Abstract
Background
Human infections with Plasmodium knowlesi, a simian malaria parasite, are more common than previously thought. They have been detected by molecular detection methods in various countries in Southeast Asia, where they were initially diagnosed by microscopy mainly as Plasmodium malariae and at times, as Plasmodium falciparum. There is a paucity of information on the morphology of P. knowlesi parasites and proportion of each erythrocytic stage in naturally acquired human infections. Therefore, detailed descriptions of the morphological characteristics and differential counts of the erythrocytic stages of P. knowlesi parasites in human infections were made, photographs were taken, and morphological features were compared with those of P. malariae and P. falciparum.
Methods
Thick and thin blood films were made prior to administration of anti-malarial treatment in patients who were subsequently confirmed as having single species knowlesi infections by PCR assays. Giemsa-stained blood films, prepared from 10 randomly selected patients with a parasitaemia ranging from 610 to 236,000 parasites per μl blood, were examined.
Results
The P. knowlesi infection was highly synchronous in only one patient, where 97% of the parasites were at the late trophozoite stage. Early, late and mature trophozoites and schizonts were observed in films from all patients except three; where schizonts and early trophozoites were absent in two and one patient, respectively. Gametocytes were observed in four patients, comprising only between 1.2 to 2.8% of infected erythrocytes. The early trophozoites of P. knowlesi morphologically resemble those of P. falciparum. The late and mature trophozoites, schizonts and gametocytes appear very similar to those of P. malariae. Careful examinations revealed that some minor morphological differences existed between P. knowlesi and P. malariae. These include trophozoites of knowlesi with double chromatin dots and at times with two or three parasites per erythrocyte and mature schizonts of P. knowlesi having 16 merozoites, compared with 12 for P. malariae.
Conclusion
Plasmodium knowlesi infections in humans are not highly synchronous. The morphological resemblance of early trophozoites of P. knowlesi to P. falciparum and later erythrocytic stages to P. malariae makes it extremely difficult to identify P. knowlesi infections by microscopy alone.
Background
Plasmodium knowlesi, a malaria parasite species commonly found in long-tailed and pig-tailed macaques (Macaca fascicularis and Macaca nemestrina, respectively) is the only malaria parasite of primates with a 24-hour erythrocytic cycle [1,2]. Humans were shown to be susceptible to P. knowlesi by blood passage soon after the parasite was first isolated in 1932 [2] and until recently, naturally acquired human infections with P. knowlesi were thought to be extremely rare. However, following a number of reports of human knowlesi malaria infections detected by molecular methods in various countries in Southeast Asia [3-9], P. knowlesi is now recognized as the fifth species of Plasmodium infecting humans [10].
Malaria diagnosis relies heavily on examination of stained blood films by microscopy, leading to parasite detection, enumeration and identification of Plasmodium species for rational decisions on appropriate patient treatment and management. The parasite species is identified by morphological characteristics and for the diagnosis of human malaria, detailed morphological descriptions for Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae and Plasmodium ovale are given in medical text books and malaria diagnostic reference literature [11,12]. Morphological descriptions of P. knowlesi in the parasitology literature are largely based on experimental infections of rhesus macaques, since P. knowlesi causes a high parasitaemia in these hosts, as opposed to low parasitaemia in its natural macaque hosts [1,13,14]. There are no detailed descriptions of the morphology of P. knowlesi in naturally-acquired human infections other than the description by Singh et al [3] in which it was reported that the early trophozoites resemble P. falciparum while the other stages are similar to those of P. malariae. These morphological similarities between P. knowlesi, P. malariae and P. falciparum have been noted previously by other workers but without accompanying detailed accounts with coloured photographs [1,15,16]. It remains unclear whether P. knowlesi parasite stages in human erythrocytes are truly indistinguishable from P. falciparum and P. malariae infections by microscopy. Therefore, the morphology of the different blood stages of P. knowlesi parasites observed in human erythrocytes from knowlesi malaria patients is described, together with quantitative estimates of the different erythrocytic stages, and compared with those of the other malaria parasites causing human disease.
Methods
Patient details and collection of blood films
Thick and thin blood films from ten patients admitted to Kapit hospital and subsequently confirmed by nested PCR assays [3] as having single P. knowlesi infections were randomly selected from films that had been prepared from 83 knowlesi malaria patients admitted to Kapit Hospital [4]. These patients were aged between 14 to 65 years, and 6 were males (patients numbered KH364, KH370, KH399, KH412, KH421, KH433) and 4 were females (KH328, KH369, KH422, KH431). Blood films were prepared at the time of admission prior to commencement of anti-malarial treatment. Informed verbal consent was obtained from each patient before blood samples were taken.
