were significantly larger for unpleasant (M = 0.59, SD = 0.79) compared to pleasant (M = −0.43, SD = 0.47) stimuli, p < 0.05. No significant difference was observed between responses to neutral (M = −0.10, SD = 0.43) compared to pleasant (p > 0.6) or unpleasant stimuli (p > 0.1). Additionally, a significant linear trend was observed: unpleasant > neutral > pleasant, F(1,9) = 9.8, p < 0.05, partial eta-squared = 0.52. There was no main effect of probe time (F(1,9) < 1, p > 0.8, partial eta-squared = 0.004) and no Picture Category × Probe Time interaction (F(2,18) < 1, p > 0.4, partial eta-squared = 0.08).
Fig. 1.
Z-transformed startle EMG responses across picture categories and instruction types. Means and standard errors are depicted.
Table 3 presents the mean log transformed SCRs and standard errors of the mean. An analysis of SCRs revealed a main effect of picture category (F(2,18) = 9.2, p < 0.01, partial eta-squared = 0.51), with larger responses to pleasant versus neutral (p < 0.05), and unpleasant versus neutral picture trials (p < 0.05). There was no difference observed between responses to pleasant and unpleasant stimuli (p = 1.00).
Table 3.
Skin conductance responses and heart rate changes, as a function of picture category and instruction type
| Neutral |
Pleasant |
Unpleasant |
|||||
|---|---|---|---|---|---|---|---|
| Measure | Passive Viewing |
Passive Viewing |
Increase | Decrease | Passive Viewing |
Increase | Decrease |
| SCRs (log [SCR + 1]) | 0.07 ± 0.02 | 0.16 ± 0.03 | 0.12 ± 0.03 | 0.09 ± 0.02 | 0.15 ± 0.02 | 0.10 ± 0.02 | 0.09 ± 0.03 |
| Heart rate change (BPM) | 0.26 ± 0.46 | −1.36 ± 0.46 | −1.09 ± 0.34 | −1.29 ± 0.49 | −2.05 ± 0.57 | −0.78 ± 0.62 | −2.86 ± 0.66 |
Note. Means and standard errors are reported. SCRs = skin conductance responses; BPM = beats per minute.
Means and standard errors of the mean for heart rate change are shown in Table 3. An analysis of heart rate during passive picture viewing revealed a main effect of picture category (F(2,18) = 5.5, p = 0.01, partial eta-squared = 0.38). Participants exhibited greater heart rate deceleration for pleasant compared to neutral trials (p < 0.05), and unpleasant compared to neutral trials (p < 0.05), but no difference was found between pleasant and unpleasant picture trials (p = 1.00).
Psychophysiological Effects During Emotion Regulation
A 2 Picture Category (pleasant, unpleasant) × 2 Probe Time (4 s, 7 s) × 2 Instruction Type (increase, decrease) analysis of startle response data revealed main effects of picture category (F(1,9) = 16.5, p < 0.01, partial eta-squared = 0.65) and instruction type (F(1,9) = 43.5, p < 0.001, partial eta-squared = 0.83). Follow-up contrasts showed that across regulation conditions, responses were larger to unpleasant (M = 0.44, SD = 0.34) compared to pleasant (M = −0.44, SD = 0.35) pictures, (p < 0.01). Across picture categories, startle responses were significantly lower in the decrease condition (M = −0.55, SD = 0.26) compared to the increase condition (M = 0.55, SD = 0.27) (p < 0.001). No main effect of probe time was observed, F(1,9) < 1, p > 0.4, partial eta-squared = 0.08. There were no significant interactions of Picture Category × Time (F(1,9) = 3.4, p = 0.1, partial eta-squared = 0.27), Picture Category × Instruction Type (F(1,9) < 1, p > 0.5, partial eta-squared = 0.05), or Time × Instruction Type (F(1,9) < 1, p > 0.8, partial eta-squared = 0.01).
A 2 Picture Category (pleasant, unpleasant) × 2 Instruction Type (increase, decrease) analysis of SCRs revealed a main effect of instruction type (F(1,9) = 10.4, p < 0.05, partial eta-squared = 0.54) but no main effect of picture category (F(1,9) < 1, p > 0.4, partial eta-squared = 0.07) or Picture Category × Instruction Type interaction (F(1,9) = 1.3, p > 0.2, partial eta-squared = 0.13). Follow-up analyses indicated that, across picture categories, responses were lower in the decrease condition compared to the increase condition (p < 0.05).