Staining of thick and thin blood films
Thin blood films were fixed with absolute methanol (BDH, England) for 10 seconds and were allowed to dry at room temperature before staining. Thick and thin blood films were stained with 3% and 10% Giemsa (BDH, England) respectively, in Gurr® buffered water, pH 7.2 (BDH, England) for 30 minutes, as described previously [11]. All thick and thin blood films were examined under the microscope at a magnification of × 1,000 with immersion oil.
Parasite counts and determination of differential parasitaemia
The parasitaemia of each patient in the study was calculated from thick blood films by counting the number of parasitized red blood cells per white blood cells in over 100 fields and calculated using the actual white blood cell count of each patient. The percentage of each of the parasite developmental stages was determined based on the number of early trophozoites, late and mature trophozoites, schizonts, and gametocytes in thin blood films. A total of 500 parasitized erythrocytes were counted for each of the 10 patients except for patients KH328 and KH422 where 50 parasitized erythrocytes were counted due to the low parasitaemia. The morphology of each of P. knowlesi parasite developmental stages was scored according to the descriptions by Garnham [1] and Coatney et al [16] as follows: early trophozoite – parasite appears as ring of cytoplasm with chromatin dot and no malaria pigment; late and mature trophozoites – parasites with denser cytoplasm, undivided nuclear chromatin, with or without malaria pigment; schizont – parasite with multiple masses of nuclear chromatin and clumps of dark brown pigment; gametocytes – parasites appear to fill most of the host erythrocyte with single large chromatin mass and scattered prominent pigment. In addition, other characteristics such as multiple infections of single erythrocytes with early and mature trophozoites, the number of chromatin dots in ring-form stages and descriptions on the abundance and location of malaria pigment were recorded. The sizes of the parasites were measured using a measuring eyepiece graticule at 1,000× magnification. The sizes of infected erythrocytes were measured relative to surrounding uninfected erythrocytes.
Results
Differential counts and morphological characteristics of P. knowlesi parasites
Thick and thin blood films were examined from ten patients with P. knowlesi, with a parasitaemia between 610 to 236,000 parasites per μl blood (Table 1). All of the developmental stages of the malaria parasite life cycle expected in the peripheral blood were observed in Giemsa-stained thin blood films and are presented (Figures 1, 2, 3, 4 and 5). The infections in all 10 patients were asynchronous, except for one patient (KH433) who had 97% of parasites in the late trophozoite stage without malaria pigment at the time of admission (Table 1; Figure 1g, i, q; Figure 5).
Table 1.
Differential parasitaemia in 10 patients with single infections of P. knowlesi.
| Percentages (%) | |||||
| Patient code | Parasitaemia (parasites per μl blood) | Early trophozoites | Late/Mature trophozoites | Schizonts | Gametocytes |
| KH433 | 7,708 | 2.0 | 97.0 | 1.0 | 0 |
| KH421 | 10,290 | 77.0 | 17.0 | 6.0 | 0 |
| KH370 | 1,908 | 72.1 | 26.1 | 0 | 1.8 |
| KH328 | 610 | 66.7 | 33.3 | 0 | 0 |
| KH369 | 9,751 | 34.4 | 20.9 | 43.2 | 1.5 |
| KH399 | 9,700 | 31.0 | 65.0 | 4.0 | 0 |
| KH412 | 2,752 | 29.0 | 67.0 | 4.0 | 0 |
| KH431 | 1,155 | 25.6 | 61.7 | 11.5 | 1.2 |
| KH364 | *236,000 | 4.4 | 82.1 | 10.7 | 2.8 |
| KH422 | 920 | 0 | 76.0 | 24.0 | 0 |
*based on examination of thin blood film
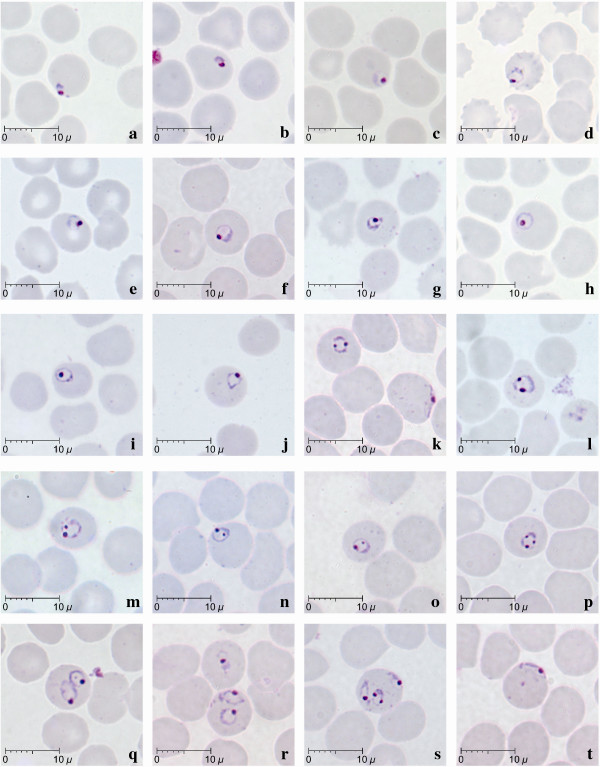
Figure 1.