Analysis of heart rate during emotion regulation revealed a significant main effect of instruction type (F(2,18) = 4.3, p < 0.05, partial eta-squared = 0.32), with greater deceleration in the decrease compared to the increase condition (p < 0.05). There was no main effect of picture category (F(1,9) = 3.3, p > 0.1, partial eta-squared = 0.27), though there was a trend toward significance for an Picture Category × Instruction Type interaction (F(1,9) = 3.9, p = 0.08, partial eta-squared = 0.30).
Difficulty Ratings
Ratings of difficulty in up- and down-regulating emotion are reported in Table 4. A comparison of difficulty ratings was carried out with a 2 Picture Category (pleasant, unpleasant) × 2 Instruction Type (increase, decrease) repeated-measures ANOVA. A main effect of picture category was observed, F(1,9) = 6.9, p < 0.05, partial eta-squared = 0.43. On a 5-point scale (with higher ratings indicating greater difficulty), participants rated the regulation of negative emotion (M = 3.10, SD = 0.57) as more difficult than regulating positive emotion (M = 2.45, SD = 0.64) (p < 0.05). There was no main effect of instruction type and no Picture Category × Instruction Type interaction.
Table 4.
Ratings of difficulty in regulating emotion, as a function of picture category and instruction type
| Picture category | ||
|---|---|---|
| Instruction type | Pleasant | Unpleasant |
| Increase | 2.6 ± 1.1 | 2.8 ± 1.0 |
| Decrease | 2.3 ± 1.2 | 3.4 ± 1.1 |
Note. Ratings are given on a 5-point scale, with higher numbers indicating greater difficulty. Means and standard deviations are reported.
Correlational analyses were conducted to examine the relationship between ratings of perceived difficulty and each psychophysiological variable. The rated difficulty of down-regulating positive emotion was found to be significantly associated with startle magnitude when attempting to decrease positive emotion, r = .78, p < 0.01. No other significant correlations between difficulty ratings and psychophysiological variables were found.
Regulation Strategies
Participant reports of strategies adopted to increase and decrease their emotional responses are described below. Although some participants reported using more than one strategy to up- or down-regulate their responses, most of the reports indicate that a few principal strategies were regularly employed. To increase positive or negative emotion, 80% of participants reported increasing the self-relevance of the stimuli (e.g., imagining that the depicted scene involved either themselves or a loved one), while 40% reported increasing attention to salient features of the stimuli (e.g., blood in pictures depicting mutilations). To decrease positive or negative emotion, 80% of participants reported decreasing attention to salient features of the stimuli, 20% reported attempting to objectify the stimuli (e.g., imagining that the scene depicted was not real), and 20% reported decreasing self-relevance.
Discussion
The primary aim of this study was to assess the relative contributions of valence and arousal to changes in psychophysiological reactivity during emotion regulation. Consistent with previous work (Dillon & LaBar, 2005), instructions to decrease (down-regulate) emotional responses resulted in reduced startle magnitude for both positive and negative emotion, compared to instructions to increase (up-regulate) emotion. Additionally, down-regulating both positive and negative emotion led to significantly reduced SCRs and heart rate, compared to up-regulating emotion. The finding that ratings of difficulty were positively associated with startle responses when down-regulating positive emotion suggests that difficulty may have limited the reduction of positive emotion to some degree. However, the startle modulation effects described above, as well as the lack of such an association for any other condition, suggest that perceived difficulty likely had limited influence on the pattern of emotion regulation effects reported here. These findings suggest that the physiological effects of voluntary emotion regulation are largely similar for both positive and negative emotion. In particular, these results indicate that arousal may contribute more than valence to changes in somatic and autonomic reactivity during attempts to regulate emotion. Across regulation instructions, however, it was found that startle responses were larger overall for unpleasant compared to pleasant stimuli, suggesting that physiological changes that occur during emotion regulation may, to some degree, reflect the modulation of valence. However, for positive versus negative emotion regulation, we found more similarities than differences in the patterns of physiological changes, which raises the possibility that both forms of regulation may share a common neural basis. Consistent with this idea, a number of functional neuroimaging studies have documented that activation in areas of the prefrontal cortex during positive (Beauregard et al., 2001) or negative emotion regulation (Ochsner et al., 2002, 2004; Phan et al., 2005) is coupled with changes in amygdala activation. A more recent study (Kim & Hamann, 2007) provides further evidence that up- and down-regulating emotion is associated with increases and decreases in amygdala activation, respectively, for both positive and negative emotion. The authors also reported greater modulation of amygdala activity during the regulation of positive emotion, possibly reflecting greater difficulty in regulating responses to negative stimuli (which subjects rated higher on arousal) or greater malleability of positive emotional responses. Together, these findings suggest that the amygdala may serve as a key neural substrate for the voluntary regulation of positive and negative emotion and also highlight potential influences of both valence- and arousal-related processes in emotion regulation.
Certain limitations of the current investigation should be noted. First, interpretation of the observed regulation effects is complicated by the lack of a baseline condition during the emotion regulation phase of the study. In particular, it is unclear to what extent the regulation data were impacted by habituation of the startle reflex over the course of the testing session. Due to this important limitation of the design, additional work will be needed to clarify the physiological effects of up- and down-regulating emotion. Second, startle responses were not significantly modulated by positive or negative emotion during passive picture viewing. Such findings likely reflect in part the small size of the sample, as well as limitations of the stimuli. In particular, the lack of pleasure-attenuated startle is not an uncommon finding, and further development of potent appetitive stimuli will likely help to advance the study of positive emotion regulation, which to date has been encumbered by the challenge of securing really effective positive stimuli in a laboratory setting. Third, the lack of a significant effect of startle probe time in the present study suggests that affective modulation effects may remain relatively stable during processing of affective stimuli. Additional work will be needed to further characterize the temporal dynamics of emotion regulation effects across physiological systems and to identify factors (e.g., regulation strategy) that may influence the time course of emotional responding.
There is evidence (e.g., Gross, 1998) that the psychophysiological effects of voluntary emotion regulation are determined in part by the regulation strategy adopted (e.g., suppression, cognitive reappraisal). Due to the small sample size in this study, the physiological effects of different regulation approaches were not examined. With regard to heart rate, it was observed that attempts to decrease emotion resulted in greater heart rate deceleration as compared to increasing emotion. When attempting to down-regulate emotion, most participants reported reducing attention to the more salient aspects of the stimuli, which appears to be incongruent with the commonly reported association between heart rate deceleration and heightened attention and sensory intake. However, it is conceivable that increased attention toward less salient features contributed to this reduction in heart rate. Additionally, the finding of a trend toward a significant interaction between condition type and picture category for heart rate raises the possibility that with a larger sample, valence-specific emotion regulation effects may become evident. While the results of the present study suggest that regulating positive and negative emotion may have similar physiological effects, further work is needed to clarify the effectiveness of different approaches to increase or decrease emotion. Finally, it is important to note that because no online measure of the experience of emotion was obtained in the current study, the effectiveness of the regulation instruction in eliciting changes in emotional experience cannot be directly verified.
Conclusions
The results from this study provide further evidence that conscious and deliberate attempts to regulate emotion are associated with reliable changes in autonomic and somatic reflex responsiveness. The observed emotion regulation effects contribute to a growing literature on the multitude of physiological effects of regulating positive and negative emotion. As noted above, functional neuroimaging studies have implicated multiple regions of the prefrontal cortex in the regulation of positive and negative emotion. It has been observed that dysfunction in such regions can lead to disturbances in emotional regulation (i.e., hypo- or hyper-emotionality) that contribute significantly to impaired real-world competencies (Anderson et al., 1999, 2006; Koenigs & Tranel, 2007; Koenigs et al., 2007) and may underlie different forms of developmental psychopathology, including autism, ADHD, and conduct disorder (Bauer & Hesselbrock, 2001; Castellanos & Tannock, 2002; Machado & Bachevalier, 2003). Further work in both normal and clinical populations could help to clarify the basic neural mechanisms by which positive and negative emotions are regulated, and lead to a better understanding of the behavioral consequences of dysfunction in this circuitry and their possible treatment.