Early trophozoites of P. knowlesi parasites in human infections. Giemsa-stained thin blood films from patients KH369-a,c,f,k,m,n,o,p,r,s,t; KH370-d,h; KH399-b; KH412-l, KH421-e,j; KH433-g,i,q.
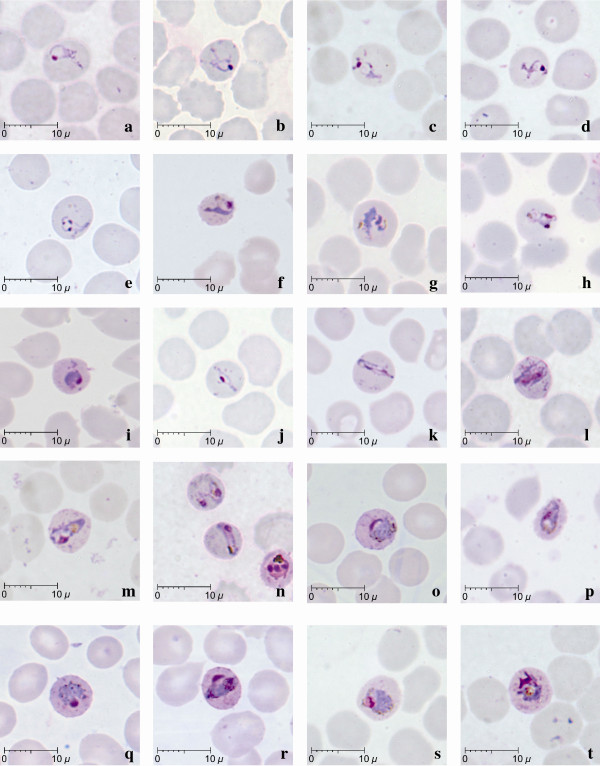
Figure 2.
Late (a-l) and mature (m-t) trophozoites of P. knowlesi parasites in human infections. Giemsa-stained thin blood films from patients KH364-n; KH369-a,g,l,s; KH370-b,j; KH399-h,p; KH412-c,m,t; KH421-f,i; KH422-o,q,r; KH433-d,e; KH431-k.
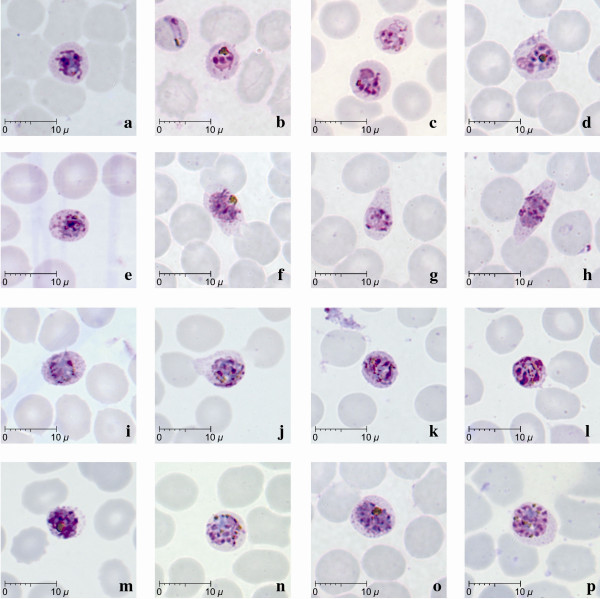
Figure 3.
Schizonts of P. knowlesi parasites in human infections. Giemsa-stained thin blood films from patients KH364-b; KH369-c,d,f,g,h,n,o,p; KH412-a; KH421-j,m; KH422-e,i; KH431-k,l.
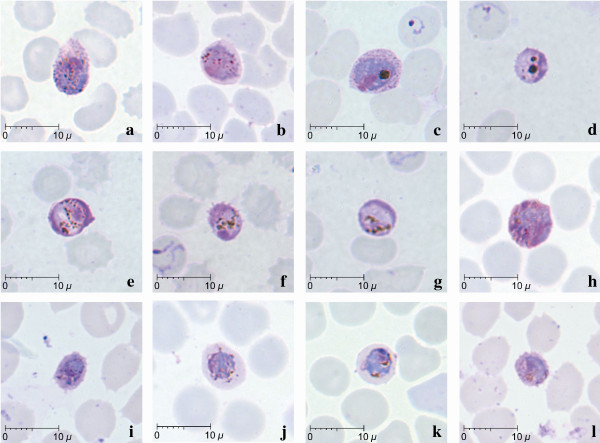
Figure 4.
Gametocytes of P. knowlesi parasites in human infections. Giemsa-stained thin blood films from patients KH370-a,h; KH369-b,c,j,k; KH364-d,e,f,g; KH431-i,l.
Figure 5.
Giemsa-stained thick blood film from patient KH433 showing late trophozoites of P. knowlesi.
Early trophozoites of P. knowlesi were seen in all patients examined except one (KH422). They were characterized by the appearance of a ring-like cytoplasm enclosing a vacuole with a dot of round nuclear chromatin projecting from the cytoplasm (Figure 1c–g). Some early trophozoites with small and non-vacuolated cytoplasm appeared as a mass of bluish stained bodies measuring between 1.5 to 2 μm (Figure 1, a–b). The ring form trophozoite of P. knowlesi measured approximately one third to half the diameter (2.5 to 4 μm) of the infected erythrocyte (Figure 1, c–s). The cytoplasm of the ring form trophozoite was slightly thicker especially on the side opposite the nuclear chromatin dot (Figure 1, c–g). A single prominent nuclear chromatin dot (Figure 1, a–j, ) was common (62.5 to 100% of ring forms in all nine patients with early trophozoites; Table 2) though double chromatin dots (Figure 1, k–o) were also frequently observed (7.8 to 37.5% of ring forms in 8 of 9 patients with early trophozoites; Table 2). Double chromatin dots were mostly situated at opposing poles of the ring form (Figure 1, k–l) but occasionally seen to be closely together (Figure 1m). In addition, a single or accessory chromatin dot lying within the vacuole was occasionally seen (Figure 1,h, i, n, o). Early trophozoites with triple chromatin dots were rare and only seen in one of the patients (Table 2; Figure 1, p). Multiply-infected erythrocytes were observed in blood films from three of the 10 patients, of which only one (KH364) had a parasitaemia above 100,000 parasites/μl blood. Single erythrocytes containing more than three parasites were observed in two patients (Table 2; Figure 1, s), and doubly infected erythrocytes (Figure 1, q, r) was the more common form of multiple infections in three patients (Table 2). Appliqué forms, that resembled those of P. falciparum, were infrequently seen, being observed in only three patients (Figure 1, k, s, t; Table 2). In all erythrocytes containing early trophozoites of P. knowlesi, enlargement of erythrocytes and stippling were not observed.
Table 2.
Characteristics of P. knowlesi parasites in naturally acquired human infections.
| Malaria pigment (%) | *Chromatin dots (%) | **Number of parasites in single erythrocyte (%) | Band form trophozoite | Appliqué form | ||||||
| Patient code | Clumped | Scattered | Single | Double | Triple | 1 | 2 | 3 | ||
| KH369 | 81.5 | 18.5 | 71.6 | 25.8 | 2.6 | 92.4 | 6.8 | 0.8 | Yes | Yes |
| KH364 | 75.0 | 25.0 | 92.2 | 7.8 | 0 | 74.9 | 20.6 | 4.5 | Yes | Yes |
| KH370 | n | n | 88.9 | 11.1 | 0 | 100.0 | - | - | Yes | - |
| KH412 | 100.0 | 0 | 62.5 | 37.5 | 0 | 100.0 | - | - | Yes | Yes |
| KH433 | n | n | 85.7 | 14.3 | 0 | 100.0 | - | - | - | - |
| KH431 | 5.6 | 94.4 | 89.3 | 10.7 | 0 | 98.2 | 1.8 | - | Yes | - |
| KH421 | 16.7 | 83.3 | 81.3 | 18.7 | 0 | 100.0 | - | - | - | - |
| KH422 | 0 | 100 | 0 | 0 | 0 | 100.0 | - | - | Yes | - |
| KH328 | 0 | 100 | 85.7 | 14.3 | 0 | 100.0 | - | - | - | - |
| KH399 | 33.3 | 66.7 | 100.0 | 0 | 0 | 100.0 | - | - | - | - |
*based on early trophozoites or ring form stages, and these were absent in patient KH422.