Acknowledgements
This work was supported by National Institute of Neurological Disorders and Stroke Program Project Grant P01 NS19632 and NIDA R01 DA022549. The authors would like to thank two anonymous reviewers for their helpful comments on this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson SW, Barrash J, Bechara A, Tranel D. Impairments of emotion and real-world complex behavior following childhood- or adult-onset damage to ventromedial prefrontal cortex. J. Int. Neuropsychol. Soc. 2006;12:224–235. doi: 10.1017/S1355617706060346. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat. Neurosci. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. CSD/BEM localization of P300 sources in adolescents “at-risk”: evidence of frontal cortex dysfunction in conduct disorder. Biol. Psychiatry. 2001;50:600–608. doi: 10.1016/s0006-3223(01)01066-6. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J. Neurosci. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: defensive and appetitive reactions in picture processing. Emot. 2001;1:276–298. [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: the self-assessment manikin and the semantic differential. J. Behav. Ther. Exp. Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Affective reactions to acoustic stimuli. Psychophysiology. 2000;37:204–215. [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat. Rev. Neurosci. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affective style, psychopathology, and resilience: brain mechanisms and plasticity. Am. Psychol. 2000;55:1196–1214. doi: 10.1037//0003-066x.55.11.1196. [DOI] [PubMed] [Google Scholar]
- Davis M, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: lesion and stimulation studies. J. Neurosci. 1982;2:791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, Filion DL. The electrodermal system. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. 3rd edn. Cambridge University Press; New York: 2007. pp. 200–223. [Google Scholar]
- Dillon DG, Labar KS. Startle modulation during conscious emotion regulation is arousal-dependent. Behav. Neurosci. 2005;119:1118–1124. doi: 10.1037/0735-7044.119.4.1118. [DOI] [PubMed] [Google Scholar]
- Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S. Regulation of emotional responses elicited by threat-related stimuli. Hum. Brain Mapp. 2007;28:409–423. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlund AJ, Cacioppo JT. Guidelines for human electromyographic research. Psychophysiology. 1986;23:567–589. doi: 10.1111/j.1469-8986.1986.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Geisser S, Greenhouse SW. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. J. Pers. Soc. Psychol. 1998;74:224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Emotional suppression: physiology, self-report, and expressive behavior. J. Pers. Soc. Psychol. 1993;64:970–986. doi: 10.1037//0022-3514.64.6.970. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Hiding feelings: the acute effects of inhibiting negative and positive emotion. J. Abnorm. Psychol. 1997;106:95–103. doi: 10.1037//0021-843x.106.1.95. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Malmstadt JR, Larson CL, Davidson RJ. Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology. 2000;37:515–522. [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. J. Cogn. Neurosci. 2007;19:776–798. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Koch M. The neurobiology of startle. Prog. Neurobiol. 1999;59:107–128. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Tranel D. Irrational economic decision-making after ventromedial prefrontal damage: evidence from the Ultimatum Game. J. Neurosci. 2007;27:951–956. doi: 10.1523/JNEUROSCI.4606-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Young L, Adolphs R, Tranel D, Cushman F, Hauser M, Damasio A. Damage to the prefrontal cortex increases utilitarian moral judgements. Nature. 2007;446:908–911. doi: 10.1038/nature05631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ. The emotion probe: studies of motivation and attention. Am. Psychol. 1995;50:371–385. doi: 10.1037//0003-066x.50.5.372. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-6. University of Florida; Gainesville, FL: 2005. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychol. Rev. 1990;97:377–395. [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. Non-human primate models of childhood psychopathology: the promise and the limitations. J. Child Psychol. Psychiatry. 2003;44:64–87. doi: 10.1111/1469-7610.00103. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Bunge SA, Gross JJ. Automatic emotion regulation. Soc. Pers. Psychol. Compass. 2007;1:146–167. [Google Scholar]
- Maxwell SE, Delaney HD. Designing Experiments and Analyzing Data: A Model Comparisons Perspective. Brooks/Cole; Pacific Grove, CA: 1990. [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an fMRI study of the cognitive regulation of emotion. J. Cogn. Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn. Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol. Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Sutton SK, Davidson RJ, Donzella B, Irwin W, Dottl DA. Manipulating affective state using extended picture presentations. Psychophysiology. 1997;34:217–226. doi: 10.1111/j.1469-8986.1997.tb02135.x. [DOI] [PubMed] [Google Scholar]
- Vasey MW, Thayer JF. The continuing problem of false positives in repeated measures ANOVA in psychophysiology: a multivariate solution. Psychophysiology. 1987;24:479–486. doi: 10.1111/j.1469-8986.1987.tb00324.x. [DOI] [PubMed] [Google Scholar]
- Vrana SR, Lang PJ. Fear imagery and the startle-probe reflex. J. Abnorm. Psychol. 1990;99:189–197. doi: 10.1037//0021-843x.99.2.189. [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Frankland PW. The acoustic startle reflex: neurons and connections. Brain Res. Brain Res. Rev. 1995;21:301–314. doi: 10.1016/0165-0173(96)00004-5. [DOI] [PubMed] [Google Scholar]