**based on early and late trophozoite stages.
n = no pigment because no mature trophozoites were observed.
Late and mature trophozoites were seen in all 10 patients examined and were characterized by denser cytoplasm compared to the ring-like cytoplasm in early trophozoites (Figure 2, a–t). The cytoplasm of the late trophozoites of P. knowlesi appeared to be slightly amoeboid and irregular in shape, while vacuoles were maintained (Figure 2, a–h). Late trophozoites measured approximately 3 to 5 μm. The cytoplasm of the parasites that extended across the erythrocyte forming "band-like" trophozoites was seen in six of the 10 patients (Figure 2, g, h, k, l, r; Table 2). Nuclear chromatin dots of late trophozoites seemed to be slightly larger in size compared to those in the early trophozoites (Figure 2). There was very little or no obvious malaria pigment in the majority of the late trophozoites stages.
Mature trophozoites of P. knowlesi appeared slightly larger compared to the late trophozoite with more solid and dense cytoplasm measuring approximately 5 to 6 μm and the presence of malaria pigment. The nuclear chromatin mass was more conspicuous but remained undivided (Figure 2, l–t) and vacuoles were small or completely absent (Figure 2, m–t). Malaria pigment was observed either in the form of scattered, fine dark brown grains (18.5 to 100% of infected erythrocytes; Table 2, Figure 2, g, h,l, m, o, p, s), or clumps of dense golden brown granules (5.6 to 100% of infected erythrocytes; Table 2, Fig 2, n, q, r, t) in mature trophozoites. Infected erythrocytes were generally not enlarged and stippling such as more recent entomological Schüffner's dots was not observed. However, irregular and sparse dots that were unevenly distributed were noticed in some infected erythrocytes with mature trophozoites (Figure 2, l, n, r, t).
Young and mature schizonts were seen in eight of the 10 patients examined, though they were found to be less common in three of these patients (<5% of total infected erythrocytes, Table 1). Young schizonts, characterized by the appearance of between 2 to 5 divided nuclear chromatin masses and abundant pigment granules, were common, occurring in eight of the 10 patients (Figure 3, a–i). They occupied at least two thirds (5 to 6 μm) of the infected erythrocyte while mature schizonts appeared to occupy nearly the whole infected erythrocyte. A maximum number of 16 merozoites in mature schizonts was observed in the blood film from patient KH369 (Figure 3, p). The divided chromatin masses and merozoites in young and mature schizonts were either irregularly scattered (Figure 3, l, m, n) or in the form of a grape-like cluster (Figure 3, o, p). Malaria pigment in both young and mature schizonts appeared to be either aggregated into many small granules (Figure 3, i, j, k, l, n, p ) or aggregated into dense clumps of brownish black round mass (Figure 3, b, d, e, f, m, o). The position of pigment aggregates was variable. Infected erythrocytes with mature schizonts were not enlarged compared to uninfected erythrocytes. Distorted and fimbriated infected erythrocytes were observed rarely in the blood film from only one patient, KH369 (Figure 3, f, g, h). Fine irregular stippling was evident in most of the erythrocytes containing schizonts (Figure 3, a, e–j, o, p), where the parasites occupied approximately two thirds of the erythrocyte.
Gametocytes, comprising only between 1.2 to 2.8% of infected erythrocytes, were observed in four of the 10 patients examined (Figure 4; Table 2). Mature gametocytes were characterized by their spherical shape occupying most of the infected erythrocyte, chromatin was compact and the dark brown pigment was irregularly scattered (Figure 4, a–h). Young gametocytes occupied about two thirds of the infected erythrocytes and were difficult to differentiate from mature trophozoites (Figure 4 i–l). The cytoplasm of macrogametocytes appeared bluish with the dense pinkish chromatin situated at the periphery of the parasite (Figure 4 a–d). In contrast, the cytoplasm of microgametocytes stained a pinkish-purple hue with a variably positioned darker large chromatin mass (Figure 4 e–h). Pigment grains in both macro and microgametocytes were seen to be irregularly distributed (Figure 4 a, b, e–l), however, macrogametocytes with clumps of dense dark-brownish malaria pigment were also observed (Figure 4 c–d). The infected erythrocytes were generally not enlarged, though some gametocytes were seen to be slightly smaller than the uninfected erythrocytes (Figure 4 d, f, i, l).
Morphological comparison between P. knowlesi and P. malariae
The morphology of P. knowlesi parasites and the characteristic of the infected erythrocyte in human infections in the present study were compared with those of P. malariae as described in the literature [1,12,16] (Table 3).
Table 3.
Comparison between P. knowlesi and P. malariae parasites in human infections
| P. malariae* | P. knowlesi | |
| HOST ERYTHROCYTE | ||
| Size | Not enlarged | Not enlarged |
| Shape | Rounded, not distorted | Rounded, generally not distorted |
| Stippling | Ziemen's stippling under certain staining conditions | Irregular dots or stippling in some erythrocytes with mature trophozoites, schizonts and gametocytes |
| PARASITE | ||
| Early trophozoite (ring form) | Ring form with dense cytoplasm and single chromatin; sometimes with accessory dot | Ring forms are compact with dense cytoplasm; single or double chromatin and rarely triple chromatin; appliqué forms; multiple parasites in single erythrocyte |
| Late trophozoite | Regular and compact cytoplasm; appearance of pigment | Dense and thick cytoplasm; Cytoplasm slightly amoeboid and irregular; band forms; varying pigmentation |
| Mature trophozoite | Compact, rounded, heavily pigmented; band forms; not amoeboid | Compact and dense cytoplasm, rounded shape with dark brown pigment; band forms; not amoeboid |
| Schizont | Occupies whole erythrocyte, contains 6–12 merozoites, usually 8; merozoites clustered around dark-brown malaria pigment forming rosette pattern | Occupies whole erythrocyte; contains up to 16 merozoites; merozoites irregularly scattered or grape-like cluster; malaria pigment scattered or collects into a single mass |
| Gametocyte | Round, compact, occupies whole erythrocyte, scattered malaria pigment; early forms are very similar to mature trophozoites | Round, compact, fills whole erythrocyte, scattered or clumped malaria pigment; early forms are very similar to mature trophozoites |
Discussion
Plasmodium knowlesi was first isolated in 1931 in India from a long-tailed macaque imported from Singapore [2]. It was studied in a number of different primate species and was also shown to be infectious to humans via blood passage in 1932 [1,2]. The detailed morphology of P. knowlesi was described by Sinton and Mulligan [14], as observed in infections in rhesus monkeys. Here, the morphological descriptions of P. knowlesi malaria parasites from naturally acquired human infections, that had all been misdiagnosed by microscopy as P. malariae, are described.
Detailed observations of Giemsa-stained thin blood films from 10 patients with varying degree of parasite load, showed that human infections were generally asynchronous at the time of admission with all stages of the erythrocytic cycle of P. knowlesi parasites present in seven of the 10 patients. Only one patient (KH433) had a highly synchronous infection with 97% of infected erythrocytes containing late trophozoites.
Early trophozoites of P. knowlesi appeared as ring forms within the infected erythrocyte and possessed characteristics that resembled the early trophozoites of P. falciparum such as double chromatin dots, multiply-infected erythrocytes and appliqué forms. The resemblance of the early trophozoites of P. knowlesi to those of P. falciparum has also been noted previously in human P. knowlesi infections [3,15] and in experimental infections in rhesus monkeys [2,14]. Human infections with P. knowlesi can be easily mistaken for falciparum malaria when the infecting parasites are predominantly at the early trophozoite or ring form developmental stage. If the blood film had been prepared earlier from the patient with the highly synchronous infection, most of the trophozoites would have appeared as ring forms and the infection would have been diagnosed by microscopy as P. falciparum.
Late and mature trophozoites, schizonts and gametocytes of P. knowlesi in human infections were generally indistinguishable from those of P. malariae. There were no specific features of the cytoplasm, nucleus and pigment of the parasites or the infected erythrocytes that could easily distinguish P. knowlesi from P. malariae, other than the presence of very amoeboid cytoplasm. However, this was observed in only some late trophozoites of P. knowlesi. Moreover, 'band form' trophozoites, which are a characteristic feature for P. malariae parasites [1,16,17] were observed in more than half of the blood films examined.
In agreement with previous descriptions of the number of merozoites in mature P. knowlesi schizonts in infections of rhesus macaques [1,14,16], a maximum of 16 merozoites per mature schizont were observed in one of the human infections, which is higher than the 12 reported for a mature schizont of P. malariae [1,16,17]. In addition, no mature schizonts of P. knowlesi were observed with symmetrical arranged merozoites surrounding clumps of malaria pigments or "rosette pattern," which was often described as a characteristic of schizonts of P. malariae [1,16,17].
The infected erythrocytes for all stages were generally not enlarged, a feature that is shared with erythrocytic stages of P. falciparum and P. malariae. Distorted infected erythrocytes, which are a common feature in P. knowlesi infections of rhesus macaques [13,14], were rarely observed in human P. knowlesi infections in the present study. No stippling was apparent that resembled Schüffner's dots, which are observed in P. vivax infected erythrocytes. However, faint stippling that appeared as light irregular dots were evident in only some of the infected erythrocytes with mature trophozoite and schizont stages. This type of stippling in erythrocytes infected with P. knowlesi is referred to as 'Sinton and Mulligan's stippling' and was noted previously in infections in rhesus monkeys [1,14,16] and humans [18]. According to Sinton and Mulligan [14], who first described this, the stippling was only consistently observed in some infected erythrocytes of macaques, when the panoptic method of Green [19] was used for staining. Similarly in P. malariae infections, the infected erythrocytes are not enlarged or distorted and may display some form of irregular fine stippling in blood films with intense staining [1].
Morphologically, and depending on the predominant circulating parasite stage, it was not possible to identify robust morphological differences to distinguish between P. knowlesi and P. malariae or P. falciparum using routine microscopy methods for malaria diagnosis. This lack of distinguishing features accounts for P. knowlesi infections being overlooked, despite major differences in the pathogenesis of each of these different lineage parasites [4,20]. Laboratory misidentification of P. knowlesi as P. malariae by microscopy is largely unavoidable, although it is difficult to rationalize how a parasitaemia greater than 100,000 per μl blood, together with signs of severe malaria, could clinically be mistaken for a P. malariae infection, except that until recently, such infections would have been relatively rare and on a background of P. falciparum transmission. Until the application of DNA based technology, the definitive identification test for P. knowlesi parasites was to inject infected blood into rhesus monkey and observe for a fatal outcome [15]. Clearly this was, and is beyond the scope of all but a few international centres with this type of facility. Readily available molecular tools, most notably the P. knowlesi-specific PCR assay [3], provide a relatively available means to properly identify P. knowlesi [3,6-9]. Molecular methods are expensive, not available in most rural diagnostic laboratories and as such, are not currently used for routine malaria diagnosis [21]. Areas of Southeast Asia, where there is a suspicion that P. knowlesi infects humans, would benefit from a PCR screening exercise to estimate the relative proportion of P. knowlesi to P. malariae infections. This information would be valuable to prime local healthcare professionals to recognize severe knowlesi malaria in ill patients with a laboratory report of P. malariae.
Despite morphological similarities with P. malariae, there are other clinical and parasitological parameters such as presentation with one or more of the WHO clinical criteria for severe malaria and a parasitaemia greater than 5,000/μl blood, that, together with a history of time spent in the forest and forest fringe areas of Southeast Asia, should strongly implicate a P. knowlesi rather than a P. malariae infection [4,20]. Given the consequence of misdiagnosis and delayed treatment for this parasite with a relatively short erythrocytic cycle, every effort should be made to accurately diagnose knowlesi malaria in populations at risk.
Conclusion
The morphology of P. knowlesi parasites in human infections closely resembled those of P. falciparum in the early trophozoite stage and P. malariae in the later stages of the erythrocytic cycle. Although there were some minor morphological differences between the blood stages of P. knowlesi and P. malariae, it would be difficult to positively identify knowlesi malaria based on morphology alone. However, even in the absence of PCR facilities, severe symptoms, a parasitaemia >5,000/μl blood, with P. malariae parasite morphology and a recent history of time spent in the forest fringe areas of Southeast Asia should be enough to make a diagnosis of knowlesi malaria. In view of what is now known of the distribution and severe manifestation of knowlesi malaria, continued misdiagnosis of this important pathogen is no longer acceptable.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
KSL, BS and JCS wrote the paper, BS and JCS conceived the study, KSL stained and examined the slides by microscopy.
Acknowledgments
Acknowledgements
This study was supported by grants from The Wellcome Trust and Universiti Malaysia Sarawak. The funding bodies had no role in the study design, in the collection, analysis and interpretation of data, in the writing of the manuscript and in the decision to submit the manuscript for publication.
Contributor Information
Kim-Sung Lee, Email: kimsunglee@gmail.com.
Janet Cox-Singh, Email: coxsingh@gmail.com.
Balbir Singh, Email: bskhaira55@gmail.com.
References
- Garnham PCC. Malaria parasites and other haemosporidia. Oxford: Blackwell Scientific Publications; 1966. [Google Scholar]
- Knowles R, Das Gupta BM. A study of monkey-malaria and its experimental transmission to man. Indian Medical Gazette. 1932;67:301–320. [PMC free article] [PubMed] [Google Scholar]
- Singh B, Sung LK, Matusop A, Radhakrishnan A, Shamsul SS, Cox-Singh J, Thomas A, Conway D. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet. 2004;363:1017–1024. doi: 10.1016/S0140-6736(04)15836-4. [DOI] [PubMed] [Google Scholar]
- Cox-Singh J, Davis TM, Lee KS, Shamsul SS, Matusop A, Ratnam S, Rahman HA, Conway DJ, Singh B. Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin Infect Dis. 2008;46:165–171. doi: 10.1086/524888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongwutiwes S, Putaporntip C, Iwasaki T, Sata T, Kanbara H. Naturally acquired Plasmodium knowlesi malaria in human, Thailand. Emerg Infect Dis. 2004;10:2211–2213. doi: 10.3201/eid1012.040293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchavez J, Espino FE, Curameng P, Espina R, Bell D, Chiodini P, Nolder D, Sutherland C, Lee KS, Singh B. Human infections with Plasmodium knowlesi, the Philippines. Emerg Infect Dis. 2008;14:811–813. doi: 10.3201/eid1405.071407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng OT, Ooi EE, Lee CC, Jarrod LP, Ng LC, Wong PS, Tu TM, Loh JP, Leo YS. Naturally acquired human Plasmodium knowlesi infection, Singapore. Emerg Infect Dis. 2008;14:814–816. doi: 10.3201/eid1405.070863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vythilingam I, NoorAzian YM, Huat TC, Jiram AI, Yusri YM, Azahari AH, NorParina I, NoorRain A, Hakim L. Plasmodium knowlesi in humans, macaques and mosquitoes in peninsular Malaysia. Parasit Vectors. 2008;1:26. doi: 10.1186/1756-3305-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu HM, Li J, Zheng H. Human natural infection of Plasmodium knowlesi. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2006;24:70–71. [PubMed] [Google Scholar]
- White NJ. Plasmodium knowlesi: the fifth human malaria parasite. Clin Infect Dis. 2008;46:172–173. doi: 10.1086/524889. [DOI] [PubMed] [Google Scholar]
- Basic malaria microscopy. Part 1. Learner's Guide: World Health Organization – Geneva; 1991. [Google Scholar]
- Warrell DA, Gilles HM. Essential Malariology. 4. Arnold; London, UK; 2002. [Google Scholar]
- Singh J, Ray AP, Nair CP. Further observations on transmission experiments with Plasmodium knowlesi. Ind J Malariol. 1950;4:317–335. [PubMed] [Google Scholar]
- Sinton JA, Mulligan HW. A critical review of the literature relating to the identification of the malarial parasites recorded from monkeys of the families Cercopithecidae and Colobidae. Records of the Malaria Survey of India. 1933;3:381–443. [Google Scholar]
- Chin W, Contacos PG, Coatney GR, Kimball HR. A naturally acquired quotidian-type malaria in man transferable to monkeys. Science. 1965;149:865. doi: 10.1126/science.149.3686.865. [DOI] [PubMed] [Google Scholar]
- Coatney RG, Collins WE, Warren M, Contacos PG. The Primate Malarias. Washington: U.S. Government Printing Office; 1971. [Google Scholar]
- Sandosham AA. Malariology with special reference to Malaya. Singapore: University of Malaya Press; 1959. [Google Scholar]
- Fong YL, Cadigan FC, Coatney GR. A presumptive case of naturally occurring Plasmodium knowlesi malaria in man in Malaysia. Trans R Soc Trop Med Hyg. 1971;65:839–840. doi: 10.1016/0035-9203(71)90103-9. [DOI] [PubMed] [Google Scholar]
- Green RTB. A malarial parasite of Malayan monkeys and its development in anopheline mosquitoes. Trans R Soc Trop Med Hyg. 1932;24:455–477. doi: 10.1016/S0035-9203(32)90142-4. [DOI] [Google Scholar]
- Cox-Singh J, Singh B. Knowlesi malaria: newly emergent and of public health importance? Trends Parasitol. 2008;24:406–410. doi: 10.1016/j.pt.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B. Molecular methods for diagnosis and epidemiological studies of parasitic infections. Int J Parasitol. 1997;27:1135–1145. doi: 10.1016/S0020-7519(97)00111-2. [DOI] [PubMed] [Google Scholar]